Abstract
Nitrate (NO3−) is a prominent atmospheric pollutant and a key chemical constituent of snow and ice, which plays a crucial role in the atmosphere and significantly impacts regional climate and environment conditions through a series of complex chemical processes. By summarizing the recent research progress on the nitrate chemical process (particularly on the isotopic measurements of NO3− (δ15N, Δ17O and δ18O)) in atmosphere and glacier snow, this study mainly investigated the chemical compositions and chemical processes, formation pathways, and photochemical reactions of nitrate in snow and atmosphere. Our results identified that the main ways of atmospheric nitrate formation are the hydrolysis of N2O5 and the reaction of ·OH with NO2; the spatial distribution of Δ17O and δ18O values of atmospheric nitrate have a significant latitudinal trend between 30° N–60° N; the study of stable isotopes (δ15N and δ18O) and the oxygen isotope anomaly (Δ17O) of nitrate have mainly been carried out over the densely populated and coastal mega cities; there exist significant gaps in the study of chemistry processes of nitrate in snow and ice and the air–snow interfaces across glaciated regions. This study provides a basic reference for more robust observations and research of nitrate in glacier areas in the future.
1. Introduction
Nitrates are widely present in the surface environment and are an essential mineral of terrestrial ecosystems. They play a crucial role in the nitrogen cycle of the Earth system [1,2,3,4].As one of the important components of atmospheric deposition, nitrate (NO3−) has a profound impact on the environment and ecosystem. Nitrate ion in the atmosphere is a key component of fine particulate matter (PM2.5), accounting for more than 20% of the total mass of PM2.5. In addition, nitrates are one of the key factors in the formation of acid rain, which has adverse effects on water quality and ecosystems [5,6,7,8]. The precursor of nitrate, NOx (NOx = NO + NO2), is an important component of atmospheric pollutants. In recent years, with the rapid social and economic development, the content of nitrogen oxides (NOx) in the atmosphere emitted by motor vehicle exhaust, fossil fuel combustion, thermal power plants, etc., has significantly increased. NOx not only exacerbates global atmospheric pollution but also gradually becomes a scientific focus of international atmosphere and environmental science community [9,10,11]. In the atmosphere, the majority of NOx undergoes various transformations to form nitric acid (HNO3), and this process has a significant impact on the pH of atmospheric rainfall. The NOx has a strong relation with ozone (O3) and hydroxyl radicals (·OH) in the atmosphere [1,12,13,14,15]. Therefore, it is crucial to study the formation pathways and chemical processes of atmospheric nitrate to improve our understanding of nitrate chemistry and advance the field of atmospheric environment. Nitrogen (N) and its cycling in nitrates in atmospheric environment and glacier snow play important roles in the natural nitrogen cycle of the Earth system, offering valuable insights into the impacts of human activities on the atmospheric environment and ecosystem.
The stable isotopes of nitrate and the oxygen isotope anomaly of nitrate are essential parameters and analysis channels in investigating atmospheric nitrate and their sources, formation pathways, and chemical processes [14,16]. The stable isotopes, including 18O, 17O, 16O, 15N, and 14N (Table 1), can be measured within the NO3− anion. Given the relatively lower abundance of heavier isotopes, the utilization of δ values is widespread for expressing the relative isotopic ratios, which is defined by the formula ‰) = () (Table 1), and according to the Vienna Standard Mean Ocean Water (VSMOW) for δ17O and δ18O, the reference is gaseous N2 for δ15N [17]. In various biological, physical, and chemical reactions, the phenomenon where isotopes of a specific element distributed in different substances in varying proportions is referred to as isotope fractionation. Isotope fractionation related to mass is called mass-dependent fractionation (MDF). In this phenomenon, there is a linear relationship between δ17O and δ18O, which is expressed as δ17O = 0.52 × δ18O [18,19]. This phenomenon is widely observed and identified in oxygen and water bodies. However, this linear relationship does not exist in the formation process of O3, which is known as mass-independent fractionation (MIF) of isotope [20,21,22,23,24]. The degree of MIF is generally determined and expressed by employing the oxygen isotopic anomaly (Δ17O): Δ17O = δ17O − 0.52 × δ18O (Table 1).

Table 1.
Stable isotopes and isotopic anomalies of nitrate ion.
The current scientific understanding of atmospheric and snow/ice chemistry remains incomplete. A large number of relevant studies primarily rely on numerical isotopic fractionation model, chemistry box model, and chemical transport model. These models have investigated oxygen isotopic anomalies in the atmosphere, the photolysis of nitrate in Antarctic ice/snow, and the formation processes of nitrate [14,25,26]. In addition, many studies on the chemical processes and formation pathways of atmospheric nitrates have been conducted in coastal cities of North America [12,13,27,28,29], some mega cities in Europe [28,30], and over the eastern coastal region of Asia [8,31,32,33]. The investigation of chemical processes of nitrate in glacier regions has significant implications for understanding atmospheric nitrogen oxide levels in the past. However, studies on nitrate chemical processes in glacier snow/ice and its formation pathways at the air–snow interface have been primarily carried out in the Antarctic ice sheet and Greenland ice sheet, while little relevant studies have been performed over mountain glaciers, leaving large gaps in understanding well the nitrate chemical processes over the cryosphere area. This study provides a comprehensive review of atmospheric nitrate chemistry and snow chemistry. Additionally, we propose the current study limitation and highlight the future research needs of nitrate chemistry of snow/ice.
2. Measurement Methods for Stable Oxygen Isotope
Currently, there are several methods available for determining the N and O isotopes of nitrates, including ion-exchange-AgNO3 method [34], pyrolysis method [35], azide method [36], and denitrification bacteria method [24,37].
The ion-exchange-AgNO3 method: The nitrate (NO3−) samples were individually combusted in a furnace and subjected to a high-temperature pyrolysis to produce N2 and CO. The separated gases were introduced into an isotope ratio mass spectrometer (IRMS) through a chromatographic column for the analysis of δ15N [38]. The oxygen isotopes (δ17O and δ18O) were determined using the pyrolysis method. After purifying the nitrate (NO3−) samples, they were converted into AgNO3 solution through ion exchange column and Ag2SO4. The dried AgNO3 powder was then heated in a high-temperature quartz reaction tube (550 °C) to generate O2, NO2, Ag, and trace amounts of N2 and NO. The generated gases were directed into a liquid nitrogen trap for enrichment, purification, and separation. Finally, the obtained oxygen gas (O2) was introduced into an IRMS for the testing of oxygen isotope ratios [35].
The azide method: The first step involves using cadmium metal to reduce the amount of NO3− converted to NO2−. The second step is to reduce the generated NO2− to N2O with the assistance of sodium azide and a weak acid buffer environment. Subsequently, the N2O gas is thermally decomposed into N2 and O2 through a high-temperature metal tube, and then, they enter an IRMS for the determination of N and O isotopes [36].
The denitrification bacteria method: This method involves the direct conversion of NO3− to N2O by denitrifying bacteria. Firstly, the cultivation of denitrifying bacteria requires the preparation of culture medium, cultivation liquid, and rinsing solution. The freeze-dried powder of golden pseudomonas bacteria is activated, followed by sealed cultivation. Afterwards, well-grown individual colonies with a golden yellow appearance are isolated and cultivated separately. Then, the cultivated denitrifying bacterial solution undergoes a series of processes, such as centrifugation, fractionation, and nitrogen blowing to remove N2O gas from the culture liquid. Finally, the sample is injected into sample vials containing the bacterial solution for reaction. The obtained gas samples are purified, concentrated, and separated, and subsequently, their δ15N, δ17O, and δ18O values are determined using an IRMS [37,39].
Among the four pre-treatment methods, the ion-exchange-AgNO3 method and the azide method require a larger quantity of sample volume (with a higher detection limit) and involve a cumbersome process. Additionally, the azide method has a shorter pre-treatment time and higher accuracy, but it involves the use of highly toxic hazardous substances in the reaction. However, the denitrification bacteria method is simple to operate but has a low detection limit and can meet the required experimental accuracy. Thus, the denitrification bacteria method is one of the most widely applied methods for the detection of stable oxygen isotope of nitrate ion (NO3−).
