3.3.1. Effects of PM2.5 and Different Components on Cell Viability
Atmospheric PM
2.5 easily absorbs toxic substances due to its large specific surface area, seriously damaging human health [
35]. Epidemiological studies have shown that PM
2.5 can cause cardiovascular and cerebrovascular diseases, lung diseases and endocrine disorders [
36,
37]. Recently, more and more attention has been paid to the role of PM
2.5 in metabolic disorders (such as insulin resistance, neuroinflammation, etc.) [
36,
38]. However, the molecular mechanism of lipid metabolism disturbance caused by PM
2.5 leading to nonalcoholic fatty liver disease (NAFLD) is less studied.
Due to different emission sources, regions, weather, time and other factors, the composition of PM
2.5 is different to some extent, and the components of PM
2.5 can also interact with each other to produce secondary pollutants with greater toxicity [
39]. The composition of PM
2.5 varies greatly, and they cause different toxicological effects. Therefore, when assessing the impact of PM
2.5 particles on liver injury, it is necessary to consider not only the PM
2.5 particles themselves, but also the impact of each component of PM
2.5.
By studying the toxic effects of PM2.5 and its components, we can determine the primary air pollution sources in a certain period in this area and provide an experimental basis for air pollution control. Therefore, to explore the main toxic components of PM2.5 particles, we divided PM2.5 into WP, OP and WSP, and established an experimental model of HepG2 cell infection. In recent years, epidemiological investigations and animal experiments have shown that PM2.5 can cause NAFLD, but the differential effects of PM2.5 and its different components on liver function are rarely reported.
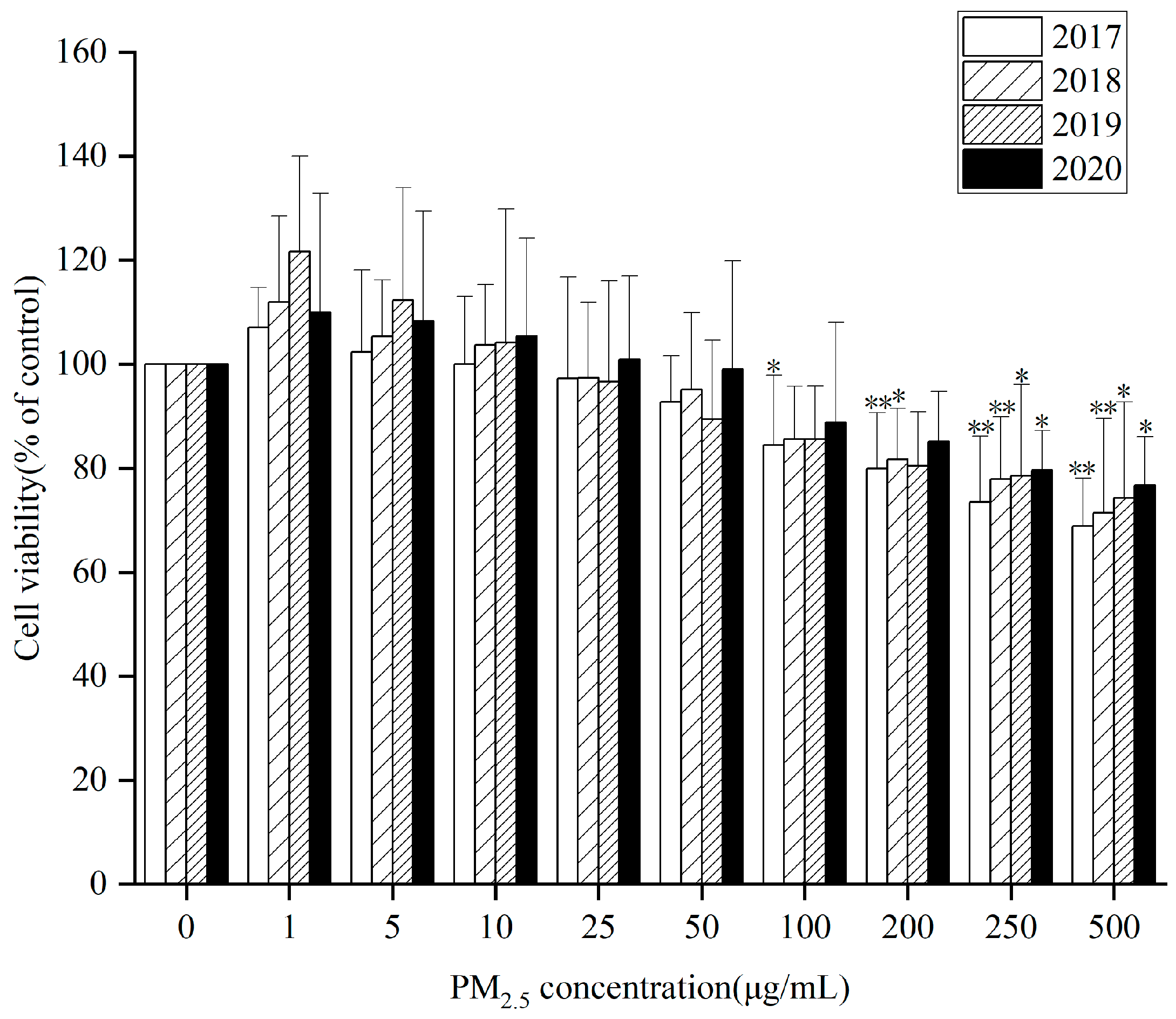
HepG2 cells were treated with three components of PM
2.5 (WP, OP and WSP) at different concentrations for 24 h from 2017 to 2020. Cell viability measured by CCK8 kit showed that after 24 h exposure, extracts (WP, OP, and WSP) from PM
2.5 samples for 4 years had significant cytotoxic effects on cells at 0, 50, 100, 200, 250, and 500 µg/mL compared to controls. Both 4-year WP (
Figure 1) and WSP (
Figure S2A) promoted cell proliferation at low concentrations (1, 5, 10 µg/mL). However, OP (
Figure S2B) inhibited cell proliferation with increasing concentration and the cell survival rate was lower at the same concentration for a particular year from 2017 to 2020. Relatively, OP was a key component in inhibiting cell proliferation. However, there was no significant difference in cell viability between different components of PM
2.5 over the years.
Next, LDH is an important enzyme in cells that is rapidly released from cells when the cell membrane is damaged and is often used to assess cell death or cytotoxicity [
40]. In this study, we found that, compared with the control group, LDH activity increased significantly at WP (
Figure 2), WSP (
Figure S3A), and OP (
Figure S3B) concentrations of 100 µg/mL from 2017 to 2020. In 2017, WP significantly increased LDH activity at a 50 µg/mL concentration. Elevating LDH, induced by WP components at 0, 25, and 50 µg/mL, showed significant differences in 2017 and 2019, while at 100 µg/mL, there were significant differences between 2017 and the other three years. When the WSP component was 100 µg/mL, LDH activity displayed remarkable differences in 2019. Of note, there was a significant difference in LDH activity between 2017 and 2019 when OP components were 50 and 100 µg/mL. This may be related to differences in physical and chemical properties due to different sources of particulate matter in 2019 [
41].
