1. Introduction
Photodissociation (photolysis) is the process in which air molecules absorb solar photons, leading to the destruction of intramolecular bonds. Photodissociation processes play a key role in atmospheric photochemistry, initiating the most important cycles of the chemical composition change in the middle atmospheric layers; see, for example, [
1] and the lower [
2] atmospheric layers. The formation and destruction of the stratospheric ozone, which protects the biosphere from harsh ultraviolet solar radiation, are associated with photodissociation processes. In particular, the chain of ozone formation in the stratosphere is initiated from the photolysis of molecular oxygen (1) and continues with the recombination of atomic and molecular oxygen with the participation of any air molecule (M) as a third body (2).
Photolysis of the ozone, which can occur in the stratosphere and troposphere, leads to the formation of both atomic oxygen in the ground O(
3P) (3) and excited atomic oxygen O(
1D) (4) states. It does not substantially affect ozone destruction since the recombination reaction (2) quickly restores ozone; however, it is important for the destruction of ozone (5) in chain reactions involving nitrogen, hydrogen, chlorine, and bromine radicals, generally designated as X (e.g., NO, Cl, Br) in (5).
Photodissociation of chlorofluorocarbons and halons in the stratosphere initiates the formation of chlorine and bromine radicals, which determine the destruction of stratospheric ozone in the chlorine and bromine catalytic cycles. The understanding of the role played by these processes in the global depletion of the ozone layer led to international agreements on phasing out the production and use of CFCs [
3].
Photolysis of ozone at wavelengths shorter than 320 nm (4) results in the formation of an excited atomic oxygen atom O(
1D), playing a decisive role in atmospheric chemistry, which leads to the formation of active hydrogen and nitrogen oxides by reactions (6) and (7).
The formation of ozone holes in the polar regions is also directly related to photolysis processes, which determine the rate of ozone destruction when the Sun returns after the polar night, during which, as a result of chlorine and bromine activation, Cl
2O
2 and Cl
2 molecules, which are sensitive to solar radiation, are formed on polar stratospheric clouds. These molecules quickly disintegrate after sunrise as a result of photodissociation processes (8) and (9), resulting in the formation of free radicals and rapid destruction of ozone in catalytic cycles (5) [
4].
Photolysis of nitrogen dioxide (10) plays an important role both in the stratosphere, where it produces active nitrogen oxides participating in the catalytic destruction of ozone, and in the troposphere, where photodissociation of NO
2 is the only channel for the atomic oxygen production since the photodissociation of molecular oxygen (1) does not occur in the troposphere because solar radiation with the wavelengths shorter than 242 nm is completely absorbed in the stratosphere [
5]. The Tropospheric ozone is, on the one hand, a greenhouse and toxic gas. It also produces excited atomic oxygen (reaction 3), which promotes the formation of hydroxyl radicals (6) and self-purification of the troposphere.
The photodissociation of nitric acid vapour HNO
3 (11), which is an important reservoir for the nitrogen group, both in the stratosphere and in the troposphere, determines the redistribution of nitrogen-containing gases within the NO
y family. In the stratosphere, as a result of photolysis (11), nitrogen dioxide can return to the catalytic destruction of ozone (5), from which it is extracted as a result of its reaction with hydroxyl radicals, and in the troposphere, after photodissociation of HNO
3, nitrogen dioxide can return to the ozone formation chain through photolysis (10), and recombination (2), which is interrupted during the formation of HNO
3.
Due to the crucial role of photodissociation processes in atmospheric photochemistry, they are an important part of any chemical transport model (CTM), and any chemical climate model (CCM) used to study the time evolution of atmospheric chemical composition. The quantitative assessment of photodissociation rates used in CTM and CCM is based on the principles of chemical kinetics. According to these principles, the rates of destruction of reactant
A, and the formation of products
B and
C of the photodissociation process (12), are proportional to the concentration of the reactant with a proportionality coefficient, depending on the solar radiation flux and the absorption properties of the atmosphere (13).
where
A is the concentration of reactant and
B and
C are the concentrations of reaction products, the proportionality coefficient
J, called the photolysis rate, is determined by the formula:
where σ
A(λ, T) and q
A(λ, T) are the absorption cross sections of gas A and the quantum yield of the reaction, which determine the absorption properties of the gas under consideration at height z and depend on the wavelength (λ) and temperature (T), and I(λ) is the actinic solar radiation flux at wavelength λ at altitude z, λ
1–λ
2 is part of the spectrum in which the molecule can dissociate. The absorption cross sections and quantum yields characterising the absorption properties of gases are based on laboratory measurements and are constantly updated and published in collections that summarise the results of many laboratory measurements, such as JPL [
6].
The total solar radiation flux at the height z is determined by the incoming direct, reflected, and scattered radiation of the Sun (15).
For an accurate calculation of the total solar radiation fluxes, it is necessary to solve the integral radiative transfer equation [
7], which can lead to a significant complication of the CTM and CCM code; therefore, the simplified methods are most often used in atmospheric models [
8,
9,
10]. At the same time, the main difficulties in calculating the total radiation are associated with the necessity to consider molecular, aerosol, and cloud scattering. Therefore, simplified methods include a simplified treatment of scattered radiation using two, four, or more stream methods [
11,
12,
13]. However, even the use of simplified methods for calculating scattered radiation with high spectral resolution, such as narrow-band methods, requires substantial computer time, given the need to increase spatial resolution in climate models. A possible solution to the problem is the use of wide-band methods that treat solar radiation fluxes and absorption parameters in rather wide spectral ranges [
14,
15], or the use of pre-calculated photodissociation rates [
16,
17,
18].
