Assessment of Atmospheric Pollution by Selected Elements and PAHs during 12-Month Active Biomonitoring of Terrestrial Mosses
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Study Area and Climate Conditions
2.3. Methods
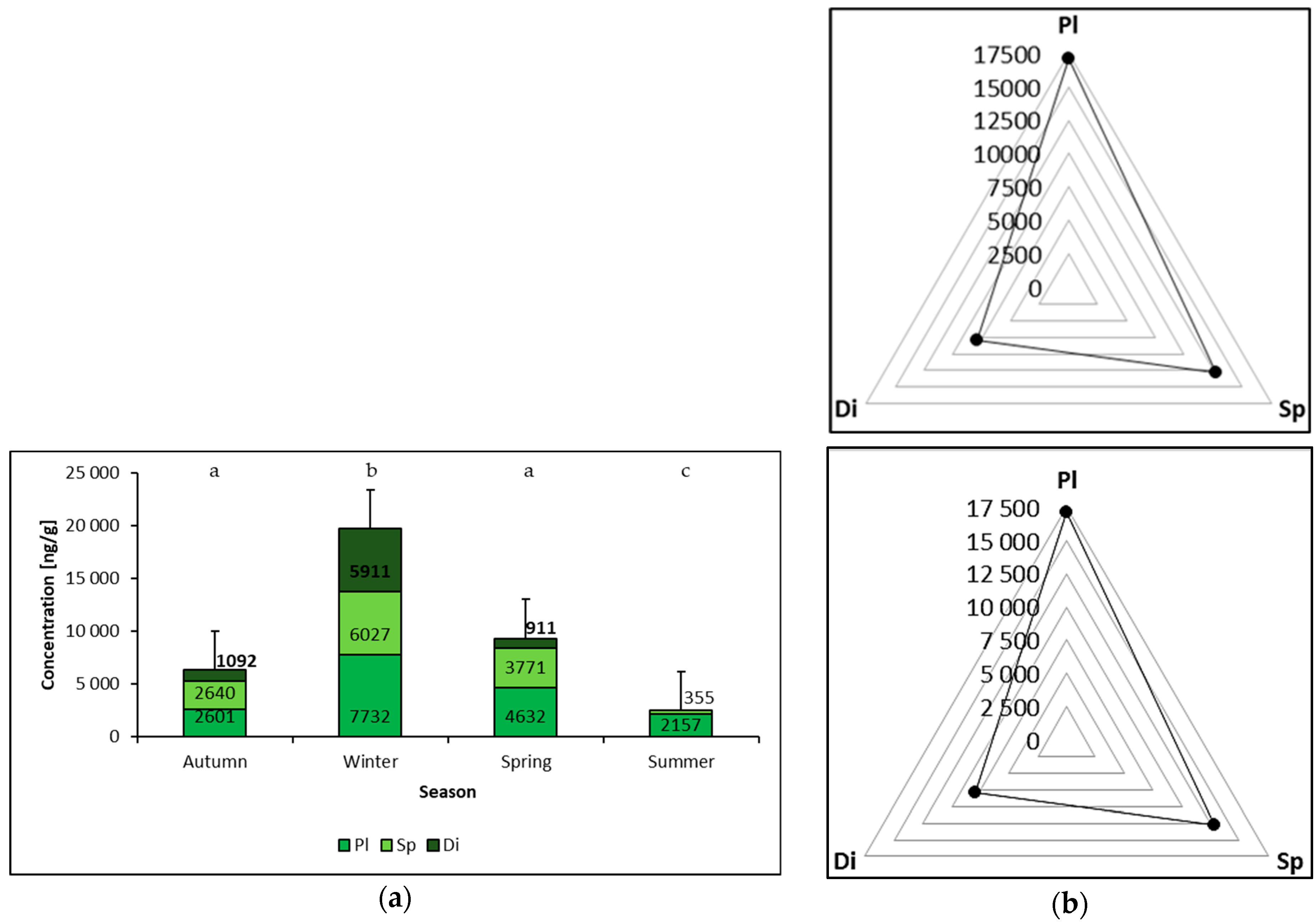
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, H.; Zhao, C.; Yang, Y. A Comprehensive Analysis of the Spatio-Temporal Variation of Urban Air Pollution in China during 2014–2018. Atmos. Environ. 2020, 220, 117066. [Google Scholar] [CrossRef]
- Yang, J.; Shi, B.; Shi, Y.; Marvin, S.; Zheng, Y.; Xia, G. Air Pollution Dispersal in High Density Urban Areas: Research on the Triadic Relation of Wind, Air Pollution, and Urban Form. Sustain. Cities Soc. 2020, 54, 101941. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Kan, H. Air Pollution: A Global Problem Needs Local Fixes. Nature 2019, 570, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The Need for Clean Air: The Way Air Pollution and Climate Change Affect Allergic Rhinitis and Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Isinkaralar, O.; Isinkaralar, K.; Bayraktar, E.P. Monitoring the Spatial Distribution Pattern According to Urban Land Use and Health Risk Assessment on Potential Toxic Metal Contamination via Street Dust in Ankara, Türkiye. Environ. Monit. Assess. 2023, 195, 1085. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Agathokleous, E.; De Marco, A.; Paoletti, E.; Calatayud, V. Urban Population Exposure to Air Pollution in Europe over the Last Decades. Environ. Sci. Eur. 2021, 33, 28. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Islam, F. The Effects of Air Pollution on COVID-19 Infection and Mortality—A Review on Recent Evidence. Front. Public Health 2020, 8, 580057. [Google Scholar] [CrossRef]
- Hernandez Carballo, I.; Bakola, M.; Stuckler, D. The Impact of Air Pollution on COVID-19 Incidence, Severity, and Mortality: A Systematic Review of Studies in Europe and North America. Environ. Res. 2022, 215, 114155. [Google Scholar] [CrossRef]
- Liang, D.; Shi, L.; Zhao, J.; Liu, P.; Sarnat, J.A.; Gao, S.; Schwartz, J.; Liu, Y.; Ebelt, S.T.; Scovronick, N.; et al. Urban Air Pollution May Enhance COVID-19 Case-Fatality and Mortality Rates in the United States. Innovation 2020, 1, 100047. [Google Scholar] [CrossRef]
- Cicala, S.; Holland, S.P.; Mansur, E.T.; Muller, N.Z.; Yates, A.J. Expected Health Effects of Reduced Air Pollution from COVID-19 Social Distancing. Atmosphere 2021, 12, 951. [Google Scholar] [CrossRef]
- Yushin, N.; Chaligava, O.; Zinicovscaia, I.; Grozdov, D.; Vergel, K. Mosses as Bioindicators of Heavy Metal Air Pollution in the Lockdown Period Adopted to Cope with the COVID-19 Pandemic. Atmosphere 2020, 11, 1194. [Google Scholar] [CrossRef]
- Harrison, R.M.; Van Vu, T.; Jafar, H.; Shi, Z. More Mileage in Reducing Urban Air Pollution from Road Traffic. Environ. Int. 2021, 149, 106329. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Massagué, J.; Alastuey, A.; Moreno, T.; Gangoiti, G.; Mantilla, E.; Duéguez, J.J.; Escudero, M.; Monfort, E.; Pérez García-Pando, C.; et al. Lessons from the COVID-19 Air Pollution Decrease in Spain: Now What? Sci. Total Environ. 2021, 779, 146380. [Google Scholar] [CrossRef]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, Ø.; Rodhe, H.; Cowling, E. Acid Rain and Air Pollution: 50 Years of Progress in Environmental Science and Policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Idrees, Z.; Zheng, L. Low Cost Air Pollution Monitoring Systems: A Review of Protocols and Enabling Technologies. J. Ind. Inf. Integr. 2020, 17, 100123. [Google Scholar] [CrossRef]