3. Chemical Processes of Atmospheric Nitrate
On a global scale, the photolytic reactions of NO and ·OH, along with the hydrolysis of N2O5, are commonly acknowledged as the dominant pathways for the formation of nitrates (Equations (1) and (2)) [26,40]. Chemical Equation (1)–(8) encompass the primary formation pathways of atmospheric nitrate (NO3−), whereas M in Equation (1) represents oxygen (O2) and/or nitrogen (N2) in the atmosphere, HC in Equation (3) represents hydrocarbons, and DMS stands for dimethyl sulfide. An improved GEOS-Chem simulation, incorporating Δ17O of nitrate from various sites, revealed that both NO2 + OH (Equation (1)) and N2O5 + H2O (Equation (2)) contribute approximately 41% to global nitrate formation, and other mechanisms contribute less than 6% to near-surface nitrate levels globally [40]. The reaction between NO2 and ·OH primarily occurs during daylight hours. The reaction between NO2 and O3 generates N2O5, which subsequently hydrolyzes to form HNO3, primarily taking place at night [26]. The contributions of different reactions vary greatly in different regions and periods. Due to the significant emissions of hydrocarbon compounds (HCS) in the atmosphere, the reaction between NO3 and HC (Equation (3)) becomes an important pathway for the formation of industrial nitrates in industrial areas [41]. In non-coastal areas, the lower mixing ratio of DMS leads to a smaller contribution to the reaction of NO3 + DMS [42]. Studies have shown that in low temperature and heavily polluted weather conditions, the absorption of N2O5 in aerosols and clouds is the primary pathway of nitrate formation [8,43]. Experiment-based research has indicated that the hydrolysis of NO2 and NO3 (Equations (4) and (5)) is not the main pathway for the formation of HNO3 in the atmosphere due to their low reaction probabilities [44,45,46]. However, recent model simulations have reported that the hydrolysis of NO2 (Equation (4)) is also an important source of HNO3 formation in winter haze weather [47]. Additionally, it has been found that the heterogeneous reactions of NO3 and N2O5 on the surface of aerosols are the main factors controlling the generation of nitrate in particulate matter during polluted weather conditions [48].
In general, there are two main oxidation pathways for NO to form NO2 in the atmosphere. One is through the reaction of NO with O3 (Equation (6)), and the other is through the oxidation of NO with OH/RO2 (Equation (7)), followed by hydrolysis to form HNO3. HO2/RO2 represents peroxyl radicals, which typically refer to a group of substances produced by reaction between certain organic groups (R) or hydrogen atoms with O2 in the atmosphere [31]. NO2 can also react with O3 and generates NO3− radical (Equation (8)). The NO3− radical can react directly with hydrocarbons (HC) and dimethyl sulfide (DMS) and then generate HNO3, or it can undergo hydrolysis on the surfaces of aerosols to form HNO3 [40,41]. The reaction of NO3 with HC/DMS predominantly occurs during the night, because NO3 is easily prone to photolysis during the daytime [49]. Nitrogen pentoxide (N2O5) is an active NOx reservoir during the night, which can react at the surfaces of airborne particles, generating HNO3 or both NO2 and nitryl chloride (ClNO2) (Equation (10)) [50]. Moreover, there are other potential mechanisms for the formation of nitrate particles, such as the hydrolysis of organic nitrates (RONO2) and halogen nitrates (XNO3), which play an important role in the formation of atmospheric nitrates in coastal areas and tropical rainforest [33]. Additionally, some halogens (X, such as Cl, Br, I) in polar regions can participate in photochemical reactions with NOx, oxidizing and producing HNO3 [51,52]. Taking bromine (Br) as an example, the halogen components typically undergo many reactions with nitric oxide and nitrate according to processes presented in Equations (11)–(14) to form nitric acid eventually [51].
NO2 + OH + M → HNO3 + M,
NO3 + HC/DMS → HNO3 +Others,
N2O5 + H2O (surface) → 2HNO3 (liquid),
2NO2 + H2O (surface) → HNO3 (liquid) +HONO,
NO3 + H2O (surface) → HNO3 (liquid) +OH,
NO + O3 → NO2+ O2,
NO + HO2 (ROx) → NO2 +OH(RO),
NO2 + O3→ NO3 + O2,
NO2 + NO3 → N2O5,
N2O5 + Cl− (surface) → NO3− (particle) +ClNO2,
Br + O3 → BrO+ O2,
NO + BrO → NO2 +Br,
NO2 + BrO + M → BrNO3+ M,
BrNO3 +H2O → HOBr +HNO3.
Reactions indicated by Equations (7) and (12) represent the oxidation and photolysis of NOx by O3, HO2, and ROx after NO is emitted into the atmosphere. The cycle/transfer between NO and NO2 occurs quite rapidly, allowing them to establish a photochemical steady state during the day. The conversion of NOx to nitrates is at least three orders of magnitude slower than the formation of NO and NO2. The primary daytime reaction for the conversion of NOx to nitrates involves the oxidation of NO2 by ·OH to form HNO3 (reaction 12, Equation (1)). Processes 5 to 11 in Figure 1 represent the formation pathways of nitrates during the nighttime. NO2 reacts with O3 (reaction 5, Equation (8)) to form NO3, which then reacts with hydrocarbons (HC) to generate HNO3 (reaction 8, Equation (3)). Alternatively, NO and NO2 can inter-react to form N2O5 (reaction 7, Equation (9)), which undergoes heterogeneous reactions on the surfaces of aerosol to form HNO3 (reaction 9, Equation (2)). Reactions 7 to 9 mainly occur at night since NO3 is highly unstable during the day and rapidly undergoes photolysis, resulting in low concentrations [19,26].
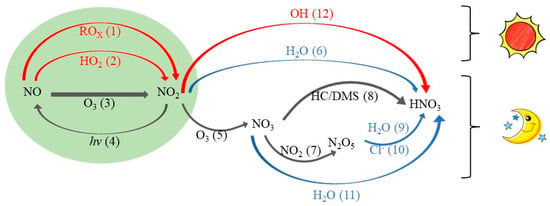
Figure 1.
Schematic diagram illustrating main pathways of NO3− formation in the atmosphere (reaction 1−reaction 12). Red font indicates reactions where the Δ17O of the oxidants is close to zero, and blue font indicates processes of heterogeneous reactions in the atmosphere. (The figure was modified from [19].
It is worth noting that heterogeneous reactions are also one of the main pathways to produce HNO3. Simulations of atmospheric chemistry in the coastal areas of California using a chemistry box model have shown that over 50% of the nitrates in the summer atmosphere are generated from the reaction of NO2 and ·OH, while over 90% of the nitrates in the winter atmosphere are produced through the heterogeneous reaction of N2O5 [14]. Nitrates in snow pits in Greenland are almost entirely generated from the reaction of NO2 and ·OH in the summer, while in the winter, they are mainly produced through the reaction of NO2 and O3. Nitrates formed from the reaction of NO3 and hydrocarbons account for about 40% of the total nitrate in the atmosphere, while the hydrolysis of N2O5 accounts for 60% of nitrate formation [19]. In addition, the reaction between N2O5 and CI− is a heterogeneous reaction. Studies have shown that N2O5 reacts with CI− released from coal-fired power plants to form ClNO2 (reaction 10, Equation (9)), resulting in high concentrations of ClNO2 in the atmosphere of North China [49,53,54]. Therefore, the reaction between N2O5 and CI− is also noteworthy in severe polluted regions. Moreover, a higher relative humidity of atmosphere during nighttime than daytime creates favorable conditions for heterogeneous reactions, such as the hydrolysis of NO3 (reaction 11, Equation (5)). These processes are crucial for the formation of nitrates during nighttime, with the pathways of (NO3 + H2O/HC) and (N2O5 + H2O/CI−) being the primary contributors. However, it should be emphasized that the heterogeneous reactions, especially the reaction (NO2 + H2O/OH), make comparable contributions to nitrate formation during both daytime and nighttime [31]. In wintertime, the low concentration of ·OH [55,56] and the high concentration of N2O5 in field measurements [57,58,59] highlight the significant contribution of N2O5 heterogeneous reactions to nitrate formation in haze weather [60,61]. Additionally, during haze periods in North China, higher relative humidity and larger aerosol surface area facilitate the heterogeneous reactions of nitrogen oxides. Therefore, the reaction pathway of (NO2 + H2O) also serves as an important potential pathway for nitrate formation during haze events [55,62,63]. Moreover, it has been identified that the reactions among biogenic volatile organic compounds (BVOCs) and NOx and NO3 produce organic nitrates (e.g., RONO2, RO2NO2) [64]. Organic nitrate aerosols have the ability of absorbing sunlight, particularly in the ultraviolet (UV) range. Intense solar radiation and high temperature can lead to the increase of NOx from nitrate photolysis, and a reduced source from organic nitrates can result in a short lifetime in the warmer air [40,65]. The formed RONO2 undergoes oxidation reactions, yielding second-generation RONO2 species. The photolysis and oxidation of those species can contribute to the recycling of NOx [66]. Additionally, particle-phase RONO2 (pRONO2) contributes to the formation of organic aerosols. RONO2 in the atmosphere is usually removed through dry/wet deposition or by subjecting it to hydrolysis to form inorganic nitrates and alcohols [67,68,69].