One of the methods using pre-calculated values of photodissociation coefficients is the “Look up table” (LUT), which is used in the SOCOL [
19], SLIMCAT/TOMCAT [
20], and LMDz-REPROBUS [
21] models. The idea of this method is that a high spectral resolution model is used to pre-calculate photolysis rates as a function of several atmospheric parameters. These pre-calculated results are then used to obtain photolysis rates by interpolating tabular values according to current parameters from a global model. Usually, cloud and aerosol effects, which are extremely important in the troposphere, are not considered in LUT calculations, and are added to the photolysis rates obtained by the LUT method only as modification factors. While in the stratosphere, the effect of tropospheric clouds can be reasonably considered by selecting the altitude and albedo of the cloud top, in the troposphere, most of the chemical processes occur within or below the cloud-aerosol layers. Therefore, when developing global models of tropospheric photochemistry, it is necessary to consider both the increase in the rate of photochemical processes above clouds and the upper cloud layers and the decrease in the rate beneath optically thick clouds and absorbing aerosols [
22,
23,
24].
A more accurate accounting of the processes associated with the propagation of solar radiation in the atmosphere became possible thanks to the development of the wide-band Cloud-J algorithm (a modified version of the Fast-J algorithm) [
25] for calculating photolysis rates in the presence of cloud and aerosol layers, considering their optical thickness, single scattering albedo, and scattering phase function. Cloud-J enables the modelling of global atmospheric photochemistry by incorporating the physical properties of scattering and absorbing particles directly [
26]. This capability enhances the accuracy of photolysis rate calculations by accounting for the complex interactions of solar radiation with various atmospheric components.
The Cloud-J algorithm demonstrates accuracy comparable to many narrow-band methods for calculating photolysis coefficients while its computational efficiency is remarkable. Typically, the time dedicated to its calculations does not exceed 10% of the total computation time needed for the climate model [
25]. When employing wide-band methods, special attention should be paid to the accuracy of accounting for the influence of different types of atmospheric scattering and reflection on the photodissociation coefficients in the troposphere and stratosphere. The spectral dependences of the parameters of Rayleigh, aerosol, and cloud scattering exhibit significant differences. In addition, the scattering and reflecting substances reside in distinct altitude layers, which influences their interactions with solar radiation. For example, the influence reflected by surface solar radiation on the photodissociation coefficients depends not only on the reflective properties of the surface but also on the attenuation of downward solar radiation in different spectral regions. Rayleigh scattering, on the one hand, is inversely proportional to the fourth power of the wavelength, which determines its strong dependence on the selected spectral ranges, and, on the other hand, depends on the air density, which decreases exponentially with height. Aerosol and cloud scattering show less variation across the spectrum but occur in narrow altitude layers (cloud in the troposphere, and aerosol in the troposphere and stratosphere) and have a scattering indicatrix strongly elongated along the beam.
Figure 1 illustrates the spectral variability of solar radiation fluxes along with the action spetra (absorption cross sections × quantum yields) of O
2 and O
3 (
Figure 1a). Additionally, it shows the action spectra of various gases considered in this study (
Figure 1b). The figure highlights the interactions between solar radiation and atmospheric constituents, which are crucial for understanding the photolysis processes discussed in the paper.
All atmospheric gases can be categorised into three distinct groups based on their absorption characteristics: (1) gases with absorption bands only in the hard ultraviolet spectrum, at wavelengths shorter than 250 nm; (2) gases with absorption bands in the entire ultraviolet range (100–410 nm); (3) gases with absorption bands in the ultraviolet and visible ranges. The first group includes molecular oxygen, water vapour, carbon dioxide, nitrous oxide, hydrochloric acid vapour, freons, and other chlorofluorocarbons. Since solar radiation at these wavelengths is entirely absorbed in the stratosphere, the photolysis of these gases occurs at altitudes above the tropopause, where they play a critical role in the photochemistry of the middle atmosphere. Furthermore, because radiation in this ultraviolet range does not reach the Earth’s surface and denser layers of the atmosphere, the photolysis rates of these gases in the troposphere are expected to be largely unaffected by surface reflection and scattering processes.
The second group of gases includes nitric acid vapours and most constituents from nitrogen, hydrogen, chlorine, and bromine groups. These gases exhibit a dependence of photodissociation coefficients on the conditions of solar radiation passage in the 290–320 nm range. In this range, both the solar radiation flux and the absorption by ozone, which determine the absorption properties of the atmosphere, change greatly across the spectrum.
These gases are the most interesting from the point of view of the reflection and scattering effects on the photodissociation rates because the solar radiation in this spectral region reaches the troposphere and the surface. (
Figure 1a). A significant aspect of this group is the photodissociation of ozone, which results in the formation of excited oxygen O(
1D), which is formed during the absorption of solar radiation up to 320 nm. Therefore, assessing the effects of tropospheric reflection and scattering on O(
1D) formation rates under varying atmospheric conditions is essential for a comprehensive understanding of photodissociation processes and their implications for atmospheric chemistry.
The primary species in the third category are ozone with the formation of atomic oxygen in the ground state O(3P), nitrogen dioxide NO2, nitrate trioxide NO3, as well as chlorine and bromine reservoirs. These gases are essential for understanding the ozone anomalies in the polar regions. It is particularly interesting to analyse the contribution of the visible range of solar radiation to their photodissociation, especially since this spectral range experiences minimal attenuation as it travels through the atmosphere.
