Assessing Risk of Emissions Generated During Illegal Waste Burning: Phytotoxicity and Bioaccumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Analytical Determinations
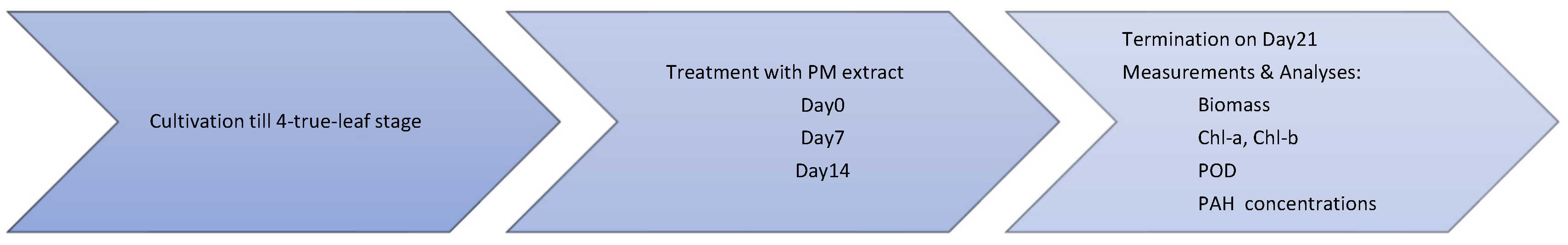
2.3. Phytotoxicity Testing
2.3.1. Treatment of Test Plants
2.3.2. Photosynthetic Pigment Measurements
2.3.3. Peroxidase (POD) Activity
2.4. Bioaccumulation Studies
2.5. Calculations and Statistical Methods
3. Results and Discussion
3.1. Composition of the Extract
3.2. Phytotoxicity Assessment
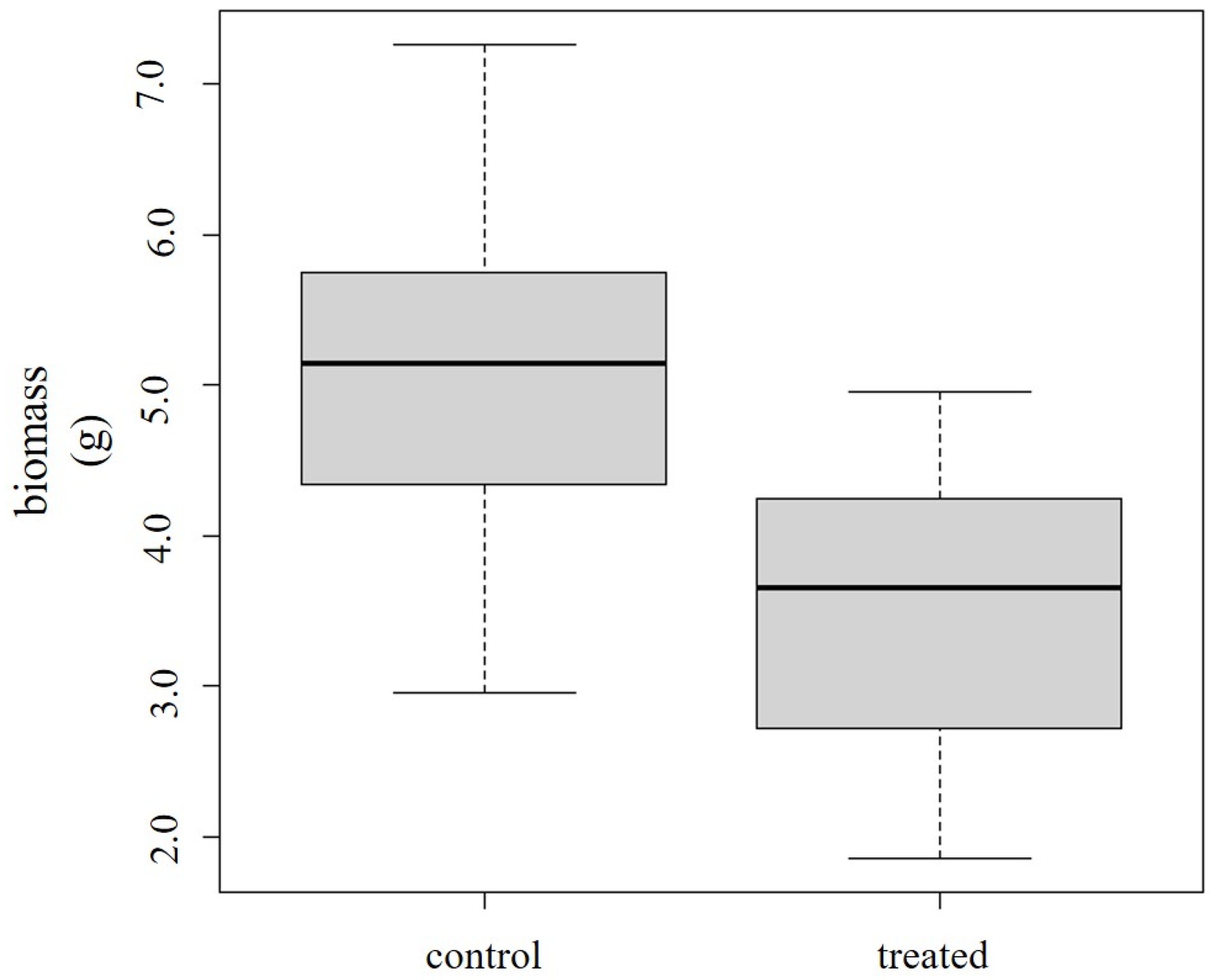
3.2.1. Decrease in Biomass
3.2.2. Chlorophyll-a and Chlorophyll-b Content
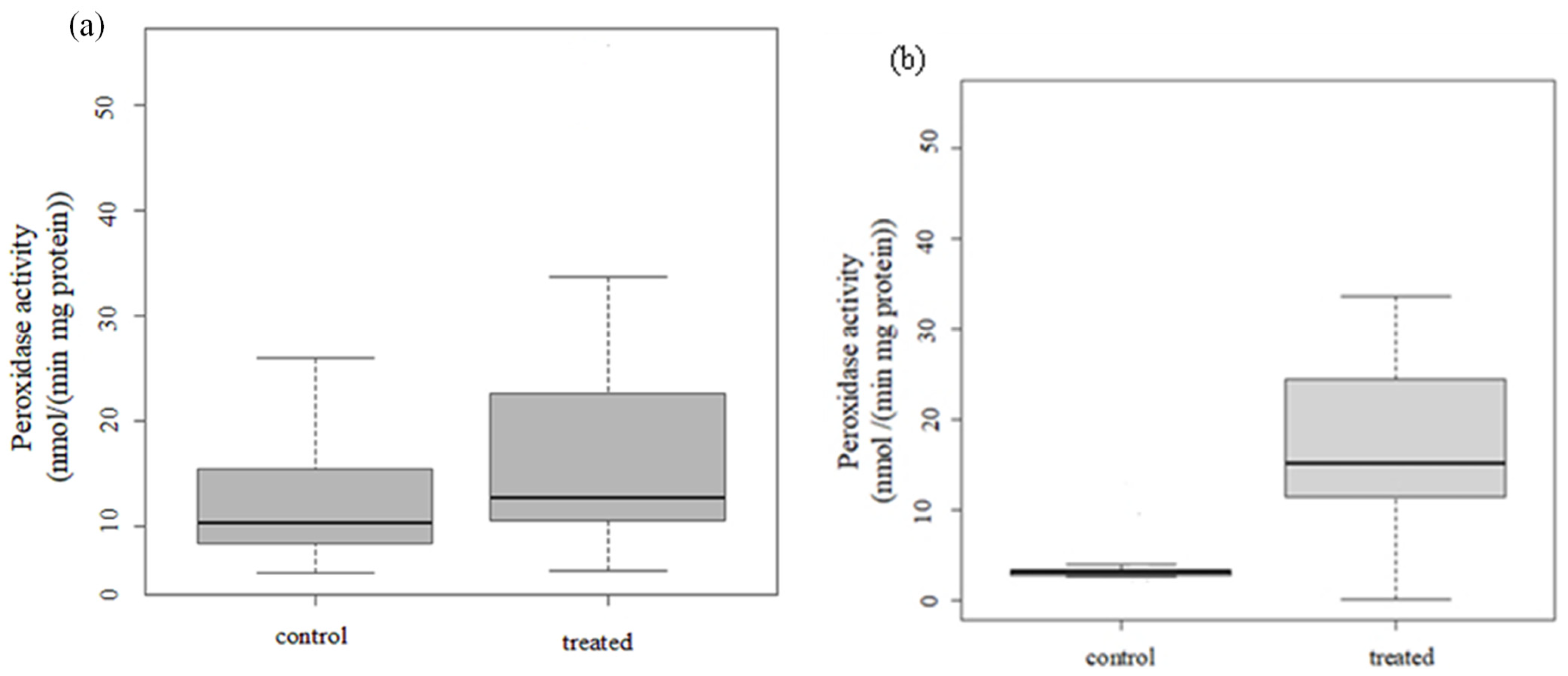
3.2.3. Peroxidase Activity
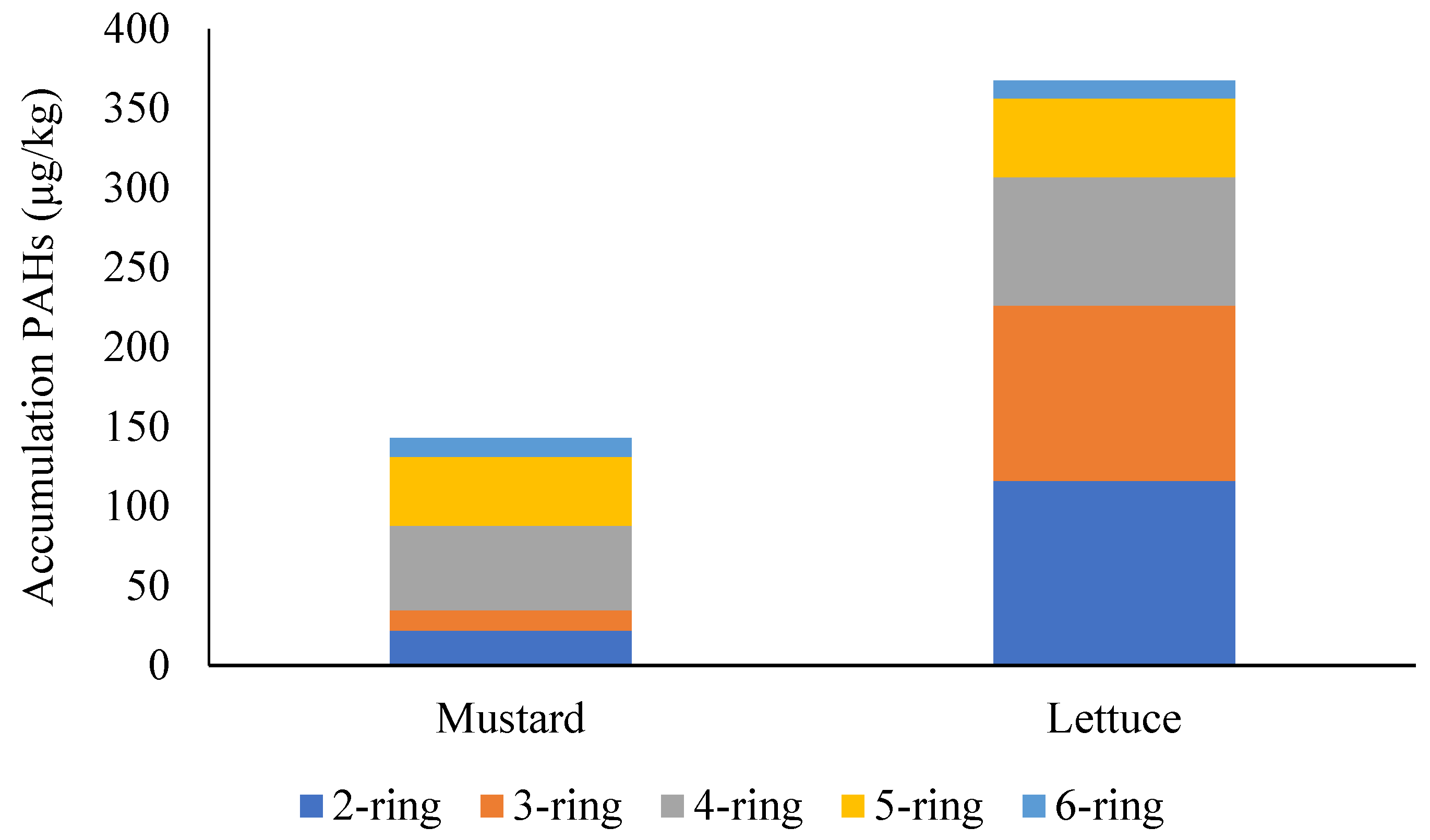
3.3. Bioaccumulation and Bioconcentration of PAHs
3.4. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Dingenen, R.; Raes, F.; Putaud, J.-P.; Baltensperger, U.; Charron, A.; Facchini, M.-C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Hansson, H.-C.; et al. A European aerosol phenomenology-1: Physical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2561–2577. [Google Scholar] [CrossRef]
- Puxbaum, H.; Caseiro, A.; Sánchez-Ochoa, A.; Kasper-Giebl, A.; Claeys, M.; Gelencsér, A.; Legrand, M.; Preunkert, S.; Pio, C. Levoglucosan levels at background sites in Europe for assessing the impact of biomass combustion on the European aerosol background. J. Geophys. Res. 2007, 112, D23S05/1–D23S05/11. [Google Scholar] [CrossRef]
