Evaluating the Impact of Increased Heavy Oil Consumption on Urban Pollution Levels through Isotope (δ13C, δ34S, 14C) Composition
Abstract
1. Introduction
2. Materials and Methods
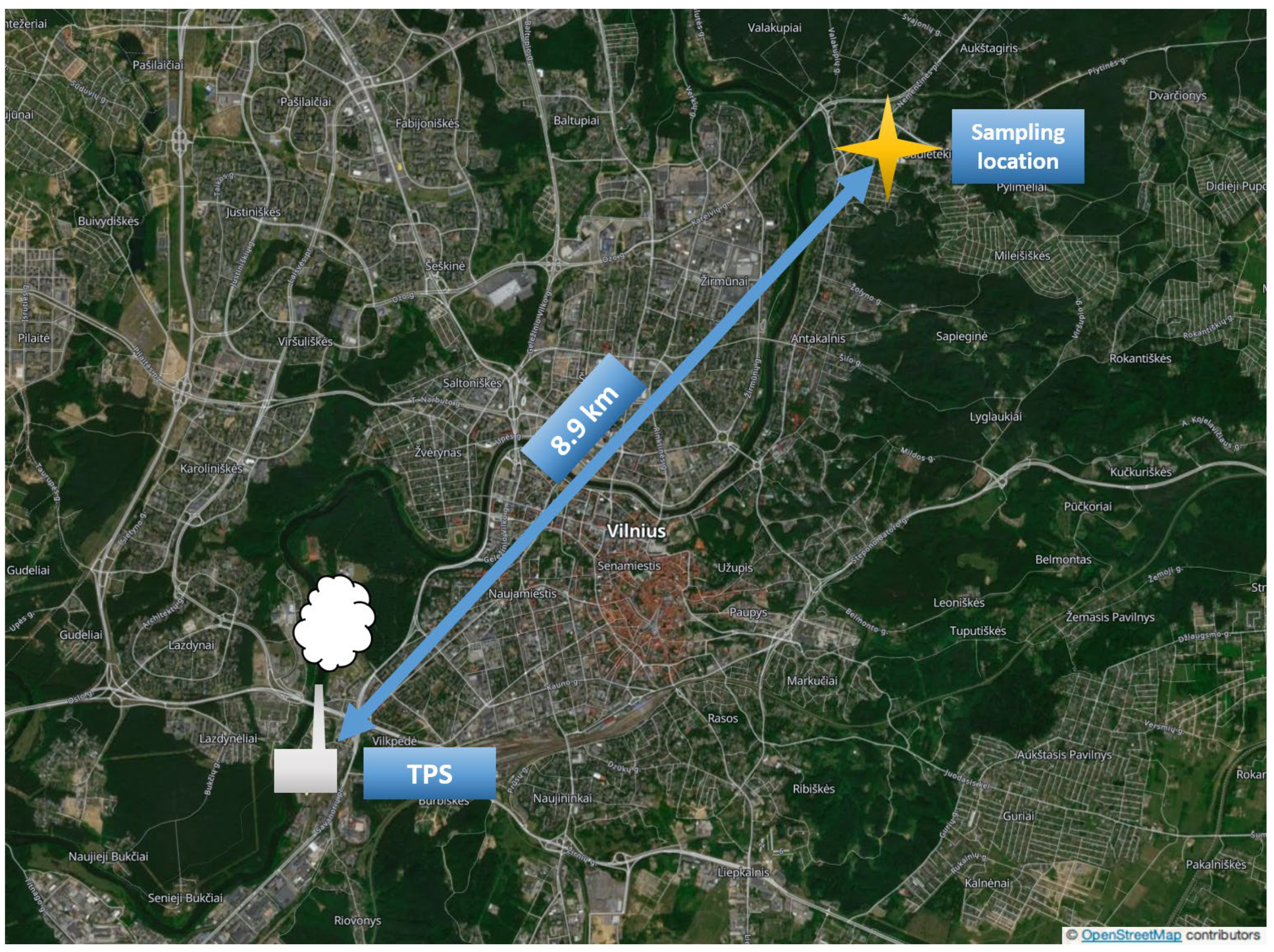
2.1. Sampling Site
2.2. Sample Collection
2.3. Stable Isotope Measurements
2.4. Radiocarbon Measurements
2.5. Water Soluble Inorganic Ions and SO2 Concentration Measurements
2.6. Meteorological Data
3. Results and Discussion
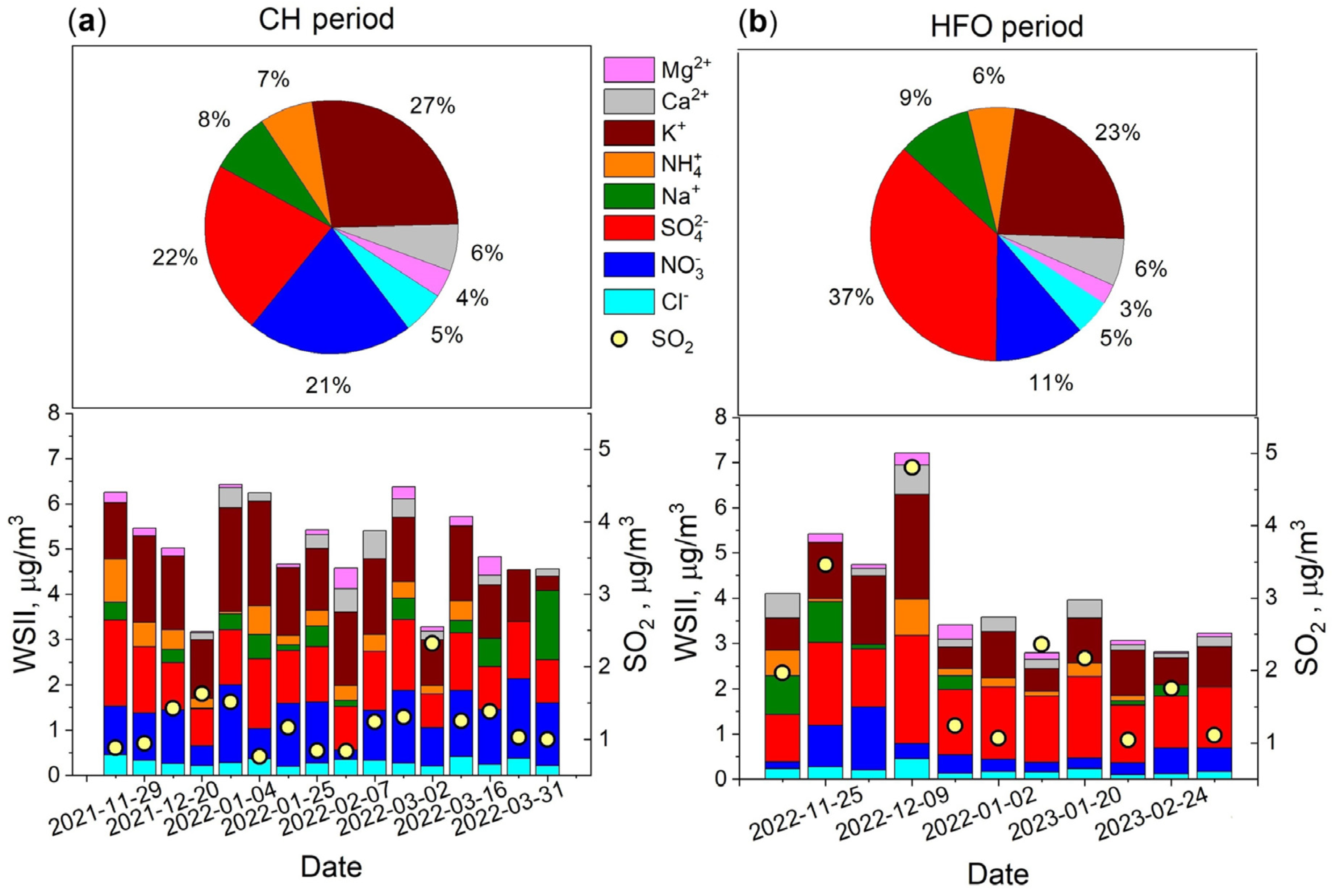
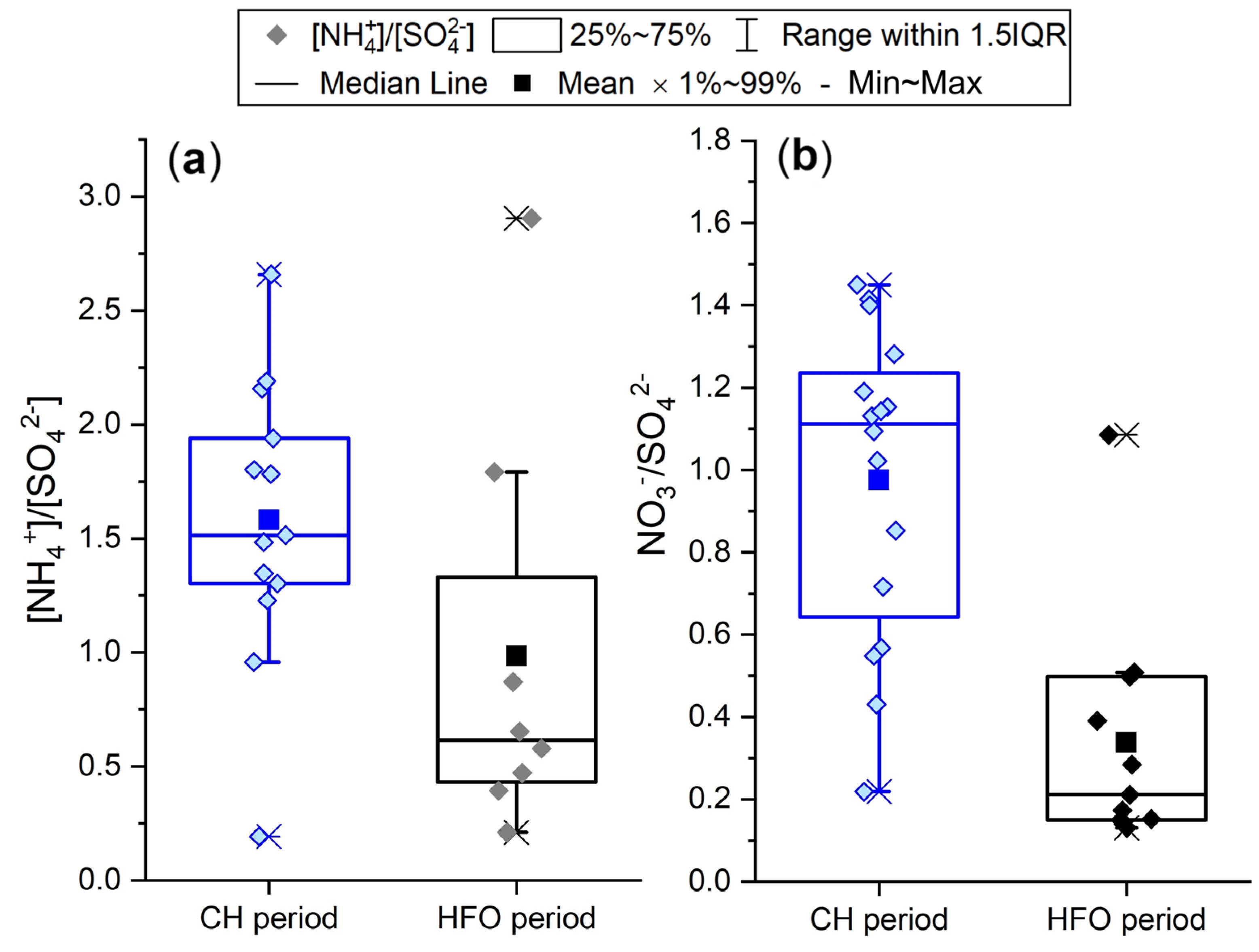
3.1. Concentration of Water-Soluble Inorganic Ions and SO2
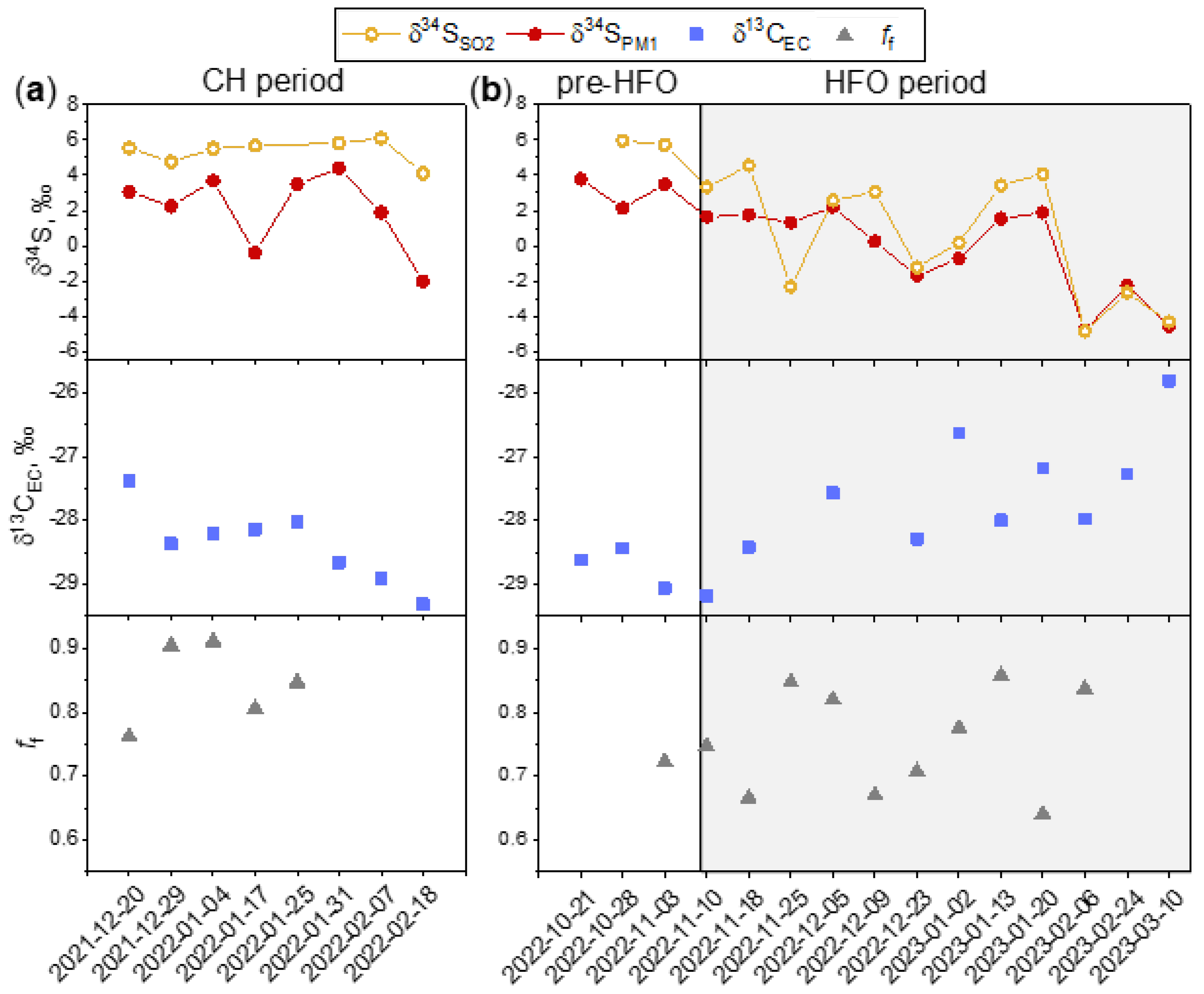
3.2. Carbon and Sulfur Isotope Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenger, J. Urban Air Quality. Atmos. Environ. 1999, 33, 4877–4900. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Squizzato, S.; Masiol, M.; Agostini, C.; Visin, F.; Formenton, G.; Harrison, R.M.; Rampazzo, G. Factors, Origin and Sources Affecting PM1 Concentrations and Composition at an Urban Background Site. Atmos. Res. 2016, 180, 262–273. [Google Scholar] [CrossRef]
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; Van Der Gon, H.D.; Facchini, M.C.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U.; et al. Particulate matter, air quality and climate: Lessons learned and future needs. Atmos. Chem. Phys. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Tomasi, C.; Fuzzi, S.; Kokhanovsky, A.A. Atmospheric Aerosols: Life Cycles and Effects on Air Quality and Climate. In Atmospheric Aerosols: Life Cycles and Effects on Air Quality and Climate; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Bressi, M.; Cavalli, F.; Putaud, J.P.; Fröhlich, R.; Petit, J.-E.; Aas, W.; Äijälä, M.; Alastuey, A.; Allan, J.; Aurela, M.; et al. A European aerosol phenomenology—7: High-time resolution chemical characteristics of submicron particulate matter across Europe. Atmos. Environ. X 2021, 10, 100108. [Google Scholar] [CrossRef]
- Shao, P.; Tian, H.; Sun, Y.; Liu, H.; Wu, B.; Liu, S.; Liu, X.; Wu, Y.; Liang, W.; Wang, Y.; et al. Characterizing remarkable changes of severe haze events and chemical compositions in multi-size airborne particles (PM1, PM2.5 and PM10) from January 2013 to 2016–2017 winter in Beijing, China. Atmos. Environ. 2018, 189, 133–144. [Google Scholar] [CrossRef]
- Huang, B.; Gan, T.; Pei, C.; Li, M.; Cheng, P.; Chen, D.; Cai, R.; Wang, Y.; Li, L.; Huang, Z.; et al. Size-segregated Characteristics and Formation Mechanisms of Water-soluble Inorganic Ions during Different Seasons in Heshan of Guangdong, China. Aerosol Air Qual. Res. 2020, 20, 1961–1973. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Espen Yttri, K.; Canonaco, F.; Eckhardt, S.; Evangeliou, N.; Fiebig, M.; Gundersen, H.; Hjellbrekke, A.-G.; Myhre, C.L.; Platt, S.M.; Prévôt, A.S.H.; et al. Trends, composition, and sources of carbonaceous aerosol at the Birkenes Observatory, northern Europe, 2001–2018. Atmos. Chem. Phys. 2021, 21, 7149–7170. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.; Ren, H.; Yang, J.; Deng, J.; Wang, D.; Sun, Y.; Wang, Z.; Kawamura, K.; Fu, P. Diurnal variations in primary and secondary organic aerosols in an eastern China coastal city: The impact of land-sea breezes. Environ. Pollut. 2023, 319, 121016. [Google Scholar] [CrossRef]
