Carbon and Water Balances in a Watermelon Crop Mulched with Biodegradable Films in Mediterranean Conditions at Extended Growth Season Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. The Site, the Crop and the Mulching
2.2. Continuous Monitoring of Agro- and Micro-Meteorological Variables
2.3. The Net Biome Exchange, the Carbon and Water Balance
2.4. The Water Use Efficiencies
3. Results and Discussion
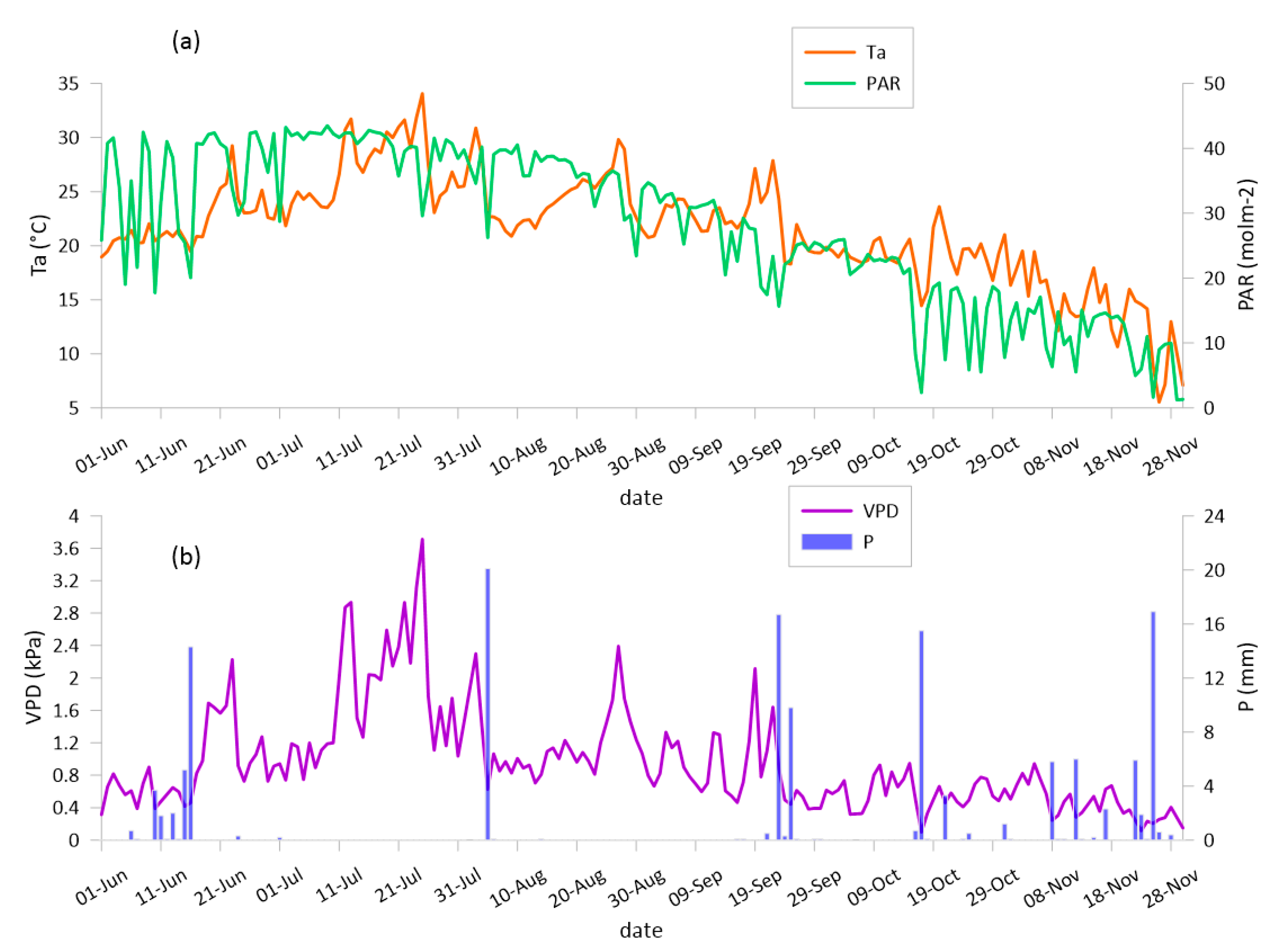
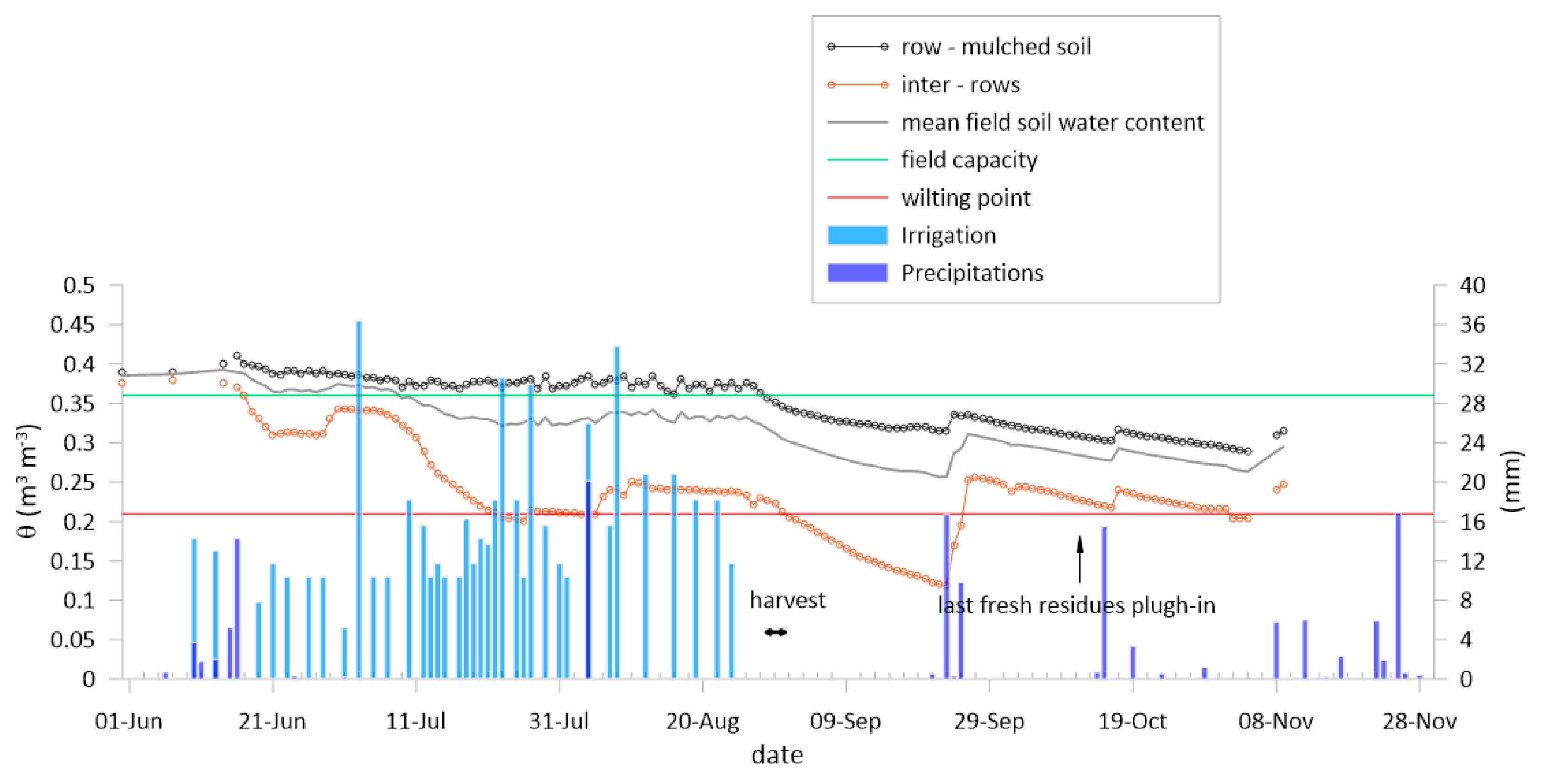
3.1. The Weather, the Soil Water Content and Crop Development
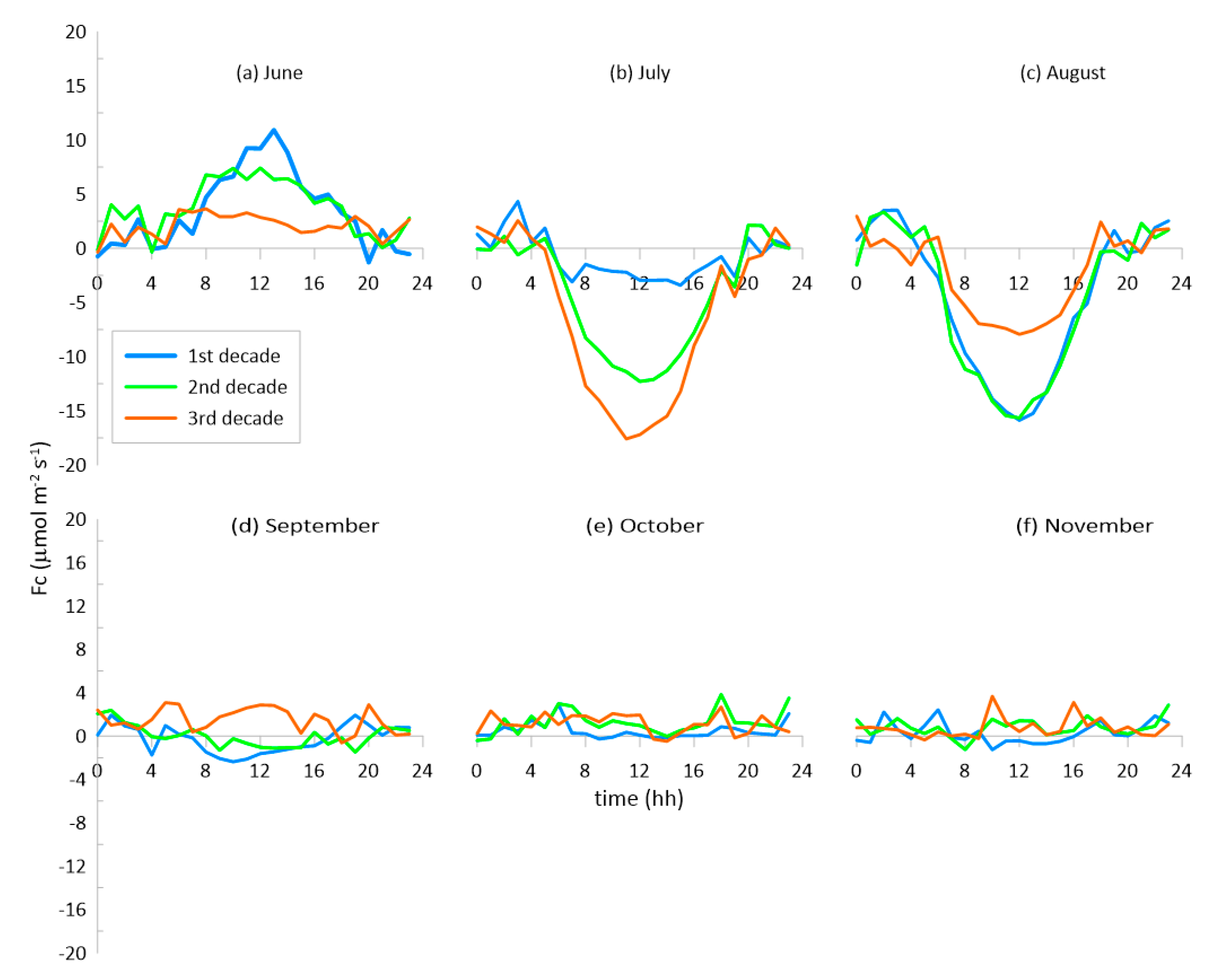