- Ly, H.B.; Le, L.M.; Van Phi, L.; Phan, V.H.; Tran, V.Q.; Pham, B.T.; Le, T.T.; Derrible, S. Development of an AI Model to Measure Traffic Air Pollution from Multisensor and Weather Data. Sensors 2019, 19, 4941. [Google Scholar] [CrossRef]
- Rohi, G.; Ejofodomi, O.; Ofualagba, G. Autonomous Monitoring, Analysis, and Countering of Air Pollution Using Environmental Drones. Heliyon 2020, 6, e03252. [Google Scholar] [CrossRef]
- Feng, Y.; Ning, M.; Lei, Y.; Sun, Y.; Liu, W.; Wang, J. Defending Blue Sky in China: Effectiveness of the “Air Pollution Prevention and Control Action Plan” on Air Quality Improvements from 2013 to 2017. J. Environ. Manag. 2019, 252, 109603. [Google Scholar] [CrossRef]
- Mahajan, S.; Kumar, P.; Pinto, J.A.; Riccetti, A.; Schaaf, K.; Camprodon, G.; Smári, V.; Passani, A.; Forino, G. A Citizen Science Approach for Enhancing Public Understanding of Air Pollution. Sustain. Cities Soc. 2020, 52, 101800. [Google Scholar] [CrossRef]
- Abad, E.; Abalos, M.; Fiedler, H. Air Monitoring with Passive Samplers for Dioxin-like Persistent Organic Pollutants in Developing Countries (2017–2019). Chemosphere 2022, 287, 131931. [Google Scholar] [CrossRef]
- Thang, P.Q.; Kim, S.J.; Lee, S.J.; Kim, C.H.; Lim, H.J.; Lee, S.B.; Kim, J.Y.; Vuong, Q.T.; Choi, S.D. Monitoring of Polycyclic Aromatic Hydrocarbons Using Passive Air Samplers in Seoul, South Korea: Spatial Distribution, Seasonal Variation, and Source Identification. Atmos. Environ. 2020, 229, 117460. [Google Scholar] [CrossRef]
- Yayla, E.E.; Sevik, H.; Isinkaralar, K. Detection of Landscape Species as a Low-Cost Biomonitoring Study: Cr, Mn, and Zn Pollution in an Urban Air Quality. Environ. Monit. Assess. 2022, 194, 687. [Google Scholar] [CrossRef]
- Giordano, S.; Spagnuolo, V.; Capozzi, F. Biomonitoring of Air Pollution. Atmosphere 2021, 12, 433. [Google Scholar] [CrossRef]
- Sorrentino, M.C.; Capozzi, F.; Wuyts, K.; Joosen, S.; Mubiana, V.K.; Giordano, S.; Samson, R.; Spagnuolo, V. Mobile Biomonitoring of Atmospheric Pollution: A New Perspective for the Moss-Bag Approach. Plants 2021, 10, 2384. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Kimbrough, S.; Krabbe, S.; Logan, R.; Isakov, V.; Baldauf, R. Identifying Air Pollution Source Impacts in Urban Communities Using Mobile Monitoring. Sci. Total Environ. 2020, 715, 136979. [Google Scholar] [CrossRef]
- Szczepaniak, K.; Astel, A.; Simeonov, V.; Tsakovski, S.; Biziuk, M.; Bode, P.; Przyjazny, A. Comparison of Dry and Living Sphagnum Palustre Moss Samples in Determining Their Biocumulative Capability as Biomonitoring Tools. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1101–1115. [Google Scholar] [CrossRef]
- van Laaten, N.; Merten, D.; von Tümpling, W.; Schäfer, T.; Pirrung, M. Comparison of Spider Web and Moss Bag Biomonitoring to Detect Sources of Airborne Trace Elements. Water Air Soil Pollut. 2020, 231, 512. [Google Scholar] [CrossRef]
- Świsłowski, P.; Kříž, J.; Rajfur, M. The Use of Bark in Biomonitoring Heavy Metal Pollution of Forest Areas on the Example of Selected Areas in Poland. Ecol. Chem. Eng. S 2020, 27, 195–210. [Google Scholar] [CrossRef]
- Chaligava, O.; Shetekauri, S.; Badawy, W.M.; Frontasyeva, M.V.; Zinicovscaia, I.; Shetekauri, T.; Kvlividze, A.; Vergel, K.; Yushin, N. Characterization of Trace Elements in Atmospheric Deposition Studied by Moss Biomonitoring in Georgia. Arch. Environ. Contam. Toxicol. 2021, 80, 350–367. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dutta, R.; Das, P. A Critical Review on Plant Biomonitors for Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Air through Solvent Extraction Techniques. Chemosphere 2020, 251, 126441. [Google Scholar] [CrossRef]
