Experimental Study on Evaporation and Micro-Explosion Characteristics of Ethanol and Diesel Blended Droplets
Abstract
1. Introduction
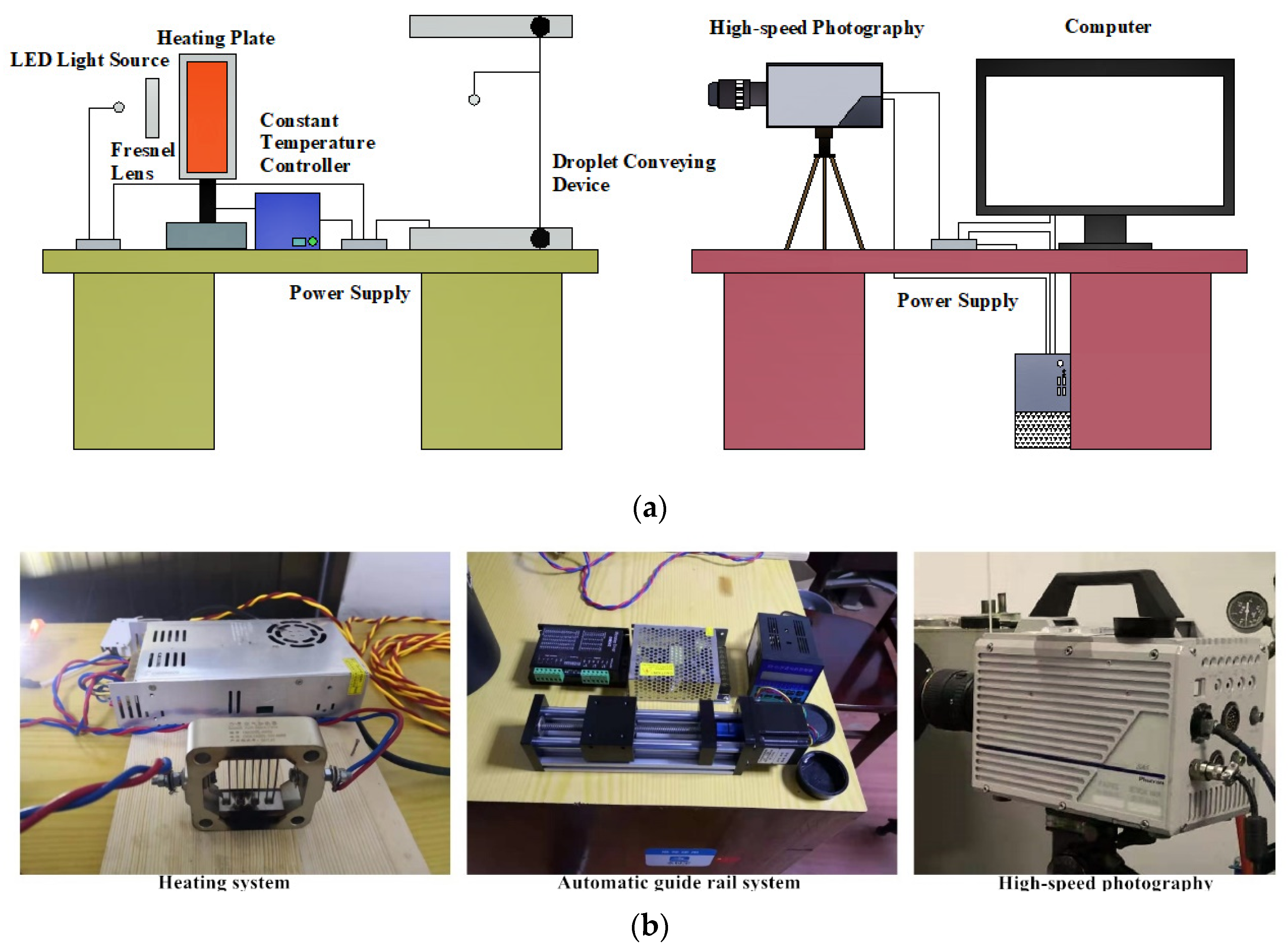
2. Experimental Device and Image Processing Method
2.1. Evaporation Device
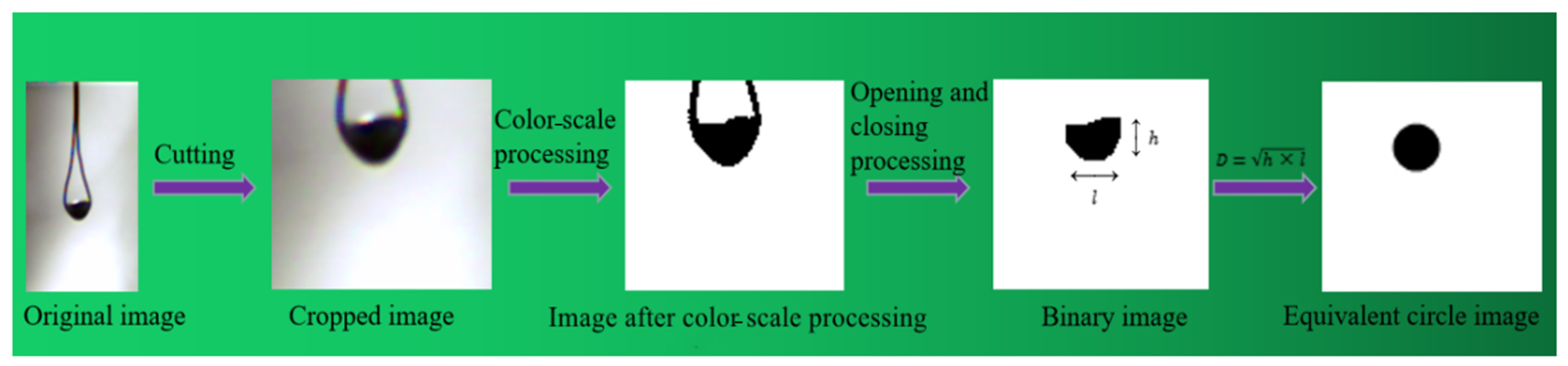
2.2. Image Processing Method
2.3. Fuel Preparation and Physical Properties
