Effects of Climate Change on Malaria Risk to Human Health: A Review
Abstract
1. Introduction
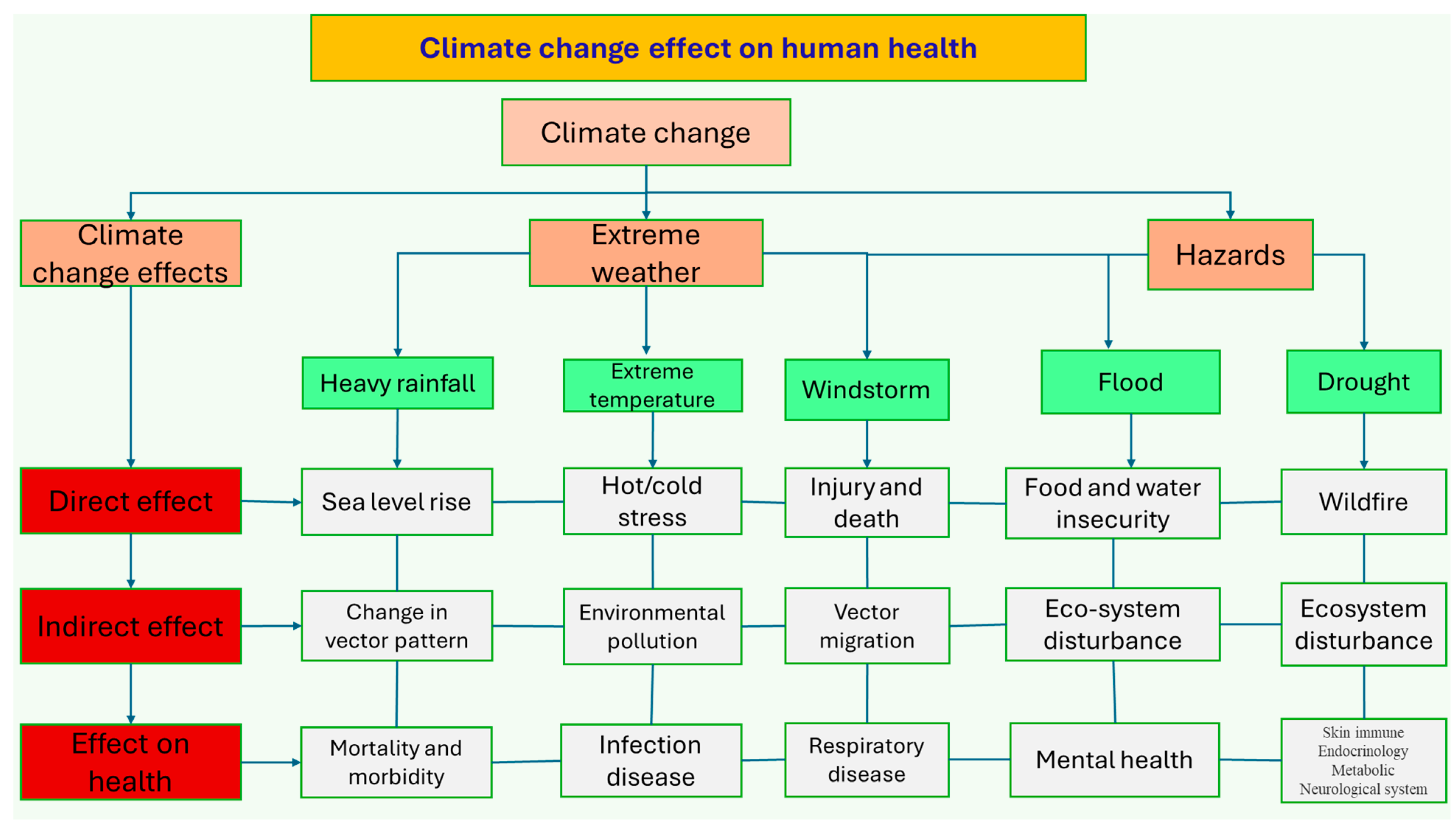
2. Climate Change Effects on Human Health
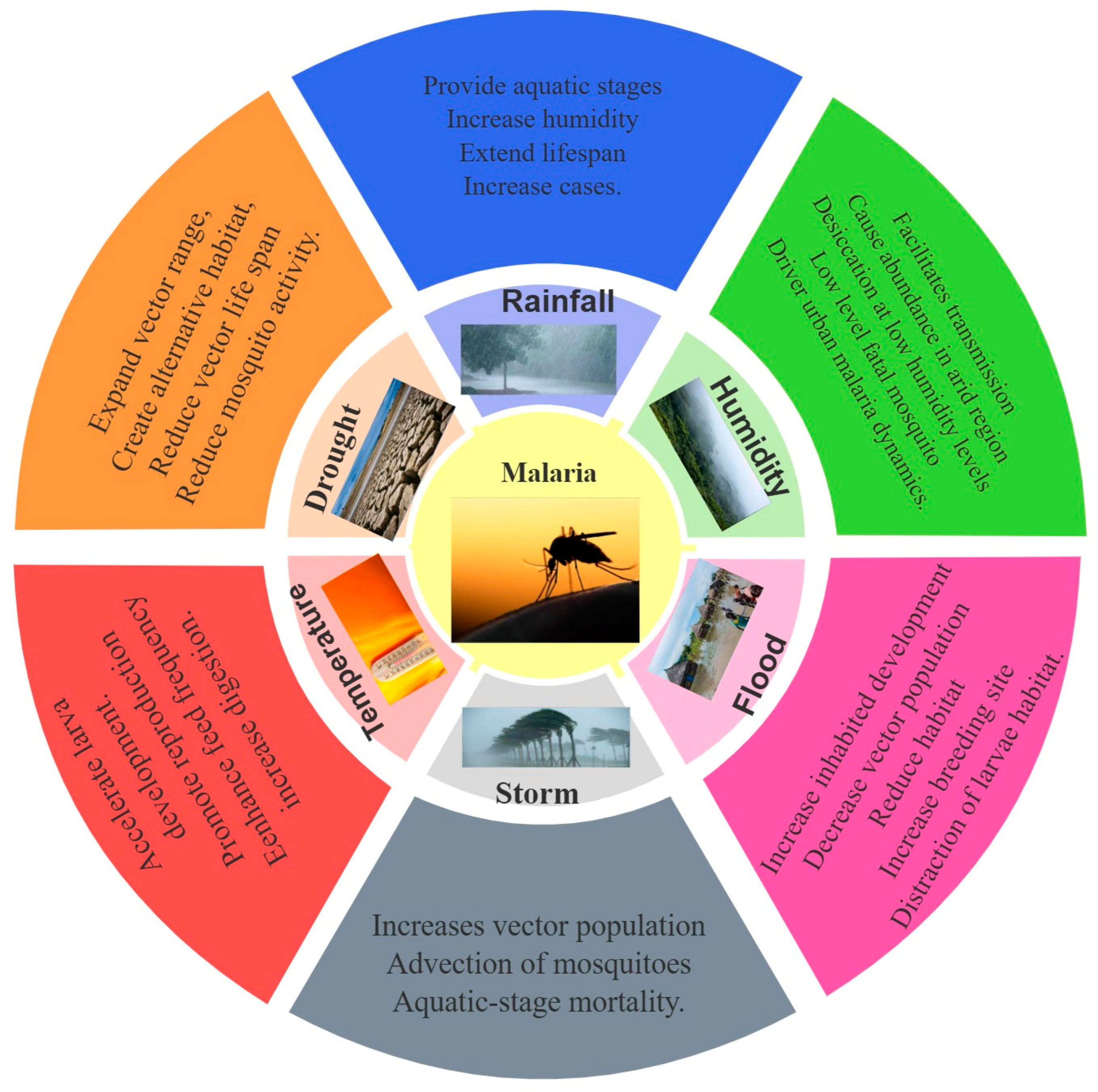
3. Climate Change Effects on Malaria
3.1. Effect of Temperature on Malaria
3.2. Effect of Rainfall on Malaria
3.3. Effect of Relative Humidity on Malaria
3.4. Effect of Extreme Weather on Malaria
3.4.1. Effect of Flood on Malaria
3.4.2. Effect of Drought on Malaria
3.4.3. Effect of Storm on Malaria
3.5. Effect of El Nino, La Nina, and Southern Oscillation on Malaria
3.6. Effect of Non-Meteorological Features on Malaria
3.6.1. Altitude
3.6.2. Land Use, Land Cover, and Land Use Change
3.6.3. Malaria Control Management
3.6.4. Population Density and Socioeconomic Condition
3.6.5. Soil Moisture
3.7. Projection of Climate Change Impacts on Malaria
3.8. Potential Pathways of Meteorological Factors Affecting Malaria
3.9. Aggravation of Climate Change Effects on Human Health
3.10. Research Gaps
3.11. Limitations and Future Perspectives
4. Conclusions and Perspectives
- Further research should adopt a multidisciplinary approach incorporating climatic, ecological, socioeconomic, and health data to develop comprehensive models that capture the complex interactions influencing malaria transmission. Collaboration between climatologists, epidemiologists, public health experts, and policymakers is essential.
- There is a need to bolster existing malaria control programs by incorporating climate change projections into planning and implementation, including scaling up vector control measures, such as insecticide-treated nets and indoor residual spraying, and expanding access to effective anti-malarial treatments.
- New mosquito control tools; collaboration between policymakers, practitioners, public health, and the population; and multisectoral risk assessments to understand the link between climate change impacts, conservation strategies, and control measures against malaria vectors are crucial.
- Continuous monitoring of climatic variables and malaria incidence is crucial for early detection and response to potential outbreaks. Integrating climate data with health surveillance systems can improve predictive modeling and timely interventions. Improved early warning and early action systems, improved forecasting of potential epidemic scenarios, and complex model simulation are important.