- Jafarova, M.; Grifoni, L.; Aherne, J.; Loppi, S. Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics. Atmosphere 2023, 14, 1007. [Google Scholar] [CrossRef]
- Szwed, M.; Żukowski, W.; Kozłowski, R. The Presence of Selected Elements in the Microscopic Image of Pine Needles as an Effect of Cement and Lime Pressure within the Region of Białe Zagłębie (Central Europe). Toxics 2021, 9, 15. [Google Scholar] [CrossRef]
- Cesa, M.; Bertossi, A.; Cherubini, G.; Gava, E.; Mazzilis, D.; Piccoli, E.; Verardo, P.; Nimis, P.L. Development of a Standard Protocol for Monitoring Trace Elements in Continental Waters with Moss Bags: Inter- and Intraspecific Differences. Environ. Sci. Pollut. Res. 2015, 22, 5030–5040. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Adamo, P.; Di Palma, A.; Aboal, J.R.; Bargagli, R.; Fernandez, J.A.; Lopez Mahia, P.; Reski, R.; Tretiach, M.; Spagnuolo, V.; et al. Sphagnum Palustre Clone vs Native Pseudoscleropodium Purum: A First Trial in the Field to Validate the Future of the Moss Bag Technique. Environ. Pollut. 2017, 225, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Aboal, J.R.; Boquete, M.T.; Carballeira, A.; Casanova, A.; Debén, S.; Fernández, J.A. Quantification of the Overall Measurement Uncertainty Associated with the Passive Moss Biomonitoring Technique: Sample Collection and Processing. Environ. Pollut. 2017, 224, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, A.; González, A.G.; Adamo, P.; Giordano, S.; Reski, R.; Pokrovsky, O.S. Biosurface Properties and Lead Adsorption in a Clone of Sphagnum Palustre (Mosses): Towards a Unified Protocol of Biomonitoring of Airborne Heavy Metal Pollution. Chemosphere 2019, 236, 124375. [Google Scholar] [CrossRef]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernández, J.A. Moss Bag Biomonitoring: A Methodological Review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best Options for the Exposure of Traditional and Innovative Moss Bags: A Systematic Evaluation in Three European Countries. Environ. Pollut. 2016, 214, 362–373. [Google Scholar] [CrossRef]
- Aničić Urošević, M.; Kuzmanoski, M.; Milićević, T.; Kodranov, I.; Vergel, K.; Popović, A. Moss Bag Sensitivity for the Assessment of Airborne Elements at Suburban Background Site during Spring/Summer Season Characterized by Saharan Dust Intrusions. Air Qual. Atmos. Health 2022, 15, 1357–1377. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of Vitality, Seasonality and Specific Leaf Area on PAH Uptake in Moss and Lichen Transplanted in Bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- Paoli, L.; Bandoni, E.; Sanità di Toppi, L. Lichens and Mosses as Biomonitors of Indoor Pollution. Biology 2023, 12, 1248. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Rajfur, M. Is Your Moss Alive during Active Biomonitoring Study? Plants 2021, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Möslinger, L.; Korjenic, A.; Streit, E.; Sulejmanovski, A.; Frank, P.N.; Hummel, E. Viability of Living Moss for Indoor Green Walls: A Study on Temperature, Humidity, and Irrigation. Sustainability 2023, 15, 15625. [Google Scholar] [CrossRef]
