Combined Effects of Ambient PM2.5 and Cold Exposure on the Development of Metabolic Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Exposure Design
2.2. Blood Pressure Measurement
2.3. Metabolic Cage
2.4. Blood Glucose Tolerance and Insulin Sensitivity Measurement
2.5. Blood Biomarkers
2.6. Serous Lipid Determination
2.7. Histological Analysis and Transmission Electron Microscopy Observation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
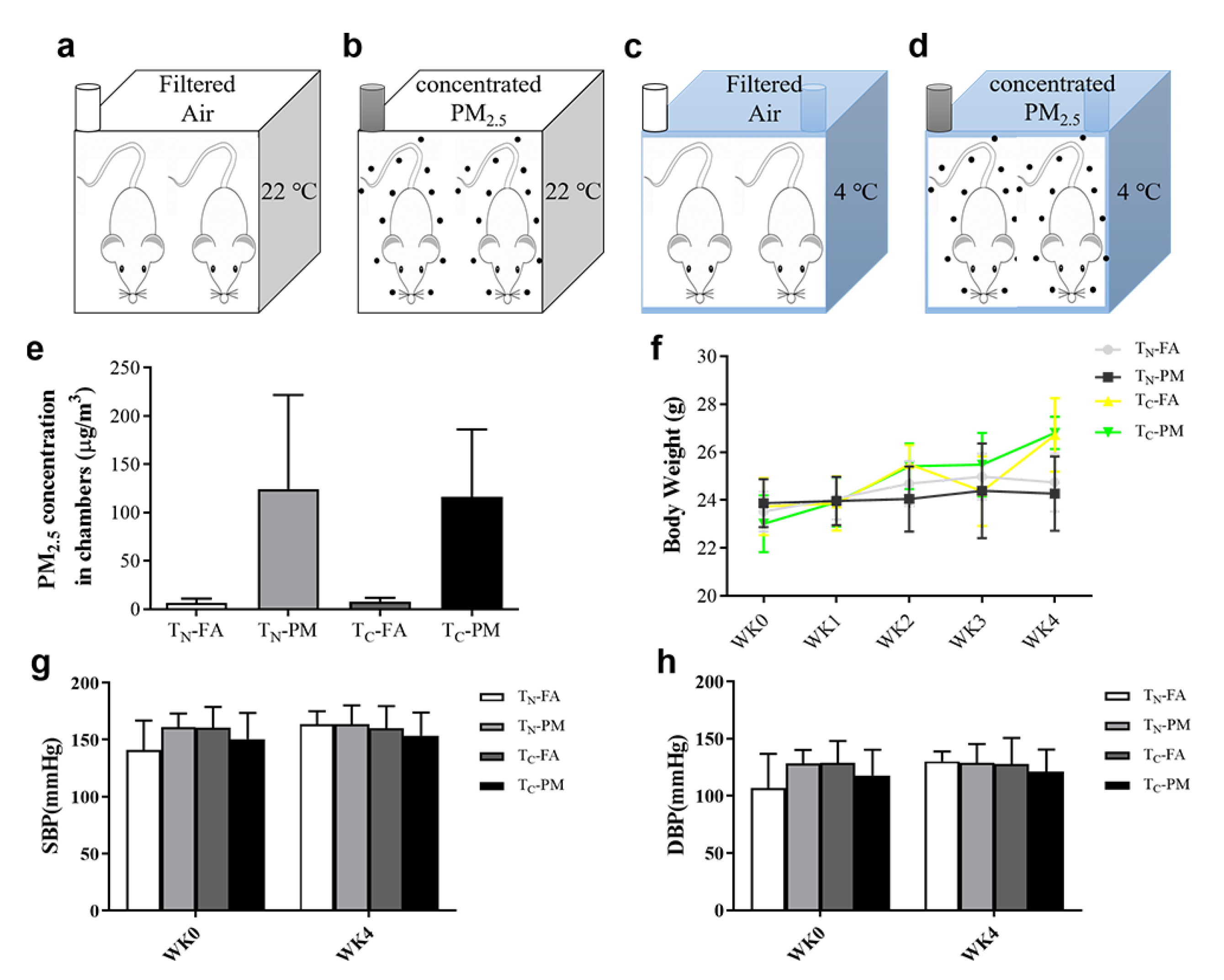
3.1. PM2.5 Concentrations and Body Weight of the Mice
3.2. Combined Effects of PM2.5 and Cold Exposure on Blood Pressure
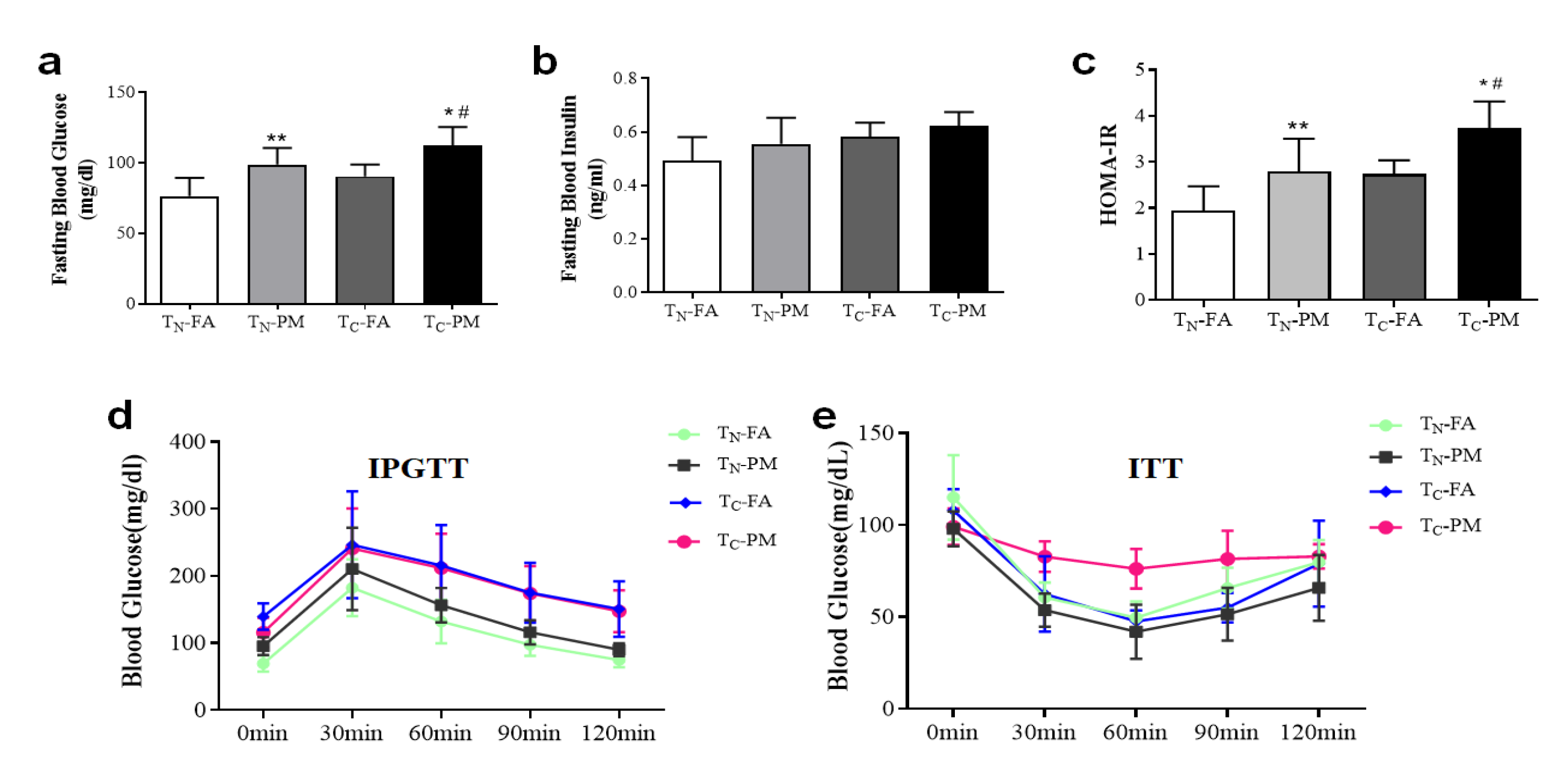
3.3. Cold Exacerbates PM2.5-Induced Glucose Tolerance and Insulin Resistance
3.4. Cold Exacerbates PM2.5-Induced Energy Imbalance
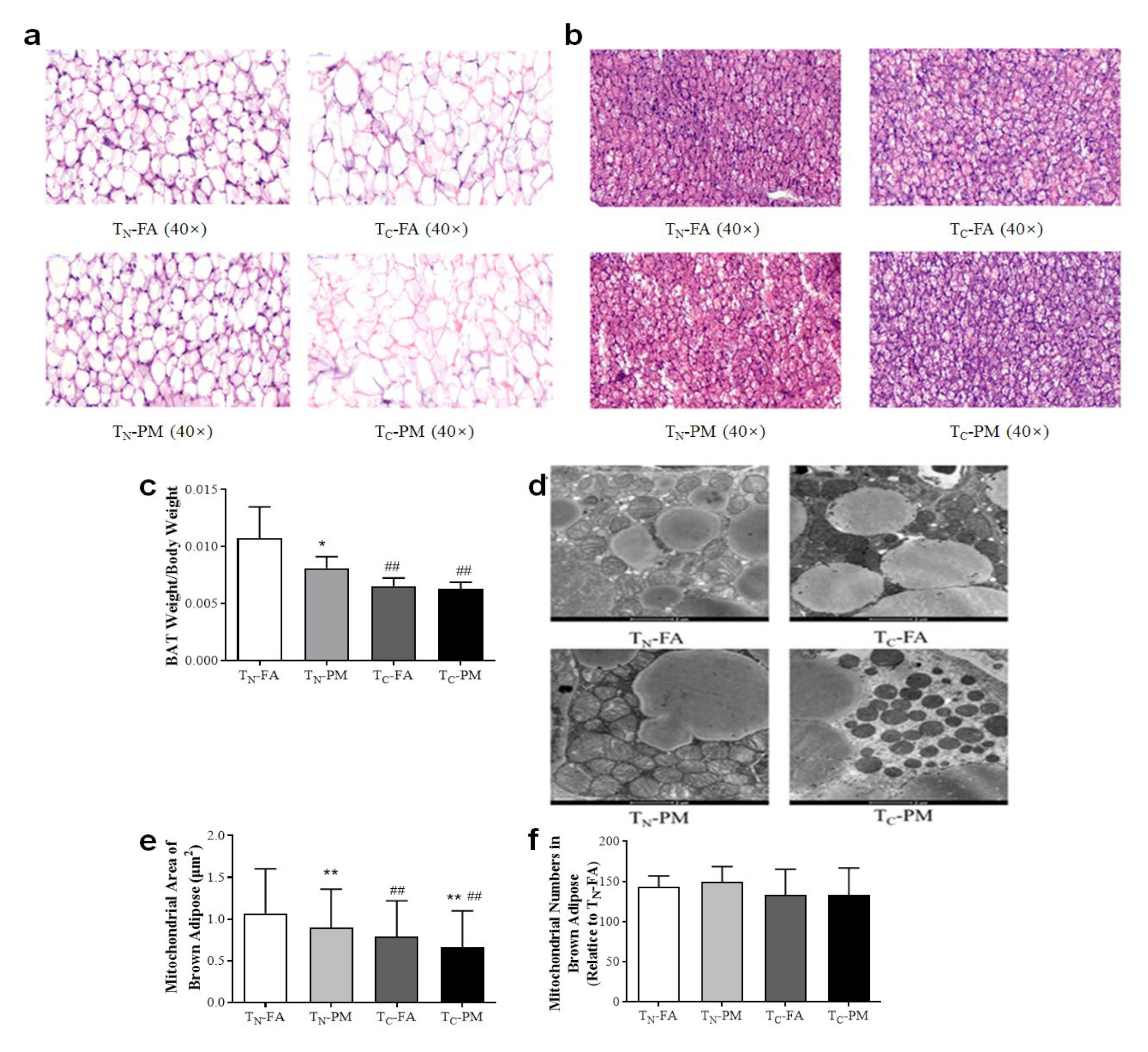
3.5. Cold Exacerbates PM2.5-Induced Pathological and Mitochondria Alteration
3.6. Cold Influences PM2.5-Induced Abnormal Lipid Metabolism
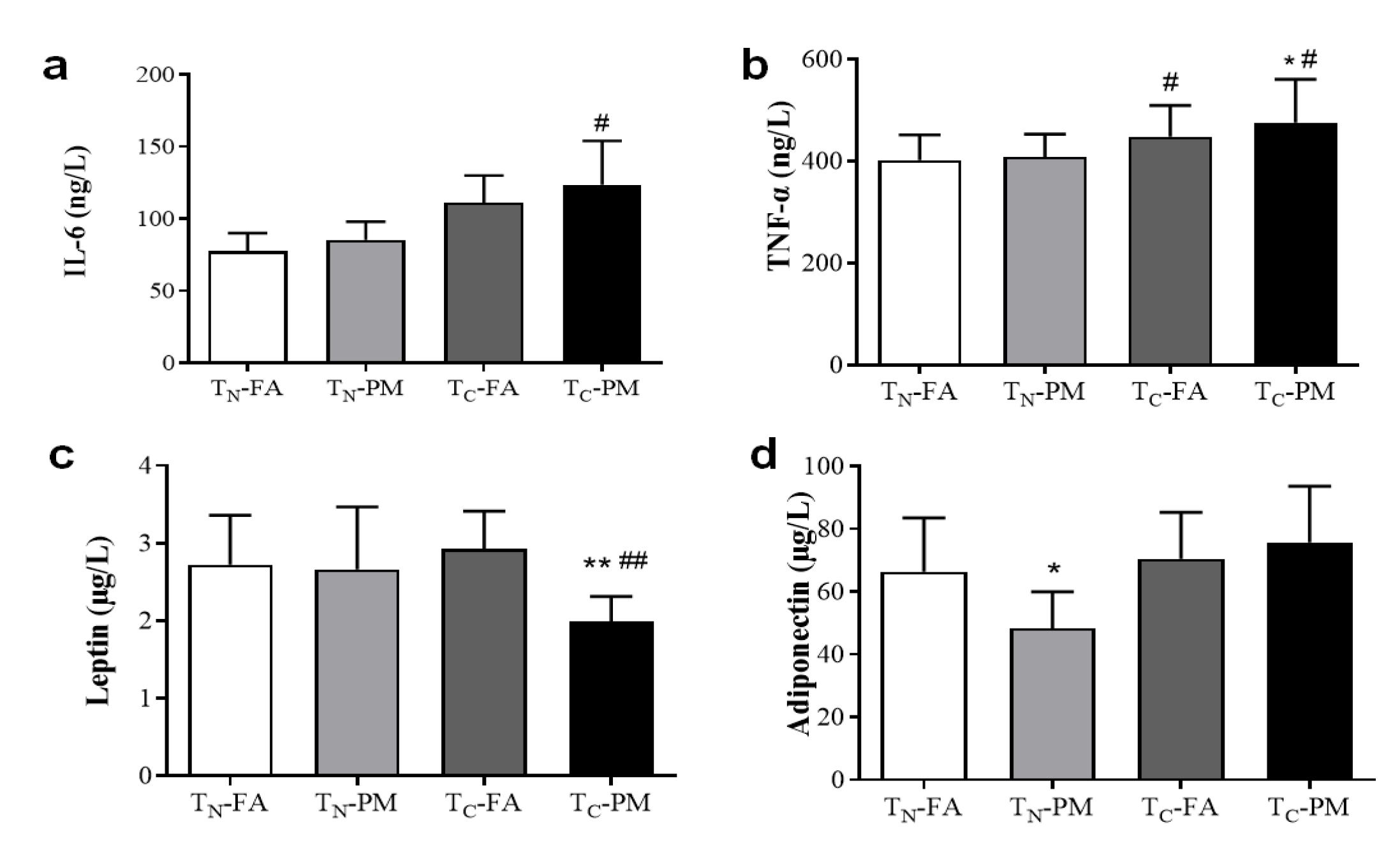
3.7. Cold Exacerbates PM2.5-Induced Inflammation and Energy Expenditure Inhibition
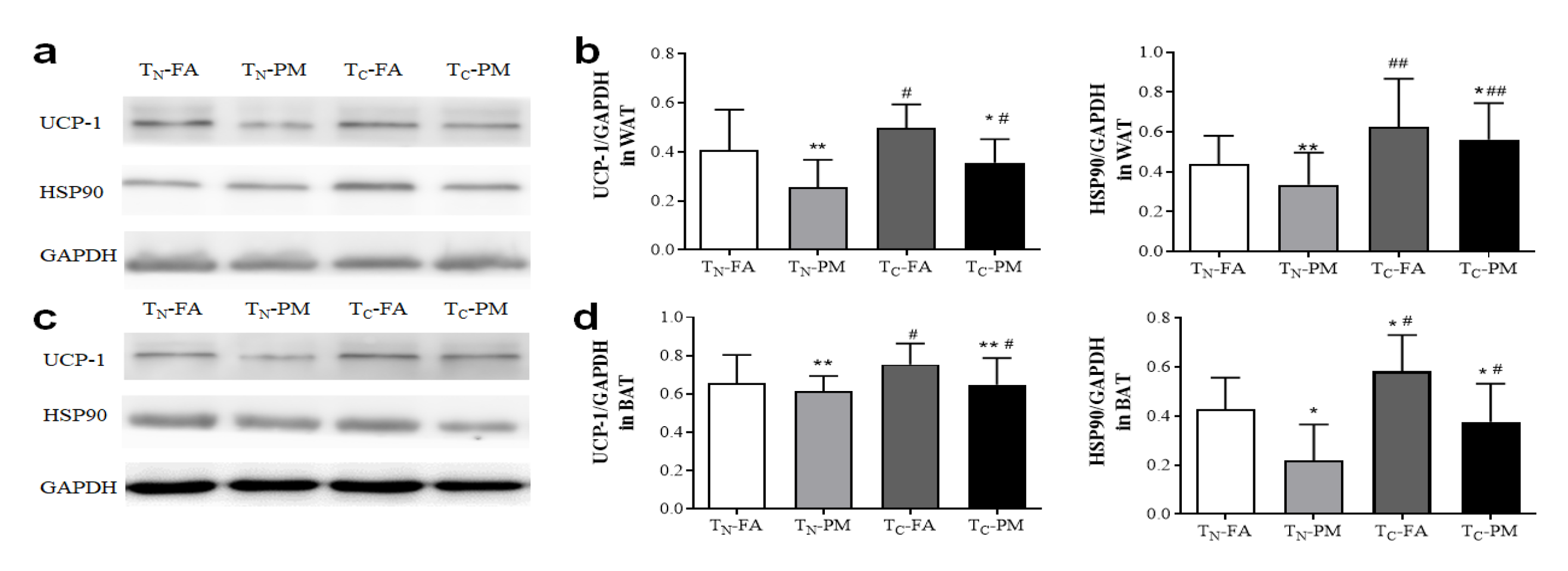
3.8. Combined Exposure to PM2.5 and the Cold Inhibits Thermogenesis and Energy Expenditure
4. Discussion
4.1. Cold Exacerbates PM2.5-Induced Glucose Metabolism Disorders
4.2. Cold Exacerbates PM2.5-Induced Lipid Metabolism Disorders
4.3. Cold Exacerbates PM2.5-Induced Protein Metabolism Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. Clean Air J. 2016, 26, 6. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Varghese, B.M.; Hansen, A.; Zhang, Y.; Driscoll, T.; Morgan, G.; Dear, K.; Gourley, M.; Capon, A.; Bi, P. Heat exposure and cardiovascular health outcomes: A systematic review and meta-analysis. Lancet Planet. Health 2022, 6, e484–e495. [Google Scholar] [CrossRef] [PubMed]
- Lakhoo, D.P.; Blake, H.A.; Chersich, M.F.; Nakstad, B.; Kovats, S. The Effect of High and Low Ambient Temperature on Infant Health: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9109. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; van Daalen, K.; Anto, J.M.; Dasandi, N.; Drummond, P.; Hamilton, I.G.; Jankin, S.; Kendrovski, V.; Lowe, R.; Rocklöv, J.; et al. Tracking progress on health and climate change in Europe. Lancet Public Health 2021, 6, e858–e865. [Google Scholar] [CrossRef]
