Low-Cost Pollen and Allergy Symptoms Monitoring with Beenose Sampler and Livepollen App: The Case Study of the Metz City, France, During Spring 2023
Abstract
:1. Introduction
2. Materials and Methods
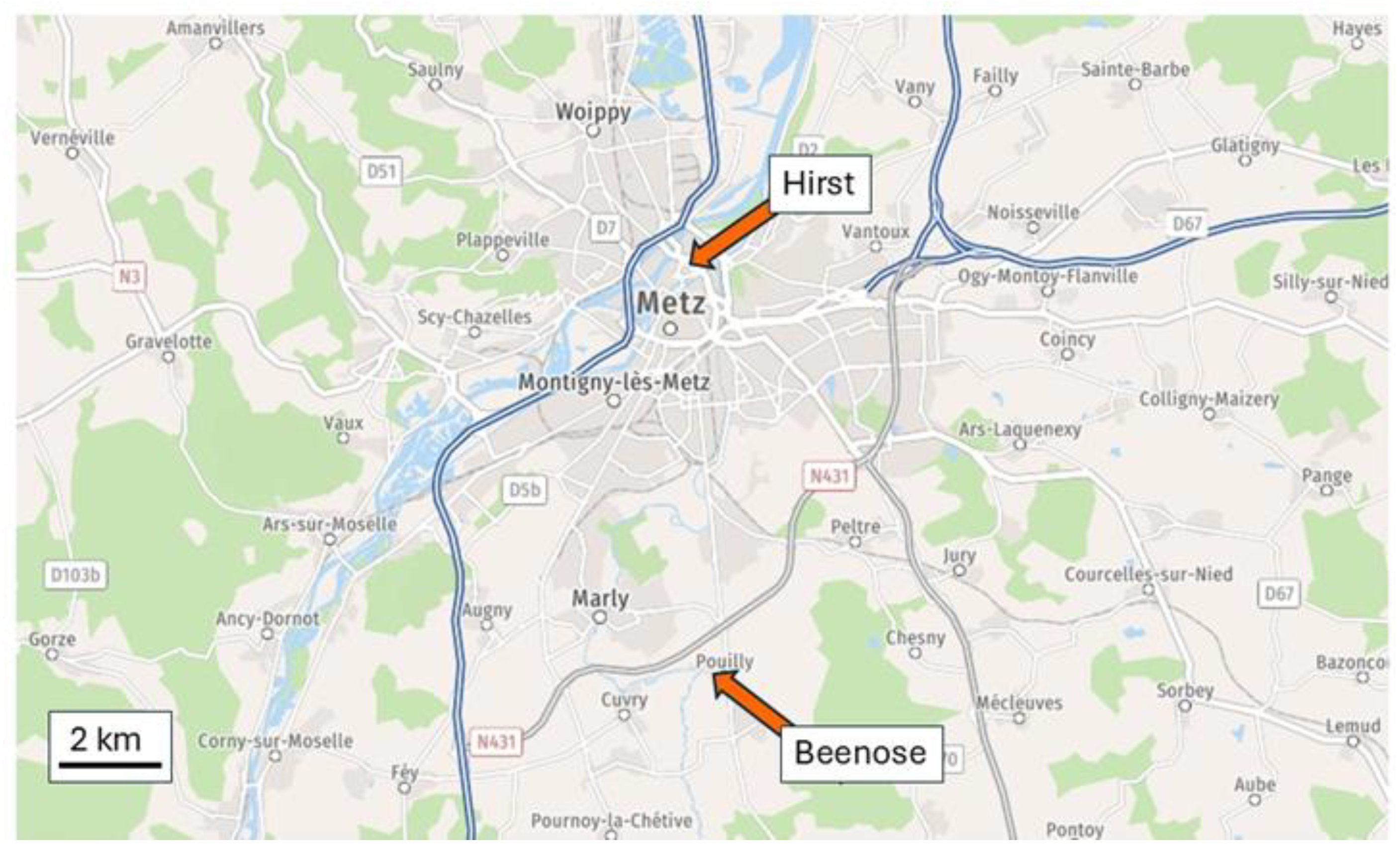
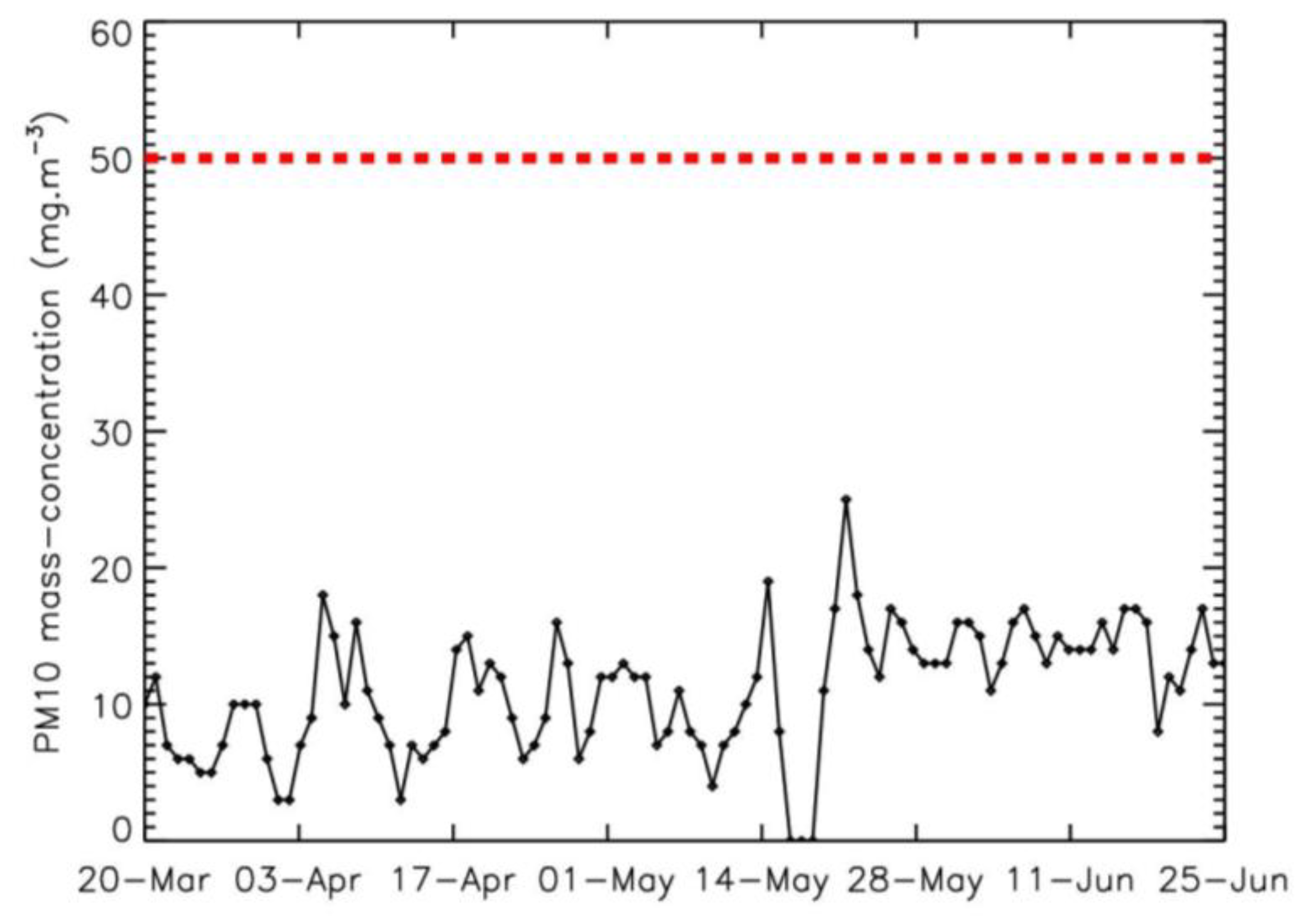
2.1. Measurements Conditions
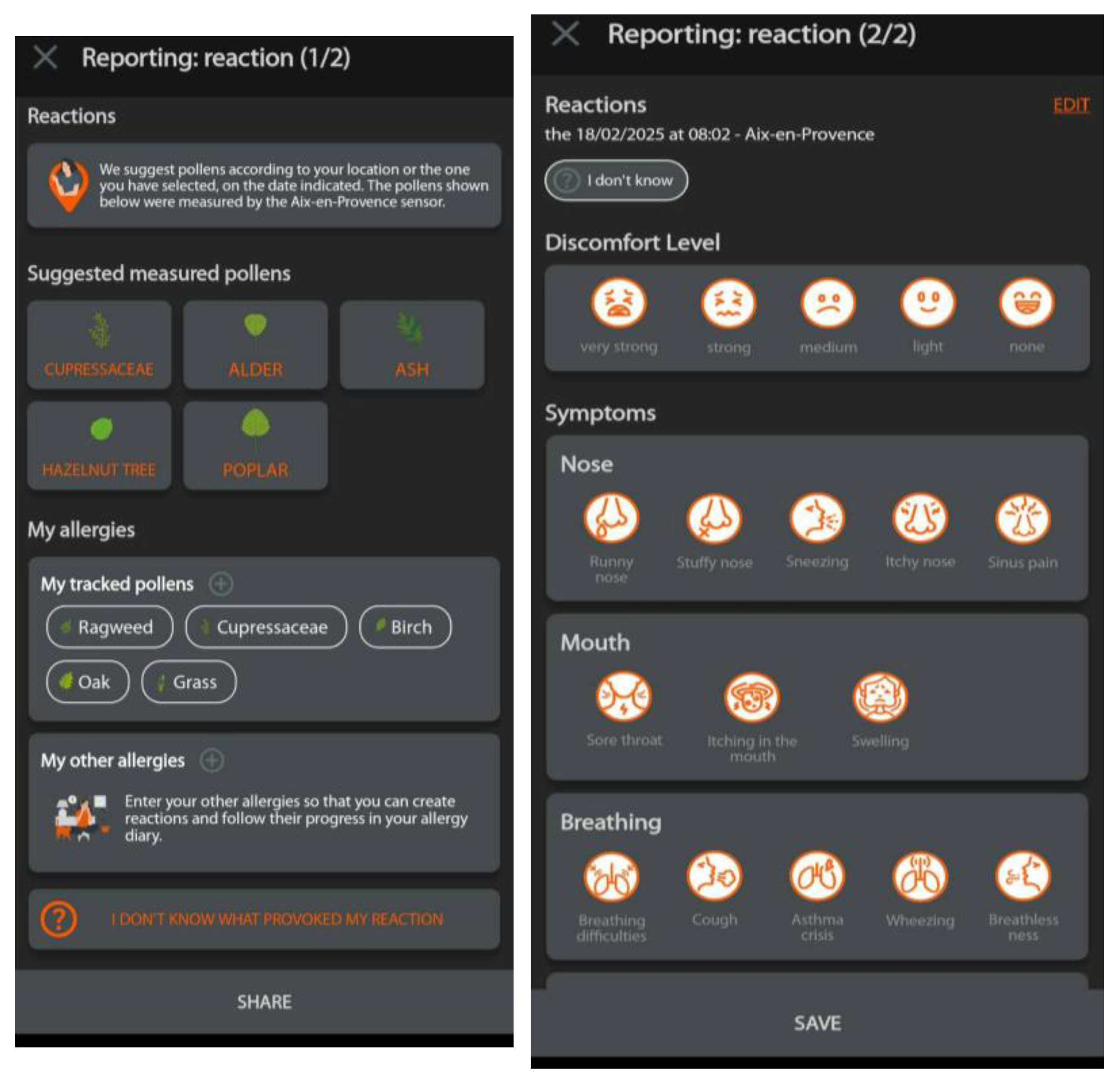
2.2. Origin of the Data
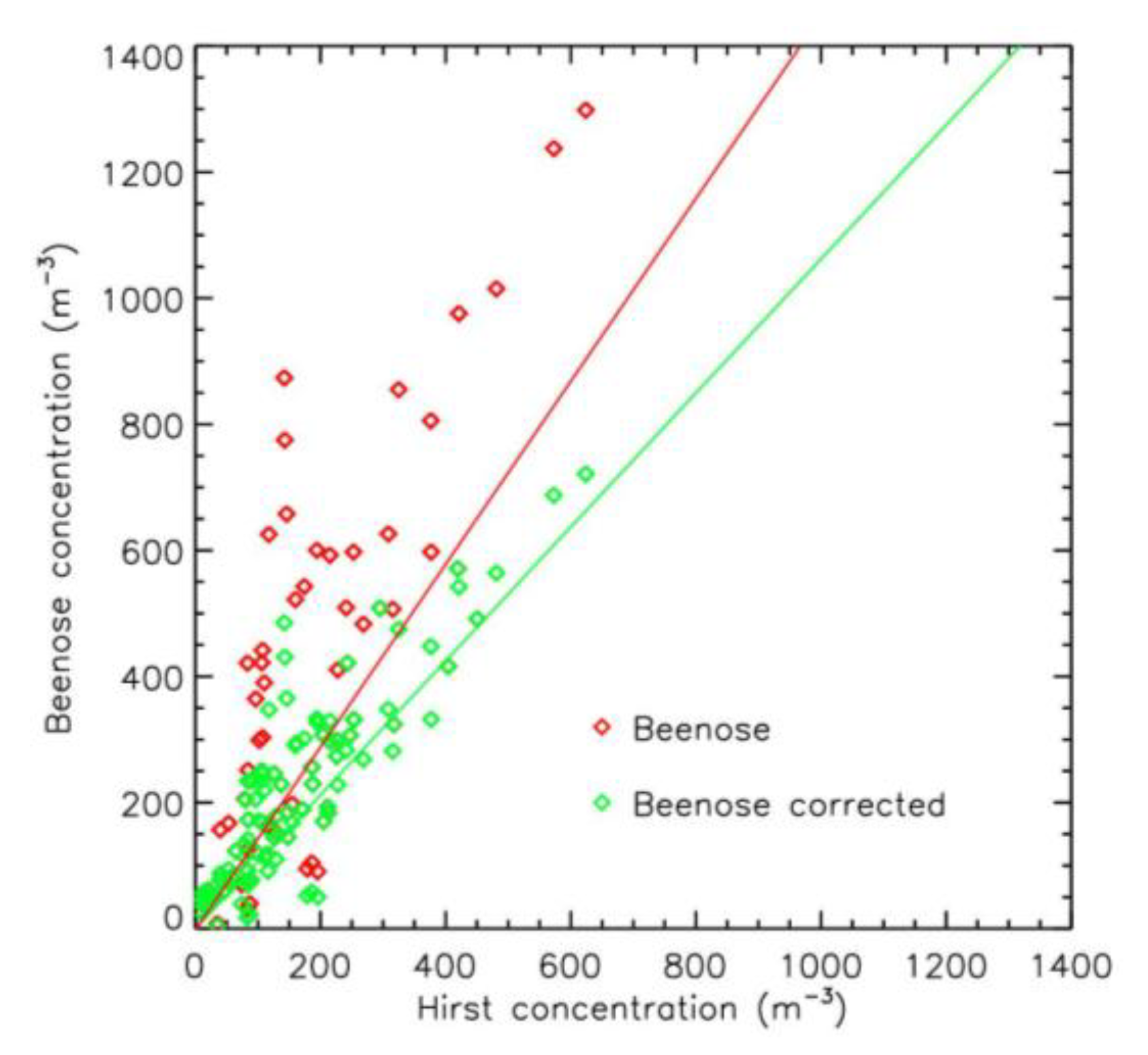
2.3. Intercomparison of Beenose and Hirst Sensors
3. Results
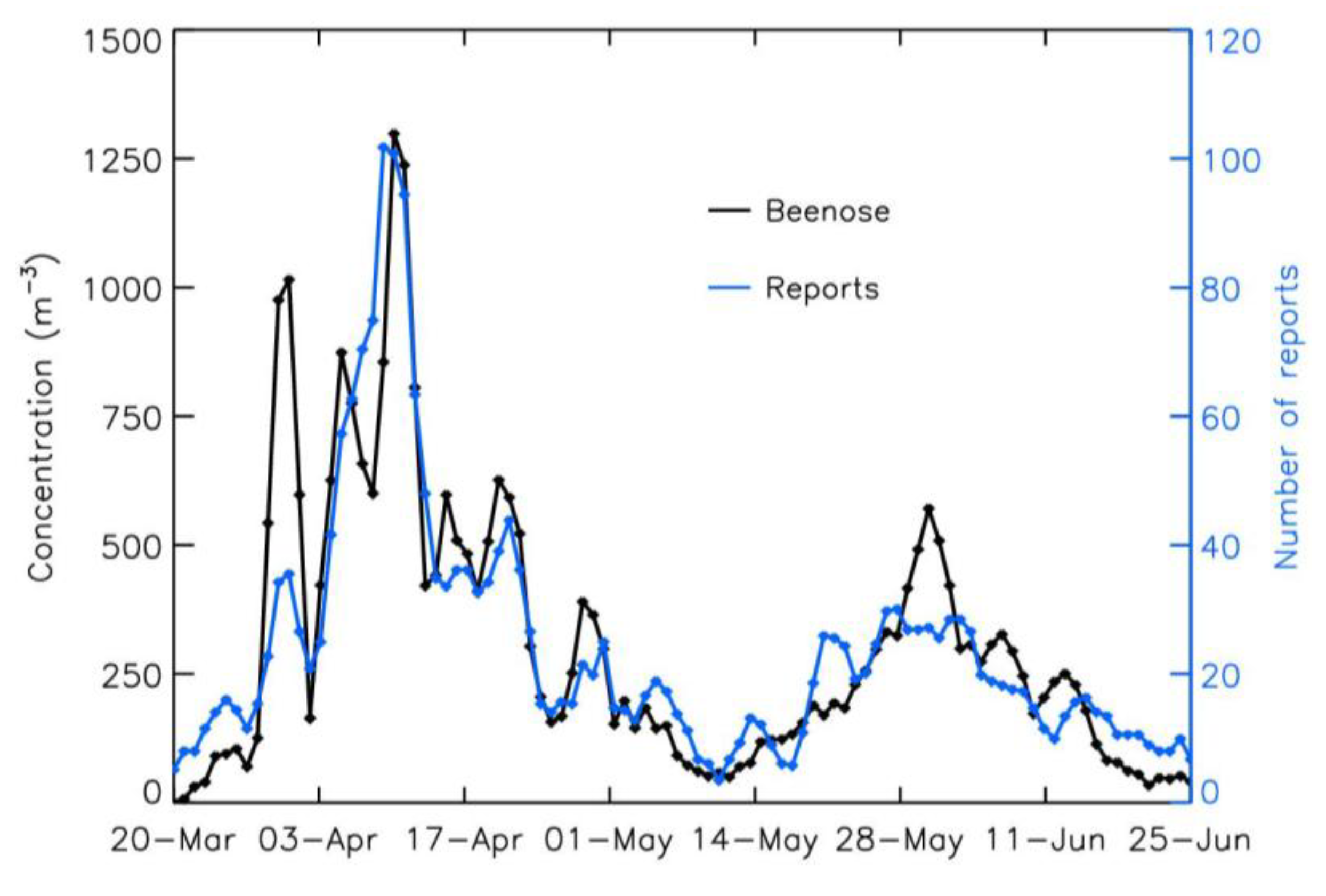
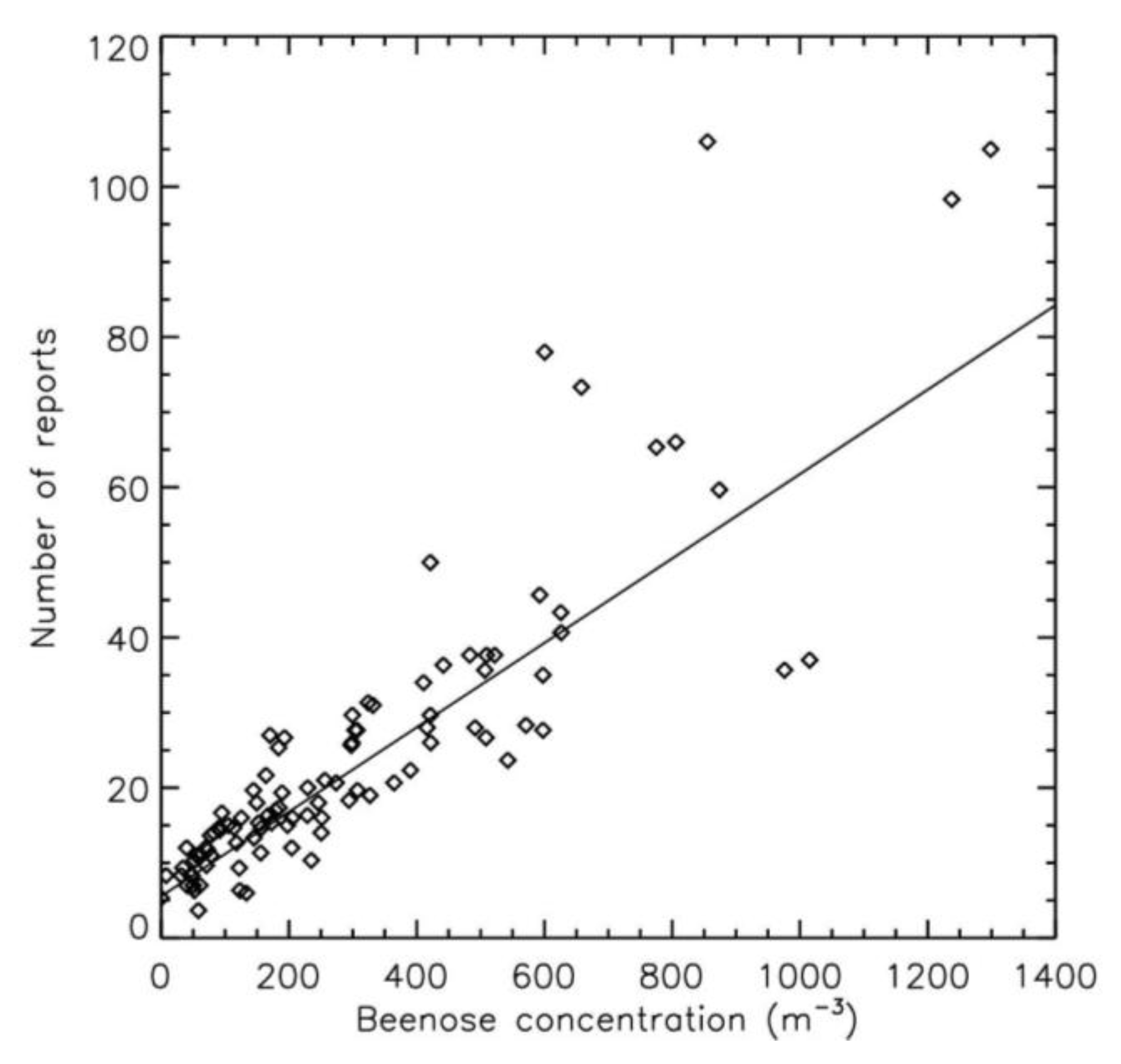
3.1. Comparison Between Pollen Concentrations and Symptoms Reports: Entire Period
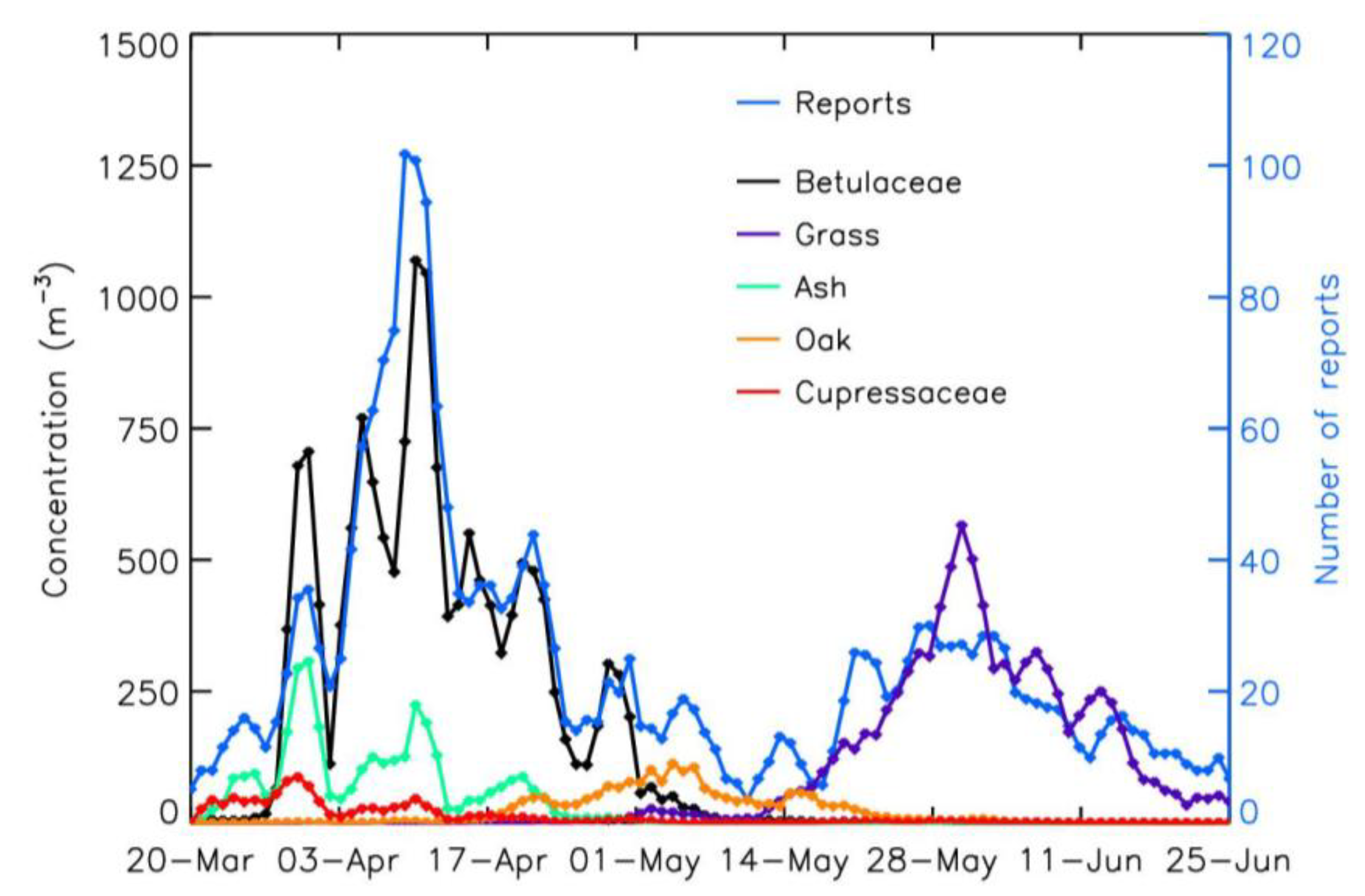
3.2. Pollen Concentrations and Symptoms Reports: Analysis by Period and Predominant Species
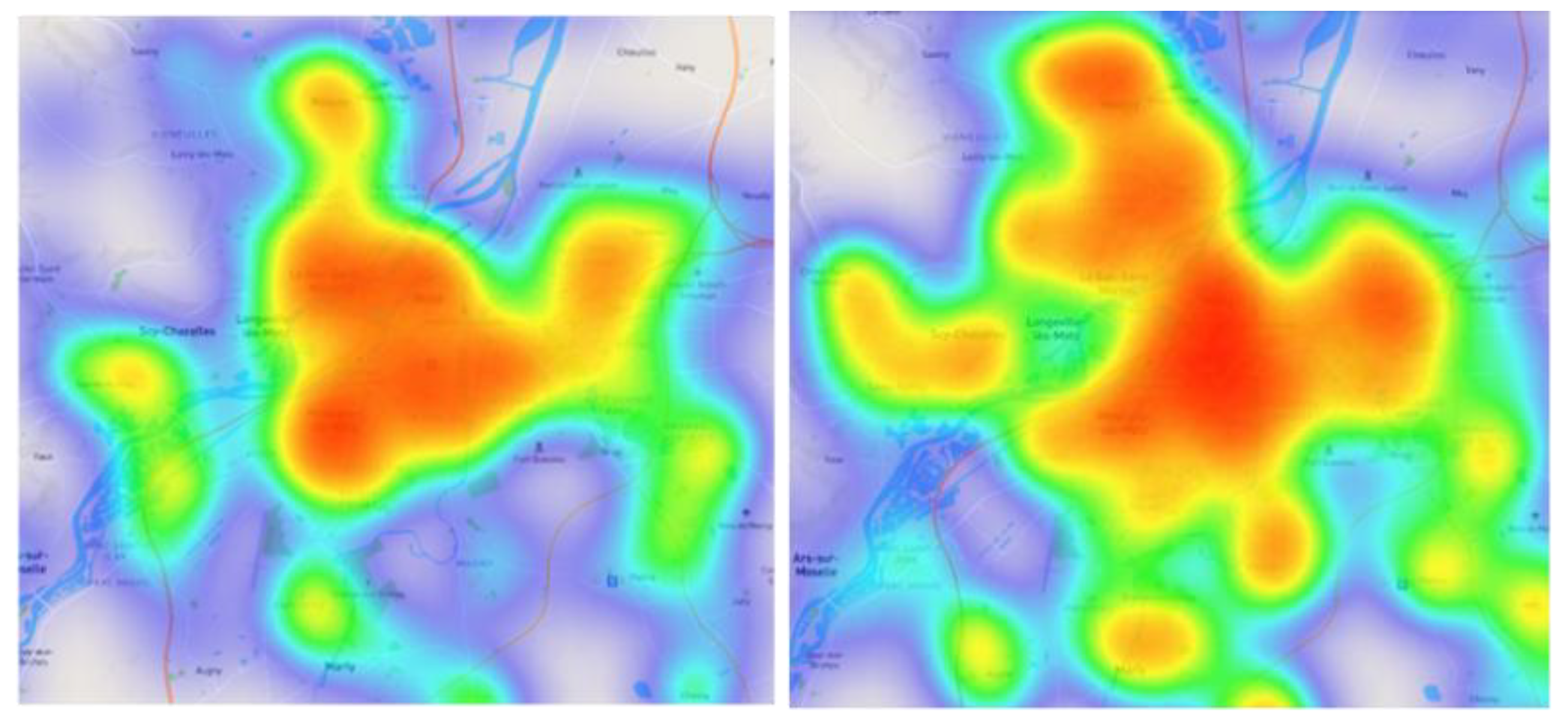
3.3. Spatial Variability of the Symptoms’ Reports
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ANSES. Etat des Connaissances sur l’impact Sanitaire lié à l’exposition de la Population générale aux Pollen présents dans l’air Ambiant. Anses. Saisine n°2011-SA-0151. 2014. Available online: https://www.anses.fr/fr/system/files/AIR2011sa0151Ra.pdf (accessed on 1 October 2024). (In French).
- Savouré, M.; Bousquet, J.; Jaakkola, J.J.K.; Jaakkola, M.S.; Jacquemin, B.; Nadif, R. Worldwide Prevalence of Rhinitis in Adults: A Review of Definitions and Temporal Evolution. Clin. Transl. Allergy 2022, 12, e12130. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lou, H.; Wang, Y.; Wang, X.; Cao, F.; Wang, K.; Chu, X.; Wang, C.; Zhang, L. Endotypes of chronic rhinitis: A cluster analysis study. Allergy 2019, 74, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Cardell, L.