- Debén, S.; Fernández, J.A.; Carballeira, A.; Aboal, J.R. Using Devitalized Moss for Active Biomonitoring of Water Pollution. Environ. Pollut. 2016, 210, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, C.; Carballeira, A.; Aboal, J.R.; Fernández, J.A. Biomonitoring Freshwater FISH Farms by Measuring Nitrogen Concentrations and the Δ15N Signal in Living and Devitalized Moss Transplants. Environ. Pollut. 2019, 245, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, V.; Fernández, J.Á.; Aboal, J.R.; Picariello, E.; De Nicola, F. Accumulation of Polycyclic Aromatic Hydrocarbons in the Devitalized Aquatic Moss Fontinalis Antipyretica: From Laboratory to Field Conditions. J. Environ. Qual. 2021, 50, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Nickel, S.; Schröder, W. Reorganisation of a Long-Term Monitoring Network Using Moss as Biomonitor for Atmospheric Deposition in Germany. Ecol. Indic. 2017, 76, 194–206. [Google Scholar] [CrossRef]
- Nekhoroshkov, P.; Peshkova, A.; Zinicovscaia, I.; Vergel, K.; Kravtsova, A. Assessment of the Atmospheric Deposition of Heavy Metals and Other Elements in the Mountain Crimea Using Moss Biomonitoring Technique. Atmosphere 2022, 13, 573. [Google Scholar] [CrossRef]
- Couto, J.A.; Fernández, J.A.; Aboal, J.R.; Carballeira, A. Annual Variability in Heavy-Metal Bioconcentration in Moss: Sampling Protocol Optimization. Atmos. Environ. 2003, 37, 3517–3527. [Google Scholar] [CrossRef]
- Sergeeva, A.; Zinicovscaia, I.; Grozdov, D.; Yushin, N. Assessment of Selected Rare Earth Elements, HF, Th, and U in the Donetsk Region Using Moss Bags Technique. Atmos. Pollut. Res. 2021, 12, 101165. [Google Scholar] [CrossRef]
- Milićević, T.; Aničić Urošević, M.; Vuković, G.; Škrivanj, S.; Relić, D.; Frontasyeva, M.V.; Popović, A. Assessment of Species-Specific and Temporal Variations of Major, Trace and Rare Earth Elements in Vineyard Ambient Using Moss Bags. Ecotoxicol. Environ. Saf. 2017, 144, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Demková, L.; Baranová, B.; Oboňa, J.; Árvay, J.; Lošák, T. Assessment of Air Pollution by Toxic Elements on Petrol Stations Using Moss and Lichen Bag Technique. Plant Soil Environ. 2017, 63, 355–361. [Google Scholar] [CrossRef]
- Culicov, O.A.; Zinicovscaia, I.; Duliu, O.G. Active Sphagnum Girgensohnii Russow Moss Biomonitoring of an Industrial Site in Romania: Temporal Variation in the Elemental Content. Bull. Environ. Contam. Toxicol. 2016, 96, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Rogova, N.; Ryzhakova, N.; Gusvitskii, K.; Eruntsov, V. Studying the Influence of Seasonal Conditions and Period of Exposure on Trace Element Concentrations in the Moss-Transplant Pylaisia Polyantha. Environ. Monit. Assess. 2021, 193, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, A.; Adamo, P.; Dohi, T.; Fujiwara, K.; Hagiwara, H.; Kitamura, A.; Sakoda, A.; Sato, K.; Iijima, K. Testing Mosses Exposed in Bags as Biointerceptors of Airborne Radiocaesium after the Fukushima Dai-Ichi Nuclear Power Station Accident. Chemosphere 2022, 308, 136179. [Google Scholar] [CrossRef]
- Saitanis, C.J.; Frontasyeva, M.V.; Steinnes, E.; Palmer, M.W.; Ostrovnaya, T.M.; Gundorina, S.F. Spatiotemporal Distribution of Airborne Elements Monitored with the Moss Bags Technique in the Greater Thriasion Plain, Attica, Greece. Environ. Monit. Assess. 2013, 185, 955–968. [Google Scholar] [CrossRef]
- De Agostini, A.; Cortis, P.; Cogoni, A. Monitoring of Air Pollution by Moss Bags around an Oil Refinery: A Critical Evaluation over 16 Years. Atmosphere 2020, 11, 272. [Google Scholar] [CrossRef]
- Fernández, J.A.; Boquete, M.T.; Carballeira, A.; Aboal, J.R. A Critical Review of Protocols for Moss Biomonitoring of Atmospheric Deposition: Sampling and Sample Preparation. Sci. Total Environ. 2015, 517, 132–150. [Google Scholar] [CrossRef]
- ICP Vegetation. Heavy Metals, Nitrogen and POPs in European Mosses: 2020 Survey. 2020. Available online: https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf (accessed on 22 November 2023).
