Potential Health Risks of Indoor Particulate Matter Heavy Metals in Resource-Constrained Settings of South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Population, and Selection of Homes
2.2. Sample Collection and Analysis
2.3. Walkthrough Indoor Assessment
2.4. Data Analysis
3. Results
3.1. Summary Statistics of Heavy Metal Levels
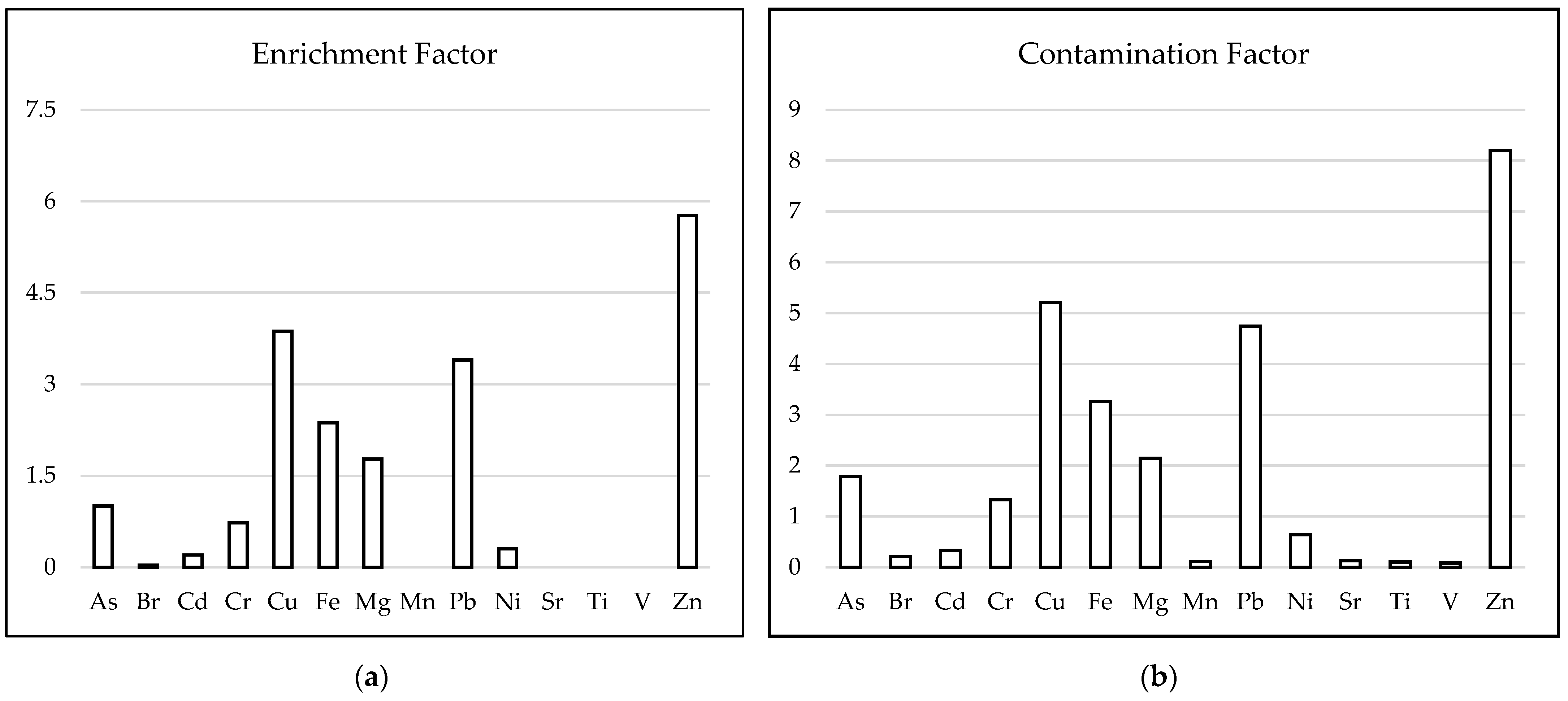
3.2. The Use of Contamination Indices to Assess the Level of Contamination in the Homes
3.3. The Relationship between Pollutant Sources and PM2.5 Heavy Metals
3.4. Analysis of Correlations among Heavy Metals in PM2.5
3.5. Identification of the Sources of Heavy Metals in Indoor PM2.5 Using PCA
3.6. Evaluation of the Health Risk for Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roomaney, R.; Wright, C.; Cairncross, E.; Abdelatif, N.; Cois, A.; Turawa, E.; Awotiwon, O.; Neethling, I.; Nojilana, B.; Pacella, R. Estimating the burden of disease attributable to household air pollution from cooking with solid fuels in South Africa for 2000, 2006 and 2012. S. Afr. Med. J. 2022, 112, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; de Oca, M.M.; Salvi, S.; Balakrishnan, K.; Hadfield, R.; Ramirez-Venegas, A.; Halpin, D.; Obianuju, B.O.; MeiLan, K.H.; Padilla, R.P. Household air pollution and COPD: Cause and effect or confounding by other aspects of poverty? Int. J. Tuberc. Lung Dis. 2022, 26, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tandjaoui-Lambiotte, Y.; Crockett, F.; Nunes, H.; Annesi-Maesano, I.; Sésé, L. Air pollution: The silent killer is also indoors. Int. J. Tuberc. Lung Dis. 2022, 26, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabai, T.; Xie, W.; Yan, L.; Zhao, L.; Carter, E.; Guo, D.; Daskalopoulou, S.S.; Chan, Q.; Elliott, P.; Ezzati, M. Household Air Pollution and Blood Pressure, Vascular Damage, and Subclinical Indicators of Cardiovascular Disease in Older Chinese Adults. Am. J. Hypertens. 2022, 35, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, R.; Zhu, Y.; Wang, T.; Fang, J.; Xie, Y.; Yuan, N.; Xu, H.; Song, X.; Huang, W. The effect of using personal-level indoor air cleaners and respirators on biomarkers of cardiorespiratory health: A systematic review. Environ. Int. 2022, 158, 106981. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Huang, X.; Zhang, J.; Lu, R.; Su, C.; Huang, C. The new model for evaluating indoor air quality based on childhood allergic and respiratory diseases in Shanghai. Build. Environ. 2022, 207, 108410. [Google Scholar] [CrossRef]
