Mitigating Ammonia Emissions from Liquid Manure Using a Commercially Available Additive Under Real-Scale Farm Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Manure Management
2.2. Slurry Analysis
2.3. Emission and Flux Determination
- There were PVC funnels floating via a suitable frame on the surface of the slurry. Each funnel had a diameter of 0.42 m and covered a surface of approximately 0.14 m2; the total covered area for each tank was approximately 0.7 m2, making it greater than the 0.5 m2 suggested by the aforementioned VERA protocol.
- There were PTFE tubes connecting each floating funnel with a corresponding “lung flask.” The flasks were hermetically sealed with rubber stoppers.
- There were two Dreschel acid traps with a volume of 500 mL, which were sealed and connected to each other. Each trap contained 300 mL of a solution of 1% boric acid.
- One flow meter was placed behind the acid trap Drechsel.
- One vacuum pump was positioned downstream of the system, with an air flux of 1.5 L/min.
- F is the NH3 flux (mg/m2,h);
- Q is the air flow (m3/h);
- Cin is the NH3 concentration in the air above the slurry surface, sampled via the funnel system (mg/m3);
- Cout is the corresponding background NH3 air concentration (mg/m3);
- A is the surface of the funnel (m2).
2.4. Statistical Analysis
3. Results
3.1. Weather Characteristics
3.2. Slurry Chemical Characteristics
3.3. Ammonia Emissions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the Atmosphere: A Review on Emission Sources, Atmospheric Chemistry and Deposition on Terrestrial Bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and Greenhouse Gas Emissions from Slurry Storage—A Review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Stelson, A.W.; Seinfeld, J.H. Thermodynamic Prediction of the Water Activity, NH4NO3 Dissociation Constant, Density and Refractive Index for the NH4NO3-(NH4)2SO4-H2O System at 25 °C. Atmos. Environ. 1982, 16, 2507–2514. [Google Scholar] [CrossRef]
- Doyle, G.J.; Tuazon, E.C.; Graham, R.A.; Mischke, T.M.; Winer, A.M., Jr.; Pitts, J.N. Simultaneous Concentrations of Ammonia and Nitric Acid in a Polluted Atmosphere and Their Equilibrium Relationship to Particulate Ammonium Nitrate; ACS Publications: Washington, DC, USA, 1979. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A Review of Ammonia Emission Mitigation Techniques for Concentrated Animal Feeding Operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Wang, S.; Nan, J.; Shi, C.; Fu, Q.; Gao, S.; Wang, D.; Cui, H.; Saiz-Lopez, A.; Zhou, B. Atmospheric Ammonia and Its Impacts on Regional Air Quality over the Megacity of Shanghai, China. Sci. Rep. 2015, 5, 15842. [Google Scholar] [CrossRef]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too Much of a Good Thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef]
- Carnevale, C.; Pisoni, E.; Volta, M. A Non-Linear Analysis to Detect the Origin of PM10 Concentrations in Northern Italy. Sci. Total Environ. 2010, 409, 182–191. [Google Scholar] [CrossRef]
- Erisman, J.W.; Schaap, M. The Need for Ammonia Abatement with Respect to Secondary PM Reductions in Europe. Environ. Pollut. 2004, 129, 159–163. [Google Scholar] [CrossRef]
- Paulot, F.; Jacob, D.J. Hidden Cost of U.S. Agricultural Exports: Particulate Matter from Ammonia Emissions. Environ. Sci. Technol. 2014, 48, 903–908. [Google Scholar] [CrossRef]
- van Donkelaar, A.; Martin, R.V.; Li, C.; Burnett, R.T. Regional Estimates of Chemical Composition of Fine Particulate Matter Using a Combined Geoscience-Statistical Method with Information from Satellites, Models, and Monitors. Environ. Sci. Technol. 2019, 53, 2595–2611. [Google Scholar] [CrossRef]
- Gautam, S.; Patra, A.K.; Kumar, P. Status and Chemical Characteristics of Ambient PM2.5 Pollutions in China: A Review. Environ. Dev. Sustain. 2019, 21, 1649–1674. [Google Scholar] [CrossRef]
- Li, Y.J.; Sun, Y.; Zhang, Q.; Li, X.; Li, M.; Zhou, Z.; Chan, C.K. Real-Time Chemical Characterization of Atmospheric Particulate Matter in China: A Review. Atmos. Environ. 2017, 158, 270–304. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, L.; Wang, S.; Wang, Y.; Sharma, S.; Shimadera, H.; Wang, X.; Bressi, M.; de Miranda, R.M.; Jiang, J.; et al. Status and Characteristics of Ambient PM2.5 Pollution in Global Megacities. Environ. Int. 2016, 89–90, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Martin, R.V.; van Donkelaar, A.; Lo, J.W.-H.; Wang, Y.; Chen, D.; Zhang, L.; Kasibhatla, P.S.; Wang, S.; Zhang, Q.; et al. Global Chemical Composition of Ambient Fine Particulate Matter for Exposure Assessment. Environ. Sci. Technol. 2014, 48, 13060–13068. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.N.; Sharma, M. Investigating the Potential Role of Ammonia in Ion Chemistry of Fine Particulate Matter Formation for an Urban Environment. Sci. Total Environ. 2010, 408, 3569–3575. [Google Scholar] [CrossRef]
- European Environment Agency. Health Impacts of Air Pollution in Europe. 2022. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2022/health-impacts-of-air-pollution (accessed on 8 April 2025).
