Abstract
Background: In end-stage HF, interventional therapy is the treatment of choice, including mechanical circulatory support and heart organ transplantation. Acute cellular rejection is considered a major impediment to the long-term survival of cardiac allografts. The aim of this study is to point out a possible relationship underlying acute cellular rejection risk in heart organ recipients. Methods: A total of 30 (25 (83%) men and 5 (17%) women) heart organ recipients with a median (Q1–Q3) age of 49 (38–60) were enrolled in the analysis. The results from repeated hospitalizations due to protocolar endomyocardial biopsies performed between one and three months following the heart transplantation in relation air pollution exposure were taken into the analysis. Results: The median (Q1–Q3) observation time after organ transplantation was 92 (82–97) days. A significant difference in PM2.5 exposure between the rejection group (16.10 (14.24–17.61)) μg/m3 and the non-rejection group (11.97 (9.85–12.97)) μg/m3 was noticed (p < 0.001). The odds ratio (95% confidence interval) for acute rejection prediction related to PM2.5 was 1.79 (1.11–2.89), p = 0.018. The reviewer operator curve for acute cellular rejection related to PM2.5 exposure was performed, and the area under the curve (AUC) was 0.873, yielding a precision of 0.600 and an f-measure of 0.545. The predicted residual plots for PM2.5 indicated a 50% increased risk for PM2.5 above 16 μg/m3 and of 91% for PM2.5 above 20 μg/m3. Conclusions: The single-center study was performed on a limited number of heart organ recipients and was related to personalized individual calculations of PM2.5 exposure. The study represents a personalized approach and indicates possible links to the hypothesis, which should be verified on a higher volume of patients.
1. Introduction
The epidemic of heart failure (HF) is reported as a current challenge, and its prevalence is increasing due to the aging of the population and improved survival with ischaemic heart disease [1]. It is considered a multi-faceted and potentially life-threatening syndrome [2]. The efficacy and efficiency of novel pharmacotherapies has been confirmed [3]. In end-stage HF, interventional therapy is the treatment of choice, including mechanical circulatory support and heart transplantation [4]. For optimal long-term results, patients are still assigned to organ transplantation. The shortage of heart donors, combined with marginal organ acceptance, limited efforts to expand the donor pool, and increased risks of acute rejection episodes, post-transplant malignancy, and renal dysfunction, has led to increasing suboptimal results.
Acute cellular rejection is considered a major impediment to the long-term survival of cardiac allografts [5]. The effectiveness of current immunosuppressive strategies that target recipient T cells has been reported, though these do not address innate immune response [6]. Acute cellular rejection (ACR) risk within the first year following heart transplantation is reported in up to 20% of organ recipients and is believed to represent the imbalance between a conventional versus regulatory CD4+ T cell alloimmune response [7].
Ambient air particles negatively affect human health, increasing cardiovascular, neurological, respiratory, and malignancy risk. These pollutants were found to elicit cytokines released by airway epithelium and activate hematopoietic and non-hematopoietic cells, including dendritic cells and fibroblasts [8]. In animal studies, ischemic renal reperfusion injury related to PM2.5 exposure was noted through local innate immune aggravation and mitochondrial dysfunction [9]. Ambient fine particles can lead to immune damage, emphasizing oxidative stress, inflammatory responses, and immune cells [10]. Short-term exposure to PM2.5 may even exaggerate the inflammatory response in autoimmune diseases [11]. The cardiotoxic effect of air pollution is believed to be mainly mediated by sympathovagal imbalance, oxidative stress amplification, and inflammatory and pro-aggregatory cascade activation [12]. Dendritic cell activation next to higher secretions of necrosis factor-α (TNF-α) 1-beta, and interleukins 6 and 8 (IL-1β, IL-6, IL-8) in response to PM2.5 has been already noticed [13]. The aggravation of chronic obturatory pulmonary disease (COPD) secondary to airborne PM2.5 particles was found to be related to the enhancement of autophagy in alveolar macrophages [14].
The aim of the study is to point out the possible relationship underlying acute cellular rejection risk in heart organ recipients.
2. Materials and Methods
2.1. Patients
A total of 30 (25 (83%) men and 5 (17%) women) heart transplant recipients with a median (Q1–Q3) age of 49 (38–60) were enrolled in the analysis.
