Pollution Characteristics, Toxicological Properties, and Health Risk Assessment of Phthalic Acid Esters in Water, Soil, and Atmosphere
Abstract
1. Introduction
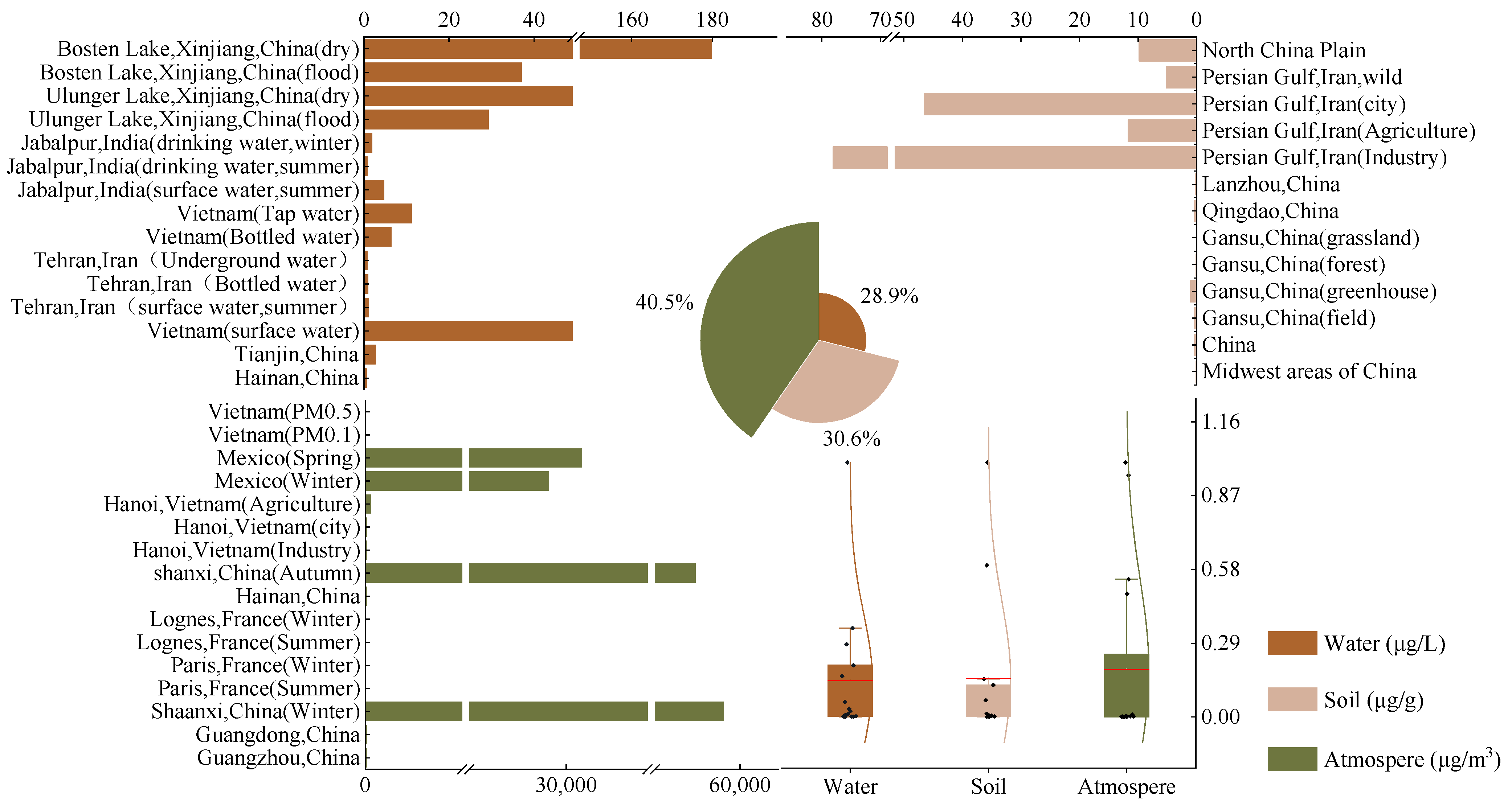
2. Sources and Pollution Status
3. Determination Techniques and Strategies
| Detection Settings | Pre-Treatment or Instrument Optimization | Detection Media | Advantage | Disadvantage | Recovery Rate (%) | Detection Limit | Quantification Limit | Linear Range | References |
|---|---|---|---|---|---|---|---|---|---|
| GC-MS | Accelerated solvent extraction and cycle extraction (two times) | Soil and atmospheric particulate matter | Fast extraction speed, small amount of organic solvent, and high automation degree | False positives, poor reproducibility, and stability are possible and costly | 65.7~108 | - | - | - | [26,48,51,52,53,54,55] |
| Ultrasonic extraction | Food contact material | - | 86.0~108.0 | - | - | 0.02~2.0 µg·mL−1 | |||
| 20 mL n-hexane extraction. Ultrasonic extraction was performed at 40 °C for 30 min | Express plastic bag | Simple pretreatment, good recovery rate, and precision | 85.0~107.2 | 2.0~10.0 mg·kg−1 | - | 0.05~1.0 µg·mL−1 | |||
| Acetone/n-hexane (1:1) was extracted twice; extraction for 10 min | Soil/agricultural products | - | 78.2 | 1.04~14.24 µg·L−1 | 0.24~0.45 µg·L−1 | ||||
| N-hexane/acetone (4:1), repeated three times | Dust | - | 76.0~130.0 | - | 10 µg·kg−1 | - | |||
| Determination of DOC, WEOC, and TOC in water by catalytic oxidation at high-temperature glass fiber membrane filtration | Water | - | 94.0~106.0 | 0.006~0.01 µg·L−1 | 0.018~0.045 µg·L−1 | - | |||
| Sediments | - | 77.0~113.0 | 0.003~0.008 mg·kg−1 | 0.009~0.024 µg·kg−1 | - | ||||
| UPLC-MS/MS | 35 °C column temperature and methanol–water mobile phase | Edible and medicinal fungi | The method is simple to operate and has high accuracy | - | 79.4~104.3 | - | 0.2~8.0 µg·kg−1 | 0.2~500 µg·mL−1 | [48] |
| Liquid chromatography | 226 nm test wavelength, 325 nm reference wave, and methanol–water mobile phase | Shoes | Short analysis time and good repeatability | Susceptible to interference from other chromatographic peaks | 90.5~97.7 | 1.9~8.9 mg·kg−1 | - | 10~100 µg·mL−1 | [50,56] |
| HPLC | Ultrasonic extraction has a maximum UV absorption wavelength of 205 nm | Soil | A wide linear range | - | 72.59~91.89 | 0.005~0.03 mg·kg−1 | - | 0.1~100 µg·mL−1 | [49] |
4. Toxicity and Potential Hazards
4.1. Non-Reproductive Toxicity
4.2. Reproductive Toxicity
5. Exposure Levels and Health Risk Assessment
| Evaluation Media | Pollutant | Evaluation Object | Risk | References |
|---|---|---|---|---|
| Drinking water | DEHP | Adults, children | Health risks | [12] |
| Surface water | ΣPAEs | Adults, children | Non-carcinogenic risks are negligible | [15] |
| Surface water | ΣPAEs | Adults, children | Potential carcinogenic risk | [15] |
| Domestic tap water | DEP, DBP | Adults, children | Non-carcinogenic risks are negligible | [20] |
| Domestic tap water | DEHP | Adults, children | Potential carcinogenic risk | [20] |
| Urban soil | ΣPAEs | Adults, children | Carcinogenic/non-carcinogenic risks are negligible | [20] |
| Drinking water | ΣPAEs | Adult male | health risks | [84] |
| Petrochemical atmosphere | ΣPAEs | Adults, children | Low health risks | [46] |
| University dormitory dust | ΣPAEs | Adults | Low non-carcinogenic risk | [35] |
| University dormitory dust | DEHP, BBP | Adults | Low carcinogenic risk | [35] |
| Industrial area atmosphere | ΣPAEs | Adults, children | Carcinogenic/non-carcinogenic risks are negligible | [38] |
| Industrial area atmosphere | ΣPAEs | Adults, children | Carcinogenic/non-carcinogenic risks are negligible | [7] |