In polar regions, cold temperatures maintain/preserve the stability of HNO4 formed from the reaction between HO2 and NO2 [70,71]. The presence of liquid HNO4 enables further reactions with various chemical substances (such as HNO2, HSO3−, Cl−, Br−, and I−) to form nitrates [51]. Therefore, heterogeneous chemical reactions play a crucial role in the formation of nitrates in polar regions. The specific reaction processes are illustrated by Equation (15)–(17), and Equation (17) specifically represents the reaction of halogens (X, such as Cl, Br, I) with HNO4 [72,73,74].
HNO2 + HNO4 → 2NO3− +2 H+,
HNO4 (gaseous) + HSO3− (gaseous) → HNO3 (gaseous) + SO42− (gaseous)+ H+,
HNO4 + X− → NO3− + HOX.
4. Spatial Distribution of Isotopes of Atmospheric Nitrate
Nitrates is a significant form of nitrogen in the natural environment and can be readily utilized by organisms [75]. The values of δ15N, δ18O, and Δ17O of nitrates have significant differences due to their different origins and sources. It has been demonstrated that the δ15N(NOx) in automobile exhaust is typically around −5‰ [76], while the δ15N(NOx) in atmospheric deposition ranges from −13‰ to 13‰ [77]. Synthetic fertilizers generally have δ15N values ranging from −4‰ to 4‰ [78]. However, δ15N alone cannot accurately indicate the different sources of nitrates. As such, the analysis of δ18O and Δ17O in nitrates becomes crucial in accurately identifying their sources. Integrating the data of δ15N, δ18O, and Δ17O allows a more precise identification of the origins, formation conditions, and transfer pathways of nitrate formation.
Study of stable isotopes and isotopic anomalies of atmospheric nitrates is mainly focused on the coastal cities of Asia and North America and the western coast of Australia (Figure 2). However, there is a lack of investigation of atmospheric nitrates in glacier areas. High δ15N values of atmospheric nitrate are distributed in the eastern coastal areas of Asia, the eastern coastal areas of North America, and the western coast of Australia. This is likely related to intensive anthropogenic emissions [76,77,79]. High Δ17O values of atmospheric nitrate could also be found in the eastern coastal areas of Asia, the western coast of Australia, and the northern regions of North America, while high δ18O values of atmospheric nitrate are spatially distributed along the western coast of Australia and the eastern United States. The different Δ17O and δ18O values of atmospheric nitrate are greatly influenced by atmospheric O3 concentration and the pathways involved in its formation [12,24,80,81,82,83].
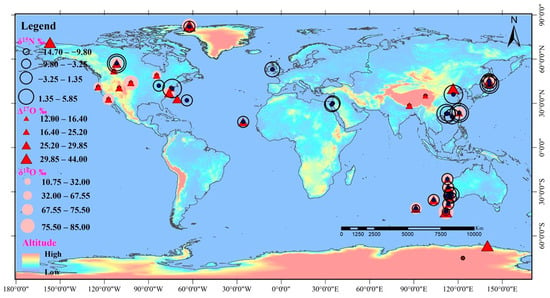
Figure 2.
Spatial distribution of stable isotopes (δ15N, δ18O) and oxygen isotope anomaly (Δ17O) in atmospheric nitrate.
4.1. Investigation of δ15N in Atmospheric NOx
Formation of NO3− in the atmosphere primarily occurs through the oxidative reactions of NOx. Atmospheric NOx has both natural and anthropogenic sources. Natural sources include lightning, wildfires, microbial activities, and stratospheric transport. Anthropogenic sources primarily consist of the following: (1) fossil fuel combustion, such as emissions from coal-fired power plants and vehicle and aircraft exhaust emissions, (2) biomass burning emissions from agricultural waste (e.g., straw, sugarcane residue, rice husk) and household heating or cooking, and (3) agricultural activities, particularly fertilizer application, which significantly impacts the release of NOx into the atmosphere [84]. Moreover, the dry and wet deposition of atmospheric NOx is the main pathway/source of nitrate content in snow and ice. Stable nitrogen isotope of nitrate can be used to determine the origins and chemical processes of atmospheric nitrogen. The calculation method of nitrogen isotope ratios is as follows:
δ15Nsample (‰) = [(15N/14N) sample/(15N/14N standard) − 1] × 1000
Different sources of NOx have different δ15N values, and emissions from fossil fuel combustion are the primary sources of NOx in the atmosphere (e.g., automobile emission and coal combustion and power plant emissions) [79,85,86]. Stable nitrogen isotopes from natural sources (such as lightning and coal combustion) typically exhibit positive values [87,88,89]. Generally, NOx from anthropogenic sources generated during the combustion process can be categorized into two types. The first is “thermal decomposition NOx,” which occurs at very high temperatures (>2000 °C). Under high oxygen and pressure conditions in the combustion chamber, nitrogen and oxygen inter-reaction occurs, resulting in a typically negative δ15N value. Examples of fuels that undergo thermal decomposition NOx include diesel, gasoline, and natural gas. The second type is “combustion NOx”, which is formed during the combustion of nitrogen oxides in the fuel itself. As the reaction temperatures are not very high (1300~1400 °C), the resulting δ15N(NOx) value is typically positive [90,91,92]. The δ15N(NOx) values contributed from the combustion of fossil fuels ranged from −17.9‰ to 19.8‰ (Figure 3) [79,85,86,93,94,95]. The δ15N values of nitrogen-containing particles generated from fuel combustion are relatively small, mostly having negative values (−19.4‰ to 2.9‰) [95]. On the other hand, the δ15N values of NOx produced from coal combustion are relatively high (5.2‰ to 19.8‰) [79,86,93]. Different combustion conditions (temperatures of combustion, pressures, oxygen levels, biomasses, fossil fuels, etc.) of fossil fuels significantly influence nitrogen isotopes and their composition [86,95]. For example, diesel and gasoline have similar chemical properties, but their nitrogen isotope values are quite different (diesel: +4.6‰ ± 0.8‰; gasoline: −7.5‰ ± 8.3‰), which is due to their different combustion processes in terms of combustion temperature and oxygen content [95]. Moreover, the δ15N values of NOx in vehicle exhaust are lower (−13‰ to −2‰) compared to the values detected from power plant emitted exhausts (6‰ to 13‰). This is primarily due to the reaction (N2 + O2 ↔2NO) (>2000 °C) that favors the formation of lighter 14N molecules, resulting in negative δ15N(NOx) values in vehicle exhaust [86]. In power plants, the reaction temperature is relatively low (1300–1400 °C), resulting in the low production of NO and a positive δ15N(NOx) value [86]. Notably, there exist significant differences in stable isotope values of nitrate from different sources. Factors contributing to these differences include combustion form, fuel type, chamber structure, and testing method. Therefore, it is necessary to conduct a detailed stable isotope analysis of nitrogen to better understand the exact sources, chemical processes, and formation pathways of nitrogen in atmosphere.
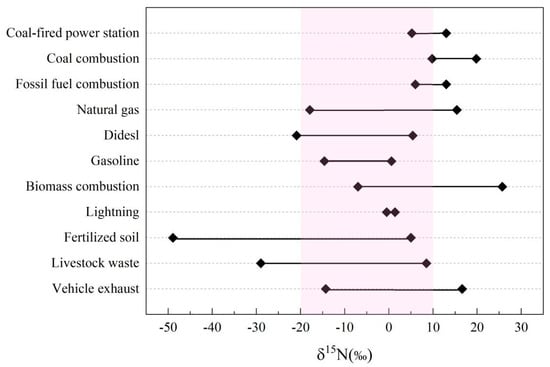
Figure 3.
Distribution of δ15N (‰) of atmospheric nitrate contributed from different sources.
4.2. Variability of δ18O and Δ17O of Atmospheric NO3−
The main oxidants that promote the conversion of NOx to NO3− in the atmosphere are O2, O3, OH, HO2/RO2, and XO. Figure 4 presents the spatial variations of δ18O and Δ17O of nitrates in the atmosphere across different latitudes at the global scale. There are two distinct patterns in both hemispheres. In the Northern Hemisphere, the δ18O values of nitrate in the atmosphere significantly increase according to latitude gradients. The variation of δ18O(NO3−) in the atmosphere is more obvious with notable fluctuations in lowlow latilatitude regions (30° N–60° N). The variation of δ18O(NO3−) with latitudes is primarily due to the gradient differences of oxygen isotope of O3 in the atmosphere [80,81,82]. It is also caused by significant spatial differences in the formation pathways of NO3− [80,81]. The variation pattern of Δ17O(NO3−) with latitude is similar to that of δ18O(NO3−) in the atmosphere. The Δ17O(NO3−) values in the atmosphere significantly increase along high latitudes (60° N–80° N) in the Northern Hemisphere. The latitude variation of Δ17O(NO3−) in the atmosphere is likely influenced by different formation pathways of nitrate [1,12,13,24,35,81].
