Abstract
Informed by the theory of developmental origins of health and disease, we conducted a survey among 66,854 mother–preschooler dyads in all kindergartens within the Longhua District of Shenzhen in China for exploring the combined effect of prenatal mosquito coil smoke (MCS) exposure and early postnatal nutritional status on obesity in preschoolers. A self-administered questionnaire was completed by mothers to collect parents’ and children’s socio-demographic characteristics, prenatal MCS exposure, child nutritional status at 1–3 years, etc., and children’s heights and weights were measured at the same time. After controlling for potential confounders, a series of logistic regression models and cross-over analyses were used to examine the independent and combination effects of prenatal MCS exposure and postnatal nutritional status on preschoolers’ obesity. We found that prenatal MCS exposure significantly increased the risk of preschoolers’ obesity (AOR = 1.14, 95%CI = 1.08–1.21). Additionally, early postnatal well-nourished nutritional status also increased the risk of preschoolers’ obesity (AOR = 1.56, 95%CI = 1.47–1.66). The cross-over analysis showed that the combination of prenatal MCS exposure with early general postnatal nutrition status (AOR = 1.22, 95%CI = 1.10–1.36) and early postnatal well-nourished nutrition status (AOR = 1.81, 95%CI = 1.65–1.97), respectively, increased the risk of preschoolers’ obesity. Our results indicated that prenatal MCS exposure and early postnatal nutritional status can independently and jointly increase the risk of preschoolers’ obesity.
1. Introduction
Childhood obesity is a critical public health concern around the world, with prevalence rates rising at an alarming rate. The World Health Organization (WHO) estimated that there were around 39 million obese or overweight children under the age of five in 2020 [1,2]. The national obesity prevalence among children aged 6–17 in China climbed from 1.8–2.4% in the 1990s to 7.9–12.7% in 2015–2019 [3]. Moreover, childhood obesity is a significant public health concern due to it being an important risk factor for non-communicable diseases such as obesity, diabetes, cardiovascular diseases and cancer [4,5,6,7], as well as premature death in adulthood [8]. Additionally, the morbidity of obesity-related illnesses and subsequent medical treatments lead to high health-care expenses [9].
According to the theory of developmental origins of health and disease (DOHaD), malnutrition and environmental hazard factors during pregnancy may influence developmental plasticity, thereby altering susceptibility to obesity in later life [10,11]. Developmental plasticity, sometimes referred to as fetal programming, is the process whereby a fetus utilizes environmental cues to adjust its individual phenotype to the current and predicted environment [12]. If a fetus is exposed to undernutrition due to maternal malnutrition or placental abnormalities, it will try to overcome these limitations by irreversible adoption of a thrifty phenotype [13,14]. However, the adaptations become detrimental when the postnatal environment, with an abundance of nutrients, differs from the undernutrition environment predicted in uterus, resulting in an increased risk of obesity [12,14].
It is well-known that the fetal nutrition environment is related not only to maternal food intake, but also to the ability of the placenta to efficiently transport nutrients [15]. Since the placenta is a transitory organ that transports nutrients and oxygen from mother to fetus and discharges waste from fetus to mother, changes in placental form and function may contribute to a change in nutrient supply [16]. In addition, a damaged placenta has been associated with adverse birth outcomes, such as low birth weight and preterm birth [17,18], which are also risk factors for obesity [19,20]. Previous findings have shown that prenatal exposure to air pollution is associated with placental changes in terms of decreased placental weight [17,18], placental size and surface [21] and increased placental vascular resistance [22], suggesting that exposure to air pollution might restrict placental exchange function and thus create an undernutrition environment for the fetus.
Recently, prenatal exposure to air pollution as a risk factor for the development of obesity has received considerable attention [23,24,25,26,27]. For example, a large urban longitudinal study revealed that prenatal exposure to outdoor air pollution was related to an increased body mass index (BMI) during the first 5 years of life [23]. Another study reported that prenatal exposure to air pollutants was linked to increased weight gain and anthropometric measurements between the first and sixth months of life [24]. Moreover, a systematic review supported a positive association between prenatal exposure to environmental tobacco smoke and childhood obesity [26]. The findings above have indicated that prenatal exposure to air pollutants is strongly linked to childhood obesity.
In China, mosquito coil smoke (MCS) is a common cause of indoor air pollution, because mosquito coil is widely used as an effective mosquito repellent [28]. MCS releases not only pyrethrin/pyrethroids [29], but also carbon monoxide (CO), particulate matter (PM) and volatile organic compounds (VOCs) (Figure 1) [30,31,32,33]. Our previous studies showed that prenatal exposure to MCS negatively affects the neurodevelopment of children [34,35]. Additionally, Hisada et al. reported a weak positive association between infant development at 18 months of age and prenatal exposure to MCS that contains pyrethroid [36]. However, the effects of prenatal MCS on physical development, especially obesity, among preschoolers has not been studied in detail. Furthermore, the possible causal mechanisms linking exposure to air pollution and childhood obesity remain unclear. One plausible mechanism is that prenatal MCS exposure could lead to childhood obesity through the mismatching of the prenatal and postnatal nutritional environments. In order to gain preliminary evidence to support this hypothesis, this study first aimed to explore the association between prenatal MCS exposure and childhood obesity. In addition, the study aimed to investigate the interactive effect of prenatal MCS exposure and early postnatal nutritional status on obesity among preschoolers.
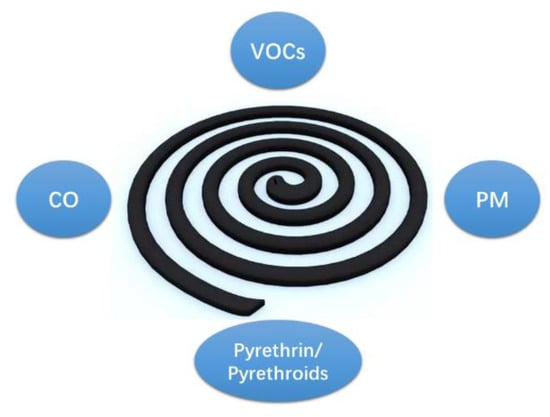
Figure 1.
Chemical contents of mosquito coil smoke. VOCs: volatile organic compounds; CO: carbon monoxide; PM: particulate matter.