-O.; Olsson, P.; Andersson, M.; Welin, K.-O.; Svensson, J.; Tennvall, G.R.; Hellgren, J. TOTALL: High cost of allergic rhinitis—A national Swedish population-based questionnaire study. NPJ Prim. Care Respir. Med. 2016, 26, 15082. [Google Scholar] [CrossRef] [PubMed]
- D’amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesia-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; Van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Vandenplas, O.; Vinnikov, D.; Blanc, P.D.; Agache, I.; Bachert, C.; Bewick, M.; Cardell, L.-O.; Cullinan, P.; Demoly, P.; Descatha, A.; et al. Impact of Rhinitis on Work Productivity: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1274–1286.e9. [Google Scholar] [CrossRef]
- Sofiev, M.; Bergmann, K. Allergenic Pollen. In A Review of the Production, Release, Distribution, and Health Impacts; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Traidl-Hoffmann, C.; Kasche, A.; Menzel, A.; Jakob, T.; Thiel, M.; Ring, J.; Behrendt, H. Impact of Pollen on Human Health, More Than Allergen Carriers? Int. Arch. Allergy Immunol. 2003, 131, 1–13. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma; a global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Bukstein, D.A. The economic impact of allergic rhinitis and current guidelines for treatment. Ann. Allergy Asthma Immunol. 2011, 106, S12–S16. [Google Scholar] [CrossRef]
- Agache, I.; Canelo-Aybar, C.; Annesi-Maesano, I.; Cecchi, L.; Biagioni, B.; Chung, F.; D’Amato, G.; Damialis, A.; Del Giacco, S.; De las Vecillas, L.; et al. The Impact of Indoor Pollution on Asthma-Related Outcomes: A Systematic Review for the EAACI Guidelines on Environmental Science for Allergic Diseases and Asthma. Allergy 2024, 79, 1761–1788. [Google Scholar] [CrossRef]