- Noblet, C.; Lestremau, F.; Collet, S.; Chatellier, C.; Beaumont, J.; Besombes, J.L.; Albinet, A. Aerosolomics based approach to discover source molecular markers: A case study for discriminating residential wood heating vs garden green waste burning emission sources. Chemosphere 2024, 352, 141242. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, C.; Bauer, H.; Dattler, A.; Hitzenberger, R.; Weissenboeck, G.; Marr, I.L.; Puxbaum, H. Chemical characterisation of particle emissions from burning leaves. Atmos. Environ. 2008, 42, 9070–9079. [Google Scholar] [CrossRef]
- Krecl, P.; de Lima, C.H.; Bosco, T.C.D.; Targino, A.C.; Hashimoto, E.M.; Oukawa, G.Y. Open waste burning causes fast and sharp changes in particulate concentrations in peripheral neighborhoods. Sci. Total Environ. 2021, 765, 142736. [Google Scholar] [CrossRef] [PubMed]
- Kováts, N.; Hubai, K.; Diósi, D.; Hoffer, A.; Teke, G. Foliar uptake and accumulation of polycyclic aromatic hydrocarbons from diesel emissions. Polycycl. Aromat. Compd. 2021, 42, 6124–6135. [Google Scholar] [CrossRef]
- Grantz, D.A.; Garner, J.H.B.; Johnson, D.W. Ecological effects of particulate matter. Environ. Int. 2003, 29, 213–239. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Sakugawa, H. Fluoranthene fumigation and exogenous scavenging of reactive oxygen intermediates (ROI) in evergreen Japanese red pine seedlings (Pinus densiflora Sieb. et. Zucc.). Chemosphere 2008, 72, 747–754. [Google Scholar] [CrossRef]
- Boutin, C.; Aya, K.L.; Carpenter, D.; Thomas, P.J.; Rowland, O. Phytotoxicity testing for herbicide regulation: Shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Sci. Total Environ. 2012, 415, 79–92. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Teke, G. Effects of urban atmospheric particulate matter on higher plants using Lycopersicon esculentum as model species. SN Appl. Sci. 2021, 3, 770. [Google Scholar] [CrossRef]
- Teke, G.; Hubai, K.; Diósi, D.; Kováts, N. Assessment of Foliar Uptake and Accumulation of Airborne Polyaromatic Hydrocarbons Under Laboratory Conditions. Bull. Environ. Contam. Toxicol. 2020, 104, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Kováts, N.; Hubai, K.; Sainnokhoi, T.A.; Teke, G. Biomonitoring of polyaromatic hydrocarbon accumulation in rural gardens using lettuce plants. J. Soils Sed. 2021, 21, 106–117. [Google Scholar] [CrossRef]
- Rabe, R.; Kreeb, K.H. Enzyme activities and chlorophyll and protein content in plants as indicators of air pollution. Environ. Pollut. 1979, 19, 119–137. [Google Scholar] [CrossRef]