Reflection and scattering processes’ in the near ultraviolet and visible spectrum are noteworthy because a significant portion of the incoming solar radiation can penetrate the lower layers of the atmosphere and the surface, where it may subsequently be reflected and scattered upward. The influence of cloud and aerosol layers and other scattering and reflection processes on the photolysis rates were considered in the works [
27,
28]. Despite the fact that many aspects related to the passage of solar radiation have been clarified, nevertheless, some spectral and altitude features of various gases are not sufficiently understood.
In this study, the Cloud-J algorithm is examined from the point of view of the accuracy of the chosen spectral resolution for the calculations of photodissociation rates in the lower and middle atmosphere. The use of such an algorithm as Cloud-J in CTM or CCM allows for effectively improving the performance in predicting photolysis rates by taking into account the influence of scattering and reflection processes in the presence of cloud and aerosol layers.
This analysis considers the characteristics of solar radiation propagation mentioned earlier and aims to evaluate the impact of reflection and scattering processes on molecules, aerosols, and clouds. At the first stage of the study, the results of calculating the photodissociation coefficients using Cloud-J v.8.0 are compared with those calculated for the same conditions by the LibRadtran method exploiting very high spectral resolution. Then, the Cloud-J algorithm is employed to obtain new estimates of the reflection and scattering processes influence in different spectral ranges on the photodissociation rates of the main gases.
2. Materials and Methods
The photolysis rates of the main gases with absorption bands in different spectral ranges (
Figure 1b), which we used as reference data, were calculated using the LibRadtran software (version 2.0.5) package that was designed to calculate radiative transfer in the Earth’s atmosphere. Its main part is the uvspec programme, a widely used tool for UV calculations that has demonstrated good accuracy in a number of validation campaigns, and which can be used to calculate luminosity, illuminance, and actinic fluxes of solar radiation [
29]. The data calculated using LibRadtran were used as reference data in article [
30], which compared different methods for calculating photolysis rates. The LibRadtran model uses a six-stream discrete ordinate method with a spectral resolution of 0.001 nm in the 121–130 nm range; the Lyman-alpha line (121.6 nm) is in this range, which requires a higher spectral resolution: 0.5 nm in the 130–175 nm range, 0.001–0.002 nm in the 175–205 nm range. This range contains the Schumann–Runge oxygen absorption band(s) (176–192.5 nm), which have a complex structure and also require a high spectral resolution of 0.5 nm in the 205–305 nm range, and 1 nm in the 350–700 nm range (a total of 25,000 spectral intervals, of which 15,000 are in the range from 175 to 700 nm).
The Cloud-J model used to study the effect of scattering and reflectance on photodissociation coefficients is a photolysis rate calculation module based on Fast-J [
25]. This new data set (Cloud-J v8.0) includes near-UV cross sections for water vapour absorption that can be used to calculate photolysis rates in atmospheric chemistry models. This code was updated, and improved versions of the last published version 7.6c [
31]. It cleans up some minor bugs and now includes the option for water vapour absorption in the ultraviolet region 290–340 nm. The new H2O cross sections are derived from [
32].
The radiative transfer calculations in Cloud-J v.8.0 are based on the Feautrier method [
33] for solving the radiative transport equations in a plane-parallel atmosphere. The Cloud-J v.8.0 spectral range (177.5–778 nm) is divided into 18 spectral intervals (bins) (see
Table 1). Photodissociation of molecular oxygen in the 177–215 nm wavelength region plays an important role in the stratosphere leading to the formation of ozone (reactions (1)–(2)). Ozone photolysis and O(
1D) formation contribute to the destruction of long-lived greenhouse and ozone-depleting gases (e.g., N
2O).
The calculation of O
2 photolysis is complicated by the fine structure of the Schumann–Runge oxygen absorption band (176–192.5 nm), which requires a high spectral resolution. Therefore, in Cloud-J v.8.0, the optical depth distribution function in the Schumann–Runge (S–R) bands [
34] was used to form the first 11 bins, covering the range of 177.5–291 nm, and spectrally unconnected wavelength ranges with the same optical depth were combined, considering the relative contribution of each bin. The spectral bins are shown in
Table 1 from [
35].
This separation of each Schumann–Runge band range resulted in a highly accurate accounting of the influence of these bands on the photolysis rates. The influence of the Lyman-alpha line, considered in earlier versions of the code, and which is important for calculating photodissociation in the mesosphere, was excluded in Cloud-J v.8.0. To speed up the calculations, Cloud-J v.8.0 uses a simple attenuation of sunlight by absorption of O
2 and O
3 for wavelengths shorter than 290 nm (bins 1–11). Detailed accounting of scattering and reflection processes is performed only in spectral intervals of 12–18, in which solar radiation can reach the lower atmosphere and surface. Spectral data (absorption cross sections and dissociation quantum yields) are calculated based on laboratory data, in particular, JPL [
36] and IUPAC [
37].