3.3.2. Effects of PM2.5 and Its Components on Inflammatory Factors and Oxidative Stress
Previous studies have illustrated that oxidative stress and abnormal upregulation of inflammation caused by irritants can aggravate liver dyslipidemia and lipid accumulation, ultimately promoting NAFLD development [
42,
43,
44]. Based on this, we evaluated markers of inflammation, oxidative stress, and lipid metabolism after exposure to HepG2 cells from different components of PM
2.5 in different years to determine their possible relationship.
Figure 3,
Figure 4 and
Figures S3 and S4 show that after exposure to different components of PM
2.5 from 2017 to 2020, the levels of inflammatory factors IL-6 and TNF-α in HepG2 cells gradually elevated with the increase of exposure concentration, suggesting that PM
2.5 and its components can trigger cellular inflammation.
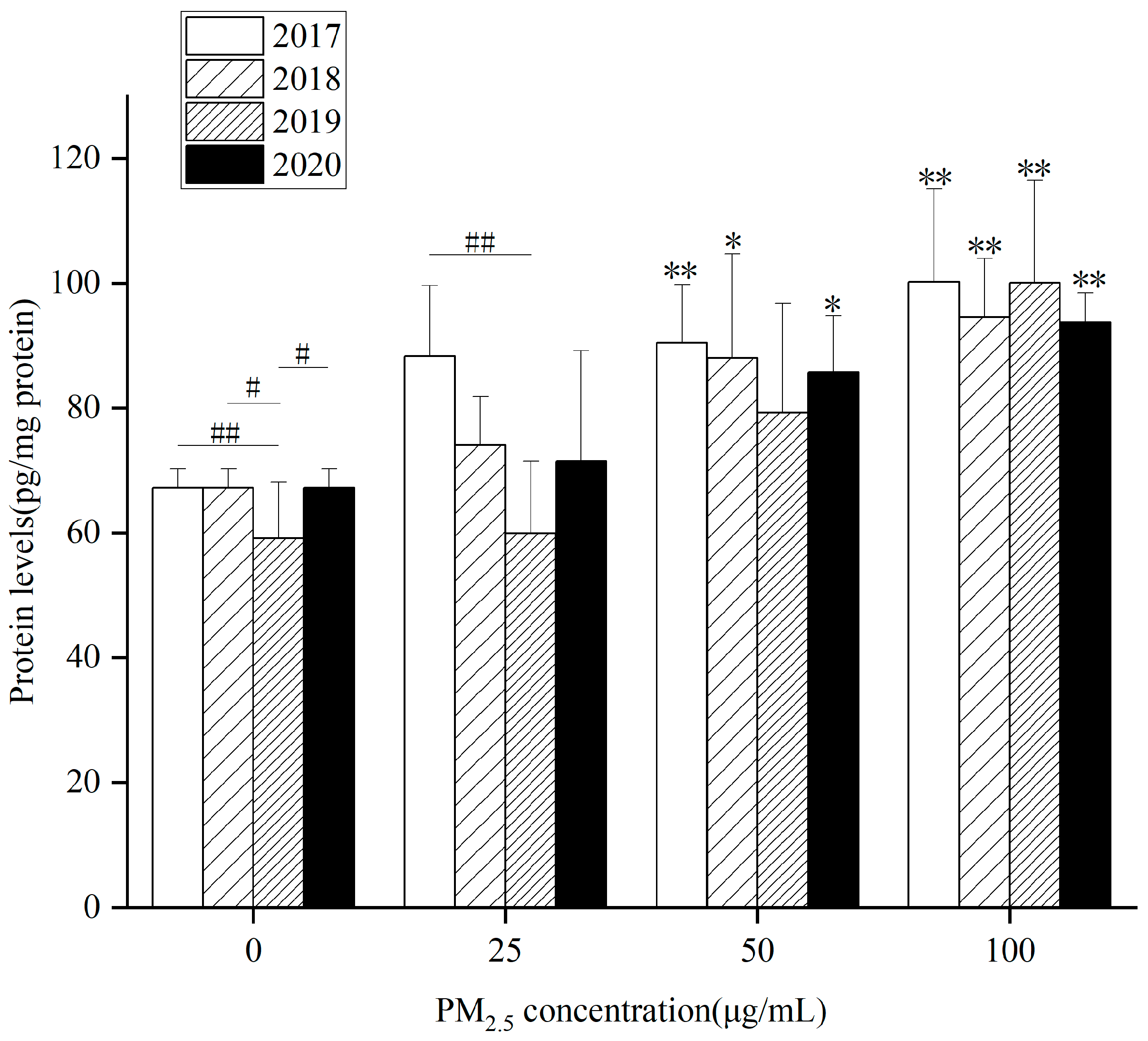
At the same concentration, each component in different years affected inflammatory factors differently. The key components that contribute to elevated levels of inflammatory factors also vary. In
Figure 3, IL-6 levels in HepG2 cells markedly raised after WP components exposure to 50 and 100 µg/mL for 24 h in 2017, 2018 and 2020. In 2019, it was only at 100 µg/mL that it was obviously higher than the control group. Compared with the control group, 4-year exposure to WSP (
Figure S4A) and OP (
Figure S4B) for 24 h remarkably up-regulated intracellular IL-6 levels at 100 µg/mL. In 2020, there was a significant difference in WSP components at 50 µg/mL. After exposure of the OP component to 50 µg/mL in 2018, the intracellular IL-6 level was observably higher than that of the control group.
When the WP component concentration was 25 µg/mL, there was a significant difference between 2017 and 2019. After exposure to OP components at concentrations of 25 and 50 µg/mL, there were significant differences in IL-6 levels in 2019 and 2020.