The results of endomyocardial biopsies (EMBs) performed for the first 6 months following heart transplantation were examined in relation to the applied pharmacotherapy and the air pollution (measured by PM2.5 air particulate matter) in habitation places. The first two biopsies performed within the first postoperative month were excluded from the analysis to rule out the possible relation of cold ischemia-related myocardial injury. The analysis did not include recurrent acute cellular rejection (ACR) patients. None of the patients reported use of air conditioning in their habitation place.
2.2. Method
Heart transplant recipients’ and donors’ demographic and clinical preoperative characteristics were examined. The results of protocolar endomyocardial biopsies performed during repeated hospitalizations between one to three months following heart transplantation were taken into the analysis, in relation air pollution exposure.
The laboratory test results, including tacrolimus (TACR) and mycophenolate mofetil (MPA) serum concentrations in combination with transthoracic echocardiography and endomyocardial biopsies (EMBs), were considered in the analysis and compared with possible exposure to air pollutants in the habitation place.
Based on acute cellular rejection confirmed with EMB, the group was divided into a rejection (Group 1) and control group (Group 2).
2.3. Endomyocardial Biopsies
All endomyocardial biopsies (EMBs) were examined in the reference center. Biopsy fragments were fixed in a 4% buffered formaldehyde solution, and then routinely dehydrated in ethyl alcohol series and transferred to paraffin wax through xylene. Tissue slices 5 microns thick were stained H&E (hematoxylin and eosin). The rejection grade was estimated using ISHLT’s working formulation [15] (Figure 1). Acute cellular rejection (ACR) was defined as stage 2 (more focus on lymphocytic infiltrate with associated myocardial damage), 3 (multifocal or diffuse lymphocytes infiltrate with myocardial damage), or stage 4 (diffuse, polymorphous infiltrate with extensive myocyte damage, edema, vasculitis, hemorrhage) [15].
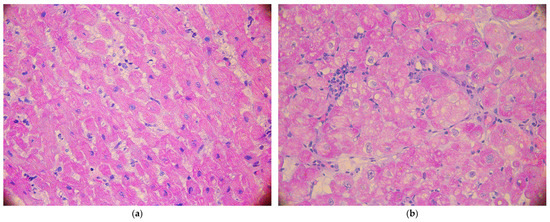
Figure 1.
The histopathologic confirmation of no-acute cellular rejection: grade 0 (a) and grade 1a (b).
2.4. Personalized Calculations of Air Pollution Exposure in Habitation Place
To assess patients’ individual exposure to PM2.5, we used all the available air quality data. The State Environmental Monitoring (SEM) data were applied to render previous reports [16]. SEM is established based on the Act of Inspection of Environmental Protection to provide reliable data on the state of the environment [17].
Calculations involved time-dependent high-resolution bottom–up emission inventory maps stored in the Central Emission Database were elaborated based on Standard Nomenclature for Air Pollution (SNAP) categories including the main air pollutant emission sectors in Poland like residential emission, energy production, industry, transport, and agriculture [18].
Outdoor exposure to air pollution was estimated based on patients’ home addresses. The mean values for air pollution within a particular period defined by the analysis time frame were calculated based on mean values between two environmental monitoring stations and the habitation place.
2.5. Statistical Analysis
The normality of the distribution of variables was tested with the Shapiro–Wilk test. The t-test, Cochran–Cox test or Mann–Whitney test, or Fisher’s exact test were used where applicable to compare the variables between the two groups. ANOVA tests evaluated the repeated measures. The odds ratio was calculated with 95% confidence intervals for acute cellular rejection in relation to PM2.5 exposure. The receiver operator curve (ROC), alongside the precision and f-measure, was calculated to predict ACR due to PM2.5. Statistical analysis was performed using JASP version 0.14.1 (University of Amsterdam, Netherlands), with the significance level set at p < 0.05 (https://jasp-stats.org, accessed on 5 February 2025).
The sample was estimated using the minimum sample size estimation tool of the PQStat software (v. 1.8.6). Based on the assumption of an undetermined target population size, α = 0.05 and β = 0.2, the minimum sample size was estimated at 24 participants. Hence, the authors decided to collect full data from at least 30 participants.
2.6. Bioethics Committee
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Poznan University of Medical Sciences, Poznan, Poland (protocol code 694/20 on 4 November 2020), for studies involving humans.