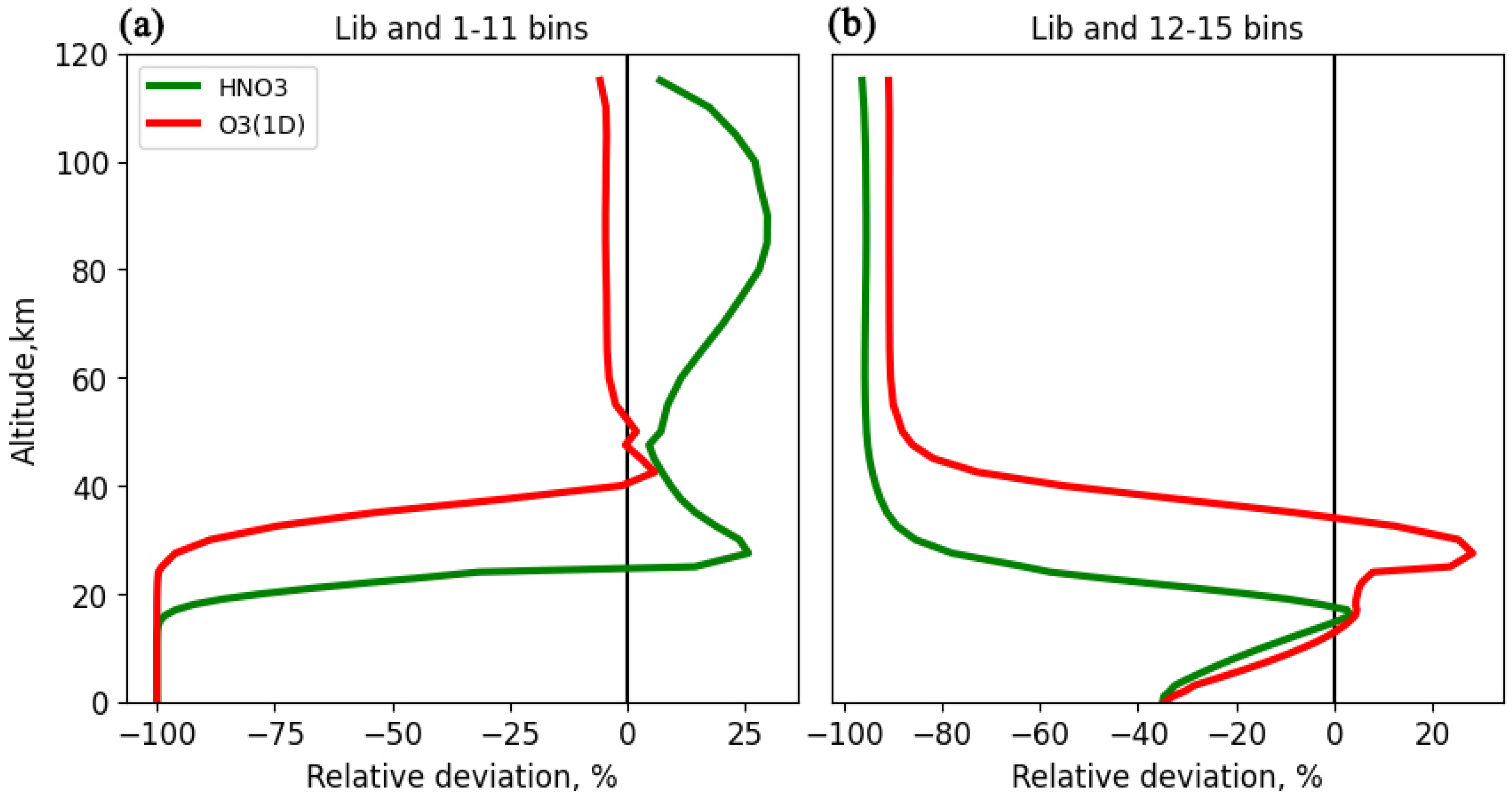
The first 11 bins cover the spectral range of solar radiation up to 290 nm, which does not reach the lower atmosphere and surface; therefore, for gases whose absorption cross sections are located only in this region, reflection from the surface and scattering in the dense layers of the troposphere should not play a significant role in calculating their photolysis coefficients.