- Kumar, G.S.; Dubey, P.S. Differential response and detoxifying mechanism of Cassia siamea Lam. and Dalbergia sissoo Roxb. of different ages to SO2 treatment. J. Environ. Biol. 1998, 9, 243–249. [Google Scholar]
- Verma, A.; Singh, S.N. Biochemical and ultrastructural changes in plant foliage exposed to auto-pollution. Environ. Monit. Assess. 2006, 120, 585–602. [Google Scholar] [CrossRef]
- Keller, T. The use of peroxidase activity for monitoring and mapping air pollution areas. Eur. J. For. Pathol. 1974, 4, 11–19. [Google Scholar] [CrossRef]
- Sarkar, R.K.; Banerjee, A.; Mukherji, S. Acceleration of Peroxidase and Catalase Activities in Leaves of Wild Dicotyledonous Plants, as an Indication of Automobile Exhaust Pollution. Environ. Pollut. Ser. A 1986, 42, 289–295. [Google Scholar] [CrossRef]
- Hoffer, A.; Jancsek-Turóczi, B.; Tóth, Á.; Kiss, G.; Naghiu, A.; Levei, E.A.; Marmureanu, L.; Machon, A.; Gelencsér, A. Emission factors for PM10 and PAHs from illegal burning of different types of municipal waste in households. Atmos. Chem. Phys. 2020, 20, 16135–16144. [Google Scholar] [CrossRef]
- MSZ 1484-6:2003; Testing of waters. Part 6: Determination of polycyclic aromatic hydrocarbons (PAH) content by gas chromatographic-mass spectrometry. Hungarian Standard Association: Budapest, Hungary, 2003.
