Abstract
Open burning of agricultural waste is a common practice in both developed and developing regions of the world, and the emissions pose serious inputs for ambient concentrations of atmospheric particulate matter (PM). In addition, when agricultural waste burning is combined with open-air burning of domestic waste such as plastic, rags, or tires, the potential risk of generating toxic emissions increases. PM samples produced via controlled burning of selected waste types were tested in our laboratory using the No. 227 OECD Guideline for the Testing of Chemicals: Terrestrial Plant Test. Comparing two recommended test species, lettuce (Lactuca sativa) and mustard (Sinapis alba), L. sativa showed significantly higher sensitivity, as treatment elucidated biomass reduction (Df = 1, F = 16.43, p = 0.000385), the biomass of the treated plants was 61.4-91.7% of the control plants. In our investigation inhibition in photosynthetic pigment activity chlorophyll-b in lettuce (Df = 1, F = 3.609, p = 0.0701) was found. The levels of the stress enzyme peroxidase increased significantly in the case of both test species (L. sativa: Df = 1, F = 6.76, p = 0.0112; S. alba: Df = 1, F = 49.99, p = 1.63 × 10−9), indicating that peroxidase could be regarded as the most sensitive indicator of air pollution. The bioaccumulation pattern was also assessed, proving the risk of significant bioaccumulation of potentially toxic compounds in edible parts of the vegetables tested. Both test plants accumulated higher molecular weight PAHs in significant quantities, as the concentration of 5-ring PAHs was 43.2 μg/kg in mustard and 49.35 μg/kg in lettuce.
1. Introduction
The 2008/50/CE Directive of the European Union defined atmospheric particulate matter (PM) as one of the most dangerous pollutants to human health. Threshold levels are set for 10 μm-diameter (PM10) and 2.5 μm-diameter (PM2.5) particles; nevertheless, these thresholds are often exceeded [1]. Biomass burning is a major contributor, especially in the cold season when biomass such as wood or pellets is used for heating. However, Puxbaum et al. demonstrated that biomass combustion tracers, such as levoglucosan, could be found at rural European sites even in the warm season [2]. Potential sources include the burning of garden waste, such as cut branches, leaves, etc. Green waste burning in gardens both in suburban and rural areas is a widespread practice in European countries [3]. Reasons for doing so can be simple convenience; however, proper waste management practices can still be lacking. Composting can be a viable alternative; however, there are certain materials that cannot be composted, for example, fruits or leaves that are infected by bacteria, fungi, or other parasites.
In Europe, open-air combustion of garden waste is not allowed in certain countries [4]. In Hungary, however, it can be practiced under strictly controlled conditions. Real environmental issues emerge when garden waste is co-combusted with household waste such as plastic, used furniture, rags, or other pieces. Burning domestic waste either in the open air or indoors in stoves is strictly prohibited; still, it is a practice that can be widely experienced.
Open burning generates significant emissions of particulate matter, especially PM2.5 and finer fractions [5]. Depending on the type of waste burned, particles bind potentially toxic heavy metals and polycyclic aromatic hydrocarbons (PAHs) [6]. As co-combustion of household and garden wastes can occur in the vegetation period, plants can be seriously impacted both in the gardens and in the surrounding natural environments.
Plants are exposed to PM and PM-bound compounds via different pathways. Particles can be deposited on plant surfaces and onto the soil, carrying potentially toxic compounds. In addition to dry deposition, wet deposition is also an important pathway [7]. In this process, toxicants are washed out by rain or snow.
Potentially toxic compounds can exert direct phytotoxic effects mostly via the formation of reactive oxygen species (ROS) [8], and they can also accumulate in the edible parts of garden plants. Consumption of impacted vegetables and crops plays an important exposure route for humans both for PAHs and heavy metals. Leafy vegetables are especially at risk as they reportedly accumulate higher concentrations due to their enlarged foliar surface [6].
To obtain a comprehensive picture of the potential impacts, this study investigates the phytotoxicity and bioaccumulation effects of particulate matter from mixed waste burning using standardized plant testing. The No. 227 OECD Guideline for the Testing of Chemicals: Terrestrial Plant Test (hereinafter referred to as ‘Guideline’) was adapted and modified to assess the phytotoxic responses of two recommended test species, lettuce (Lactuca sativa) and mustard (Sinapis alba). The Guideline was originally designed to test the phytotoxicity of general chemicals, biocides, and crop protection products used in agriculture [9]. As the protocol described by the Guideline uses foliar application of test substances, it has also been proven applicable to investigate the effect of water-soluble components of PM [10]. In addition, foliar treatment could also be used to follow the bioaccumulation of PM-bound materials [11]. While diverse studies are already available to examine the deleterious effects of potentially toxic PM-bound compounds, this protocol makes it possible to separately investigate phytotoxicity posed by wet deposition.
The Guideline uses the following end-points: biomass, shoot length, and visual symptoms for phytotoxicity assessment. However, biochemical end-points have also shown good discriminative power when deleterious effects of urban PM were quantified on roadside plants [12] following the treatment protocol described by the Guideline. Biochemical end-points involve photosynthetic pigment levels and antioxidant or stress enzymes. Inhibition of photosynthetic activity has also been a traditionally applied end-point in air pollution studies, as earlier papers have reported decreased levels of chlorophyll-a and -b [13]. Air pollutants have been demonstrated to cause inhibitory effects on protein synthesis [14], and foliar protein content is a sensitive and easy-to-measure indicator of air pollution [15]. Antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) play a key role in the antioxidant defense system of plants. They have an old and established use in the assessment of airborne pollution [16,17].
2. Materials and Methods
2.1. Sampling and Sample Preparation
PM10 samples from the controlled burning of a model domestic waste were collected on quartz filters. Experimental conditions and detailed procedures are given in Hoffer et al. [18]. Shortly, each waste specimen is combusted in a commercially available cast-iron stove (Servant S 114, with a heating capacity of 5 kW). This model is generally used in Hungary. Prior to each combustion experiment, the stove was preheated with charcoal for about 1 h. The temperature of the flue gas was regularly checked, and the mean temperature value was 299 °C. PM10 samples were collected on 150 mm diameter quartz filters (Advantec QR100 quartz fiber—Frisenette Aps, Knebel, Denmark, binder-free) using a high-volume aerosol sampler (Kalman System Co., Budapest, Hungary; flow rate: 32 m3/h).