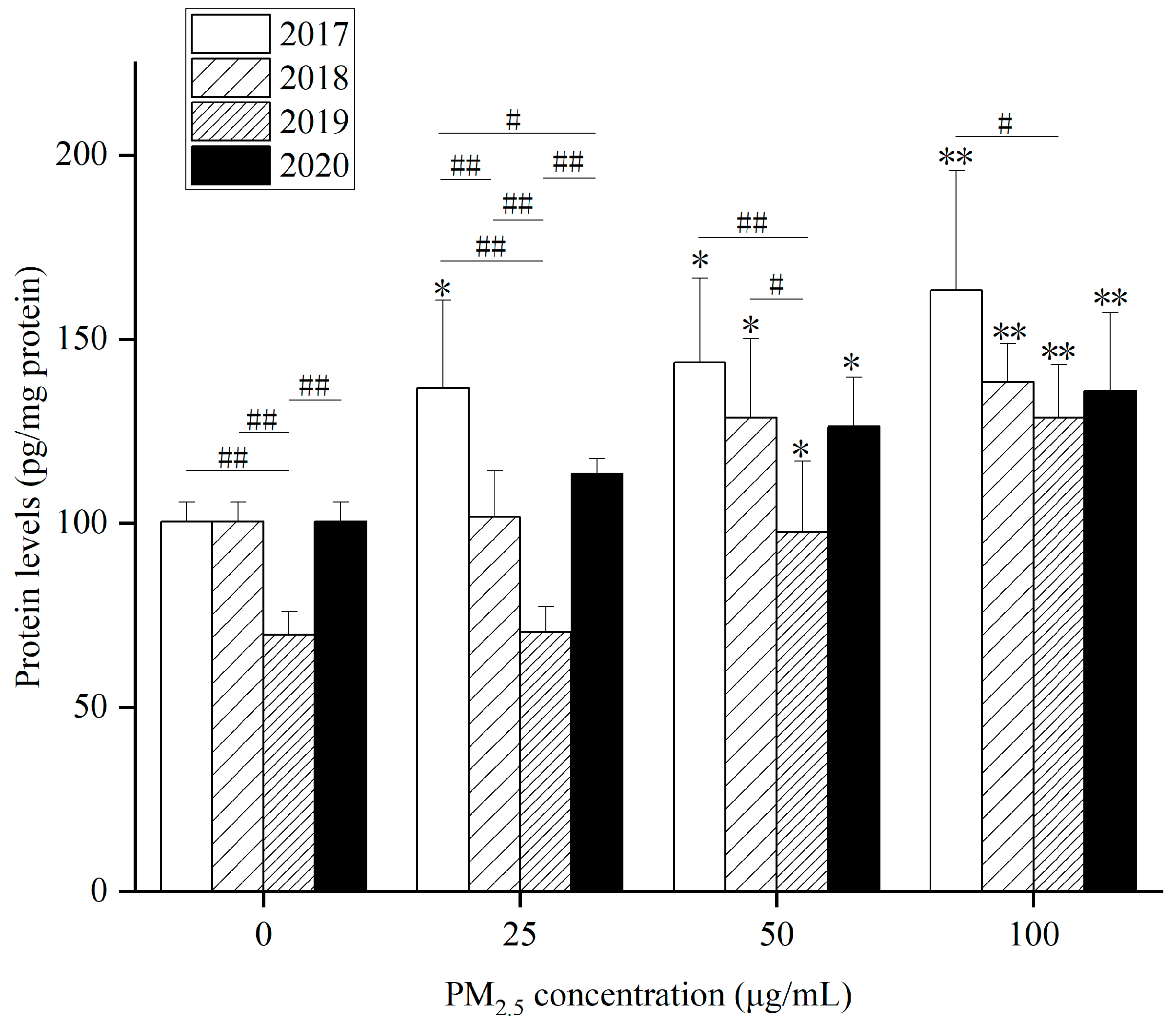
The level of TNF-α in HepG2 cells increased after WP (
Figure 4), WSP (
Figure S5A) and OP (
Figure S5B) exposure for 24 h. Compared with the control group, 24 h after WP exposure from 2017 to 2020, intracellular TNF-α levels were markedly raised at 50 and 100 µg/mL concentrations. After 24 h exposure to WSP, the level of TNF-α went up significantly only when the concentration was 100 µg/mL. After OP exposure, TNF-α levels in HepG2 cells increased markedly at a 100 µg/mL concentration at 4 years. At a 50 µg/mL concentration, there was only a significant increase in HepG2 cells in 2017 and 2020.
The interannual differences in TNF-α levels in different years after exposure to the three components are as follows: (1) TNF-α levels induced by WP component at 0 and 25 µg/mL in 2019 showed significant differences from the other three years. At 25 µg/mL, TNF-α levels in 2017 differed significantly from those in 2018 and 2020. At 50 µg/mL, TNF-α levels in 2019 presented a significant difference relative to 2017 and 2018. At 100 µg/mL, there was only a significant difference in TNF-α levels between 2017 and 2019. (2) TNF-α levels induced by WSP component at 25 and 50 µg/mL caused significant differences between 2019 and 2020. At 50 µg/mL, 2020 also differed from 2018. At 100 µg/mL, intracellular TNF-α levels significantly differed in 2019 compared to 2018 and 2020. (3) OP components at four concentrations (0, 25, 50, 100 µg/mL) were obviously different in TNF-α levels in 2019 and the other three years.
Oxidative stress is the critical mechanism of NAFLD. Various hepatotoxic substances will lead to the production of ROS in hepatocytes. Then, the interaction of oxygen free radicals with cell membranes will result in lipid peroxy reaction to form lipid free radicals, which will jointly cause liver cell damage [
45,
46]. As displayed in
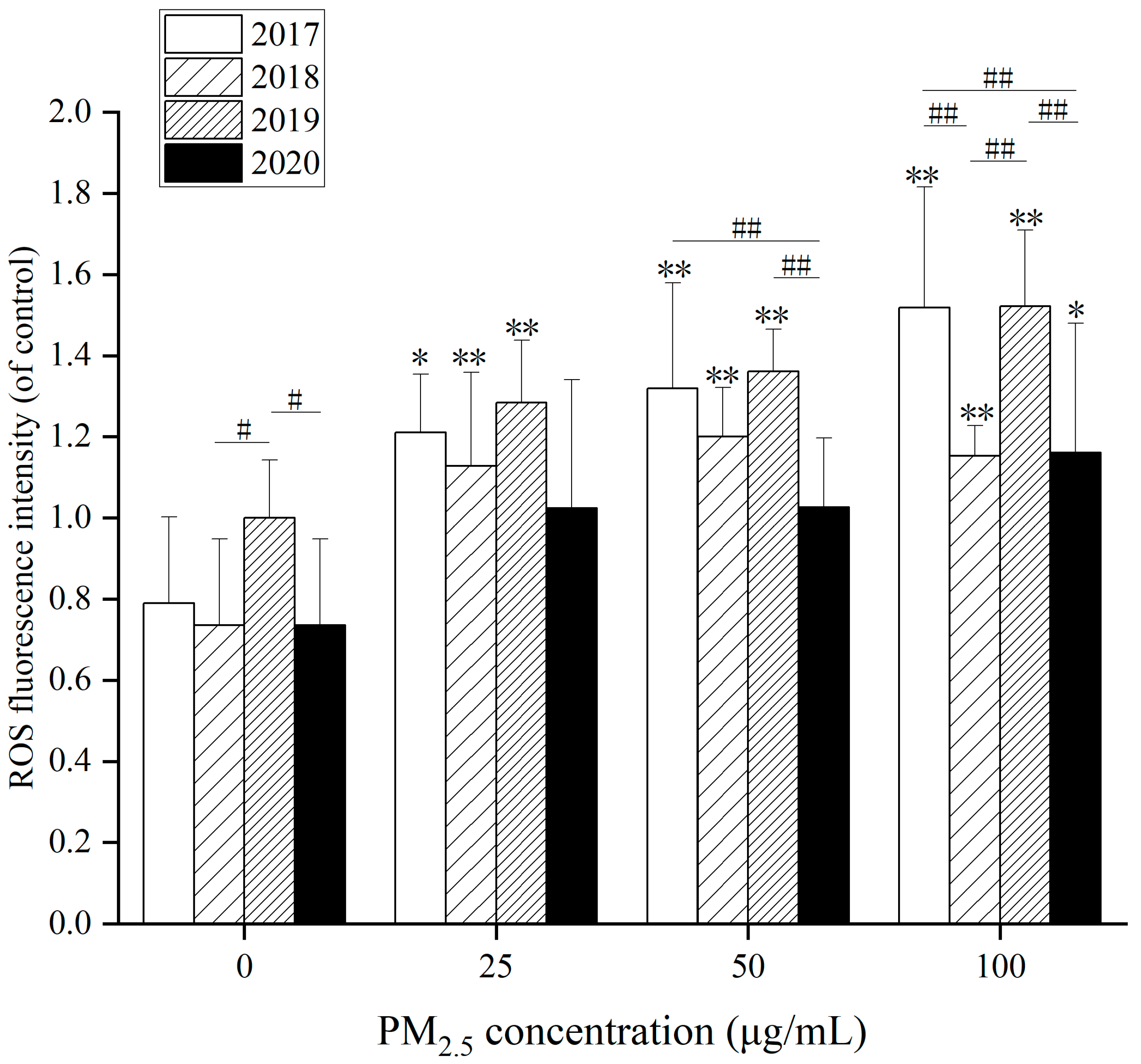
Figure 5, after 24 h exposure to WP at different concentrations, ROS levels in HepG2 cells raised to 100 µg/mL in 2020 compared with the control group. In the other three years, there was a markedly when WP concentration went up at 25, 50 and 100 µg/mL. Besides, in
Figure 5, WP was markedly augmented at 25 µg/mL after action compared to the control group. In 2020, due to reduced pollution, it did not become significant until 100 µg/mL. The effects of WSP and OP also cause significant changes in ROS.