- Community engagement and education and policy integration can foster local resilience and support behavior change for reduction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babaie, J.; Barati, M.; Azizi, M.; Ephtekhari, A.; Sadat, S.J. A systematic evidence review of the effect of climate change on malaria in Iran. BMJ 2018, 42, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Moazami, A.; Nik, V.M.; Carlucci, S.; Geving, S. Impacts of future weather data typology on building energy performance–Investigating long-term patterns of climate change and extreme weather conditions. Appl. Energy 2019, 238, 696–720. [Google Scholar] [CrossRef]
- Kumar, V.; Ranjan, D.; Verma, K. Global climate change: The loop between cause and impact. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 187–211. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, J.; Wang, S.; Yue, C.; Shen, H. Global warming, human-induced carbon emissions, and their uncertainties. Sci. China Earth Sci. 2011, 54, 1458–1468. [Google Scholar] [CrossRef]
- Manabe, S. Role of greenhouse gas in climate change. Tellus A 2019, 71, 1620078. [Google Scholar] [CrossRef]
- Akhtar, R. Introduction: Extreme weather events and human health: A global perspective. In Extreme Weather Events and Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–11. [Google Scholar] [CrossRef]
- Sam, A.S.; Padmaja, S.S.; Kächele, H.; Kumar, R.; Müller, K. Climate change, drought and rural communities: Understanding people’s perceptions and adaptations in rural eastern India. IJDRR 2020, 44, 101436. [Google Scholar] [CrossRef]
- Islam, S.M.F.; Karim, Z.J.D. World’s demand for food and water: The consequences of climate change. In Desalination-Challenges and Opportunities; IntechOpen: Rijeka, Croatia, 2019; pp. 1–27. [Google Scholar] [CrossRef]
- Mera, G.A. Drought and its impacts in Ethiopia. Weather Clim. Extrem. 2018, 22, 24–35. [Google Scholar] [CrossRef]
- Rocque, R.J.; Beaudoin, C.; Ndjaboue, R.; Cameron, L.; Poirier-Bergeron, L.; Poulin-Rheault, R.A.; Fallon, C.; Tricco, A.C.; Witteman, H.O. Health effects of climate change: An overview of systematic reviews. BMJ Open 2021, 11, e046333. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. Int. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Vermeulen, S.J.; Aggarwal, P.K.; Corner-Dolloff, C.; Girvetz, E.; Loboguerrero, A.M.; Ramirez-Villegas, J.; Rosenstock, T.; Sebastian, L.; Rosenstock, T.; et al. Reducing risks to food security from climate change. Glob. Food Secur. Agr. 2016, 11, 34–43. [Google Scholar] [CrossRef]
- Romanello, M.; McGushin, A.; Di Napoli, C.; Drummond, P.; Hughes, N.; Jamart, L.; Kennard, H.; Lampard, P.; Solano Rodriguez, B.; Arnell, N.; et al. The 2021 report of the Lancet Countdown on health and climate change: Code red for a healthy future. Lancet 2021, 398, 1619–1662. [Google Scholar] [CrossRef]
- Barry, N.; Toe, P.; Pare Toe, L.; Lezaun, J.; Drabo, M.; Dabire, R.K.; Diabate, A. Motivations and expectations driving community participation in entomological research projects: Target Malaria as a case study in Bana, Western Burkina Faso. Malar. J. 2020, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Fears, R.; Canales-Holzeis, C.; Caussy, D.; Harper, S.L.; Hoe, V.C.W.; McNeil, J.N.; Mogwitz, J.; ter Meulen, V.; Haines, A. Climate action for health: Inter-regional engagement to share knowledge to guide mitigation and adaptation actions. Glob. Policy 2023, 15, 75–96. [Google Scholar] [CrossRef]
- Béguin, A.; Hales, S.; Rocklöv, J.; Åström, C.; Louis, V.R.; Sauerborn, R. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Glob. Environ. Change 2011, 21, 1209–1214. [Google Scholar] [CrossRef]
- Frischknecht, F.; Matuschewski, K. Plasmodium sporozoite biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025478. [Google Scholar] [CrossRef]
- Egbendewe-Mondzozo, A.; Musumba, M.; McCarl, B.A.; Wu, X. Climate change and vector-borne diseases: An economic impact analysis of malaria in Africa. Int. J. Environ. Res. Public Health 2011, 8, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Rossi, S.; Montecchini, S.; Dell’Anna, M.L.; De Conto, F.; Medici, M.C.; Chezzi, C.; Arcangeletti, M.C.; et al. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar. J. 2013, 12, 321. [Google Scholar] [CrossRef]
- Tseha, S.T. Plasmodium species and drug resistance. In Plasmodium Species and Drug Resistance; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Patz, J.A.; Olson, S.H. Climate change and health: Global to local influences on disease risk. Ann. Trop Med. Parasitol. 2006, 100, 535–549. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, M.; Kovats, R.S.; Livermore, M.T.J.; Martens, P. Climate change and malaria: Analysis of the SRES climate and socio-economic scenarios. Glob. Environ. Change 2004, 14, 87–99. [Google Scholar] [CrossRef]
- Dabaro, D.; Birhanu, Z.; Negash, A.; Hawaria, D.; Yewhalaw, D. Effects of rainfall, temperature and topography on malaria incidence in elimination targeted district of Ethiopia. Malar. J. 2021, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, M.; Ahmad, S.; Iqbal, J. Current Status and the Epidemiology of Malaria in the Middle East Region and Beyond. Microorganisms 2021, 9, 338. [Google Scholar] [CrossRef]
- van Eijk, A.M.; Hill, J.; Noor, A.M.; Snow, R.W.; ter Kuile, F.O. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2015, 3, e617–e628. [Google Scholar] [CrossRef]
- Fouque, F.; Reeder, J.C. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araujo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Raimi, M.O.; Odubo, T.V.; Omidiji, A.O. Creating the Healthiest Nation: Climate Change and Environmental Health Impacts in Nigeria: A Narrative Review. Sustain. Environ. 2021, 6, 61–122. [Google Scholar] [CrossRef]
- Leal Filho, W.; Bönecke, J.; Spielmann, H.; Azeiteiro, U.M.; Alves, F.; de Carvalho, M.L.; Nagy, G. Climate change and health: An analysis of causal relations on the spread of vector-borne diseases in Brazil. J. Clean. Prod. 2018, 177, 589–596. [Google Scholar] [CrossRef]
- Gautam, R.; Mishra, S.; Milhotra, A.; Nagpal, R.; Mohan, M.; Singhal, A.; Kumari, P. Challenges with Mosquito-borne Viral Diseases: Outbreak of the Monsters. Curr. Top. Med. Chem. 2017, 17, 2199–2214. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Malaria Policy Advisory Group (MPAG) Meeting Report, 18–20 April 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240074385 (accessed on 26 November 2024).