- Boquete, M.T.; Aboal, J.R.; Carballeira, A.; Fernández, J.A. Effect of Age on the Heavy Metal Concentration in Segments of Pseudoscleropodium Purum and the Biomonitoring of Atmospheric Deposition of Metals. Atmos. Environ. 2014, 86, 28–34. [Google Scholar] [CrossRef]
- Lazo, P.; Kika, A.; Qarri, F.; Bekteshi, L.; Allajbeu, S.; Stafilov, T. Air Quality Assessment by Moss Biomonitoring and Trace Metals Atmospheric Deposition. Aerosol Air Qual. Res. 2022, 22, 220008. [Google Scholar] [CrossRef]
- The Minister of Environment of the Republic of Poland. Regulation of the Minister of Environment of October 9, 2014 on the Protection of Plant Species; 2014. Available online: https://dziennikustaw.gov.pl/DU/rok/2014/pozycja/1409 (accessed on 22 November 2023).
- Dołęgowska, S.; Gałuszka, A.; Migaszewski, Z.M. Significance of the Long-Term Biomonitoring Studies for Understanding the Impact of Pollutants on the Environment Based on a Synthesis of 25-Year Biomonitoring in the Holy Cross Mountains, Poland. Environ. Sci. Pollut. Res. 2021, 28, 10413–10435. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A. Distribution Patterns of PAHs and Trace Elements in Mosses Hylocomium splendens (Hedw.) B.S.G. and Pleurozium schreberi (Brid.) Mitt. from Different Forest Communities: A Case Study, South-Central Poland. Chemosphere 2007, 67, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Hrabák, P.; Wacławek, S.; Liskova, K.; Antos, V.; Rajfur, M.; Ząbkowska-Wacławek, M. The Application of Active Biomonitoring with the Use of Mosses to Identify Polycyclic Aromatic Hydrocarbons in an Atmospheric Aerosol. Molecules 2021, 26, 7258. [Google Scholar] [CrossRef] [PubMed]
- WeatherSpark Year-Round Climate and Average Weather Conditions in Końskie. Available online: https://pl.weatherspark.com/y/86349/Średnie-warunki-pogodowe-w:-Końskie-Polska-w-ciągu-roku#Sections-Precipitation (accessed on 4 January 2024).
- Przybytniowski, J.W.; Dziekański, P. Synthetic Measurement Used As Assessment of Spatial Disparities of the Natural Environment. Zesz. Nauk. Uniw. Przyr. Siedlcach. Ser. Adm. Zarządzanie 2020, 49, 89–98. [Google Scholar] [CrossRef]
- Świsłowski, P.; Vergel, K.; Zinicovscaia, I.; Rajfur, M.; Wacławek, M. Mosses as a Biomonitor to Identify Elements Released into the Air as a Result of Car Workshop Activities. Ecol. Indic. 2022, 138, 108849. [Google Scholar] [CrossRef]
- Chief Inspectorate of Environmental Protection. Annual Assessment of Air Quality in the Swietokrzyskie Voivodeship. Voivodship Report for 2022; Chief Inspectorate of Environmental Protection: Kielce, Poland, 2023.
- García-Seoane, R.; Fernández, J.A.; Chilà, A.; Aboal, J.R. Improving the Uptake of Pollutants in Moss Bags: The Wind Effect. Ecol. Indic. 2019, 107, 105577. [Google Scholar] [CrossRef]
- ISO 15587-2:2002; DIN—German Institute for Standardization Water Quality—Digestion for the Determination of Elements in Water—Part 2: Nitric Acid Digestion. ISO: Geneva, Switzerland, 2002.
- ISO 17294-2:2003; DIN—German Institute for Standardization Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of 62 Elements. ISO: Geneva, Switzerland, 2004.
- ISO/TS 13530:2009; International Organization for Standardization Water Quality—Guidance on Analytical Quality Control for Chemical and Physicochemical Water Analysis. ISO: Geneva, Switzerland, 2009.
- DIN EN 17503:2020; DIN - German Institute for Standardization Environmental Solid Matrices - Determination of Polycyclic Aromatic Hydrocarbons (PAH) by Gas Chromatography (GC) and High Performance Liquid Chromatography (HPLC); German and English Version PrEN 17503:2020 (DIN EN 17503:2020-06) 2020. ISO: Geneva, Switzerland, 2020.
