Organic Vapors from Residential Biomass Combustion: Emission Characteristics and Conversion to Secondary Organic Aerosols
Abstract
1. Introduction
2. Materials and Methods
2.1. Combustion and Aging Experiments
2.2. Sampling and Analysis
2.3. Parameterizations of NMOC Volatility
3. Results and Discussion
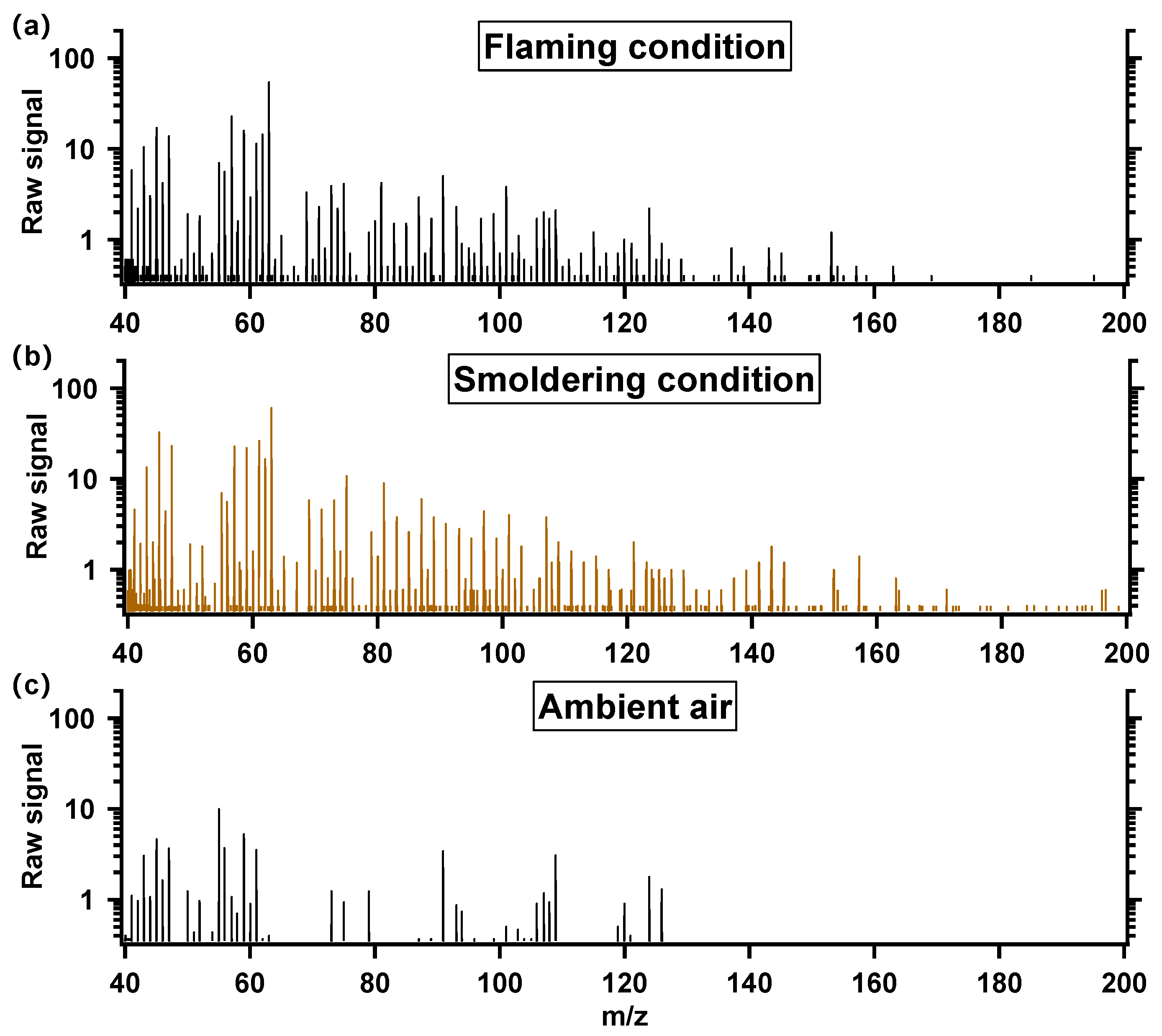
3.1. NMOC Spectrum of Residential Biomass Combustion Emissions
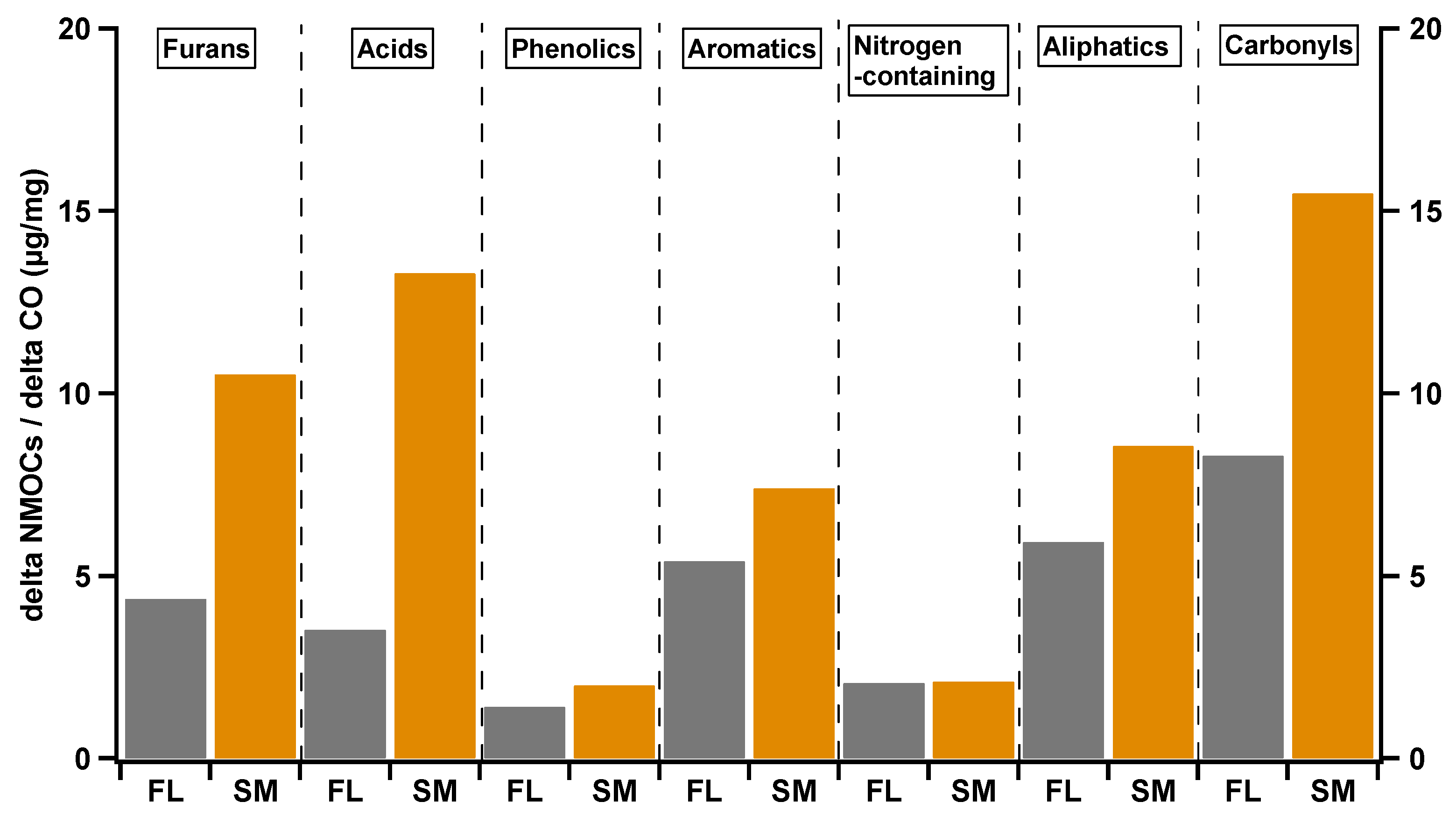
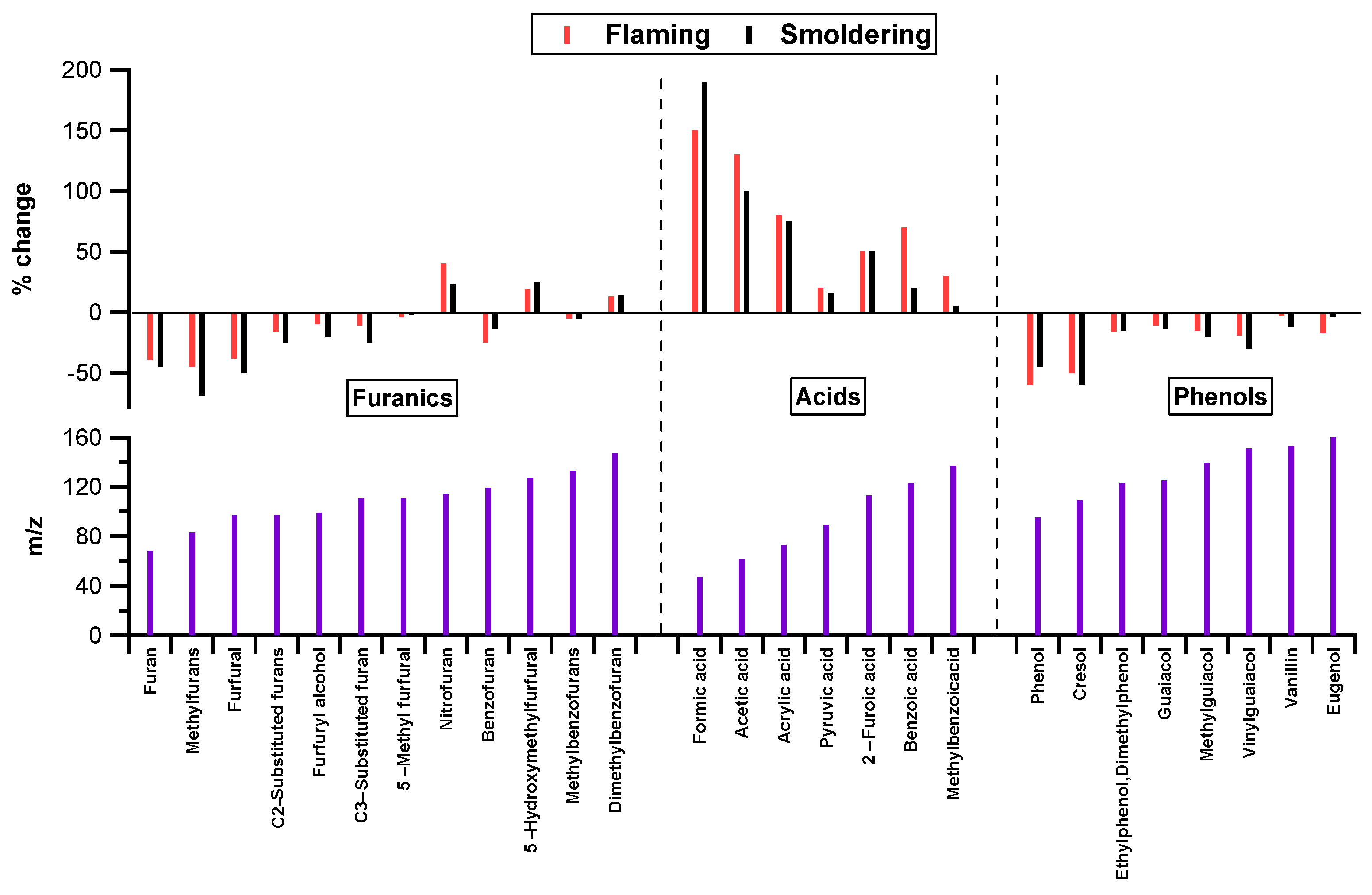
3.2. Emission Characteristics of NMOCs at Different Combustion Conditions
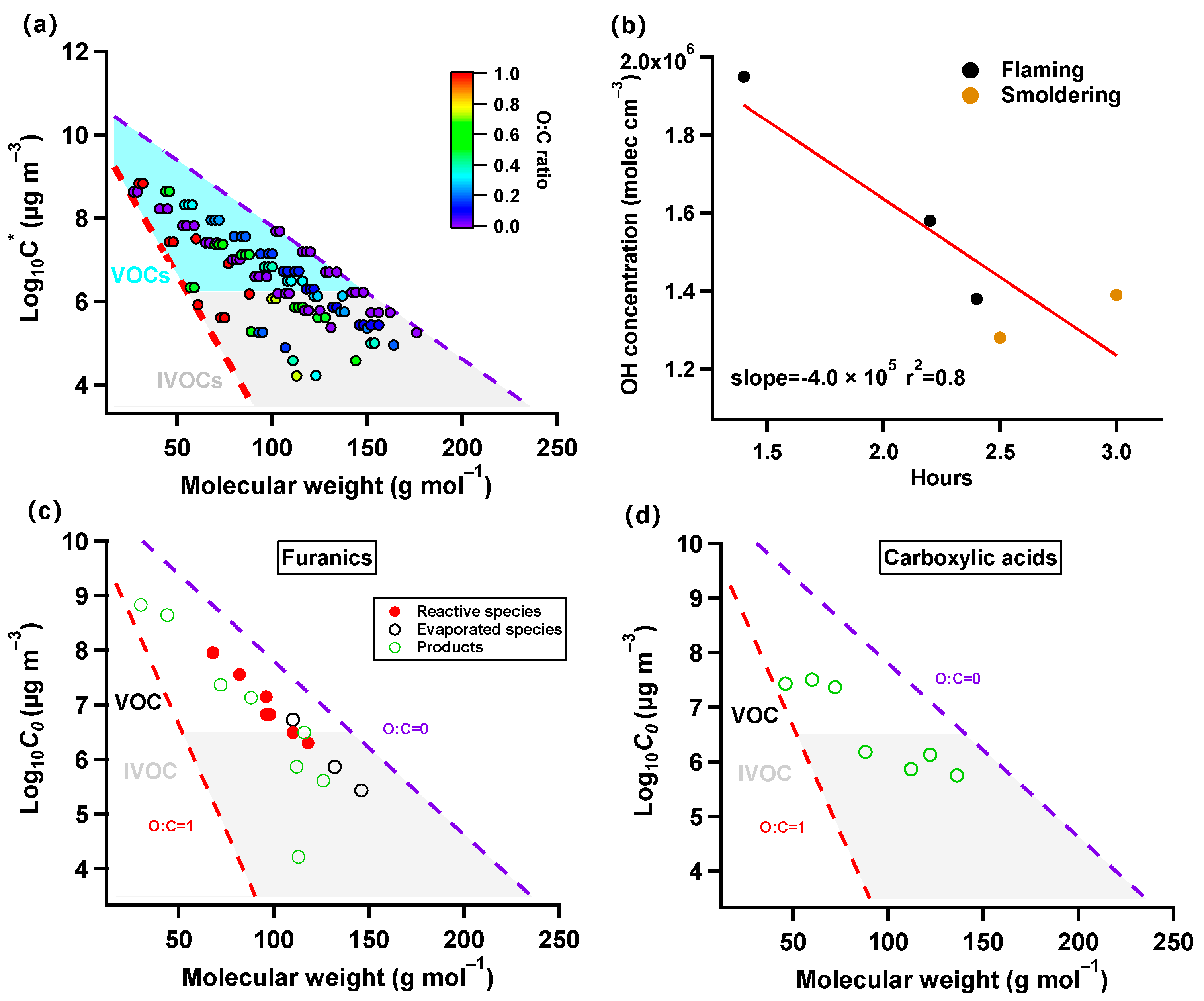
3.3. Transformation of NMOCs during Photochemical Aging
3.4. Implications for Residential Biomass Burning SOA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jathar, S.H.; Gordon, T.D.; Hennigan, C.J.; Pye, H.O.; Pouliot, G.; Adams, P.J.; Donahue, N.M.; Robinson, A.L. Unspeciated organic emissions from combustion sources and their influence on the secondary organic aerosol budget in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 10473–10478. [Google Scholar] [CrossRef] [PubMed]
- Butt, E.W.; Rap, A.; Schmidt, A.; Scott, C.E.; Pringle, K.J.; Reddington, C.L.; Richards, N.A.D.; Woodhouse, M.T.; Ramirez-Villegas, J.; Yang, H.; et al. The impact of residential combustion emissions on atmospheric aerosol, human health, and climate. Atmos. Chem. Phys. 2016, 16, 873–905. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef]
- Cai, S.; Wang, Y.; Zhao, B.; Wang, S.; Chang, X.; Hao, J. The impact of the “Air Pollution Prevention and Control Action Plan” on PM2.5 concentrations in Jing-Jin-Ji region during 2012–2020. Sci. Total. Environ. 2017, 580, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.J.S.; Lea-Langton, A.R.; Jones, J.M.; Williams, A.; Layden, P.; Johnson, R. The impact of fuel properties on the emissions from the combustion of biomass and other solid fuels in a fixed bed domestic stove. Fuel Process Technol. 2016, 142, 115–123. [Google Scholar] [CrossRef]