- Szidat, S.; Ruff, M.; Perron, N.; Wacker, L.; Synal, H.-A.; Hallquist, M.; Shannigrahi, A.S.; Yttri, K.E.; Dye, C.; Simpson, D. Fossil and non-fossil sources of organic carbon (OC) and elemental carbon (EC) in Göteborg, Sweden. Atmos. Chem. Phys. 2009, 9, 1521–1535. [Google Scholar] [CrossRef]
- Ivančič, M.; Gregorič, A.; Lavrič, G.; Alföldy, B.; Ježek, I.; Hasheminassab, S.; Pakbin, P.; Ahangar, F.; Sowlat, M.; Boddeker, S.; et al. Two-year-long high-time-resolution apportionment of primary and secondary carbonaceous aerosols in the Los Angeles Basin using an advanced total carbon–black carbon (TC-BC(λ)) method. Sci. Total Environ. 2022, 848, 157606. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Huang, R.-J.; Cosijn, M.M.; Yang, L.; Guo, J.; Cao, J.; Dusek, U. Measurement report: Dual-carbon isotopic characterization of carbonaceous aerosol reveals different primary and secondary sources in Beijing and Xi’an during severe haze events. Atmos. Chem. Phys. 2020, 20, 16041–16053. [Google Scholar] [CrossRef]
- Meusinger, C.; Dusek, U.; King, S.M.; Holzinger, R.; Rosenørn, T.; Sperlich, P.; Julien, M.; Remaud, G.S.; Bilde, M.; Röckmann, T.; et al. Chemical and isotopic composition of secondary organic aerosol generated by α-pinene ozonolysis. Atmos. Chem. Phys. 2017, 17, 6373–6391. [Google Scholar] [CrossRef]
- Cappa, C.D.; Onasch, T.B.; Massoli, P.; Worsnop, D.R.; Bates, T.S.; Cross, E.S.; Davidovits, P.; Hakala, J.; Hayden, K.L.; Jobson, B.T.; et al. Radiative Absorption Enhancements Due to the Mixing State of Atmospheric Black Carbon. Science 2012, 337, 1078–1081. [Google Scholar] [CrossRef]
- Samset, B.H.; Myhre, G.; Herber, A.; Kondo, Y.; Li, S.-M.; Moteki, N.; Koike, M.; Oshima, N.; Schwarz, J.P.; Balkanski, Y.; et al. Modelled black carbon radiative forcing and atmospheric lifetime in AeroCom Phase II constrained by aircraft observations. Atmos. Chem. Phys. 2014, 14, 12465–12477. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan City, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.; Cheng, S.; Gao, R.; Zhou, Y.; Xue, L.; Shou, Y.; Wang, J.; Wang, X.; Nie, W.; et al. Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: Temporal variations and source apportionments. Atmos. Environ. 2011, 45, 6048–6056. [Google Scholar] [CrossRef]
- Yuan, C.-S.; Lee, C.-G.; Liu, S.-H.; Chang, J.-C.; Yuan, C.; Yang, H.-Y. Correlation of atmospheric visibility with chemical composition of Kaohsiung aerosols. Atmos. Res. 2006, 82, 663–679. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Cai, T.; Fang, D.; Wang, Y.; Song, J.; Hu, M.; Zhang, Y. Concentrations and chemical compositions of fine particles (PM2.5) during haze and non-haze days in Beijing. Atmos. Res. 2016, 174-175, 62–69. [Google Scholar] [CrossRef]
- Sun, Z.; Mu, Y.; Liu, Y.; Shao, L. A comparison study on airborne particles during haze days and non-haze days in Beijing. Sci. Total Environ. 2013, 456–457, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crippa, M.; Janssens-Maenhout, G.; Dentener, F.; Guizzardi, D.; Sindelarova, K.; Muntean, M.; Van Dingenen, R.; Granier, C. Forty years of improvements in European air quality: Regional policy-industry interactions with global impacts. Atmos. Chem. Phys. 2016, 16, 3825–3841. [Google Scholar] [CrossRef]