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Child, G.B.D.; Adolescent Health, C.; Reiner, R.C., Jr.; Olsen, H.E.; Ikeda, C.T.; Echko, M.M.; Ballestreros, K.E.; Manguerra, H.; Martopullo, I.; Kassebaum, N.J.; et al. Diseases, Injuries, and Risk Factors in Child and Adolescent Health, 1990 to 2017: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2017 Study. JAMA Pediatr. 2019, 173, e190337. [Google Scholar] [CrossRef]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Patterson, C.; et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet 2009, 373, 1693–1733. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Biswas, B.K. Effect of climate change on vector-borne disease. In Emerging Issues in Climate Smart Livestock Production; Elsevier: Amsterdam, The Netherlands, 2022; pp. 263–316. [Google Scholar] [CrossRef]
- Aladenika, S.S.; Fadairo, J.K.; Iniekong, P.; Nnedu, E.B.; Onyemelukwe, N.F. Some Haemostatic Parameters among Malaria Infected Patients in Semi-urban Setting of Malaria Endemic Region. Afr. J. Health Sci. Technol 2020, 2, 113–119. Available online: https://academicjournals.org/journal/AJHST/article-full-text-pdf/FAC328B68976 (accessed on 26 November 2024).
- Patz, J.A.; Grabow, M.L.; Limaye, V.S. When it rains, it pours: Future climate extremes and health. Ann. Glob. Health 2014, 80, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Panzi, E.K.; Okenge, L.N.; Kabali, E.H.; Tshimungu, F.; Dilu, A.K.; Mulangu, F.; Kandala, N.B. Geo-climatic factors of malaria morbidity in the democratic Republic of Congo from 2001 to 2019. Int. J. Environ. Res. Public Health 2022, 19, 3811. [Google Scholar] [CrossRef]
- Midekisa, A.; Beyene, B.; Mihretie, A.; Bayabil, E.; Wimberly, M.C. Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasites Vectors 2015, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Hasyim, H.; Nursafingi, A.; Haque, U.; Montag, D.; Groneberg, D.A.; Dhimal, M.; Kuch, U.; Muller, R. Spatial modelling of malaria cases associated with environmental factors in South Sumatra, Indonesia. Malar. J. 2018, 17, 87. [Google Scholar] [CrossRef]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Obeagu, G. Emerging public health strategies in malaria control: Innovations and implications. Ann. Med. Surg. 2024, 86, 6576–6584. [Google Scholar] [CrossRef]
- Rozi, I.E.; Syahrani, L.; Permana, D.H.; Asih, P.B.; Hidayati, A.P.; Kosasih, S.; Dewayanti, F.K.; Risandi, R.; Zubaidah, S.; Bangs, M.J.; et al. Human behavior determinants of exposure to Anopheles vectors of malaria in Sumba, Indonesia. PLoS ONE 2022, 17, e0276783. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109 (Suppl. S1), 141–161. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, T.P.; Botwe, P.K.; Arko-Mensah, J.; Issah, I.; Acquah, A.A.; Hogarh, J.N.; Dwomoh, D.; Robins, T.G.; Fobil, J.N.; Dwomoh, D.; et al. A systematic review of the effects of temperature on Anopheles mosquito development and survival: Implications for malaria control in a future warmer climate. Int. J. Environ. Res. Public Health 2021, 18, 7255. [Google Scholar] [CrossRef] [PubMed]
- Coates, S.J.; Enbiale, W.; Davis, M.D.; Andersen, L.K. The effects of climate change on human health in Africa, a dermatologic perspective: A report from the International Society of Dermatology Climate Change Committee. Int. J. Dermatol. 2020, 59, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Eikenberry, S.E.; Gumel, A.B. Mathematical modeling of climate change and malaria transmission dynamics: A historical review. J. Math. Biol. 2018, 77, 857–933. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.; Fonseca, B.R.; Caminade, C.; Thiaw, W.M.; Deme, A.; Morse, A.P.; Ndione, J.A.; Gaye, A.T.; Diaw, A.; Ngom Ndiaye, M.K. Climate variability and malaria over West Africa. Am. J. Trop. Med. Hyg. 2020, 102, 1037. [Google Scholar] [CrossRef]
- Kipruto, E.K.; Ochieng, A.O.; Anyona, D.N.; Mbalanya, M.; Mutua, E.N.; Onguru, D.; Nyamongo, I.K.; Estambale, B.B. Effect of climatic variability on malaria trends in Baringo County, Kenya. Malar. J. 2017, 16, 220. [Google Scholar] [CrossRef]