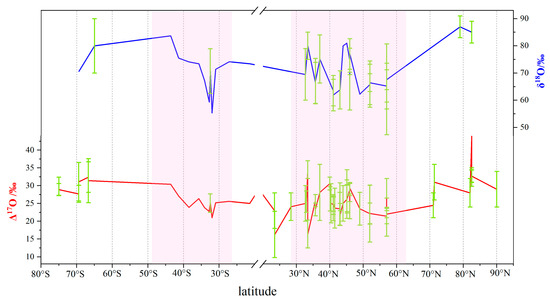
Figure 4.
Latitude variations of Δ17O(NO3−) and δ18O(NO3−) in the atmosphere.
The characteristic values of Δ17O(NO3−) and δ18O(NO3−) in the atmosphere are both positive, with Δ17O values being smaller than δ18O values (15~30‰) (Figure 4). Δ17O(NO3−) and δ18O(NO3−) in the atmosphere exhibit clear seasonal variations [96]. The Δ17O(NO3−) and δ18O(NO3−) values in summer/spring are lower than those in winter. The seasonal variations of ozone content in the atmosphere have significant impacts on the Δ17O and δ18O values [1,15,97] . The correlation between Δ17O and δ18O values mainly lies in their similar seasonal variations. However, the driving factors behind the seasonal variations of Δ17O and δ18O are different [97]. The seasonal variation of δ18O(NO3−) in the atmosphere is primarily influenced by the different oxidation pathways of NO3− in winter and summer, and the seasonal variation of O3 concentration and light intensity. The δ18O value of the O3 pathway is higher than that of the ·OH pathway. In the summer months, there is strong solar radiation, leading to a high degree of decomposition of volatile organic compounds (VOCs). As a consequence, the O3 production rate decreases, leading to a greater generation of NO3− through the ·OH pathway in the summer atmosphere. Conversely, during the winter months, there is relatively weaker solar radiation and a lower degree of VOCs decomposition, and the O3 production rate increases, causing a higher proportion of NO3− produced through the O3 pathway in the winter atmosphere [12,13,14]. The seasonal variation of Δ17O(NO3−) values is due to seasonal differences in the oxidation pathways of NOx [15,19]. Due to the photolysis of N2O5, NO3− is primarily generated through the (NO2 + ·OH) pathway during the day, while at night, it is mainly produced through the hydrolysis of N2O5 or the reaction of NO3− radicals with HC/DMS. Notably, the Δ17O values in the N2O5 hydrolysis or the reaction of NO3− radicals with HC/DMS to form NO3− are higher than those in the ·OH pathway [14,31]. In summer, there is a stronger radiation intensity and a longer duration of sunlight compared to winter, which results in the significant photolysis of N2O5 and indirectly leads to higher concentrations of ·OH. Consequently, lower Δ17O values are observed in summer, while higher Δ17O values are observed in winter. In addition, the seasonal variation of atmospheric water vapor content can impact the oxidation formation of NO3−. Higher water vapor content can facilitate the generation of NO3− through the hydrolysis pathway of N2O5, thus influencing the value of Δ17O [32]. Currently, our understanding of the reaction processes involving NO3− in the atmosphere is still limited. The relative contributions of each process may vary; thus, more in-depth research employing chemical models is necessary to investigate the underlying mechanisms of the seasonal variations in Δ17O(NO3−) and δ18O(NO3−).
The generation process of nitrate ion (NO3−) in the atmosphere is rather complex. It is difficult to accurately determine the chemical pathways of nitrate solely through measuring Δ17O of atmospheric nitrate. Currently, many studies combined measured Δ17O(NO3−) with atmospheric chemistry models to further analyze the chemical pathways and formation mechanisms of atmospheric nitrate in various regions and seasons. Atmospheric chemistry models usually include chemistry box models, GEOS-Chem chemical transport models, and photochemical steady-state (PSS) [14,26,33,97]. For example, a chemistry box model has been employed to simulate the presence of nitrate in coastal aerosols in California, USA, and its simulation results were in good agreement with the experimental data during the spring and winter seasons [14]. However, a divergence emerged during the late summer and autumn, where the simulated values were found to be 2‰–4‰ higher than the experimental measurements. This discrepancy can be attributed to the oversight of nitrate transport in the box model, highlighting the importance and necessity of considering the transport processes in precisely predicting atmospheric nitrate in coastal aerosols [14]. The results simulated from the chemistry box model indicate that during the spring season, the formation of nitrate primarily occurs through the hydrolysis of N2O5 and with high Δ17O values, whereas during the summer, the higher oxidation of HO2/RO2 results in lower Δ17O values. Simulations of Δ17O values in the tropical marine boundary layer show that PSS more accurately stimulates Δ17O variations, while GEOS-Chem still suffers from some errors due to the utilization of the photochemical steady-state equation at night [33,97].
5. Characteristics of Nitrogen–Oxygen Isotopes of Nitrates in Snow and Ice
Research on nitrates in snow and ice is primarily focused on the Antarctic region [25,81,98,99]. The concentration of NO3− in surface snow and ice in Antarctica ranges from 0.5 to 4.0 μeq L−1. It increases gradually from coastal regions towards the interior of Antarctica. The NO3− concentration in snow and ice from Southwestern Antarctic is lower compared to that of the Southeastern Antarctic region. Emerging evidence from earlier research suggests an inverse correlation between NO3− concentration in Antarctic snow/ice and the rate of snow accumulation [100]. In addition, as the thickness of snow increases, NO3− concentration gradually decreases, which is attributed to wind drift and sastrugi formation in the vicinity of the sampling site [25]. The concentration changes of NO3− after deposition and isotopic fractionation is influenced by the evaporation of HNO3, the photolysis of NO3−, and the wind-driven redistribution of surface snow, causing a rapid decrease in the surface concentration of NO3− [101]. However, δ15N(NO3−) values displayed an inverse trend with snow thickness and NO3− concentration. Strong enrichment of δ15N(NO3−) in snow with increasing snow depth due to isotopic fractionation is caused by the mass loss of NO3− in the upper snowpack [102]. The variations of δ18O and δ15N along the vertical profiles of snow layer are opposite, as the snow depth increases, δ18O values gradually decrease. The variation of Δ17O along the depths of snow has a similar trend to that of δ18O, which is due to the fact that in NO3− deposition, the NO3− is formed secondarily after NO3− photolysis, and the isotopes of ·OH and H2O are exchanged, resulting in a decrease in δ18O values, whereas the Δ17O values of ·OH and H2O are close to zero, and the Δ17O value decreases during the secondary NO3− formation [25,98]. This trend of NO3− and its isotopic composition usually can be observed in the low-accumulation regions of interior Antarctica and is mainly driven by the photolysis and recycling processes of NO3− [25,81,98,99,102] Isotope analysis suggested that δ15N(NO3−) in Antarctic snow ranges from −19.4‰ to 36.6‰. The significant variation of δ15N(NO3−) values likely indicates the fractionation of nitrogen isotopes in the snow of Antarctica [81,103]. The NO3− and its δ15N(NO3−) values exhibit similar spatial variation, namely, their values gradually increase from the coasts of East Antarctica towards/to the inland of Antarctica [81,99]. The δ18O(NO3−) values in Antarctic snow and ice range from 12‰ to 101‰ (Table S1). The spatial variation of δ18O(NO3−) is different compared with that of δ15N, and in areas with high snow accumulation rates along the Antarctic coast, the δ18O(NO3−) values are higher, whereas in areas with lower accumulation rates in the inland, the δ18O values are lower [99]. The spatial variation of δ18O values of nitrate in Antarctic snow/ice is primarily influenced by the formation pathways of NO3− in snow layers. Additionally, once NOx is deposited in snow layers, they will undergo consequent physical and chemical transformations. The products of NO3− photolysis undergo secondary oxidation reactions, leading to the regeneration of NO3− in surface snow and ice [103,104]. Moreover, it was reported that the Δ17O(NO3−) values in Antarctic snow range from 13.2‰ to 44‰ [99,105]. The variation amplitude of Δ17O(NO3−) is smaller compared to those of δ15N and δ18O, suggesting that the spatial variation of Δ17O(NO3−) in Antarctic snow is not obvious. The trend of Δ17O(NO3−) is similar to that of δ18O, i.e., it gradually decreases from coastal areas to inland Antarctica [106].
6. Optical Properties of Nitrate in Snow and Ice
Higher concentrations of nitrate in snow and ice can lead to increased light absorption, affecting the albedo of surface snow/ice, which in turn can impact the energy exchange and flux between the Earth’s surface and the atmosphere [25,107]. The deposition of NO3− from the atmosphere onto the surface of snow layers causes a series of complex physical and chemical reactions, which occur at the air–snow interface. During this process, in addition to NO3− concentration changes, the fractionation of nitrogen and oxygen isotopes in NO3− also takes place. The photochemical processes of NO3− in snow and ice can be described as follows, with reactions (19) and (20) being the main reactions of photolysis [108].