- Burns, A.M.; Chandler, G.; Dunham, K.J.; Carlton, A.G. Data Gap: Air Quality Networks Miss Air Pollution from Concentrated Animal Feeding Operations. Environ. Sci. Technol. 2023, 57, 20718–20725. [Google Scholar] [CrossRef]
- Bauer, S.E.; Tsigaridis, K.; Miller, R. Significant Atmospheric Aerosol Pollution Caused by World Food Cultivation. Geophys. Res. Lett. 2016, 43, 5394–5400. [Google Scholar] [CrossRef]
- Han, X.; Zhu, L.; Liu, M.; Song, Y.; Zhang, M. Numerical Analysis of Agricultural Emissions Impacts on PM2.5 in China Using a High-Resolution Ammonia Emission Inventory. Atmos. Chem. Phys. 2020, 20, 9979–9996. [Google Scholar] [CrossRef]
- Final Assessment Report on Ammonia. Available online: https://unece.org/fileadmin/DAM/env/documents/2020/AIR/WGSR/Final_Assessment_Report_on_Ammonia_v2_20201126_b.pdf (accessed on 8 April 2025).
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; Vries, W.; Oenema, O.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution. In Global Overview on Nutrient Management; UNEP, Ed.; Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- Air Pollution in Europe. Available online: https://www.eea.europa.eu/publications/national-emission-reduction-commitments-directive-2023 (accessed on 8 April 2025).
- Van Damme, M.; Clarisse, L.; Franco, B.; Sutton, M.A.; Erisman, J.W.; Wichink Kruit, R.; van Zanten, M.; Whitburn, S.; Hadji-Lazaro, J.; Hurtmans, D.; et al. Global, Regional and National Trends of Atmospheric Ammonia Derived from a Decadal (2008–2018) Satellite Record. Environ. Res. Lett. 2021, 16, 055017. [Google Scholar] [CrossRef]
- Kupper, T.; Eugster, R.; Sintermann, J.; Häni, C. Ammonia Emissions from an Uncovered Dairy Slurry Storage Tank over Two Years: Interactions with Tank Operations and Meteorological Conditions. Biosyst. Eng. 2021, 204, 36–49. [Google Scholar] [CrossRef]
- Sommer, S.G.; Zhang, G.Q.; Bannink, A.; Chadwick, D.; Misselbrook, T.; Harrison, R.; Hutchings, N.J.; Menzi, H.; Monteny, G.J.; Ni, J.Q.; et al. Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2006; Volume 89, pp. 261–335. [Google Scholar] [CrossRef]
- Zilio, M.; Orzi, V.; Chiodini, M.E.; Riva, C.; Acutis, M.; Boccasile, G.; Adani, F. Evaluation of Ammonia and Odour Emissions from Animal Slurry and Digestate Storage in the Po Valley (Italy). Waste Manag. 2020, 103, 296–304. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Besson, C.; Larson, R.A. Modeling Ammonia Emissions from Manure in Conventional, Organic, and Grazing Dairy Systems and Practices to Mitigate Emissions. J. Dairy Sci. 2024, 107, 359–382. [Google Scholar] [CrossRef]
- Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and Repealing Directive 2001/81/EC. Available online: https://eur-lex.europa.eu/eli/dir/2016/2284/oj/eng (accessed on 28 October 2025).