3. Results and Discussion
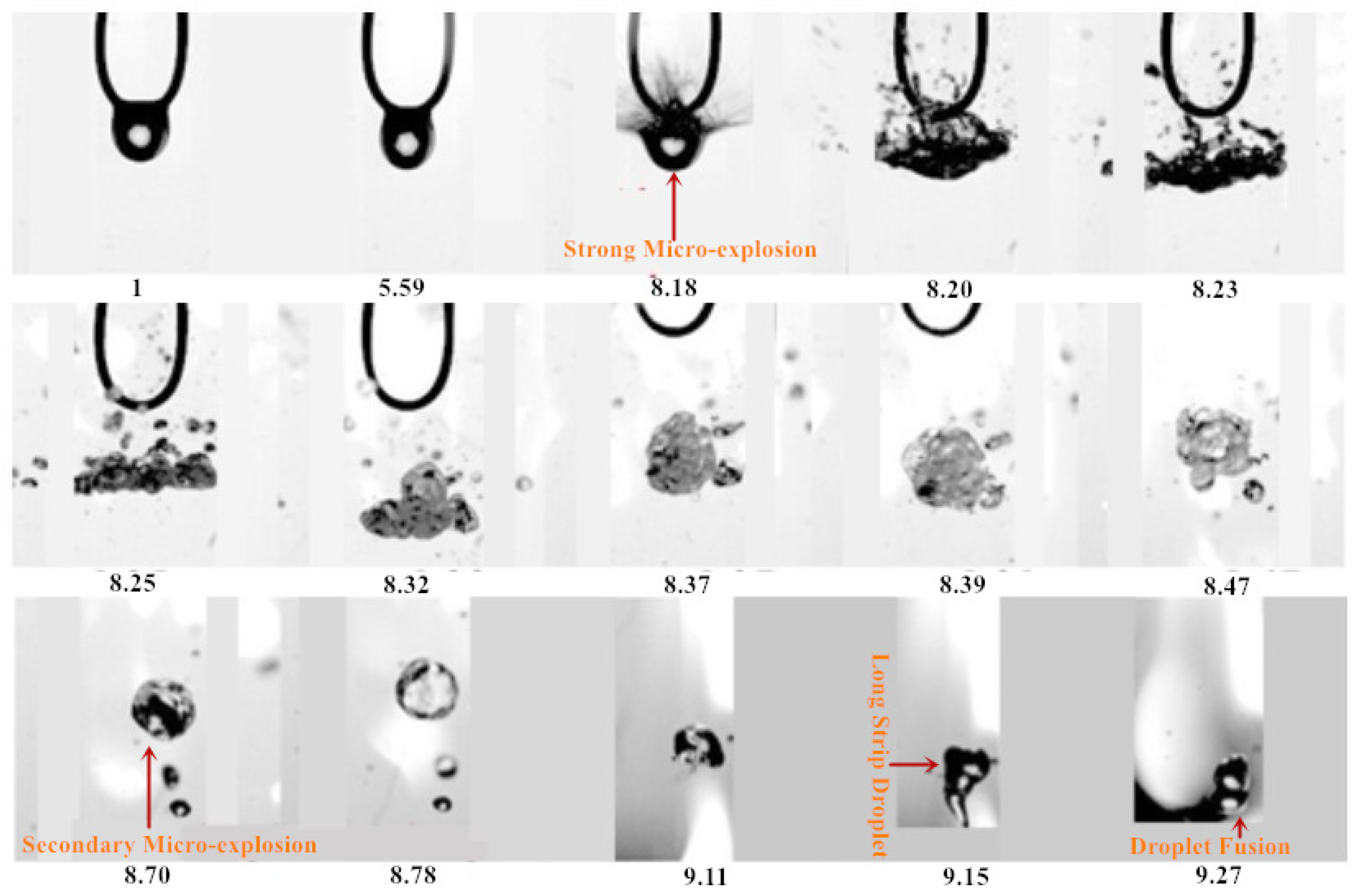
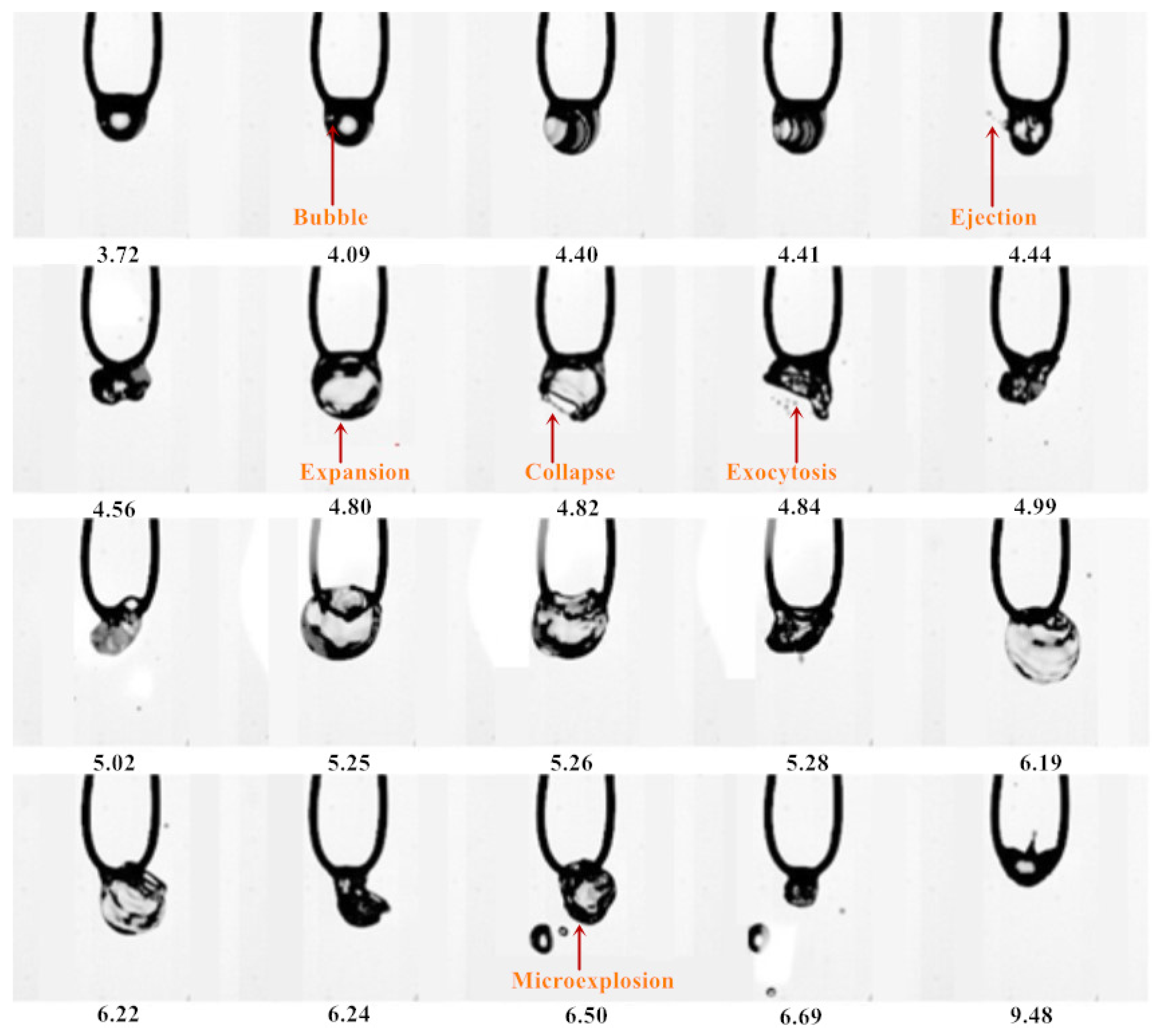
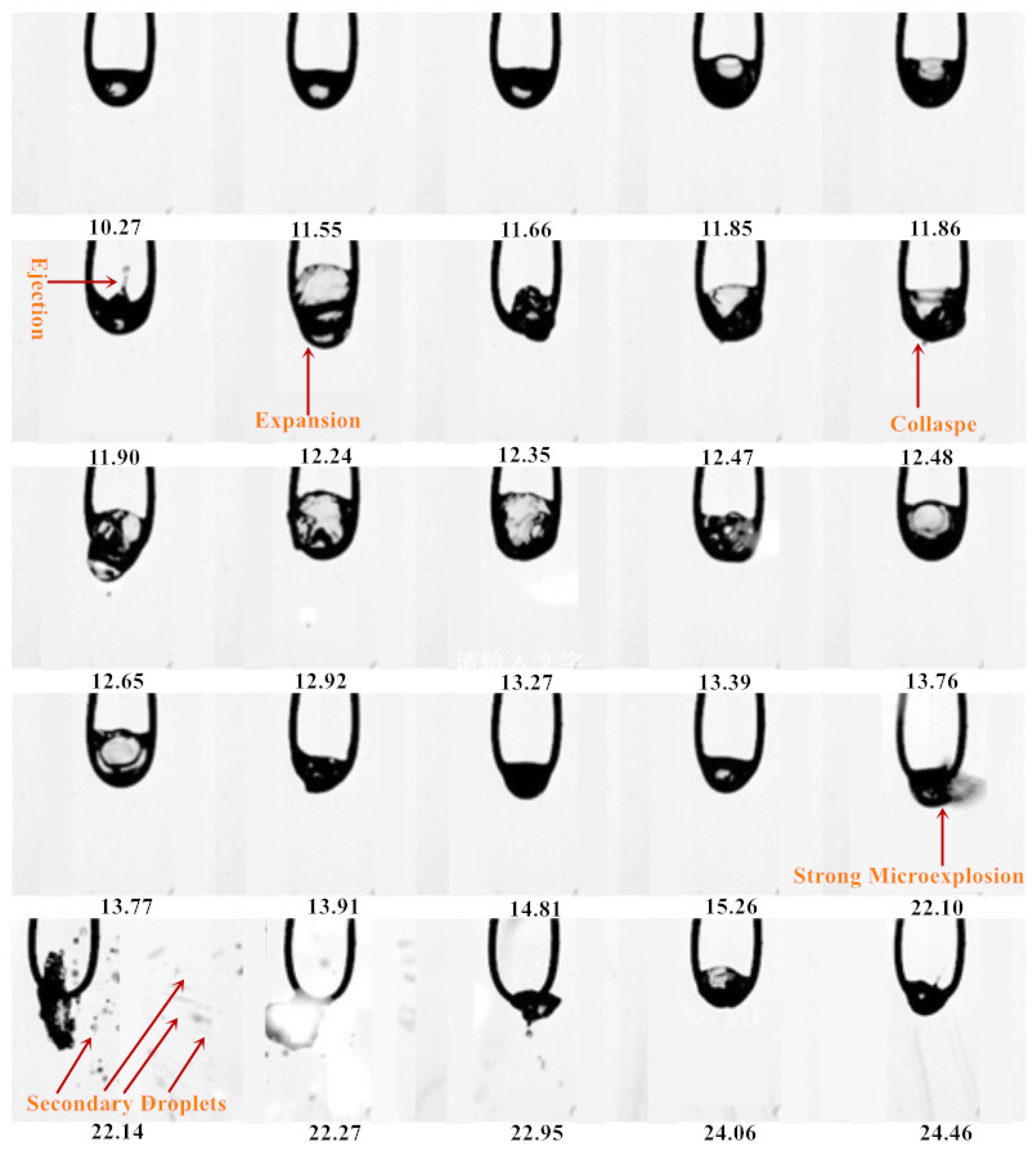
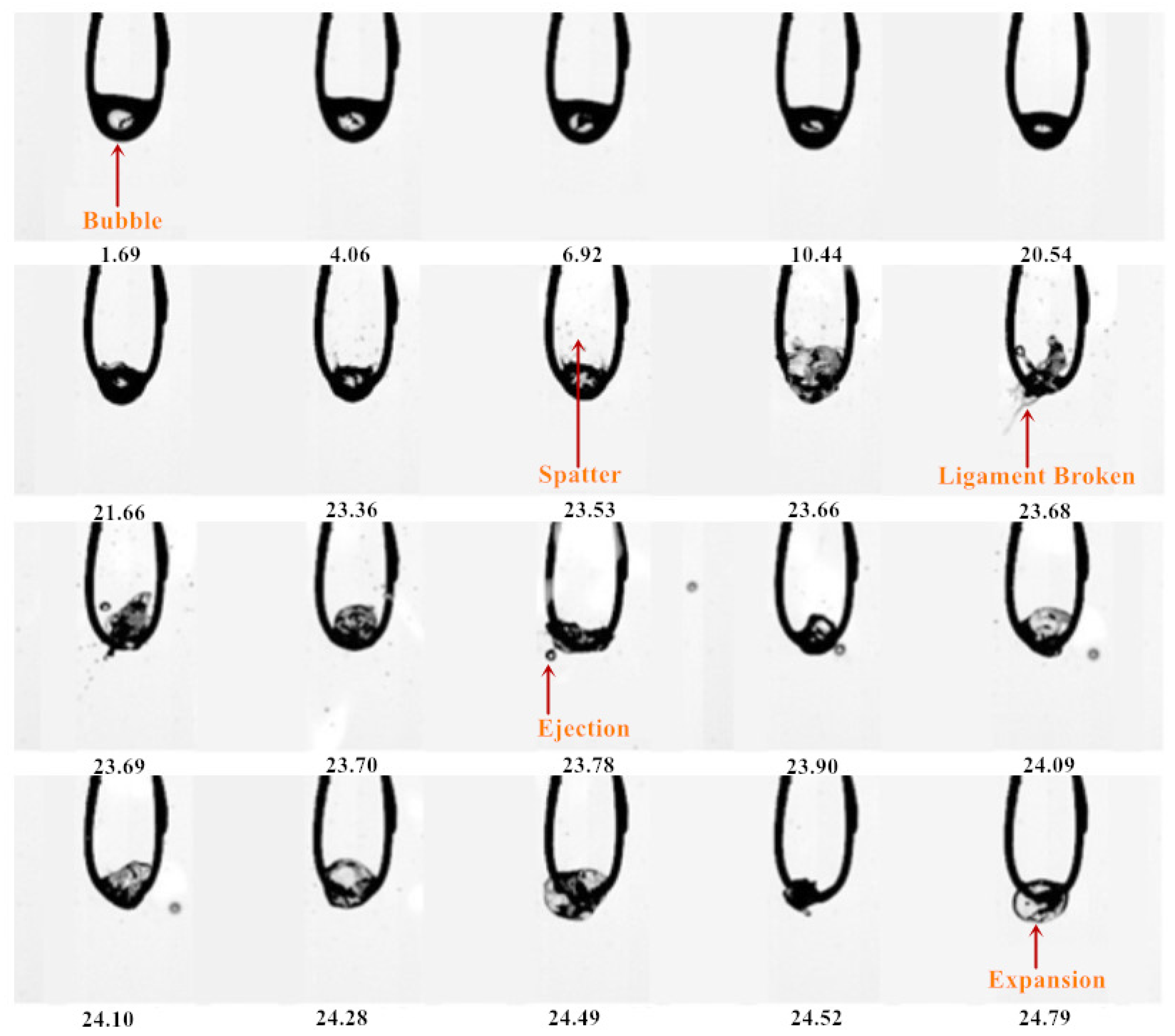
3.1. Evaporation Sequence Diagram of Mixed Droplets
3.1.1. Evaporation Sequence of the DE20 Droplet
3.1.2. Evaporation Sequence of the DE40 Droplet
3.1.3. Evaporation Sequence of the DE60 Droplet