- ISO/IEC 17025:2018; Magyar Szabvány. Vizsgáló- és kalibrálólaboratóriumok felkészültségének általános követelményei. Vizsgálólaboratóriumok akkreditálási programja. International Organization for Standardization: Geneva, Switzerland, 2018.
- Kováts, N.; Fábián, V.A.; Hubai, K.; Diósi, D.; Sainnokhoi, T.A.; Békéssy, Z.; Teke, G. Seasonal differences in rural particulate matter ecotoxicity. Aerosol Sci. Eng. 2020, 4, 169–177. [Google Scholar] [CrossRef]
- Bag, N.; Palni, L.; Chandra, S.; Nandi, S. Somatic embryogenesis in ’maggar’ bamboo (Dendrocalamus hamiltonii) and field performance of regenerated plants. Curr. Sci. 2012, 102, 1279–1287. [Google Scholar]
- Imberty, A.; Goldberg, R.; Catesson, A.-M. Tetramethylbenzidine and p-phenylenediamine-pyrocat-echol for peroxidase histochemistry and biochemistry: Two new, non-carcinogenic chromogens for investigating lignification process. Plant Sci. Lett. 1984, 35, 103–108. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustoš, P. The uptake of persistent organic pollutants by plants. Cent. Eur. J. Biol. 2011, 6, 223–235. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Kwarteng, L.; Devasurendra, A.M.; Laskaris, Z.; Arko-Mensah, J.; Nti, A.A.A.; Takyi, S.; Acquah, A.A.; Dwomoh, D.; Basu, N.; Robins, T.; et al. Occupational exposures to particulate matter and PM2. 5-associated polycyclic aromatic hydrocarbons at the Agbogbloshie waste recycling site in Ghana. Environ. Int. 2022, 158, 106971. [Google Scholar] [CrossRef]
- Noblet, C.; Besombes, J.L.; Lemire, M.; Pin, M.; Jaffrezo, J.L.; Favez, O.; Aujay-Plouzeau, R.; Dermigny, A.; Karoski, N.; Van Elsuve, D.; et al. Emission factors and chemical characterization of particulate emissions from garden green waste burning. Sci. Total Environ. 2021, 798, 149367. [Google Scholar] [CrossRef]
- Kakareka, S.V.; Kukharchyk, T.I. PAH emission from the open burning of agricultural debris. Sci. Total Environ. 2003, 308, 257–261. [Google Scholar] [CrossRef]
- Capozzi, F.; Di Palma, A.; Adamo, P.; Spagnuolo, V.; Giordano, S. Monitoring chronic and acute PAH atmospheric pollution using transplants of the moss Hypnum cupressiforme and Robinia pseudacacia leaves. Atmos. Environ. 2017, 150, 45–54. [Google Scholar] [CrossRef]
- Mentes, D.; Tóth, C.E.; Nagy, G.; Muránszky, G.; Póliska, C. Investigation of gaseous and solid pollutants emitted from waste tire combustion at different temperatures. Waste Manag. 2022, 149, 302–312. [Google Scholar] [CrossRef]
- Filella, M. Antimony and PET bottles: Checking facts. Chemosphere 2020, 261, 127732. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Astrup, T.F. Characterisation of source-separated, rigid plastic waste and evaluation of recycling initiatives: Effects of product design and source-separation system. Waste Manag. 2019, 87, 161–172. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.A.; Hinojosa-Reyes, L.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.; Maya-Treviño, L.; Guzmán-Mar, J.L. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Li, Z.F.; Shen, J.; Wu, K.Y.; Zhao, P.P.; Wu, Z.; Liu, Z.; Yang, J.; Liu, H.; Rensing, C.; et al. Toxicity of different forms of antimony to rice plants: Photosynthetic electron transfer, gas exchange, photosynthetic efficiency, and carbon assimilation combined with metabolome analysis. J. Hazard. Mater. 2022, 437, 129433. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Filella, M. The influence of additives on the fate of plastics in the marine environment, exemplified with barium sulphate. Mar. Pollut. Bull. 2020, 158, 111352. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cardoso, A.A.; Monteiro, F.A. Sulfur supply reduces barium toxicity in Tanzania guinea grass (Panicum maximum) by inducing antioxidant enzymes and proline metabolism. Ecotoxicol. Environ. Saf. 2021, 208, 111643. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Iliopoulos, N.; Gotsis, G.; Fiotakis, K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard. Mater. 2008, 156, 277–284. [Google Scholar] [CrossRef]