NO3− + hv →NO2−+ O(3P)
NO3− + hv → NO2 + O−
NO2− + hν → NO + O−
NO2− + OH→NO2 + OH−
These photochemical reactions significantly affect NO3− concentrations in snow and play an important role in the transformation of nitrogen species and impact snowpack chemistry [108,109]. The nitrogen and oxygen isotope fractionation coefficients (18ε = −34‰ and 15ε = −48‰) of NO3− photo-decomposition in Dome C ice core have been quantified [25,110]. Comparative results with the Raleigh fractionation model indicate that 15ε has been underestimated. Snow samples exposed to ultraviolet radiation and placed in a closed laboratory environment greatly impact the photolysis and photo-decomposition of NO3− in snow. In this setting, the photolytic products such as NO2 and OH were involved in subsequent NO3− generation and deposition, leading to the dilution of the isotopic ratios [102]. The secondary production of NO3−, as a result of the photolysis of NO3−, and the by-products (such as NO2 and NO) of photolysis react with oxidants in the atmosphere, results in the variation of nitrogen isotopes [104,111]. Additionally, the photochemical decomposition of NO3− significantly affects the isotope changes in snow. Investigating and understanding the photochemical processes of NO3− in snow and ice is crucial for evaluating its environmental fate and impact on atmospheric composition.
7. Conclusions
The stable isotopes and isotopic anomalies of nitrate can be used to robustly trace the sources of NO3− and determine the oxidation pathways of NO3− and to understand the chemical cycle of NO3− in multiple environmental media. This study mainly reviewed and synthesized the chemical processes of nitrate in atmosphere and snow/ice. The conclusions that can be drawn are as follows:
- (1)
- The formation of nocturnal atmospheric nitrates primarily occurs through the hydrolysis of N2O5. During the daytime, the nitrates are mainly generated through the oxidation of NO2 by OH radicals, resulting in the formation of HNO3. In summer, nitrates are mainly formed through the reaction of OH radicals with NO2, while in winter, the hydrolysis of N2O5 is the main process for the formation of atmospheric nitrate.
- (2)
- Research on stable isotopes and isotopic anomaly of atmospheric nitrate in glacier regions is scarce, especially in high-altitude glacier regions.
- (3)
- The Δ17O and δ18O values of nitrate in the atmosphere increase with latitude. The variation of δ18O(NO3−) is more obvious with notable fluctuations in low-latitude regions (30° N–60° N).
- (4)
- From the coastal areas to inland Antarctica, δ18O(NO3−) values in snow and ice gradually decrease and δ15N(NO3−) values increase. Photochemical reactions significantly facilitate the interaction of NOx and NO3− at the air–snow interface, and greatly affect the NO3− concentration.
A study of the chemical processes of atmospheric nitrate and snow–ice nitrate in mountain glaciers is very rare and is currently in its initial stage. The study on the oxidation process of nitrate at the air–snow interface and in snow and ice of mountain glaciers has not been performed yet. Stable isotopes and isotopic anomaly of nitrate, as emerging techniques for the study of formation pathway and chemical processes of nitrate in atmosphere and snow/ice, can be used to examine/investigate the contribution of oxidation pathways of nitrate in high-altitude mountain glaciers. The study of nitrate isotopes in ice cores has not been conducted yet, which is an obvious research gap of cryosphere chemistry and can be bridged with the fields of climate study and environmental records. The study of chemical processes of nitrate in snow pits, ice cores, and the air–snow interface on glaciers needs a combination of numerical model simulation and extensive field observations of nitrate. Further study on the chemical processes of nitrate in the atmosphere and snow/ice is also needed to improve the understanding of its sources, formation mechanisms, and spatial–temporal variations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos15010059/s1, Table S1: Summary of values of Δ17O(NOx), δ18O(NOx), and δ15N(NOx) in Antarctic snow [81,99,112].
Author Contributions
Conceptualization, M.C.; methodology, Y.X.; software, Y.X.; formal analysis, M.C.; investigation, Y.X.; data curation, Y.X.; writing—original draft, M.C.; writing—review and editing, H.N.; visualization, H.N.; supervision, Y.X.; project administration, H.N.; funding acquisition, H.N.; validation, M.C.; Resources, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number: 41971080, and Youth Innovation Promotion Association CAS, grant number: 2021429.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41971080). We also thank the Youth Innovation Promotion Association CAS (2021429) for its support. We would also like to thank the experts for their support and help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savarino, J.; Kaiser, J.; Morin, S.; Sigman, D.M.; Thiemens, M.H. Nitrogen and oxygen isotopic constraints on the origin of atmospheric nitrate in coastal Antarctica. Atmos. Chem. Phys. 2007, 7, 1925–1945. [Google Scholar] [CrossRef]
- Gao, Z.M.; Zhang, F.Z. Research progress and perspectives on nitrogen cycle and pollution in the environment. Environ. Sci. Ser. 1982, 4, 7–12. (In Chinese) [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Savarino, J.; Frey, M.M.; Yan, N.; Bekki, S.; Bottenheim, J.W.; Martins, J.M.F. Tracing the Origin and Fate of NOx in the Arctic Atmosphere Using Stable Isotopes in Nitrate. Science 2008, 322, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.F.; Chen, Y.; Hao, Q.J.; Wang, H.B.; Yang, F.M.; Zhao, Q.; Bo, Y.; He, K.B.; Yao, Y.G. Seasonal variation and source analysis of the water-soluble inorganic ions in fine particulate matter in Suzhou. Environ. Sci. 2016, 37, 4482–4489. (In Chinese) [Google Scholar]
- Fan, M.Y.; Cao, F.; Zhang, Y.Y.; Bao, M.Y.; Liu, X.Y.; Zhang, W.Q.; Gao, S.; Zhang, Y.L. Characteristics and source of water soluble inorganic ions in fine particulate matter during winter in Xuzhou. Environ. Sci. 2017, 38, 4478–4485. (In Chinese) [Google Scholar]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Xie, Z.; Chi, X.; Yu, X.; Fan, S.; Kang, H.; Liu, C.; Zhan, H. Atmospheric Δ17O (NO3−) reveals nocturnal chemistry dominates nitrate production in Beijing haze. Atmos. Chem. Phys. 2018, 18, 14465–14476. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhang, Y.L. Oxygen isotope anomaly (Δ17O) in atmospheric nitrate: A review. Chin. Sci. Bull. 2019, 64, 649–662. (In Chinese) [Google Scholar] [CrossRef]
- Lamsal, L.N.; Martin, R.V.; Padmanabhan, A.; Zhang, Q.; Sioris, C.E.; Chance, K.; Kurosu, T.P.; Newchurch, M.J. Application of satellite observations for timely updates to global anthropogenic NOx emission inventories. Geophys. Res. Lett. 2011, 38, 185–193. [Google Scholar] [CrossRef]
- Kato, N.; Akimoto, H. Anthropogenic emissions of SO2 and NOx in Asia: Emission inventories. Atmos. Environ. Part A Gen. Top. 1992, 26, 2997–3017. [Google Scholar] [CrossRef]
- Savard, M.M.; Cole, A.; Vet, R.; Smirnoff, A. The Δ17O and δ18O values of simultaneously collected atmospheric nitrates from anthropogenic sources—Implications for polluted air masses. Atmos. Chem. Phys. 2018, 18, 10373–10389. [Google Scholar] [CrossRef]