3. Results
There were neither fatal events nor clinical presentations of acute cellular rejection in the presented group throughout the study period. The median (Q1–Q3) observation time after organ transplantation was 92 (82–97) days.
Laboratory tests combined with transthoracic echocardiography were performed prior to EMB at the following intervals: 21 (17–28) days, 35 (31–42) days, 69 (57–77) days, and 92 (82–97) days after transplantation. If the EMB confirmed ACR, the standard protocol included a 3000 mg parenteral infusion of methylprednisolone, followed by an increase in oral steroid therapy and a control EMB within 7 days. Only the original results, without one week post-ACR EMB steroid therapy effects, were considered in the analysis.
There were eight patients with EMB-confirmed acute myocardial rejection, and they were treated with standard steroid therapy, including parenteral infusion of an overall amount of three grams of methylprednisolone within three days. Based on acute cellular rejection confirmed with EMB, the group was divided into a rejection (Group 1) and control group (Group 2). The demographic and clinical characteristics of the heart organ recipients in both subgroups are presented in Table 1.

Table 1.
Group demographic and clinical characteristics.
Four consecutive protocolar EMB results in relation to applied anti-rejection pharmacotherapy were considered, as presented in Table 2.

Table 2.
Laboratory, echocardiographic, and endomyocardial biopsy (EMB) results.
There were no significant changes in tacrolimus serum concentrations in the whole group (p = 0.017), nor between the rejection and non-rejection subpopulations (p = 0.554), as presented in Figure 2.
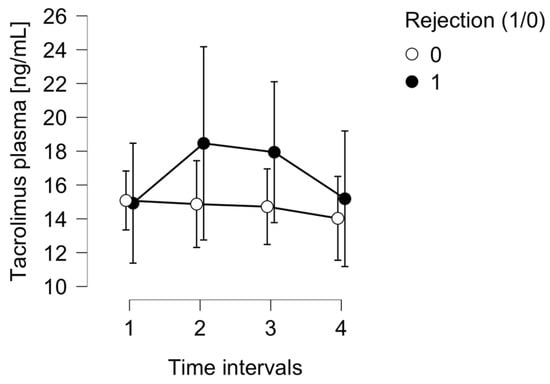
Figure 2.
The tacrolimus serum concentration in the ACR group (1) and control group (0).
There were significant changes in mycophenolate mofetil acid serum concentrations within the whole group (p = 0.447) and also between rejection and non-rejection subpopulations (p = 0.059), as presented in Figure 3.
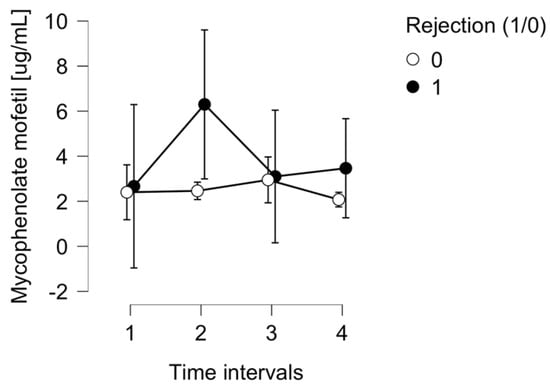
Figure 3.
The mycophenolate mofetil serum concentration in the ACR group (1) and control group (0).
The peripheral blood count results, including myocardial injury markers such as Troponin-I serum concentration, were considered in analysis and found to be inconclusive for ACR prediction within the whole group (p = 0.412) and also between rejection and non-rejection subgroups (p = 0.955).
Inflammatory markers like C-reactive protein (CRP) were analyzed throughout the study period. They did not reveal any significant differences for ACR prediction between the rejection and non-rejection subgroups (p = 0.647), as shown in Figure 4.
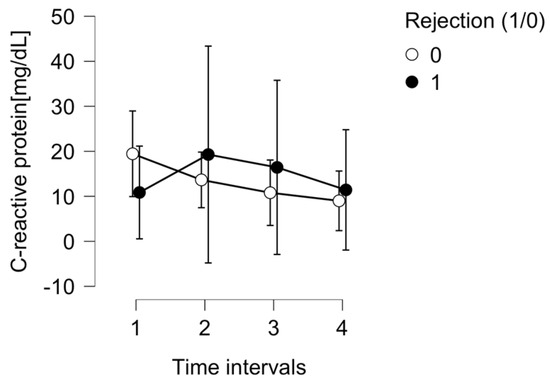
Figure 4.