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Available online: https://eur-lex.europa.eu/eli/dir/2010/75/oj/eng (accessed on 28 October 2025).
- Directive (EU) 2024/1785 of the European Parliament and of the Council of 24 April 2024 Amending Directive 2010/75/EU of the European Parliament and of the Council on Industrial Emissions (Integrated Pollution Prevention and Control) and Council Directive 1999/31/EC on the Landfill of Waste. Available online: https://eur-lex.europa.eu/eli/dir/2024/1785/oj/eng (accessed on 30 October 2025).
- DECRETO LEGISLATIVO n. 152—Norme in Materia Ambientale. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:decreto.legislativo:2006-04-03;152 (accessed on 28 October 2025).
- U.S. Environmental Protection Agency. Emissions from Animal Feeding Operations; U.S. EPA: Washington, DC, USA, 2005. [Google Scholar]
- Code 359; Conservation Practice Standard Waste Treatment Lagoon. USDA: Washington, DC, USA, 2017.
- Directive (EU) 2024/1785 of the European Parliament and of the Council of 24 April 2024 Amending Directive 2010/75/EU of the European Parliament and of the Council on Industrial Emissions (Integrated Pollution Prevention and Control) and Council Directive 1999/31/EC on the Landfill of Waste (Text with EEA Relevance). 2024. Available online: http://data.europa.eu/eli/dir/2024/1785/oj/eng (accessed on 28 October 2025).
- Zapletal, M.; Mikuska, P. Ammonia Emissions and Dry Deposition in the Vicinity of Dairy Farms. Atmósfera 2019, 32, 337–350. [Google Scholar] [CrossRef]
- Grant, R.H.; Boehm, M.T. Ammonia Emissions from Differing Manure Storage Facilities at Two Midwestern Free-Stall Dairies. Atmosphere 2020, 11, 1108. [Google Scholar] [CrossRef]
- ten Hoeve, M.; Nyord, T.; Peters, G.M.; Hutchings, N.J.; Jensen, L.S.; Bruun, S. A Life Cycle Perspective of Slurry Acidification Strategies under Different Nitrogen Regulations. J. Clean. Prod. 2016, 127, 591–599. [Google Scholar] [CrossRef]
- Soluzioni Innovative per i Reflui Degli Allevamenti. Regione Piemonte. Available online: https://www.regione.piemonte.it/web/pinforma/notizie/soluzioni-innovative-per-reflui-degli-allevamenti (accessed on 28 May 2025).
- Wang, Y.; Xu, H.; Wang, Y.; Wang, W.; Wang, X.; Zhang, H.; Zhu, Z.; Dong, H. Achieving Overall Low Greenhouse Gas and Ammonia Emissions from Digested Pig Slurry during Storage and after Field Application by Maintaining a Micro-Oxygen Level at the Storage Stage. J. Environ. Chem. Eng. 2025, 13, 116712. [Google Scholar] [CrossRef]
- Owusu-Twum, M.Y.; Kelleghan, D.; Gleasure, G.; Connolly, S.; Forrestal, P.; Lanigan, G.J.; Richards, K.G.; Krol, D.J. Mitigation of Ammonia and Methane Emissions with Manure Amendments during Storage of Cattle Slurry. Waste Manag. Res. 2025, 43, 568–579. [Google Scholar] [CrossRef]
- Maris, S.C.; Capra, F.; Ardenti, F.; Chiodini, M.E.; Boselli, R.; Taskin, E.; Puglisi, E.; Bertora, C.; Poggianella, L.; Amaducci, S.; et al. Reducing N Fertilization without Yield Penalties in Maize with a Commercially Available Seed Dressing. Agronomy 2021, 11, 407. [Google Scholar] [CrossRef]
- Meggio, F.; Chiodini, M.E.; Broggio, M.; Poggianella, L.; Acutis, M. Responses on Vegetative Growth, Yield and Quality with the Application of Resonant® Fortify White Fertilizer in Vitis vinifera L. Cv. Chardonnay. Italus Hortus 2024, 31, 29. [Google Scholar] [CrossRef]
- Borgonovo, F.; Conti, C.; Lovarelli, D.; Ferrante, V.; Guarino, M. Improving the Sustainability of Dairy Slurry by A Commercial Additive Treatment. Sustainability 2019, 11, 4998. [Google Scholar] [CrossRef]
- Peterson, C.B.; El Mashad, H.M.; Zhao, Y.; Pan, Y.; Mitloehner, F.M. Effects of SOP Lagoon Additive on Gaseous Emissions from Stored Liquid Dairy Manure. Sustainability 2020, 12, 1393. [Google Scholar] [CrossRef]
- Chiodini, M.E.; Costantini, M.; Zoli, M.; Bacenetti, J.; Aspesi, D.; Poggianella, L.; Acutis, M. Real-Scale Study on Methane and Carbon Dioxide Emission Reduction from Dairy Liquid Manure with the Commercial Additive SOP LAGOON. Sustainability 2023, 15, 1803. [Google Scholar] [CrossRef]
- SOP Farm. Available online: https://www.sopfarm.com/en (accessed on 28 May 2025).