- Syrek-Gerstenkorn, Z.; Syrek-Gerstenkorn, B.; Paul, S. A Comparative Study of SOx, NOx, PM2.5 and PM10 in the UK and Poland from 1970 to 2020. Appl. Sci. 2024, 14, 3292. [Google Scholar] [CrossRef]
- Celasun, O.; Mineshima, A.; Arregui, N.; Mylonas, V.; Ari, A.; Teodoru, I.; Black, S.; Zhunussova, K.; Iakova, D.; Parry, I. Surging Energy Prices in Europe in the Aftermath of the War: How to Support the Vulnerable and Speed up the Transition Away from Fossil Fuels; IMF Working Papers; IMF: Washington, DC, USA, 2022; Volume 2022. [Google Scholar] [CrossRef]
- Wu, D.; Li, Q.; Ding, X.; Sun, J.; Li, D.; Fu, H.; Teich, M.; Ye, X.; Chen, J. Primary Particulate Matter Emitted from Heavy Fuel and Diesel Oil Combustion in a Typical Container Ship: Characteristics and Toxicity. Environ. Sci. Technol. 2018, 52, 12943–12951. [Google Scholar] [CrossRef]
- Garaniya, V.; McWilliam, D.; Goldsworthy, L.; Ghiji, M. Extensive chemical characterization of a heavy fuel oil. Fuel 2018, 227, 67–78. [Google Scholar] [CrossRef]
- Pei, X.; Jameel, A.G.A.; Chen, C.; AlGhamdi, I.A.; AlAhmadi, K.; AlBarakati, E.; Saxena, S.; Roberts, W.L. Swirling Flame Combustion of Heavy Fuel Oil: Effect of Fuel Sulfur Content. J. Energy Resour. Technol. Trans. ASME 2020, 143, 082103. [Google Scholar] [CrossRef]
- Tao, L.; Fairley, D.; Kleeman, M.J.; Harley, R.A. Effects of Switching to Lower Sulfur Marine Fuel Oil on Air Quality in the San Francisco Bay Area. Environ. Sci. Technol. 2013, 47, 10171–10178. [Google Scholar] [CrossRef] [PubMed]
- Saario, A.; Rebola, A.; Coelho, P.J.; Costa, M.; Oksanen, A. Heavy fuel oil combustion in a cylindrical laboratory furnace: Measurements and modeling. Fuel 2005, 84, 359–369. [Google Scholar] [CrossRef]
- Allouis, C.; Beretta, F.; D’alessio, A. Structure of inorganic and carbonaceous particles emitted from heavy oil combustion. Chemosphere 2003, 51, 1091–1096. [Google Scholar] [CrossRef]
- Alonso-Hernández, C.M.; Bernal-Castillo, J.; Bolanos-Alvarez, Y.; Gómez-Batista, M.; Diaz-Asencio, M. Heavy metal content of bottom ashes from a fuel oil power plant and oil refinery in Cuba. Fuel 2011, 90, 2820–2823. [Google Scholar] [CrossRef]
- Oeder, S.; Kanashova, T.; Sippula, O.; Sapcariu, S.C.; Streibel, T.; Arteaga-Salas, J.M.; Passig, J.; Dilger, M.; Paur, H.-R.; Schlager, C.; et al. Particulate Matter from Both Heavy Fuel Oil and Diesel Fuel Shipping Emissions Show Strong Biological Effects on Human Lung Cells at Realistic and Comparable In Vitro Exposure Conditions. PLoS ONE 2015, 10, e0126536. [Google Scholar] [CrossRef]
- AB Vilniaus Šilumos Tinklai. Available online: https://chc.lt/lt/gn/44/vilniaus-silumos-tinklai-nuo-kovo-pabaigos-nebenaudos-mazasierio-mazuto-grizta-prie-atpigusiu-gamtiniu-duju:757 (accessed on 12 May 2024).
- Masalaite, A.; Holzinger, R.; Remeikis, V.; Röckmann, T.; Dusek, U. Characteristics, sources and evolution of fine aerosol (PM 1) at urban, coastal and forest background sites in Lithuania. Atmos. Environ. 2017, 148, 62–76. [Google Scholar] [CrossRef]