Intervals from 12 to 15 cover the spectral range from 290 to 320 nm, in which, on the one hand, with an increase in wavelength, a rapid increase in the solar radiation flux is noted, and, on the other hand, the ozone absorption cross section also quickly decreases, which in this range, determines the transmission of radiation and its passage to the surface and lower troposphere (
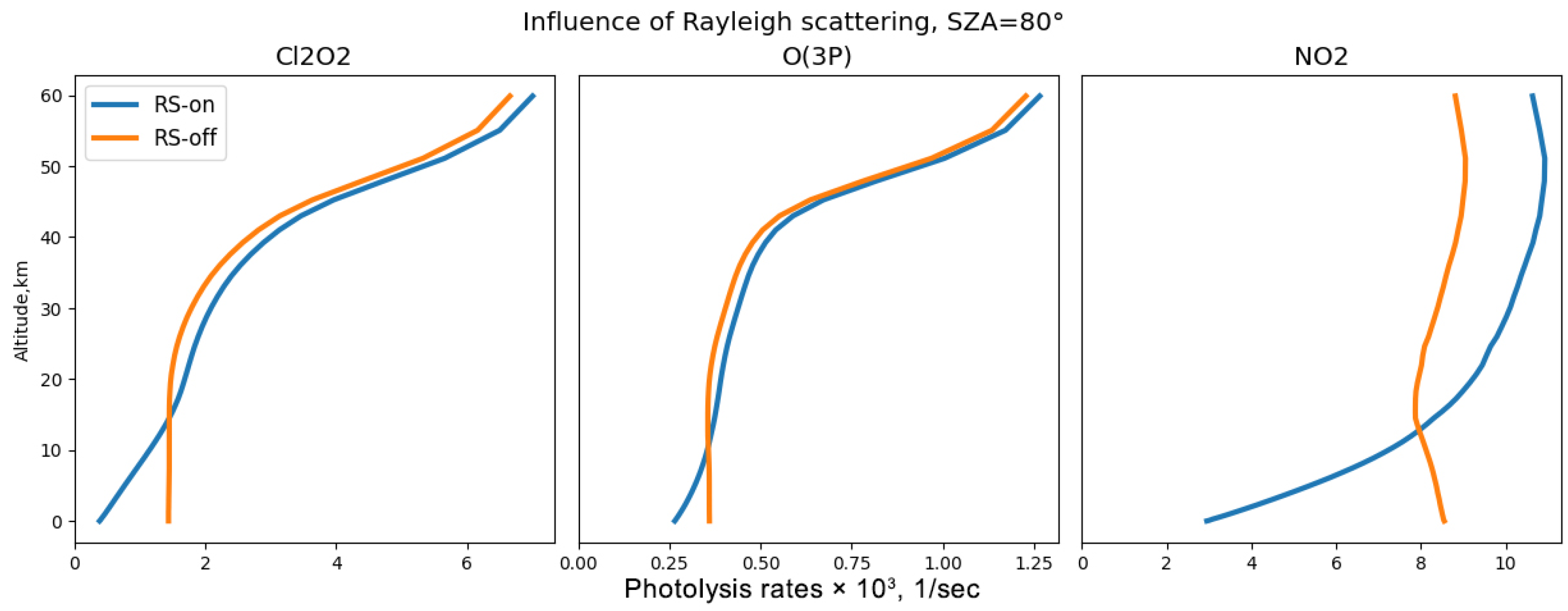
Figure 1). Rayleigh scattering, which is inversely proportional to the 4th power of the wavelength, is most effective in this spectral range since here, the radiation reaches the dense layers of the atmosphere, and the Rayleigh scattering coefficient remains significant.
In bin 16 (320–345 nm), the maximum sensitivity of the ozone absorption cross sections to wavelength changes is observed, and the solar radiation flux is characterised by an oscillatory change without a general tendency to increase or decrease (
Figure 1a). Most of the solar radiation in this range reaches the lower troposphere and the surface, where it is reflected by the surface and scattered by dense layers of the atmosphere near the surface. Reflected and scattered radiation is also slightly attenuated, propagating upward from the surface and lower layers. Thus, due to the short wavelengths in this range, molecular scattering can effectively affect the photodissociation coefficients of gases both in the troposphere and in the stratosphere.
Bin 17 (345–485 nm) is characterised by minimal values of the ozone absorption cross sections, i.e., almost all solar radiation reaches the surface. At the same time, an increase in the intensity of solar radiation is noted (
Figure 1a), which increases the significance of Rayleigh scattering in this range; since the scattering coefficient for these wavelengths is still quite high, the attenuation of radiation is small, and the solar radiation fluxes are already quite high. In the 18th bin from (485–778 nm), the solar radiation intensity reaches its maximum values and changes little, and the ozone absorption cross sections also increase slightly and change little, but the wavelength is already large, as a result of which the Rayleigh scattering coefficient is already smaller than in the ranges with shorter wavelengths and the role of this range for Rayleigh scattering becomes small, even though the fact that the radiation fluxes reaching the Earth’s surface reach their maximum values.
Cloud-J v.8.0 calculates photolysis rates in the presence of aerosol layers considering their optical properties: optical attenuation thickness, single scattering albedo, and scattering phase function. Cloud-J v.8.0 includes scattering phase functions for a wide range of atmospheric aerosols. Except for ice clouds, all of these scattering functions are calculated using the Mie theory. To account for the effects of clouds, Cloud-J v.8.0 uses the quadrature method developed by Neu et al. (2007) [
38], which is based on the assumption that the calculation of the radiative field from a large number of atmospheric columns can be approximated by selecting a few representative atmospheric columns, each with its cloud distribution in each layer. Up to four independent columns are identified in the model and the photolysis rates calculated for them are averaged to determine the final values for the model cell considered. The inclusion of Rayleigh scattering effects is determined by the pseudo-Rayleigh absorption cross section (% scattering cross section), which considers the loss of radiation in the lower stratosphere due to Rayleigh scattering [
35]. The contribution of molecularly scattered radiation to the total radiative field used in Formulas (14) and (15) is considered using an eight-flux approximation with an equal distribution of fluxes over four upward and four downward angles. A complete set of equations, a description of the methods for accounting for processes associated with the passage of solar radiation, and a detailed description of the spectral range can be found in [
25,
35].
Using LibRadtran v.2.0.5 and Cloud-J v.8.0, the photolysis rates of the most important constituents with absorption bands in different spectral ranges (
Figure 1b)—O
2, O
3(O
1D), O
3(O
1D + O
3P), HNO
3, Cl
2O
2, and NO
2—were calculated using the spectral solar irradiance (SSI) calculated by the COSI model, which reproduces, with high accuracy, the spectral irradiance measured by the SOLSTICE (up to 320 nm) and SIM (starting from 320 nm) satellites on board the SORCE satellite during the solar minimum of 2008, as well as SOLSPEC during the ATLAS 3 mission in 1994. COSI is used to model the variability of solar irradiance, and also provides SIR with very high spectral resolution. A more detailed description of COSI is given in [
39]. In the work of [
30], COSI data were used to compare several modules for calculating photolysis rates.