- Rajkumar, S.; Young, B.N.; Clark, M.L.; Benka-Coker, M.L.; Bachand, A.M.; Brook, R.D.; Nelson, T.L.; Volckens, J.; Reynolds, S.J.; L’Orange, C.; et al. Household air pollution from biomass-burning cookstoves and metabolic syndrome, blood lipid concentrations, and waist circumference in Honduran women: A cross-sectional study. Environ. Res. 2019, 170, 46–55. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Park, B.; Palanivel, R.; Vinayachandran, V.; Deiuliis, J.A.; Gangwar, R.S.; Das, L.; Yin, J.; Choi, Y.; Al-Kindi, S.; et al. Metabolic effects of air pollution exposure and reversibility. J. Clin. Investig. 2020, 130, 6034–6040. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, S.; Fan, P.; Li, L.; Feng, S.; Xiao, H.; Zhu, L. The Roles of Liver Inflammation and the Insulin Signaling Pathway in PM2.5 Instillation-Induced Insulin Resistance in Wistar Rats. Dis. Markers 2021, 2021, 2821673. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Tang, S.L.; Liu, T.; Wang, Y.; Xu, X.J.; Xiao, N.; Li, C.; Xu, Y.J.; He, Z.X.; Ma, S.L.; et al. Effects of long-term PM(2.5) exposure on metabolic syndrome among adults and elderly in Guangdong, China. Environ. Health A Glob. Access Sci. Source 2022, 21, 84. [Google Scholar] [CrossRef]
- Liang, X.; Pan, J.; Cao, C.; Zhang, L.; Zhao, Y.; Fan, Y.; Li, K.; Tao, C.; Wang, Y. Transcriptional Response of Subcutaneous White Adipose Tissue to Acute Cold Exposure in Mice. Int. J. Mol. Sci. 2019, 20, 3968. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Sugimoto, S.; Mena, H.A.; Sansbury, B.E.; Kobayashi, S.; Tsuji, T.; Wang, C.-H.; Yin, X.; Huang, T.L.; Kusuyama, J.; Kodani, S.D.; et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 2022, 4, 775–790. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 27007. [Google Scholar] [CrossRef]