- Standard Methods for the Examination of Water and Wastewater—Laura Bridgewater, American Public Health Association, American Water Works Association, Water Environment Federation—Google Libri. Available online: https://books.google.it/books/about/Standard_Methods_for_the_Examination_of.html?id=dd2juAAACAAJ&redir_esc=y (accessed on 14 December 2022).
- VERA Test Protocol. Available online: https://www.vera-verification.eu/app/uploads/sites/9/2019/05/VERA_Test_protocol_slurryseparation_v3_2018_final.pdf (accessed on 8 April 2025).
- Balsari, P.; Airoldi, G.; Dinuccio, E.; Gioelli, F. Ammonia Emissions from Farmyard Manure Heaps and Slurry Stores—Effect of Environmental Conditions and Measuring Methods. Biosyst. Eng. 2007, 97, 456–463. [Google Scholar] [CrossRef]
- Finzi, A.; Mattachini, G.; Lovarelli, D.; Riva, E.; Provolo, G. Technical, Economic, and Environmental Assessment of a Collective Integrated Treatment System for Energy Recovery and Nutrient Removal from Livestock Manure. Sustainability 2020, 12, 2756. [Google Scholar] [CrossRef]
- Open Meteo. Available online: https://open-meteo.com/ (accessed on 8 August 2025).
- Martínez-Suller, L.; Azzellino, A.; Provolo, G. Analysis of Livestock Slurries from Farms across Northern Italy: Relationship between Indicators and Nutrient Content. Biosyst. Eng. 2008, 99, 540–552. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to Sulfuric Acid for Slurry Acidification: Impact on Slurry Composition and Ammonia Emissions during Storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse Gas and Ammonia Emissions from Slurry Storage: Impacts of Temperature and Potential Mitigation through Covering (Pig Slurry) or Acidification (Cattle Slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E.; Christensen, B.T. Effects of Temperature, Wind Speed and Air Humidity on Ammonia Volatilization from Surface Applied Cattle Slurry. J. Agric. Sci. 1991, 117, 91–100. [Google Scholar] [CrossRef]
- Ni, J. Mechanistic Models of Ammonia Release from Liquid Manure: A Review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Montes, F.; Rotz, C.A.; Chaoui, H.I. Process Modeling of Ammonia Volatilization from Ammonium Solution and Manure Surfaces: A Review with Recommended Models. Trans. ASABE 2009, 52, 1707–1720. [Google Scholar] [CrossRef]