- Thomas, S.; Ravishankaran, S.; Justin, N.J.A.; Asokan, A.; Kalsingh, T.M.J.; Mathai, M.T.; Valecha, N.; Montgomery, J.; Thomas, M.B.; Eapen, A. Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of Plasmodium vivax and Plasmodium falciparum: A study from a malaria-endemic urban setting, Chennai in India. Malar. J. 2018, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Nyawanda, B.O.; Beloconi, A.; Khagayi, S.; Bigogo, G.; Obor, D.; Otieno, N.A.; Lange, S.; Franke, J.; Sauerborn, R.; Utzinger, J.; et al. The relative effect of climate variability on malaria incidence after scale-up of interventions in western Kenya: A time-series analysis of monthly incidence data from 2008 to 2019. Parasite Epidem. Cont. 2023, 21, e00297. [Google Scholar] [CrossRef] [PubMed]
- Mbouna, A.D.; Tompkins, A.M.; Lenouo, A.; Asare, E.O.; Yamba, E.I.; Tchawoua, C. Modelled and observed mean and seasonal relationships between climate, population density and malaria indicators in Cameroon. Malar. J. 2019, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Wangdi, K.; Wetzler, E.; Cox, H.; Marchesini, P.; Villegas, L.; Canavati, S. Spatial patterns and climate drivers of malaria in three border areas of Brazil, Venezuela and Guyana, 2016–2018. Sci. Rep. 2022, 12, 10995. [Google Scholar] [CrossRef] [PubMed]
- Moukam Kakmeni, F.M.; Guimapi, R.Y.A.; Ndjomatchoua, F.T.; Pedro, S.A.; Mutunga, J.; Tonnang, H.E. Spatial panorama of malaria prevalence in Africa under climate change and interventions scenarios. Int. J. Health Geogr. 2018, 17, 2. [Google Scholar] [CrossRef]
- Edman, J.D.; Spielman, A. Blood-feeding by vectors: Physiology, ecology, behavior, and vertebrate defense. In The Arboviruses; CRC Press: Boca Raton, FL, USA, 2020; pp. 153–190. [Google Scholar] [CrossRef]
- Colon-Gonzalez, F.J.; Sewe, M.O.; Tompkins, A.M.; Sjodin, H.; Casallas, A.; Rocklov, J.; Caminade, C.; Lowe, R. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: A multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health 2021, 5, e404–e414. [Google Scholar] [CrossRef]
- Ewing, D.A.; Cobbold, C.A.; Purse, B.V.; Nunn, M.A.; White, S.M. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J. Theor. Biol. 2016, 400, 65–79. [Google Scholar] [CrossRef]
- Neddermeyer, J.H.; Parise, K.L.; Dittmar, E.; Kilpatrick, A.M.; Foster, J.T. Nowhere to fly: Avian malaria is ubiquitous from ocean to summit on a Hawaiian island. Biol. Conserv. 2023, 279, 109943. [Google Scholar] [CrossRef]
- Shapiro, L.L.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef] [PubMed]
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [PubMed]
- Ototo, E.N.; Ogutu, J.O.; Githeko, A.; Said, M.Y.; Kamau, L.; Namanya, D.; Simiyu, S.; Mutimba, S. Forecasting the potential effects of climate change on malaria in the Lake Victoria Basin using regionalized climate projections. Acta Parasitol. 2022, 67, 1535–1563. [Google Scholar] [CrossRef]
- Lubinda, J.; Haque, U.; Bi, Y.; Hamainza, B.; Moore, A.J. Near-term climate change impacts on sub-national malaria transmission. Sci. Rep. 2021, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Ingholt, M.M.; Chen, T.T.; Hildebrandt, F.; Pedersen, R.K.; Simonsen, L. Temperate climate malaria in nineteenth century Denmark. BMC Infect. Dis. 2022, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Tapias-Rivera, J.; Gutierrez, J.D. Environmental and socio-economic determinants of the occurrence of malaria clusters in Colombia. Acta Trop. 2023, 241, 106892. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.S.; Booth, M.; Nikulin, G.; McCreesh, N. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospat. Health 2016, 11 (Suppl. S1), 406. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, G.J.; Maharaj, R.; Witbooi, P.; Okosun, K.O. Modelling the influence of temperature and rainfall on the population dynamics of Anopheles arabiensis. Malar. J. 2016, 15, 364. [Google Scholar] [CrossRef]
- Petrić, M. “Modelling the Influence of Meteorological Conditions on Mosquito Vector Population Dynamics (Diptera, Culicidae)”. University of Novi Sad (Serbia). 2020. Available online: https://www.proquest.com/openview/f742f8c2bd2fc96b4b9f80fefc1996ad/1?pq-origsite=gscholar&cbl=2026366&diss=y (accessed on 25 November 2024).