2. Materials and Methods
2.1. Study Population
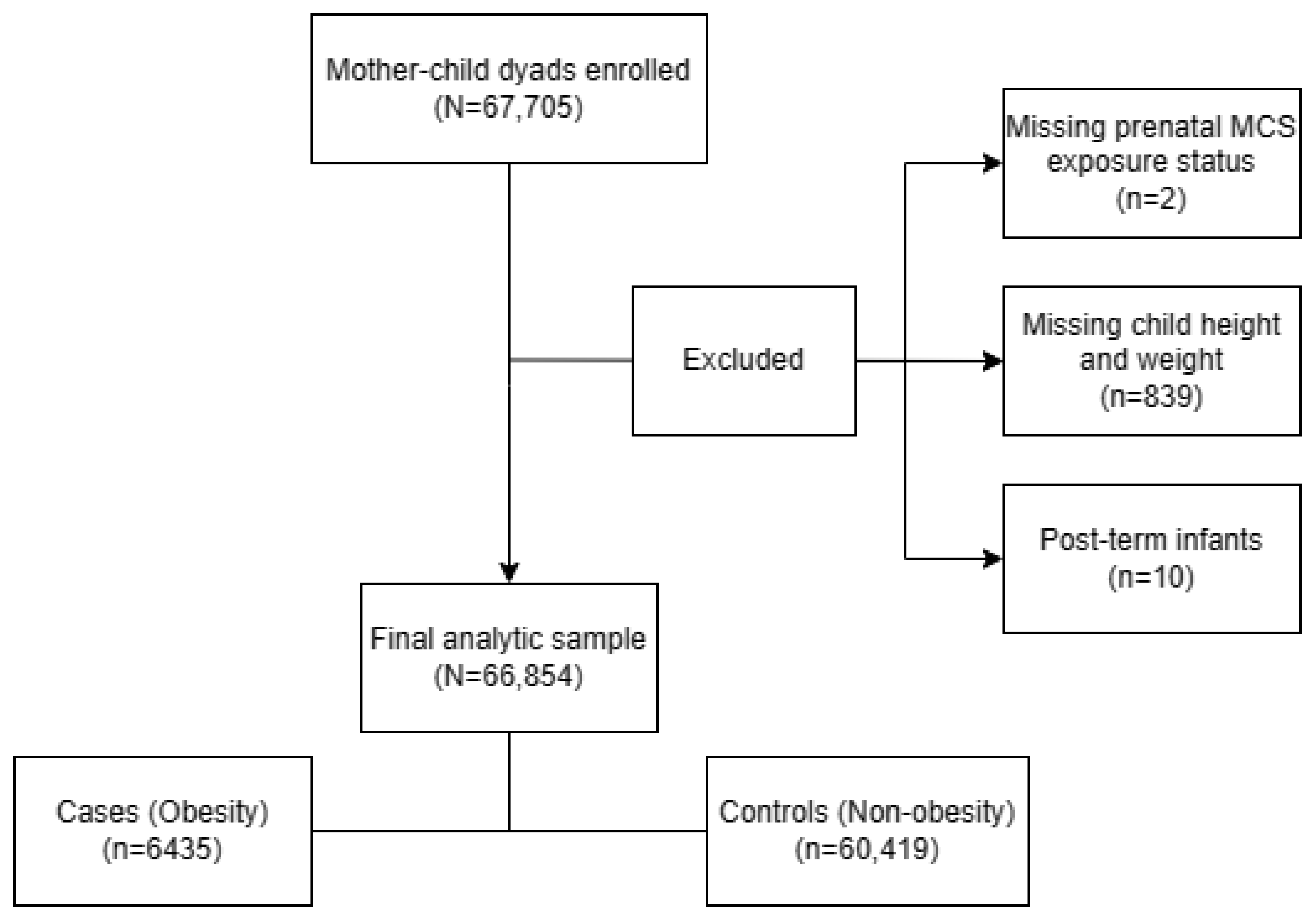
A population-based survey was carried out from October until December 2021 in 235 kindergartens in the Longhua District of Shenzhen, China, with a total of 67,705 mother–child dyads enrolled. After excluding children: (1) whose mothers did not report their prenatal MCS exposure status (n = 2), (2) who did not participate in physical examinations causing a lack of child height and weight data (n = 839) and (3) who were post-term infants (gestational age >42 weeks) (n = 10). The remaining 66,854 (98.74%) mother–child dyads were included in the final sample for analyses (Figure 2). Within this sample, we utilized multiple imputation (MI) to impute the missing data for covariates among 5277 (7.89%) questionnaires which lacked information on at least one selected covariate. The study was approved by the Ethic Committee of the School of Public Health of Sun Yat-sen University, and written informed consent was obtained from the mothers of all the children involved in the study, in accordance with the Declaration of Helsinki.
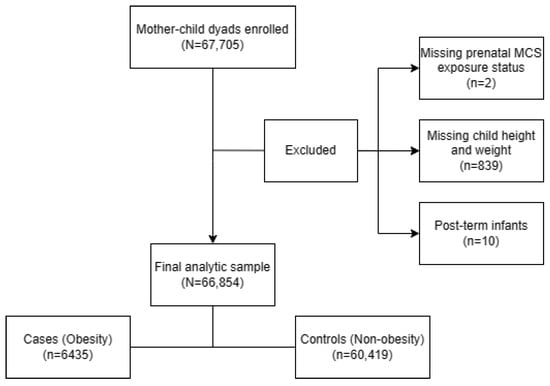
Figure 2.
Flow chart of the analytic sample selection process.
2.2. Data Collection
The well-trained doctors in kindergartens directed the mothers to fulfil a self-administered structured questionnaire to collect the following information: (1) the socio-demographic characteristics of the parents including age at conception, education level, household income and marital status; (2) maternal pre-pregnancy weight and height, and weight gain during pregnancy; (3) maternal household air pollution exposure during pregnancy including environmental tobacco smoke exposure, mosquito coil smoke exposure, incense smoke exposure and cooking fuel type; (4) maternal heavy metal exposure and benzene exposure; (5) birth-related variables of gestational age and birth weight; (6) child’s birth date and gender; (7) maternal recall of feeding pattern (breastfeeding, bottle feeding and mixed feeding), child nutritional status at 1–3 years old (general, poor-nourished or well-nourished), child physical activity frequency at 1–3 years old (0, 1, 2–3, 4–6, 7 days/week) and child sleep duration at 1–3 years old (<9 h/day, 9–16 h/day, >16 h/day).
2.3. Prenatal MCS Exposure Measurement
The following question was asked of mothers to measure the prenatal MCS exposure throughout their entire pregnancy: “During your pregnancy, did you or members of your family burn mosquito coils at home?” (Two response options: “No” or “Yes”.) If the answer was “Yes”, prenatal MCS exposure during the entire pregnancy was considered as being present. The following questions were used to measure MCS exposure during each of the three trimesters of pregnancy and its frequency: (1) “During your pregnancy in 1–13 weeks (first trimester)/ in 14–27 weeks (second trimester)/ after 28 weeks (third trimester), did you or members of your family burn mosquito coils at home?” (Two response options: “No” or “Yes”.) Prenatal MCS exposure was deemed to have occurred in each trimester if the response was “Yes.” (2) “During your pregnancy in 1–13 weeks (first trimester)/ in 14–27 weeks (second trimester)/ after 28 weeks (third trimester), how many times did your family burn mosquito coils per week at home?” (Three response options: “0 time/week”, “1 time/week” or “≥2 times/week”.).
2.4. Early Postnatal Nutritional Status Measurement
Early postnatal nutritional status for children (1–3 years old) was measured by asking mothers the following question: “Which of the following nutritional status most closely matched your child’s situation during the age of 1–3 years?” (Three response options: “Poor-nourished”, “General” or “Well-nourished”.) The answer was based on the parents’ subjective assessment of their child.
2.5. Measurement and Definition of Obesity
At the Longhua Maternity & Child Healthcare Hospital, skilled nurses took standardized measurements of each child’s height and weight. A portable electronic weight scale with a fractional value of 0.01 kg was used to measure the child’s weight. The scale was placed on level ground and each preschooler was asked to stand in the center of the scale bareheaded, barefooted and dressed in light clothing that was close to their body. The nurses read the measurement and accurately recorded it up to 0.1 kg after the value stabilized. A human column altimeter was used to measure height (fractional value = 0.1 cm). On horizontal ground, the altimeter was positioned vertically against the wall. Each preschooler was instructed to stand barefoot and bareheaded on the pedal, with their heels close together, feet spaced at an angle of 60 degrees, chest raised, abdomen pulled in and eyes looking straight ahead. With their line of sight the same height as the slide board, the nurses read the measurements by sliding the slider to the apex of the measured child’s skull.