Based on the data of the (Hungarian) National Statistics Agency on the typical composition of domestic waste in Hungary, the following ratios were applied (Table 1).

Table 1.
Composition of the model waste burned during the experiment.
Filters were processed to prepare aqueous extract as follows. Each filter was cut into small pieces and placed in a beaker containing 200 mL of high-purity water. The beaker was covered and kept at room temperature for 24 h. During that time, pieces were stirred several times. The sample was filtered through a 0.45-µm pore size filter. One portion was used immediately for the first treatment, and the remaining extract was stored at −18 °C.
2.2. Analytical Determinations
PAHs were measured following MSZ (Hungarian Standard) 1484-6:2003 [19] using an Agilent 6890GC 5973E MSD GC-MS. The equipment used was an HP-6890 gas chromatograph coupled to an HP 5973 quadrupole mass spectrometer (low-resolution single MS, Agilent Technologies, Palo Alto, CA, USA).
During our measurements, one composite sample was made from each batch of test plants (mustard vs. lettuce, control vs. treated plants). First, 10 g of plant material was homogenized with 10 g of anhydrous sodium sulfate in a ceramic mortar and extracted using an ultrasonic extraction method with 20 mL n-hexane three times for 20 min. Then, 10 mL of acetone and 100 μL of 0.01 μg/mL deuterated PAH surrogate mixture (Naphtalene-d8, Acenaphthene-d10, Phenanthrene-d10, Chrysene-d12, Benzo(a)pyrene-d12, Perylene-d12; Restek Corporation, Bellefonte, PA, USA) were added to the extract and it was concentrated in a dry nitrogen stream to 1 mL and the dissolvent was changed to hexane. Next, 100 μL of 0.01 μg/mL internal standard mixture (2-floro-biphenyl and p-terphenyl-d14) was added (the final concentration was 4 μg/L water). The GC column was 30 m × 0.25 mm i.d., film thickness 0.25 μm, ZB-Semivolatiles (Phenomenex, Torrance, CA, USA).
A five-level internal calibration was used. The first level was the limit of quantitation, and the last level was 80% of the linear range. The limits of detection (LOD) values of accumulated PAHs in plant samples were 0.01 μg/kg.
Heavy metal concentrations were determined according to EPA 6010C: 2007 using ICP-OES Thermo iCAP 6300 (Kromat Ltd., Budapest, Hungary). HNO3 acid was used to acidify water samples before the measurement. The LOD were Hg 0.2 μg/L; Cd, Sb 0.5 μg/L; Au, As, Co, Sn, Pb, Cr, Ti, Se 1 μg/L; Mo, Ni 2 μg/L; Fe, Mn, Cu, Zn 5 μg/L; Al, B, Ba, Sr 10 μg/L. The linearity of the calibration curve was checked daily.
Quality control: Analysis of the samples was carried out in the testing laboratory at the Laboratory of the ELGOSCAR-2000 Environmental Technology and Water Management Ltd. Balatonfűzfő, Hungary, accredited by the National Accreditation Authority, registration number NAH-1-1278/2015. The measurements were performed according to laboratory accreditation procedures in compliance with ISO/IEC 17025:2018 [20] and with the laboratory’s internal quality management system guidelines.
2.3. Phytotoxicity Testing
Treatment of selected test species, L. sativa and S. alba, was performed based on the No. 227 OECD Guideline for the Testing of Chemicals: Terrestrial Plant Test protocol. In order to assess the phytotoxicity of the sample, the following end-points were used: biomass (as the measure of growth impairment), photosynthetic pigments, and peroxidase activity.
The steps of the test procedure are summarized in Figure 1.

Figure 1.
Steps of the phytotoxicity testing procedure.
2.3.1. Treatment of Test Plants
Mustard and lettuce seeds were purchased from Rédei Kertimag Zrt. In the case of lettuce, the ’King of May’ variety was selected. A total of 25–25 seeds were sown in pots of 15 cm diameter in commercial soil (pH: 6.8 ± 0.5; N (m/m%): min 0.3; P2O5 (m/m%): min 0.1; K2O (m/m%): min 0.3). Cultivation of the test plants was performed in a glasshouse, and the environmental conditions were as follows: temperature: 22 °C ± 10 °C; humidity: 70% ± 25%; photoperiod: minimum 16 h light; light intensity: 350 ± 50 µE/m2 s. These conditions were in harmony with the requirements of the Guideline.
Three consecutive treatments were applied at 1-week intervals. The first treatment (Day 0) was carried out when the plants reached the 4 true leaf stage; additional treatments were applied on Day 7 and Day 14.
Treatment implied that the test substance (extract) was sprayed on the surface of the test plants using a CONXIN Q1P-CX01-380 portable electric paint spray gun (CONXIN Ltd., Zhejiang, China) with an application volume of 5 mL/pot/treatment [21]. The test was terminated on Day 21. Above-ground parts were cut with a pre-washed scissor and were immediately taken to the laboratory. They were washed with ionic load-free water and kept in the freezer (−20 °C) until analysis.
Control groups were also used, where seedlings received foliar spraying with tap water on the same day as treated plants; 10-10 replicates (10-10 pots) were included both in the control and treatment groups.
2.3.2. Photosynthetic Pigment Measurements
Photosynthetic pigment contents were determined by the spectrophotometric method [22]. A 0.2 g sample was taken from the leaf of each plant (10-10 plants in each group). The sample was homogenized with 15 mL of 80% acetone (Fisher Chemicals, ≥99.8%, ACS; VWR International Ltd., Debrecen, Hungary) and centrifuged at 4500 rpm for 10 min (Mistral 2000 MSE, DJB Labcare Ltd. Newport Pagnell, Buckinghamshire, UK). The supernatant was separated and diluted with 80% acetone to a final volume of 25 mL. Absorbance was measured at 440 nm, 645 nm, 663 nm, and 750 nm using a UV-VIS spectrophotometer (Metertech SP8001, ABL&E-JASCO, Budapest, Hungary).
The following equations were used for final calculations:
where E: extinction values at wavelengths; V: final volume (25 mL); W = mass of sample (0.2 g).