- Katz, D.S.W.; Zigler, C.M.; Bhavnani, D.; Balcer-Whaley, S.; Matsui, E.C. Pollen and Viruses Contribute to Spatio-Temporal Variation in Asthma-Related Emergency Department Visits. Environ. Res. 2024, 257, 119346. [Google Scholar] [CrossRef]
- Fried, J.; Yuen, E.; Li, A.; Zhang, K.; Nguyen, S.A.; Gudis, D.A.; Rowan, N.R.; Schlosser, R.J. Rhinologic disease and its impact on sleep: A systematic review. Int. Forum Allergy Rhinol. 2021, 11, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, G.; Kiotseridis, H.; Romberg, K.; Dahl, Å.; Bjermer, L.; Lindgren, M.; Aronsson, D.; Tunsäter, A.; Tufvesson, E. Cognitive dysfunction and quality of life during pollen season in children with seasonal allergic rhinitis. Pediatr. Allergy Immunol. 2021, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, K.S.; Reitsma, S.; Fokkens, W.J. Direct and indirect costs of allergic and non-allergic rhinitis in the Netherlands. Allergy 2020, 75, 2993–2996. [Google Scholar] [CrossRef]
- Neumann, J.E.; Anenberg, S.C.; Weinberger, K.R.; Amend, M.; Gulati, S.; Crimmins, A.; Roman, H.; Fann, N.; Kinney, P.L. Estimates of Present and Future Asthma Emergency Department Visits Associated With Exposure to Oak, Birch, and Grass Pollen in the United States. GeoHealth 2019, 3, 11–27. [Google Scholar] [CrossRef]
- Naclerio, R.; Ansotegui, I.J.; Bousquet, J.; Canonica, G.W.; D’Amato, G.; Rosario, N.; Pawankar, R.; Peden, D.; Bergmann, K.-C.; Bielory, L.; et al. International Expert Consensus on the Management of Allergic Rhinitis (AR) Aggravated by Air Pollutants: Impact of Air Pollution on Patients with AR: Current Knowledge and Future Strategies. World Allergy Organ. J. 2020, 13, 100106. [Google Scholar] [CrossRef]