- Tomas, J.M.; Pourquie, M.J.B.M.; Jonker, H.J.J. Stable Stratification Effects on Flow and Pollutant Dispersion in Boundary Layers Entering a Generic Urban Environment. Bound.-Layer Meteorol. 2016, 159, 221–239. [Google Scholar] [CrossRef]
- Steeneveld, G.J.; van de Wiel, B.J.H.; Holtslag, A.A.M. Diagnostic Equations for the Stable Boundary Layer Height: Evaluation and Dimensional Analysis. J. Appl. Meteorol. Clim. 2007, 46, 212–225. [Google Scholar] [CrossRef]
- Phillips, S.B.; Arya, S.P.; Aneja, V.P. Ammonia Flux and Dry Deposition Velocity from Near-Surface Concentration Gradient Measurements over a Grass Surface in North Carolina. Atmos. Environ. 2004, 38, 3469–3480. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, W.; Zhu, Z.; Yang, J.; Li, X.; Tian, Z.; Dong, H.; Zou, G. Mitigating Ammonia Emissions from Typical Broiler and Layer Manure Management—A System Analysis. Waste Manag. 2019, 93, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; Zheng, G.; Ma, C. Characterization of Odorous Pollution and Health Risk Assessment of Volatile Organic Compound Emissions in Swine Facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- VanderZaag, A.C.; Gordon, R.J.; Glass, V.M.; Jamieson, R.C. Floating Covers to Reduce Gas Emissions from Liquid Manure Storages: A Review. Appl. Eng. Agric. 2008, 24, 657–671. [Google Scholar] [CrossRef]
- VanderZaag, A.; Amon, B.; Bittman, S.; Kuczyński, T. Ammonia Abatement with Manure Storage and Processing Techniques. In Costs of Ammonia Abatement and the Climate Co-Benefits; Reis, S., Howard, C., Sutton, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 75–112. [Google Scholar] [CrossRef]
- Symeon, G.K.; Akamati, K.; Dotas, V.; Karatosidi, D.; Bizelis, I.; Laliotis, G.P. Manure Management as a Potential Mitigation Tool to Eliminate Greenhouse Gas Emissions in Livestock Systems. Sustainability 2025, 17, 586. [Google Scholar] [CrossRef]