- Kamal, R.; Srivastava, A.K.; Kesavachandran, C.N.; Bihari, V.; Singh, A. Chronic obstructive pulmonary disease (COPD) in women due to indoor biomass burning: A meta analysis. Int. J. Environ. Health Res. 2022, 32, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Kangevari, M.; Malekpour, M.-R.; Masinaei, M.; Moghaddam, S.S.; Ghamari, S.-H.; Abbasi-Kangevari, Z.; Rezaei, N.; Rezaei, N.; Mokdad, A.H.; Naghavi, M. Effect of air pollution on disease burden, mortality, and life expectancy in North Africa and the Middle East: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Planet. Health 2023, 7, e358–e369. [Google Scholar] [CrossRef]
- Momtazan, M.; Geravandi, S.; Rastegarimehr, B.; Valipour, A.; Ranjbarzadeh, A.; Yari, A.R.; Dobaradaran, S.; Bostan, H.; Farhadi, M.; Darabi, F. An investigation of particulate matter and relevant cardiovascular risks in Abadan and Khorramshahr in 2014–2016. Toxin Rev. 2018, 38, 290–297. [Google Scholar] [CrossRef]
- Moradi, M.; Mokhtari, A.; Mohammadi, M.J.; Hadei, M.; Vosoughi, M. Estimation of long-term and short-term health effects attributed to PM 2.5 standard pollutants in the air of Ardabil (using Air Q+ model). Environ. Sci. Pollut. Res. 2022, 29, 21508–21516. [Google Scholar] [CrossRef]
- Khaefi, M.; Geravandi, S.; Hassani, G.; Yari, A.R.; Soltani, F.; Dobaradaran, S.; Moogahi, S.; Mohammadi, M.J.; Mahboubi, M.; Alavi, N. Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, southwest Iran during 2009-2013. Aerosol Air Qual. Res. 2017, 17, 230–237. [Google Scholar] [CrossRef]
- Effatpanah, M.; Effatpanah, H.; Jalali, S.; Parseh, I.; Goudarzi, G.; Barzegar, G.; Geravandi, S.; Darabi, F.; Ghasemian, N.; Mohammadi, M.J. Hospital admission of exposure to air pollution in Ahvaz megacity during 2010–2013. Clin. Epidemiol. Glob. Health 2020, 8, 550–556. [Google Scholar] [CrossRef]
- Dastoorpoor, M.; Sekhavatpour, Z.; Masoumi, K.; Mohammadi, M.J.; Aghababaeian, H.; Khanjani, N.; Hashemzadeh, B.; Vahedian, M. Air pollution and hospital admissions for cardiovascular diseases in Ahvaz, Iran. Sci. Total Environ. 2019, 652, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Indoor Particulate Matter. 2022. Available online: https://www.epa.gov/indoor-air-quality-iaq/indoor-particulate-matter (accessed on 9 January 2023).