- Masalaite, A.; Holzinger, R.; Ceburnis, D.; Remeikis, V.; Ulevičius, V.; Röckmann, T.; Dusek, U. Sources and atmospheric processing of size segregated aerosol particles revealed by stable carbon isotope ratios and chemical speciation. Environ. Pollut. 2018, 240, 286–296. [Google Scholar] [CrossRef]
- Cachier, H.; Buat-Menard, P.; Fontugne, M.; Rancher, J. Source terms and source strengths of the carbonaceous aerosol in the tropics. J. Atmos. Chem. 1985, 3, 469–489. [Google Scholar] [CrossRef]
- Fisseha, R.; Saurer, M.; Jäggi, M.; Siegwolf, R.T.W.; Dommen, J.; Szidat, S.; Samburova, V.; Baltensperger, U. Determination of primary and secondary sources of organic acids and carbonaceous aerosols using stable carbon isotopes. Atmos. Environ. 2008, 43, 431–437. [Google Scholar] [CrossRef]
- Garbarienė, I.; Šapolaitė, J.; Garbaras, A.; Ežerinskis, Ž.; Pocevičius, M.; Krikščikas, L.; Plukis, A.; Remeikis, V. Origin Identification of Carbonaceous Aerosol Particles by Carbon Isotope Ratio Analysis. Aerosol. Air Qual. Res. 2016, 16, 1356–1365. [Google Scholar] [CrossRef]
- Garbaras, A.; Šapolaitė, J.; Garbarienė, I.; Ežerinskis, Ž.; Mašalaitė-Nalivaikė, A.; Skipitytė, R.; Plukis, A.; Remeikis, V. Aerosol source (biomass, traffic and coal emission) apportionment in Lithuania using stable carbon and radiocarbon analysis. Isot. Environ. Health Stud. 2018, 54, 463–474. [Google Scholar] [CrossRef]
- Dusek, U.; Ten Brink, H.M.; Meijer, H.A.J.; Kos, G.; Mrozek, D.; Röckmann, T.; Holzinger, R.; Weijers, E.P. The contribution of fossil sources to the organic aerosol in the Netherlands. Atmos. Environ. 2013, 74, 169–176. [Google Scholar] [CrossRef]
- Widory, D. Combustibles, fuels and their combustion products: A view through carbon isotopes. Combust. Theory Model. 2006, 10, 831–841. [Google Scholar] [CrossRef]
- Aguilera, J.; Whigham, L.D. Using the 13C/12C carbon isotope ratio to characterise the emission sources of airborne particulate matter: A review of literature. Isot. Environ. Health Stud. 2018, 54, 573–587. [Google Scholar] [CrossRef]
- Górka, M.; Skrzypek, G.; Hałas, S.; Jędrysek, M.-O.; Strąpoć, D. Multi-seasonal pattern in 5-year record of stable H, O and S isotope compositions of precipitation (Wrocław, SW Poland). Atmos. Environ. 2017, 158, 197–210. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, K.; Qiu, P.; Xu, M.; Guo, Z. Quantifying SO2 oxidation pathways to atmospheric sulfate using stable sulfur and oxygen isotopes: Laboratory simulation and field observation. Atmos. Chem. Phys. 2024, 24, 2195–2205. [Google Scholar] [CrossRef]
- Kawamura, H.; Matsuoka, N.; Tawaki, S.; Momoshima, N. Sulfur Isotope Variations in Atmospheric Sulfur Oxides, Particulate Matter and Deposits Collected at Kyushu Island, Japan. Water Air Soil. Pollut. 2001, 130, 1775–1780. [Google Scholar] [CrossRef]