- Buters, J.T.M.; Antunes, C.; Galveias, A.; Bergmann, K.C.; Thibaudon, M.; Galán, C.; Schmidt-Weber, C.; Oteros, J. Pollen and Spore Monitoring in the World. Clin. Transl. Allergy 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Buters, J.; Clot, B.; Galán, C.; Gehrig, R.; Gilge, S.; Hentges, F.; O’Connor, D.; Sikoparija, B.; Skjoth, C.; Tummon, F.; et al. Automatic Detection of Airborne Pollen: An Overview. Aerobiologia 2024, 40, 13–37. [Google Scholar] [CrossRef]
- Hirst, J.M. An Automatic Volumetric Spore Trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Tummon, F.; Adams-Groom, B.; Antunes, C.M.; Bruffaerts, N.; Buters, J.; Cariñanos, P.; Celenk, S.; Choël, M.; Clot, B.; Cristofori, A.; et al. The Role of Automatic Pollen and Fungal Spore Monitoring Across Major End-User Domains. Aerobiologia 2024, 40, 57–75. [Google Scholar] [CrossRef]
- Giesecke, T.; Fontana, S.L.; van der Knaap, W.O.; Pardoe, H.S.; Pidek, I.A. From early pollen trapping experiments to the Pollen Monitoring Programme. Veg. Hist. Archaeobotany 2010, 19, 247–258. [Google Scholar] [CrossRef]
- Dell’Anna, R.; Lazzeri, P.; Frisanco, M.; Monti, F.; Malvezzi Campeggi, F.; Gottardini, E.; Bersani, M. Pollen discrimination and classification by Fourier transform infrared (FT-IR) microspectroscopy and machine learning. Anal. Bioanal. Chem. 2009, 394, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Šaulien, I.; Šukien, L.; Daunys, G.; Valiulis, G.; Vaitkevicius, L.; Matavulj, P.; Brdar, S.; Panic, M.; Sikoparija, B.; Clot, B.; et al. Automatic pollen recognition with the Rapid-E particle counter; the first-level procedure; experience and next steps. Atmos. Meas. Tech. 2019, 12, 3435–3452. [Google Scholar] [CrossRef]