The C-reactive protein (CRP) level in the ACR group (1) and control group (0).
The air pollution exposure related to particulate matter PM2.5 was individually calculated for each heart transplant recipient. The mean values of the air PM2.5 concentration throughout the study period were examined and compared between both groups. A significant difference in PM2.5 exposure between the rejection group (16.10 (14.24–17.61)) μg/m3 and the non-rejection group (11.97 (9.85–12.97)) μg/m3 was noticed (p < 0.001) (Figure 5).
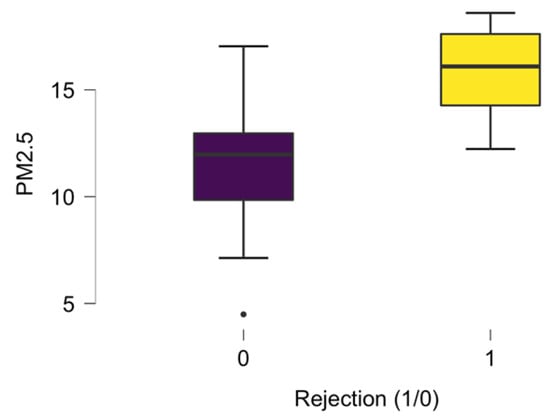
Figure 5.
The PM2.5 median exposure in the ACR group (1) and control group (0).
The possible relation between air pollution particles and acute rejection risk was examined for PM2.5, PM10, and NO2, as presented in Table 3.

Table 3.
The predictive value of air pollutants on acute rejection episodes.
The odds ratio (95% confidence interval) for acute rejection prediction related to PM2.5 was 1.79 (1.11–2.89), p = 0.018. The reviewer operator curve for acute cellular rejection related to PM2.5 exposure was performed, and the area under the curve (AUC) was 0.873, yielding a precision of 0.600 and an f-measure of 0.545. The predicted residual plots for PM2.5 indicated a 50% increased risk for PM2.5 above 16 μg/m3 and 91% for PM2.5 above 20 μg/m3, respectively, as presented in Figure 6.
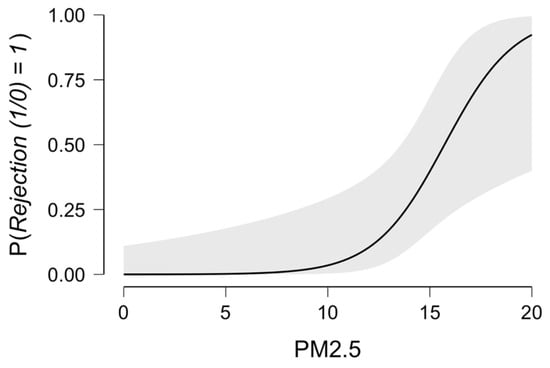
Figure 6.
ACR prediction in relation to PM2.5 exposure.
4. Discussion
The novelty of our analysis is based on the possible relationship between increased acute cellular rejection risk following heart organ transplantation and ambient air pollution. This personalized analysis suggests that environmental factors may possibly trigger an inflammatory host response that results in ACR. According to our results, ambient pollution related to PM2.5 may influence ACR risk, especially when the PM2.5 concentration exceeds 20 μg/m3.
This analysis indicates the possible role of non-traditional acute rejection factors linked to excessive immunological activation.
Heart organ transplantation is considered the optimal therapeutic resort for patients with end-stage heart failure. Among the main post-transplant complications, acute rejection episodes, even with satisfactory serum levels of immunosuppression, followed by opportunistic infection risk and eminent malignancy risk, are reported [19]. Noninvasive markers for clinically silent graft rejection have been proposed [20,21].