3.1.4. Evaporation Sequence of the DE80 Droplet
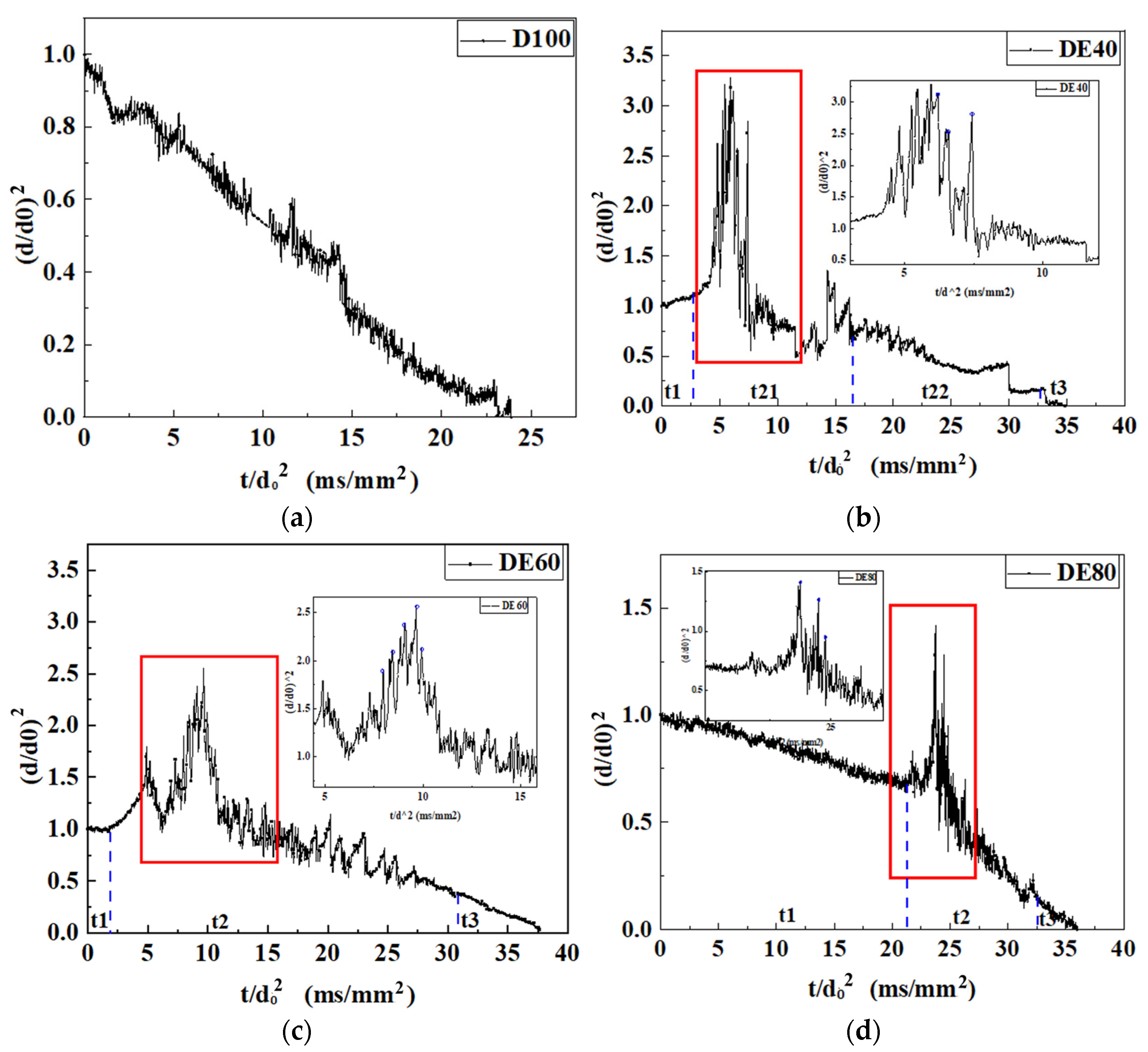
3.2. Evaporation Mode
3.3. The Influence of Mix Proportion on the Droplet Diameter
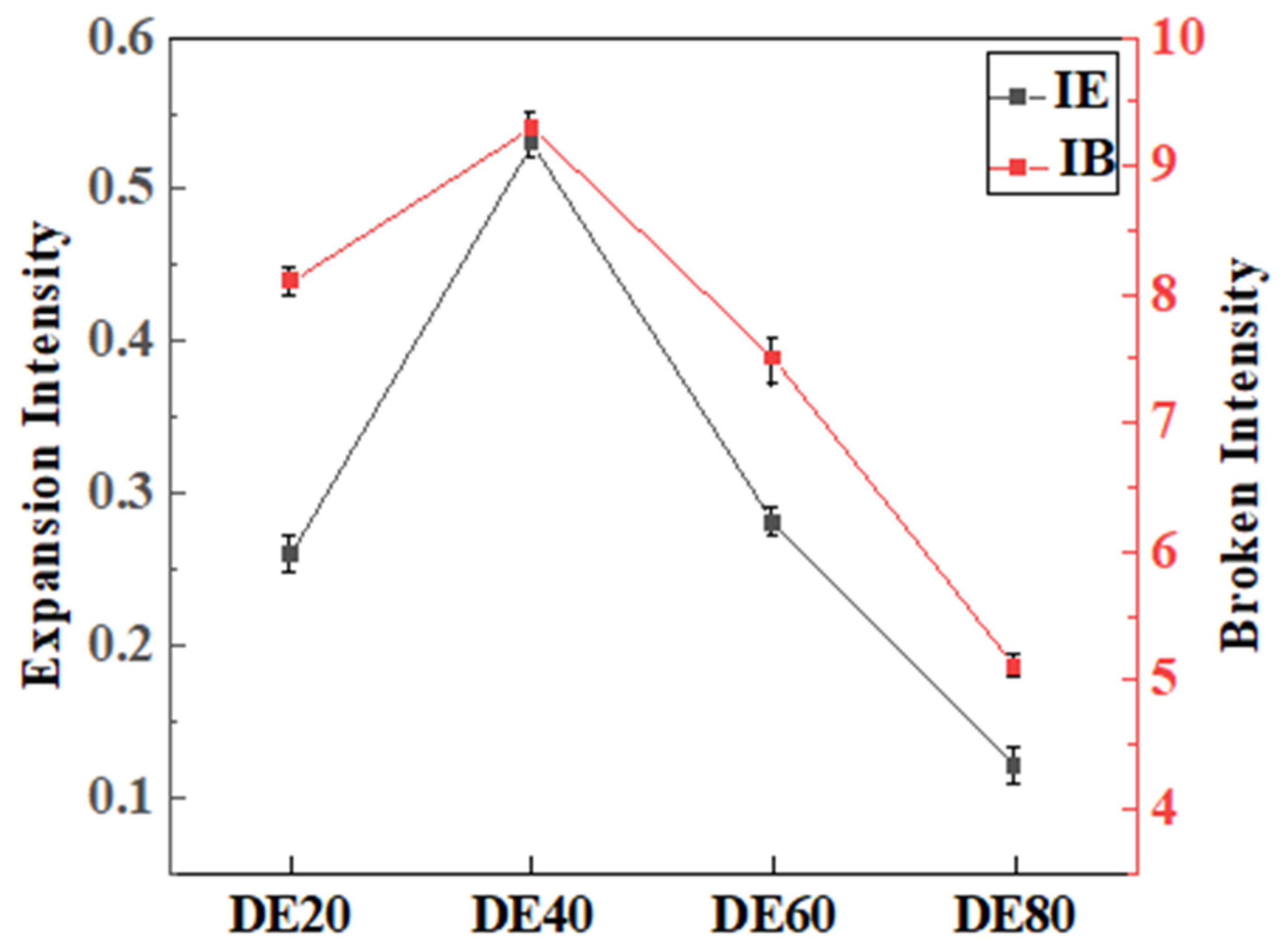
3.4. Expansion Intensity and Crushing Intensity
3.5. Time Percentage of Different Evaporation Stages
4. Conclusions
- (1)
- With the increase in ethanol blending ratio in mixed droplets (the proportion of ethanol gradually increased from 20% to 80%), the encapsulation mode of droplets changed from water-in-oil mode to oil-in-water mode. In water-in-oil mode, due to the high content of heavy components, the oil film directly covered the surface of the droplet, and the light components vaporized inside the droplet after they absorbed heat, and then gathered inside the droplet, resulting in ejection and micro-explosion. In the early stage of oil-in-water mixed droplets, light components were wrapped on the surface of the droplet, which needed to undergo evaporation for a period of time and then turned into water-in-oil mixed droplet. The droplet diameter changed in this stage, satisfying the d2 law.