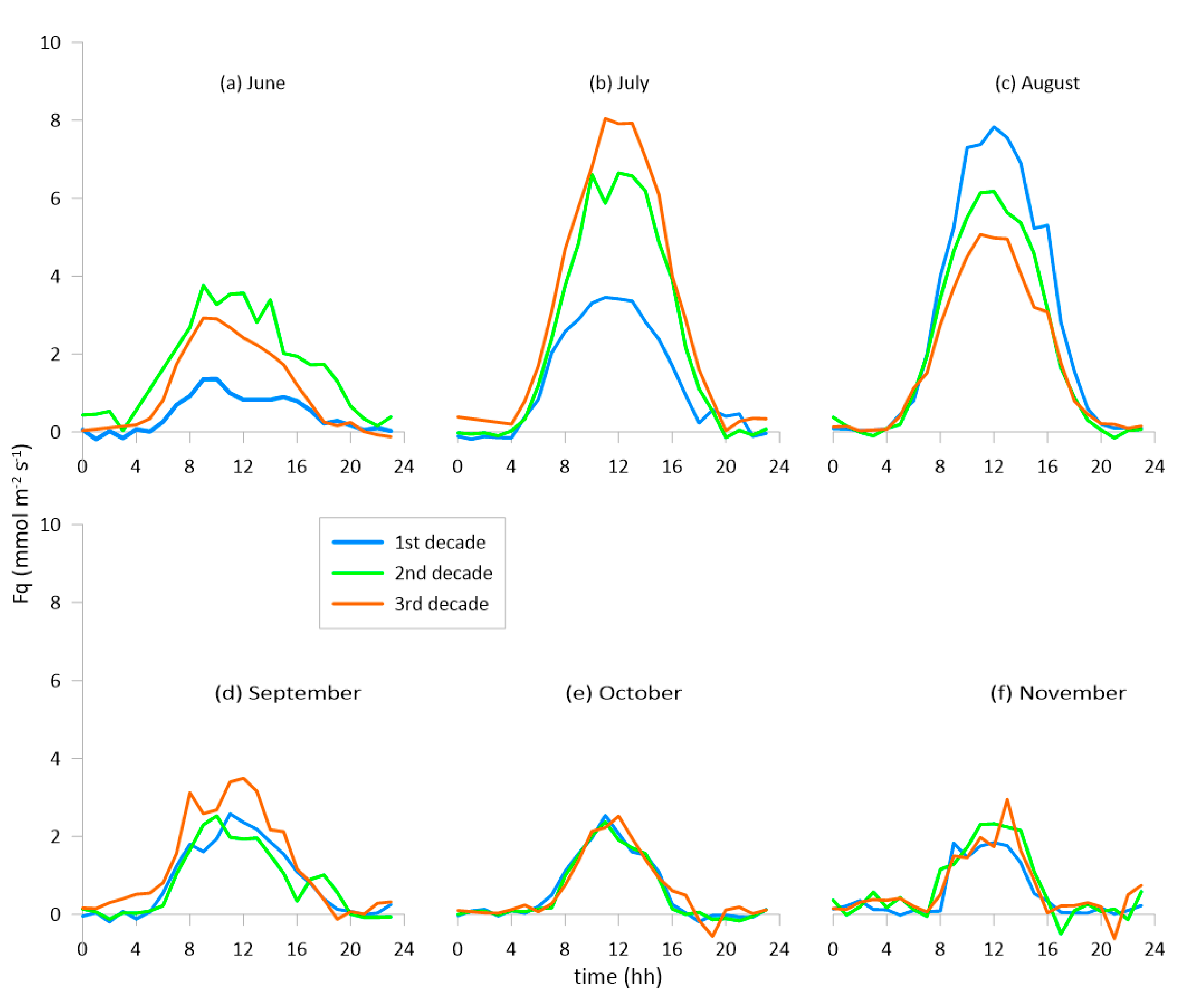
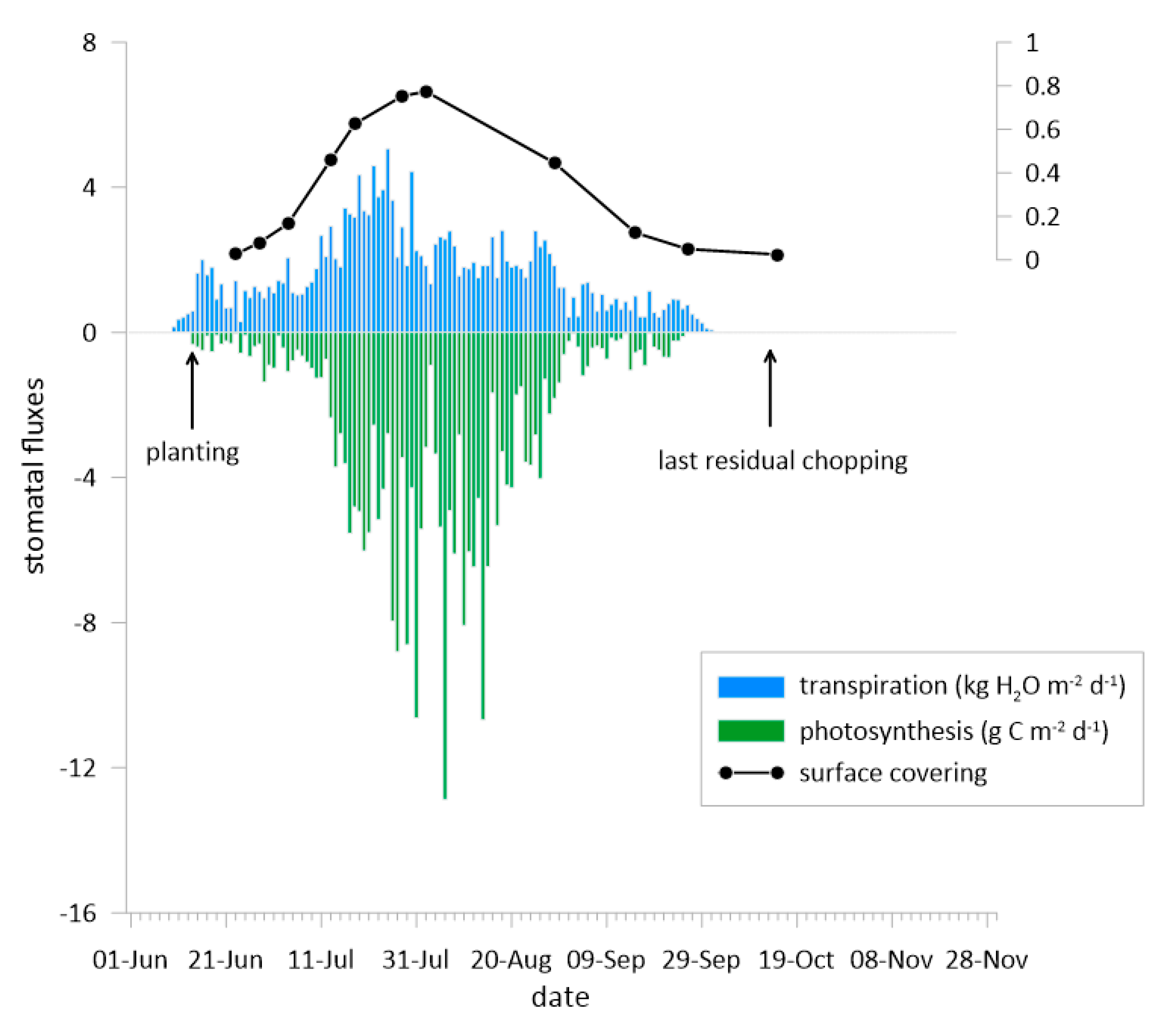
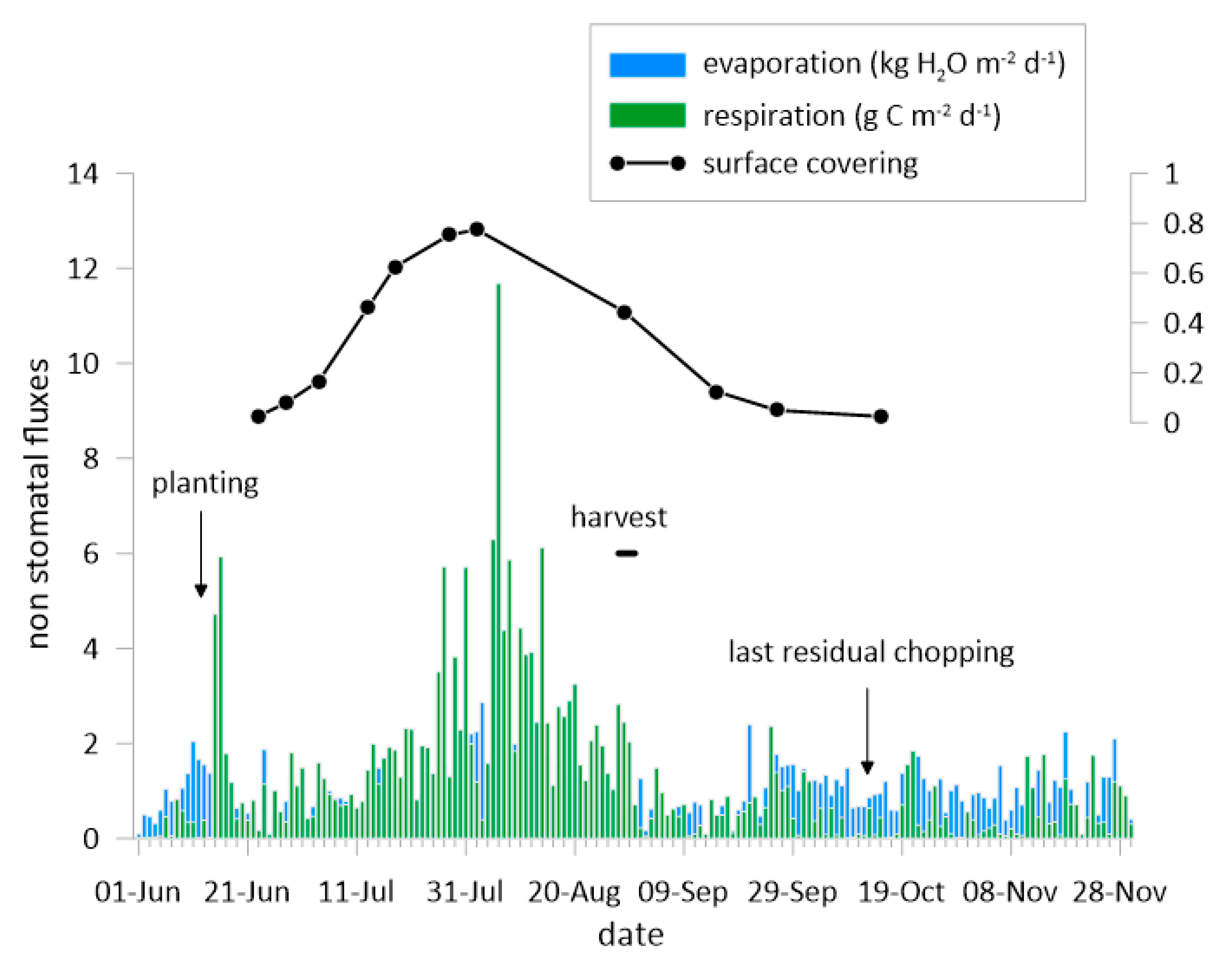
3.2. The Fluxes of CO2 and H2O
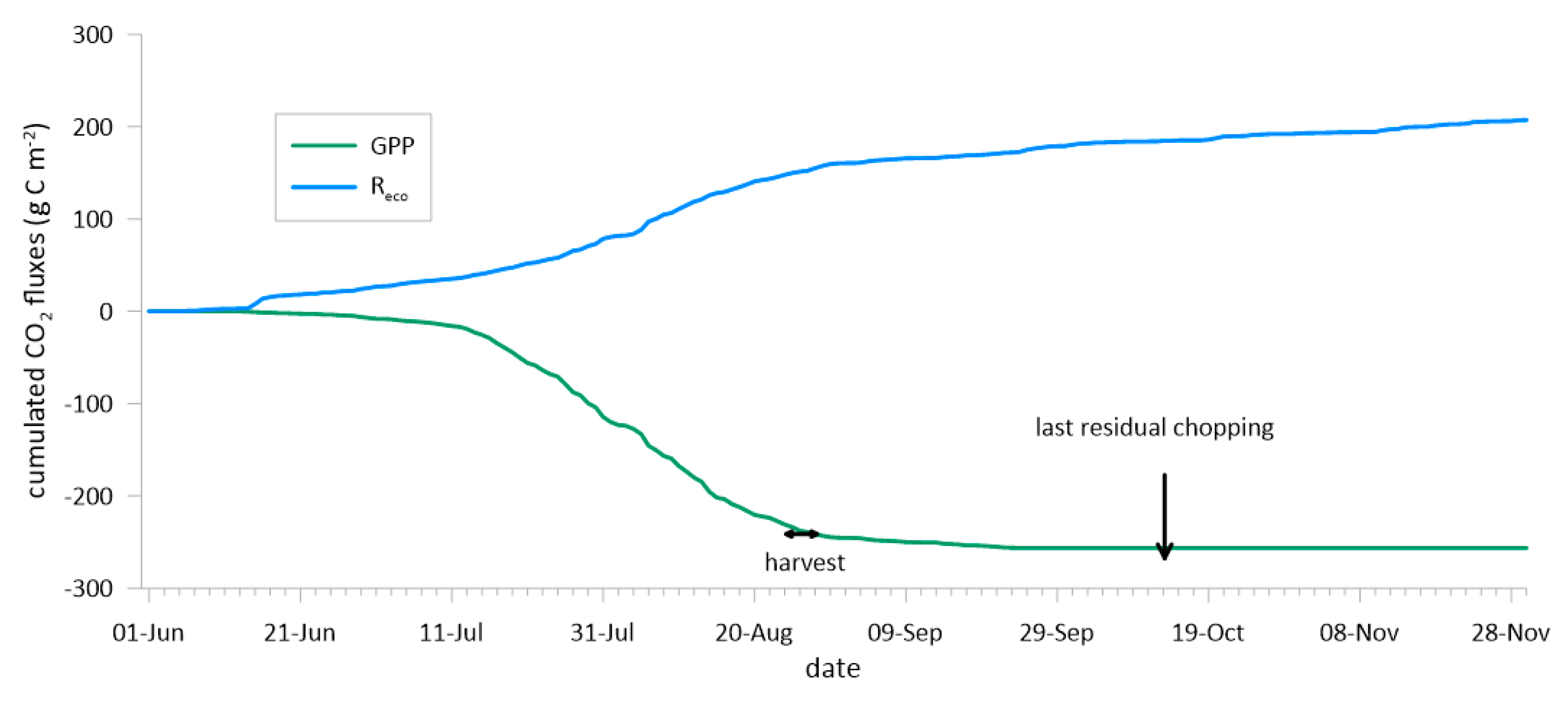
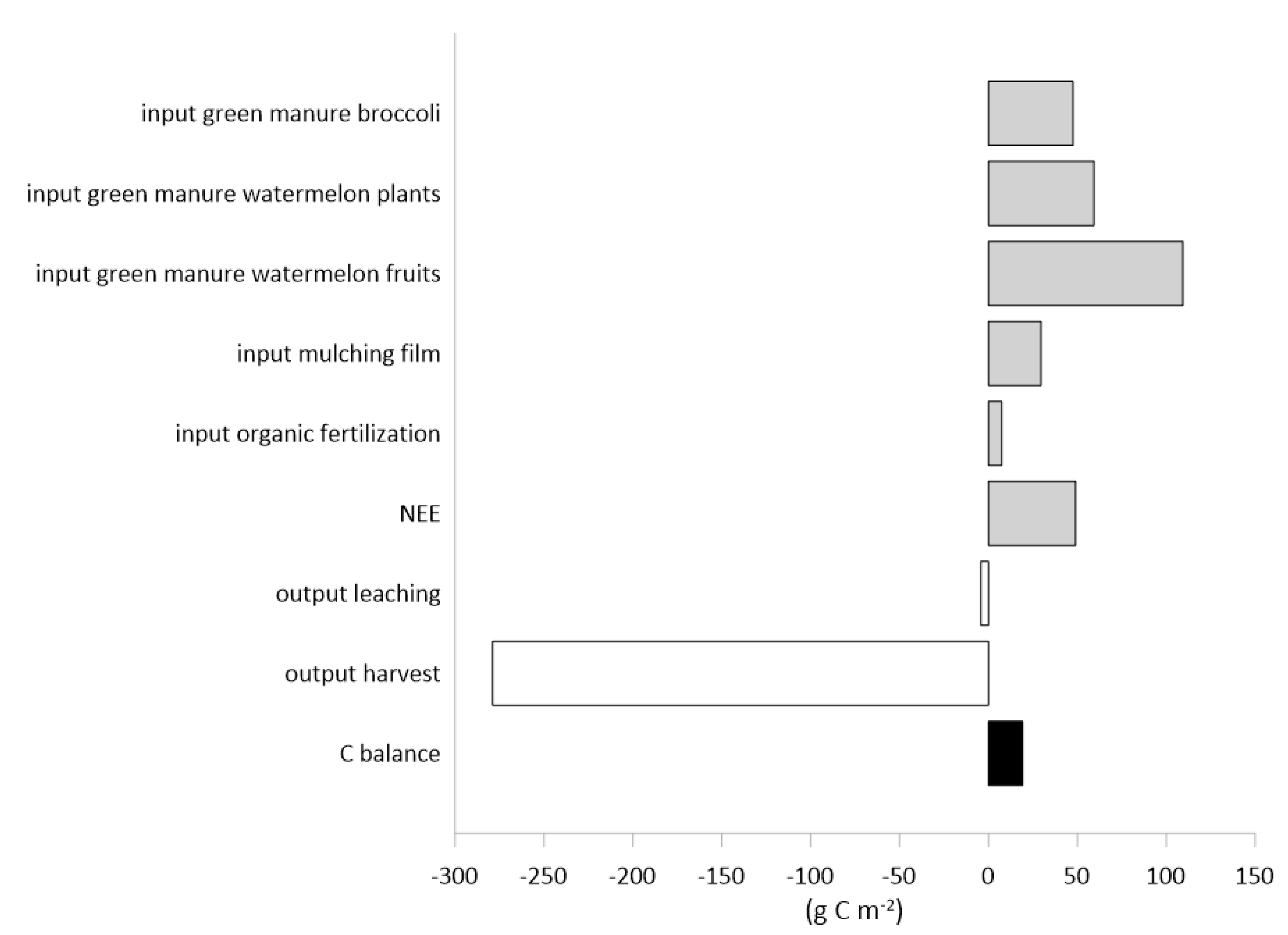
3.3. The Carbon and Water Balances and the Water Use Efficiencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Website. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 November 2023).