- Yu, P.; Xu, R.; Li, S.; Yue, X.; Chen, G.; Ye, T.; Coêlho, M.; Saldiva, P.H.N.; Sim, M.R.; Abramson, M.J.; et al. Exposure to wildfire-related PM2.5 and site-specific cancer mortality in Brazil from 2010 to 2016: A retrospective study. PLoS Med. 2022, 19, e1004103. [Google Scholar] [CrossRef]
- Benmarhnia, T.; Oulhote, Y.; Petit, C.; Lapostolle, A.; Chauvin, P.; Zmirou-Navier, D.; Deguen, S. Chronic air pollution and social deprivation as modifiers of the association between high temperature and daily mortality. Environ. Health A Glob. Access Sci. Source 2014, 13, 53. [Google Scholar] [CrossRef]
- Li, J.; Woodward, A.; Hou, X.-Y.; Zhu, T.; Zhang, J.; Brown, H.; Yang, J.; Qin, R.; Gao, J.; Gu, S.; et al. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2017, 575, 1556–1570. [Google Scholar] [CrossRef]
- Shaposhnikov, D.; Revich, B.; Bellander, T.; Bedada, G.B.; Bottai, M.; Kharkova, T.; Kvasha, E.; Lezina, E.; Lind, T.; Semutnikova, E.; et al. Mortality related to air pollution with the moscow heat wave and wildfire of 2010. Epidemiology 2014, 25, 359–364. [Google Scholar] [CrossRef]
- Wang, Q.; Li, B.; Benmarhnia, T.; Hajat, S.; Ren, M.; Liu, T.; Knibbs, L.D.; Zhang, H.; Bao, J.; Zhang, Y.; et al. Independent and Combined Effects of Heatwaves and PM2.5 on Preterm Birth in Guangzhou, China: A Survival Analysis. Environ. Health Perspect. 2020, 128, 17006. [Google Scholar] [CrossRef]
- De Sario, M.; Katsouyanni, K.; Michelozzi, P. Climate change, extreme weather events, air pollution and respiratory health in Europe. Eur. Respir. J. 2013, 42, 826. [Google Scholar] [CrossRef]
- Du, X.; Zeng, X.; Pan, K.; Zhang, J.; Song, L.; Zhou, J.; Chen, R.; Xie, Y.; Sun, Q.; Zhao, J.; et al. Metabolomics analysis of urine from healthy wild type mice exposed to ambient PM2.5. Sci. Total Environ. 2020, 714, 136790. [Google Scholar] [CrossRef]