| Plastic lunch box | ΣPAEs | Adults, children | Carcinogenic/non-carcinogenic risks are negligible | [84] |
| Surface water | DEHP | Adults, children | Low health risks | [85] |
| Menstrual pad | ΣPAEs | Adults, children | Non-carcinogenic risks are negligible | [85] |
| Menstrual pad | DEHP | Adults, children | Potential carcinogenic risk | [85] |
6. Prevention and Control of PAE Pollution
| Year | Nations/Organizations | Substance | Requirement | References |
|---|---|---|---|---|
| 1999 | EU | DiNP, DEHP DBP, DiBP DOP, BBP | Prohibited in the production of toys and childcare products for children under 3 years of age. | [86] |
| 2009 | US. CPSIA | DEHP, DBP BBP | Permanent ban on the use, distribution, and import of these substances in children’s toys and childcare products. Restricted use: 0.1%. | [87] |
| 2009 | US. CPSIA | DiNP, DiDP DnOP | Can be used in children’s oral products and care products. Restricted use: 0.1%. | [87] |
| 2009 | CHINA | DEHP, DBP BBP | DEHP, DBP, and BBP sum less than or equal to 0.1%. | [88] |
| 2009 | CHINA | DiNP, DiDP DnOP | DiNP, DiDP, and DnOP sum less than or equal to 0.1%. | [88] |
| 2011 | EU | BBP | Quantity contained less than 0.1%. Migration less than 0.3 mg·kg−1. | [90] |
| 2011 | EU | DEHP | Quantity contained less than 0.1%. Migration less than 30 mg/kg. | [90] |
| 2011 | EU | DiDP | Quantity contained less than 0.1%. The sum of DiDP and DiNP is less than 9 mg·kg−1. | [90] |
| 2011 | EU | DiNP | Quantity contained less than 0.1%. The sum of DiNP and DiNP is less than | [90] |
| 2012 | US. EPA | DEHP | C water ≤ 6 µg·L−1 | [90] |
| 2018 | EU. REACH | DEHP, DBP BBP, DiBP | 1. Substances or mixtures that may not be used as toys or childcare products. 2. Individual or combined concentration of four PAEs less than or equal to 0.1%. | [13] |
| 2018 | EU. REACH | DEHP DBP BBP | 1. Substances or mixtures that may not be used as toys or childcare products. 2. Individual or combined concentration of three PAEs less than or equal to 0.1%. | [13] |
| 2022 | WHO | DEHP | C water ≤ 8 | [89] |
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Zhou, X.; Miao, M.; Li, D.-K.; Wang, Z.; Li, R.; Liang, H.; Yuan, W. Association of Bisphenol A Exposure with LINE-1 Hydroxymethylation in Human Semen. Int. J. Environ. Res. Public Health 2018, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yan, Z.; Feng, C.; Wang, J.; Bai, Y.; Wu, F. Research Focus Analysis of Endocrine Disrupting Chemicals (EDCs) Based on Bibliometrics. Res. Environ. Sci. 2022, 35, 424–434. [Google Scholar] [CrossRef]
- Schneider, M.; Pons, J.L.; Labesse, G.; Bourguet, W. In Silico Predictions of Endocrine Disruptors Properties. Endocrinology 2019, 160, 2790. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Wei, Q.; Jin, Z.; Wei, D.; Jin, Y. Research progress on reproductive toxicity and reproductive system tumors induced by environmental endocrine disrupting chemicals. J. Environ. Occup. Med. 2022, 39, 833–839. [Google Scholar]
- Lu, K.; Ye, X. The effects and mechanisms of environmental endocrine disruptors exposure on pubertal development in females. Environ. Chem. 2023, 42, 358–369. [Google Scholar]
- Cao, Y.; Gu, W.; Cui, Q.; Zhuang, S.; Chen, Y.; Zhang, X.; Dong, X.; Tang, S. Pollution characteristics and health risk assessments of four phthalate esters in PM2.5 during heating and non-heating periods in Chaoyang District of Beijing. J. Environ. Hyg. 2022, 12, 95–101. [Google Scholar] [CrossRef]