The pharmacological immunosuppressive agents applied after organ transplantation either inhibit cytokine release or the cell cycle. The anti-rejection regimen following heart transplantation is calcineurin inhibitor-based therapy, which blocks interleukin 2 transcription via FKBP12 [22,23]. Interleukin 2 and its receptors play a substantial role in cell-mediated immunity, particularly T lymphocyte proliferation and activation. Typically, calcineurin inhibitors combined with mycophenolate mofetil and steroids are considered the first-line therapy of choice. Mycophenolate mophetil suppresses T-cell proliferation by restraining guanosine nucleotide synthesis [24]. The mycophenolate is administered as a pro-drug [25]. The third drugs included in the standard immunosuppressive protocol are corticosteroids. They display immunomodulatory and anti-inflammatory characteristics by decreasing the number of circulating CD4+ T lymphocytes, interacting with antigen-presenting dendritic cells [26]. We thoroughly investigated the applied drug dosages and levels when estimating the rejection risk. The therapeutic serum levels of anti-rejection drugs were achieved throughout the monitoring period in the analyzed group [27,28].
Ambient particulate matter below 2.5 microns in diameter (PM2.5) comprises solid and liquid particles that are mostly the product of industrial activity, gasoline combustion, cigarette smoke, and biomass burning [29]. The two possible methods of immunological system irritation by PM2.5 inhalation are related to inflammatory cell activation, which releases various mediators, and particle translocation via the pulmonary epithelium to the bloodstream, leading to oxidative stress and vascular dysfunction [30]. Our analysis suggests a 50% increase in rejection risk among heart recipients exposed to ambient PM2.5 above 15 µg/m3. The acute cellular rejection risk among patients chronically exposed to PM2.5 above 20 µg/m3 increased to over 90%.
The respiratory system is particularly vulnerable to PM2.5 as these particles may trigger oxidative stress via mitochondrial dysfunction, oxidases, and metabolic enzyme activation [31]. PM2.5-induced lipid peroxidation and endothelial cell injury are believed to recruit innate immunity cells and propagate atherosclerosis [32]. Our previous analysis revealed the relationship between air pollutant exposure and premature epicardial artery disease development [33,34]. The beneficiary role of immunosuppressive drugs on PM2.5-generated inflammatory activation has been reported in animal studies [35].
There is a growing awareness of the possible modulatory role of ambient pollution on organ recipient outcomes. Previous reports [36] suggested a relationship between 1-year acute rejection in kidney recipients and PM2.5, highlighting the importance of environmental exposure on post-transplant outcomes. A recent epidemiological study [37] indicated an association between ambient PM2.5 levels and an increased risk of either graft failure or fatal events in lung transplant recipients. In the cohort study by Chan et al. [38], PM2.5 exposure was reported as an independent risk factor associated with adverse outcomes following kidney transplantation. Airborne fine particles may enact their negative impact on solid organ recipients as early as in the first three postoperative months [39]. Al-Kindi et al. [40] investigated long-term survival in heart transplant patients, pointing out an estimated mortality hazard ratio per 10 μg/m3 increment in annual exposure to PM2.5.
In the analyzed population, the rejection group was older. A higher rejection rate is generally associated with younger patients, as the innate and adaptive immunological response declines with age [41,42]. The presented possible relation to air pollution may be even more extensive in older heart recipients, which should be confirmed through studies with a higher volume of participants.
Air pollutant exposure can be related to geographical factors, as we found significant differences in coronary artery disease risk, secondary to inflammatory reactions triggered related to habitation place urbanization [43]. As we did not include patients who are professionally active or living in air-conditioned accommodations in the analysis, the presented results can be regarded free from influence from these factors. A multivariable analysis in a larger cohort of patients is required to confirm the presented phenomenon.
Study Limitations
The single-center study was performed on a limited number of heart organ recipients and was related to personalized individual calculations of PM2.5 exposure. The study represents a personalized approach and indicates possible links to the hypothesis that should be verified in a longitudinal multi-center, high-volume study that incorporates additional biomarkers in order to better to understand the immune response to air pollutant particles and to confirm the suggested relationship using multivariable models.
This study was performed as a single-center analysis on a relatively small sample size, which limits its statistical power and generalizability. The relatively short observation period might not have captured the long-term effects of PM2.5 exposure. The analysis was based on outdoor air pollutant exposure in habitation places. We did not investigate the long-term consequences of acute rejection episodes on chronic graft dysfunction or mortality.
5. Conclusions
This study’s results present the hypothesis of a possible relation between acute rejection episodes in heart transplant recipients and personalized PM2.5 exposure. This hypothesis may help to understand the phenomenon of ACR in solid-organ donors undergoing adequate immunosuppressive therapy. Further studies are required to confirm the proposed link.