Statistical significance in ROS levels, especially after the WSP treatment, existed in different years. After 24 h exposure to WSP at different concentrations (
Figure S6A), ROS levels in HepG2 cells elevated at 50 and 100 µg/mL in 2018 compared with controls. ROS levels in HepG2 cells augmented at 100 µg/mL in 2020. After 24 h of OP exposure (
Figure S6B), ROS levels in HepG2 cells increased at 25, 50, and 100 µg/mL in 2017 compared with controls. ROS levels mounted up dramatically at 100 µg/mL in 2018. In 2019 and 2020, the ROS levels in HepG2 cells were elevated at 50 and 100 µg/mL. After WSP exposure in 2017, intracellular ROS levels were lower than in the remaining three years. ROS levels were higher after exposure to WP components in 2018 and 2020. In 2017 and 2019, it was higher after total particle exposure. After OP component exposure, intracellular ROS levels in 2019 were lower than those in the other three years.
In
Figure 6, after 24 h exposure to WP at different concentrations, SOD activity in HepG2 cells decreased at 50 and 100 µg/mL at 4 years compared with control. At 100 µg/mL, there is a significant difference between 2019 and 2020. Besides, after 24 h exposure to WSP at different concentrations (
Figure S7A), SOD activity in HepG2 cells decreased significantly at 100 µg/mL in 2020 compared with the control group. At 100 µg/mL, there is a significant difference between 2018 and 2019. Additionally, after 24 h exposure to different concentrations of OP (
Figure S7B), SOD activity in HepG2 cells decreased significantly at 25, 50 and 100 µg/mL in 2017 compared with control. In 2018, SOD activity significantly reduced at 50 and 100 µg/mL. SOD activity in HepG2 cells decreased significantly at 100 µg/mL OP in 2019 and 2020. When the concentration of OP is 0, there is a significant difference between 2019 and the other three years. At 25 µg/mL of OP, SOD activity in 2019 was significantly different from that in 2018 and 2020. At 100 µg/mL of OP, there was a significant difference in SOD activity between 2017 and 2018, 2018 and 2020, 2017 and 2019, 2019 and 2020.
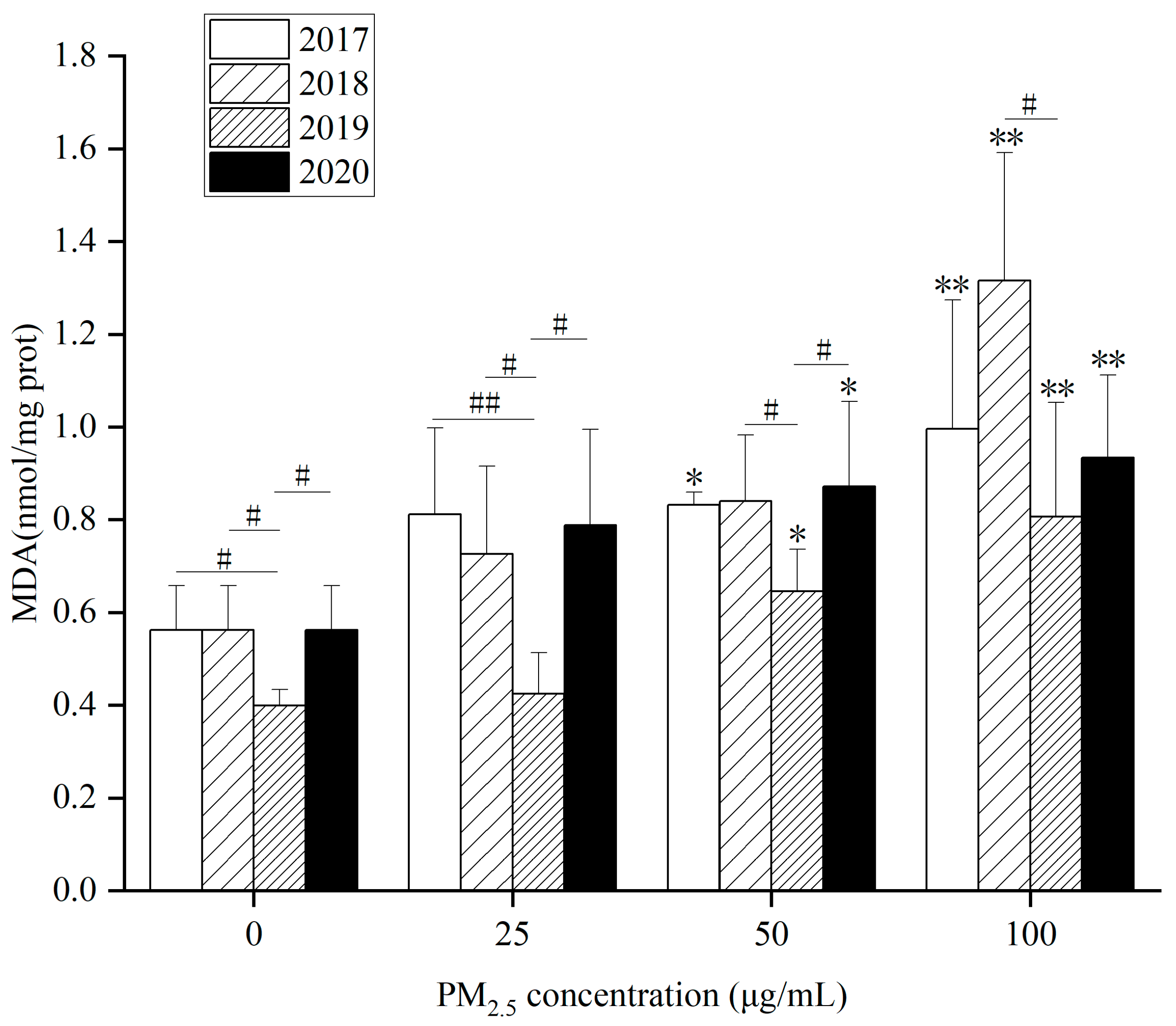
As for intracellular MDA content, (1)
Figure 7 showed that MDA content in HepG2 cells elevated dramatically at 50 and 100 µg/mL in 2017, 2019 and 2020 after WP exposure at different concentrations for 24 h. When WP exposure concentration was 100 µg/mL in 2018, MDA content in HepG2 cells went up. (2) In
Figure S8A, MDA content in HepG2 cells was remarkably raised at 100 µg/mL at 4 years after exposure to WSP at different concentrations for 24 h. (3) In