- García-Garcinuño, R.; Vallecillos, L.; Marcé, R.M.; Borrull, F. Occurrence of high production volume chemicals and polycyclic aromatic hydrocarbons in urban sites close to industrial areas. Human exposure and risk assessment. Chemosphere 2024, 351, 141167. [Google Scholar] [CrossRef]
- Farzanehfar, V.; Naderi, N.; Kobarfard, F.; Faizi, M. Determination of dibutyl phthalate neurobehavioral toxicity in mice. Food Chem. Toxicol. 2016, 94, 221–226. [Google Scholar] [CrossRef]
- Li, C.; Yan, Z.; Li, L.; Zhang, Y. Associations between Childhood Asthma and Phthalate Exposure. J. Environ. Occup. Med. 2013, 30, 563–568. [Google Scholar] [CrossRef]
- Gao, H.; Yang, M.; Qin, C.; Wei, Z.; Xia, L. Research progress on the mechanism of male reproductive toxicity induced by Phthalic acid esters exposure. J. Toxicol. 2021, 35, 77–82. [Google Scholar] [CrossRef]
- Nie, Y.; Qi, L.; Gao, S.; Ning, J.; Li, G. Hazard assessment of the effects of Di-(2-ethylhexyl) phthalate (DEHP) on health. J. Toxicol. 2021, 35, 361–366. [Google Scholar] [CrossRef]
- Kumawat, M.; Sharma, P.; Pal, N.; James, M.M.; Verma, V.; Tiwari, R.R.; Shubham, S.; Sarma, D.K.; Manoj, K. Occurrence and seasonal disparity of emerging endocrine disrupting chemicals in a drinking water supply system and associated health risk. Sci. Rep. 2022, 12, 9252. [Google Scholar] [CrossRef] [PubMed]
- China Chamber of Commerce for Import and Export of Textiles. Revision of Phthalic acid esters in Appendix XVII of the EU REACH regulation will soon come into force. Light Text. Ind. Fujian 2019, 1, 4–5. [Google Scholar] [CrossRef]
- Jaime, D.; Abrahan, M.; Pabel, C.; Jürgen, M. Groundwater contamination pathways of Phthalic acid esters and bisphenol A: Origin, characteristics, transport, and fate–A review. Environ. Int. 2022, 170, 107550. [Google Scholar]
- Mi, Q. Pollution Characteristics and Health Risk Assessment of Phthalate Esters in Typical Lakes. Master’s Thesis, Chinese Research Academy of Environmental Sciences, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Han, W.; Zhao, Y.; Dang, J.; Xiong, L. Distribution and ecological risk evaluation of phthalate esters in Fenhe River Basin. Environ. Chem. 2017, 36, 1377–1387. [Google Scholar]
- Hu, X.; Han, Z.; Zhuo, Y.; Wang, W. Distribution characteristics and risk assessment of phthalate esters in surface water of Huangpu River. Environ. Chem. 2007, 2, 258–259. [Google Scholar]
- Kong, H.; Liu, H. Identification the key pollutants of phthalic acid esters in surface water of China and ecological. Environ. Chem. 2021, 40, 706–716. [Google Scholar]
- Le, T.M.; Nguyen, H.M.; Nguyen, V.K.; Nguyen, A.V.; Vu, N.D.; Yen, N.T.; Hoang, A.Q.; Minh, T.B.; Kannan, K.; Tran, T.M. Profiles of phthalic acid esters (PAEs) in bottled water, tap water, lake water, and wastewater samples collected from Hanoi, Vietnam. Sci. Total Environ. 2021, 788, 147831. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zheng, J.; Liang, J.; Li, R.; Gong, Z. Source tracing and health risk assessment of phthalate esters in household tap-water: A case study of the urban area of Quanzhou, Southeast China. Ecotoxicol. Environ. Saf. 2022, 248, 114277. [Google Scholar] [CrossRef]
- Du, Q.; Chen, Q.; Chen, X.; Li, W.; Zhao, X.; Gao, W.; Tu, K.; Liu, Y. Concentration Distribution and Health Risk Assessment of Phthalate Esters (PAEs) in Various Environmental Media in China. Environ. Sci. 2024, 22, 3620–3630. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, L.; Zhang, P.; Zhang, D.; Li, X. Pollution characteristics and health risk assessment of phthalate esters (PAEs) in soils of typical agricultural areas in Qingdao. Environ. Chem. 2024, 43, 4622–4629. [Google Scholar]