- Du, X.; Zeng, X.; Zhang, J.; Pan, K.; Song, L.; Zhou, J.; Zhou, L.; Xie, Y.; Sun, Q.; Ge, W.; et al. Ambient fine particulate matter induced the elevation of blood pressure through ACE2/Ang(1–7) pathway: The evidence from urine metabolites. Ecotoxicol. Environ. Saf. 2020, 203, 111044. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Jiang, S.; Du, X.; Zeng, X.; Zhang, J.; Song, L.; Zhou, J.; Kan, H.; Sun, Q.; Xie, Y.; et al. AMPK activation attenuates inflammatory response to reduce ambient PM2.5-induced metabolic disorders in healthy and diabetic mice. Ecotoxicol. Environ. Saf. 2019, 179, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Jerrett, M.; Anderson, H.R.; Burnett, R.T.; Stone, V.; Derwent, R.; Atkinson, R.W.; Cohen, A.; Shonkoff, S.B.; Krewski, D.; et al. Public health benefits of strategies to reduce greenhouse-gas emissions: Health implications of short-lived greenhouse pollutants. Lancet 2009, 374, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of The Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Goettems-Fiorin, P.B.; Grochanke, B.S.; Baldissera, F.G.; dos Santos, A.B.; Homem de Bittencourt, P.I.; Ludwig, M.S.; Rhoden, C.R.; Heck, T.G. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: Stress response and extracellular to intracellular HSP70 ratio analysis. J. Physiol. Biochem. 2016, 72, 643–656. [Google Scholar] [CrossRef]
- Hansen, A.B.; Ravnskjær, L.; Loft, S.; Andersen, K.K.; Bräuner, E.V.; Baastrup, R.; Yao, C.; Ketzel, M.; Becker, T.; Brandt, J.; et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ. Int. 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Liu, C.; Fonken, L.K.; Wang, A.; Maiseyeu, A.; Bai, Y.; Wang, T.Y.; Maurya, S.; Ko, Y.A.; Periasamy, M.; Dvonch, T.; et al. Central IKKβ inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part. Fibre Toxicol. 2014, 11, 53. [Google Scholar] [CrossRef]
- Pearson, J.F.; Bachireddy, C.; Shyamprasad, S.; Goldfine, A.B.; Brownstein, J.S. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care 2010, 33, 2196–2201. [Google Scholar] [CrossRef]
- Zanobetti, A.; Dominici, F.; Wang, Y.; Schwartz, J.D. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health A Glob. Access Sci. Source 2014, 13, 38. [Google Scholar] [CrossRef]
- Askari, S.; Asghari, G.; Ghanbarian, A.; Khazan, M.; Alamdari, S.; Azizi, F. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. Arch. Iran. Med. 2014, 17, 441–443. [Google Scholar]