- Stenglein, R.M.; Clanton, C.J.; Schmidt, D.R.; Jacobson, L.D.; Janni, K.A. Covers for Mitigating Odor and Gas Emissions in Animal Agriculture: An Overview. Air Qual. Educ. Anim. Agric. 2011, 1–9. Available online: https://lpelc.org/wp-content/uploads/2019/03/Covers-overview-FINAL_1.pdf (accessed on 28 October 2025).
- Nicolai, R.; Pohl, S.; Schmidt, D. Covers for Manure Storage Units; SDSU: San Diego, CA, USA, 2004. [Google Scholar]
- European Commission; Joint Research Centre. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office: Luxembourg, 2017. [Google Scholar]
- Guarino, M.; Fabbri, C.; Brambilla, M.; Valli, L.; Navarotto, P. Evaluation of Simplified Covering Systems to Reduce Gaseous Emissions from Livestock Manure Storage. Trans. ASABE 2006, 49, 737–747. [Google Scholar] [CrossRef]
- Vanderzaag, A.; Gordon, R.; Jamieson, R.; Burton, D.; Stratton, G. Gas Emissions from Straw Covered Liquid Dairy Manure During Summer Storage and Autumn Agitation. Trans. ASABE 2009, 52, 599–608. [Google Scholar] [CrossRef]
- Hansen, R.R.; Nielsen, D.A.; Schramm, A.; Nielsen, L.P.; Revsbech, N.P.; Hansen, M.N. Greenhouse Gas Microbiology in Wet and Dry Straw Crust Covering Pig Slurry. J. Environ. Qual. 2009, 38, 1311–1319. [Google Scholar] [CrossRef]
- Smith, K.; Cumby, T.; Lapworth, J.; Misselbrook, T.; Williams, A. Natural Crusting of Slurry Storage as an Abatement Measure for Ammonia Emissions on Dairy Farms. Biosyst. Eng. 2007, 97, 464–471. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of Animal Slurry—A Review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Cocolo, G.; Jonassen, K.; Abildgaard, L.; Sommer, S.G. Continuous In-House Acidification Affecting Animal Slurry Composition. Biosyst. Eng. 2015, 132, 56–60. [Google Scholar] [CrossRef]
- Eriksen, J.; Sørensen, P.; Elsgaard, L. The Fate of Sulfate in Acidified Pig Slurry during Storage and Following Application to Cropped Soil. J. Environ. Qual. 2008, 37, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-S.; Park, H.-J.; Hao, X.; Lee, S.-I.; Jeon, B.-J.; Kwak, J.-H.; Choi, W.-J. Nitrogen, Carbon, and Dry Matter Losses during Composting of Livestock Manure with Two Bulking Agents as Affected by Co-Amendments of Phosphogypsum and Zeolite. Ecol. Eng. 2017, 102, 280–290. [Google Scholar] [CrossRef]