- Shezi, B.; Jafta, N.; Sartorius, B.; Naidoo, R. Developing a predictive model for fine particulate matter concentrations in low socio-economic households in Durban, South Africa. Indoor Air 2018, 28, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shezi, B.; Jafta, N.; Asharam, K.; Tularam, H.; Barregård, L.; Naidoo, R.N. Predictors of urban household variability of indoor PM 2.5 in low socio-economic communities. Environ. Sci. Process. Impacts 2020, 22, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Jafta, N.; Jeena, P.; Barregard, L.; Naidoo, R. Childhood tuberculosis and exposure to indoor air pollution: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2015, 19, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Borsi, S.H.; Goudarzi, G.; Sarizadeh, G.; Dastoorpoor, M.; Geravandi, S.; Shahriyari, H.A.; Mohammadi, Z.A.; Mohammadi, M.J. Health endpoint of exposure to criteria air pollutants in ambient air of on a populated in Ahvaz City, Iran. Front. Public Health 2022, 10, 869656. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Jafari, A.J.; Gholami, M.; Arfaeinia, H.; Shahsavani, A.; Fanaei, F. Characterization, possible sources and health risk assessment of PM2. 5-bound Heavy Metals in the most industrial city of Iran. J. Environ. Health Sci. Eng. 2021, 19, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Eto, Y.; Aikawa, M. Risk assessment and management of PM2. 5-bound heavy metals in the urban area of Kitakyushu, Japan. Sci. Total Environ. 2021, 795, 148748. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- WHO. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution. 2007. Available online: https://www.who.int/publications/i/item/9789289071796#:~:text=Cadmium%20has%20also%20been%20identified,elevate%20blood%20pressure%20in%20adultsWe (accessed on 17 January 2022).
- Gruszecka-Kosowska, A. Deposited particulate matter enrichment in heavy metals and related health risk: A case study of Krakow, Poland. Proceedings 2020, 44, 1. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, W.; Shu, Y.; Tian, Y.; Feng, Y.; Zhang, T.; Chen, W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ. Pollut. 2019, 254, 113113. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, L.T.; Owoade, O.K.; Hopke, P.K.; Olise, F.S. Heavy metals in industrially emitted particulate matter in Ile-Ife, Nigeria. Environ. Res. 2017, 156, 320–325. [Google Scholar] [CrossRef] [PubMed]
- EThekwini Municipality. About Ethekwini. 2022. Available online: https://www.durban.gov.za/pages/government/about-ethekwini (accessed on 4 May 2023).
- StatsSA. Mid-Year Population Estimates. 2021. Available online: https://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed on 4 May 2023).
- Niranjan, I. A Case Study of Environmental Health in the South Durban Basin; University of Kwazulu-Natal: Durban, South Africa, 2005. [Google Scholar]
- Bell, F.G.; Maud, R.R. Landslides associated with the colluvial soils overlying the Natal Group in the greater 651 Durban region of Natal, South Africa. Environ. Geol. 2000, 39, 1029–1038. [Google Scholar] [CrossRef]
- Shezi, B.; Mathee, A.; Cele, N.; Ndabandaba, S.; Street, R.A. Occupational exposure to fine particulate matter (PM4 and PM2. 5) during hand-made cookware operation: Personal, indoor and outdoor levels. Int. J. Environ. Res. Public Health 2020, 17, 7522. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Batterman, S.; Parker, E.; Godwin, C.; Chin, J.-Y.; O’Toole, A.; Robins, T.; Brakefield-Caldwell, W.; Lewis, T. Particle concentrations and effectiveness of free-standing air filters in bedrooms of children with asthma in Detroit, Michigan. Build. Environ. 2011, 46, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Enamorado-Báez, S.; Gómez-Guzmán, J.; Chamizo, E.; Abril, J. Levels of 25 trace elements in high-volume air filter samples from Seville (2001–2002): Sources, enrichment factors and temporal variations. Atmos. Res. 2015, 155, 118–129. [Google Scholar] [CrossRef]
- Bern, C.R.; Walton-Day, K.; Naftz, D.L. Improved enrichment factor calculations through principal component analysis: Examples from soils near breccia pipe uranium mines, Arizona, USA. Environ. Pollut. 2019, 248, 90–100. [Google Scholar] [CrossRef]
- Tomlinson, D.; Wilson, J.; Harris, C.; Jeffrey, D. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund, Human Health Evaluation Manual Part A; USEPA: Washington, DC, USA, 1989.