- Sauvageat, E.; Zeder, Y.; Tummon, F.; Clot, B.; Crouzy, B.; Konzelmann, T.; Lieberherr, G.; Tummon, F.; Vasilatou, K. Online pollen monitoring using digital holography. Atmos. Meas. Tech. 2020, 13, 1539–1550. [Google Scholar] [CrossRef]
- Tummon, F.; Bruffaerts, N.; Celenk, S.; Choël, M.; Clot, B.; Crouzy, B.; Galán, C.; Gilge, S.; Hajkova, L.; Mokin, V.; et al. Towards standardisation of automatic pollen and fungal spore monitoring: Best practices and guidelines. Aerobiologia 2024, 40, 39–55. [Google Scholar] [CrossRef]
- Oteros, J.; Pusch, G.; Weichenmeier, I.; Heimann, U.; Möller, R.; Röseler, S.; Traidl-Hoffmann, C.; Schmidt-Weber, C.B.; Buters, J. Automatic and Online Pollen Monitoring. Int. Arch. Allergy Immunol. 2015, 167, 158–166. [Google Scholar] [CrossRef]
- Renard, J.-B.; El Azari, H.; Lauthier, J.; Surcin, J. Spatial Variation of Airborne Pollen Concentrations Locally around Brussels City, Belgium, during a Field Campaign in 2022–2023, Using the Automatic Sensor Beenose. Sensors 2024, 24, 3731. [Google Scholar] [CrossRef]
- Montagne, R.; Pham-Thi, N.; Demoly, P. Aggravation des pollinoses: Résultats d’une enquête nationale auprès des médecins allergologues francophones. Rev. Française D’allergologie 2024, 64, 103905. (In French) [Google Scholar] [CrossRef]
- Brake, D.R.; Yaman, R.N.; Camargo, A.R.; Marks, L.A.; Maddux, J.T.; Ochkur, S.I.; Rank, M.A. Meteorological and environmental factors that impact pollen counts, allergenicity, and thresholds: A scoping review. Allergy Asthma Proc. 2023, 44, 229–236. [Google Scholar] [CrossRef]