- Morin, S.; Savarino, J.; Bekki, S.; Gong, S.; Bottenheim, J.W. Signature of Arctic surface ozone depletion events in the isotope anomaly (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 2007, 7, 1451–1469. [Google Scholar] [CrossRef]
- Michalski, G.; Scott, Z.; Kabiling, M.; Thiemens, M.H. First measurements and modeling of Δ17O in atmospheric nitrate. Geophys. Res. Lett. 2003, 30, GL017015. [Google Scholar] [CrossRef]
- Morin, S.; Savarino, J.; Frey, M.M.; Domine, F.; Jacobi, H.W.; Kaleschke, L.; Martns, J.M.F. Comprehensive isotopic composition of atmospheric nitrate in the Atlantic Ocean boundary layer from 65° S to 79° N. J. Geophys. Res. Atmos. 2009, 114, D05303. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 1973. [Google Scholar]
- Criss, R.E. Principles of Stable Isotope Distribution; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Matsuhisa, Y.; Goldsmith, J.R.; Clayton, R.N. Mechanisms of hydrothermal crystallization of quartz at 250-degrees-C and 15 Kbar. Geochim. Cosmochim. Acta 1978, 42, 173. [Google Scholar] [CrossRef]
- Kunasek, S.A.; AlexandeL, B.; Steig, E.J.; Hastings, M.G.; Gleason, D.J.; Jarvis, J.C. Measurements and modeling of Δ17O of nitrate in snowpits from Summit, Greenland. J. Geophys Res. 2008, 113, JD010103. [Google Scholar] [CrossRef]
- Miller, M.F. Isotopic fractionation and the quantification of 17O anomalies in the oxygen three-isotope system: An appraisal and geochemical significance. Geochim. Cosmochim. Acta 2002, 66, 1881–1889. [Google Scholar] [CrossRef]
- Meijer HA, J.; Li, W.J. The use of electrolysis for accurate Δ17O and δ18O isotope measurements in water. Isot. Environ. Health Stud. 1998, 34, 349–369. [Google Scholar] [CrossRef]
- Luz, B.; Barkan, E. The isotopic ratios 17O/16O and 18O/16O in molecular oxygen and their significance in biogeochemistry. Geochim. Cosmochim. Acta 2005, 69, 1099–1110. [Google Scholar] [CrossRef]
- Farquhar, J.; Savarino, J.; Jackson, T.L.; Thiemens, M.H. Evidence of atmospheric sulphur in the Martian regolith from sulphur isotopes in meteorites. Nature 2000, 404, 50–52. [Google Scholar] [CrossRef]
- Kaiser, J.; Hastings, M.G.; Houlton, B.Z.; Röckmann, T.; Sigman, D.M. Triple oxygen isotope analysis of nitrate using the denitrifier method and thermal decomposition of N2O. Anal. Chem. 2007, 79, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.M.; Savarino, J.; Morin, S.; Erbland, J.; Martins JM, F. Photolysis imprint in the nitrate stable isotope signal in snow and atmosphere of East Antarctica and implications for reactive nitrogen cycling. Atmos. Chem. Phys. 2009, 9, 8681–8696. [Google Scholar] [CrossRef]
- Alexander, B. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 2009, 9, 5043–5056. [Google Scholar] [CrossRef]
- Patris, N.; Cliff, S.S.; Quinn, P.K.; Kasem, M.; Thiemens, M.H. Isotopic analysis of aerosol sulfate and nitrate during ITCT-2k2: Determination of different formation pathways as a function of particle size. J. Geophys. Res. Atmos. 2007, 112, D23301. [Google Scholar] [CrossRef]
- Savarino, J.; Morin, S.; Erbland, J.; Grannec, F.; Patey, M.D.; Vicars, W.; Alexander, B.; Achterberg, E.P. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl. Acad. Sci. USA 2013, 110, 17668–17673. [Google Scholar] [CrossRef] [PubMed]
- Vicars, W.C.; Morin, S.; Savarino, J.; Wagner, N.L.; Erbland, J.; Vince, E.; Martins, J.M.F.; Lerner, B.M.; Quinn, P.K.; Coffman, D.J.; et al. Spatial and diurnal variability in reactive nitrogen oxide chemistry as reflected in the isotopic composition of atmospheric nitrate: Results from the CalNex 2010 field study. J. Geophys Res. Atmos. 2013, 118, 10567–10588. [Google Scholar] [CrossRef]
- Bourgeois, I.; Savarino, J.; Caillon, N.; Angot, H.; Barbero, A.; Delbart, F.; Voisin, D.; Clement, J.C. Tracing the fate of atmospheric nitrate in a subalpine watershed using Δ17O. Environ. Sci. Technol. 2018, 52, 5561–5570. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, W.; Fan, M.Y.; Li, J.; Fang, H.; Cao, F.; Lin, Y.C.; Wilkins, B.P.; Liu, X.; Bao, M.; et al. A diurnal story of Δ17O(NO3−) in urban Nanjing and its implication for nitrate aerosol formation. npj Clim. Atmos. Sci. 2022, 5, 50. [Google Scholar] [CrossRef]
- Qin, R.; Shi, G.T.; Chen, Z.L. Review of the study on the stable isotopes of nitrogen and oxygen in atmospheric nitrate. Adv. Earth Sci. 2019, 34, 124–139. (In Chinese) [Google Scholar] [CrossRef]
- Nelson, D.M.; Tsunogai, U.; Ding, D.; Ohyama, T.; Komatsu, D.D.; Nakagawa, F.; Noguchi, I.; Yamaguchi, T. Triple oxygen isotopes indicate urbanization affects sources of nitrate in wet and dry atmospheric deposition. Atmos. Chem. Phys. 2018, 18, 6381–6392. [Google Scholar] [CrossRef]
- Chang, C.; Langston, J.; Riggs, M.; Campbell, D.H.; Silva, S.R.; Kendall, C. A method for nitrate collection for delta δ15N and δ18O analysis from waters with low nitrate concentrations. Can. J. Fish. Aquat. Sci. 1999, 56, 1856–1864. [Google Scholar] [CrossRef]
- Michalski, G.; Savarino, J.; Böhlke, J.K.; Thiemens, M. Determination of the total oxygen isotopic composition of nitrate and the calibration of a Δ17O nitrate reference material. Anal. Chem. 2002, 74, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- McIlvin, M.R.; Altabet, M.A. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal. Chem. 2005, 77, 5589–5595. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A Bacterial Method for the Nitrogen Isotopic Analysis of Nitrate in Seawater and Freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef]
- Soto, D.X.; Koehler, G.; Hobson, K.A. Combining Denitrifying Bacteria and Laser Spectroscopy for Isotopic Analyses (δ15N, δ18O) of Dissolved Nitrate. Anal. Chem. 2015, 87, 7000–7005. [Google Scholar] [CrossRef] [PubMed]
- Casciotti, K.L.; Sigman, D.M.; Hastings, M.G.; Böhlke, J.K.; Hilkert, A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 2002, 4, 4905–4912. [Google Scholar] [CrossRef]
- Alexander, B. Global inorganic nitrate production mechanisms: Comparison of a global model with nitrate isotope observations. Atmos. Chem. Phys. 2020, 20, 3859–3877. [Google Scholar] [CrossRef]
- Brown, S.S. Budgets for nocturnal VOC oxidation by nitrate radicals aloft during the 2006 Texas Air Quality Study. J. Geophys. Res. Atmos. 2011, 116, D24305. [Google Scholar] [CrossRef]
- Preunkert, S.; Jourdain, B.; Legrand, M.; Udisti, R.; Becagli, S.; Cerri, O. Seasonality of sulfur species (dimethyl sulfide, sulfate, and methanesulfonate) in Antarctica: Inland versus coastal regions. J. Geophys. Res. Atmos. 2008, 113, D15302. [Google Scholar] [CrossRef]
- Fan, M.-Y.; Zhang, Y.; Lin, Y.; Cao, F.; Zhao, Z.; Sun, Y.; Qiu, Y.; Fu, P.; Wang, Y. Changes of emission sources to nitrate aerosols in Beijing after the clean air actions: Evidence from dual isotope compositions. J. Geophys. Res. Atmos. 2020, 125, e2019JD031998. [Google Scholar] [CrossRef]
- Burkholder, J.B.; Sander, S.P.; Abbatt, J.P.D.; Barker, J.R.; Huie, R.E.; Kolb, C.E.; Kurylo, M.J.; Orkin, V.L.; Wilmouth, D.M.; Wine, P.H. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation Number 18; California Institute of Technology: Pasadena, CA, USA, 2015).