- USEPA. US Environmental Protection Agency’s Integrated Risk Information System 2011. Available online: http://www.epa.gov/iris/ (accessed on 7 January 2023).
- Taylor, A.A.; Tsuji, J.S.; McArdle, M.E.; Adams, W.J.; Goodfellow, W.L., Jr. Recommended Reference Values for Risk Assessment of Oral Exposure to Copper. Risk Anal. 2022, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Batterman, S.; Su, F.-C.; Jia, C.; Naidoo, R.N.; Robins, T.; Naik, I. Manganese and lead in children’s blood and airborne particulate matter in Durban, South Africa. Sci. Total Environ. 2011, 409, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.F.; Dobaradaran, S.; Saeedi, R.; Nabipour, I.; Nazmara, S.; Abadi, D.R.V.; Arfaeinia, H.; Ramavandi, B.; Spitz, J.; Mohammadi, M.J. Levels and ecological and health risk assessment of PM 2.5-bound heavy metals in the northern part of the Persian Gulf. Environ. Sci. Pollut. Res. 2020, 27, 5305–5313. [Google Scholar] [CrossRef] [PubMed]
- Debipersadh, S.; Sibanda, T.; Selvarajan, R.; Naidoo, R. Investigating toxic metal levels in popular edible fishes from the South Durban basin: Implications for public health and food security. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vetrimurugan, E.; Shruti, V.; Jonathan, M.; Roy, P.D.; Kunene, N.; Villegas, L.E.C. Metal concentration in the tourist beaches of South Durban: An industrial hub of South Africa. Mar. Pollut. Bull. 2017, 117, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, G.; Alavi, N.; Geravandi, S.; Idani, E.; Behrooz, H.R.A.; Babaei, A.A.; Alamdari, F.A.; Dobaradaran, S.; Farhadi, M.; Mohammadi, M.J. Health risk assessment on human exposed to heavy metals in the ambient air PM10 in Ahvaz, southwest Iran. Int. J. Biometeorol. 2018, 62, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- MalAmiri, N.; Rashki, A.; Hosseinzadeh, S.R.; Kaskaoutis, D.G. Mineralogical, geochemical, and textural characteristics of soil and airborne samples during dust storms in Khuzestan, southwest Iran. Chemosphere 2022, 286, 131879. [Google Scholar] [CrossRef] [PubMed]
- Asvad, S.R.; Esmaili-Sari, A.; Bahramifar, N.; Behrooz, R.D.; Paschalidou, A.K.; Kaskaoutis, D.G. Heavy metals contamination status and health risk assessment of indoor and outdoor dust in Ahvaz and Zabol cities, Iran. Atmos. Pollut. Res. 2023, 14, 101727. [Google Scholar] [CrossRef]
- Tian, X.; Cui, K.; Sheu, H.-L.; Hsieh, Y.-K.; Yu, F. Effects of Rain and Snow on the Air Quality Index, PM2.5 Levels, and Dry Deposition Flux of PCDD/Fs. Aerosol Air Qual. Res. 2021, 21, 210158. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef]
- Swaringen, B.F.; Gawlik, E.; Kamenov, G.D.; McTigue, N.E.; Cornwell, D.A.; Bonzongo, J.-C.J. Children’s exposure to environmental lead: A review of potential sources, blood levels, and methods used to reduce exposure. Environ. Res. 2022, 204, 112025. [Google Scholar] [CrossRef]