- Elsasser, M.; Busch, C.; Orasche, J.; Schön, C.; Hartmann, H.; Schnelle-Kreis, J.; Zimmermann, R. Dynamic Changes of the Aerosol Composition and Concentration during Different Burning Phases of Wood Combustion. Energy Fuel 2013, 27, 4959–4968. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Galbally, I.E. Known and unexplored organic constituents in the earth’s atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Berkemeier, T.; Schilling-Fahnestock, K.A.; Seinfeld, J.H.; Pöschl, U. Molecular corridors and kinetic regimes in the multiphase chemical evolution of secondary organic aerosol. Atmos. Chem. Phys. 2014, 14, 8323–8341. [Google Scholar] [CrossRef]
- Donahue, N.M.; Robinson, A.L.; Stanier, C.O.; Pandis, S.N. Coupled partitioning, dilution, and dhemical aging of semivolatile organics. Environ. Sci. Technol. 2006, 40, 2635–2643. [Google Scholar] [CrossRef]
- Kroll, J.H.; Smith, J.D.; Che, D.L.; Kessler, S.H.; Worsnop, D.R.; Wilson, K.R. Measurement of fragmentation and functionalization pathways in the heterogeneous oxidation of oxidized organic aerosol. Phys. Chem. Chem. Phys. 2009, 11, 8005–8014. [Google Scholar] [CrossRef] [PubMed]
- Jathar, S.; Cappa, C.; Wexler, A.; Seinfeld, J.; Kleeman, M.J. Multi-generational oxidation model to simulate secondary organic aerosol in a 3-D air quality model. Geosci. Model. Dev. 2015, 8, 1857–1891. [Google Scholar] [CrossRef]
- He, K.; Fu, T.; Zhang, B.; Xu, H.; Sun, J.; Zou, H.; Zhang, Z.; Hang Ho, S.S.; Cao, J.; Shen, Z. Examination of long-time aging process on volatile organic compounds emitted from solid fuel combustion in a rural area of China. Chemosphere 2023, 333, 138957. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Mukhopadhyay, K.; Thangaswamy, D.; Natarajan, A.; Chakraborty, D. Characterisation of indoor volatile organic compounds and its association with respiratory symptoms among children living in solid fuel using households in Tamil Nadu, India. Mapan 2022, 37, 565–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, S.; Sheng, J.; Zhao, D.; Ding, D.; Yao, L.; Zheng, H.; Wu, J.; Cheng, Y.; Yan, Q.; et al. Real-time emission and stage-dependent emission factors/ratios of specific volatile organic compounds from residential biomass combustion in China. Atmos. Res. 2021, 248, 105189. [Google Scholar] [CrossRef]