- Yavuz, D.; Seymen, M.; Suheri, S.; Yavuz, N.; Türkmen, Ö.; Kurtar, E.S. How do rootstocks of citron watermelon (Citrullus lanatus var. citroides) affect the yield and quality of watermelon under deficit irrigation? Agric. Water Manag. 2020, 241, 106351. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; Bilotto, M.; Petriccione, M.; Ferrara, E.; Mori, M.; Morra, L. Assessing Yield and Quality of Melon (Cucumis melo L.) Improved by Biodegradable Mulching Film. Plants 2023, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Rana, G.; Katerji, N. Measurement and estimation of actual evapotranspiration in the field under Mediterranean climate: A review. Eur. J. Agron. 2000, 13, 125–153. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual-Seva, N.; Nájera, I.; Giner, A.; Baixaul, C.; Pascual, B. Yield response of seedless watermelon to different drip irrigation strategies under Mediterranean conditions. Agric. Water Manag. 2019, 212, 99–110. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y.; Dong, X. Strip tillage improves soil biological activity, fruit yield and sugar content of triploid watermelon. Soil Tillage Res. 2016, 163, 266–273. [Google Scholar] [CrossRef]
- Zribi, W.; Aragüés, R.; Medina, E.; Faci, J.M. Efficiency of inorganic and organic mulching materials for soil evaporation control. Soil Tillage Res. 2015, 148, 40–45. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, A.; Liu, F.; Niu, W.; Siddique, K.H.M. Effect of film mulching on crop yield and water use efficiency in drip irrigation systems: A meta-analysis. Soil Tillage Res. 2022, 221, 105392. [Google Scholar] [CrossRef]
- Mudare, S.; Li, M.; Kanomanyanga, J.; Lamichhane, J.R.; Lakshmanan, P.; Cong, W.-F. Ecosystem services of organic versus inorganic ground cover in peach orchards: A meta-analysis. Food Energy Secur. 2023, 12, e463. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Yan, C.; Wu, Q.; Gong, D.; He, W.; Liu, H.; Wang, J.; Mei, X. Mitigation of greenhouse gas emissions and improved yield by plastic mulching in rice production. Sci. Total Environ. 2023, 880, 162984. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, H.; Li, Y. Relationships between carbon fluxes and environmental factors in a drip-irrigated, film- mulched cotton field in arid region. PLoS ONE 2018, 13, e0192467. [Google Scholar] [CrossRef]
- Gong, D.; Hao, W.; Mei, X.; Gao, X.; Liu, Q.; Caylor, K. Warmer and Wetter Soil Stimulates Assimilation More than Respiration in Rainfed Agricultural Ecosystem on the China Loess Plateau: The Role of Partial Plastic Film Mulching Tillage. PLoS ONE 2015, 10, e0136578. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Guo, R.; Liu, Y.; Naseer, M.A.; Hussain, S.; Liu, D.; Zhang, P.; Chen, X.; Ren, X. Biodegradable film mulching combined with straw incorporation can significantly reduce global warming potential with higher spring maize yield. Agric. Ecosyst. Environ. 2022, 340, 108181. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, X.; Xiao, M.; Zhao, C.; Yao, H. A meta-analysis of film mulching cultivation effects on soil organic carbon and soil greenhouse gas fluxes. Catena 2021, 206, 105483. [Google Scholar] [CrossRef]
- Ming, G.; Hu, H.; Tian, F.; Khan, M.Y.A.; Zhang, Q. Carbon budget for a plastic-film mulched and drip-irrigated cotton field in an oasis of Northwest China. Catena 2021, 206, 105483. [Google Scholar] [CrossRef]
- Xiong, L.; Liang, C.; Ma, B.; Shah, F.; Wu, W. Carbon footprint and yield performance assessment under plastic film mulching for winter wheat production. J. Clean. Prod. 2020, 270, 122468. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F. Multi-site assessment of the effects of plastic-film mulch on the soil organic carbon balance in semiarid areas of China. Agric. For. Meteorol. 2016, 228, 42–51. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Li, M.; Yang, Y.; Li, F.-M. Does long-term plastic film mulching really decrease sequestration of organic carbon in soil in the Loess Plateau? Eur. J. Agron. 2017, 89, 53–60. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Zhao, F.; Sainju, U.M. Response of soil carbon fractions and dryland maize yield to mulching. Soil Sci. Soc. Am. J. 2018, 82, 371–381. [Google Scholar] [CrossRef]
- Yin, M.; Li, Y.; Fang, H.; Chen, P. Biodegradable mulching film with an optimum degradation rate improves soil environment and enhances maize growth. Agric. Water Manag. 2019, 216, 127–137. [Google Scholar] [CrossRef]
- Birkhofer, K.; Diehl, E.; Andersson, J.; Ekroos, J.; Früh-Müller, A.; Machnikowski, F.; Mader, V.L.; Nilsson, L.; Sasaki, K.; Rundlöf, M.; et al. Ecosystem services—Current challenges and opportunities for ecological research. Front. Ecol. Evol. 2015, 2, 87. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, C.; Martinez-Aguirre, A.; Perez-Garcia, B.; Martinez-Urreaga, J.; de la Orden, M.U.; Fonseca-Velero, C. Use of residual agricultural plastics and cellulose fibers for obtaining sustainable eco-composites prevents waste generation. J. Clean. Prod. 2014, 83, 228–237. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Munoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Cai, H.; Fang, H.; Li, Y.; Chen, P.; Li, Y. Effects of degradable film mulching on crop yield and water use efficiency in China: A meta-analysis. Soil Tillage Res. 2020, 202, 104676. [Google Scholar] [CrossRef]
- Lee, X.; Massman, W.; Law, B.E. Handbook of micrometeorology: A guide for surface flux measurement and analysis. In Atmospheric and Oceanographic Sciences Library; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of net ecosystem exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Sahu, P. On the correlation structure of water vapor and carbon dioxide in the atmospheric surface layer: A basis for flux partitioning. Water Resour. Res. 2008, 44, W10418. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Kustas, W.P. Partitioning carbon dioxide and water vapor fluxes using correlation analysis. Agric. For. Meteorol. 2010, 150, 89–99. [Google Scholar] [CrossRef]