- Martı, A.C.; Rivero, V.C.; Marı, M.L. Contamination by heavy metals in soils in the neighbourhood of a scrapyard of discarded vehicles. Sci. Total Environ. 1998, 212, 145–152. [Google Scholar]
- Yadav, S.; Rajamani, V. Geochemistry of aerosols of northwestern part of India adjoining the Thar Desert. Geochim. Cosmochim. Acta 2004, 68, 1975–1988. [Google Scholar] [CrossRef]
- Möller, A.; Müller, H.; Abdullah, A.; Abdelgawad, G.; Utermann, J. Urban soil pollution in Damascus, Syria: Concentrations and patterns of heavy metals in the soils of the Damascus Ghouta. Geoderma 2005, 124, 63–71. [Google Scholar] [CrossRef]
- Yongming, H.; Peixuan, D.; Junji, C.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar] [CrossRef]
- Zarandi, S.M.; Shahsavani, A.; Khodagholi, F.; Fakhri, Y. Concentration, sources and human health risk of heavy metals and polycyclic aromatic hydrocarbons bound PM2. 5 ambient air, Tehran, Iran. Environ. Geochem. Health 2019, 41, 1473–1487. [Google Scholar] [CrossRef]
- Shezi, B.; Street, R.A.; Webster, C.; Kunene, Z.; Mathee, A. Heavy Metal Contamination of Soil in Preschool Facilities around Industrial Operations, Kuils River, Cape Town (South Africa). Int. J. Environ. Res. Public Health 2022, 19, 4380. [Google Scholar] [CrossRef]
- Behrooz, R.D.; Kaskaoutis, D.G.; Grivas, G.; Mihalopoulos, N. Human health risk assessment for toxic elements in the extreme ambient dust conditions observed in Sistan, Iran. Chemosphere 2021, 262, 127835. [Google Scholar] [CrossRef]
- Tashakor, M.; Behrooz, R.D.; Asvad, S.R.; Kaskaoutis, D.G. Tracing of Heavy Metals Embedded in Indoor Dust Particles from the Industrial City of Asaluyeh, South of Iran. Int. J. Environ. Res. Public Health 2022, 19, 7905. [Google Scholar] [CrossRef]


| Parameter | ||
|---|---|---|
| Ingestion rate (mg/day) | IngR | 200 |
| Exposure rate (365 days/year) | EF | 365 |
| Duration of exposure (days) | ED | 6 |
| Childs body weight (kg) | BW | 15 |
| Mean duration for carcinogens (days) | AT | 25,550 |
| Mean duration for non-carcinogens (days) | AT | 2190 |
| Inhalation rate (mg/cm2) | Irair | 10 |
| Dermal surface area (cm2) | SA | 2100 |
| Soil adherence factor (mg/cm2) | AF | 0.2 |
| Dermal absorption factor | ABS | 0.1 |
| Dermal exposure ratio | FE | 0.61 |
| Particulate emission factor (m3/kg) | PEF | 1,300,000,000 |
| Conversion factor (kg/mg−1) | CF | 0.000001 |
| ng/m3 | Al * | As | Br | Cd | Cr | Cu | Ni | Fe | Mg | Mn | Pb | Sr | Ti | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 18 | 15 | 3 | 38 | 4 | 48 | 4 | 18 | 29 | 1 | 49 | 1.8 | 1 | 1 | 78 |
| Max | 836 | 315 | 123 | 261 | 641 | 859 | 163 | 1456 | 1169 | 39 | 854 | 72 | 37 | 46 | 1596 |