- Mukai, H.; Tanaka, A.; Fujii, T.; Zeng, Y.; Hong, Y.; Tang, J.; Guo, S.; Xue, H.; Sun, Z.; Zhou, J.; et al. Regional Characteristics of Sulfur and Lead Isotope Ratios in the Atmosphere at Several Chinese Urban Sites. Environ. Sci. Technol. 2001, 35, 1064–1071. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Farquhar, J.; Kaufman, A.J.; Wu, N.; Li, C.; Dickerson, R.R.; Wang, P. Identification of sources and formation processes of atmospheric sulfate by sulfur isotope and scanning electron microscope measurements. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Inomata, Y.; Ohizumi, T.; Take, N.; Sato, K.; Nishikawa, M. Transboundary transport of anthropogenic sulfur in PM2.5 at a coastal site in the Sea of Japan as studied by sulfur isotopic ratio measurement. Sci. Total Environ. 2016, 553, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.L.; Barrie, L.A.; Toom-Sauntry, D.; Sirois, A.; Krouse, H.R.; Li, S.M.; Sharma, S. Sources of aerosol sulphate at Alert: Apportionment using stable isotopes. J. Geophys. Res. Atmos. 1999, 104, 11619–11631. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Yu, M.; Xie, F.; Zhang, Y. Anthropogenic Emission Sources of Sulfate Aerosols in Hangzhou, East China: Insights from Isotope Techniques with Consideration of Fractionation Effects between Gas-to-Particle Transformations. Environ. Sci. Technol. 2022, 56, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, Q.; Liu, C.; Fu, P.; Strauss, H.; Yang, J.; Hu, J.; Wei, L.; Ren, H.; Peters, M.; et al. Using stable isotopes to trace sources and formation processes of sulfate aerosols from Beijing, China. Sci. Rep. 2016, 6, 29958. [Google Scholar] [CrossRef]
- Dusek, U.; Hitzenberger, R.; Kasper-Giebl, A.; Kistler, M.; Meijer, H.A.J.; Szidat, S.; Wacker, L.; Holzinger, R.; Röckmann, T. Sources and formation mechanisms of carbonaceous aerosol at a regional background site in the Netherlands: Insights from a year-long radiocarbon study. Atmos. Chem. Phys. 2017, 17, 3233–3251. [Google Scholar] [CrossRef]
- Genberg, J.; Hyder, M.; Stenström, K.; Bergström, R.; Simpson, D.; Fors, E.O.; Jönsson, J.; Swietlicki, E. Source apportionment of carbonaceous aerosol in southern Sweden. Atmos. Chem. Phys. 2011, 11, 11387–11400. [Google Scholar] [CrossRef]
- Zotter, P.; Ciobanu, V.G.; Zhang, Y.L.; El-Haddad, I.; Macchia, M.; Daellenbach, K.R.; Salazar, G.A.; Huang, R.-J.; Wacker, L.; Hueglin, C.; et al. Radiocarbon analysis of elemental and organic carbon in Switzerland during winter-smog episodes from 2008 to 2012—Part 1: Source apportionment and spatial variability. Atmos. Chem. Phys. 2014, 14, 13551–13570. [Google Scholar] [CrossRef]
- Garbaras, A.; Rimšelyte, I.; Kvietkus, K.; Remeikis, V. Δ13C Values in Size-Segregated Atmospheric Carbonaceous Aerosols at a Rural Site in Lithuania. Lith. J. Phys. 2009, 49, 229–236. [Google Scholar] [CrossRef][Green Version]
- Schmid, H.; Laskus, L.; Jürgen Abraham, H.; Baltensperger, U.; Lavanchy, V.; Bizjak, M.; Burba, P.; Cachier, H.; Crow, D.; Chow, J.; et al. Results of the “carbon conference” international aerosol carbon round robin test stage I. Atmos. Environ. 2001, 35, 2111–2121. [Google Scholar] [CrossRef]
- Kawamura, K.; Kobayashi, M.; Tsubonuma, N.; Mochida, M.; Watanabe, T.; Lee, M. Organic and Inorganic Compositions of Marine Aerosols from East Asia: Seasonal Variations of Water-Soluble Dicarboxylic Acids, Major Ions, Total Carbon and Nitrogen, and Stable C and N Isotopic Composition. Geochem. Soc. Spec. Publ. 2004, 9, S1873–S9881. [Google Scholar]