- Tobiszewski, M.; Namieśnik, J. PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Concha-Graña, E.; Piñeiro-Iglesias, M.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Proposal of a Procedure for the Analysis of Atmospheric Polycyclic Aromatic Hydrocarbons in Mosses. Talanta 2015, 134, 239–246. [Google Scholar] [CrossRef]
- Turgut, E.T.; Gaga, E.O.; Jovanović, G.; Odabasi, M.; Artun, G.; Ari, A.; Urošević, M.A. Elemental Characterization of General Aviation Aircraft Emissions Using Moss Bags. Environ. Sci. Pollut. Res. 2019, 26, 26925–26938. [Google Scholar] [CrossRef]
- De Nicola, F.; Alfani, A.; Maisto, G. Polycyclic Aromatic Hydrocarbon Contamination in an Urban Area Assessed by Quercus Ilex Leaves and Soil. Environ. Sci. Pollut. Res. 2014, 21, 7616–7623. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Rajfur, M. The Influence of Environmental Conditions on the Lifespan of Mosses under Long-Term Active Biomonitoring. Atmos. Pollut. Res. 2021, 12, 101203. [Google Scholar] [CrossRef]
- Tretiach, M.; Pittao, E.; Crisafulli, P.; Adamo, P. Influence of Exposure Sites on Trace Element Enrichment in Moss-Bags and Characterization of Particles Deposited on the Biomonitor Surface. Sci. Total Environ. 2011, 409, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Sabovljević, M.S.; Weidinger, M.; Sabovljević, A.D.; Stanković, J.; Adlassnig, W.; Lang, I. Metal Accumulation in the Acrocarp Moss Atrichum Undulatum under Controlled Conditions. Environ. Pollut. 2020, 256, 113397. [Google Scholar] [CrossRef]
- Cortis, P.; Vannini, C.; Cogoni, A.; De Mattia, F.; Bracale, M.; Mezzasalma, V.; Labra, M. Chemical, Molecular, and Proteomic Analyses of Moss Bag Biomonitoring in a Petrochemical Area of Sardinia (Italy). Environ. Sci. Pollut. Res. 2016, 23, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Culicov, O.A.; Yurukova, L. Comparison of Element Accumulation of Different Moss- and Lichen-Bags, Exposed in the City of Sofia (Bulgaria). J. Atmos. Chem. 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Makholm, M.M.; Mladenoff, D.J. Efficacy of a Biomonitoring (Moss Bag) Technique for Determining Element Deposition Trends on a Mid-Range (375 Km) Scale. Environ. Monit. Assess. 2005, 104, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Monaci, F.; Ancora, S.; Bianchi, N.; Bonini, I.; Paoli, L.; Loppi, S. Combined Use of Native and Transplanted Moss for Post-Mining Characterization of Metal(Loid) River Contamination. Sci. Total Environ. 2021, 750, 141669. [Google Scholar] [CrossRef] [PubMed]
- Shvetsova, M.S.; Kamanina, I.Z.; Frontasyeva, M.V.; Madadzada, A.I.; Zinicovscaia, I.I.; Pavlov, S.S.; Vergel, K.N.; Yushin, N.S. Active Moss Biomonitoring Using the “Moss Bag Technique” in the Park of Moscow. Phys. Part. Nucl. Lett. 2019, 16, 994–1003. [Google Scholar] [CrossRef]
- Vuković, G.; Urošević, M.A.; Škrivanj, S.; Vergel, K.; Tomašević, M.; Popović, A. The First Survey of Airborne Trace Elements at Airport Using Moss Bag Technique. Environ. Sci. Pollut. Res. 2017, 24, 15107–15115. [Google Scholar] [CrossRef]
- Abdo, S.; Koroleva, Y. Mapping and Spatial Prediction of (Na, Cl, Ca, and Mg) Atmospheric Deposition in Moss Samples: A Study in the Kaliningrad Region, Russia. Arab. J. Geosci. 2023, 16, 651. [Google Scholar] [CrossRef]
- Sergeeva, A.; Zinicovscaia, I.; Vergel, K.; Yushin, N.; Urošević, M.A. The Effect of Heavy Industry on Air Pollution Studied by Active Moss Biomonitoring in Donetsk Region (Ukraine). Arch. Environ. Contam. Toxicol. 2021, 80, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Khiem, L.H.; Sera, K.; Hosokawa, T.; Nam, L.D.; Quyet, N.H.; Frontasyeva, M.; My, T.T.T.; My, N.T.B.; Zinicovscaia, I.; Nghia, N.T.; et al. Active Moss Biomonitoring Technique for Atmospheric Elemental Contamination in Hanoi Using Proton Induced X-Ray Emission. J. Radioanal. Nucl. Chem. 2020, 325, 515–525. [Google Scholar] [CrossRef]