- Kunlong, H.; Jun, T.; Yini, C.; Bei, X.; Wenbing, T. Accumulation of phthalates under high versus low nitrogen addition in a soil-plant system with sludge organic fertilizers instead of chemical fertilizers. Environ. Pollut. 2021, 291, 118193. [Google Scholar]
- Wang, Y.; Qu, D.; Zhou, X.; Lei, C.; Ma, Y.; Wang, B.; Chen, Y.; Dai, L.; Chen, J. Pollution Characteristics of Phthalic acid esters in Lanzhou Urban Soil and Health Risk Assessment of Population. Environ. Prot. Sci. 2023, 49, 107–114. [Google Scholar]
- Jin, T.; Xu, D.; Xue, Y.H.; Xi, B.; Sun, J.; Peng, K.; Zhang, J.; Jin, D. Analysis of phthalate esters pollution in 59 topsoils of long-term film mulching plots in China. Asian J. Ecotoxicol. 2022, 17, 462–471. [Google Scholar]
- Feng, Y.; Ying, R.; Wang, G.; Zhang, Y.; Du, J.; Lin, Y. pollution characteristics and risk of phthalic acid esters in soils and agro-products in the Midwest areas of China. Environ. Chem. 2022, 41, 1591–1602. [Google Scholar]
- Shi, C.; Wu, Q.; Liu, Q.; Zhao, J.; Zhou, J.; Ren, J.; Liu, Y. Spatial Distributions and Factors of Phthalic Acid Esters in Agricultural Soils in China: A Review. J. Ecol. Rural. Environ. 2024, 40, 23–35. [Google Scholar]
- Hu, S. Pollution Characteristics and Ecological Risk Assessment of Microplastics and Six Phthalate Esters in Farmland of Shanghai Suburb. J. Ecol. Rural. Environ. 2024, 40, 975–984. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Zhang, Y.; Li, M.; Hao, J.; Xiong, Y.; Li, C.; Cao, J. Accumulation Characteristics and Sources of PAEs in Agricultural Soils in Gansu Province. Environ. Sci. 2022, 43, 4622–4629. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Qiao, Y. Study on partition of Phthalic acid esters among indoor air, PM2.5 and house dust based on field monitoring data. Environ. Chem. 2021, 40, 3706–3713. [Google Scholar]
- Yang, Q.; Shen, B.; Cai, J.; Liu, Z.; Li, Y.; Feng, S.; Zhou YLu, S.; Zhao, H.; Ye, Z.; Xiong, J. Distribution and exposure assessment of phthalic acid esters (PAE) sin indoor dust of Shanghai. Shanghai J. Prev. Med. 2022, 34, 247–251+264. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Liu, Y.; Sun, Y.; Wu, M.; Ma, J. Comparative assessment of human exposure to phthalate esters and bisphenol A from different indoor dust. J. Shanghai Univ. 2019, 25, 282–292. [Google Scholar]
- Teil, M.J.; Moreau-Guigon, E.; Blanchard, M.; Alliot, F.; Gasperi, J.; Cladière, M.; Mandin, C.; Moukhtar, S.; Chevreuil, M. Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors. Chemosphere 2016, 146, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Belmares, R.O.; Krais, A.M.; Esfahani, B.K.; Rosas-Pérez, I.; Mucs, D.; López-Marure, R.; Bergman, Å.; Alfaro-Moreno, E. Phthalate esters on urban airborne particles: Levels in PM10 and PM2.5 from Mexico City and theoretical assessment of lung exposure. Environ. Res. 2018, 161, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Li, N.; Pan, J.; Deng, N. Phthalate exposure and health risks from dust in university dormitories in Changchun City. Environ. Chem. 2024, 43, 2465–2476. [Google Scholar]
- Zeng, D.; Chen, Z.; Zhang, B. Dermal risk assessment of PAEs contained in household dust. Environ. Sci. Technol. 2021, 44, 185–193. [Google Scholar]
- Pei, J.; Sun, Y.; Yin, Y. The effect of air change rate and temperature on phthalate concentration in house dust. Sci. Total Environ. 2018, 639, 760–768. [Google Scholar] [CrossRef]
- Joel, S.; Natalia, N.; Jorge, M.; Isabel, T.; Soledad, M.; Purificación, L. Multi-class organic pollutants in atmospheric particulate matter (PM2.5) from a Southwestern Europe industrial area: Levels, sources and human health risk. Environ. Res. 2022, 214, 114195. [Google Scholar]