- Masalaite, A.; Remeikis, V.; Zenker, K.; Westra, I.; Meijer, H.; Dusek, U. Seasonal changes of sources and volatility of carbonaceous aerosol at urban, coastal and forest sites in Eastern Europe (Lithuania). Atmos. Environ. 2020, 225, 117374. [Google Scholar] [CrossRef]
- Šapolaitė, J.; Garbarienė, I.; Garbaras, A.; Bučinskas, L.; Pabedinskas, A.; Remeikis, V.; Ežerinskis, Ž. Development of graphitization method for low carbon aerosol filter samples with Automated Graphitization System AGE-3. Appl. Radiat. Isot. 2022, 190, 110461. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Discussion Reporting of 14C Data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Niu, Z.; Feng, X.; Zhou, W.; Wang, P.; Liu, Y.; Lu, X.; Du, H.; Fu, Y.; Li, M.; Mei, R.; et al. Tree-ring Δ14C time series from 1948 to 2018 at a regional background site, China: Influences of atmospheric nuclear weapons tests and fossil fuel emissions. Atmos. Environ. 2020, 246, 118156. [Google Scholar] [CrossRef]
- Romano, S.; Pichierri, S.; Fragola, M.; Buccolieri, A.; Quarta, G.; Calcagnile, L. Characterization of the PM2.5 aerosol fraction monitored at a suburban site in south-eastern Italy by integrating isotopic techniques and ion beam analysis. Front. Environ. Sci. 2022, 10, 971204. [Google Scholar] [CrossRef]
- Heal, M.R.; Naysmith, P.; Cook, G.T.; Xu, S.; Duran, T.R.; Harrison, R.M. Application of 14C analyses to source apportionment of carbonaceous PM2.5 in the UK. Atmos. Environ. 2011, 45, 2341–2348. [Google Scholar] [CrossRef]
- Levin, I.; Hesshaimer, V. Radiocarbon—A Unique Tracer of Global Carbon Cycle Dynamics. Radiocarbon 2000, 42, 69–80. [Google Scholar] [CrossRef]
- NOAA. Climate Data Online (CDO). Available online: https://www.ncei.noaa.gov/cdo-web/ (accessed on 17 March 2024).
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT Atmospheric Transport and Dispersion Modeling System. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Davuliene, L.; Jasineviciene, D.; Garbariene, I.; Andriejauskiene, J.; Ulevicius, V.; Bycenkiene, S. Long-term air pollution trend analysis in the South-eastern Baltic region, 1981–2017. Atmos. Res. 2020, 247, 105191. [Google Scholar] [CrossRef]
- AB Vilniaus šilumos tinklai. PEOPLE CREATE HEAT. 2021. Available online: https://chc.lt/data/public/uploads/2022/06/ifrs-vst-en-2021-final.pdf (accessed on 12 May 2024).
- Harrison, R.M.; Jones, A.M.; Beddows, D.C.S.; Derwent, R.G. The effect of varying primary emissions on the concentrations of inorganic aerosols predicted by the enhanced UK Photochemical Trajectory Model. Atmos. Environ. 2013, 69, 211–218. [Google Scholar] [CrossRef]
- Tsimpidi, A.P.; Karydis, V.A.; Pandis, S.N. Response of Inorganic Fine Particulate Matter to Emission Changes of Sulfur Dioxide and Ammonia: The Eastern United States as a Case Study. J. Air Waste Manag. Assoc. 2007, 57, 1489–1498. [Google Scholar] [CrossRef]
- Pathak, R.K.; Yao, X.; Chan, C.K. Sampling Artifacts of Acidity and Ionic Species in PM2.5. Environ. Sci. Technol. 2003, 38, 254–259. [Google Scholar] [CrossRef]