Chl-a = [9.78 × E663 − 0.99 × E645] [V/1000 × W]
Chl-b = [21.4 × E645 − 465 × E663] × [V/1000 × W]
Car = [4.69 × E440 − 0.268 × (5.13 × E663 + 20.41 × E645)] × [V/1000 × W]
2.3.3. Peroxidase (POD) Activity
A total of 0.2 g leaf material per plant was taken, and the sample was frozen and homogenized in an ice-cold mortar and pestle in phosphate buffer (50 mmol/L, pH 7) containing 1 mmol/L EDTA and 0.5 mmol/L Phenylmethylsulfonyl fluoride (PMSF). Prepared samples were centrifuged for 20 min at 15,000× g at 4 °C. Tetramethylbenzidine (TMB) peroxidase activity (A 654) was measured following the protocol described in Imberty et al. [23] with minor modifications.
2.4. Bioaccumulation Studies
Lettuce and white mustard test plants were grown and treated according to the 227 OECD Guideline simultaneously with the vegetative vigor test. Similarly to the vegetative vigor test, plants received 3 treatments. Plants were cut on Day 28, and freshly cut leaves were washed with ionic-free water and kept in a freezer (−20 °C) until analysis; 10-10 plants of each species were used, also 10-10 control plants were grown. They received spraying with tap water.
2.5. Calculations and Statistical Methods
The analyses were carried out using one-way ANOVA in the R Statistical Environment. Statistical analyses were performed using the R 4.0.0 program (http://cran.r-project.org/src/base/R-4/R-4.0.0.tar.gz, accessed on 24 August 2024) packages Rcmdr, ssplines; RcmdrMisc; car; carData; sandwich; effects.
Bioconcentration Factors (BCF) were calculated according to the formula given by Kacálková and Tlustoš [24]:
3. Results and Discussion
3.1. Composition of the Extract
A total of 19 PAHs were found in the extract, including the 16 priority EPA PAHs (Table 2). These are defined by the US Environmental Protection Agency (EPA) as posing the highest environmental risk [25].

Table 2.
Concentration of PAHs in the waste PM10 extract.
The dominant PAHs in the extract were phenanthrene (47.2 µg/L), fluoranthene (22 µg/L), and pyrene (19.3 µg/L). Considering their potential sources, their occurrence cannot be regarded as specific, as different burning activities resulted in the emission of these PAHs. Kwarteng et al. detected high concentrations of phenanthrene, fluoranthene, and pyrene when occupational exposures to PM2.5-associated PAHs were monitored at a waste recycling site in Ghana [26]. Phenanthrene was also a dominant PAH when emissions from green waste burning [27] or from the open burning of agricultural debris [28] were characterized.
The 5-ring dibenzo(a.h)anthracene and 6-ring benzo(g.h.i)perylene were detected in relatively high concentrations, 0.379 and 0.477 µg/L. One potential source can be, for example, polyurethane (PU). Kováts et al. found similarly high concentrations of these PAHs when PU was burned under similar controlled conditions [6]. The joint presence of benzo(a)pyrene and indeno1.2.3CD-pyrene can be attributed to waste tire components. Capozzi et al. measured PAH accumulation in Robinia pseudoacacia leaves in areas where waste burnings randomly occur and attributed the occurrence of these PAHs to tire burning [29]. Mentes et al. measured PAH emission from waste tire combustion at different temperatures. A wide range of PAHs were detected in the samples; total PAH concentration decreased with increasing temperature from 18.83 mg total PAH kg−1 fuel burned at 650 °C to 1.91 mg total PAH kg−1 fuel burned at 900 °C [30].
Of potentially toxic heavy metals, Sb and Zn were detected in considerable concentrations (Table 3). One of the potential sources of Sb can be the polyethylene terephthalate (PET) content of the waste burned. PET has become the most used material for food and beverage packaging [31] but also represents an important share of household waste [32]. Antimony trioxide (Sb2O3) is used as a catalyst during the manufacture of PET, leaving a residual antimony content reaching as high as 300 mg/kg [33]. Sb has demonstrated phytotoxicity. Zhu et al. reported that in higher plants, Sb disturbed the ROS balance [34]. Ba also occurred in high concentration, 23 µg/L. Different Ba compounds are used in various plastics; BaSO4 is most commonly applied as an inert white filler and extender [35]. Barium is a nonessential element for plants, and it causes reported toxicity only in excess concentrations [36].

Table 3.
Concentration of heavy metals in the PM10 extract.
Zn was detected in PM10 samples when different plastic materials were burned under controlled conditions in a comparative study by Kováts et al. Zn concentrations were in the range of 8.23–78.8 μg/kg, with polyvinyl chloride (PVC) emitting the highest value [6]. Valavanidis et al. measured potentially toxic heavy metal concentrations in particulate soot emissions from the controlled combustion of six different plastic materials such as polyvinyl chloride, low- and high-density polyethylene, polystyrene, polypropylene, and polyethylene terephthalate [37]. In comparison to all other samples, PET burning emissions contained high amounts of Al, Pb, Cu, Ni, and Zn. Hama et al. reported that in PM2.5 samples from solid waste burning, Zn and Pb were found in high concentrations [38]. Peter et al. detected a wide range of toxic heavy metals such as As, Cd, Cu, Cr, Ni, Pb, Se, and Zn in PM2.5 samples in a municipal waste dumpsite affected by accidental or deliberate burning [39]. Heavy metals were even detected in firewater samples from experimentally burned waste dumps [40].
Zn has been experimentally shown to induce antioxidative responses [41]. In heavy metal-polluted soils, Zn was found to be the principal cause of the phytotoxic effects [42].
3.2. Phytotoxicity Assessment
Table 4 gives a summary of phytotoxicity test results, comparing the two test plants. L. sativa showed higher sensitivity, as 3 test end-points indicated a toxic effect.

Table 4.
Summary of phytotoxicity test results. +: significant effect recorded; 0: effect recorded was non-significant.
3.2.1. Decrease in Biomass
Considering biomass reduction after the treatment, lettuce showed a significant response (Df = 1, F = 16.43, p = 0.000385) (Figure 2). Mustard was non-responsive (Df = 1, F = 0.156, p = 0.694).