Author Contributions
Conceptualization, T.U. and K.S.; methodology, K.S., D.K.-M., R.S., J.N. and J.B.; validation, K.S. and J.B.; formal analysis, T.U., K.S. and J.B.; investigation, J.S., H.W.-B., J.K., M.M., D.K.-M., R.S. and J.N.; resources, H.W.-B., J.S., J.K. and M.M.; data curation, K.S., J.K., M.M., E.S.-M. and M.J.; writing—original draft preparation, T.U.; writing—review and editing, K.S., H.W.-B., R.S., J.S., J.K., M.M., E.S.-M., J.N. and M.J.; visualization, T.U., D.K.-M., R.S. and J.N.; supervision, M.J.; project administration, T.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Poznan University of Medical Sciences, Poznan, Poland (protocol code 694/20 on 4 November 2020) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the obtained results will be available upon reasonable request via the corresponding authors’ e-mail contact within 3 years following the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Siopi, S.A.; Antonitsis, P.; Karapanagiotidis, G.T.; Tagarakis, G.; Voucharas, C.; Anastasiadis, K. Cardiac Failure and Cardiogenic Shock: Insights into Pathophysiology, Classification, and Hemodynamic Assessment. Cureus 2024, 16, e72106. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Barge-Caballero, E. Advanced Heart Failure: Definition, Epidemiology, and Clinical Course. Heart Fail. Clin. 2021, 17, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Huang, S.; Miao, X.; Li, J.; Wu, Z.; Shi, Y.; Liu, H.; Jiang, Y.; Yu, X.; Xie, M.; et al. The dynamic cellular landscape of grafts with acute rejection after heart transplantation. J. Heart Lung Transplant. 2023, 42, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, B.J.; Dun, H.; Amrute, J.M.; Lin, C.Y.; Bredemeyer, A.L.; Terada, Y.; Bayguinov, P.O.; Koenig, A.L.; Frye, C.C.; Fitzpatrick, J.A.J.; et al. Donor Macrophages Modulate Rejection After Heart Transplantation. Circulation 2022, 146, 623–638. [Google Scholar] [CrossRef]
- Kim, J.V.; Assadian, S.; Hollander, Z.; Burns, P.; Shannon, C.P.; Lam, K.; Toma, M.; Ignaszewski, A.; Davies, R.A.; Delgado, D.; et al. Regulatory T Cell Biomarkers Identify Patients at Risk of Developing Acute Cellular Rejection in the First Year Following Heart Transplantation. Transplantation 2023, 107, 1810–1819. [Google Scholar] [CrossRef]
- Duchesne, M.; Okoye, I.; Lacy, P. Epithelial cell alarmin cytokines: Frontline mediators of the asthma inflammatory response. Front. Immunol. 2022, 13, 975914. [Google Scholar] [CrossRef]
- Sanches, T.R.; Parra, A.C.; Sun, P.; Graner, M.P.; Itto, L.Y.U.; Butter, L.M.; Claessen, N.; Roelofs, J.J.; Florquin, S.; Veras, M.M.; et al. Air pollution aggravates renal ischaemia-reperfusion-induced acute kidney injury. J. Pathol. 2024, 263, 496–507. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Xu, B.; Tian, H. Exercise-Mediated Protection against Air Pollution-Induced Immune Damage: Mechanisms, Challenges, and Future Directions. Biology 2024, 13, 247. [Google Scholar] [CrossRef]
- Bellinato, F.; Adami, G.; Vaienti, S.; Benini, C.; Gatti, D.; Idolazzi, L.; Fassio, A.; Rossini, M.; Girolomoni, G.; Gisondi, P. Association Between Short-term Exposure to Environmental Air Pollution and Psoriasis Flare. JAMA Dermatol. 2022, 158, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Argacha, J.F. Effets de la pollution de l’air sur les évènements cardiovasculaires en unité de soins intensifs cardiologiques [Effects of air pollution on cardiovascular events in cardiac intensive care units]. Ann. Cardiol. Angeiol. 2023, 72, 101663. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Okuda, T.; Nagao, M.; Miyasaka, N.; Tanaka, M.; Takano, H. PM2.5 collected using cyclonic separation causes stronger biological responses than that collected using a conventional filtration method. Environ. Res. 2021, 198, 110490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Yue, H.; Zhao, P.; Li, J. Effective-components combination alleviates PM2.5-induced inflammation by evoking macrophage autophagy in COPD. J. Ethnopharmacol. 2024, 321, 117537. [Google Scholar] [CrossRef]
- Billingham, M.E.; Cary, N.R.; Hammond, M.E.; Kemnitz, J.; Marboe, C.; McCallister, H.A.; Snovar, D.C.; Winters, G.L.; Zerbe, A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J. Heart Transplant. 1990, 9, 587–593. [Google Scholar]
- Urbanowicz, T.; Skotak, K.; Olasińska-Wiśniewska, A.; Filipiak, K.J.; Bratkowski, J.; Wyrwa, M.; Sikora, J.; Tyburski, P.; Krasińska, B.; Krasiński, Z.; et al. Long-Term Exposure to PM10 Air Pollution Exaggerates Progression of Coronary Artery Disease. Atmosphere 2024, 15, 216. [Google Scholar] [CrossRef]
- Available online: https://powietrze.gios.gov.pl/pjp/maps/modeling (accessed on 20 July 2018).