- Wang, X.; Zhang, Y.; Huang, B.; Chen, Z.; Zhong, M.; Wang, W.; Liu, X.; Fan, Y.; Hu, W. Atmospheric phthalate pollution in plastic agricultural greenhouses in Shaanxi Province, China. Environ. Pollut. 2021, 269, 116096. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of Phthalic acid esters in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in tehran, Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- Mantuo, H.; Yuqi, Z.; Kesong, L.; Bingyan, L.; Jiwen, L.; Lixuan, Z.; Yuan, K. Inhalation bioacessibility and lung cell penetration of indoor PM2.5-bound PAEs and its implication in risk assessment. Environ. Pollut. 2023, 322, 121216. [Google Scholar]
- Wu, Q.; Lei, J.; Wang, L. Pollution Characteristics and Exposure Risk of PAEs in PM2.5 of Indoor Environment on University Campus. Res. Environ. Sci. 2021, 34, 2525–2535. [Google Scholar] [CrossRef]
- Wang, M. Pollution Characteristics and Risk Assessment of Phthalate Esters in Typical Lake of Xinjiang, China. Master’s Thesis, Shihezi University, Shihezi, China, 2023. [Google Scholar]
- Yan, L.; Guanhua, H.; Lei, Z.; Hua, G.; Chunhua, L.; Hang, Z.; Honglu, L. Phthalate esters (PAEs) in soil and vegetables in solar greenhouses irrigated with reclaimed water. Environ. Sci. Pollut. Res. Int. 2020, 27, 22658–22669. [Google Scholar]
- Arfaeinia, H.; Fazlzadeh, M.; Taghizadeh, F.; Saeedi, R.; Spitz, J.; Dobaradaran, S. Phthalate acid esters (PAEs) accumulation in coastal sediments from regions with different land use configuration along the Persian Gulf. Ecotoxicol. Environ. Saf. 2019, 169, 496–506. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Y.; Fan, Y.; Chen, S.; Mai, B. Phthalic acid esters in Atmospheric Particulate Matter at Typical Petrochemical Regions of Southern China: Pollution Characteristics, Risk Assessment and Influencing Factors. J. Instrum. Anal. 2022, 41, 1130–1137. [Google Scholar] [CrossRef]
- Huong, L.; Thi, P.T.P.; Minh, B.; Dat, n.; Thao, N.; My, N.; Hikari, S.; Akira, K.; Mui, L.; Trung, N. Comprehensive Analysis of Organic Micropollutants in Fine Particulate Matter in Hanoi Metropolitan Area, Vietnam. Atmosphere 2022, 13, 2088. [Google Scholar] [CrossRef]
- Zhao, T. Establishment and Validation of Detection Methods for Triterpenes, Sterols, and Phthalates in Edible and Medicinal Fungi. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Lin, J.; Sun, Y.; Wang, J.; Gao, Y. Study on the high-performance liquid chromatography detection method of Phthalic Acid Esters in soils. China Environ. Sci. 2023, 43, 756–763. [Google Scholar]
- Feng, X.; Bai, Z.; Huang, Z.; Lu, C.; Yang, M. Determination of Six Phthalic acid esters in Footwear by High Performance Liquid Chromatography. Leather Sci. Eng. 2022, 32, 58–61. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Zhang, P. Study on determination and migration rule of phthalate esters plasticizers in plasticizers in food contact meterials. J. Shanxi Univ. Sci. Technol. 2022, 40, 49–54. [Google Scholar] [CrossRef]
- Bai, R. Determination of Eighteen Phthalic acid esters in Plastic Express Bags by Gas Chromatography Mass Spectrometry. Plast. Sci. Technol. 2022, 50, 80–84. [Google Scholar] [CrossRef]
- Xu, S.; Xiang, K.; Liu, Z.; Zeng, Y.; Chen, S. Determination of Polycyclic Aromatic Hydrocarbons and Phthalic acid esters in Soil and Atmospheric Particulates by Accelerated Solvent Extraction. J. Anal. Sci. 2023, 1, 47–53. [Google Scholar]
- Qin, X.L.; Zhang, T.; Sun, H.W. Occurrence of phthalate esters in indoor and outdoor dust in China: Distribution and risk assessment. Asian J. Ecotoxicol. 2016, 11, 231–237. (In Chinese) [Google Scholar]