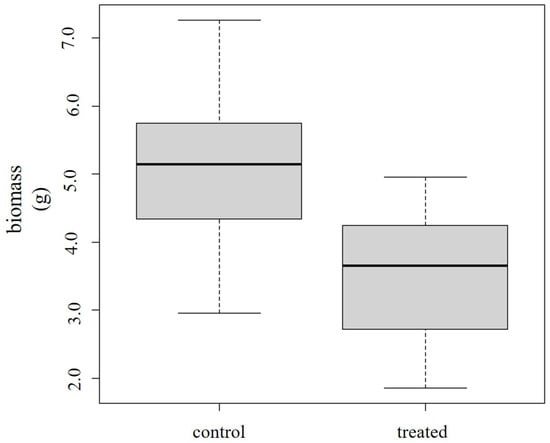
Figure 2.
Decreased biomass in treated lettuce plants in comparison to the control.
Reduced biomass in treated groups seems to be a general symptom when the deleterious effects of air pollution are examined. Kováts et al. evaluated the phytotoxic effects of urban PM2.5 extract on 13 common roadside plants and found biomass reduction to have the highest discriminative power [12]. Biomass was reported as the most sensitive end-point in the study of Storch-Böhm et al. when test plants were exposed to diesel engine exhaust [43]. Pavlík et al. treated Lactuca sativa plants with atmospheric PM suspensions. A wide range of symptoms were detected, including reduced biomass in treated plants [44].
Treatment with individual PAHs also triggered growth inhibition in several studies. Foliar application of anthracene resulted in reduced biomass and a decrease in photosynthetic performance in lettuce plants [45]. Similar treatment with anthracene and benzo(k)fluoranthene caused biomass reduction in celery plants [46]. Phytotoxicity of atmospheric phenanthrene was linked with biomass decrease in clover [47]. Foliar exposure to phenanthrene caused a concentration-dependent reduction in growth, photosynthesis, and chlorophyll contents in the study of Ahammed et al. [48].
3.2.2. Chlorophyll-a and Chlorophyll-b Content
As indicated in Table 4, the only measurable effect was experienced in the case of chlorophyll-b in lettuce (Df = 1, F = 3.609, p = 0.0701). White mustard did not show any significant response.
A decrease in chlorophyll content in polluted sites can be associated with the inhibition of chlorophyll biosynthesis, chloroplast damage, or enhanced chlorophyll degradation [49]. These will, in turn, negatively influence photosynthetic rates and productivity [50].
Reduction in photosynthetic pigment content has been considered as an ultimate answer to air pollution. Total chlorophyll content was used as a measure of plant tolerance to air pollution in a wide range of studies [51,52,53,54]. Different photosynthetic pigments, however, might show different and species-dependent sensitivity without clear tendency. Giri et al. assessed the impact of air pollution on Azadirachta indica, Nerium oleander, Mangifera indica, and Dalbergia sissoo. In the case of N. oleander, Chl-a showed a higher reduction than Chl-b but in the case of M. indica, the trend was just the opposite [55]. Azadirachta indica, Nerium oleander, Mangifera indica, and Ficus bengalensis were used in a similar study [56]. Chl-a showed a higher reduction than Chl- b in polluted sites in the case of A. indica, N. oleander, and F. benghalensis; however, M. indica showed a different response again, similarly to the previously cited study. Interestingly, carotenoid reduction showed very similar values for all species. Joshi and Swami [57] also reported species-dependent differences in the sensitivity of Chl-a and Chl-b when the effects of traffic-related air pollution were assessed on 6 different tree species.
In our study, white mustard did not show significant responses in any of the photosynthetic pigments. Joshi et al., on the contrary, detected changes in all pigments when white mustard was collected from clean and urban/industrial sites in India. The study, however, used exposure covering several months, much longer than the 21 days of our test [58].
3.2.3. Peroxidase Activity
Increased activities of peroxidase were found in both test plants (Figure 3). The differences between the control and treated groups were significant (in the case of lettuce, Df = 1, F = 6.76, p = 0.0112, while in the case of mustard, Df = 1, F = 49.99, p = 1.63 × 10−9).
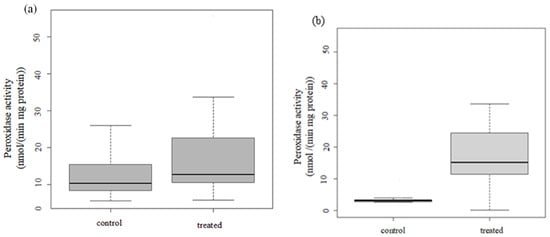
Figure 3.
Changes in peroxidase activity in (a) lettuce and (b) mustard.
In our study, increasing POD activities were measured. The changes in POD activity can be considered as a general and sensitive indicator of plant stress [59]. Earlier studies used this biomarker for assessing the impact of air pollution on plants [16]. Both atmospheric PAHs and heavy metals have been reported to elucidate oxidative stress via the overproduction of reactive oxygen species (ROS). Excess ROS triggers the activation of antioxidant enzymes such as peroxidases [60].
In previous studies, POD was found to be the most sensitive end-point in plant stress experiments assessing the phytotoxicity of both heavy metals [61] and PAH exposure [62]. Our results are in harmony with the results of other investigations, where raised activity of peroxidase has been reported as a general response to PM-bound toxic compounds [63].
3.3. Bioaccumulation and Bioconcentration of PAHs
All PAHs measured in the extract (Table 2) were accumulated in the leaves of both the mustard and lettuce test plants. Table 5 gives their concentration in the PM extract as well as in the plants. Additionally, calculated BCFs are given.

Table 5.
Concentration of PAHs in the test plants and calculated BCF values.
Comparing the accumulation capacity of the two test plants, lettuce had a better accumulation rate. Total PAHs amounted to 367 µg/kg, while the concentration of total PAHs was 141.3 µg/kg in mustard. Lettuce has a high foliar surface, with a thin cuticula, making the plant very sensitive to foliar deposition of contaminants. It has been proven to have outstanding bioaccumulation potential [64,65]. In a lab-scale accumulation study, lettuce test plants were treated with diesel exhaust PM2.5 extract, and these test plants accumulated 18 PAHs being present in the sample [11]. Field studies also indicate that lettuce generally accumulates a wide range of PAHs [12].