- Tan, F.; Tong, S.; Jing, B.; Hou, S.; Liu, Q.; Li, K.; Zhang, Y.; Ge, M. Heterogeneous reactions of NO2 with CaCO3–(NH4)2SO4 mixtures at different relative humidities. Atmos. Chem. Phys. 2016, 16, 8081–8093. [Google Scholar] [CrossRef]
- Crowley, J.N.; Ammann, M.; Cox, R.A.; Hynes, R.G.; Jenkin, M.E.; Mellouki, A.; Rossi, M.J.; Troe, J.; Wallington, T.J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume V—Heterogeneous reactions on solid substrates. Atmos. Chem. Phys. 2010, 10, 9059–9223. [Google Scholar] [CrossRef]
- Chan, Y.; Evans, M.J.; He, P.; Holmes, C.D.; Jaeglé, L.; Kasibhatla, P.; Liu, X.; Sherwen, T.; Thornton, J.A.; Wang, X.; et al. Heterogeneous nitrate production mechanisms in intense haze events in the North China Plain. J. Geophys. Res. Atmos. 2021, 126, e2021JD034688. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Chen, H.; Yang, X.; Chen, J.; Geng, F. Particulate nitrate formation in a highly polluted urban area: A case study by single-particle mass spectrometry in Shanghai. Environ. Sci. Technol. 2009, 43, 3061–3066. [Google Scholar] [CrossRef]
- Wang, H.; Lu, K.; Guo, S.; Wu, Z.; Shang, D.; Tan, Z.; Wang, Y.; Le Breton, M.; Lou, S.; Tang, M.; et al. Efficient N2O5 uptake and NO3 oxidation in the outflow of urban Beijing. Atmos. Chem. Phys. 2018, 18, 9705–9721. [Google Scholar] [CrossRef]
- Thornton, J.A.; Kercher, J.P.; Riedel, T.P.; Wagner, N.L.; Cozic, J.; Holloway, J.S.; Dubé, W.P.; Wolfe, G.M.; Quinn, P.K.; Middlebrook, A.M.; et al. A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature 2010, 464, 271–274. [Google Scholar] [CrossRef]
- Evans, M.J. Coupled evolution of BrOx-ClOx-HOx-NOx chemistry during bromine-catalyzed ozone depletion events in the Arctic boundary layer. J. Geophys. Res. 2003, 108, 8368. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Plane, J.M.C.; Mahajan, A.S.; Anderson, P.S.; Bauguitte, S.J.-B.; Jones, A.E.; Roscoe, H.K.; Salmon, R.A.; Bloss, W.J.; Lee, J.D.; et al. On the vertical distribution of boundary layer halogens over coastal Antarctica: Implications for O3, HOx, NOx and the Hg lifetime. Atmos. Chem. Phys. 2008, 8, 887–900. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, J.; Ouyang, B.; Mehra, A.; Xu, W.; Wang, Y.; Bannan, T.J.; Worrall, S.D.; Priestley, M.; Bacak, A.; et al. Production of N2O5 and ClNO2 in summerm urban Beijing, China. Atmos. Chem. Phys. Discuss. 2018, 349, 11581–11597. [Google Scholar] [CrossRef]
- Tham, Y.J.; Wang, Z.; Li, Q.; Yun, H.; Wang, W.; Wang, X.; Xue, L.; Lu, K.; Ma, N.; Bohn, B.; et al. Significant concentrations of nitryl chloride sustained in the morning investigations of the causes and impacts on ozone production in a polluted region of northern China. Atmos. Chem. Phys. 2016, 16, 14959–14977. [Google Scholar] [CrossRef]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D.J.A.C.; et al. Exploring the severe winter haze in Beijing the impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, Z.; Liang, H.; Huang, L.; Huang, D. CarbonyI compounds over urban Beijing Concentrations on haze and non-haze days and effects on radical chemistry. Atmos. Environ. 2016, 124, 207–216. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Lu, K. Development of a portable cavity—Enhanced absorption spectrometer for the measurement ambient NO3 and N2O5 experimental setup, lab characterizations, and field applications in a polluted urban environment. Atmos. Meas. Tech. 2017, 10, 1465. [Google Scholar] [CrossRef]
- Wang, H.; Lu, K.; Chen, X.; Zhu, Q.; Chen, Q.; Guo, S.; Jiang, M.; Li, X.; Shang, D.; Tan, Z.; et al. High N2O5 concentrations observed in urban Beijing Implications of a large nitrate formation pathway. Environ. Sci. Technol. Lett. 2017, 4, 416–420. [Google Scholar] [CrossRef]
- Li, Z.; Hu, R.; Xie, P.; Wang, H.; Lu, K.; Wang, D. Intercomparison of in situ CRDS and CEAS for measurements of atmospheric N2O5 in Beijing, China. Sci. Total Environ. 2018, 613, 131–139. [Google Scholar] [CrossRef]
- Su, X.; Tie, X.; Li, G.; Cao, J.; Huang, R.; Feng, T.; Long, X.; Xu, R. Effect of hydrolysis of N2O5 on nitrate and ammonium formation m Beijing China WRF—Chem model simulation. Sci. Total Environ. 2017, 579, 221–229. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wu, W.S.; Wang, T. Summertime PM2.5 ionic species in four major cities of China nitrate formation in an 631 ammonia deficient atmosphere. Atmos. Chem. Phys. 2009, 9, 1711–1722. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Guo, J.; Wang, Z.; Zhang, M. Observation of nitrous acid (HONO) in Beijing, China Seasonal variation, nocturnal formation and daytime budget. Sci. Total Environ. 2017, 587, 350–359. [Google Scholar] [CrossRef]
- Tong, S.; Hou, S.; Zhang, Y.; Chu, B.; Liu, Y.; He, H.; Zhao, P.; Ge, M. Comparisons of measured nitrous acid (HONO) concentrations in a pollution period at urban and suburban Beijing, in autumn of 2014, in autumn of 2014. Sci. China Chem. 2015, 58, 1393–1402. [Google Scholar] [CrossRef]
- Browne, E.C.; Cohen, R.C. Effects of biogenic nitrate chemistry on the NOx lifetime in remote continental regions. Atmos. Chem. Phys. 2012, 12, 11917–11932. [Google Scholar] [CrossRef]
- Kasibhatla, P.; Sherwen, T.; Evans, M.J.; Carpenter, L.J.; Reed, C.; Alexander, B.; Chen, Q.; Sulprizio, M.P.; Lee, J.D.; Read, K.A.; et al. Global impact of nitrate photolysis in sea-salt aerosol on NOx, OH, and O3 in the marine boundary layer. Atmos. Chem. Phys. 2018, 18, 11185–11203. [Google Scholar] [CrossRef]
- Müller, J.F.; Peeters, J.; Stavrakou, T. Fast photolysis of carbonyl nitrates from isoprene. Atmos. Chem. Phys. 2014, 14, 2497–2508. [Google Scholar] [CrossRef]
- Xu, L.; Guo, H.; Boyd, C.M.; Klein, M.; Bougiatioti, A.; Cerully, K.M.; Hite, J.R.; Isaacman-VanWertz, G.; Kreisberg, N.M.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. P. Natl. Acad. Sci. USA. 2015, 112, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rindelaub, J.D.; McAvey, K.M.; Shepson, P.B. The photochemical production of organic nitrates from α-pinene and loss via acid-dependent particle phase hydrolysis. Atmos. Environ. 2015, 100, 193–201. [Google Scholar] [CrossRef]
- Jacobs, M.I.; Burke, W.J.; Elrod, M.J. Kinetics of the reactions of isoprene-derived hydroxynitrates: Gas phase epoxide formation and solution phase hydrolysis. Atmos. Chem. Phys. 2014, 14, 8933–8946. [Google Scholar] [CrossRef]
- Legrand, M.; Preunkert, S.; Frey, M.; Bartels-Rausch, T.; Kukui, A.; King, M.D.; Savarino, J.; Kerbrat, M.; Jourdain, B. Large mixing ratios of atmospheric nitrous acid (HONO) at Concordia (east Antarctic plateau) in summer: A strong source from surface snow? Atmos. Chem. Phys. 2014, 14, 9963–9976. [Google Scholar] [CrossRef]
- Dentener, F.; Williams, J.; Metzger, S. Aqueous phase reaction of HNO4: The impact on tropospheric chemistry. J. Geophys. Res. Atmos. 2002, 41, 109–133. [Google Scholar]
- Régimbal, J.M.; Mozurkewich, M. Peroxynitric acid decay mechanisms and kinetics at low PH. J. Phys. Chem. A 1997, 101, 8822–8829. [Google Scholar] [CrossRef]
- Løgager, T.; Sehested, K. Formation and decay of peroxynitrous acid: A pulse radiolysis study. J. Phys. Chem. 1993, 97, 6664–6669. [Google Scholar] [CrossRef]
- Amel, D.F. Trends in the structure of federally insured depository institutions. Fed. Reserv. Bull. 1996, 82, 1984–1994. [Google Scholar]
- Mayer, B.; Boyer, E.W.; Goodale, C.; Jaworski, N.A.; van Breemen, N.; Howarth, R.W.; Seitzinger, S.; Billen, G.; Lajtha, K.; Nadelhoffer, K.; et al. Sources of nitrate in rivers draining sixteen watersheds in the northeastern U.S.: Isotopic constraints. Biogeochemistry 2002, 57, 171–197. [Google Scholar]