Figure S8B, after exposure to OP at different concentrations for 24 h, MDA content in HepG2 cells at 50 and 100 µg/mL in 2017, 2018 and 2019 were observably augmented. In 2020, HepG2 cells’ MDA content went up significantly at 100 µg/mL. After WP, WSP exposure in 2019, the intracellular MDA content was reduced. However, MDA content after OP exposure was greater than that in the other three years.
WP, OP and WSP significantly increased intracellular MDA content, indicating that many lipid free radicals formed in cells after exposure to PM
2.5. The decrease in intracellular SOD content suggested that the activity of the major antioxidant enzyme SOD decreased under the action of PM
2.5, revealing that PM
2.5 and its components not only promoted the formation of oxygen free radicals and lipid free radicals, but also inhibited the antioxidant system of cells (
Figure 6,
Figure 7 and
Figures S7 and S8). Xie et al. found that after PM
2.5 acted on HepG2 cells, intracellular ROS level and MDA content increased while SOD activity reduced, which was consistent with our results [
47].
3.3.3. Effects of PM2.5 and Different Components on Cellular Lipids
As a raw material of lipids and a product of lipolysis, FFA is a potential mediator in the development of NAFLD. Under physiological conditions, FFA can be lipidated to produce TG (a major component of lipids) and can also be oxidized to provide energy [
48]. However, hepatic FFA overload may promote oxidative stress and produce ROS, which may be key to the progression of simple steatosis to steatohepatitis [
49].
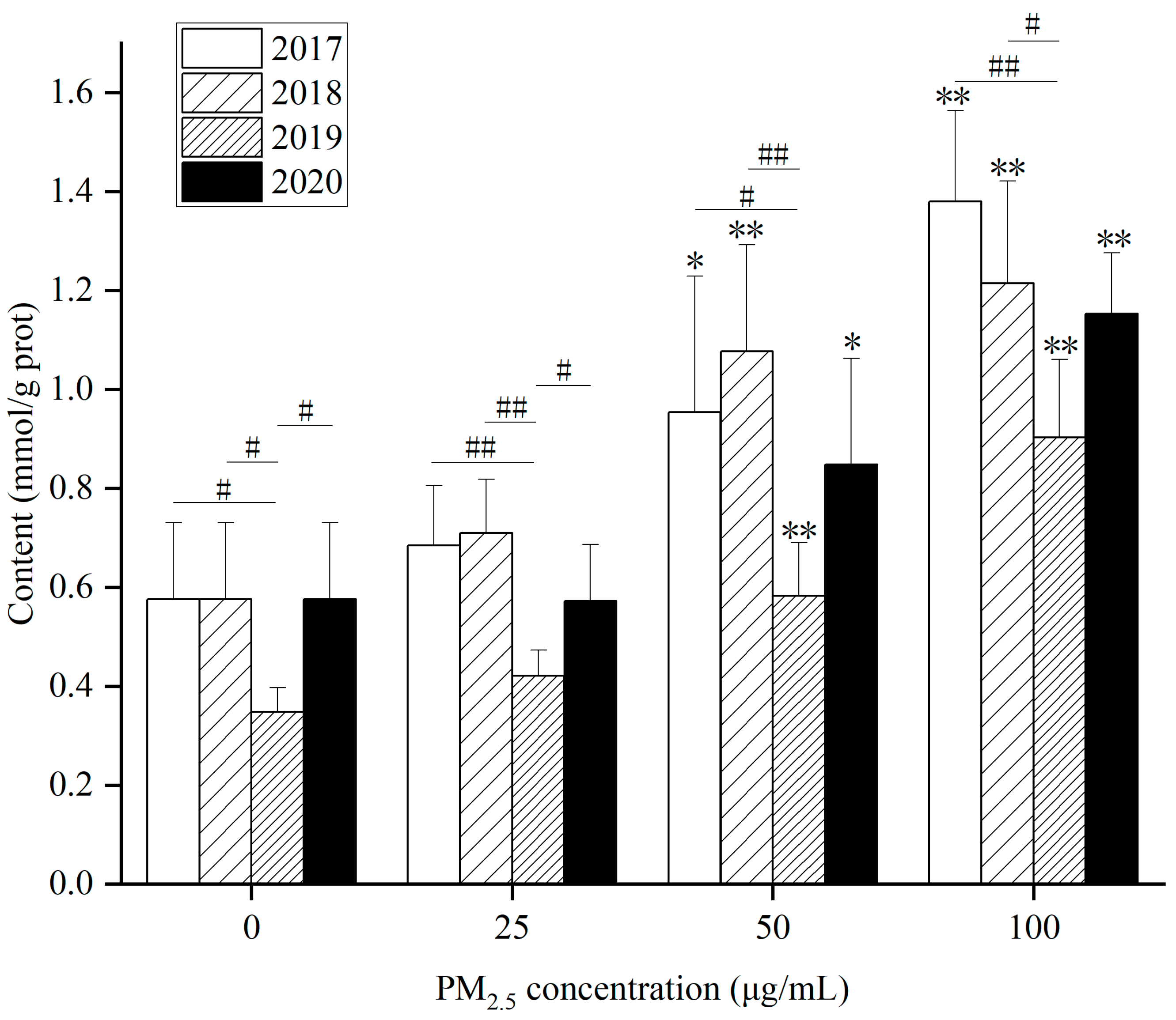
As for intracellular TG content after PM
2.5 exposure, (1) WP significantly elevated TG content in HepG2 cells at 50 and 100 µg/mL after exposure for 24 h compared with the control for 4 years (
Figure 8). (2) After exposure to WSP (
Figure S9A), TG levels in HepG2 cells in 2017 and 2020 were markedly augmented at 50 and 100 µg/mL. In 2018 and 2019, TG levels rose, obviously only at 100 µg/mL. (3) After exposure to OP (
Figure S9B), TG levels increased remarkably in 2017 at concentrations of 50 and 100 µg/mL. TG levels at 25 µg/mL exposure in 2018 were observably higher than those in the control. In 2019 and 2020, TG content increased significantly at 100 µg/mL. After exposure to the WP component, TG content was lower in 2019 compared to three years. On the contrary, TG content was higher in 2019 after WSP exposure.