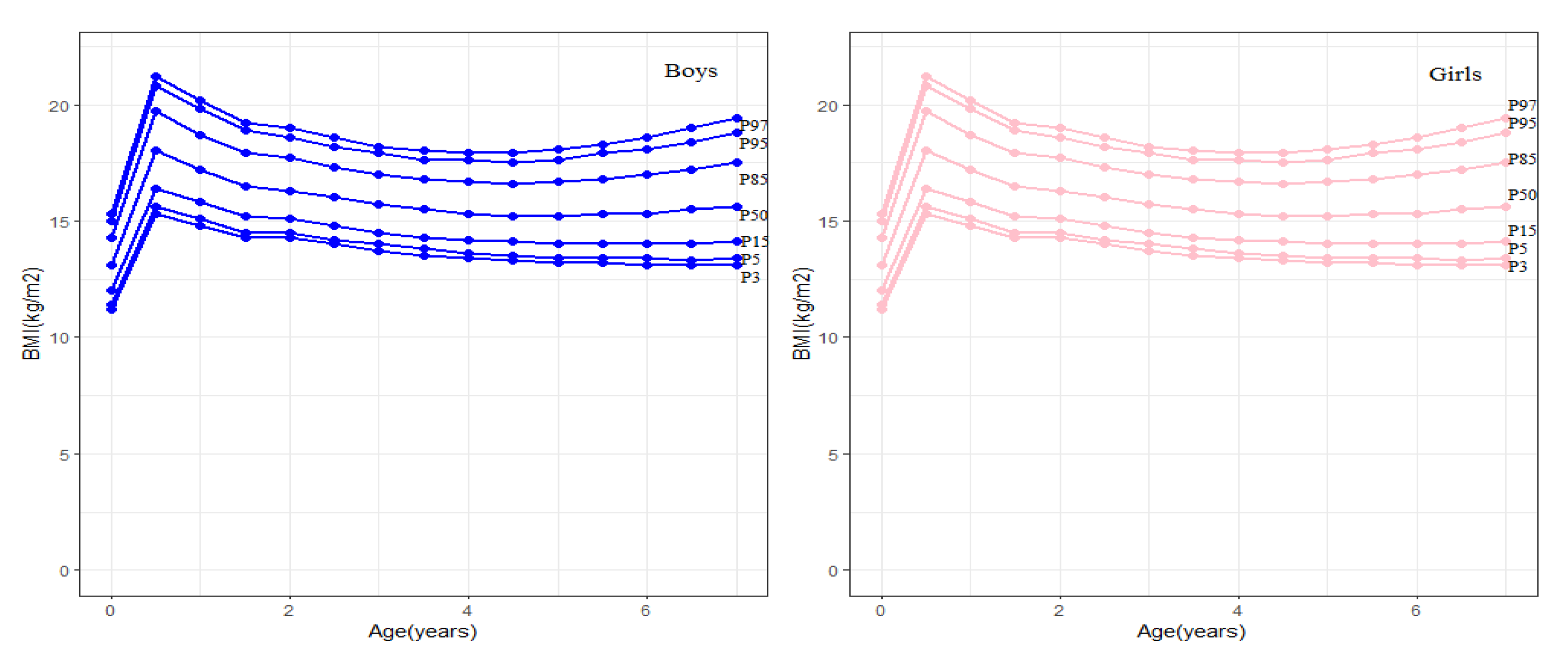
Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared (kg/m2). Childhood obesity was defined as the BMI ≥ the cut-offs for age and sex according to the BMI growth curves for Chinese children (Figure 3) [37].
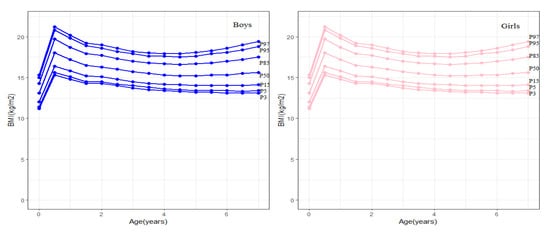
Figure 3.
BMI standard reference graph for 0–8 year old boys and girls.
2.6. Potential Confounding Variables
According to the previous literature [26,38,39,40,41,42,43], and based upon the feasibility of data collection, a range of potential confounders which could impact a child’s BMI were selected as covariates in this study. These included the child’s gender and age, gestational age, birth weight, maternal age at conception and pre-pregnancy BMI, maternal weight gain during pregnancy, parental education level and marital status, household income, prenatal exposure to environmental tobacco smoke and incense smoke, cooking fuel type, prenatal exposure to heavy metal and benzene, the child’s feeding pattern, nutritional status, physical activity frequency and sleep duration.
2.7. Statistical Analyses
Categorical variables were presented as frequencies and percentages and analyzed using the chi-square test.
Univariate and multivariate logistic regression models were used to evaluate the association of prenatal MCS and early postnatal child nutritional status with childhood obesity before and after adjusting for the selected potential covariates. First, the independent impacts of prenatal MCS exposure during the whole pregnancy and early postnatal child nutritional status on childhood obesity were identified. Second, the effects of prenatal MCS exposure during different trimesters of pregnancy on the risk of childhood obesity were estimated to discover which trimester of pregnancy might be the sensitive period for the association between prenatal MCS exposure and childhood obesity. We further conducted a cross-over analysis to verify the sensitive period based on different permutations of exposure (Yes) versus no exposure (No) in each trimester during pregnancy. Third, we estimated the association between the frequency of MCS exposure during different trimesters of pregnancy with childhood obesity. The results of the univariate logistic regression were reported as odds ratios (OR) and 95% confidence intervals (CI). All the multivariate logistic regression models above were performed with adjustment for the aforementioned potential confounding variables with adjusted OR (AOR) and 95% CI calculated.
A cross-over analysis was performed to assess the additive interaction effects on obesity and modification effects between prenatal MCS exposure and early postnatal child nutritional status; with OR, the relative excess risk due to interaction (RERI) and the attributable proportion due to interaction (AP) were used to implicate the strength of these effects, respectively. In addition, their multiplicative interaction effects on obesity were tested by establishing multiplicative terms in logistic regression models and the interaction of odds ratio (IOR) was used to implicate the strength of the multiplicative interaction effects.
Additionally, we conducted stratified analyses by sex to evaluate potential sex-difference in the independent and combined effects of prenatal MCS exposure and early postnatal child nutritional status on childhood obesity. Furthermore, the sensitivity analyses were conducted to assess whether the above associations were robust among 50,013 participants without prenatal exposure to environmental tobacco smoke, incense smoke and cooking fuel with coal.
All statistical analyses were performed with R statistical software (version 4.1.3). All two-sided p-values < 0.05 were deemed statistically significant.
3. Results
3.1. Population Characteristics
The characteristics of the study participants are shown in Table 1. Of the 66,854 included preschoolers, 6435 (9.63%) were obese and 60,419 (90.37%) were non-obese. The prenatal MCS exposure rate was 30.34% and the child well-nourished rate was 66.81%.

Table 1.
Characteristics of the study participants.
Statistically significant differences in the prevalence of childhood obesity were observed in the following characteristics: gender and age of children, birth weight, preterm birth, maternal pre-pregnancy BMI and weight gain during pregnancy, education level and marital status of parents, monthly household income, prenatal exposure to indoor air pollution (including MCS and incense smoke), cooking fuel type, the child’s feeding pattern, postnatal nutritional status, physical activity frequency and sleep duration.