- (2)
- The evaporation of droplets was divided into the following four modes: strong micro-explosion mode, ejection mode, exocytosis mode, and tensile crushing mode, and the explosion intensity decreased in turn.
- (3)
- The vapor cloud phenomenon was found in the evaporation process of the DE60 droplet, which was due to the high evaporation rate on the droplet surface, which led to the mixture of vapor and ambient gas, and the non-isothermal condensation phenomenon of a droplet under the condition of uneven heating.
- (4)
- Notably, our findings reveal the positive impact of increased ethanol content in reducing emissions such as particulates and NOx from diesel engines. However, as highlighted in the research by Nord, A.J., J.T. Hwang, and W.F. Northrop [50], while e-diesel can reduce particulate emissions, there remains controversy over its effects on NOx, CO, and HC emissions. Additionally, the low flash point of e-diesel could pose safety challenges.
- (5)
- Although diesel is compositionally complex, its evaporation process at 723 K can be regarded as the evaporation of a single-component droplet, satisfying the d2 law. The evaporation process of ethanol–diesel mixed droplets is divided into three phases, as follows: instantaneous heating, fluctuating evaporation, and equilibrium evaporation stages. During the fluctuating evaporation stage, droplets can produce ejections and micro-explosions of various intensities. The number and velocity of secondary droplets are positively correlated with the expansion and micro-explosion intensities of the droplets; the greater the intensity, the more numerous the secondary droplets.
- (6)
- Based on the experimental phenomena and comprehensive analysis, a model for the expansion and crushing intensities of mixed fuels was proposed that better conforms to actual laws. This model was used to calculate the expansion and crushing intensities of ethanol–diesel mixed droplets at different blend ratios. With the increase in ethanol blend ratio, both expansion and crushing intensities first increase and then decrease. This trend is consistent with the phenomena observed experimentally, where the expansion and crushing intensities were highest for the DE40 droplets.
- (7)
- Combining our experiments with those of Chen, Chan-Cheng, and Horng-Jang Liaw [51] comparing typical diesel fuel, we found that ethanol has a significantly higher spontaneous combustion temperature (AIT) of 368.8 °C, which significantly affects the ignition and combustion process under typical engine operating conditions. This higher AIT indicates that the use of ethanol–diesel mixtures may require the adjustment of engine settings or additional preheating measures, to ensure efficient and safe combustion under a variety of environmental conditions. Therefore, the development and optimization of ethanol–diesel mixtures as an alternative fuel requires not only consideration of its environmental properties, but also evaluation of its feasibility and safety in practical applications.
- (8)
- There is a clear positive correlation between the high microburst intensity observed in this study and the evaporation rate of droplets. Droplets with higher micro-explosion intensity promote more uniform and complete combustion by increasing the contact area between fuel and air. This improved combustion efficiency significantly reduces engine emissions of solid particles and unburned hydrocarbons, making it environmentally friendly. Particularly in internal combustion engines using diesel–ethanol blends, ethanol’s high oxygen content helps to reduce emissions of harmful gases such as nitrogen oxides (NOx), as the additional oxygen molecules promote a more complete combustion reaction. Future research will use techniques such as spectral analysis to quantitatively analyze this relationship to verify the specific impact between evaporation rate and environmental emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Economics, B.E. BP Energy Outlook; BP Plc: London, UK, 2018. [Google Scholar]
- Taghizadeh-Alisaraei, A.; Rezaei-Asl, A. The effect of added ethanol to diesel fuel on performance, vibration, combustion and knocking of a CI engine. Fuel 2016, 185, 718–733. [Google Scholar] [CrossRef]
- Mukhtar, M.; Hagos, F.Y.; Noor, M.; Mamat, R.; Abdullah, A.A. Tri-fuel emulsion with secondary atomization attributes for greener diesel engine–A critical review. Renew. Sustain. Energy Rev. 2019, 111, 490–506. [Google Scholar] [CrossRef]
- Shi, X.; Pang, X.; Mu, Y.; He, H.; Shuai, S.; Wang, J.; Chen, H.; Li, R. Emission reduction potential of using ethanol–biodiesel–diesel fuel blend on a heavy-duty diesel engine. Atmos. Environ. 2006, 40, 2567–2574. [Google Scholar] [CrossRef]
- Lee, T.H.; Hansen, A.C.; Li, G.; Lee, T. Effects of isopropanol-butanol-ethanol and diesel fuel blends on combustion characteristics in a constant volume chamber. Fuel 2019, 254, 115613. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; Kayatas, Z.; Takayama, R.; He, Z.; Kandasamy, S.; Kosaka, H. Combustion and emission characteristics of RCEM and common rail diesel engine working with diesel fuel and ethanol/hydrous ethanol injected in the intake and exhaust port: Assessment and comparison. Energy Convers. Manag. 2020, 205, 112453. [Google Scholar] [CrossRef]
- Fernando, S.; Hanna, M. Development of a novel biofuel blend using ethanol− biodiesel− diesel microemulsions: EB-diesel. Energy Fuels 2004, 18, 1695–1703. [Google Scholar] [CrossRef]