- Iwen, K.A.; Backhaus, J.; Cassens, M.; Waltl, M.; Hedesan, O.C.; Merkel, M.; Heeren, J.; Sina, C.; Rademacher, L.; Windjäger, A.; et al. Cold-Induced Brown Adipose Tissue Activity Alters Plasma Fatty Acids and Improves Glucose Metabolism in Men. J. Clin. Endocrinol. Metab. 2017, 102, 4226–4234. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, X.; Du, X.; Pan, K.; Song, L.; Song, W.; Xie, Y.; Zhao, J. Parental PM2.5 Exposure-Promoted Development of Metabolic Syndrome in Offspring Is Associated with the Changes of Immune Microenvironment. Toxicol. Sci. 2019, 170, 415–426. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, F.; Xu, J.; Peng, L.; Ye, X.; Yang, D.; Zhao, J.; Sun, Q. PM2.5 exposure and cold stress exacerbates asthma in mice by increasing histone acetylation in IL-4 gene promoter in CD4+ T cells. Toxicol. Lett. 2019, 316, 147–153. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, J.; Geng, F.; Peng, L.; Ye, X.; Yang, D.; Zhao, J.; Sun, Q. Childhood co-exposure of cold stress and PM2.5 aggravates the susceptibility and severity of asthma in adulthood of mice. Environ. Toxicol. 2021, 36, 177–184. [Google Scholar] [CrossRef]
- Liu, G.; Sun, B.; Yu, L.; Chen, J.; Han, B.; Liu, B.; Chen, J. Short-term exposure to ambient air pollution and daily atherosclerotic heart disease mortality in a cool climate. Environ. Sci. Pollut. Res. 2019, 26, 23603–23614. [Google Scholar] [CrossRef]
- Watts, N.; Adger, W.N.; Agnolucci, P.; Blackstock, J.; Byass, P.; Cai, W.; Chaytor, S.; Colbourn, T.; Collins, M.; Cooper, A.; et al. Health and climate change: Policy responses to protect public health. Lancet 2015, 386, 1861–1914. [Google Scholar] [CrossRef]
- Ji, D.; Cui, Y.; Li, L.; He, J.; Wang, L.; Zhang, H.; Wang, W.; Zhou, L.; Maenhaut, W.; Wen, T.; et al. Characterization and source identification of fine particulate matter in urban Beijing during the 2015 Spring Festival. Sci. Total Environ. 2018, 628–629, 430–440. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cao, F. Fine particulate matter (PM2.5) in China at a city level. Sci. Rep. 2015, 5, 14884. [Google Scholar] [CrossRef]