- Caillaud, D.M.; Martin, S.; Ségala, C.; Vidal, P.; Lecadet, J.; Pellier, S.; Rouzaire, P.; Tridon, A.; Evrard, B. Airborne pollen levels and drug consumption for seasonal allergic rhinoconjunctivitis: A 10-year study in France. Allergy 2015, 70, 99–106. [Google Scholar] [CrossRef]
- Guillam, M.T.; Ségala, C. Pollen et effets sanitaires: Synthése des études épidémiologiques. Rev. Française D’allergologie D’immunologie Clin. 2008, 48, 14–19. [Google Scholar] [CrossRef]
- Caillaud, D.; Martin, S.; Segala, C.; Besancenot, J.-P.; Clot, B.; Thibaudon, M. Effects of Airborne Birch Pollen Levels on Clinical Symptoms of Seasonal Allergic Rhinoconjunctivitis. Int. Arch. Allergy Immunol. 2014, 163, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Karatzas, K.; Bastl, K.; Berger, U.; Buters, J.; Darsow, U.; Demoly, P.; Durham, S.R.; Galán, C.; Gehrig, R.; et al. Pollen season is reflected on symptom load for grass and birch pollen-induced allergic rhinitis in different geographic areas—An EAACI Task Force Report. Allergy 2020, 75, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Vaidyanathan, A.; Lo, F.; Brown, C.; Hess, J.J. Short Term Physician Visits and Medication Prescriptions for Allergic Disease Associated with Seasonal Tree, Grass, and Weed Pollen Exposure Across the United States. Environ. Health 2021, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Luyten, A.; Bürgler, A.; Glick, S.; Kwiatkowski, M.; Gehrig, R.; Beigi, M.; Hartmann, K.; Eeftens, M. Ambient pollen exposure and pollen allergy symptom severity in the EPOCHAL study. Allergy 2024, 79, 1908–1920. [Google Scholar] [CrossRef]