While mustard is a widely used test species, rather scarce information is available considering its bioaccumulation potential. Other Brassicaceae species have been mentioned in literature studies, but in comparative studies, they generally show lower accumulation potential than lettuce. Chen et al. measured the concentration of 16 PAHs in home gardens affected by straw burning. These concentrations were in the range of 208.7 to 269.5 μg/kg in lettuce but reached lower values in cabbage: 114.5 to 175.1 μg/kg. Accumulation in Chinese cabbage was similar to lettuce, ranging from 200.8 to 295.1 μg/kg [66]. Jia et al. collected different vegetable samples from near industrial areas of Shanghai and found highest concentrations in romaine (up to 458.0 μg/kg), followed by Chinese cabbage (up to 348.1 μg/kg), lettuce (up to 319 μg/kg), and finally in Shanghai green cabbage (284 μg/kg) [67]. Rocket (Eruca sativa Mill.) was found to show lower PAH accumulation than lettuce in a comparative study by Kováts et al. when foliar uptake of PAHs from an aqueous extract of diesel aerosol sample was assessed using different test plants [6].
Differences were found both in the total accumulation capacity of the two plants and in the accumulation pattern (Figure 4). Considering lower molecular weight (LMW) PAHs, mustard accumulated in total 22 μg/kg of 2-ring PAHs, 12.8 μg/kg of 3-ring PAHs, and 53.2 μg/kg of 4-ring PAHs, while these figures were 116 μg/kg, 110.4 μg/kg, and 80.4 μg/kg in case of lettuce. However, when the accumulation of higher molecular weight (HMW) PAHs is compared, values fall very close to each other. Mustard accumulated 43.2 μg/kg of 5-ring PAHs, and lettuce 49.35 μg/kg. Similarly, mustard accumulated 11.9 μg/kg of 5-ring PAHs, and lettuce 11.1 μg/kg.
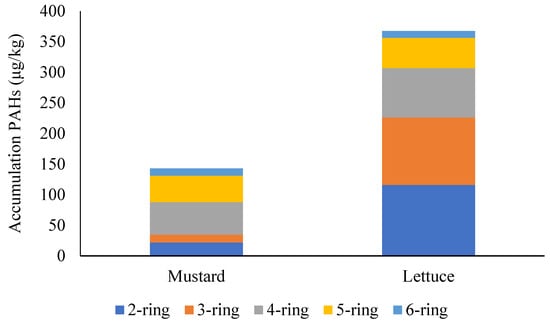
Figure 4.
Accumulation pattern of different molecular weight PAH groups in the test plants.
Most studies agree that bioaccumulation is more effective for LMW PAHs. However, different plant species reportedly have different bioaccumulation capacities. Teke et al., for example, found a significant correlation between PAH BCFs and molecular weights in lettuce [11]. Mustard, on the other hand, showed higher bioconcentration for HMW PAHs [68]. According to Chen et al., the ratio between LMW and HMW PAHs was 65.5% and 34.5% in lettuce, 80% and 20% in cabbage, and 66.8% and 33.2% in Chinese cabbage when the effects of biomass burning were assessed in home gardens [66].
Accumulation of PAHs in vegetables might pose human health risks as well, considering that dietary uptake is the major exposure route of these compounds in comparison to inhalation [69]. The International Agency for Research on Cancer overviewed the potential carcinogenicity of 16 polycyclic aromatic compounds present in food (reviewed by Sampaio et al. [70]). According to this classification, benzo(a)pyrene is carcinogenic to humans, benzanthracene, benzo(b)fluoranthene, benzo(k)fluoranthene, and dibenzo[a.h]anthracene are probably carcinogenic to humans, finally, chrysene and the 6-ring indeno1.2.3CD-pyrene and benzo(g.h.i)perylene are possibly carcinogenic to humans. These compounds were found in the tested plants in measurable quantities (Table 5), e.g., the concentration of the probably carcinogenic benzo(b)fluoranthene amounted to 16.9 µg/kg in mustard and 17.8 µg/kg in lettuce.
3.4. Limitations of the Study
Although open burning of domestic waste is a worldwide problem, the composition of the waste shows geographical differences. The model waste used in our burning experiment was based on Hungarian statistical data, with the highest ratio of plastics (15%), followed by textiles (13%) and treated wood (12%). The dominance of plastics was reported in studies from South Africa [71] and from Nepal, as well [72]. A US study, however, also included glass (e.g., bottles) and metals (e.g., cans) simulating so-called barrel burning [73]. The composition of the waste burned will influence the quality of emissions. Our study concentrated on compounds with well-known phytotoxic potential, such as PAHs and heavy metals, but other studies address compounds that are potentially hazardous for human health, such as polychlorinated dibenzo-p-dioxins and dibenzofurans [74] or endocrine disrupters [75]. The major limitation of the study, however, is that, at present, the magnitude of the problem (e.g., the number of households following this illegal practice in Hungary) is not known, which might identify a gap both for scientists and policy-makers.
4. Conclusions
Open burning of domestic waste is a worldwide problem. Though more and more studies are addressing the possible impacts emissions might pose to human health, investigations concerning ecosystem-level impacts are still rare. In this study, the phytotoxic effects of particulate matter originating from controlled burning of garden and household waste were assessed. The sensitivity of two test plants, mustard and lettuce, was also compared. While lettuce showed moderate phytotoxic effects, mustard was less responsive as only peroxidase level reduction indicated the deleterious effect of the treatments. Amongst all biological end-points, the stress enzyme peroxidase proved the most sensitive. Bioaccumulation of particle-bound polycyclic aromatic hydrocarbons was also evaluated. In lettuce, a significant accumulation of 16 EPA PAHs was experienced; this test species showed, in general, a higher accumulation capacity than mustard. However, bioaccumulation of heavier (5- and 6-ring PAHs) was very similar in both plants. As consumption of PAH-contaminated food is the most significant exposure pathway for humans, these results will most possibly indicate the human health risk open burning of waste might pose. While burning household waste is illegal in Hungary (similar to many countries in Europe), more stringent educational campaigns might be needed to stop this kind of practice. In addition, the role of local governments needs to be emphasized as they also play a vital role in more effective control of such behavioral practices.