- Vakkari, V.; Kerminen, V.-M.; Beukes, J.P.; Tiitta, P.; van Zyl, P.G.; Josipovic, M.; Venter, A.D.; Jaars, K.; Worsnop, D.R.; Kulmala, M.; et al. Rapid changes in biomass burning aerosols by atmospheric oxidation. Geophys. Res. Lett. 2014, 41, 2644–2651. [Google Scholar] [CrossRef]
- Ortega, A.M.; Day, D.A.; Cubison, M.J.; Brune, W.H.; Bon, D.; de Gouw, J.A.; Jimenez, J.L. Secondary organic aerosol formation and primary organic aerosol oxidation from biomass-burning smoke in a flow reactor during FLAME-3. Atmos. Chem. Phys. 2013, 13, 11551–11571. [Google Scholar] [CrossRef]
- Shrivastava, M.; Cappa, C.D.; Fan, J.; Goldstein, A.H.; Guenther, A.B.; Jimenez, J.L.; Kuang, C.; Laskin, A.; Martin, S.T.; Ng, N.L.; et al. Recent advances in understanding secondary organic aerosol: Implications for global climate forcing. Rev. Geophys. 2017, 55, 509–559. [Google Scholar] [CrossRef]
- Niu, X.; Li, J.; Wang, Q.; Ho, S.S.H.; Sun, J.; Li, L.; Cao, J.; Ho, K.F. Characteristics of fresh and aged volatile organic compounds from open burning of crop residues. Sci. Total. Environ. 2020, 726, 138545. [Google Scholar] [CrossRef]
- Romanias, M.N.; Coggon, M.M.; Al Ali, F.; Burkholder, J.B.; Dagaut, P.; Decker, Z.; Warneke, C.; Stockwell, C.E.; Roberts, J.M.; Tomas, A.; et al. Emissions and atmospheric chemistry of furanoids from biomass burning: Insights from laboratory to atmospheric observations. ACS Earth Space Chem. 2024, 8, 857–899. [Google Scholar] [CrossRef]
- Gilman, J.B.; Lerner, B.M.; Kuster, W.C.; Goldan, P.D.; Warneke, C.; Veres, P.R.; Roberts, J.M.; de Gouw, J.A.; Burling, I.R.; Yokelson, R.J. Biomass burning emissions and potential air quality impacts of volatile organic compounds and other trace gases from fuels common in the US. Atmos. Chem. Phys. 2015, 15, 13915–13938. [Google Scholar] [CrossRef]
- Hatch, L.E.; Luo, W.; Pankow, J.F.; Yokelson, R.J.; Stockwell, C.E.; Barsanti, K.C. Identification and quantification of gaseous organic compounds emitted from biomass burning using two-dimensional gas chromatography–time-of-flight mass spectrometry. Atmos. Chem. Phys. 2015, 15, 1865–1899. [Google Scholar] [CrossRef]