- Renard, J.-B.; El Azari, H.; Richard, J.; Lauthier, J.; Surcin, J. Towards an Automatic Pollen Detection System in Ambient Air Using Scattering Functions in the Visible Domain. Sensors 2022, 22, 4984. [Google Scholar] [CrossRef]
- El Azari, H.; Renard, J.-B.; Lauthier, J.; Dudok deWitt, T. A Laboratory Evaluation of the new automated pollen sensor Beenose: Pollen discrimination using machine learning techniques. Sensors 2023, 23, 2964. [Google Scholar] [CrossRef]
- Adamov, S.; Lemonis, N.; Clot, B.; Crouzy, B.; Gehrig, R.; Graber, M.J.; Sallin, C.; Tummon, F. On the measurement uncertainty of Hirst-type volumetric pollen and spore samplers. Aerobiologia 2021, 40, 77–91. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- Suarez-Suarez, M.; Maya-Manzano, J.M.; Clot, B.; Graber, M.J.; Sallin, C.; Tummon, F.; Buters, J. Accuracy of a hand-held resistance-free flowmeters for flow adjustments of Hirst-Type pollen traps. Aerobiologia 2023, 39, 143–148. [Google Scholar] [CrossRef]
- Andersson, K.; Lidholm, J. Characteristics and immunobiology of grass pollen allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef]
- Kiotseridis, H.; Cilio, C.M.; Bjermer, L.; Tunsäter, A.; Jacobsson, H.; Dahl, Å. Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin. Transl. Allergy 2013, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Sedghy, F.; Varasteh, A.R.; Sankian, M.; Moghadam, M. Interaction Between Air Pollutants and Pollen Grains, the Role on the Rising Trend in Allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar] [PubMed]
- Berger, M.; Bastl, M.; Bouchal, J.; Dirr, L.; Berger, U. The influence of air pollution on pollen allergy sufferers. Allergol. Sel. 2021, 5, 345–348. [Google Scholar] [CrossRef] [PubMed]

| Ash | Betulaceae | Oak | Grass | |