Author Contributions
Conceptualization, S.T. and K.H.; methodology and investigation, S.T., B.E.-V., G.T. and K.H.; data curation, K.H.; visualization, S.T. and K.H.; writing—original draft preparation, N.K.; supervision, project administration, funding acquisition, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the NKFIH-872 project ‘Establishment of a National Multidisciplinary Laboratory for Climate Change’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data analyzed during this study are included in this published article.
Acknowledgments
This work was supported by the ’National Multidisciplinary Laboratory for Climate Change’ project (National Research, Development, and Innovation Office, NKFIH-872). The authors thank the ELGOSCAR-2000 Environmental Technology and Water Management Ltd. (Head Office: 164 Soroksari u. H-1095 Budapest, Laboratory: H-8184 Balatonfuzfo) for conducting analytical measurements and the HUN-REN–PE Air Chemistry Research Group (University of Pannonia) for providing the samples.
Conflicts of Interest
Gábor Teke is an employee of ELGOSCAR-2000 Environmental Technology and Water Management Ltd. The paper reflects the views of the scientists and not the company.
References
- Van Dingenen, R.; Raes, F.; Putaud, J.-P.; Baltensperger, U.; Charron, A.; Facchini, M.-C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Hansson, H.-C.; et al. A European aerosol phenomenology-1: Physical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2561–2577. [Google Scholar] [CrossRef]
- Puxbaum, H.; Caseiro, A.; Sánchez-Ochoa, A.; Kasper-Giebl, A.; Claeys, M.; Gelencsér, A.; Legrand, M.; Preunkert, S.; Pio, C. Levoglucosan levels at background sites in Europe for assessing the impact of biomass combustion on the European aerosol background. J. Geophys. Res. 2007, 112, D23S05/1–D23S05/11. [Google Scholar] [CrossRef]
- Noblet, C.; Lestremau, F.; Collet, S.; Chatellier, C.; Beaumont, J.; Besombes, J.L.; Albinet, A. Aerosolomics based approach to discover source molecular markers: A case study for discriminating residential wood heating vs garden green waste burning emission sources. Chemosphere 2024, 352, 141242. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, C.; Bauer, H.; Dattler, A.; Hitzenberger, R.; Weissenboeck, G.; Marr, I.L.; Puxbaum, H. Chemical characterisation of particle emissions from burning leaves. Atmos. Environ. 2008, 42, 9070–9079. [Google Scholar] [CrossRef]
- Krecl, P.; de Lima, C.H.; Bosco, T.C.D.; Targino, A.C.; Hashimoto, E.M.; Oukawa, G.Y. Open waste burning causes fast and sharp changes in particulate concentrations in peripheral neighborhoods. Sci. Total Environ. 2021, 765, 142736. [Google Scholar] [CrossRef] [PubMed]
- Kováts, N.; Hubai, K.; Diósi, D.; Hoffer, A.; Teke, G. Foliar uptake and accumulation of polycyclic aromatic hydrocarbons from diesel emissions. Polycycl. Aromat. Compd. 2021, 42, 6124–6135. [Google Scholar] [CrossRef]
- Grantz, D.A.; Garner, J.H.B.; Johnson, D.W. Ecological effects of particulate matter. Environ. Int. 2003, 29, 213–239. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Sakugawa, H. Fluoranthene fumigation and exogenous scavenging of reactive oxygen intermediates (ROI) in evergreen Japanese red pine seedlings (Pinus densiflora Sieb. et. Zucc.). Chemosphere 2008, 72, 747–754. [Google Scholar] [CrossRef]
- Boutin, C.; Aya, K.L.; Carpenter, D.; Thomas, P.J.; Rowland, O. Phytotoxicity testing for herbicide regulation: Shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Sci. Total Environ. 2012, 415, 79–92. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Teke, G. Effects of urban atmospheric particulate matter on higher plants using Lycopersicon esculentum as model species. SN Appl. Sci. 2021, 3, 770. [Google Scholar] [CrossRef]
- Teke, G.; Hubai, K.; Diósi, D.; Kováts, N. Assessment of Foliar Uptake and Accumulation of Airborne Polyaromatic Hydrocarbons Under Laboratory Conditions. Bull. Environ. Contam. Toxicol. 2020, 104, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Kováts, N.; Hubai, K.; Sainnokhoi, T.A.; Teke, G. Biomonitoring of polyaromatic hydrocarbon accumulation in rural gardens using lettuce plants. J. Soils Sed. 2021, 21, 106–117. [Google Scholar] [CrossRef]
- Rabe, R.; Kreeb, K.H. Enzyme activities and chlorophyll and protein content in plants as indicators of air pollution. Environ. Pollut. 1979, 19, 119–137. [Google Scholar] [CrossRef]
- Kumar, G.S.; Dubey, P.S. Differential response and detoxifying mechanism of Cassia siamea Lam. and Dalbergia sissoo Roxb. of different ages to SO2 treatment. J. Environ. Biol. 1998, 9, 243–249. [Google Scholar]
- Verma, A.; Singh, S.N. Biochemical and ultrastructural changes in plant foliage exposed to auto-pollution. Environ. Monit. Assess. 2006, 120, 585–602. [Google Scholar] [CrossRef]
- Keller, T. The use of peroxidase activity for monitoring and mapping air pollution areas. Eur. J. For. Pathol. 1974, 4, 11–19. [Google Scholar] [CrossRef]
- Sarkar, R.K.; Banerjee, A.; Mukherji, S. Acceleration of Peroxidase and Catalase Activities in Leaves of Wild Dicotyledonous Plants, as an Indication of Automobile Exhaust Pollution. Environ. Pollut. Ser. A 1986, 42, 289–295. [Google Scholar] [CrossRef]
- Hoffer, A.; Jancsek-Turóczi, B.; Tóth, Á.; Kiss, G.; Naghiu, A.; Levei, E.A.; Marmureanu, L.; Machon, A.; Gelencsér, A. Emission factors for PM10 and PAHs from illegal burning of different types of municipal waste in households. Atmos. Chem. Phys. 2020, 20, 16135–16144. [Google Scholar] [CrossRef]
- MSZ 1484-6:2003; Testing of waters. Part 6: Determination of polycyclic aromatic hydrocarbons (PAH) content by gas chromatographic-mass spectrometry. Hungarian Standard Association: Budapest, Hungary, 2003.