- Miller, D.J.; Wojtal, P.K.; Clark, S.C.; Hastings, M.G. Vehicle NOx emission plume isotopic signatures: Spatial variability across the eastern United States. J. Geophys. Res. Atmos. 2017, 8, 4698–4717. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing Anthropogenic Inputs of Nitrogen to Ecosystems. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Blackwell: Oxford, UK, 2007. [Google Scholar]
- Wassenaar, L.I. Evaluation of the origin and fate of nitrate in the abbotsford aquifer using the isotopes of 15N and 18O in NO3−. Appl. Geochem. 1995, 10, 391–405. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M.; Shaw, S.L. Nitrogen isotopic composition of coal-fired power plant NOx: Influence of emission controls and implications for global emission inventories. Environ. Sci. Technol. 2012, 46, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Freyer, H.D. Seasonal variation of 15N/14N ratios in atmospheric nitrate species. Tellus Ser. B—Chem. Phys. Meteorol. 1991, 43, 30–44. [Google Scholar] [CrossRef]
- Shi, G.; Buffen, A.; Ma, H.; Hu, Z.; Sun, B.; Li, C.; Yu, J.; Ma, T.; An, C.; Jiang, S.; et al. Distinguishing summertime atmospheric production of nitrate across the East Antarctic Ice Sheet. Geochim. Cosmochim. Acta. 2018, 231, 1–14. [Google Scholar] [CrossRef]
- Lyons, J.R. Transfer of mass-independent fractionation in ozone to other oxygen-containing radicals in the atmosphere. Geophys. Res. Lett. 2001, 28, 3231–3234. [Google Scholar] [CrossRef]
- Vicars, W.C.; Savarino, J. Quantitative constraints on the 17O-excess (Δ17O) signature of surface ozone: Ambient measurements from 50° N to 50° S using the nitrite-coated filter technique. Geochim. Cosmochim. Acta 2014, 135, 270–287. [Google Scholar] [CrossRef]
- Li, D.J.; Wang, X.M. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application. Atmos. Environ. 2008, 42, 4747–4754. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures. Atmos. Environ. 2014, 92, 359–366. [Google Scholar] [CrossRef]
- Heaton, T.H.E. 15N/14N ratios of NOx from vehicle engines and coal-fired power stations. Tellus B 1990, 42, 304–307. [Google Scholar] [CrossRef]
- Hoering, T. The isotope composition of the ammonia and nitrateion in rain. Geochim. Et Cosmochim. Acta 1958, 12, 97–102. [Google Scholar] [CrossRef]
- Fibiger, D.L.; Hastings, M.G. First Measurements of the nitrogen isotopic composition of NOx from biomass burning. Environ. Sci. Technol. 2016, 50, 11569–11574. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.G. Evaluating source, chemistry and climate change based upon the isotopic composition of nitrate in ice cores. IOP Conf. Ser. Earth Environ. Sci. 2010, 9, 012002. [Google Scholar] [CrossRef]
- Bowman, C.T. Kinetics of pollutant formation and destruction in combustion. Prog. Energy Combust. Sci. 1975, 1, 33–45. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Vince, I.M. Nitric oxide formation from N2 in flames: The importance of “prompt” NO. Prog. Energy Combust. Sci. 1980, 6, 35–51. [Google Scholar] [CrossRef]
- Toof, J.L. A Model for the Prediction of Thermal, Prompt, and Fuel NOx Emissions From Combustion Turbines. J. Eng. Gas Turbines Power 1986, 108, 340–347. [Google Scholar] [CrossRef]
- Heaton, T.H.E. 15N/14N ratios of nitrate and ammonium in rain at Pretoria, South Africa. Atmos. Environ. 1987, 21, 843–852. [Google Scholar] [CrossRef]
- Walters, W.W.; Tharp, B.D.; Fang, H.; Kozak, B.J.; Michalski, G. Nitrogen isotope composition of thermally produced NOX from various fossil-fuel combustion sources. Environ. Sci. Technol. 2015, 49, 11363–11371. [Google Scholar] [CrossRef]
- Widory, D. Nitrogen isotopes: Tracers of origin and processes affecting PM10 in the atmosphere of Paris. Atmos. Environ. 2007, 41, 2382–2390. [Google Scholar] [CrossRef]
- Guha, T.; Lin, C.T.; Bhattacharya, S.K.; Mahajan, A.S.; Ou-Yang, C.F.; Lan, Y.P.; Hsu, S.C.; Liang, M.C. Isotopic ratios of nitrate in aerosol samples from Mt. Lulin, a high-altitude station in central Taiwan. Atmos. Environ. 2017, 154, 53–69. [Google Scholar] [CrossRef]
- Morin, S.; Sander, R.; Savarino, J. Simulation of the diurnal variations of the oxygen isotope anomaly (Δ17O) of reactive at-mospheric species. Atmos. Chem. Phys. 2011, 11, 3653. [Google Scholar] [CrossRef]
- Berhanu, T.A.; Meusinger, C.; Erbland, J.; Jost, R.; Bhattacharya, S.K.; Johnson, M.S.; Savarino, J. Laboratory study of nitrate photolysis in Antarctic snow. II. Isotopic effects and wavelength dependence. J. Chem. Phys. 2014, 140, 244306. [Google Scholar] [CrossRef] [PubMed]
- Erbland, J.; Vicars, W.C.; Savarino, J.; Morin, S.; Frey, M.M.; Frosini, D.; Vince, E.; Martins, J.M.F. Air-snow transfer of nitrate on the East Antarctic Plateau—Part 1: Isotopic evidence for a photolytically driven dynamic equilibrium in summer. Atmos. Chem. Phys. 2013, 13, 6403–6419. [Google Scholar] [CrossRef]
- Arthern, R.J.; Winebrenner, D.P.; Vaughan, D.G. Antarctic snow accumulation mapped using polarization of 4.3 cm wavelength microwave emission. Atmospheres 2006, 111. [Google Scholar] [CrossRef]
- Frey, M.M.; Stewart, R.W.; McConnell, J.R.; Bales, R.C. Atmospheric hydroperoxides in West Antarctica: Links to stratospheric ozone and atmospheric oxidation capacity. J. Geophys. Res. 2005, 110, D23301. [Google Scholar] [CrossRef]
- Blunier, T.; Floch, G.L.; Jacobi, H.W.; Quansah, E. Isotopic view on nitrate loss in Antarctic surface snow. Geophys. Res. Lett. 2005, 32, L13501. [Google Scholar] [CrossRef]
- Shi, G.T.; Qin, R.; Ma, H.M.; Hu, Z.Y.; An, C.L.; Jiang, S.; Li, Y.S. A Review of the stable isotopic composition of nitrate in Antarctic snow and ice east. Chin. J. Polar Res. 2019, 31, 117–127. (In Chinese) [Google Scholar]
- McCabe, J.R.; Boxe, C.S.; Colussi, A.J.; Hoffman, M.R.; Thiemens, M.H. Oxygen isotopic fractionation in the photochemistry of nitrate in water and ice. J. Geophys. Res. 2005, 110, D15310. [Google Scholar] [CrossRef]
- Buffen, A.; Hastings, M.G. The isotopic composition of nitrate in west Antarctica at present and since the last glacial stage. In Proceedings of the American Geophysical Union, Fall Meeting 2014, San Francisco C31C-0310 (2014), San Francisco, TX, USA, 15–19 December 2014. [Google Scholar]
- Zatko, M.; Geng, L.; Alexander, B.; Sofen, E.; Klein, K. The impact of snow nitrate photolysis on boundary layer chemistry and the recycling and redistribution of reactive nitrogen across Antarctica and Greenland in a global chemical transport model. Atmos. Chem. Phys. 2016, 16, 2819–2842. [Google Scholar] [CrossRef]
- Ginot, P.; Kull, C.; Schwikowski, M.; Schotterer, U.; Gäggeler, H.W. Effects of post depositional processes on snowcomposition of asubtropical glacier (Cerro Tapado, Chilean Andes). J. Geophys. Res. Atmos. 2001, 106, 32375–32386. [Google Scholar]
- Mcfall, A.S.; Edwards, K.C.; Anastasio, C. Nitrate photochemistry at the air-ice interface and in other ice reservoirs. Environ. Sci. Technol. 2018, 52, 5710–5717. [Google Scholar] [CrossRef] [PubMed]
- Dibb, J.E.; Arsenault, M.; Peterson, M.C.; Honrath, R.E. Fast nitrogen oxide photochemistry in Summit, Greenland snow. Atmos. Environ. 2002, 36, 2501–2511. [Google Scholar] [CrossRef]
- Miller, C.E.; Yung, Y.L. Photo-induced isotopic fractionation. J. Geophys. Res. 2000, 105, 29039–29051. [Google Scholar] [CrossRef]
- Anastasio, C.; Galbavy, E.S.; Hutterli, M.A.; Burkhart, J.F.; Friel, D.K. Photoformation of hydroxyl radical on snow grains at Summit, Greenland. Atmos. Environ. 2007, 41, 5110–5121. [Google Scholar] [CrossRef]
- Noro, K.; Hattori, S.; Uemura, R.; Fukui, K.; Hirabayashi, M.; Kawamura, K.; Motoyama, H.; Takenaka, N.; Yoshida, N. Spatial variation of isotopic compositions of snowpack nitrate related to post-depositional processes in eastern Dronning Maud Land, East Antarctica. Geochem. J. 2018, 52, e7–e14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).