- Steinfeld, J.I. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. Environ. Sci. Policy Sustain. Dev. 1998, 40, 26. [Google Scholar] [CrossRef]
- Niemi, J.V.; Tervahattu, H.; Vehkamäki, H.; Kulmala, M.; Koskentalo, T.; Sillanpää, M.; Rantamäki, M. Characterization and source identification of a fine particle episode in Finland. Atmos. Environ. 2004, 38, 5003–5012. [Google Scholar] [CrossRef]
- Viana, M.; Reche, C.; Amato, F.; Alastuey, A.; Querol, X.; Moreno, T.; Lucarelli, F.; Nava, S.; Calzolai, G.; Chiari, M.; et al. Evidence of biomass burning aerosols in the Barcelona urban environment during winter time. Atmos. Environ. 2013, 72, 81–88. [Google Scholar] [CrossRef]
- Li, J.; Pósfai, M.; Hobbs, P.V.; Buseck, P.R. Individual aerosol particles from biomass burning in southern Africa: 2, Compositions and aging of inorganic particles. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Liu, X.; Van Espen, P.; Adams, F.; Cafmeyer, J.; Maenhaut, W. Biomass Burning in Southern Africa: Individual Particle Characterization of Atmospheric Aerosols and Savanna Fire Samples. J. Atmos. Chem. 2000, 36, 135–155. [Google Scholar] [CrossRef]
- Christensen, K.A.; Stenholm, M.; Livbjerg, H. The formation of submicron aerosol particles, HCl and SO2 in straw-fired boilers. J. Aerosol Sci. 1998, 29, 421–444. [Google Scholar] [CrossRef]
- Lee, C.; Martin, R.V.; Van Donkelaar, A.; Lee, H.; Dickerson, R.R.; Hains, J.C.; Krotkov, N.; Richter, A.; Vinnikov, K.; Schwab, J.J. SO2 emissions and lifetimes: Estimates from inverse modeling using in situ and global, space-based (SCIAMACHY and OMI) observations. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Sawlani, R.; Agnihotri, R.; Sharma, C.; Patra, P.K.; Dimri, A.P.; Ram, K.; Verma, R.L. The severe Delhi SMOG of 2016: A case of delayed crop residue burning, coincident firecracker emissions, and atypical meteorology. Atmos. Pollut. Res. 2018, 10, 868–879. [Google Scholar] [CrossRef]
- Norman, A.; Belzer, W.; Barrie, L. Insights into the biogenic contribution to total sulphate in aerosol and precipitation in the Fraser Valley afforded by isotopes of sulphur and oxygen. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Bučinskas, L.; Garbarienė, I.; Mašalaitė, A.; Šapolaitė, J.; Ežerinskis, Ž.; Jasinevičienė, D.; Remeikis, V.; Garbaras, A. Dual carbon and sulfur isotopes as tracers of PM1 pollution sources after COVID-19 confinement in Vilnius, Lithuania. Urban. Clim. 2024, 55, 101894. [Google Scholar] [CrossRef]
- Bertelsen, N.; Mathiesen, B.V. EU-28 Residential Heat Supply and Consumption: Historical Development and Status. Energies 2020, 13, 1894. [Google Scholar] [CrossRef]
- Igliński, B.; Piechota, G.; Buczkowski, R. Development of biomass in polish energy sector: An overview. Clean. Technol. Environ. Policy 2015, 17, 317–329. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Kang, S.; Yan, F.; Hu, Z.; Chen, P.; Huang, G.; Li, C.; Stubbins, A. Stable Carbon Isotope Signatures of Carbonaceous Aerosol Endmembers in the Tibetan Plateau. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Xu, H.; Wu, D.; Liu, Y.; Yang, H. Isotopic compositions of elemental carbon in smoke and ash derived from crop straw combustion. Atmos. Environ. 2014, 92, 303–308. [Google Scholar] [CrossRef]