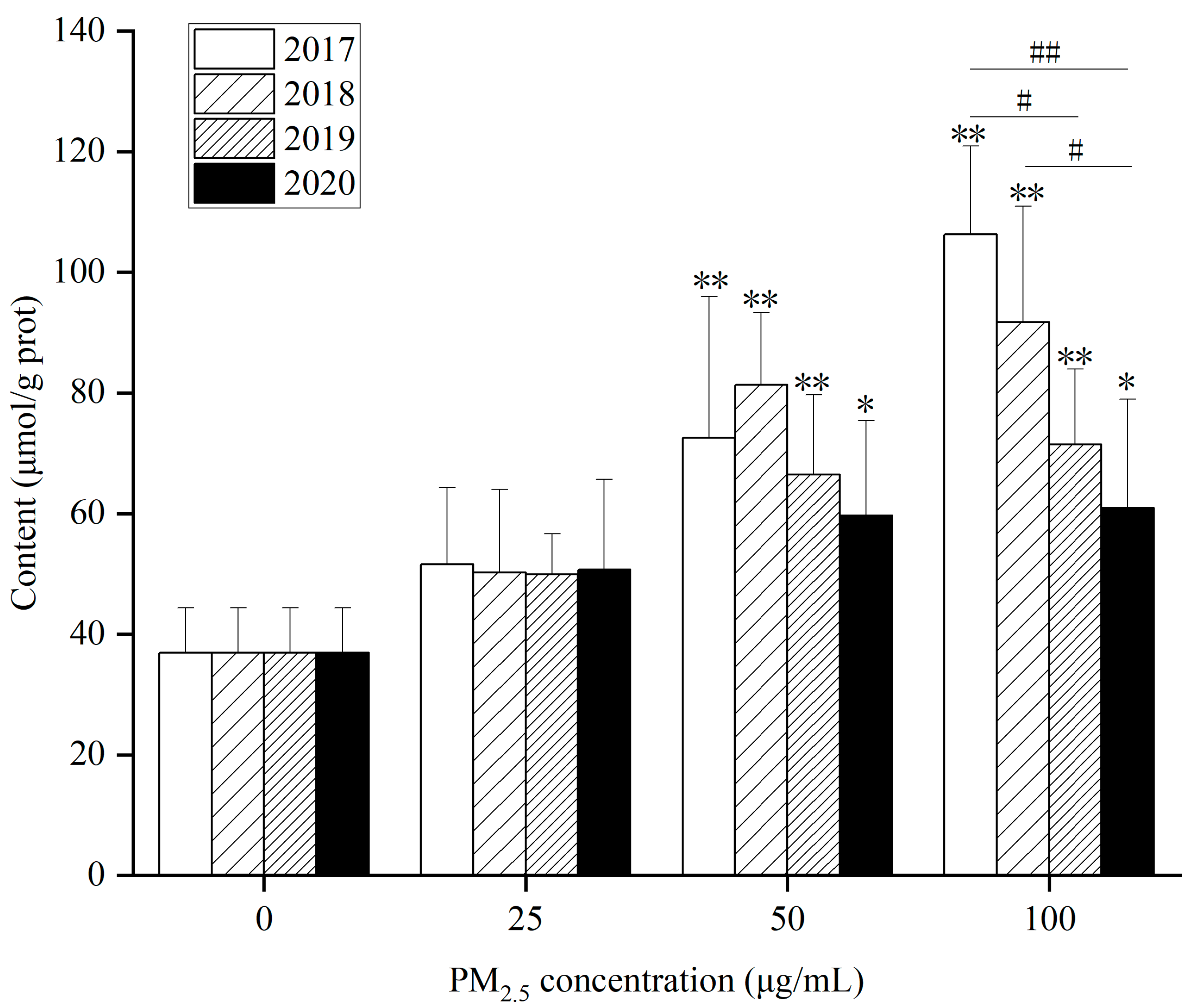
As for FFA content, (1) in
Figure 9, FFA content in 4-year HepG2 cells at 50 and 100 µg/mL after WP exposure for 24 h rose remarkably compared with the control. (2) After exposure to WSP (
Figure S10A), FFA content in HepG2 cells significantly augmented at 100 µg/mL for 4 years. (3) After exposure to OP (
Figure S10B), FFA levels in 2017 elevated significantly at concentrations of 25, 50, and 100 µg/mL. FFA content increased dramatically at 100 µg/mL in the remaining three years compared with the control. After 100 µg/mL WP exposure, intracellular FFA levels decreased yearly from 2017–2020. After OP exposure, intracellular FFA levels were higher in 2017 compared to the other three years.
As seen in
Figure 8,
Figure 9 and
Figures S9 and S10, after exposure to different components, the intracellular TG content varied significantly between years, especially WSP, while FFA content varied little between years. Due to the important role of FFA in lipid metabolism, increased FFA content and flux in the liver can reflect metabolic dysfunction. Therefore, lipid accumulation, especially TG accumulation caused by imbalances in lipogenesis and lipolysis, as well as metabolic changes associated with FFA, including lipid synthesis, lipid uptake, lipid oxidation, and lipid output, are critical to understanding the molecular mechanisms of PM
2.5-induced lipid dysregulation [
50].