- Rana, G.; Palatella, L.; Scanlon, T.M.; Martinelli, N.; Ferrara, R.M. CO2 and H2O flux partitioning in a Mediterranean cropping system. Agric. For. Meteorol. 2018, 260–261, 118–130. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality Control and Flux Sampling Problems for Tower and Aircraft Data. J. Atmos. Ocean. Technol. 1997, 14, 515–526. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.; Leuning, R. Correction of flux measurements fordensity effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Campi, P.; Mastrorilli, M.; Stellacci, A.M.; Modugno, F.; Palumbo, A.D. Increasing the effective use of water in green asparagus through deficit irrigation strategies. Agric. Water Manag. 2019, 217, 119–130. [Google Scholar] [CrossRef]
- Searles, P.S.; Saravia, D.A.; Rousseaux, M.C. Root length density and soil water distribution in drip irrigated olive orchards in Argentina under arid conditions. Crop Pasture Sci. 2009, 60, 280–288. [Google Scholar] [CrossRef]
- Autovino, D.; Rallo, G.; Provenzano, G. Predicting soil and plant water status dynamic in olive orchards under different irrigation systems with Hydrus-2D: Model performance and scenario analysis. Agric. Water Manag. 2018, 203, 225–235. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.; Woodwell, G.; Randerson, J.; Rastetter, E.; Lovett, G.; Baldocchi, D.; Clark, D.; Harmon, M.; Schimel, D.; Valentini, R.; et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Rana, G.; Ferrara, R.M.; Vitale, D.; D’Andrea, L.; Palumbo, A.D. Carbon assimilation and water use efficiency of a perennial bioenergy crop (Cynara cardunculus L.) in Mediterranean environment. Agric. For. Meteorol. 2016, 217, 137–150. [Google Scholar] [CrossRef]
- Loubet, B.; Laville, P.; Lehuger, S.; Larmanou, E.; Fléchard, C.; Mascher, N.; Genermont, S.; Roche, R.; Ferrara, R.M.; Stella, P.; et al. Carbon, nitrogen and Greenhouse gases budgets over a four years crop rotation in northern France. Plant Soil 2011, 343, 109–137. [Google Scholar] [CrossRef]
- Miller, G.; Khalilian, H.; Adelberg, J.W.; Farahani, H.J.; Hassell, R.L.; Wells, C.E. Grafted Watermelon Root Length Density and Distribution under Different Soil Moisture Treatments. HortScience 2013, 48, 1021–1026. [Google Scholar] [CrossRef]
- Vitti, C.; Stellacci, A.M.; Leogrande, R.; Mastrangelo, M.; Cazzato, E.; Ventrella, D. Assessment of organic carbon in soils: A comparison between the Springer–Klee wet digestion and the dry combustion methods in Mediterranean soils (Southern Italy). Catena 2016, 137, 113–119. [Google Scholar] [CrossRef]
- Herbrich, M.; Gerke, H.H.; Bens, O.; Sommer, M. Water balance and leaching of dissolved organic and inorganic carbon of eroded Luvisols using high precision weighing lysimeters. Soil Tillage Res. 2017, 165, 144–160. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Campi, P.; Muschitiello, C.; Leogrande, R.; Vonella, A.V.; Ventrella, D.; Rana, G. Soil respiration during three cropping cycles of durum wheat under different tillage conditions in a Mediterranean environment. Soil Use Manag. 2022, 38, 1547–1563. [Google Scholar] [CrossRef]
- Rana, G.; Katerji, N. A Measurement Based Sensitivity Analysis of the Penman-Monteith actual evapotranspiration model for crops of different height and in contrasting water status. Theor. Appl. Climatol. 1998, 60, 141–149. [Google Scholar] [CrossRef]
- Palatella, L.; Rana, G.; Vitale, D. Toward a flux-partitioning procedure based on the direct use of high-frequency eddy-covariance data. Bound. Layer Meteorol. 2014, 153, 327–337. [Google Scholar] [CrossRef]
- Skaggs, T.H.; Anderson, R.G.; Alfieri, J.G.; Scanlon, T.M.; Kustas, W.P. Fluxpart: Open-source software for partitioning carbon dioxide and water vapor fluxes. Agric. For. Meteorol. 2018, 253–254, 218–224. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Rana, G. Water use efficiency of crops cultivated in Mediterranean region: Review and analysis. Eur. J. Agron. 2008, 28, 493–507. [Google Scholar] [CrossRef]
- Fernández, J.-E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water use indicators and economic analysis for on farm irrigation decision: A case study of a super high density olive tree orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Bruno, M.R.; Campi, P.; Camposeo, S.; De Carolis, G.; Gaeta, L.; Martinelli, N.; Mastrorilli, M.; Modugno, A.F.; Mongelli, T.; et al. Water use of a super high-density olive orchard submitted to regulated deficit irrigation in Mediterranean environment over three contrasted years. Irrig. Sci. 2024, 42, 57–73. [Google Scholar] [CrossRef]
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.F.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Glob. Biogeochem. Cycles 2009, 23, GB2018. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Liu, M.; Zhang, C.; Sun, G.; Lu, C.; Xu, X.; Ren, W.; Pan, S.; Chappelka, A. Model estimates of net primary productivity, evapotranspiration, andwater use efficiency in the terrestrial ecosystems of the southern United Statesduring 1895–2007. For. Ecol. Manag. 2010, 259, 1311–1327. [Google Scholar] [CrossRef]
- Cabral, O.M.R.; Rocha, H.R.; Gash, J.H.; Ligo, M.A.V.; Ramos, N.P.; Packer, A.P.; Batista, E.R. Fluxes of CO2 above a sugarcane plantation in Brazil. Agric. For. Meteorol. 2013, 182–183, 54–66. [Google Scholar] [CrossRef]
- Baldocchi, D. A comparative study of mass and energy exchange rates over a closed C3(wheat) and an open C4(corn) crop: II. CO2 exchange and water use efficiency. Agric. For. Meteorol. 1994, 67, 291–321. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Raes, D.; Fereres, E. AquaCrop—The FAO Crop Model to Simulate Yield Response to Water: I. Concepts and Underlying Principles. Agron. J. 2009, 101, 426–437. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, J.; Qian, C.; Liu, B.; Ni, L. Applicability of Biodegradable Mulch in Maize Production in Northeastern Yunnan. Agric. Sci. Tech. 2015, 16, 2324–2330. [Google Scholar]