- Bhattu, D.; Zotter, P.; Zhou, J.; Stefenelli, G.; Klein, F.; Bertrand, A.; Temime-Roussel, B.; Marchand, N.; Slowik, J.G.; Baltensperger, U.; et al. Effect of stove technology and combustion conditions on gas and particulate emissions from residential biomass combustion. Environ. Sci. Technol. 2019, 53, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.A.; Slowik, J.G.; El Haddad, I.; Kilic, D.; Klein, F.; Dommen, J.; Temime-Roussel, B.; Marchand, N.; Baltensperger, U.; Prévôt, A.S.H. Characterization of gas-phase organics using proton transfer reaction time-of-flight mass spectrometry: Fresh and aged residential wood combustion emissions. Atmos. Chem. Phys. 2017, 17, 705–720. [Google Scholar] [CrossRef]
- Desservettaz, M.; Pikridas, M.; Stavroulas, I.; Bougiatioti, A.; Liakakou, E.; Hatzianastassiou, N.; Sciare, J.; Mihalopoulos, N.; Bourtsoukidis, E. Emission of volatile organic compounds from residential biomass burning and their rapid chemical transformations. Sci. Total. Environ. 2023, 903, 166592. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, C.E.; Veres, P.R.; Williams, J.; Yokelson, R.J. Characterization of biomass burning emissions from cooking fires, peat, crop residue, and other fuels with high-resolution proton-transfer-reaction time-of-flight mass spectrometry. Atmos. Chem. Phys. 2015, 15, 845–865. [Google Scholar] [CrossRef]
- Yokelson, R.J.; Burling, I.R.; Gilman, J.B.; Warneke, C.; Stockwell, C.E.; de Gouw, J.; Akagi, S.K.; Urbanski, S.P.; Veres, P.; Roberts, J.M.; et al. Coupling field and laboratory measurements to estimate the emission factors of identified and unidentified trace gases for prescribed fires. Atmos. Chem. Phys. 2013, 13, 89–116. [Google Scholar] [CrossRef]