- Fecchio, A.; Wells, K.; Bell, J.A.; Tkach, V.V.; Lutz, H.L.; Weckstein, J.D.; Clegg, S.M.; Clark, N.J. Climate variation influences host specificity in avian malaria parasites. Ecol. Lett. 2019, 22, 547–557. [Google Scholar] [CrossRef]
- Lamy, K.; Tran, A.; Portafaix, T.; Leroux, M.; Baldet, T. Impact of regional climate change on the mosquito vector Aedes albopictus in a tropical island environment: La Réunion. Sci. Total Environ. 2023, 875, 162484. [Google Scholar] [CrossRef]
- Hertig, E. Distribution of Anopheles vectors and potential malaria transmission stability in Europe and the Mediterranean area under future climate change. Parasites Vectors 2019, 12, 18. [Google Scholar] [CrossRef]
- Matthew, O.J. Investigating climate suitability conditions for malaria transmission and impacts of climate variability on mosquito survival in the humid tropical region: A case study of Obafemi Awolowo University Campus, Ile-Ife, south-western Nigeria. Int. J. Biometeorol. 2020, 64, 355–365. [Google Scholar] [CrossRef]
- Wudil, A.H.; Usman, M.; Rosak-Szyrocka, J.; Pilař, L.; Boye, M.; Health, P. Reversing years for global food security: A review of the food security situation in Sub-Saharan Africa (SSA). Int. J. Environ. Res. 2022, 19, 14836. [Google Scholar] [CrossRef]
- Haileselassie, W.; Parker, D.M.; Taye, B.; David, R.E.; Zemene, E.; Lee, M.C.; Zhong, D.; Zhou, G.; Alemu, T.; Tadele, G.; et al. Burden of malaria, impact of interventions and climate variability in Western Ethiopia: An area with large irrigation based farming. BMC Public Health 2022, 22, 196. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, C.J.B. Climate change and vector-borne diseases in China: A review of evidence and implications for risk management. Biology 2022, 11, 370. [Google Scholar] [CrossRef]
- Srimath-Tirumula-Peddinti, R.C.P.K.; Neelapu, N.R.R.; Sidagam, N. Association of climatic variability, vector population and malarial disease in district of Visakhapatnam, India: A modeling and prediction analysis. PLoS ONE 2015, 10, e0128377. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Zhang, Y.; Xiang, J.; Tong, M.X.; Gao, Q.; Zhang, Y.; Sun, S.; Liu, Q.; Jiang, B.; et al. Effect of temperature and its interactions with relative humidity and rainfall on malaria in a temperate city Suzhou, China. Environ. Sci. Pollut. Res. 2021, 28, 16830–16842. [Google Scholar] [CrossRef] [PubMed]
- Baril, C.; Pilling, B.G.; Mikkelsen, M.J.; Sparrow, J.M.; Duncan, C.A.; Koloski, C.W.; LaZerte, S.E.; Cassone, B.J. The influence of weather on the population dynamics of common mosquito vector species in the Canadian Prairies. Parasites Vectors 2023, 16, 153. [Google Scholar] [CrossRef]
- Masood, W.; Aquil, S.; ullah, H.; Nadeem, A.; Mehmood, H.; Islam, Z.; Essar MYAhmad, S. Impact of climate change on health in Afghanistan amidst a humanitarian crisis. J. Clim. Change Health 2022, 6, 100139. [Google Scholar] [CrossRef]
- Semenza, J.C.; Rocklov, J.; Ebi, K.L. Climate Change and Cascading Risks from Infectious Disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef]
- Oscar Júnior, A.C.; de Assis Mendonça, F. Climate change and risk of arboviral diseases in the state of Rio de Janeiro (Brazil). Theor. Appl. Climatol. 2021, 145, 731–745. [Google Scholar] [CrossRef]
- Rey, J.R.; Walton, W.E.; Wolfe, R.J.; Connelly, C.R.; O’Connell, S.M.; Berg, J.; Sakolsky-Hoopes, G.E.; Laderman, A.D. North American wetlands and mosquito control. Int. J. Environ. Res. Public Health 2012, 9, 4537–4605. [Google Scholar] [CrossRef] [PubMed]
- Moyo, E.; Nhari, L.G.; Moyo, P.; Murewanhema, G.; Dzinamarira, T. Health effects of climate change in Africa: A call for an improved implementation of prevention measures. Eco Environ. Health 2023, 2, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, P.; Mahendran, R.; Huang, W.; Gao, Y.; Yang, Z.; Ye, T.; Wen, B.; Wu, Y.; Li, S.; et al. Global climate change and human health: Pathways and possible solutions. Eco Environ. Health 2022, 1, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Al Noman, A.; Das, D.; Nesa, Z.; Tariquzzaman, M.; Sharzana, F.; Rakibul Hasan, M.; Riaz, B.K.; Sharower, G. Rahman, M.M. Importance of Wolbachia-mediated biocontrol to reduce dengue in Bangladesh and other dengue-endemic developing countries. Biosaf. Health 2023, 5, 69–77. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dwivedi, S. Technology, Impact of El Niño–Southern Oscillation and Indian Ocean Dipole on malaria transmission over India in changing climate. Int. J. Environ. Sci. Technol. 2024, 21, 91–100. [Google Scholar] [CrossRef]