| Mean (SD) | 214 (167) | 271 (52) | 37 (26) | 140 (76) | 197 (164) | 792 (142) | 98 (32) | 513 (361) | 384 (212) | 16 (9) | 723 (138) | 22 (21) | 17 (9) | 12 (9) | 1235 (247) |
| Median | 166 | 277 | 32 | 131 | 179 | 810 | 92 | 467 | 367 | 15 | 715 | 12 | 15 | 10 | 1246 |
| %>Sample mean | - | 60%% | 43% | 50% | 43% | 90% | 43% | 43% | 47% | 43% | 63% | 41% | 40% | 40% | 57% |
| %>background value | - | 77% | 0% | 50% | 40% | 97% | 18% | 87% | 90% | 0% | 97% | 0% | 0% | 0% | 100% |
| WHO annual limits [39] | 6 | 5 | 20 | 500 |
| As | Cr | Cu | Mn | Ni | Pb | Ti | V | Zn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Constant | 5.38 | 4.87–5.89 | 4.34 | 3.27–5.50 | 3.00 | 2.14–3.87 | 4.37 | 3.75–5.00 | 6.37 | 5.90–6.84 | 2.37 | 1.60–3.12 | 2.89 | 1.98–3.81 | 6.91 | 6.44–7.39 | ||
| Proximity to industry | 0.53 | −0.01–1.07 | ||||||||||||||||
| Use of incense/ candles | 1.12 | 0.04–2.20 | ||||||||||||||||
| Presence of windows | 1.02 | −0.01–2.05 | 1.03 | 0.05–2.01 | 1.00 | 0.02–1.92 | ||||||||||||
| Cross Ventilation | −0.91 | −1.79–(−0.02) | −0.88 | −1.72–(−0.03) | ||||||||||||||
| Home heating | −0.91 | −1.66– (0.18) | −0.78 | −1.56–0.01 | ||||||||||||||
| Proximity to pollutant generating activities | 0.93 | 0.14–1.72 | 1.71 | −0.02–0.81 | 0.98 | 0.23–1.73 | 1.51 | 0.17–2.85 | 1.30 | 0.32–2.27 | 0.86 | 0.13–1.60 | 1.59 | 0.17–3.01 | 0.99 | 0.24–1.73 | ||
| Wall type: corrugated iron/wood) | −1.06 | −1.62–(−0.49) | −1.07 | −1.60–(−0.53) | −1.22 | −1.91–(−0.52) | −0.98 | −1.50–(−0.46) | −0.99 | −1.52–(−0.46) | ||||||||
| Age of the house: ≤25 years | −0.33 | −0.68–0.01 | −0.33 | −0.67–(−0.00) | −0.32 | −0.64–(−0.00) | −0.75 | −1.39–(−0.11) | −0.36 | −0.68–(−0.03) | ||||||||
| As | Br | Cd | Cr | Cu | Fe | Mg | Mn | Ni | Pb | Sr | Ti | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | |||||||||||||
| Br | 0.29 | 1 | ||||||||||||
| Cd | 0.30 | 0.33 | 1 | |||||||||||
| Cr | *** 0.08 | 0.14 | 0.22 | 1 | ||||||||||
| Cu | 0.92 | 0.27 | 0.22 | *** 0.30 | 1 | |||||||||
| Fe | *** 0.34 | 0.18 | 0.26 | *** 0.98 | 0.34 | 1 | ||||||||
| Mg | 0.31 | * 0.20 | 0.04 | 0.40 | ** 0.36 | 0.49 | 1 | |||||||
| Mn | 0.34 | * 0.20 | 0.35 | * 0.16 | *** 0.38 | ** 0.20 | *** 0.31 | 1 | ||||||
| Ni | *** 0.40 | 0.23 | 0.20 | *** 0.90 | *** 0.38 | *** 0.88 | 0.46 | ** 0.30 | 1 | |||||
| Pb | *** 0.92 | 0.40 | * 0.50 | 0.14 | *** 0.91 | 0.20 | *** 0.35 | 0.42 | 0.46 | 1 | ||||
| Sr | 0.24 | −0.03 | 0.08 | −0.01 | 0.16 | 0.01 | 0.26 | 0.32 | 0.11 | 0.19 | 1 | |||
| Ti | 0.39 | 0.10 | 0.08 | −0.00 | 0.33 | 0.16 | *** 0.38 | ** 0.09 | 0.02 | 0.39 | 0.29 | 1 | ||
| V | 0.16 | * −0.18 | −0.30 | 0.11 | 0.24 | 0.05 | −0.08 | ** 0.29 | * 0.24 | 0.17 | −0.02 | −0.25 | 1 | |
| Zn | 0.83 | 0.33 | ** 0.40 | 0.44 | *** 0.89 | 0.48 | *** 0.38 | ** 0.39 | 0.68 | 0.85 | 0.18 | * 0.31 | 0.25 | 1 |