- Donahue, N.M.; Robinson, A.L.; Pandis, S.N. Atmospheric organic particulate matter: From smoke to secondary organic aerosol. Atmos. Environ. 2009, 43, 94–106. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.; Donahue, N.; Prevot, A.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.J.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wu, Y.; Hu, K.; Jiang, X.; Tian, P.; Sheng, J.; Pan, B.; Zhao, D. Aging effects on residential biomass burning emissions under quasi-real atmospheric conditions. Environ. Pollut. 2023, 337, 122615. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Hu, D.; Kong, S.; Wu, Y.; Ding, S.; Cheng, Y.; Qiu, H.; Zheng, S.; Yan, Q.; et al. Evolution of organic aerosol from wood smoke influenced by burning phase and solar radiation. J. Geophys. Res.-Atmos. 2021, 126, e2021JD034534. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, D.; Tian, P.; Sheng, J.; Liu, Q.; Li, R.; Hu, K.; Jiang, X.; Li, S.; Bi, K.; et al. Tracing the formation of secondary aerosols influenced by solar radiation and relative humidity in suburban environment. J. Geophys. Res.-Atmos. 2022, 127, e2022JD036913. [Google Scholar] [CrossRef]
- Bruns, E.A.; El Haddad, I.; Slowik, J.G.; Kilic, D.; Klein, F.; Baltensperger, U.; Prévôt, A.S.H. Identification of significant precursor gases of secondary organic aerosols from residential wood combustion. Sci. Rep. 2016, 6, 27881. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, A.; Yli-Pirilä, P.; Tiitta, P.; Leskinen, A.; Kortelainen, M.; Orasche, J.; Schnelle-Kreis, J.; Lehtinen, K.E.J.; Zimmermann, R.; Jokiniemi, J.; et al. Volatile organic compounds from logwood combustion: Emissions and transformation under dark and photochemical aging conditions in a smog chamber. Environ. Sci. Technol. 2018, 52, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Kautzman, K.E.; Loza, C.L.; Schilling, K.A.; Coggon, M.M.; Chhabra, P.S.; Chan, M.N.; Chan, A.W.H.; Hersey, S.P.; Crounse, J.D.; et al. Secondary organic aerosol formation from biomass burning intermediates: Phenol and methoxyphenols. Atmos. Chem. Phys. 2013, 13, 8019–8043. [Google Scholar] [CrossRef]