3.2. Association between Prenatal MCS Exposure and Obesity
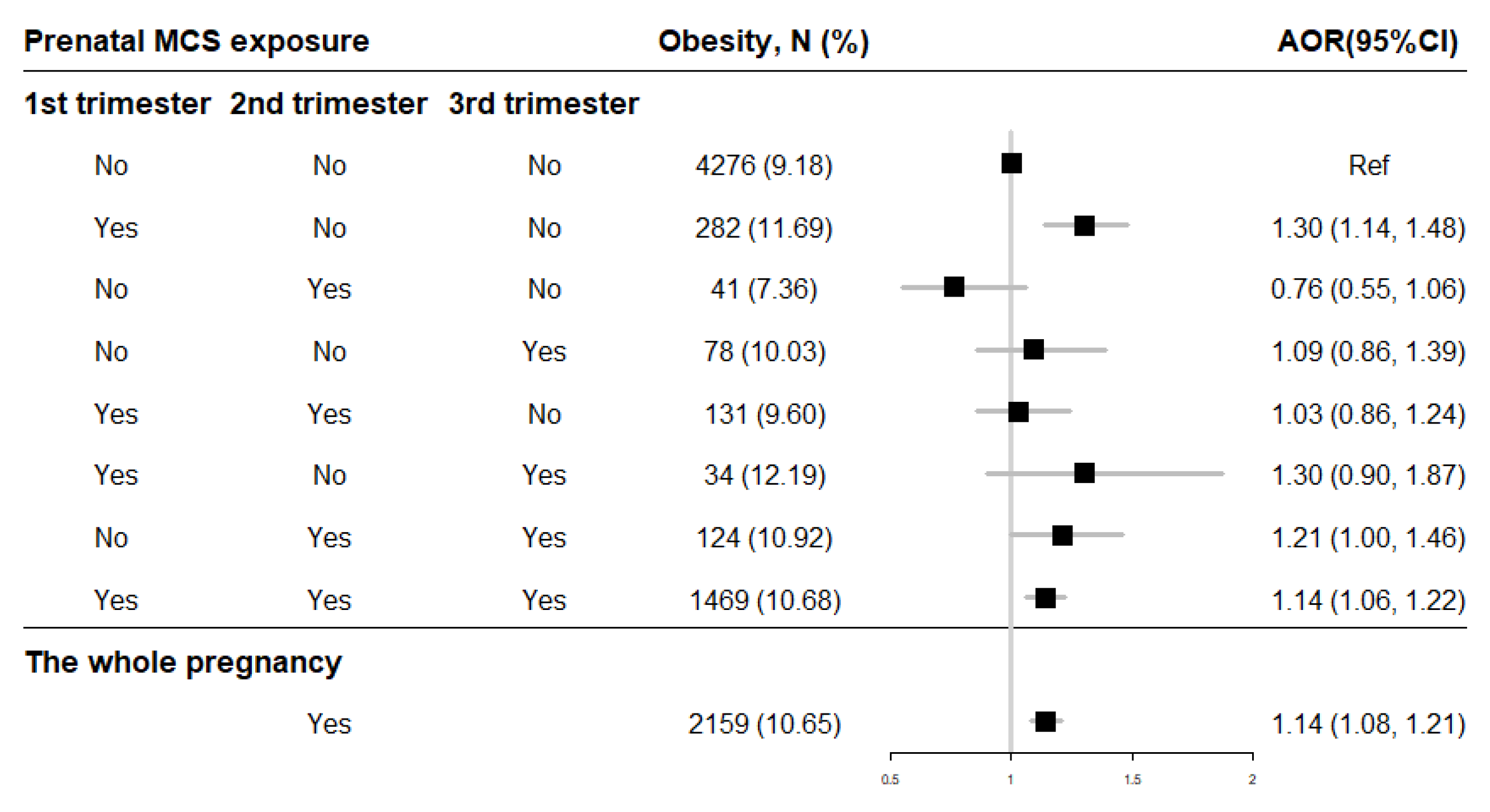
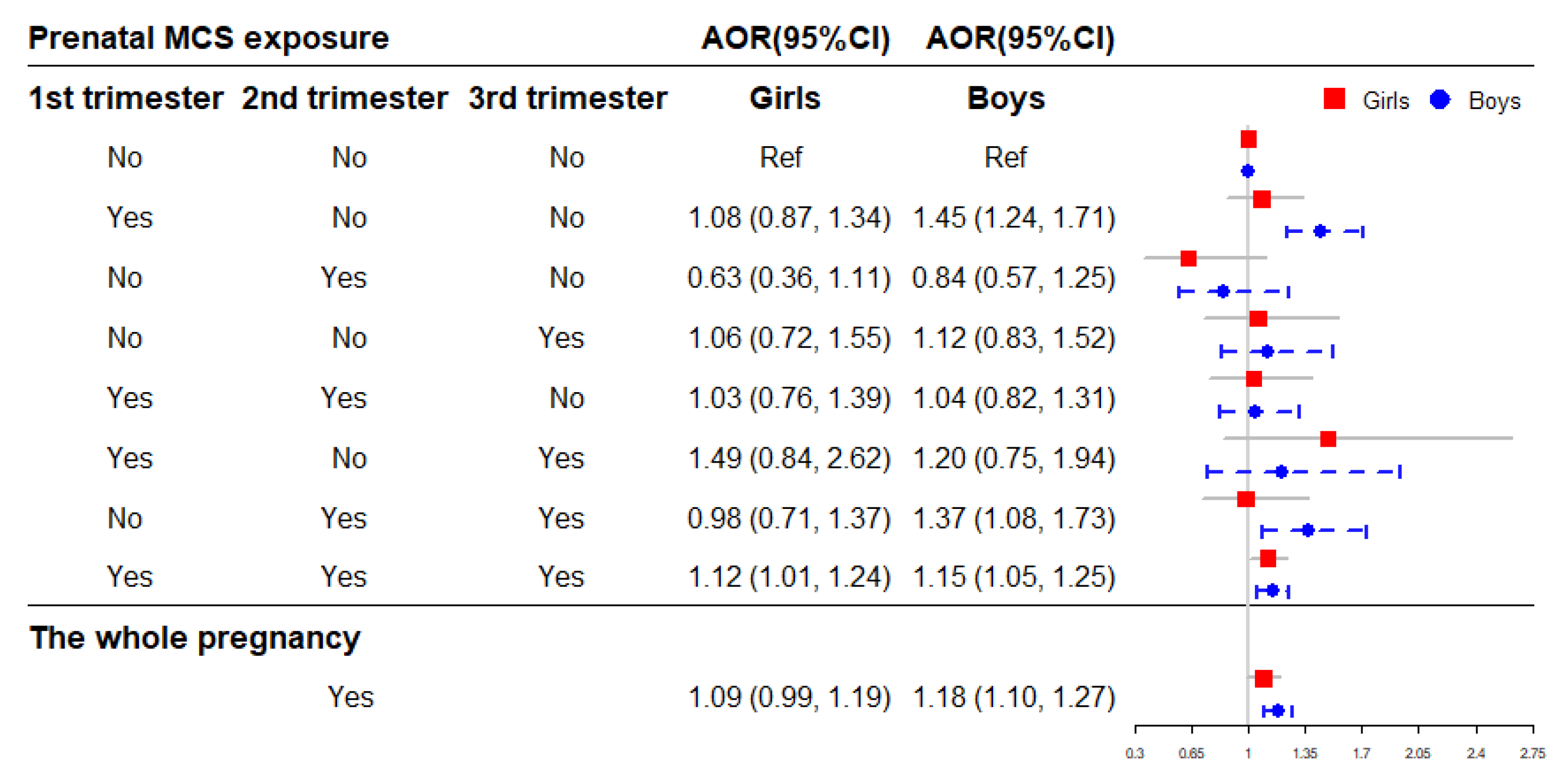
After adjusting for potential confounders, the binary logistic regressions showed that prenatal exposure to MCS during the whole pregnancy (AOR = 1.14, 95% CI = 1.08–1.21) increased the risk of offspring obesity compared to mothers without exposure (Figure 4). And the risk of childhood obesity increased incrementally with the frequency (0 time/week, 1 time/week, ≥2 times/week) of MCS exposure in each trimester (Table 2). Moreover, the cross-over analysis indicated that prenatal MCS exposures in the first trimester (AOR = 1.30, 95% CI = 1.14–1.48), in both the second and second trimesters (AOR = 1.21, 95% CI = 1.00–1.46) as well as through all three trimesters (AOR = 1.14, 95% CI = 1.06–1.22) were significantly associated with higher childhood obesity risk compared with the no MCS exposure participants (Figure 4).
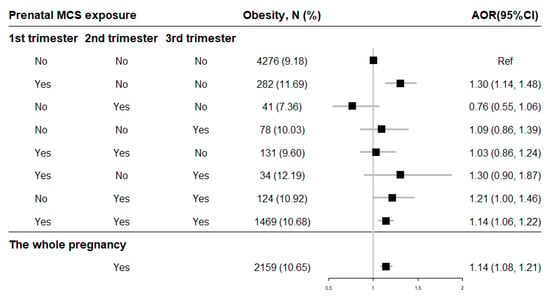
Figure 4.