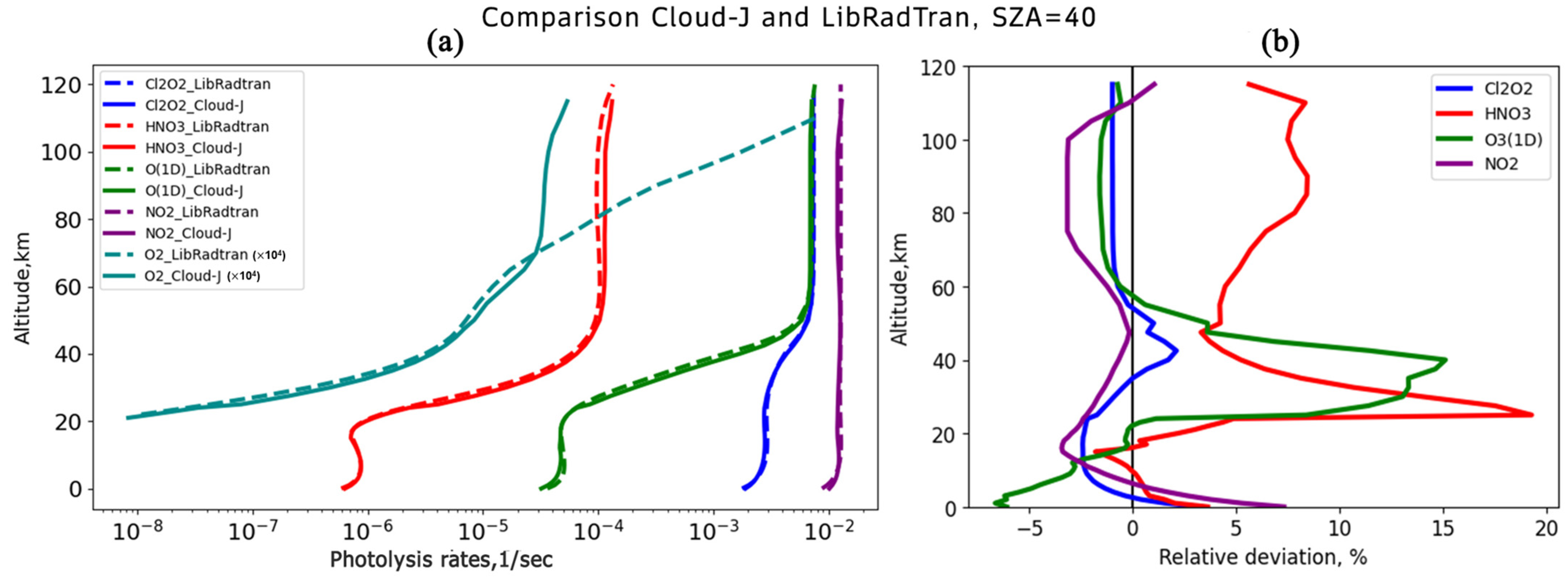
For comparison, Cloud-J v.8.0 and LibRadtran v.2.0.5 were calculated using a tropical standard atmosphere with 50 vertical levels from 0 to 115 km, for cloudless and aerosol-free conditions, for a solar zenith angle of 40° and a surface albedo of 0.1.
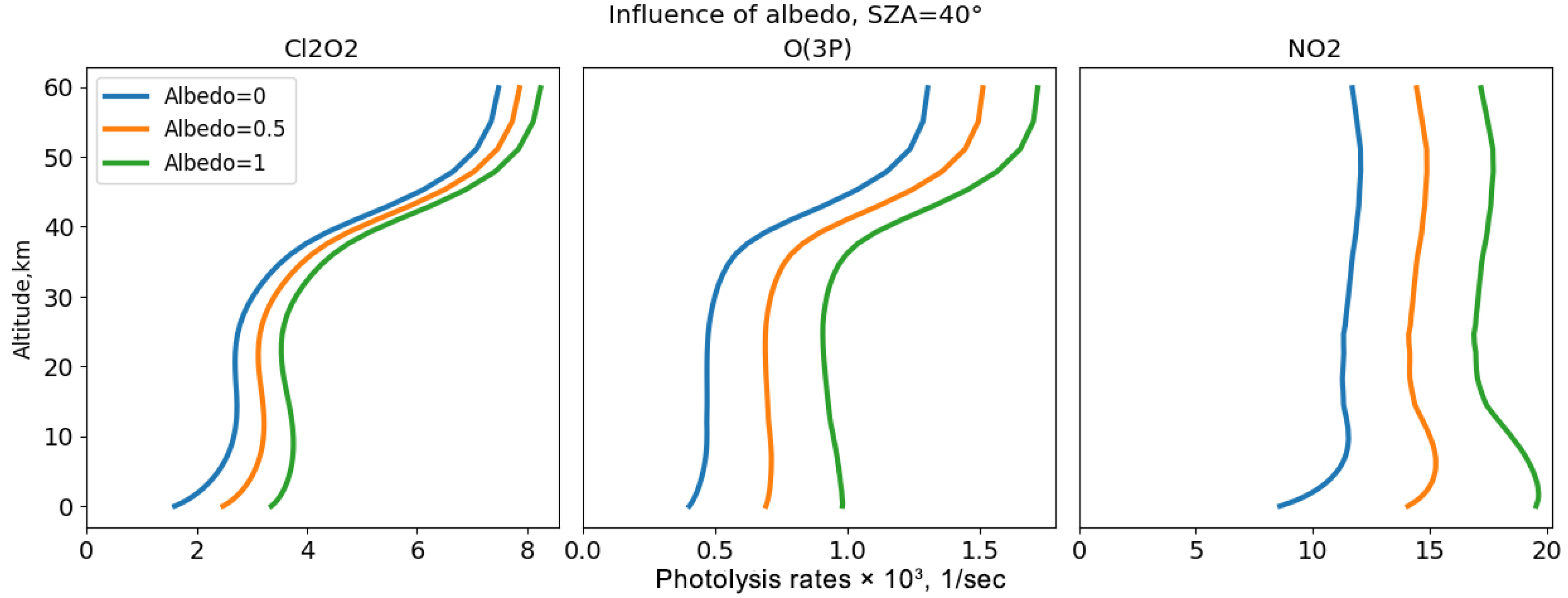
To analyse the influence of reflection and scattering processes across different spectral ranges on the photodissociation rates of key atmospheric gases, a series of experiments were conducted. These experiments considered various factors, including the effects of radiation reflection by the surface with different albedo values, Rayleigh molecular scattering, and the scattering of solar radiation by clouds and aerosols. The experimental design allowed these processes to be individually enabled or disabled, providing a clear understanding of their impact. For these experiments, atmospheric parameters were derived from the Cloud-J code. The analysis was performed over 57 vertical levels, extending from the Earth’s surface up to 60 km in altitude. This comprehensive approach aims to elucidate how these scattering and reflection phenomena affect the photodissociation rates at various wavelengths, thereby contributing to a more accurate modelling of atmospheric chemical processes.
4. Conclusions