- Fang, Z.; Deng, W.; Zhang, Y.; Ding, X.; Tang, M.; Liu, T.; Hu, Q.; Zhu, M.; Wang, Z.; Yang, W.; et al. Open burning of rice, corn and wheat straws: Primary emissions, photochemical aging, and secondary organic aerosol formation. Atmos. Chem. Phys. 2017, 17, 14821–14839. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Kautzman, K.E.; Chhabra, P.S.; Surratt, J.D.; Chan, M.N.; Crounse, J.D.; Kürten, A.; Wennberg, P.O.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from photooxidation of naphthalene and alkylnaphthalenes: Implications for oxidation of intermediate volatility organic compounds (IVOCs). Atmos. Chem. Phys. 2009, 9, 3049–3060. [Google Scholar] [CrossRef]
- Donahue, N.M.; Epstein, S.A.; Pandis, S.N.; Robinson, A.L. A two-dimensional volatility basis set: 1. organic-aerosol mixing thermodynamics. Atmos. Chem. Phys. 2011, 11, 3303–3318. [Google Scholar] [CrossRef]
- Li, Y.; Pöschl, U.; Shiraiwa, M. Molecular corridors and parameterizations of volatility in the chemical evolution of organic aerosols. Atmos. Chem. Phys. 2016, 16, 3327–3344. [Google Scholar] [CrossRef]
- Akagi, S.K.; Yokelson, R.J.; Wiedinmyer, C.; Alvarado, M.J.; Reid, J.S.; Karl, T.; Crounse, J.D.; Wennberg, P.O. Emission factors for open and domestic biomass burning for use in atmospheric models. Atmos. Chem. Phys. 2011, 11, 4039–4072. [Google Scholar] [CrossRef]
- Sinha, P.; Hobbs, P.V.; Yokelson, R.J.; Bertschi, I.T.; Blake, D.R.; Simpson, I.J.; Gao, S.; Kirchstetter, T.W.; Novakov, T. Emissions of trace gases and particles from savanna fires in southern Africa. J. Geophys. Res.-Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Stefenelli, G.; Jiang, J.; Bertrand, A.; Bruns, E.A.; Pieber, S.M.; Baltensperger, U.; Marchand, N.; Aksoyoglu, S.; Prévôt, A.S.H.; Slowik, J.G.; et al. Secondary organic aerosol formation from smoldering and flaming combustion of biomass: A box model parametrization based on volatility basis set. Atmos. Chem. Phys. 2019, 19, 11461–11484. [Google Scholar] [CrossRef]