- Di Palma, A.; Capozzi, F.; Spagnuolo, V.; Giordano, S.; Adamo, P. Atmospheric Particulate Matter Intercepted by Moss-Bags: Relations to Moss Trace Element Uptake and Land Use. Chemosphere 2017, 176, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.; Urošević, M.A.; Pergal, M.; Janković, M.; Goryainova, Z.; Tomašević, M.; Popović, A. Residential Heating Contribution to Level of Air Pollutants (PAHs, Major, Trace, and Rare Earth Elements): A Moss Bag Case Study. Environ. Sci. Pollut. Res. 2015, 22, 18956–18966. [Google Scholar] [CrossRef] [PubMed]
- Paliulis, D. Evaluation of Zinc Accumulation in Moss (Pylaisia Polyantha) Growing Near Intensive Traffic Street Based on Modelling and Experimental Data. Rocz. Ochr. Sr. 2021, 23, 198–213. [Google Scholar] [CrossRef]
- Hu, R.; Yan, Y.; Zhou, X.; Wang, Y.; Fang, Y. Monitoring Heavy Metal Contents with Sphagnum Junghuhnianum Moss Bags in Relation to Traffic Volume in Wuxi, China. Int. J. Environ. Res. Public Health 2018, 15, 374. [Google Scholar] [CrossRef]
- Donovan, M.; Norman, A.L.; Reid, M.L. Local Vehicles Add Nitrogen to Moss Biomonitors in a Low-Traffic Protected Wilderness Area as Revealed by a Long-Term Isotope Study. J. Nat. Conserv. 2022, 70, 126292. [Google Scholar] [CrossRef]
- Foan, L.; Domercq, M.; Bermejo, R.; Santamaría, J.M.; Simon, V. Mosses as an Integrating Tool for Monitoring PAH Atmospheric Deposition: Comparison with Total Deposition and Evaluation of Bioconcentration Factors. A Year-Long Case-Study. Chemosphere 2015, 119, 452–458. [Google Scholar] [CrossRef]
- Sucharová, J.; Holá, M. PAH and PCB Determination of the Concentration Gradient in Moss Pleurozium Schreberi near a Highway, and Seasonal Variability at the Background Reference Site. Int. J. Environ. Anal. Chem. 2014, 94, 712–727. [Google Scholar] [CrossRef]
- Huang, S.; Dai, C.; Zhou, Y.; Peng, H.; Yi, K.; Qin, P.; Luo, S.; Zhang, X. Comparisons of Three Plant Species in Accumulating Polycyclic Aromatic Hydrocarbons (PAHs) from the Atmosphere: A Review. Environ. Sci. Pollut. Res. 2018, 25, 16548–16566. [Google Scholar] [CrossRef]
- Gómez-Arroyo, S.; Zavala-Sánchez, M.Á.; Alonso-Murillo, C.D.; Cortés-Eslava, J.; Amador-Muñoz, O.; Jiménez-García, L.F.; Morton-Bermea, O. Moss (Hypnum amabile) as Biomonitor of Genotoxic Damage and as Bioaccumulator of Atmospheric Pollutants at Five Different Sites of Mexico City and Metropolitan Area. Environ. Sci. Pollut. Res. 2021, 28, 9849–9863. [Google Scholar] [CrossRef] [PubMed]
- Çabuk, H.; Kılıç, M.S.; Ören, M. Biomonitoring of Polycyclic Aromatic Hydrocarbons in Urban and Industrial Environments of the Western Black Sea Region, Turkey. Environ. Monit. Assess. 2014, 186, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.; Urošević, M.A.; Goryainova, Z.; Pergal, M.; Škrivanj, S.; Samson, R.; Popović, A. Active Moss Biomonitoring for Extensive Screening of Urban Air Pollution: Magnetic and Chemical Analyses. Sci. Total Environ. 2015, 521–522, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.; Calabrese, S.; D’Alessandro, W.; Planer-Friedrich, B. Active Moss Monitoring Allows to Identify and Track Distribution of Metal(Loid)s Emitted from Fumaroles on Vulcano Island, Italy. J. Volcanol. Geotherm. Res. 2014, 280, 30–39. [Google Scholar] [CrossRef]
- Stogiannidis, E.; Laane, R. Source Characterization of Polycyclic Aromatic Hydrocarbons by Using Their Molecular Indices: An Overview of Possibilities. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 49–133. ISBN 978-3-319-10638-0. [Google Scholar]
- Clément, N.; Muresan, B.; Hedde, M.; François, D. PAH Dynamics in Roadside Environments: Influence on the Consistency of Diagnostic Ratio Values and Ecosystem Contamination Assessments. Sci. Total Environ. 2015, 538, 997–1009. [Google Scholar] [CrossRef]