|---|---|---|---|---|
| Pearson coefficient | 0.94 | 0.86 | 0.88 | 0.95 |
| Without Entire Season | Shift from 2 April 2023 | With One Entire Season | Day Shift from 2 April 2023 | |
|---|---|---|---|---|
| Pearson coefficient | 0.81 | 0.89 | 0.87 | 0.93 |
| Spearman coefficient | 0.86 | 0.88 | 0.92 | 0.92 |
| Betulaceae | Grass | |
|---|---|---|
| Pearson coefficient | 0.87 | 0.79 |
| Spearman coefficient | 0.92 | 0.82 |
| Betulaceae | Grass | |
|---|---|---|
| Pearson coefficient | 061 | 0.84 |
| Spearman coefficient | 0.81 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renard, J.-B.; Lefèvre, S.; Glévarec, G. Low-Cost Pollen and Allergy Symptoms Monitoring with Beenose Sampler and Livepollen App: The Case Study of the Metz City, France, During Spring 2023. Atmosphere 2025, 16, 271. https://doi.org/10.3390/atmos16030271
Renard J-B, Lefèvre S, Glévarec G. Low-Cost Pollen and Allergy Symptoms Monitoring with Beenose Sampler and Livepollen App: The Case Study of the Metz City, France, During Spring 2023. Atmosphere. 2025; 16(3):271. https://doi.org/10.3390/atmos16030271
Chicago/Turabian StyleRenard, Jean-Baptiste, Sébastien Lefèvre, and Gaëlle Glévarec. 2025. "Low-Cost Pollen and Allergy Symptoms Monitoring with Beenose Sampler and Livepollen App: The Case Study of the Metz City, France, During Spring 2023" Atmosphere 16, no. 3: 271. https://doi.org/10.3390/atmos16030271
APA StyleRenard, J.-B., Lefèvre, S., & Glévarec, G. (2025). Low-Cost Pollen and Allergy Symptoms Monitoring with Beenose Sampler and Livepollen App: The Case Study of the Metz City, France, During Spring 2023. Atmosphere, 16(3), 271. https://doi.org/10.3390/atmos16030271