- Parandaman, A.; Kumar, M.; Francisco, J.S.; Sinha, A. Organic acid formation from the atmospheric oxidation of gem diols: Reaction mechanism, energetics, and rates. J. Phys. Chem. A 2018, 122, 6266–6276. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-M.; Jacob, D.J.; Wittrock, F.; Burrows, J.P.; Vrekoussis, M.; Henze, D.K. Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols. J. Geophys. Res.-Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Kawamura, K.; Okuzawa, K.; Aggarwal, S.G.; Irie, H.; Kanaya, Y.; Wang, Z. Determination of gaseous and particulate carbonyls (glycolaldehyde, hydroxyacetone, glyoxal, methylglyoxal, nonanal and decanal) in the atmosphere at Mt. Tai. Atmos. Chem. Phys. 2013, 13, 5369–5380. [Google Scholar] [CrossRef]
- Andreae, M.O.; Merlet, P. Emission of trace gases and aerosols from biomass burning. Glob. Biogeochem. Cycles 2001, 15, 955–966. [Google Scholar] [CrossRef]
- Henze, D.K.; Seinfeld, J.H.; Ng, N.L.; Kroll, J.H.; Fu, T.M.; Jacob, D.J.; Heald, C.L. Global modeling of secondary organic aerosol formation from aromatic hydrocarbons: High- vs. low-yield pathways. Atmos. Chem. Phys. 2008, 8, 2405–2420. [Google Scholar] [CrossRef]
- Phousongphouang, P.T.; Arey, J. Rate constants for the gas-phase reactions of a series of alkylnaphthalenes with the OH radical. Environ. Sci. Technol. 2002, 36, 1947–1952. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef]
- Ng, N.L.; Kroll, J.H.; Chan, A.W.H.; Chhabra, P.S.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 2007, 7, 3909–3922. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, M.; Li, X.; Zong, T.; Xu, N.; Hu, S.; Zeng, L.; Chen, S.; Song, Y.; Guo, S.; et al. Aqueous secondary organic aerosol formation attributed to phenols from biomass burning. Sci. Total. Environ. 2022, 847, 157582. [Google Scholar] [CrossRef] [PubMed]
- Hatch, L.E.; Yokelson, R.J.; Stockwell, C.E.; Veres, P.R.; Simpson, I.J.; Blake, D.R.; Orlando, J.J.; Barsanti, K.C. Multi-instrument comparison and compilation of non-methane organic gas emissions from biomass burning and implications for smoke-derived secondary organic aerosol precursors. Atmos. Chem. Phys. 2017, 17, 1471–1489. [Google Scholar] [CrossRef]