- Kreppel, K.; Caminade, C.; Govella, N.; Morse, A.P.; Ferguson, H.M.; Baylis, M. Impact of ENSO 2016–17 on regional climate and malaria vector dynamics in Tanzania. Environ. Res. Lett. 2019, 14, 075009. [Google Scholar] [CrossRef]
- Harp, R.D.; Colborn, J.M.; Candrinho, B.; Colborn, K.L.; Zhang, L.; Karnauskas, K.B. Interannual climate variability and malaria in Mozambique. GeoHealth 2021, 5, e2020GH000322. [Google Scholar] [CrossRef]
- Sellers, S.; Ebi, K.L.; Hess, J. Climate Change, Human Health, and Social Stability: Addressing Interlinkages. Environ. Health Perspect. 2019, 127, 45002. [Google Scholar] [CrossRef]
- Trájer, A.J. The potential effects of climate change on the populations of Aedes punctor (Diptera: Culicidae) in Hungary. J. Insect Conserv. 2022, 26, 205–217. [Google Scholar] [CrossRef]
- Kim, J.E.; Bae, Y.J.; Lee, H.G.; Kim, D.G. Analysis of habitat characteristics of mosquitoes in Danwon-gu, Ansan city, Korea, based on civil complaint data. Entomol. Res. 2018, 48, 540–549. [Google Scholar] [CrossRef]
- Kweka, E.J.; Kimaro, E.E.; Munga, S. Effect of Deforestation and Land Use Changes on Mosquito Productivity and Development in Western Kenya Highlands: Implication for Malaria Risk. Front. Public Health 2016, 4, 238. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; Vittor, A.Y. Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2018, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gowelo, S.; Chirombo, J.; Koenraadt, C.J.M.; Mzilahowa, T.; van den Berg, H.; Takken, W.; McCann, R.S. Characterisation of anopheline larval habitats in southern Malawi. Acta Trop. 2020, 210, 105558. [Google Scholar] [CrossRef]
- Caputo, A.; Garavelli, P.L. Climate, environment and transmission of malaria. Infez Med. 2016, 2, 93–104. Available online: https://infezmed.it/media/journal/Vol_24_2_2016_1.pdf (accessed on 26 November 2024).
- Wu, Q.; Guo, C.; Li, X.-k.; Yi, B.-Y.; Li, Q.-L.; Guo, Z.-M.; Lu, J.-H. Meta-transcriptomic study of mosquito virome and blood feeding patterns at the human-animal-environment interface in Guangdong Province, China. One Health 2023, 16, 100493. [Google Scholar] [CrossRef] [PubMed]
- Mironova, V.; Shartova, N.; Beljaev, A.; Varentsov, M.; Grishchenko, M. Effects of climate change and heterogeneity of local climates on the development of malaria parasite (Plasmodium vivax) in Moscow megacity region. Int. J. Environ. Res. Public Health 2019, 16, 694. [Google Scholar] [CrossRef]
- Vanwambeke, S.O.; Lambin, E.F.; Eichhorn, M.P.; Flasse, S.P.; Harbach, R.E.; Oskam, L.; Somboon, P.; Van Beers, S.; Van Benthem, B.H.; Walton, C.; et al. Impact of land-use change on dengue and malaria in northern Thailand. EcoHealth 2007, 4, 37–51. [Google Scholar] [CrossRef]
- Ermert, V.; Fink, A.H.; Morse, A.P.; Paeth, H. The impact of regional climate change on malaria risk due to greenhouse forcing and land-use changes in tropical Africa. Environ. Health Perspect. 2012, 120, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gafna, D.J.; Obando, J.A.; Kalwij, J.M.; Dolos, K.; Schmidtlein, S. Climate Change Impacts on the Availability of Anti-malarial Plants in Kenya. Clim. Change Ecol. 2023, 5, 100070. [Google Scholar] [CrossRef]
- Fletcher, I.K.; Stewart-Ibarra, A.M.; Sippy, R.; Carrasco-Escobar, G.; Silva, M.; Beltran-Ayala, E.; Ordoñez, T.; Adrian, J.; Saenz, F.E.; Drakeley, C.; et al. The relative role of climate variation and control interventions on malaria elimination efforts in El Oro, Ecuador: A modeling study. Front. Environ. Sci. 2020, 8, 135. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Castro, M.C. Challenges for malaria elimination in Brazil. Malar. J. 2016, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.L.J.; Zewotir, T. Evaluating the effects of climate and environmental factors on under-5 children malaria spatial distribution using generalized additive models (GAMs). J. Epidemiol. Glob. Health 2020, 10, 304–314. [Google Scholar] [CrossRef]
- Krsulovic, F.A.M.; Moulton, T.P.; Lima, M.; Jaksic, F. Epidemic malaria dynamics in Ethiopia: The role of self-limiting, poverty, HIV, climate change and human population growth. Malar. J. 2022, 21, 135. [Google Scholar] [CrossRef]