| Variable | PC1: Household Sources, Traffic Emissions and Industries | PC2: Industries and Natural Sources (i.e., Natural Soil and Marine Aerosols) | PC3: Natural Sources (i.e., Marine Soil and Natural Soil) | PC4: Natural Sources (i.e., Natural Soil) |
|---|---|---|---|---|
| As | 0.488 | |||
| Br | −0.38 | |||
| Cd | 0.31 | |||
| Cr | 0.569 | |||
| Cu | 0.461 | |||
| Fe | 0.556 | |||
| Mg | 0.351 | |||
| Mn | 0.385 | 0.310 | ||
| Ni | 0.464 | |||
| Pb | 0.487 | |||
| Sr | 0.666 | |||
| Ti | 0.321 | −0.381 | ||
| V | 0.701 | |||
| Zn | 0.419 | |||
| Eigenvalue | 5.5 | 2.3 | 1.6 | 1.2 |
| % of variance | 39.6 | 16.2 | 11.1 | 8.0 |
| Cumulative % | 39.6 | 55.8 | 66.9 | 75.4 |
| ADIing | ADIinh | ADIderm | HQing | HQinh | HQderm | HI | LCR | |
|---|---|---|---|---|---|---|---|---|
| As | 3.5 × 10–3 | 1.3 × 10−7 | 4.4 × 10−4 | 1.155 × 101 | 4.4 × 10−4 | 1.5 × 100 | 1.3 × 101 | 2.0 × 101 |
| Br | - | - | - | - | - | - | - | - |
| Cd | 1.8 × 10−3 | 6.9 × 10−8 | 2.3 × 10−4 | 3.6 × 100 | 6.9 × 10−3 | 2.3 × 101 | 2.7 × 101 | - |
| Cr | 2.5 × 10−3 | 9.7 × 10−8 | 3.2 × 10−4 | 8.4 × 100 | 9.7 × 10−4 | 5.4 × 100 | 1.4 × 101 | - |
| Cu | 1.0 × 10−2 | 3.9 × 10−7 | 1.3 × 10−3 | 2.5 × 10−1 | 9.7 × 10−5 | 1.1 × 10−1 | 3.6 × 10−1 | - |
| Fe | 6.7 × 10−3 | 2.5 × 10−7 | 8.4 × 10−4 | 9.4 × 10−1 | 1.1 × 10−3 | 1.2 × 10−2 | 9.5 × 10−1 | - |
| Mg | - | - | - | - | - | - | - | - |
| Mn | 2.1 × 10−4 | 7.9 × 10−9 | 2.6 × 10−5 | 1.5 × 10−1 | 5.6 × 10−6 | 1.95 × 10−2 | 1.6 × 101 | - |
| Ni | 1.3 × 10−3 | 4.8 × 10−8 | 1.6 × 10−4 | 6.3 × 10−2 | 5.4 × 10−4 | 3.0 × 10−2 | 9.3 × 10−2 | - |
| Pb | 9.2 × 10−3 | 3.6 × 10−7 | 1.2 × 10−3 | 2.6 × 100 | 2.5 × 10−4 | 8.5 × 10−1 | 3.8 × 101 | 3.2 × 10−1 |
| Sr | - | - | - | - | - | - | - | - |
| Ti | - | - | - | - | - | - | - | - |
| V | - | - | - | - | - | - | - | - |
| Zn | 1.6 × 10−2 | 6.1 × 10−7 | 2.0 × 10−3 | 5.3 × 10−2 | 2.0 × 10−6 | 2.7 × 10−2 | 8.0 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shezi, B.; Jafta, N.; Naidoo, R.N. Potential Health Risks of Indoor Particulate Matter Heavy Metals in Resource-Constrained Settings of South Africa. Atmosphere 2024, 15, 911. https://doi.org/10.3390/atmos15080911
Shezi B, Jafta N, Naidoo RN. Potential Health Risks of Indoor Particulate Matter Heavy Metals in Resource-Constrained Settings of South Africa. Atmosphere. 2024; 15(8):911. https://doi.org/10.3390/atmos15080911
Chicago/Turabian StyleShezi, Busisiwe, Nkosana Jafta, and Rajen N Naidoo. 2024. "Potential Health Risks of Indoor Particulate Matter Heavy Metals in Resource-Constrained Settings of South Africa" Atmosphere 15, no. 8: 911. https://doi.org/10.3390/atmos15080911
APA StyleShezi, B., Jafta, N., & Naidoo, R. N. (2024). Potential Health Risks of Indoor Particulate Matter Heavy Metals in Resource-Constrained Settings of South Africa. Atmosphere, 15(8), 911. https://doi.org/10.3390/atmos15080911