- Hameed, S.; Pan, K.; Su, W.; Trupp, M.; Mi, L.; Zhao, J. Label-free detection and quantification of ultrafine particulate matter in lung and heart of mouse and evaluation of tissue injury. Part. Fibre Toxicol. 2022, 19, 51. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Bai, Y.; Wang, T.Y.; Rao, X.; Wang, A.; Sun, L.; Ying, Z.; Gushchina, L.; Maiseyeu, A.; et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: Influence of CCR2 pathways in mice. Environ. Health Perspect. 2014, 122, 17–26. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Brook, R.D. Air Pollution and Type 2 Diabetes: Mechanistic Insights. Diabetes 2012, 61, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants with Hypertension: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Campolim, C.M.; Weissmann, L.; Ferreira, C.K.d.O.; Zordão, O.P.; Dornellas, A.P.S.; de Castro, G.; Zanotto, T.M.; Boico, V.F.; Quaresma, P.G.F.; Lima, R.P.A.; et al. Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice. Sci. Rep. 2020, 10, 10160. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, J.A.M.; et al. Primary Prevention of Acute Coronary Events with Lovastatin in Men and Women with Average Cholesterol LevelsResults of AFCAPS/TexCAPS. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Fortmann, S.P.; Krauss, R.M. Association of Small Low-Density Lipoprotein Particles with the Incidence of Coronary Artery Disease in Men and Women. JAMA 1996, 276, 875–881. [Google Scholar] [CrossRef]
- Araujo, J.A.; Barajas, B.; Kleinman, M.; Wang, X.; Bennett, B.J.; Gong, K.W.; Navab, M.; Harkema, J.; Sioutas, C.; Lusis, A.J.; et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008, 102, 589–596. [Google Scholar] [CrossRef]
- Yitshak Sade, M.; Kloog, I.; Liberty, I.F.; Schwartz, J.; Novack, V. The Association between Air Pollution Exposure and Glucose and Lipids Levels. J. Clin. Endocrinol. Metab. 2016, 101, 2460–2467. [Google Scholar] [CrossRef]