- Hama, S.; Kumar, P.; Alam, M.S.; Rooney, D.J.; Bloss, W.J.; Shi, Z.; Harrison, R.M.; Crilley, L.R.; Khare, M.; Gupta, S.K. Chemical source profiles of fine particles for five different sources in Delhi. Chemosphere 2021, 274, 129913. [Google Scholar] [CrossRef]
- Peter, A.E.; Nagendra, S.M.S.; Nambi, I.M. Comprehensive analysis of inhalable toxic particulate emissions from an old municipal solid waste dumpsite and neighborhood health risks. Atmos. Pollut. Res. 2018, 9, 1021–1031. [Google Scholar] [CrossRef]
- Kucerova, R.; Zavoral, M.; Mudrunka, J.; Takac, D.; Marcalikova, L. Analysis of Firewater Samples from Simulated Fires in Illegal Waste Dumps. Eng. Proc. 2023, 57, 14. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of nano-zinc oxide to Tomato plant (L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Beyer, W.N.; Green, C.E.; Beyer, M.; Chaney, R.L. Phytotoxicity of zinc and manganese to seedlings grown in soil contaminated by zinc smelting. Environ. Pollut. 2013, 179, 167–176. [Google Scholar] [CrossRef]
- Storch-Böhm, R.F.; Somensi, C.A.; Cotelle, S.; Deomar-Simoes, M.J.; Poyer-Radetski, L.; Dalpiaz, F.L.; Pimentel-Almeida, W.; Férard, J.-F.; Radetski, C.M. Sensitivity of different parameters for selection of higher plants in urban afforestation: Exposure of Guabiroba (Campomanesia xanthocarpa O. Berg.) to diesel engine exhaust. Environ. Pollut. 2020, 265, 114675. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, M.; Pavlíková, D.; Zemanová, V.; Hnilička, F.; Urbanová, V.; Szákova, J. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotoxicol. Environ. Saf. 2012, 79, 101–107. [Google Scholar] [CrossRef]
- Wieczorek, J.K.; Wieczorek, Z.J. Phytotoxicity and accumulation of anthracene applied to the foliage and sandy substrate in lettuce and radish plants. Ecotoxicol. Environ. Saf. 2007, 66, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Sienkiewicz, S.; Pietrzak, M.; Wieczorek, Z. Uptake and phytotoxicity of anthracene and benzo[k]fluoranthene applied to the leaves of celery plants (Apium graveolens var. secalinum L.). Ecotoxicol. Environ. Saf. 2015, 115, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Desalme, D.; Binet, P.; Epron, D.; Bernard, N.; Gilbert, D.; Toussaint, M.-L.; Plain, C.; Chiapusio, G. Atmospheric phenanthrene pollution modulates carbon allocation in red clover (Trifolium pratense L.). Environ. Pollut. 2011, 159, 2759–2765. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, M.M.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress. Ecotoxicol. Environ. Saf. 2012, 80, 132–139. [Google Scholar] [CrossRef]
- Paull, N.J.; Krix, D.; Irga, P.J.; Torpy, F.R. Green wall plant tolerance to ambient urban air pollution. Urban. For. Urban. Green. 2021, 63, 127201. [Google Scholar] [CrossRef]
- Kaur, M.; Nagpal, A.K. Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ. Sci. Pollut. Res. 2017, 24, 18881–18895. [Google Scholar] [CrossRef]
- Karmakar, D.; Padhy, P.K. Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef]
- Zhang, P.Q.; Liu, Y.J.; Chen, X.; Yang, Z.; Zhu, M.H.; Li, Y.P. Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicol. Environ. Saf. 2016, 132, 212–223. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Gong, J.; Yang, B.; Zhang, Z.; Wang, B.; Zhu, C.; Shi, J.; Yue, K. Comparison of the suitability of plant species for greenbelt construction based on particulate matter capture capacity, air pollution tolerance index, and antioxidant system. Environ. Pollut. 2020, 263, 114615. [Google Scholar] [CrossRef]