The association of prenatal MCS exposure with childhood obesity. Adjusted for child’s gender and age, birth weight, gestational age, maternal age at conception and pre-pregnancy BMI, maternal weight gain during pregnancy, parental education levels and marital status, household income, prenatal exposure to environmental tobacco smoke and incense smoke, cooking fuel type, prenatal exposure to heavy metal and benzene, child’s feeding pattern, nutritional status, physical activity frequency and sleep duration in models. AOR: adjusted OR.

Table 2.
The association between frequency of prenatal MCS exposure and childhood obesity.
3.3. Association between Early Postnatal Child Nutritional Status and Obesity among Preschoolers
After controlling for potential confounders, obesity was significant positively associated with early postnatal well-nourished nutritional status (AOR = 1.56, 95% CI = 1.47–1.66) (Table 3).

Table 3.
The association of early postnatal nutritional status with childhood obesity.
3.4. Combination Effect of Prenatal MCS Exposure and Early Postnatal Child Nutritional Status on Obesity among Preschoolers
Table 4 presents the results of the combined effect of prenatal MCS exposure and early postnatal child nutritional status on childhood obesity among preschoolers. Compared to the combination of no prenatal exposure to MCS and general nutritional status, the combination of MCS exposure during the whole pregnancy with early general postnatal nutritional status (AOR = 1.22, 95% CI = 1.10–1.36) and early postnatal well-nourished nutritional status (AOR = 1.81, 95% CI = 1.65–1.97), respectively, increased the risk of obesity among preschoolers (Table 4).

Table 4.
The combined effect of MCS exposure during the whole pregnancy and early postnatal nutritional status on childhood obesity.
3.5. Stratified Analyses by Sex
Figure 5 shows the association of prenatal MCS exposure with childhood obesity stratified by sex. The results indicated that exposure to MCS during the whole pregnancy significantly increase the risk of childhood obesity in boys (AOR = 1.18, 95% CI = 1.10–1.27), and the significant association was observed in girls exposed to MCS in all three trimesters (AOR = 1.12, 95% CI = 1.01–1.24) (Figure 5).
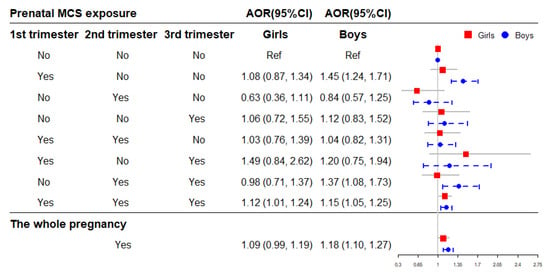
Figure 5.
The association of prenatal MCS exposure with childhood obesity stratified by sex. Adjusted for child’s age, birth weight, gestational age, maternal age at conception and pre-pregnancy BMI, maternal weight gain during pregnancy, parental education levels and marital status, household income, prenatal exposure to environmental tobacco smoke and incense smoke, cooking fuel type, pre-natal exposure to heavy metal and benzene, child’s feeding pattern, nutritional status, physical activity frequency and sleep duration in models. AOR: adjusted OR.
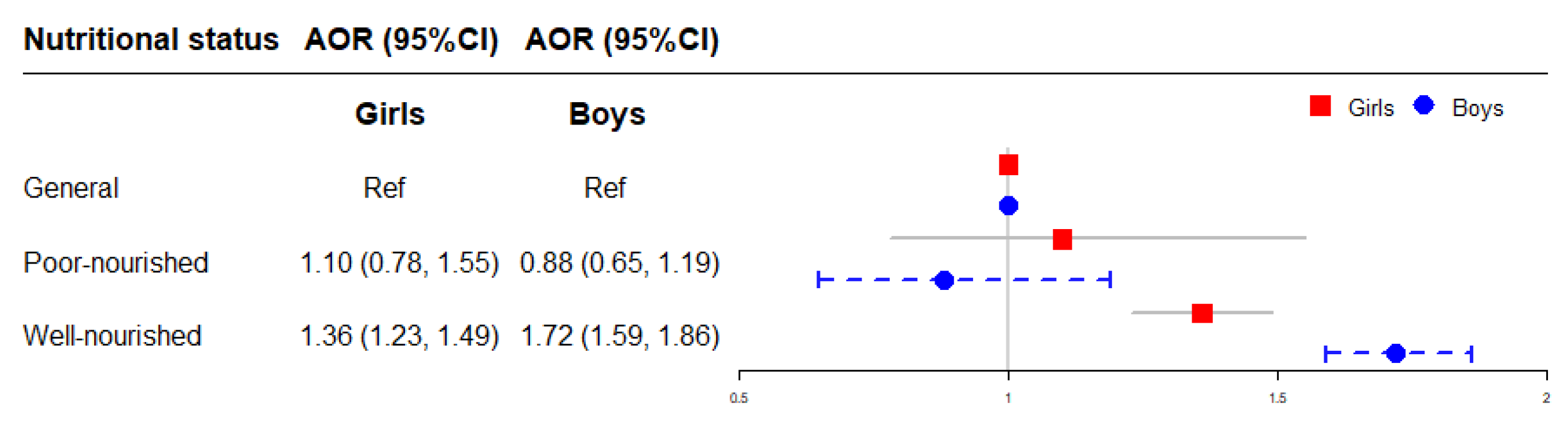
The association between early postnatal nutritional status and childhood obesity stratified by sex is illustrated in Figure 6. Compared to children with general nutritional status in each sex respectively, the risk of obesity was higher in boys (AOR = 1.72, 95% CI = 1.59–1.86) with well-nourished nutritional status than in girls (AOR = 1.36, 95% CI = 1.23–1.49) (Figure 6).
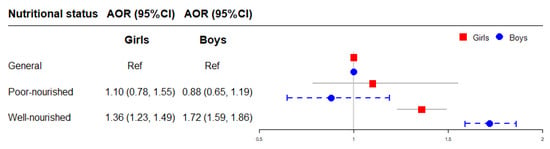
Figure 6.
The association of early postnatal nutritional status with childhood obesity stratified by sex. Adjusted for child’s age, birth weight, gestational age, maternal age at conception and pre-pregnancy BMI, maternal weight gain during pregnancy, parental education levels and marital status, household income, prenatal exposure to mosquito coil smoke, environmental tobacco smoke and incense smoke, cooking fuel type, prenatal exposure to heavy metal and benzene, child’s feeding pattern, physical activity frequency and sleep duration in models. AOR: adjusted OR.
Table 5 presents the combined effect of MCS exposure during the whole pregnancy and early postnatal nutritional status on childhood obesity stratified by sex. Compared to the combination of no prenatal exposure to MCS and general nutritional status, the combination of MCS exposure during the whole pregnancy with early well-nourished postnatal nutritional status increased the higher risk of obesity in boys (AOR = 2.05, 95% CI = 1.83–2.30) than in girls (AOR = 1.49, 95% CI = 1.30–1.72) (Table 5).

Table 5.
The combined effect of MCS exposure during the whole pregnancy and early postnatal nutritional status on childhood obesity stratified by sex.
3.6. Sensitivity Analysis
We conducted the sensitivity analyses after excluding participants with any prenatal exposure to environmental tobacco smoke, incense smoke and cooking fuel with coal (n = 16,841), and still obtained similar results with the aforementioned findings. More details are presented in Tables S1–S4.