- Graña-Baumgartner, A.; Dukkipati, V.S.R.; Biggs, P.J.; Kenyon, P.R.; Blair, H.T.; López-Villalobos, N.; Ross, A.B. Mass Spectrometry-Based Lipidomics of Brown Adipose Tissue and Plasma of New-Born Lambs Subjected to Short-Term Cold Exposure. Animals 2022, 12, 2762. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Zhang, R.; Zhan, S.; Zhong, T.; Guo, J.; Wang, Y.; Cao, J.; Li, L.; Zhang, H.; et al. Cold exposure induces lipid dynamics and thermogenesis in brown adipose tissue of goats. BMC Genom. 2022, 23, 528. [Google Scholar] [CrossRef]
- Khedoe, P.P.S.J.; Hoeke, G.; Kooijman, S.; Dijk, W.; Buijs, J.T.; Kersten, S.; Havekes, L.M.; Hiemstra, P.S.; Berbée, J.F.P.; Boon, M.R.; et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J. Lipid Res. 2015, 56, 51–59. [Google Scholar] [CrossRef]
- Du, X.; Jiang, S.; Zeng, X.; Zhang, J.; Pan, K.; Zhou, J.; Xie, Y.; Kan, H.; Song, W.; Sun, Q.; et al. Air pollution is associated with the development of atherosclerosis via the cooperation of CD36 and NLRP3 inflammasome in ApoE−/− mice. Toxicol. Lett. 2018, 290, 123–132. [Google Scholar] [CrossRef]
- Wei, Q.; Lee, J.H.; Wang, H.; Bongmba, O.Y.N.; Wu, C.-S.; Pradhan, G.; Sun, Z.; Chew, L.; Bajaj, M.; Chan, L.; et al. Adiponectin is required for maintaining normal body temperature in a cold environment. BMC Physiol. 2017, 17, 8. [Google Scholar] [CrossRef]
- Wolf, G. Adiponectin: A regulator of energy homeostasis. Nutr. Rev. 2003, 61, 290–292. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Xu, Z.; Tzan, K.; Zhong, M.; Wang, A.; Lippmann, M.; Chen, L.C.; Rajagopalan, S.; Sun, Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 124, 88–98. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of Acute Cold Stress after Long-Term Cold Stimulation on Antioxidant Status, Heat Shock Proteins, Inflammation and Immune Cytokines in Broiler Heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 Chaperone Machinery*. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef]
- Dezwaan, D.C.; Freeman, B.C. HSP90: The Rosetta stone for cellular protein dynamics? Cell Cycle 2008, 7, 1006–1012. [Google Scholar] [CrossRef]