- Bharti, S.K.; Trivedi, A.; Kumar, N. Air pollution tolerance index of plants growing near an industrial site. Urb. Clim. 2018, 24, 820–829. [Google Scholar] [CrossRef]
- Giri, S.; Shrivastava, D.; Deshmukh, K.; Dubey, P. Effect of Air Pollution on Chlorophyll Content of Leaves. Curr. Agric. Res. J. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Pimple, N.S. Adverse effect of air pollutants on the chlorophyll content in leaves from Pune, Maharashtra (India). Int. J. Pharm. Sci. Rev. Res. 2017, 44, 131. [Google Scholar]
- Joshi, P.C.; Swami, A. Air pollution induced changes in the photosynthetic pigments of selected plant species. J. Environ. Biol. 2009, 30, 295–298. [Google Scholar]
- Joshi, N.; Chauhan, A.; Joshi, P.C. Impact of industrial air pollutants on some biochemical parameters and yield in wheat and mustard plants. Environmentalist 2009, 29, 398–404. [Google Scholar] [CrossRef]
- Czégény, G.; Rácz, A. Phenolic peroxidases: Dull generalists or purposeful specialists in stress responses? J. Plant Physiol. 2023, 280, 153884. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and metabolic plant responses toward polycyclic aromatic hydrocarbons and heavy metals present in atmospheric pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Ye, Y.B.; Cui, B.; Huang, Y.H.; Colón-Carmona, A.; Wang, Z.H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Schreck, E.; Bonnard, R.; Laplanche, C.; Leveque, T.; Foucault, Y.; Dumat, C. DECA: A new model for assessing the foliar uptake of atmospheric lead by vegetation, using Lactuca sativa as an example. J. Environ. Manag. 2012, 112, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreck, E.; Laplanche, C.; Le Guédard, M.; Bessoule, J.-J.; Austruy, A.; Xiong, T.; Foucault, Y.; Dumat, C. Influence of fine process particles enriched with metals and metalloids on Lactuca sativa L. leaf fatty acid composition following air and/or soil-plant field exposure. Environ. Pollut. 2013, 179, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Zhang, J.; Zhou, M.; Li, F.; Liu, X. Accumulation characteristics and potential risk of PAHs in vegetable system grow in home garden under straw burning condition in Jilin, Northeast China. Ecotoxicol. Environ. Saf. 2018, 162, 647–654. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.; Jin, X.; Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ. Pollut. 2018, 241, 750–758. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Eck-Varanka, B.; Tumurbaatar, S.; Teke, G. Accumulation of Atmospheric PAHs in White Mustard–Can the Seeds Be Affected? Bull. Environ. Contam. Toxicol. 2024, 112, 76. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Wang, X.; Firouzkouhi, H.; Chow, J.C.; Watson, J.G.; Carter, W.; De Vos, A.S. Characterization of gas and particle emissions from open burning of household solid waste from South Africa. Atmos. Chem. Phys. 2023, 23, 8921–8937. [Google Scholar] [CrossRef]
- Das, B.; Bhave, P.V.; Sapkota, A.; Byanju, R.M. Estimating emissions from open burning of municipal solid waste in municipalities of Nepal. Waste Manag. 2018, 79, 481–490. [Google Scholar] [CrossRef]
- Gullett, B.K.; Lemieux, P.M.; Lutes, C.C.; Winterrowd, C.K.; Winters, D.L. Emissions of PCDD/F from uncontrolled, domestic waste burning. Chemosphere 2001, 43, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.; Gullett, B.; Striebich, R.; Klosterman, J.; Contreras, J.; DeVito, M. Endocrine disrupting chemical emissions from combustion sources: Diesel particulate emissions and domestic waste open burn emissions. Atmos. Environ. 2005, 39, 801–811. [Google Scholar] [CrossRef]