- Sarkar, S.; Singh, P.; Lingala, M.A.L.; Verma, P.; Dhiman, R.C. Malaria risk map for india based on climate, ecology and geographical modelling. Geospat. Health 2019, 14, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Le, P.V.; Kumar, P.; Ruiz, M.O.; Mbogo, C.; Muturi, E.J. Predicting the direct and indirect impacts of climate change on malaria in coastal Kenya. PLoS ONE 2019, 14, e0211258. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dwivedi, S. Understanding the effect of climate change in the distribution and intensity of malaria transmission over India using a dynamical malaria model. Int. J. Biometeorol. 2021, 65, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Karypidou, M.C.; Almpanidou, V.; Tompkins, A.M.; Mazaris, A.D.; Gewehr, S.; Mourelatos, S.; Katragkou, E. Projected shifts in the distribution of malaria vectors due to climate change. Clim. Change 2020, 163, 2117–2133. [Google Scholar] [CrossRef]
- Bhattarai, S.; Blackburn, J.K.; Ryan, S.J. Malaria transmission in Nepal under climate change: Anticipated shifts in extent and season, and comparison with risk definitions for intervention. Malar. J. 2022, 21, 390. [Google Scholar] [CrossRef]
- Diouf, I.; Adeola, A.M.; Abiodun, G.J.; Lennard, C.; Shirinde, J.M.; Yaka, P.; Ndione, J.-A.; Gbobaniyi, E.O. Impact of future climate change on malaria in West Africa. Theor. Appl. Climatol. 2022, 147, 853–865. [Google Scholar] [CrossRef]
- Marques, R.; Krüger, R.F.; Cunha, S.K.; Silveira, A.S.; Alves, D.M.; Rodrigues, G.D.; Peterson, A.T.; Jiménez-García, D. Climate change impacts on Anopheles (K.) cruzii in urban areas of Atlantic Forest of Brazil: Challenges for malaria diseases. Acta Trop. 2021, 224, 106123. [Google Scholar] [CrossRef]
- Nili, S.; Asadgol, Z.; Dalaei, H.; Khanjani, N.; Bakhtiari, B.; Jahani, Y. The effect of climate change on malaria transmission in the southeast of Iran. Int. J. Biometeorol. 2022, 66, 1613–1626. [Google Scholar] [CrossRef]
- Li, C.; Managi, S. Global malaria infection risk from climate change. Environ. Res. 2022, 214, 114028. [Google Scholar] [CrossRef] [PubMed]
- Fall, P.; Diouf, I.; Deme, A.; Diouf, S.; Sene, D.; Sultan, B.; Famien, A.M.; Janicot, S. Bias-corrected CMIP5 projections for climate change and assessments of impact on Malaria in Senegal under the VECTRI Model. Trop. Med. Infect. 2023, 8, 310. [Google Scholar] [CrossRef]
- Akpan, G.E.; Adepoju, K.A.; Oladosu, O.R. Potential distribution of dominant malaria vector species in tropical region under climate change scenarios. PLoS ONE 2019, 14, e0218523. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, C.; Ren, Z.; Wang, S. Predicting the Geographical Distribution of Malaria-Associated Anopheles dirus in the South-East Asia and Western Pacific Regions Under Climate Change Scenarios. Front. Environ. Sci. 2022, 10, 841966. [Google Scholar] [CrossRef]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, L.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2021, 42, 293–315. [Google Scholar] [CrossRef]
- Sheehan, M.C. 2021 Climate and Health Review - Uncharted Territory: Extreme Weather Events and Morbidity. Int. J. Health Serv. 2022, 52, 189–200. [Google Scholar] [CrossRef]
- Maron, M.; McAlpine, C.A.; Watson, J.E.M.; Maxwell, S.; Barnard, P.; Andersen, A. Climate-induced resource bottlenecks exacerbate species vulnerability: A review. Divers. Distrib. 2015, 21, 731–743. [Google Scholar] [CrossRef]
- Tanser, F.C.; Sharp, B.; le Sueur, D. Potential effect of climate change on malaria transmission in Africa. Lancet 2003, 362, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colon-Gonzalez, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Muttarak, R. Vulnerability to climate change and adaptive capacity from a demographic perspective. In International Handbook of Population and Environment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–86. [Google Scholar] [CrossRef]
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370, 20130552. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megersa, D.M.; Luo, X.-S. Effects of Climate Change on Malaria Risk to Human Health: A Review. Atmosphere 2025, 16, 71. https://doi.org/10.3390/atmos16010071
Megersa DM, Luo X-S. Effects of Climate Change on Malaria Risk to Human Health: A Review. Atmosphere. 2025; 16(1):71. https://doi.org/10.3390/atmos16010071
Chicago/Turabian StyleMegersa, Dereba Muleta, and Xiao-San Luo. 2025. "Effects of Climate Change on Malaria Risk to Human Health: A Review" Atmosphere 16, no. 1: 71. https://doi.org/10.3390/atmos16010071
APA StyleMegersa, D. M., & Luo, X.-S. (2025). Effects of Climate Change on Malaria Risk to Human Health: A Review. Atmosphere, 16(1), 71. https://doi.org/10.3390/atmos16010071