- Tagaris, E.; Sotiropoulou, R.E.P.; Gounaris, N.; Andronopoulos, S.; Vlachogiannis, D. Effect of the Standard Nomenclature for Air Pollution (SNAP) categories on air quality over Europe. Atmosphere 2015, 6, 1119–1128. [Google Scholar] [CrossRef]
- Chrysakis, N.; Magouliotis, D.E.; Spiliopoulos, K.; Athanasiou, T.; Briasoulis, A.; Triposkiadis, F.; Skoularigis, J.; Xanthopoulos, A. Heart Transplantation. J. Clin. Med. 2024, 13, 558–576. [Google Scholar] [CrossRef]
- Agbor-Enoh, S.; Shah, P.; Tunc, I.; Hsu, S.; Russell, S.; Feller, E.; Shah, K.; Rodrigo, M.E.; Najjar, S.S.; Kong, H.; et al. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation 2021, 143, 1184–1197. [Google Scholar] [CrossRef]
- Pérez-Carrillo, L.; Giménez-Escamilla, I.; Sánchez-Lázaro, I.; Triviño, J.C.; Feijóo-Bandín, S.; Lago, F.; González-Juanatey, J.R.; Martínez-Dolz, L.; Portolés, M.; Tarazón, E.; et al. Alpha-cardiac Actin Serum Expression Levels Detect Acute Cellular Rejection in Heart Transplant Patients. Transplantation 2023, 107, 466–474. [Google Scholar] [CrossRef]
- Sutaria, N.; Sylvia, L.; DeNofrio, D. Immunosuppression and Heart Transplantation. Handb. Exp. Pharmacol. 2022, 272, 117–137. [Google Scholar] [PubMed]
- Paintner, P.; Lehner, A.; Riley, R.; Fischer, M.; Kozlik-Feldmann, R.; Rosenthal, L.; Orban, M.; Jakob, A.; Haas, N.; Ulrich, S. Comparison of the Prolonged- and Immediate-Release Tacrolimus Capsule Formulation: The Patient’s View and Medication Satisfaction of Patients After Pediatric Heart Transplantation. Transplant. Proc. 2023, 55, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Panackel, C.; Mathew, J.F.; Fawas, N.M.; Jacob, M. Immunosuppressive Drugs in Liver Transplant: An Insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Straburzyńska-Migaj, E.; Casadei, V.; Bociański, M.; Jemielity, M. Different Routes of Proton Pumps Inhibitors Co-Administration have Significant Impact on Mycophenolate Acid (MPA) Serum Levels in Heart Transplant Recipients. Ann. Transplant. 2020, 25, e920225. [Google Scholar] [CrossRef]
- Barshes, N.R.; Goodpastor, S.E.; Goss, J.A. Pharmacologic immunosuppression. Front. Biosci. 2004, 9, 411–420. [Google Scholar] [CrossRef]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Zhang, B.; Weuve, J.; Langa, K.M.; D’Souza, J.; Szpiro, A.; Faul, J.; Mendes de Leon, C.; Gao, J.; Kaufman, J.D.; Sheppard, L.; et al. Comparison of Particulate Air Pollution from Different Emission Sources and Incident Dementia in the US. JAMA Intern. Med. 2023, 183, 1080–1089. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511–7533. [Google Scholar] [CrossRef]
- Hou, T.; Zhu, L.; Wang, Y.; Peng, L. Oxidative stress is the pivot for PM2.5-induced lung injury. Food Chem. Toxicol. 2024, 184, 114362. [Google Scholar] [CrossRef]