- Abrantes, I.; Ferreira, A.F.; Silva, A.; Costa, M. Sustainable aviation fuels and imminent technologies—CO2 emissions evolution towards 2050. J. Clean. Prod. 2021, 313, 127937. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Yang, L.; Cui, H.; Wang, H.; Li, C.; Tang, B.-J. Strategies to Mitigate Carbon Emissions for Sustainable Aviation: A Critical Review From a Life-cycle Perspective. Sustain. Prod. Consum. 2022, 33, 788–808. [Google Scholar] [CrossRef]
- Li, F.; Tian, J.; Han, K.; Bao, L.; Meng, K.; Lin, Q. Expansion and combustion of droplets that contain long-chain alcohol alternative fuels. Phys. Fluids 2021, 33, 017117. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.; Cai, W.; Berrocal, E.; Aldén, M.; Li, Z. Volume expansion and micro-explosion of combusting iron particles analyzed using magnified holographic imaging. Powder Technol. 2023, 420, 118412. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Karmakar, S.; Som, S. Puffing and micro-explosion behavior in the combustion of butanol/Jet A-1 and acetone-butanol-ethanol (ABE)/Jet A-1 fuel droplets. Combust. Sci. Technol. 2017, 189, 1796–1812. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Basu, S. Phenomenology of disruptive breakup mechanism of a levitated evaporating emulsion droplet. Exp. Therm. Fluid Sci. 2020, 115, 110086. [Google Scholar]
- Jing, Q.; Wang, D.; Shi, C. Effects of aluminum powder additives on deflagration and detonation performance of JP-10/DEE mixed fuel under weak and strong ignition conditions. Appl. Energy 2023, 331, 120477. [Google Scholar] [CrossRef]
- Meng, K.; Huang, Z.; Zhang, X.; Li, L.; Li, R.; Lin, Q. Effect of micro-explosion of biodiesel and ethanol droplets on evaporation: A three-stage mixed fuel droplet evaporation model. Phys. Fluids 2022, 34, 032113. [Google Scholar] [CrossRef]
- Meng, K.; Li, L.; Zhang, X.; Huang, Z.; Wang, F.; Li, R.; Lin, Q. Comparison of combustion and micro-explosion characteristics of droplet group of biodiesel/ethanol and biodiesel/RP-3/ethanol. Phys. Fluids 2022, 34, 061903. [Google Scholar] [CrossRef]
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A. Characteristics of child droplets during micro-explosion and puffing of suspension fuel droplets. Int. J. Heat Mass Transf. 2023, 209, 124106. [Google Scholar] [CrossRef]
- Gallier, S. Aluminum combustion in strong convective flows. Combust. Flame 2023, 249, 112598. [Google Scholar] [CrossRef]
- Meng, K.; Fu, W.; Lei, Y.; Zhao, D.; Lin, Q.; Wang, G. Study on micro-explosion intensity characteristics of biodiesel, RP-3 and ethanol mixed droplets. Fuel 2019, 256, 115942. [Google Scholar] [CrossRef]
- Lasheras, J.; Fernandez-Pello, A.; Dryer, F. Experimental observations on the disruptive combustion of free droplets of multicomponent fuels. Combust. Sci. Technol. 1980, 22, 195–209. [Google Scholar] [CrossRef]
- Li, H.; Rosebrock, C.D.; Wu, Y.; Wriedt, T.; Mädler, L. Single droplet combustion of precursor/solvent solutions for nanoparticle production: Optical diagnostics on single isolated burning droplets with micro-explosions. Proc. Combust. Inst. 2019, 37, 1203–1211. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Wang, B.; Wei, Y. Superheat limit and micro-explosion in droplets of hydrous ethanol-diesel emulsions at atmospheric pressure and diesel-like conditions. Energy 2018, 154, 535–543. [Google Scholar] [CrossRef]
- Chao, C.-Y.; Tsai, H.-W.; Pan, K.-L.; Hsieh, C.-W. On the microexplosion mechanisms of burning droplets blended with biodiesel and alcohol. Combust. Flame 2019, 205, 397–406. [Google Scholar] [CrossRef]
- Botero, M.; Huang, Y.; Zhu, D.; Molina, A.; Law, C. Synergistic combustion of droplets of ethanol, diesel and biodiesel mixtures. Fuel 2012, 94, 342–347. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Ganippa, L.C.; Xia, J.; Megaritis, A. Puffing and micro-explosion of diesel–biodiesel–ethanol blends. Fuel 2016, 166, 59–66. [Google Scholar] [CrossRef]
- Yi, P.; Li, T.; Fu, Y.; Xie, S. Transcritical evaporation and micro-explosion of ethanol-diesel droplets under diesel engine-like conditions. Fuel 2021, 284, 118892. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Tan, I.M. Water-in-diesel emulsion and its micro-explosion phenomenon-review. In Proceedings of the 2011 IEEE 3rd International Conference on Communication Software and Networks, Xi’an, China, 27–29 May 2011; pp. 314–318. [Google Scholar]
- Antonov, D.V.; Strizhak, P.A. Heating, evaporation, fragmentation, and breakup of multi-component liquid droplets when heated in air flow. Chem. Eng. Res. Des. 2019, 146, 22–35. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Qiao, X.; Ju, D.; Lin, Z. Effect of ambient temperature on the micro-explosion characteristics of soybean oil droplet: The phenomenon of evaporation induced vapor cloud. Int. J. Heat Mass Transf. 2019, 139, 736–746. [Google Scholar] [CrossRef]
- Shinjo, J.; Xia, J. Combustion characteristics of a single decane/ethanol emulsion droplet and a droplet group under puffing conditions. Proc. Combust. Inst. 2017, 36, 2513–2521. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Yuxin, W.; Qiao, X.; Ju, D.; Sun, C.; Zhang, Q. Experimental study on evaporation and micro-explosion characteristics of biodiesel/n-propanol blended droplet. Energy 2020, 205, 118031. [Google Scholar] [CrossRef]
- Mikami, M.; Kojima, N. An experimental and modeling study on stochastic aspects of microexplosion of binary-fuel droplets. Proc. Combust. Inst. 2002, 29, 551–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Huang, R.; Huang, S.; Ma, Y.; Xu, S.; Wang, Z. A new puffing model for a droplet of butanol-hexadecane blends. Appl. Therm. Eng. 2018, 133, 633–644. [Google Scholar] [CrossRef]