- Tubail, K.; Chen, L.; Michel, F.C., Jr.; Keener, H.M.; Rigot, J.F.; Klingman, M.; Kost, D.; Dick, W.A. Gypsum Additions Reduce Ammonia Nitrogen Losses During Composting of Dairy Manure and Biosolids. Compos. Sci. Util. 2008, 16, 285–293. [Google Scholar] [CrossRef]
- Febrisiantosa, A.; Ravindran, B.; Choi, H.L. The Effect of Co-Additives (Biochar and FGD Gypsum) on Ammonia Volatilization during the Composting of Livestock Waste. Sustainability 2018, 10, 795. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Li, G.; Wang, K.; Gong, X. Performance of Phosphogypsum and Calcium Magnesium Phosphate Fertilizer for Nitrogen Conservation in Pig Manure Composting. Bioresour. Technol. 2018, 250, 53–59. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Shi, H.; Wang, Y. Effects of Phosphogypsum and Superphosphate on Compost Maturity and Gaseous Emissions during Kitchen Waste Composting. Waste Manag. 2015, 36, 70–76. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Pezzuolo, A.; Sartori, L.; Chiumenti, R. Ammonia and Greenhouse Gas Emissions from Slatted Dairy Barn Floors Cleaned by Robotic Scrapers. Res. Agric. Eng. 2018, 64, 26–33. [Google Scholar] [CrossRef]
- Loide, V.; Saue, T.; Võsa, T.; Tamm, K. The Effect of Acidified Slurry on Crop Uptake and Leaching of Nutrients from a Loamy Topsoil. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 31–38. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. [Google Scholar] [CrossRef]
- Holtkamp, F.; Clemens, J.; Trimborn, M. Calcium Cyanamide Reduces Methane and Other Trace Gases during Long-Term Storage of Dairy Cattle and Fattening Pig Slurry. Waste Manag. 2023, 161, 61–71. [Google Scholar] [CrossRef]
- Rocha, A.S.; Morales, B.; El Mashad, H.M.; Pan, Y.; Zhao, Y.; Mitloehner, F.M. Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater. Sustainability 2024, 16, 5778. [Google Scholar] [CrossRef]
- Holly, M.; Larson, R. Effects of Manure Storage Additives on Manure Composition and Greenhouse Gas and Ammonia Emissions. Trans. ASABE 2017, 60, 449–456. [Google Scholar] [CrossRef]


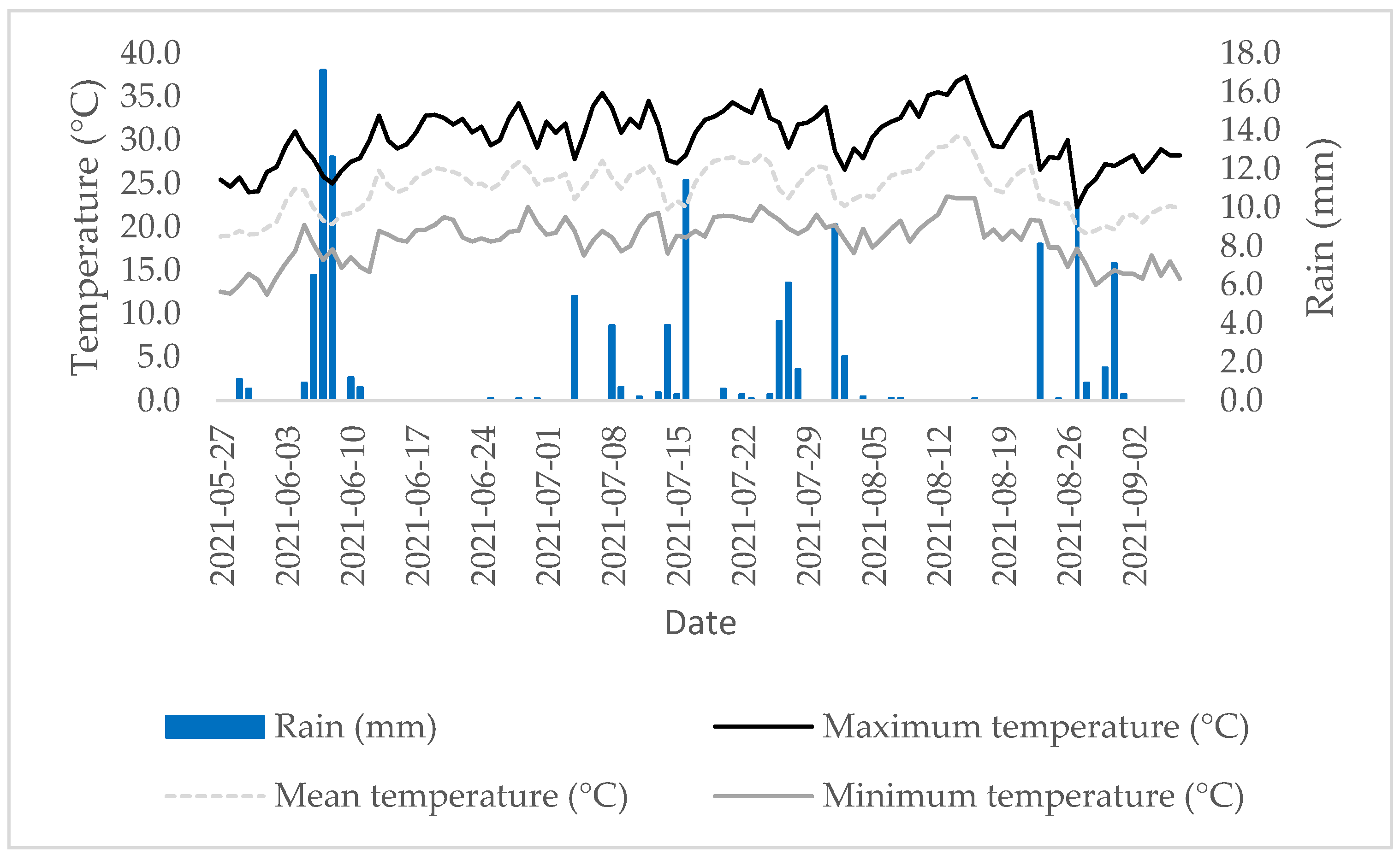

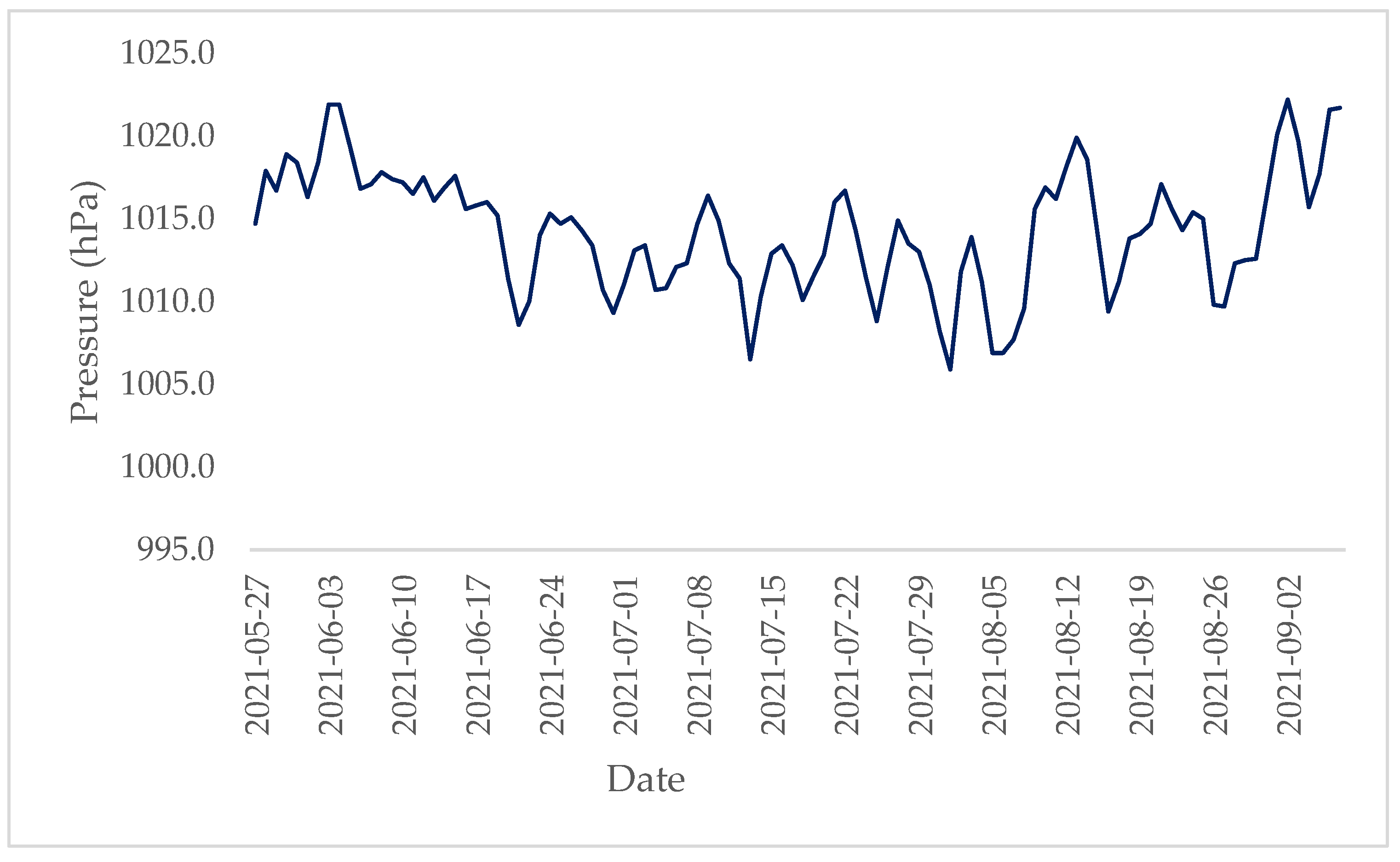
| Variable | Value |
|---|---|