4. Discussion
To the best of our knowledge, this is the first study on the combined effect of prenatal MCS exposure and early postnatal nutritional status on obesity in a large sample of Chinese preschoolers. Our study found that prenatal MCS exposure was associated with childhood obesity, and the first trimester might be the sensitive period for prenatal MCS exposure leading to childhood obesity. Moreover, early postnatal well-nourished nutritional status significantly increased the risk of obesity among preschoolers. Furthermore, prenatal MCS exposure and early postnatal nutritional status jointly affect the increased risk of childhood obesity, with a discernible gender disparity in this joint effect.
The association between prenatal exposure to air pollution and obesity has been reported in previous studies. For example, a study from the United States study found that prenatal exposure to nitrogen dioxide (NO2) was associated with greater infant weight gain and total subcutaneous fat, and PM was associated with a change in umbilical circumference and total subcutaneous fat, respectively, among infants [24]. Another USA study provided evidence of a positive association between prenatal polycyclic aromatic hydrocarbon exposure and childhood obesity (relative risk = 1.79, 95% CI = 1.09–2.96) [44]. In addition, a Spanish study found that exposures to NO2, PM10 and PM2.5 in prenatal and early postnatal periods were associated with slight increases in BMI growth [23]. Moreover, a systematic review reported an association between prenatal exposure to environmental tobacco smoke and childhood obesity (OR = 1.91, 95% CI = 1.23–2.94) [26]. Consistent with these findings, our study showed that prenatal MCS exposure is also associated with obesity in preschoolers. Interestingly, our study further identified that prenatal MCS exposure in the first trimester, rather than exposure in the other two trimesters, was associated with a significant risk of childhood obesity. This finding is consistent with a developmental model of the origin of disease [10]. In line with this finding, an America study indicated that the first and second trimesters might be the sensitive windows for prenatal PM2.5 exposure leading to childhood obesity [45]. These findings support the possibility that the first trimester of pregnancy might be the sensitive period for prenatal MCS exposure causing childhood obesity.
The imbalance between the amount of energy consumed and the amount of energy expended has been the focus of conventional approaches to the study of obesity. As such, early feeding habits and nutrition have been identified as modifiable risk factors for childhood overweight and obesity [46]. For instance, a birth cohort study found that early postnatal high-energy-dense food intake was associated with being overweight later in childhood [41]. Similarly, another cohort study showed that a dietary pattern characterized by high sugar and fat foods was related to greater adiposity and obesity risk in childhood [47]. This early postnatal high-energy dietary pattern represents one explanation for accelerated weight gain in early life resulting in higher childhood fat mass [48]. Consistent with these findings, our study also showed that early a postnatal well-nourished nutritional status significantly increases the risk of childhood obesity.
According to the DOHaD theory, the development of obesity is not only the result of postnatal factors but also a function of prenatal factors [49]. For example, Wang et al. [50] reported that elevated prenatal mercury exposure was associated with a higher risk of childhood obesity, and such risk was reduced by adequate folate ingestion. Zulkifli et al. [51] found that prenatal bisphenol A exposure significantly affected a rat’s offspring’s physiological parameters and intestinal function, and that there might be compensatory responses to postnatal trans-fat diets in the combined bisphenol A group. Another animal trial conducted using a swine model indicated that the propensity for obesity of pig fetuses exposed to prenatal malnutrition might be moderated by controlled food intake [52]. Consistent with these findings, our study showed that preschool children exposed to prenatal MCS were more susceptible to obesity in both early postnatal general nutritional and well-nourished status. This is in contrast to the children not exposed to MCS only being susceptible to obesity when exposed to a well-nourished feeding environment postnatally. These findings indicate that prenatal and postnatal factors could exert an interactive effect on childhood obesity.
The mechanism for a combined effect of prenatal hazardous factors exposure and early postnatal nutritional status on obesity may be explained as the following: First, prenatal exposure to hazardous factors such as air pollution might negatively affect placental development including decreased placental weight [17,18], placental size and surface [21], and increased placental vascular resistance [22]. Considering that MCS is generated from the incomplete combustion of mosquito coils, releasing not only the pyrethrin/pyrethroids [47], but also CO, PM and VOCs [30,31,32,33], there might be a heightened chance for prenatal MCS exposure to impair the placenta. This impairment may restrict the placenta in transferring sufficient nutrients to the fetus and cause a poorly-nourished fetal environment that leads the fetus to develop a metabolism adapted to survival under poor nutrition conditions, hypothesized as the “Thrifty Phenotype” [13,53]. Second, even if the child meets a sufficiently abundant nutrient environment after birth, he/she may still maintain a low rate in energy metabolism as programmed in uteri, which may cause the excess energy consumed to be transformed into fat stored in the body. Such a scenario would represent a “developmental mismatch” [54]. These two proposed mechanisms may explain our finding that preschoolers with prenatal MCS exposure and postnatal general nutrition or well-nourished status had a higher risk of obesity than those without prenatal MCS exposure or who experienced a poor postnatal nutrition status.
According to the sex-stratified analyses, the independent effects of prenatal exposure to MCS and early well-nourished postnatal nutritional status on obesity were more pronounced in boys than girls. Furthermore, the sex difference was also observed in the combined effect of these two factors on obesity. Previous studies have also found sex differences in the relationship between prenatal exposure to air pollution and childhood obesity. For example, an American birth cohort study observed that prenatal PM2.5 exposure was associated with increased BMI and body fat in boys and waist circumference in girls [45]. Additionally, an animal study also found a positive correlation between prenatal exposure to PM2.5 and body weight as well as food intake in male mice [55]. The endocrine-disrupting effects of prenatal exposure to air pollution and the effects of sex hormones on obesity might explain the sex difference [27]. A Shanghai animal study found that prenatal PM2.5 exposure was able to alter methylation in the promoter region of the leptin gene, resulting in increased susceptibility to obesity in offspring male mice [56]. Moreover, boys and girls are inherently biologically different in the first place, with women consuming less energy daily than men [57]. At the behavioral level, girls might pay more attention to food intake choices and their impact on health as well as weight control [58]. Further studies are required to confirm and explain the sex difference in the combined effect of prenatal exposure to MCS and early well-nourished postnatal nutritional status on obesity.
The findings of this study need to be interpreted in consideration of the following limitations: First, prenatal MCS exposure was retrospectively self-reported by mothers. It is therefore possible that this measure was influenced by recall bias, especially for the exact timing of exposure during the trimesters. Second, we did not assess the average duration of mothers’ MCS exposure each time, which prevented us investigating the association of the duration of MCS exposure with childhood obesity. Third, the assessment of early postnatal nutritional status was only based on the subjective judgement and recall of the parents. Future prospective research should include a validated measurement method such as the food frequency questionnaire (FFQ) to measure more exact energy and nutrient intakes of children during early childhood. Fourth, all the participants were selected from Longhua District of Shenzhen, which may limit the generalizability and extension of our findings due to variations in prenatal MCS exposure across other areas. Fifth, although a range of covariates were included, there were still unmeasured potential confounders such as household ventilation conditions and ambient air pollution exposure during pregnancy, contents of mosquito coils and drinking water quality, which might influence the findings. Sixth, retrospective studies provide weaker causal relationship evidence for the combined effect of prenatal and postnatal factors on childhood obesity, therefore, prospective cohort and experimental studies are needed to replicate these results.
5. Conclusions
In summary, this study indicated that prenatal MCS exposure and postnatal nutritional status can independently and jointly increase the risk of obesity in preschool children, with a discernible gender disparity. In addition, the first trimester may be the sensitive period for prenatal MCS exposure causing childhood obesity. Our findings provide preliminary epidemiological evidence for the DOHaD hypothesis of a mismatch between prenatal and postnatal nutritional environments being a risk factor for childhood obesity. In light of these findings, mosquito coil usage should be avoided during pregnancy. In addition, more research is required to identify an appropriate diet for young children that may mitigate the effects of prenatal exposure to MCS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos14061004/s1, Table S1: The association of prenatal MCS exposure with childhood obesity among preschoolers without prenatal exposure to environmental tobacco smoke, incense smoke and cooking fuel with coal.; Table S2: The association between frequency of prenatal MCS exposure with childhood obesity among pre-schoolers without prenatal exposure to environmental tobacco smoke, incense smoke and cooking fuel with coal; Table S3: The association of early postnatal nutritional status with childhood obesity among preschoolers without prenatal exposure to environmental tobacco smoke, incense smoke and cooking fuel with coal; Table S4: The combined effect of MCS exposure during the whole pregnancy and early postnatal nutritional status on childhood obesity among preschoolers without prenatal exposure to environmental to-bacco smoke, incense smoke and cooking fuel with coal.