3.3.4. Effects of PM2.5 and Its Components on Lipid Metabolism in Cells
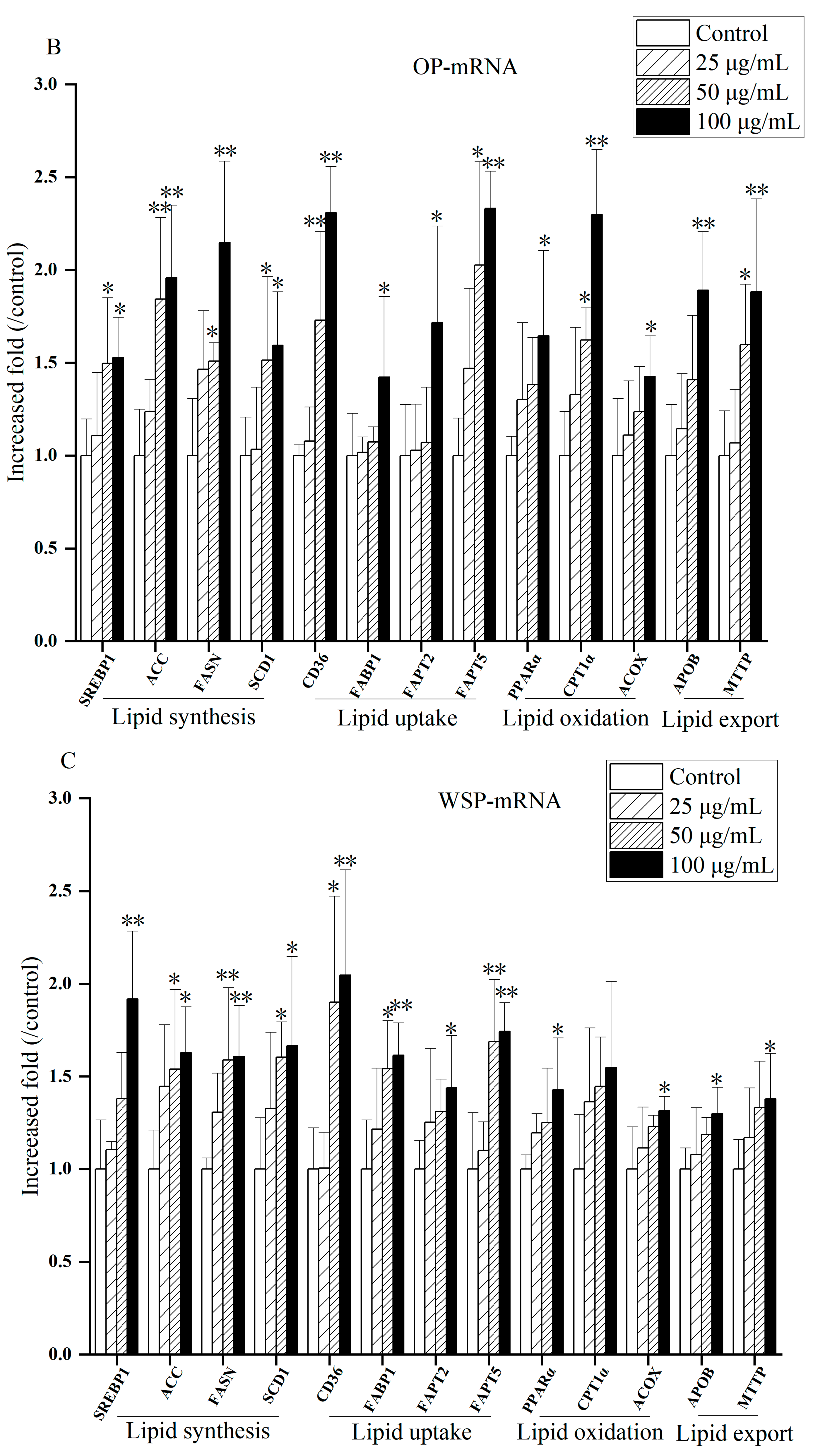
Hepatic dysfunction caused by oxidative stress and inflammatory response can interfere with lipid metabolism, which may promote the process of hepatic dyslipidemia and increase the risk of NAFLD. Therefore, we evaluated changes in mRNA levels of lipid synthesis, uptake, oxidation, and output, which are indicators of lipid metabolism in the liver.
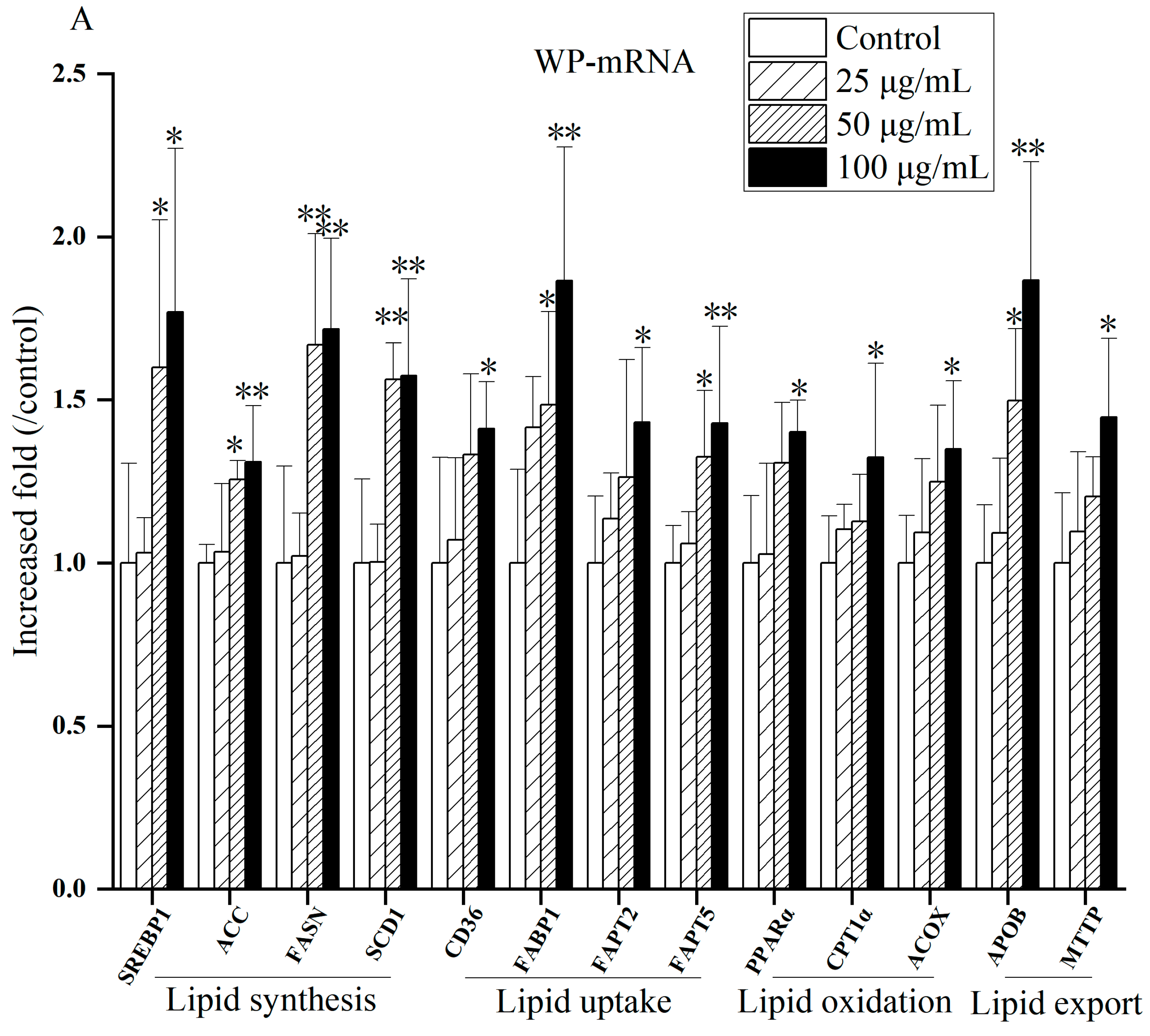
Figure 10 and
Figures S11–S13 present gene expression related to lipid metabolism after exposure to different components of PM
2.5 in 2017. As exposure levels raised, compared to control, the mRNA expressions of lipid synthesis genes (SREBP1, ACC, FASN, SCD1), lipid uptake genes (CD36, FABP1, FAPT2, FAPT5), lipid oxidation genes (PPAR-α, CPTα, ACOX1), and lipid transport genes (APOB, MTTP) were up-regulated. The mRNA expression of some genes (SREBP1, ACC, FASN, SCD1, CD36, FABP1, FAPT2, FAPT5, PPAR-α, ACOX1, APOB, MTTP) at high concentrations (50 or 100 µg/mL) of PM
2.5 were significant (
p < 0.05 or
p < 0.01), suggesting that PM
2.5 may cause lipid metabolism disturbances. It is worth noting that the mRNA expression of lipid oxidation genes of WP and OP components in 2018 and three components (WP, WSP and OP) in 2019 elucidated at 100 µg/mL. The mRNA expression of some lipid oxidation genes decreased after exposure to OP and WSP at a concentration of 100 µg/mL in 2020. The mRNA expression of the lipid transport gene MTTP also lessened after WP exposure.
In this study, we first analyzed the mRNA expression levels of genes related to lipid synthesis treated with PM
2.5 and its components. SREBP1 regulates the expression of ACC, FASN and SCD1 [
51,
52]. ACC is a rate-limiting enzyme that catalyzes the first step of fatty acid anabolism [
53]. FASN is a crucial enzyme in de novo synthesis of fatty acids [
54]. SCD1 is a rate-limiting enzyme that catalyzes the synthesis of unsaturated fats [
55]. Our results found that the mRNA expression of lipid synthesis genes (SREBP1, ACC, FASN, SCD1) augmented observably under the influence of PM
2.5 and its components from 2017 to 2020 compared with the control group. These results indicate that exposure to PM
2.5 and its components induces lipid synthesis in HepG2 cells, and the greater the concentration of PM
2.5 and its components, the greater the impact on lipid synthesis, which is consistent with previous findings [
56].
CD36, FABP1, FAPT2 and FAPT5 are key gene molecules that regulate FFA uptake in the liver [
57]. CD36 is a membrane protein with multiple metabolic functions that mediates FFA transmembrane transport to hepatocytes via FATPs [
58,
59]. FABP can enhance fatty acid intake [
60]. In this study, the mRNA expression of lipid uptake genes (CD36, FABP1, FAPT2, FAPT5) was obviously raised under the influence of PM