| TS (%) | 8.43 ± 0.12 |
| VS (% TS) | 74.25 ± 0.50 |
| TKN (g/kg) | 3.70 ± 0.06 |
| TAN (g/kg) | 1.69 ± 0.03 |
| TAN (% TKN) | 0.46 ± 0.01 |
| Organic Carbon (% DM) | 40.20 ± 0.20 |
| Date | |||||
|---|---|---|---|---|---|
| Treatment | 5/27 | 6/30 | 7/27 | 8/28 | 9/7 |
| UTC | 6.75 Aa ± 0.10 | 7.02 Aa ± 0.09 | 6.73 Aa ± 0.15 | 7.30 Aa ± 0.16 | 6.25 Ab ± 0.09 |
| SL | 7.60 Aa ± 0.16 | 7.31 Aa ± 0.15 | 7.70 Ba ± 0.11 | 7.32 Aa ± 0.14 | 7.43 Ba ± 0.22 |
| Treatment | Date | ||||
|---|---|---|---|---|---|
| 5/27 | 6/30 | 7/27 | 8/27 | 9/7 | |
| mgNH3/m2, h | mgNH3/m2, h | mgNH3/m2, h | mgNH3/m2, h | mgNH3/m2, h | |
| UTC | 7.63 Aa ± 0.50 | 6.52 Aa ± 2.26 | 5.03 Aa ± 1.68 | 5.96 Aa ± 1.37 | 3.94 Aa ± 0.51 |
| SL | 7.63 Aa ± 0.50 | 1.76 Bb ± 0.48 | 2.55 Aa ± 0.48 | 1.91 Ba ± 0.28 | 1.00 Bb ± 0.27 |
| Variable | UTC | SL |
|---|---|---|
| Temperature | +0.11 | +0.21 |
| Humidity | −0.01 | +0.71 |
| Wind | +0.97 | +0.21 |
| Pressure | −0.99 | −0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiodini, M.E.; Costantini, M.; Zoli, M.; Aspesi, D.; Poggianella, L.; Bacenetti, J. Mitigating Ammonia Emissions from Liquid Manure Using a Commercially Available Additive Under Real-Scale Farm Conditions. Atmosphere 2025, 16, 1289. https://doi.org/10.3390/atmos16111289
Chiodini ME, Costantini M, Zoli M, Aspesi D, Poggianella L, Bacenetti J. Mitigating Ammonia Emissions from Liquid Manure Using a Commercially Available Additive Under Real-Scale Farm Conditions. Atmosphere. 2025; 16(11):1289. https://doi.org/10.3390/atmos16111289
Chicago/Turabian StyleChiodini, Marcello Ermido, Michele Costantini, Michele Zoli, Daniele Aspesi, Lorenzo Poggianella, and Jacopo Bacenetti. 2025. "Mitigating Ammonia Emissions from Liquid Manure Using a Commercially Available Additive Under Real-Scale Farm Conditions" Atmosphere 16, no. 11: 1289. https://doi.org/10.3390/atmos16111289
APA StyleChiodini, M. E., Costantini, M., Zoli, M., Aspesi, D., Poggianella, L., & Bacenetti, J. (2025). Mitigating Ammonia Emissions from Liquid Manure Using a Commercially Available Additive Under Real-Scale Farm Conditions. Atmosphere, 16(11), 1289. https://doi.org/10.3390/atmos16111289