- Mishra, D.; Patyal, A. Effects of initial droplet diameter and pressure on burning of ATF gel propellant droplets. Fuel 2012, 95, 226–233. [Google Scholar] [CrossRef]
- Mikami, M.; Yagi, T.; Kojima, N. Occurrence Probability of Microexplosion in Droplet Combustion of Miscible Binary Fuels, Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1933–1941. [Google Scholar]
- Meng, K.; Fu, W.; Li, F.; Lei, Y.; Lin, Q. Expansion, injection and micro-explosion phenomena during the full combustion cycle of mixed fuel droplets of ethyl alcohol and biodiesel. Combust. Theory Model. 2020, 24, 810–828. [Google Scholar] [CrossRef]
- Ismael, M.A.; Aziz, A.R.A.; Mohammed, S.E.; Baharom, M.B.; Hagos, F.Y. Macroscopic and microscopic spray structure of water-in-diesel emulsions. Energy 2021, 223, 120040. [Google Scholar] [CrossRef]
- Coughlin, B.; Hoxie, A. Combustion characteristics of ternary fuel Blends: Pentanol, butanol and vegetable oil. Fuel 2017, 196, 488–496. [Google Scholar] [CrossRef]
- Ismael, M.A.; Heikal, M.; Aziz, A.A.; Crua, C.; El-Adawy, M.; Nissar, Z.; Baharom, M.; Zainal, A. Investigation of Puffing and Micro-Explosion of Water-in-Diesel Emulsion Spray Using Shadow Imaging. Energies 2018, 11, 2281. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K.; Ali, G.; Cho, S.O. Evaporation characteristics of kerosene droplets with dilute concentrations of ligand-protected aluminum nanoparticles at elevated temperatures. Combust. Flame 2013, 160, 2955–2963. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental understanding on the dynamics of micro-explosion and puffing in ternary emulsion droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Tarlet, D.; Mura, E.; Josset, C.; Bellettre, J.; Allouis, C.; Massoli, P. Distribution of thermal energy of child-droplets issued from an optimal micro-explosion. Int. J. Heat Mass Transf. 2014, 77, 1043–1054. [Google Scholar] [CrossRef]
- Valiullin, T.; Vershinina, K.Y.; Kuznetsov, G.; Strizhak, P. An experimental investigation into ignition and combustion of groups of slurry fuel droplets containing high concentrations of water. Fuel Process. Technol. 2020, 210, 106553. [Google Scholar] [CrossRef]
- Antonov, D.; Shlegel, N.; Strizhak, P. Secondary atomization of gas-saturated liquid droplets as a result of their collisions and micro-explosion. Chem. Eng. Res. Des. 2020, 162, 200–211. [Google Scholar] [CrossRef]
- Meng, K.; Bao, L.; Shi, Y.; Han, K.; Lin, Q.; Wang, C. Experimental investigation on ignition, combustion and micro-explosion of RP-3, biodiesel and ethanol blended droplets. Appl. Therm. Eng. 2020, 178, 115649. [Google Scholar] [CrossRef]
- Meng, K.; Bao, L.; Li, F.; Wang, C.; Lin, Q. Experimental understanding on combustion and micro-explosion characteristics of mixed droplets of aviation fuel, biodiesel and ethanol. J. Energy Inst. 2021, 97, 169–179. [Google Scholar] [CrossRef]
- Mura, E.; Calabria, R.; Califano, V.; Massoli, P.; Bellettre, J. Emulsion droplet micro-explosion: Analysis of two experimental approaches. Exp. Therm. Fluid Sci. 2014, 56, 69–74. [Google Scholar] [CrossRef]
- Langmuir, I. The Evaporation of Small Spheres. Phys. Rev. 1918, 12, 368–370. [Google Scholar] [CrossRef]
- Pan, K.-L.; Chiu, M.-C. Droplet combustion of blended fuels with alcohol and biodiesel/diesel in microgravity condition. Fuel 2013, 113, 757–765. [Google Scholar] [CrossRef]
- Nord, A.J.; Hwang, J.T.; Northrop, W.F. Emissions from a Diesel Engine Operating in a Dual-Fuel Mode Using Port-Fuel Injection of Heated Hydrous Ethanol. In Proceedings of the ASME 2015 Internal Combustion Engine Division Fall Technical Conference ICEF2015 ICEF2015-1067, Houston, TX, USA, 8–11 November 2015. [Google Scholar]
- Chen, C.-C.; Liaw, H.-J.; Shu, C.-M.; Hsieh, Y.-C. Autoignition Temperature Data for Methanol, Ethanol, Propanol, 2-Butanol, 1-Butanol, and 2-Methyl-2,4-Pentanediol. J. Chem. Eng. Data 2010, 55, 5059–5064. [Google Scholar] [CrossRef]



| Composition of Fuel Mixture (Volume Basis) | Designated Nomenclature |
|---|---|
| 20% ethanol, 78% diesel, 2% n-decanol | DE20 |
| 40% ethanol, 58% diesel, 2% n-decanol | DE40 |
| 60% ethanol, 38% diesel, 2% n-decanol | DE60 |
| 80% ethanol, 18% diesel, 2% n-decanol | DE80 |
| Properties | Diesel | Ethanol | DE20 | DE40 | DE60 | DE80 |
|---|---|---|---|---|---|---|
| Density (20 °C) [kg/L] | 0.822 | 0.789 | 0.816 | 0.811 | 0.805 | 0.799 |
| Viscosity (20 °C) [mm2/s] | 2.93 | 1.20 | 1.95 | 1.82 | 1.72 | 1.63 |
| Surface tension (mN/m) | 27.84 | 22.32 | 23.41 | 23.25 | 23.09 | 22.98 |
| Boiling point (K) | 550–650 | 351 | — | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Meng, K.; Bao, L.; Lin, Q.; Pavlova, S. Experimental Study on Evaporation and Micro-Explosion Characteristics of Ethanol and Diesel Blended Droplets. Atmosphere 2024, 15, 604. https://doi.org/10.3390/atmos15050604
Zhang Y, Meng K, Bao L, Lin Q, Pavlova S. Experimental Study on Evaporation and Micro-Explosion Characteristics of Ethanol and Diesel Blended Droplets. Atmosphere. 2024; 15(5):604. https://doi.org/10.3390/atmos15050604
Chicago/Turabian StyleZhang, Yixuan, Kesheng Meng, Lin Bao, Qizhao Lin, and Svitlana Pavlova. 2024. "Experimental Study on Evaporation and Micro-Explosion Characteristics of Ethanol and Diesel Blended Droplets" Atmosphere 15, no. 5: 604. https://doi.org/10.3390/atmos15050604
APA StyleZhang, Y., Meng, K., Bao, L., Lin, Q., & Pavlova, S. (2024). Experimental Study on Evaporation and Micro-Explosion Characteristics of Ethanol and Diesel Blended Droplets. Atmosphere, 15(5), 604. https://doi.org/10.3390/atmos15050604