- ISO/IEC 17025:2018; Magyar Szabvány. Vizsgáló- és kalibrálólaboratóriumok felkészültségének általános követelményei. Vizsgálólaboratóriumok akkreditálási programja. International Organization for Standardization: Geneva, Switzerland, 2018.
- Kováts, N.; Fábián, V.A.; Hubai, K.; Diósi, D.; Sainnokhoi, T.A.; Békéssy, Z.; Teke, G. Seasonal differences in rural particulate matter ecotoxicity. Aerosol Sci. Eng. 2020, 4, 169–177. [Google Scholar] [CrossRef]
- Bag, N.; Palni, L.; Chandra, S.; Nandi, S. Somatic embryogenesis in ’maggar’ bamboo (Dendrocalamus hamiltonii) and field performance of regenerated plants. Curr. Sci. 2012, 102, 1279–1287. [Google Scholar]
- Imberty, A.; Goldberg, R.; Catesson, A.-M. Tetramethylbenzidine and p-phenylenediamine-pyrocat-echol for peroxidase histochemistry and biochemistry: Two new, non-carcinogenic chromogens for investigating lignification process. Plant Sci. Lett. 1984, 35, 103–108. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustoš, P. The uptake of persistent organic pollutants by plants. Cent. Eur. J. Biol. 2011, 6, 223–235. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Kwarteng, L.; Devasurendra, A.M.; Laskaris, Z.; Arko-Mensah, J.; Nti, A.A.A.; Takyi, S.; Acquah, A.A.; Dwomoh, D.; Basu, N.; Robins, T.; et al. Occupational exposures to particulate matter and PM2. 5-associated polycyclic aromatic hydrocarbons at the Agbogbloshie waste recycling site in Ghana. Environ. Int. 2022, 158, 106971. [Google Scholar] [CrossRef]
- Noblet, C.; Besombes, J.L.; Lemire, M.; Pin, M.; Jaffrezo, J.L.; Favez, O.; Aujay-Plouzeau, R.; Dermigny, A.; Karoski, N.; Van Elsuve, D.; et al. Emission factors and chemical characterization of particulate emissions from garden green waste burning. Sci. Total Environ. 2021, 798, 149367. [Google Scholar] [CrossRef]
- Kakareka, S.V.; Kukharchyk, T.I. PAH emission from the open burning of agricultural debris. Sci. Total Environ. 2003, 308, 257–261. [Google Scholar] [CrossRef]
- Capozzi, F.; Di Palma, A.; Adamo, P.; Spagnuolo, V.; Giordano, S. Monitoring chronic and acute PAH atmospheric pollution using transplants of the moss Hypnum cupressiforme and Robinia pseudacacia leaves. Atmos. Environ. 2017, 150, 45–54. [Google Scholar] [CrossRef]
- Mentes, D.; Tóth, C.E.; Nagy, G.; Muránszky, G.; Póliska, C. Investigation of gaseous and solid pollutants emitted from waste tire combustion at different temperatures. Waste Manag. 2022, 149, 302–312. [Google Scholar] [CrossRef]
- Filella, M. Antimony and PET bottles: Checking facts. Chemosphere 2020, 261, 127732. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Astrup, T.F. Characterisation of source-separated, rigid plastic waste and evaluation of recycling initiatives: Effects of product design and source-separation system. Waste Manag. 2019, 87, 161–172. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.A.; Hinojosa-Reyes, L.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.; Maya-Treviño, L.; Guzmán-Mar, J.L. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Li, Z.F.; Shen, J.; Wu, K.Y.; Zhao, P.P.; Wu, Z.; Liu, Z.; Yang, J.; Liu, H.; Rensing, C.; et al. Toxicity of different forms of antimony to rice plants: Photosynthetic electron transfer, gas exchange, photosynthetic efficiency, and carbon assimilation combined with metabolome analysis. J. Hazard. Mater. 2022, 437, 129433. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Filella, M. The influence of additives on the fate of plastics in the marine environment, exemplified with barium sulphate. Mar. Pollut. Bull. 2020, 158, 111352. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cardoso, A.A.; Monteiro, F.A. Sulfur supply reduces barium toxicity in Tanzania guinea grass (Panicum maximum) by inducing antioxidant enzymes and proline metabolism. Ecotoxicol. Environ. Saf. 2021, 208, 111643. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Iliopoulos, N.; Gotsis, G.; Fiotakis, K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard. Mater. 2008, 156, 277–284. [Google Scholar] [CrossRef]
- Hama, S.; Kumar, P.; Alam, M.S.; Rooney, D.J.; Bloss, W.J.; Shi, Z.; Harrison, R.M.; Crilley, L.R.; Khare, M.; Gupta, S.K. Chemical source profiles of fine particles for five different sources in Delhi. Chemosphere 2021, 274, 129913. [Google Scholar] [CrossRef]
- Peter, A.E.; Nagendra, S.M.S.; Nambi, I.M. Comprehensive analysis of inhalable toxic particulate emissions from an old municipal solid waste dumpsite and neighborhood health risks. Atmos. Pollut. Res. 2018, 9, 1021–1031. [Google Scholar] [CrossRef]
- Kucerova, R.; Zavoral, M.; Mudrunka, J.; Takac, D.; Marcalikova, L. Analysis of Firewater Samples from Simulated Fires in Illegal Waste Dumps. Eng. Proc. 2023, 57, 14. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of nano-zinc oxide to Tomato plant (L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Beyer, W.N.; Green, C.E.; Beyer, M.; Chaney, R.L. Phytotoxicity of zinc and manganese to seedlings grown in soil contaminated by zinc smelting. Environ. Pollut. 2013, 179, 167–176. [Google Scholar] [CrossRef]
- Storch-Böhm, R.F.; Somensi, C.A.; Cotelle, S.; Deomar-Simoes, M.J.; Poyer-Radetski, L.; Dalpiaz, F.L.; Pimentel-Almeida, W.; Férard, J.-F.; Radetski, C.M. Sensitivity of different parameters for selection of higher plants in urban afforestation: Exposure of Guabiroba (Campomanesia xanthocarpa O. Berg.) to diesel engine exhaust. Environ. Pollut. 2020, 265, 114675. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, M.; Pavlíková, D.; Zemanová, V.; Hnilička, F.; Urbanová, V.; Szákova, J. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotoxicol. Environ. Saf. 2012, 79, 101–107. [Google Scholar] [CrossRef]