- Sajad, S.; Jiang, S.; Anwar, M.; Dai, Q.; Luo, Y.; Hassan, M.A.; Tetteh, C.; Song, J. Genome-Wide Study of Hsp90 Gene Family in Cabbage (Brassica oleracea var. capitata L.) and Their Imperative Roles in Response to Cold Stress. Front. Plant Sci. 2022, 13, 908511. [Google Scholar] [CrossRef]
- Krishna, P.; Sacco, M.; Cherutti, J.F.; Hill, S. Cold-Induced Accumulation of hsp90 Transcripts in Brassica napus. Plant Physiol. 1995, 107, 915–923. [Google Scholar] [CrossRef]
- Watterson, T.L.; Hamilton, B.; Martin, R.; Coulombe, R.A., Jr. Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 112, 111–122. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Xu, X.; Sun, L.; Wang, A.; Wang, T.-Y.; Maurya, S.K.; Periasamy, M.; Morishita, M.; Harkema, J.; et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part. Fibre Toxicol. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jiang, S.; Pan, K.; Du, X.; Zeng, X.; Zhang, J.; Zhou, J.; Sun, Q.; Xie, Y.; Zhao, J. AMPK activation ameliorates fine particulate matter-induced hepatic injury. Environ. Sci. Pollut. Res. 2020, 27, 21311–21319. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, J.; Fan, D.; Wang, G.; Wu, B.; Lei, L.; Wang, L.; Zhao, J.; Chen, J. Combined Effects of Ambient PM2.5 and Cold Exposure on the Development of Metabolic Disorder. Atmosphere 2023, 14, 1157. https://doi.org/10.3390/atmos14071157
Liu Z, Zhang J, Fan D, Wang G, Wu B, Lei L, Wang L, Zhao J, Chen J. Combined Effects of Ambient PM2.5 and Cold Exposure on the Development of Metabolic Disorder. Atmosphere. 2023; 14(7):1157. https://doi.org/10.3390/atmos14071157
Chicago/Turabian StyleLiu, Zhixiu, Jia Zhang, Dongxia Fan, Ge Wang, Biao Wu, Lei Lei, Lina Wang, Jinzhuo Zhao, and Jianmin Chen. 2023. "Combined Effects of Ambient PM2.5 and Cold Exposure on the Development of Metabolic Disorder" Atmosphere 14, no. 7: 1157. https://doi.org/10.3390/atmos14071157
APA StyleLiu, Z., Zhang, J., Fan, D., Wang, G., Wu, B., Lei, L., Wang, L., Zhao, J., & Chen, J. (2023). Combined Effects of Ambient PM2.5 and Cold Exposure on the Development of Metabolic Disorder. Atmosphere, 14(7), 1157. https://doi.org/10.3390/atmos14071157