- Wang, L.; Liu, Y.; Zhang, Y.; Chen, S.; Zhang, N.; Wang, Z.; Liu, H. Estimation and potential ecological risk assessment of multiphase PAEs in mangrove wetlands in Dongzhai Harbor, Hainan. Sci. Total Environ. 2023, 870, 161835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Jiang, W.J.; Hu, R.; Wu, X. Study of the method for the determination of bisphenol a in vegetable oil by high performance liquid chromatography. Sci. Technol. Cereals Oils Foods 2022, 30, 153–158. [Google Scholar]
- Zhu, D. Effect of Prenatal Phthalic Acid Esters Exposure on Preschooler Intelligence and Neuroinflammatory Mechanisms. Master’s Thesis, Anhui Medical University, Hefei, China, 2020. [Google Scholar] [CrossRef]
- Liu, J.X.; Fu, C.X.; Dai, L.; Jin, H.; Xu, Y. Effects of combined exposure of diethylhexyl phthalate and bisphenol A on learning and memory impairment and amyloid precursor protein processing pathway in mice. Asian J. Ecotoxicol. 2022, 17, 255–265. (In Chinese) [Google Scholar]
- Zhang, Y.; Wang, S.; Zhao, T.; Yang, L.; Guo, S.; Shi, Y.; Zhang, X.; Zhou, L.; Ye, L. Mono-2-ethylhexyl phthalate (MEHP) promoted lipid accumulation via JAK2/STAT5 and aggravated oxidative stress in BRL-3A cells. Ecotoxicol. Environ. Saf. 2019, 184, 109611. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Harmfulness of plasticizer phthalate esters on human health. Textitle Aux. 2016, 33, 1–7. [Google Scholar]
- Yu, C.; Wang, Q. Health risk assessment of exposure to Phthalic acid esters among children. J. Environ. Hyg. 2022, 12, 223–227. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Ye, L. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicol. Environ. Saf. 2019, 170, 391–398. [Google Scholar] [CrossRef]
- Melanie, C.; Buser, H. Edward Murray, Franco Scinicariello. Age and sex differences in childhood and adulthood obesity association with Phthalic acid esters: Analyses of Nhanes 2007–2010. Int. J. Hyg. Environ. Health 2014, 217, 687–694. [Google Scholar]
- Zhang, Y.; Meng, X.; Chen, L.; Li, D.; Zhao, L.; Zhao, Y.; Li, L.; Shi, H. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS ONE 2014, 9, e104852. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, Q.; Song, Q.; Zhao, Y.; Zhou, Y.; Zhang, Y. Metabolomics study on the effect of phthalate metabolites on overweight/obesity in school-age children. In Proceedings of the Professional Committee of Reproductive Pharmacology, Chinese Pharmacological Society. The 10th National Symposium on Basic and Clinical Reproductive Pharmacology of Chinese Pharmacological Association & 2017 Yuying Reproductive Pharmacology Forum Papers Compilation, Wenzhou, China, 3–5 November 2017; Volume 2. [Google Scholar]
- Xie, Y.; Zhu, Y.; Tao, F. Obesity in children and adolescents and maternal phthalate exposure during pregnancy. Chin. J. Sch. Health 2019, 40, 1434–1437. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Cui, T.; Wang, S. Advances in studies of the effects Phthalic acid esters on male reproductive health. Chin. J. Fam. Plan. 2021, 29, 629–634. [Google Scholar]
- Czernych, R.; Chraniuk, M.; Zagozdzon, P.; Wolska, L. Characterization of estrogenic and androgenic activity of Phthalic acid esters by the XenoScreen YES/YAS in vitro assay. Environ. Toxicol. Pharmacol. 2017, 53, 95–104. [Google Scholar] [CrossRef]
- Liu, S.; Chang, W. Advances on toxicity of phthalate esters on female reproductive system and its mechanism. J. Environ. Health 2013, 30, 374–377. [Google Scholar] [CrossRef]