- Song, S.; Zhou, X.; Guo, C.; Zhang, H.; Zeng, T.; Xie, Y.; Liu, J.; Zhu, C.; Sun, X. Emission characteristics of polychlorinated, polybrominated and mixed polybrominated/chlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs, PBDD/Fs, and PBCDD/Fs) from waste incineration and metallurgical processes in China. Ecotoxicol. Environ. Saf. 2019, 184, 109608. [Google Scholar] [CrossRef] [PubMed]
| Biological | 46% |
|---|---|
| Plastics | 15% |
| Textile | 13% |
| Treated wood | 12% |
| Paper/cardboard | 8% |
| Rubber | 4% |
| Used oil | 2% |
| Number of Rings | PAHs | PM10 Extract (µg/L) |
|---|---|---|
| two rings | Naphthalene | 0.931 |
| 2-methyl-naphthalene | 0.377 | |
| 1-methyl-naphthalene | 0.323 | |
| three rings | Acenaphthylene | 7.41 |
| Acenaphthene | 4.65 | |
| Fluorene | 6.13 | |
| Phenanthrene | 47.2 | |
| Anthracene | 8.97 | |
| four rings | Fluoranthene | 22 |
| Pyrene | 19.3 | |
| Benzanthracene | 1.55 | |
| Chrysene | 2.9 | |
| five rings | Benzo(b)fluoranthene | 1.03 |
| Benzo(k)fluoranthene | 0.38 | |
| Benzo(e)pyrene | 0.271 | |
| Benzo(a)pyrene | 0.603 | |
| Dibenzo(a.h)anthracene | 0.379 | |
| six rings | Indeno1.2.3CD-Pyrene | 0.108 |
| Benzo(g.h.i)perylene | 0.477 | |
| Total PAHs | 125 |
| Waste PM10 Extract (µg/L) | |
|---|---|
| Ag | <1.000 |
| Al | <10.0 |
| As | <1.000 |
| B | <10.0 |
| Ba | 23 |
| Cd | <0.500 |
| Co | <1.000 |
| Cr | <1.000 |
| Cu | <5.00 |
| Mo | <2.00 |
| Ni | <2.00 |
| Pb | <1.000 |
| Se | <1.000 |
| Sb | 6.2 |
| Sn | <1.000 |
| Zn | 219 |
| Na | <0.500 |
| Hg | <0.200 |
| Standard | End-Point | Lactuca sativa | Sinapis alba |
|---|---|---|---|
| OECD 227 | Biomass | + | 0 |
| Chl-a | 0 | 0 | |
| Chl-b | + | 0 | |
| Carotene | 0 | 0 | |
| POD | + | + |
| PAHs | Treated Mustard (µg/kg) | BCF | Treated Lettuce (µg/kg) | BCF |
|---|---|---|---|---|
| Naphthalene | 19.5 | 20.9 | 102 | 109.6 |
| 2-methyl-naphthalene | 1.4 | 3.7 | 8.7 | 23.1 |
| 1-methyl-naphthalene | 1.1 | 3.4 | 5.3 | 16.4 |
| Acenaphthylene | 2.2 | 0.3 | 3.58 | 0.5 |
| Acenaphthene | 0.8 | 0.2 | 2.22 | 0.5 |
| Fluorene | 2.5 | 0.4 | 7.8 | 1.3 |
| Phenanthrene | 4.5 | 0.1 | 88 | 1.9 |
| Anthracene | 2.8 | 0.3 | 8.8 | 1.0 |
| Fluoranthene | 14.3 | 0.7 | 37.5 | 1.7 |
| Pyrene | 10.2 | 0.5 | 25.2 | 1.3 |
| Benzanthracene | 6.3 | 4.1 | 15.8 | 10.2 |
| Chrysene | 22.4 | 7.7 | 2.14 | 0.7 |
| Benzo(b)fluoranthene | 16.9 | 16.4 | 17.8 | 17.3 |
| Benzo(k)fluoranthene | 4.6 | 12.1 | 7.7 | 20.3 |
| Benzo(e)pyrene | 8.9 | 32.8 | 8 | 29.5 |
| Benzo(a)pyrene | 4.1 | 6.8 | 8 | 13.3 |
| Dibenzo[a.h]anthracene | 8.7 | 23.0 | 7.85 | 20.7 |
| Indeno1.2.3CD-Pyrene | 2.6 | 24.1 | 2.6 | 24.1 |
| Benzo(g.h.i)perylene | 9.3 | 19.5 | 8.5 | 17.8 |
| Total PAHs | 143.1 | 367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumurbaatar, S.; Kováts, N.; Eck-Varanka, B.; Teke, G.; Hubai, K. Assessing Risk of Emissions Generated During Illegal Waste Burning: Phytotoxicity and Bioaccumulation. Atmosphere 2025, 16, 164. https://doi.org/10.3390/atmos16020164
Tumurbaatar S, Kováts N, Eck-Varanka B, Teke G, Hubai K. Assessing Risk of Emissions Generated During Illegal Waste Burning: Phytotoxicity and Bioaccumulation. Atmosphere. 2025; 16(2):164. https://doi.org/10.3390/atmos16020164
Chicago/Turabian StyleTumurbaatar, Selenge, Nora Kováts, Bettina Eck-Varanka, Gábor Teke, and Katalin Hubai. 2025. "Assessing Risk of Emissions Generated During Illegal Waste Burning: Phytotoxicity and Bioaccumulation" Atmosphere 16, no. 2: 164. https://doi.org/10.3390/atmos16020164
APA StyleTumurbaatar, S., Kováts, N., Eck-Varanka, B., Teke, G., & Hubai, K. (2025). Assessing Risk of Emissions Generated During Illegal Waste Burning: Phytotoxicity and Bioaccumulation. Atmosphere, 16(2), 164. https://doi.org/10.3390/atmos16020164