| Pl | ∑LMW/∑HMW | FL/(FL + PYR) | ANT/(ANT + PHE) | FLA/(FLA + PYR) | BaA/(BaA + CHR) | IcdP/(IcdP + BghiP) | BaP/BghiP |
|---|---|---|---|---|---|---|---|
| 1 | 0.201 | n.d. | 0.091 | 0.582 | 0.155 | 0.268 | 0.356 |
| 2 | 0.379 | 0.034 | 0.043 | 0.603 | 0.211 | 0.536 | 0.423 |
| 3 | 0.277 | 0.034 | 0.076 | 0.600 | 0.239 | 0.486 | 0.596 |
| 4 | 0.199 | 0.017 | 0.054 | 0.614 | 0.219 | 0.512 | 0.629 |
| 5 | 0.276 | 0.033 | 0.028 | 0.638 | 0.060 | 0.545 | 0.652 |
| 6 | 0.330 | 0.042 | 0.028 | 0.643 | 0.140 | 0.533 | 0.699 |
| 7 | 0.222 | 0.021 | 0.034 | 0.629 | 0.134 | 0.523 | 0.732 |
| 8 | 0.149 | 0.017 | 0.055 | 0.625 | 0.145 | 0.522 | 0.992 |
| 9 | 0.136 | 0.033 | 0.100 | 0.613 | 0.149 | 0.532 | 1.02 |
| 10 | 0.170 | 0.053 | 0.179 | 0.607 | 0.167 | 0.495 | 0.909 |
| 11 | 0.171 | 0.049 | 0.154 | 0.606 | 0.174 | 0.464 | 0.862 |
| 12 | 0.221 | 0.043 | 0.147 | 0.588 | 0.205 | 0.486 | 1.04 |
| Av. | 0.228 | 0.031 | 0.082 | 0.612 | 0.166 | 0.492 | 0.743 |
| Sp | ∑LMW/∑HMW | FL/(FL + PYR) | ANT/(ANT + PHE) | FLA/(FLA + PYR) | BaA/(BaA + CHR) | IcdP/(IcdP + BghiP) | BaP/BghiP |
| 1 | 0.182 | n.d. | 0.001 | 0.658 | 0.115 | 0.139 | n.d. |
| 2 | 0.439 | 0.033 | 0.024 | 0.622 | 0.202 | 0.248 | 0.142 |
| 3 | 0.232 | n.d. | 0.032 | 0.631 | 0.224 | 0.350 | 0.206 |
| 4 | 0.238 | 0.014 | 0.028 | 0.639 | 0.190 | 0.438 | 1.80 |
| 5 | 0.452 | n.d. | n.d. | 0.674 | 0.130 | 0.309 | 0.146 |
| 6 | 0.344 | 0.011 | 0.002 | 0.670 | 0.153 | 0.425 | 0.402 |
| 7 | 0.193 | 0.008 | 0.005 | 0.676 | 0.130 | 0.448 | 0.412 |
| 8 | 0.085 | n.d. | 0.005 | 0.685 | 0.124 | 0.415 | 0.462 |
| 9 | n.d. | n.d. | n.d. | 0.714 | 0.129 | 0.403 | 0.464 |
| 10 | 0.048 | 0.242 | n.d. | 0.765 | 0.179 | 0.306 | 0.490 |
| Av. | 0.246 | 0.061 | 0.014 | 0.673 | 0.158 | 0.348 | 0.503 |
| Di | ∑LMW/∑HMW | FL/(FL + PYR) | ANT/(ANT + PHE) | FLA/(FLA + PYR) | BaA/(BaA + CHR) | IcdP/(IcdP + BghiP) | BaP/BghiP |
| 1 | 20.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 0.169 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4 | n.d. | n.d. | n.d. | 0.775 | n.d. | n.d. | n.d. |
| 5 | 0.224 | n.d. | n.d. | 0.764 | n.d. | n.d. | n.d. |
| 6 | 0.458 | n.d. | 0.014 | 0.697 | 0.075 | n.d. | n.d. |
| 7 | n.d. | n.d. | n.d. | 0.954 | n.d. | n.d. | n.d. |
| 8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 11 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 12 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Av. | 5.32 | n.d. | 0.014 | 0.798 | 0.075 | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajfur, M.; Stoica, A.-I.; Świsłowski, P.; Stach, W.; Ziegenbalg, F.; Mattausch, E.M. Assessment of Atmospheric Pollution by Selected Elements and PAHs during 12-Month Active Biomonitoring of Terrestrial Mosses. Atmosphere 2024, 15, 102. https://doi.org/10.3390/atmos15010102
Rajfur M, Stoica A-I, Świsłowski P, Stach W, Ziegenbalg F, Mattausch EM. Assessment of Atmospheric Pollution by Selected Elements and PAHs during 12-Month Active Biomonitoring of Terrestrial Mosses. Atmosphere. 2024; 15(1):102. https://doi.org/10.3390/atmos15010102
Chicago/Turabian StyleRajfur, Małgorzata, Anca-Iulia Stoica, Paweł Świsłowski, Wolfgang Stach, Falko Ziegenbalg, and Eva Maria Mattausch. 2024. "Assessment of Atmospheric Pollution by Selected Elements and PAHs during 12-Month Active Biomonitoring of Terrestrial Mosses" Atmosphere 15, no. 1: 102. https://doi.org/10.3390/atmos15010102
APA StyleRajfur, M., Stoica, A.-I., Świsłowski, P., Stach, W., Ziegenbalg, F., & Mattausch, E. M. (2024). Assessment of Atmospheric Pollution by Selected Elements and PAHs during 12-Month Active Biomonitoring of Terrestrial Mosses. Atmosphere, 15(1), 102. https://doi.org/10.3390/atmos15010102