- Garbaras, A.; Garbarienė, I.; Bučinskas, L.; Šapolaitė, J.; Ežerinskis, Ž.; Matijošius, J.; Rimkus, A.; Remeikis, V. Characterization of Particulate Matter Emissions from Internal Combustion Engines Using Δ13C Values: Impact of Engine Operation Conditions and Fuel Type on PM10 Isotopic Composition. Atmos. Pollut. Res. 2023, 14, 101868. [Google Scholar] [CrossRef]
- Yao, P.; Huang, R.-J.; Ni, H.; Kairys, N.; Yang, L.; Meijer, H.A.J.; Dusek, U. 13C signatures of aerosol organic and elemental carbon from major combustion sources in China compared to worldwide estimates. Sci. Total Environ. 2022, 810, 151284. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Haneishi, Y. Effects of Combustion Emissions from the Eurasian Continent in Winter on Seasonal Δ13C of Elemental Carbon in Aerosols in Japan. Atmos. Environ. 2012, 46, 568–579. [Google Scholar] [CrossRef]
- Szidat, S.; Jenk, T.M.; Gäggeler, H.W.; Synal, H.-A.; Fisseha, R.; Baltensperger, U.; Kalberer, M.; Samburova, V.; Wacker, L.; Saurer, M.; et al. Source Apportionment of Aerosols by 14C Measurements in Different Carbonaceous Particle Fractions. Proc. Radiocarb. 2004, 46, 475–484. [Google Scholar] [CrossRef]
- Bernardoni, V.; Calzolai, G.; Chiari, M.; Fedi, M.; Lucarelli, F.; Nava, S.; Piazzalunga, A.; Riccobono, F.; Taccetti, F.; Valli, G.; et al. Radiocarbon analysis on organic and elemental carbon in aerosol samples and source apportionment at an urban site in Northern Italy. J. Aerosol Sci. 2013, 56, 88–99. [Google Scholar] [CrossRef]
- Becker, S.; Hirner, A.V. Characterisation of Crude Oils by Carbon and Sulphur Isotope Ratio Measurements as a Tool for Pollution Control. Isot. Environ. Health Stud. 1998, 34, 255–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bučinskas, L.; Garbarienė, I.; Mašalaitė, A.; Šapolaitė, J.; Ežerinskis, Ž.; Jasinevičienė, D.; Garbaras, A. Evaluating the Impact of Increased Heavy Oil Consumption on Urban Pollution Levels through Isotope (δ13C, δ34S, 14C) Composition. Atmosphere 2024, 15, 883. https://doi.org/10.3390/atmos15080883
Bučinskas L, Garbarienė I, Mašalaitė A, Šapolaitė J, Ežerinskis Ž, Jasinevičienė D, Garbaras A. Evaluating the Impact of Increased Heavy Oil Consumption on Urban Pollution Levels through Isotope (δ13C, δ34S, 14C) Composition. Atmosphere. 2024; 15(8):883. https://doi.org/10.3390/atmos15080883
Chicago/Turabian StyleBučinskas, Laurynas, Inga Garbarienė, Agnė Mašalaitė, Justina Šapolaitė, Žilvinas Ežerinskis, Dalia Jasinevičienė, and Andrius Garbaras. 2024. "Evaluating the Impact of Increased Heavy Oil Consumption on Urban Pollution Levels through Isotope (δ13C, δ34S, 14C) Composition" Atmosphere 15, no. 8: 883. https://doi.org/10.3390/atmos15080883
APA StyleBučinskas, L., Garbarienė, I., Mašalaitė, A., Šapolaitė, J., Ežerinskis, Ž., Jasinevičienė, D., & Garbaras, A. (2024). Evaluating the Impact of Increased Heavy Oil Consumption on Urban Pollution Levels through Isotope (δ13C, δ34S, 14C) Composition. Atmosphere, 15(8), 883. https://doi.org/10.3390/atmos15080883