- Ferrara, R.M.; Carozzi, M.; Decuq, C.; Loubet, B.; Finco, A.; Marzuoli, R.; Gerosa, G.; Di Tommasi, P.; Magliulo, V.; Rana, G. Ammonia, nitrous oxide, carbon dioxide, and water vapor fluxes after green manuring of faba bean under Mediterranean climate. Agric. Ecosyst. Environ. 2021, 315, 107439. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Martinelli, N.; Rana, G. CO2 and H2O fluxes due to green manuring under Mediterranean conditions. Ital. J. Agrometeorol. 2020, 45–53. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Farina, R.; Marchetti, A.; Francaviglia, R.; Napoli, R.; Di Bene, C. Modeling regional soil C stocks and CO2 emissions under Mediterranean cropping systems and soil types. Agric. Ecosyst. Environ. 2017, 238, 128–141. [Google Scholar] [CrossRef]
- Aubinet, M.; Moureaux, C.; Bodson, B.; Dufranne, D.; Heinesch, B.; Suleau, M.; Vancutsem, F.; Vilret, A. Carbon sequestration by a crop over a 4-year sugar beet/winter wheat/seed potato/winter wheat rotation cycle. Agric. For. Meteorol. 2009, 149, 407–418. [Google Scholar] [CrossRef]
- Osborne, B.; Saunders, M.; Walmsley, D.; Jones, M.; Smith, P. Key questions and uncertainties associated with the assessment of the cropland greenhouse gas balance. Agric. Ecosyst. Environ. 2010, 139, 293–301. [Google Scholar] [CrossRef]
- Lamichhane, S.; Kumar, L.; Wilson, B. Digital soil mapping algorithms and covariates for soil organic carbon mapping and their implications: A review. Geoderma 2019, 352, 395–413. [Google Scholar] [CrossRef]
- O’Rourke, S.M.; Angers, D.A.; Holden, N.M.; McBratney, A.B. Soil organic carbon across scales. Glob. Change Biol. 2015, 21, 3561–3574. [Google Scholar] [CrossRef] [PubMed]
- Funes, I.; Savé, R.; Rovira, P.; Molowny-Horas, R.; Alcañiz, J.M.; Ascaso, E.; Herms, I.; Herrero, C.; Boixadera, J.; Vayred, J. Agricultural soil organic carbon stocks in the north-eastern Iberian Peninsula: Drivers and spatial variability. Sci. Total Environ. 2019, 668, 283–294. [Google Scholar] [CrossRef]
- Mishra, U.; Riley, W.I. Scaling impacts on environmental controls and spatial heterogeneity of soil organic carbon stocks. Biogeosciences 2015, 12, 3993–4004. [Google Scholar] [CrossRef]
- Ventrella, D.; Di Giacomo, E.; Fiorentino, C.; Giglio, L.; Lopez, R.; Guastaferro, F.; Castrignanò, A. Soil Water Balance and Irrigation Strategies in an Agricultural District of Southern Italy. Ital. J. Agron. 2010, 5, 193–204. [Google Scholar] [CrossRef]
- Ren, D.; Xu, X.; Hao, Y.; Huang, G. Modeling and assessing field irrigation water use in a canal system of Hetao, upper Yellow River basin: Application to maize, sunflower and watermelon. J. Hydrol. 2016, 532, 122–139. [Google Scholar] [CrossRef]
- AlKhader, A.M.F.; Qaryouti, M.M.; Okasheh, T.Y.M. Effect of nitrogen on yield, quality, and irrigation water use efficiency of drip fertigated grafted watermelon (Citrullus lanatus) grown on a calcareous soil. J. Plant Nutr. 2019, 42, 737–748. [Google Scholar] [CrossRef]
- Fuentes, C.; Enciso, J.; Nelson, S.D.; Anciso, J.; Setamou, M.; Elsayed-Farag, S. Yield Production and Water Use Efficiency under Furrow and Drip Irrigation Systems for Watermelon in South Texas. Subtrop. Agric. Environ. 2018, 69, 1–7. [Google Scholar]
- Wang, X.-C.; Liu, R.; Luo, J.; Zhu, P.; Wang, Y.; Pan, X.; Shu, L. Effects of water and NPK fertigation on watermelon yield, quality, irrigation-water, and nutrient use efficiency under alternate partial root-zone drip irrigation. Agric. Water Manag. 2022, 271, 107785. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, S.; Zhang, H.; Lei, L.; Liang, C.; Chen, L.; Su, D.; Li, X. Deficit mulched drip irrigation improves yield, quality, and water use efficiency of watermelon in a desert oasis region. Agric. Water Manag. 2023, 277, 108103. [Google Scholar] [CrossRef]

| Climate | WUEagro [kg m−3] | WUEirr [kg m−3] | References |
|---|---|---|---|
| Mediterranean | 21.28 | [5] | |
| Mediterranean | 26.76 | [69] | |
| Semi-arid | 27.60 | 60.00 | [70] |
| Warm-temperate-humid | 33.40 | [71] | |
| Arid oasis desert | 39.00 | [72] | |
| Mediterranean | 38.38 | 15.82 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, R.M.; Azzolini, A.; Ciurlia, A.; De Carolis, G.; Mastrangelo, M.; Minorenti, V.; Montaghi, A.; Piarulli, M.; Ruggieri, S.; Vitti, C.; et al. Carbon and Water Balances in a Watermelon Crop Mulched with Biodegradable Films in Mediterranean Conditions at Extended Growth Season Scale. Atmosphere 2024, 15, 945. https://doi.org/10.3390/atmos15080945
Ferrara RM, Azzolini A, Ciurlia A, De Carolis G, Mastrangelo M, Minorenti V, Montaghi A, Piarulli M, Ruggieri S, Vitti C, et al. Carbon and Water Balances in a Watermelon Crop Mulched with Biodegradable Films in Mediterranean Conditions at Extended Growth Season Scale. Atmosphere. 2024; 15(8):945. https://doi.org/10.3390/atmos15080945
Chicago/Turabian StyleFerrara, Rossana M., Alessandro Azzolini, Alessandro Ciurlia, Gabriele De Carolis, Marcello Mastrangelo, Valerio Minorenti, Alessandro Montaghi, Mariagrazia Piarulli, Sergio Ruggieri, Carolina Vitti, and et al. 2024. "Carbon and Water Balances in a Watermelon Crop Mulched with Biodegradable Films in Mediterranean Conditions at Extended Growth Season Scale" Atmosphere 15, no. 8: 945. https://doi.org/10.3390/atmos15080945
APA StyleFerrara, R. M., Azzolini, A., Ciurlia, A., De Carolis, G., Mastrangelo, M., Minorenti, V., Montaghi, A., Piarulli, M., Ruggieri, S., Vitti, C., Martinelli, N., & Rana, G. (2024). Carbon and Water Balances in a Watermelon Crop Mulched with Biodegradable Films in Mediterranean Conditions at Extended Growth Season Scale. Atmosphere, 15(8), 945. https://doi.org/10.3390/atmos15080945