The comparison of the photolysis rates calculated using Cloud-J v.8.0 shows their good agreement with the reference data from the LibRadtran v.2.0.5 model. However, it is important to highlight the peculiarities of calculating the photolysis rates for oxygen. Since the Lyman-Alpha band, crucial for oxygen photodissociation in the mesosphere, is not considered in Cloud-J v.8.0, this process should be treated with other methods.
The analysis regarding reflection and scattering effects in different spectral ranges reveals that atmospheric gases respond differently to perturbations of the atmospheric conditions. This variability is associated with the spectral ranges in which these gases have absorption bands and the ability to photodissociate. Gases like NO2, Cl2O2, and O3(O3P), which absorb in the long-wave UV (greater than 290 nm) and visible regions, are more affected by reflection and scattering processes, as this radiation can penetrate to the dense tropospheric layers and reflective surfaces.
Rayleigh scattering is critically significant in photolysis rate calculations, particularly for gases with an absorption region in the visible spectrum. It can both enhance photolysis rates in the troposphere and reduce them at large zenith angles.
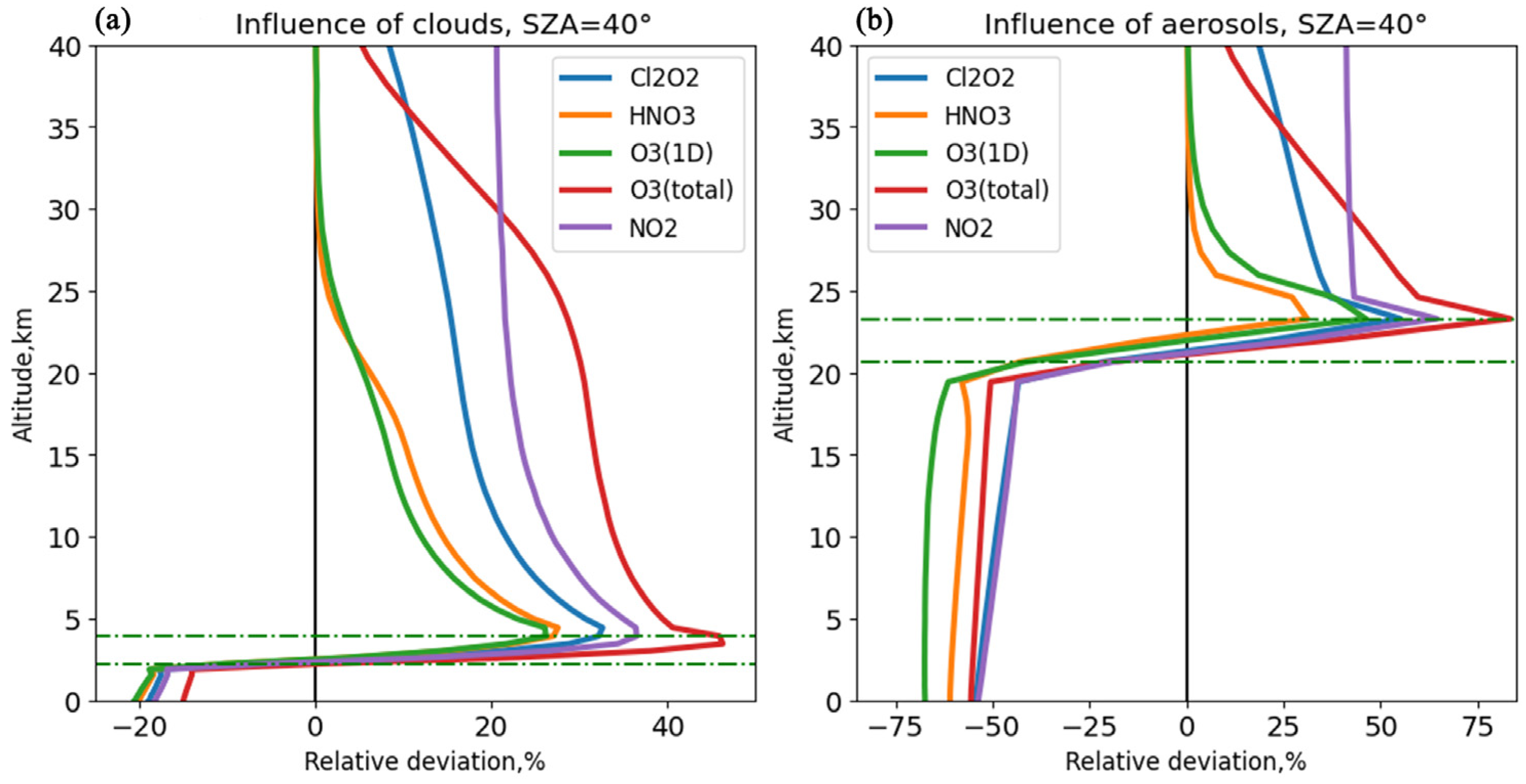
It has been shown that the presence of cloud layers can increase photolysis rates by up to 40% above the cloud layer while decreasing them by up to 20% below the cloud layer. The presence of volcanic aerosol can enhance photolysis rates in the upper parts of the atmosphere and above by up to 75% but may decrease them by the same amount in the lower atmosphere.