- Jing, P.; Zou, J.; Zhao, D.; Wang, C. The Effect of Phthalic acid esters in Uterus or Environment on Sex Hormone Level and Reproductive Development of Children at 6 Years Old. Genom. Appl. Biol. 2017, 36, 3328–3333. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y. Impact of phthalates on human health: Epidemiological evidences and plausible mechanisms of action. J. Environ. Occup. Med. 2019, 36, 295–299. [Google Scholar]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.B.; Jia, P.P.; Junaid, M.; Yang, L.; Lu, C.J.; Pei, D.S. Reproductive effects linked to DNA methylation in male zebrafish chronically exposed to environmentally relevant concentrations of di-(2-ethylhexyl) phthalate. Environ. Pollut. 2018, 237, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Wang, D.; You, H. Study on the mechanism of action between dimethyl phthalate and herring sperm DNA at molecular level. J. Environ. Sci. Health. Part. B Pestic. Food Contam. Agric. Wastes 2016, 51, 553–557. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Gao, D.; Li, Y.; Gou, T.; Yuan, W.; Chen, M.; Chen, L.; Zhang, Y.; Ma, Q.; et al. Exploring the associations between phthalate exposure and cardiometabolic risk factors clustering among children: The potential mediating role of insulin-resistant-related genes DNA methylation. J. Hazard. Mater. 2023, 461, 132578. [Google Scholar] [CrossRef]
- Wu, S. Role of DNA Methylation in the Pathogenesis of the DEHP-Induced Cryptorchidism. Ph.D. Thesis, Chongqing Medical University, Chongqing, China, 2011. [Google Scholar]
- Cheng, H.; Qin, C.; Yang, B.; Hu, X.; Waigi, M.G.; Vasilyeva, G.K.; Gao, Y. Non-covalent binding interaction between phthalic acid esters and DNA. Environ. Int. 2022, 161, 107095. [Google Scholar] [CrossRef]
- Ma, B.; Wang, L.; Tao, W.; Liu, M.; Zhang, P.; Zhang, S.; Li, X.; Lu, X. Phthalate esters in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, Northwest China: Pollution characteristics, sources, health risks, and relationships with meteorological factors. Chemosphere 2020, 242, 125226. [Google Scholar] [CrossRef] [PubMed]
- US EPA (United States Environmental Protection Agency). Soil Screening Guidance: Technical Background Document; Office of Solid Waste and Emergency Response: Washington, DC, USA, 1996; EPA/540/R-95/128.
- US EPA (United States Environmental Protection Agency). Regional Screening Level (RSL) Summary Table; Washington, DC, USA, 2024. Regional Screening Levels (RSLs)—Generic Tables|US EPA. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 30 August 2024).
- Bahare, D.; Mahnaz, N.; Mehdi, M.A.; Mohammasi, F. Health Risk Assessment of Exposure to Bisphenol A in Polymeric Baby Bottles. Environ. Health Insights 2023, 17, 11786302231151531. [Google Scholar]
- Lan, M.; Li, H.; Ying, G. On the Migration Risk of Phthalic acid esters in Plastic Containers. J. South China Norm. Univ. 2022, 54, 52–62. [Google Scholar]
- Wu, J.; Yao, H.; Huo, Z.; Zhao, H.; Song, X.; Feng, X. Determination of phthalate esters in different water bodies of Huai ‘an City by solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry and its pollution characteristics. Phys. Test. Chem. Anal. 2021, 57, 436–443. [Google Scholar]
- Chai, M.; Han, X.; Zhong, F.; Wang, Y.; Tang, Z. Contamination and health risk of phthalate esters in marketed sanitary napkins from China. China Environ. Sci. 2017, 37, 1954–1960. [Google Scholar]
- European Commission. Commission Decision 1999/815/EC of 7 December 1999; European Commission: Brussels, Belgium, 1999. [Google Scholar]
- Chen, H. Interpretation of the Latest Version of United States Consumer Product Safety Improvement Act. China Text. Lead. 2009, 7, 4. [Google Scholar] [CrossRef]
- GB 24613-2009; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China State Standardization Administration of China. China Standard Publishing House: Beijing, China, 2009.