Author Contributions
Conceptualization, W.-Q.C. and Y.L.; resources, W.-Q.C.; methodology, W.-Q.C. and Y.L.; formal analysis, Y.L.; investigation, Y.L., Q.L. and X.-C.L.; data curation, X.-C.L. and B.-J.H.; writing—original draft preparation, Y.L.; writing—review and editing, W.-Q.C. and E.S.; visualization, Y.L.; supervision, W.-Q.C.; project administration, W.-Q.C. and B.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Science and Technology Planning Project of Guangdong Province provided funding for this study under grant number 2019A1515011915. The non-profit scientific research management and academic institutions that provided the funding did not participate in the study’s design, execution, analyses, data interpretation or decision to submit results.
Institutional Review Board Statement
The Institutional Review Board of the School of Public Health at Sun Yat-sen University in Guangzhou, China, approved this study in accordance with the Declaration of Helsinki (protocol code: No. 2015–016).
Informed Consent Statement
All participants in the study gave their informed consent. Written consent for children was received from their parents or guardians.
Data Availability Statement
Due to participant privacy concerns, the datasets created and/or analyzed in this study cannot be accessed by the general public. However, they can be obtained from the corresponding author upon reasonable request.
Acknowledgments
The Longhua Maternity & Child Healthcare Hospital clinicians who assisted in recruiting participants and collecting data are sincerely appreciated by the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Obesity and Overweight: Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 1289 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Carli, M.M.; Sabo, R.T.; Sun, S.S. Childhood waist growth curves and adult diabetes. J. Dev. Orig. Health Dis. 2021, 13, 656–662. [Google Scholar] [CrossRef]
- Brady, T.M. Obesity-Related Hypertension in Children. Front. Pediatr. 2017, 5, 197. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Lassandro, G.; Valente, F.; D’Amato, G.; Portincasa, P.; Giordano, P. The Cardiovascular Disease (CVD) Risk Continuum from Prenatal Life to Adulthood: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8282. [Google Scholar] [CrossRef]
- Weihe, P.; Spielmann, J.; Kielstein, H.; Henning-Klusmann, J.; Weihrauch-Blüher, S. Childhood Obesity and Cancer Risk in Adulthood. Curr. Obes. Rep. 2020, 9, 204–212. [Google Scholar] [CrossRef]
- Lindberg, L.; Danielsson, P.; Persson, M.; Marcus, C.; Hagman, E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med. 2020, 17, e1003078. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Lynch, F.; Lewis, S.; Macciocca, I.; Craig, J.M. Epigenetics and DOHaD: How translation to predictive testing will require a better public understanding. J. Dev. Orig. Health Dis. 2022, 13, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 2007, 61, 5r–10r. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef]
- Wells, J.C. The thrifty phenotype: An adaptation in growth or metabolism? Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2011, 23, 65–75. [Google Scholar] [CrossRef]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal undernutrition influences placental-fetal development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.E1–S6.E4. [Google Scholar] [CrossRef]
- Hu, P.; Fan, L.; Ding, P.; He, Y.H.; Xie, C.; Niu, Z.; Tian, F.Y.; Yuan, S.; Jia, D.; Chen, W.Q. Association between prenatal exposure to cooking oil fumes and full-term low birth weight is mediated by placental weight. Environ. Res. 2018, 167, 622–631. [Google Scholar] [CrossRef]
- Hu, P.; Wang, C.; Ding, P.; He, Y.H.; Xie, C.; Tian, F.Y.; Yuan, S.; Jia, D.; Chen, W.Q. Placental weight mediates association between prenatal exposure to cooking oil fumes and preterm birth. J. Matern. Fetal Neonatal Med. 2021, 35, 7248–7258. [Google Scholar] [CrossRef]
- Yuan, Z.P.; Yang, M.; Liang, L.; Fu, J.F.; Xiong, F.; Liu, G.L.; Gong, C.X.; Luo, F.H.; Chen, S.K.; Zhang, D.D.; et al. Possible role of birth weight on general and central obesity in Chinese children and adolescents: A cross-sectional study. Ann. Epidemiol. 2015, 25, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Vasylyeva, T.L.; Barche, A.; Chennasamudram, S.P.; Sheehan, C.; Singh, R.; Okogbo, M.E. Obesity in prematurely born children and adolescents: Follow up in pediatric clinic. Nutr. J. 2013, 12, 150. [Google Scholar] [CrossRef]
- Rahmalia, A.; Giorgis-Allemand, L.; Lepeule, J.; Philippat, C.; Galineau, J.; Hulin, A.; Charles, M.A.; Slama, R. Pregnancy exposure to atmospheric pollutants and placental weight: An approach relying on a dispersion model. Environ. Int. 2012, 48, 47–55. [Google Scholar] [CrossRef]
- Ouidir, M.; Tekola-Ayele, F.; Canty, T.; Grantz, K.L.; Sciscione, A.; Tong, D.; Jones, R.R.; Sundaram, R.; Williams, A.; Stevens, D.; et al. Acute ambient air pollution exposure and placental Doppler results in the NICHD fetal growth studies—Singleton cohort. Environ. Res. 2021, 202, 111728. [Google Scholar] [CrossRef] [PubMed]
- De Bont, J.; Hughes, R.; Tilling, K.; Díaz, Y.; de Castro, M.; Cirach, M.; Fossati, S.; Nieuwenhuijsen, M.; Duarte-Salles, T.; Vrijheid, M. Early life exposure to air pollution, green spaces and built environment, and body mass index growth trajectories during the first 5 years of life: A large longitudinal study. Environ. Pollut. 2020, 266, 115266. [Google Scholar] [CrossRef]
- Patterson, W.B.; Glasson, J.; Naik, N.; Jones, R.B.; Berger, P.K.; Plows, J.F.; Minor, H.A.; Lurmann, F.; Goran, M.I.; Alderete, T.L. Prenatal exposure to ambient air pollutants and early infant growth and adiposity in the Southern California Mother’s Milk Study. Environ. Health A Glob. Access. Sci. Source 2021, 20, 67. [Google Scholar] [CrossRef]
- Bloemsma, L.D.; Dabelea, D.; Thomas, D.S.K.; Peel, J.L.; Adgate, J.L.; Allshouse, W.B.; Martenies, S.E.; Magzamen, S.; Starling, A.P. Prenatal exposure to ambient air pollution and traffic and indicators of adiposity in early childhood: The Healthy Start study. Int. J. Obes. 2022, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Jadotte, Y.; Zha, P.; Porter, S.A.; Holly, C.; Salmond, S.; Watkins, E.A. The association between prenatal exposure to environmental tobacco smoke and childhood obesity: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 1643–1662. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Cui, H.; Zhang, Y.; Jiang, M. Exposure to outdoor and indoor air pollution and risk of overweight and obesity across different life periods: A review. Ecotoxicol. Environ. Saf. 2022, 242, 113893. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, X.; Stevanovic, S.; Xiang, Z.; Liu, J.; Shi, H.; Liu, J.; Yu, M.; Zhu, C. Characterizing pollutant emissions from mosquito repellents incenses and implications in risk assessment of human health. Chemosphere 2018, 191, 962–970. [Google Scholar] [CrossRef]