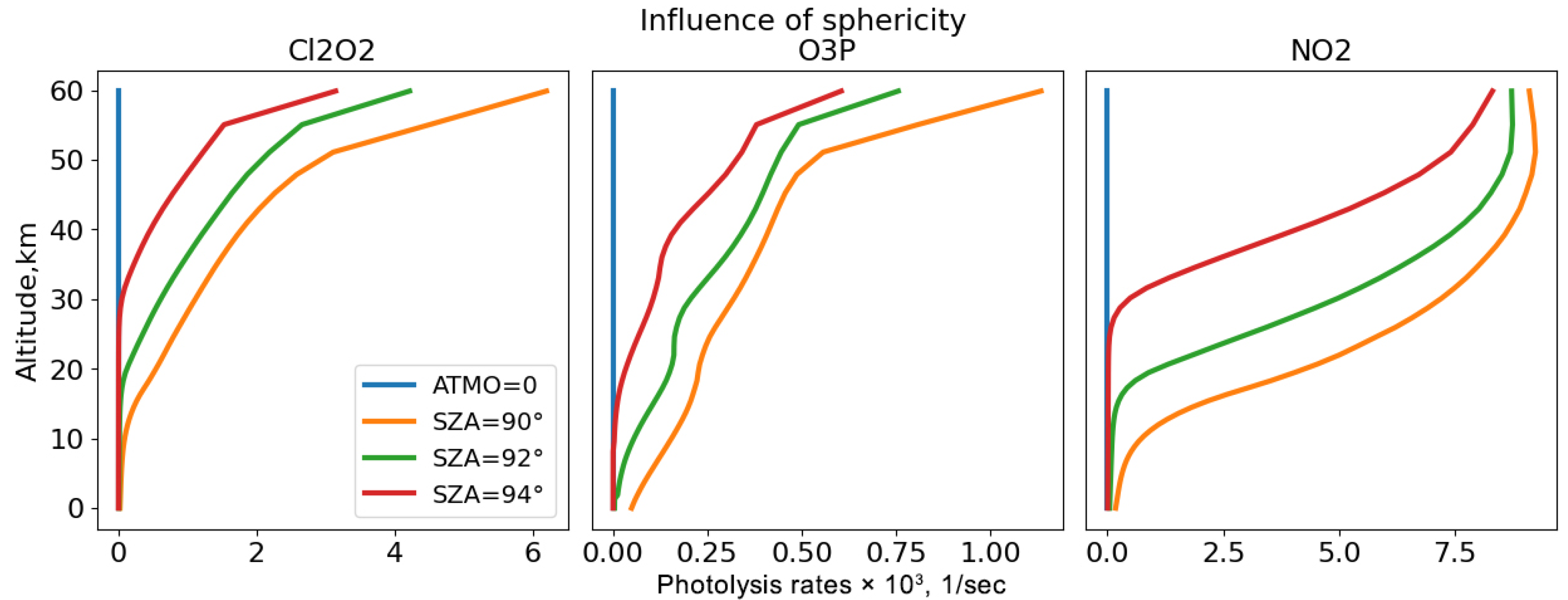
Taking into account the sphericity of the Earth becomes relevant in the upper layers of the atmosphere, where the Sun’s rays are still able to penetrate at large zenith angles. For gases such as O(3P), the influence of sphericity extends to a height of 15 km; for gases such as Cl2O2 or NO2, it extends up to 30 km.
Taking into account the processes of reflection and scattering of solar radiation plays an important role in calculating the rates of photolysis. Thus, the use of Cloud-J v.8.0 will contribute to solving complex geophysical scientific problems, such as the effects of volcanic eruptions and aerosol emissions of geoengineering.
Further research will be devoted to studying the influence of scattering and reflection processes on the concentrations of trace gases in the atmosphere in the framework of 1-D and 3-D chemistry-climate models.