- Coggon, M.M.; Lim, C.Y.; Koss, A.R.; Sekimoto, K.; Yuan, B.; Gilman, J.B.; Hagan, D.H.; Selimovic, V.; Zarzana, K.J.; Brown, S.S.; et al. OH chemistry of non-methane organic gases (NMOGs) emitted from laboratory and ambient biomass burning smoke: Evaluating the influence of furans and oxygenated aromatics on ozone and secondary NMOG formation. Atmos. Chem. Phys. 2019, 19, 14875–14899. [Google Scholar] [CrossRef]
- Arathala, P.; Tangtartharakul, C.B.; Sinha, A. Atmospheric ring-closure and dehydration reactions of 1,4-hydroxycarbonyls in the gas phase: The impact of catalysts. J. Phys. Chem. A 2021, 125, 5963–5975. [Google Scholar] [CrossRef] [PubMed]
- Koss, A.R.; Sekimoto, K.; Gilman, J.B.; Selimovic, V.; Coggon, M.M.; Zarzana, K.J.; Yuan, B.; Lerner, B.M.; Brown, S.S.; Jimenez, J.L.; et al. Non-methane organic gas emissions from biomass burning: Identification, quantification, and emission factors from PTR-ToF during the FIREX 2016 laboratory experiment. Atmos. Chem. Phys. 2018, 18, 3299–3319. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, D.D.; Cheung, H.Y.; Lee, A.K.Y.; Chan, C.K. Aqueous-phase photochemical oxidation and direct photolysis of vanillin—A model compound of methoxy phenols from biomass burning. Atmos. Chem. Phys. 2014, 14, 2871–2885. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, S.; Jiang, X.; Wu, Y.; Hu, K. Organic Vapors from Residential Biomass Combustion: Emission Characteristics and Conversion to Secondary Organic Aerosols. Atmosphere 2024, 15, 692. https://doi.org/10.3390/atmos15060692
Li R, Li S, Jiang X, Wu Y, Hu K. Organic Vapors from Residential Biomass Combustion: Emission Characteristics and Conversion to Secondary Organic Aerosols. Atmosphere. 2024; 15(6):692. https://doi.org/10.3390/atmos15060692
Chicago/Turabian StyleLi, Ruijie, Siyuan Li, Xiaotong Jiang, Yangzhou Wu, and Kang Hu. 2024. "Organic Vapors from Residential Biomass Combustion: Emission Characteristics and Conversion to Secondary Organic Aerosols" Atmosphere 15, no. 6: 692. https://doi.org/10.3390/atmos15060692
APA StyleLi, R., Li, S., Jiang, X., Wu, Y., & Hu, K. (2024). Organic Vapors from Residential Biomass Combustion: Emission Characteristics and Conversion to Secondary Organic Aerosols. Atmosphere, 15(6), 692. https://doi.org/10.3390/atmos15060692