2.5 and its components compared with the control group during 2017–2020. This suggests that exposure to PM
2.5 and its components affects lipid uptake in HepG2 cells. Studies have illustrated that activated CD36, FABPs and FATPs promoted lipid uptake and transport, while alleviated gene expression can improve lipid accumulation in hepatocytes [
59,
61], which is consistent with our results.
The activation of PPARα target gene promotes the oxidation of fatty acids, promotes decomposition, and reduces the synthesis and secretion of TG. CPT1α and ACOX1 are key rate-limiting enzymes in lipid oxidation, catalyzing mitochondrial and peroxisomal β oxidation [
62]. The over-expression of lipid oxidation genes and the dysfunction of lipid oxidation can cause the production of excessive ROS, which may induce diseases through oxidative stress [
59].In this study, under the influence of PM
2.5 and its components, mRNA expressions of lipid oxidation genes (PPAR-α, CPT1α and ACOX1) were significantly elevated in 2017 compared with the control group, which was consistent with the results of Ding et al. [
58]. We also found that the mRNA expression of the above lipid oxidation genes decreased from 2018 to 2020. Giving that the mRNA expressions of PPAR-α, CPT1α and ACOX1 decreased under short-term exposure to PM
2.5, which is related to lipidolysis [
51]. And the inhibited lipolytic genes and the weakened oxidation capacity promote lipid deposition, related to down-regulated PPARα and its regulated CPT1 and ACOX1 [
50]. Thus, the research results on the role of lipid oxidation in NAFLD are not uniform, and further research is needed.
Elevated very low-density lipoprotein (VLDL) in plasma is also a characteristic of NAFLD [
63]. VLDL are endogenous triglycerides mainly synthesized by liver cells. Newborn VLDLs produced in the liver are surrounded by phospholipids, cholesterol esters, and specific apolipoproteins, of which APOB is the most important [
64]. APOB cotranslates lipidization in the endoplasmic reticulum lumen via MTTP and further lipidization in the Golgi apparatus [
65]. Excess TG can be secreted as VLDL to relieve TG accumulation [
66]. In this study, mRNA expression of lipid exporting gene MTTP was significantly reduced in cells after WP treatment in 2020. As a lipid transporter, MTTP is mainly involved in VLDL transport and secretion formed by the combination of free fatty acids and APOB [
67]. When MTTP is reduced or dysfunction occurs, the output of TG in hepatocytes diminishes, and lipid droplet formation increases, resulting in hepatic lipid deposition [
68]. In the present study, from 2017 to 2019, the mRNA expression of lipid export genes (APOB and MTTP) was markedly raised after PM
2.5 and its components were treated compared with the control, indicating that more lipids were removed from liver tissues. Still, the role of lipid synthesis may be more significant, leading to intracellular FFA and TG accumulation.