- Henning, R.J. Particulate Matter Air Pollution is a Significant Risk Factor for Cardiovascular Disease. Curr. Probl. Cardiol. 2024, 49, 102094. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Skotak, K.; Olasińska-Wiśniewska, A.; Szczepański, K.; Tykarski, A.; Jemielity, M. Five-year mortality disparities across urban and rural areas in patients treated with coronary artery bypass grafting. Pol. Arch. Intern. Med. 2024, 134, 16847–16852. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Skotak, K.; Olasińska-Wiśniewska, A.; Filipiak, K.J.; Płachta-Krasińska, A.; Piecek, J.; Krasińska, B.; Krasiński, Z.; Tykarski, A.; Jemielity, M. The Possible Role of PM2.5 Chronic Exposure on 5-Year Survival in Patients with Left Ventricular Dysfunction Following Coronary Artery Bypass Grafting. Toxics 2024, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Liu, Y.; Wang, Z.; Wang, X.; Han, Z.; Yan, X.; Meng, A. PM2.5 activated NLRP3 inflammasome and IL-1β release in MH-S cells by facilitating autophagy via activating Wnt5a. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221137464. [Google Scholar] [CrossRef]
- Feng, Y.; Jones, M.R.; Ahn, J.B.; Garonzik-Wang, J.M.; Segev, D.L.; McAdams-DeMarco, M. Ambient air pollution and posttransplant outcomes among kidney transplant recipients. Am. J. Transplant. 2021, 21, 3333–3345. [Google Scholar] [CrossRef]
- Amubieya, O.; Weigt, S.; Shino, M.Y.; Jackson, N.J.; Belperio, J.; Ong, M.K.; Norris, K. Ambient Air Pollution Exposure and Outcomes in Patients Receiving Lung Transplant. JAMA Netw. Open 2024, 7, e2437148. [Google Scholar] [CrossRef]
- Chang, S.H.; Merzkani, M.; Murad, H.; Wang, M.; Bowe, B.; Lentine, K.L.; Al-Aly, Z.; Alhamad, T. Association of Ambient Fine Particulate Matter Air Pollution with Kidney Transplant Outcomes. JAMA Netw. Open 2021, 4, e2128190. [Google Scholar] [CrossRef]
- Choi, D.; North, M.; Ahmed, M.; Belousova, N.; Vasileva, A.; Matelski, J.; Singer, L.G.; Wu, J.K.Y.; Jeong, C.H.; Evans, G.; et al. Pollution exposure in the first 3 months post transplant is associated with lower baseline FEV1 and higher CLAD risk. J. Heart Lung Transplant. 2024, 43, 1987–1997. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Sarode, A.; Zullo, M.; Brook, J.; Burnett, R.; Oliveira, G.H.; Huang, W.; Brook, R.; Rajagopalan, S. Ambient Air Pollution and Mortality After Cardiac Transplantation. J. Am. Coll. Cardiol. 2019, 74, 3026–3035. [Google Scholar] [CrossRef]
- Gemelli, M.; Doulamis, I.P.; Tzani, A.; Rempakos, A.; Kampaktsis, P.; Alvarez, P.; Guariento, A.; Xanthopoulos, A.; Giamouzis, G.; Spiliopoulos, K.; et al. Rejection Requiring Treatment within the First Year following Heart Transplantation: The UNOS Insight. J. Pers. Med. 2023, 14, 52–64. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Hesselink, D.A.; van Gelder, T. Pharmacokinetics and pharmacodynamics of immunosuppressive drugs in elderly kidney transplant recipients. Transplant. Rev. 2015, 29, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Skotak, K.; Olasińska-Wiśniewska, A.; Filipiak, K.J.; Bratkowski, J.; Krasińska, B.; Krasiński, Z.; Tykarski, A.; Jemielity, M. The Interplay between Dyslipidemia and Neighboring Developments in Coronary Artery Disease Progression: A Personalized Approach. J. Pers. Med. 2024, 14, 237–251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).