- Li, H.; Lydy, M.J.; You, J. Pyrethroids in indoor air during application of various mosquito repellents: Occurrence, dissipation and potential exposure risk. Chemosphere 2016, 144, 2427–2435. [Google Scholar] [CrossRef]
- Elehinafe, F.B.; Okedere, O.B.; Adesanmi, A.J.; Jimoh, E.M. Assessment of Indoor Levels of Carbon Monoxide Emission from Smoldering Mosquito Coils Used in Nigeria. Environ. Health Insights 2022, 16, 1–6. [Google Scholar] [CrossRef]
- Salvi, D.; Limaye, S.; Muralidharan, V.; Londhe, J.; Madas, S.; Juvekar, S.; Biswal, S.; Salvi, S. Indoor Particulate Matter < 2.5 μm in Mean Aerodynamic Diameter and Carbon Monoxide Levels during the Burning of Mosquito Coils and Their Association with Respiratory Health. Chest 2016, 149, 459–466. [Google Scholar] [CrossRef]
- Dubey, J.; Banerjee, A.; Meena, R.K.; Kumari, K.M.; Lakhani, A. Characterization of polycyclic aromatic hydrocarbons in emissions of different mosquito coils. Bull. Environ. Contam. Toxicol. 2014, 92, 650–654. [Google Scholar] [CrossRef]
- Lu, F.; Li, S.; Shen, B.; Zhang, J.; Liu, L.; Shen, X.; Zhao, R. The emission characteristic of VOCs and the toxicity of BTEX from different mosquito-repellent incenses. J. Hazard. Mater. 2020, 384, 121428. [Google Scholar] [CrossRef]
- Yang, J.H.; Strodl, E.; Wu, C.A.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Chen, J.Y.; Chen, Y.J.; Chen, J.; et al. Association between prenatal exposure to indoor air pollution and autistic-like behaviors among preschool children. Indoor Air 2022, 32, e12953. [Google Scholar] [CrossRef]
- Fang, X.Y.; Strodl, E.; Liu, B.Q.; Liu, L.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Jiang, H.; Jing, J.; et al. Association between prenatal exposure to household inhalants exposure and ADHD-like behaviors at around 3 years of age: Findings from Shenzhen Longhua Child Cohort Study. Environ. Res. 2019, 177, 108612. [Google Scholar] [CrossRef]
- Hisada, A.; Yoshinaga, J.; Zhang, J.; Kato, T.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Ariki, N.; Komine, Y.; Shirakawa, M.; et al. Maternal Exposure to Pyrethroid Insecticides during Pregnancy and Infant Development at 18 Months of Age. Int. J. Environ. Res. Public Health 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, C.Y.; Zong, X.N.; Zhang, Y.Q. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Chin. J. Pediatr. 2009, 47, 493–498. [Google Scholar]
- Abrego Del Castillo, K.Y.; Dennis, C.L.; Wamithi, S.; Briollais, L.; McGowan, P.O.; Dol, J.; Lye, S.J. Maternal BMI, breastfeeding and perinatal factors that influence early childhood growth trajectories: A scoping review. J. Dev. Orig. Health Dis. 2022, 13, 541–549. [Google Scholar] [CrossRef]
- Hemmingsson, E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr. Obes. Rep. 2018, 7, 204–209. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef] [PubMed]
- Sirkka, O.; Fleischmann, M.; Abrahamse-Berkeveld, M.; Halberstadt, J.; Olthof, M.R.; Seidell, J.C.; Corpeleijn, E. Dietary Patterns in Early Childhood and the Risk of Childhood Overweight: The GECKO Drenthe Birth Cohort. Nutrients 2021, 13, 2046. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Sahle, B.W.; Owusu-Addo, E.; Chen, W.; Morton, R.L.; Renzaho, A.M.N. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int. J. Obes. 2021, 45, 2404–2418. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.; Hoepner, L.; Hassoun, A.; Oberfield, S.; Freyer, G.; Holmes, D.; Reyes, M.; Quinn, J.; Camann, D.; Perera, F.; et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am. J. Epidemiol. 2012, 175, 1163–1172. [Google Scholar] [CrossRef]
- Chiu, Y.M.; Hsu, H.L.; Wilson, A.; Coull, B.A.; Pendo, M.P.; Baccarelli, A.; Kloog, I.; Schwartz, J.; Wright, R.O.; Taveras, E.M.; et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ. Res. 2017, 158, 798–805. [Google Scholar] [CrossRef]
- Dattilo, A.M.; Birch, L.; Krebs, N.F.; Lake, A.; Taveras, E.M.; Saavedra, J.M. Need for early interventions in the prevention of pediatric overweight: A review and upcoming directions. J. Obes. 2012, 2012, 123023. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.C.; Thompson, J.M.D.; Dalrymple, K.V.; Wall, C.; Begum, S.; Pallippadan Johny, J.; Cutfield, W.S.; North, R.; McCowan, L.M.E.; Godfrey, K.M.; et al. Childhood dietary patterns and body composition at age 6 years: The Children of SCOPE study. Br. J. Nutr. 2020, 124, 217–224. [Google Scholar] [CrossRef]
- Samaranayake, D.; Lanerolle, P.; Waidyatilaka, I.; de Lanerolle-Dias, M.; Hills, A.P.; Wickremasinghe, A.R.; Wickramasinghe, V.P. Association between early weight gain and later adiposity in Sri Lankan adolescents. J. Dev. Orig. Health Dis. 2021, 12, 250–259. [Google Scholar] [CrossRef]
- Larqué, E.; Labayen, I.; Flodmark, C.E.; Lissau, I.; Czernin, S.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. From conception to infancy—Early risk factors for childhood obesity. Nat. Rev. Endocrinol. 2019, 15, 456–478. [Google Scholar] [CrossRef]
- Wang, G.; DiBari, J.; Bind, E.; Steffens, A.M.; Mukherjee, J.; Bartell, T.R.; Bellinger, D.C.; Hong, X.; Ji, Y.; Wang, M.C.; et al. In utero exposure to mercury and childhood overweight or obesity: Counteracting effect of maternal folate status. BMC Med. 2019, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, S.; Mohd Nor, N.S.; Sheikh Abdul Kadir, S.H.; Mohd Ranai, N.; Mohd Kornain, N.K.; Wan Mohd Zain, W.N.I.; Abdul Aziz, M. Prenatal Bisphenol a Exposure and Postnatal Trans Fat Diet Alter Small Intestinal Morphology and Its Global DNA Methylation in Male Sprague-Dawley Rats, Leading to Obesity Development. Nutrients 2022, 14, 2382. [Google Scholar] [CrossRef]
- Barbero, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Perez-Solana, M.L.; Ayuso, M.; Garcia-Real, I.; Gonzalez-Bulnes, A. Prenatal programming of obesity in a swine model of leptin resistance: Modulatory effects of controlled postnatal nutrition and exercise. J. Dev. Orig. Health Dis. 2014, 5, 248–258. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Low, F.M. Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180109. [Google Scholar] [CrossRef]
- Xie, P.; Zhao, C.; Huang, W.; Yong, T.; Chung, A.C.K.; He, K.; Chen, X.; Cai, Z. Prenatal exposure to ambient fine particulate matter induces dysregulations of lipid metabolism in adipose tissue in male offspring. Sci. Total Environ. 2019, 657, 1389–1397. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Hu, Z.; Zhou, H.; Xu, Y.; Qiu, L.; Qin, X.; Zhang, Y.; Ying, Z. Programming of mouse obesity by maternal exposure to concentrated ambient fine particles. Part. Fibre Toxicol. 2017, 14, 20. [Google Scholar] [CrossRef]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Haase, A.M.; Steptoe, A.; Nillapun, M.; Jonwutiwes, K.; Bellisle, F. Gender differences in food choice: The contribution of health beliefs and dieting. Ann. Behav. Med. A Publ. Soc. Behav. Med. 2004, 27, 107–116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).