- Word Health Organization. Guidelines for Drinking-Water Quality. Incorporating the 1st Addendum, 4th ed.; WHO: Geneva, Swizerland, 2017.
- The European Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food (Text with EEA Relevance). 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX%3A32011R0010 (accessed on 30 August 2024).
- Tao, Y.; Cui, Y.; Zhu, G.; Zhong, G.; Zhang, Y. Fate, ecotoxicity, and remediation of phthalic acid ester in soils. Curr. Opin. Environ. Sci. Health 2023, 32, 100440. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Jiang, N.; Lv, H.; Liang, C.; Yang, H.; Yao, X.; Wang, J. Occurrence, source, ecological risk, and mitigation of Phthalic acid esters (PAEs) in agricultural soils and the environment: A review. Environ. Res. 2022, 220, 115196. [Google Scholar] [CrossRef]
- Salaudeen, T.; Okoh, O.; Agunbiade, F.; Okoh, A. Fate and impact of Phthalic acid esters in activated sludge treated municipal wastewater on the water bodies in the Eastern Cape, South Africa. Ecol. Environ. Conserv. 2018, 203, 336–344. [Google Scholar]
- Li, P.; Zhou, X.; Wang, J.; Gao, Y. Biodegradation of Phthalic acid esters degrading bacteria in agriculture soil and its application. China Environ. Sci. 2024, 44, 1542–1553. [Google Scholar] [CrossRef]
- Chen, Q.; Xue, J. Study on the distribution patterns and removal approaches of Phthalic acid esters in urban wastewater treatment plants. Hei Long Jiang Environ. J. 2024, 37, 16–18. [Google Scholar]
- Zhao, Z.; Zheng, Q. Research progress of bacterial degradation of plasticizer Phthalic acid esters. J. Weifang Univ. 2023, 23, 39–42. [Google Scholar]
- Wu, Q.; Zhang, X.; Ren, W.; Li, S.; Duan, Y. Advances in Pollution Status and Microbio-Degradation of Phthalic acid esters. J. Microbiol. 2018, 38, 122–128. [Google Scholar]
- Liu, S.; Zhang, C.; Li, S.; Yang, X.; Zhou, Y.; Li, Y. Phthalic Acid Esters Degradation by Rhizosphere Microorganisms of Herbaceous Plants in Surface Water Environment. Chin. Agric. Sci. Bull. 2022, 38, 44–51. [Google Scholar]
- Zhao, Z. Pathway and Molecular Mechanism of Phthalic Acid Esters Biodegradation by Microbe. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, F.; Ren, Y.; Ji, Y.; Li, J.; Zhang, H.; Wu, Z.; Gao, R.; Bi, F.; Liu, Z.; Li, H. Pollution Characteristics, Toxicological Properties, and Health Risk Assessment of Phthalic Acid Esters in Water, Soil, and Atmosphere. Atmosphere 2024, 15, 1071. https://doi.org/10.3390/atmos15091071
Long F, Ren Y, Ji Y, Li J, Zhang H, Wu Z, Gao R, Bi F, Liu Z, Li H. Pollution Characteristics, Toxicological Properties, and Health Risk Assessment of Phthalic Acid Esters in Water, Soil, and Atmosphere. Atmosphere. 2024; 15(9):1071. https://doi.org/10.3390/atmos15091071
Chicago/Turabian StyleLong, Fangyun, Yanqin Ren, Yuanyuan Ji, Junling Li, Haijie Zhang, Zhenhai Wu, Rui Gao, Fang Bi, Zhengyang Liu, and Hong Li. 2024. "Pollution Characteristics, Toxicological Properties, and Health Risk Assessment of Phthalic Acid Esters in Water, Soil, and Atmosphere" Atmosphere 15, no. 9: 1071. https://doi.org/10.3390/atmos15091071
APA StyleLong, F., Ren, Y., Ji, Y., Li, J., Zhang, H., Wu, Z., Gao, R., Bi, F., Liu, Z., & Li, H. (2024). Pollution Characteristics, Toxicological Properties, and Health Risk Assessment of Phthalic Acid Esters in Water, Soil, and Atmosphere. Atmosphere, 15(9), 1071. https://doi.org/10.3390/atmos15091071