- Wieczorek, J.K.; Wieczorek, Z.J. Phytotoxicity and accumulation of anthracene applied to the foliage and sandy substrate in lettuce and radish plants. Ecotoxicol. Environ. Saf. 2007, 66, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Sienkiewicz, S.; Pietrzak, M.; Wieczorek, Z. Uptake and phytotoxicity of anthracene and benzo[k]fluoranthene applied to the leaves of celery plants (Apium graveolens var. secalinum L.). Ecotoxicol. Environ. Saf. 2015, 115, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Desalme, D.; Binet, P.; Epron, D.; Bernard, N.; Gilbert, D.; Toussaint, M.-L.; Plain, C.; Chiapusio, G. Atmospheric phenanthrene pollution modulates carbon allocation in red clover (Trifolium pratense L.). Environ. Pollut. 2011, 159, 2759–2765. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, M.M.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress. Ecotoxicol. Environ. Saf. 2012, 80, 132–139. [Google Scholar] [CrossRef]
- Paull, N.J.; Krix, D.; Irga, P.J.; Torpy, F.R. Green wall plant tolerance to ambient urban air pollution. Urban. For. Urban. Green. 2021, 63, 127201. [Google Scholar] [CrossRef]
- Kaur, M.; Nagpal, A.K. Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ. Sci. Pollut. Res. 2017, 24, 18881–18895. [Google Scholar] [CrossRef]
- Karmakar, D.; Padhy, P.K. Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef]
- Zhang, P.Q.; Liu, Y.J.; Chen, X.; Yang, Z.; Zhu, M.H.; Li, Y.P. Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicol. Environ. Saf. 2016, 132, 212–223. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Gong, J.; Yang, B.; Zhang, Z.; Wang, B.; Zhu, C.; Shi, J.; Yue, K. Comparison of the suitability of plant species for greenbelt construction based on particulate matter capture capacity, air pollution tolerance index, and antioxidant system. Environ. Pollut. 2020, 263, 114615. [Google Scholar] [CrossRef]
- Bharti, S.K.; Trivedi, A.; Kumar, N. Air pollution tolerance index of plants growing near an industrial site. Urb. Clim. 2018, 24, 820–829. [Google Scholar] [CrossRef]
- Giri, S.; Shrivastava, D.; Deshmukh, K.; Dubey, P. Effect of Air Pollution on Chlorophyll Content of Leaves. Curr. Agric. Res. J. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Pimple, N.S. Adverse effect of air pollutants on the chlorophyll content in leaves from Pune, Maharashtra (India). Int. J. Pharm. Sci. Rev. Res. 2017, 44, 131. [Google Scholar]
- Joshi, P.C.; Swami, A. Air pollution induced changes in the photosynthetic pigments of selected plant species. J. Environ. Biol. 2009, 30, 295–298. [Google Scholar]
- Joshi, N.; Chauhan, A.; Joshi, P.C. Impact of industrial air pollutants on some biochemical parameters and yield in wheat and mustard plants. Environmentalist 2009, 29, 398–404. [Google Scholar] [CrossRef]
- Czégény, G.; Rácz, A. Phenolic peroxidases: Dull generalists or purposeful specialists in stress responses? J. Plant Physiol. 2023, 280, 153884. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and metabolic plant responses toward polycyclic aromatic hydrocarbons and heavy metals present in atmospheric pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Ye, Y.B.; Cui, B.; Huang, Y.H.; Colón-Carmona, A.; Wang, Z.H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Schreck, E.; Bonnard, R.; Laplanche, C.; Leveque, T.; Foucault, Y.; Dumat, C. DECA: A new model for assessing the foliar uptake of atmospheric lead by vegetation, using Lactuca sativa as an example. J. Environ. Manag. 2012, 112, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreck, E.; Laplanche, C.; Le Guédard, M.; Bessoule, J.-J.; Austruy, A.; Xiong, T.; Foucault, Y.; Dumat, C. Influence of fine process particles enriched with metals and metalloids on Lactuca sativa L. leaf fatty acid composition following air and/or soil-plant field exposure. Environ. Pollut. 2013, 179, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Zhang, J.; Zhou, M.; Li, F.; Liu, X. Accumulation characteristics and potential risk of PAHs in vegetable system grow in home garden under straw burning condition in Jilin, Northeast China. Ecotoxicol. Environ. Saf. 2018, 162, 647–654. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.; Jin, X.; Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ. Pollut. 2018, 241, 750–758. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Eck-Varanka, B.; Tumurbaatar, S.; Teke, G. Accumulation of Atmospheric PAHs in White Mustard–Can the Seeds Be Affected? Bull. Environ. Contam. Toxicol. 2024, 112, 76. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Wang, X.; Firouzkouhi, H.; Chow, J.C.; Watson, J.G.; Carter, W.; De Vos, A.S. Characterization of gas and particle emissions from open burning of household solid waste from South Africa. Atmos. Chem. Phys. 2023, 23, 8921–8937. [Google Scholar] [CrossRef]
- Das, B.; Bhave, P.V.; Sapkota, A.; Byanju, R.M. Estimating emissions from open burning of municipal solid waste in municipalities of Nepal. Waste Manag. 2018, 79, 481–490. [Google Scholar] [CrossRef]
- Gullett, B.K.; Lemieux, P.M.; Lutes, C.C.; Winterrowd, C.K.; Winters, D.L. Emissions of PCDD/F from uncontrolled, domestic waste burning. Chemosphere 2001, 43, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.; Gullett, B.; Striebich, R.; Klosterman, J.; Contreras, J.; DeVito, M. Endocrine disrupting chemical emissions from combustion sources: Diesel particulate emissions and domestic waste open burn emissions. Atmos. Environ. 2005, 39, 801–811. [Google Scholar] [CrossRef]
- Song, S.; Zhou, X.; Guo, C.; Zhang, H.; Zeng, T.; Xie, Y.; Liu, J.; Zhu, C.; Sun, X. Emission characteristics of polychlorinated, polybrominated and mixed polybrominated/chlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs, PBDD/Fs, and PBCDD/Fs) from waste incineration and metallurgical processes in China. Ecotoxicol. Environ. Saf. 2019, 184, 109608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).