Application of Hollow Fiber Membrane for the Separation of Carbon Dioxide from Atmospheric Air and Assessment of Its Distribution Pattern in a Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Hollow Fiber Membrane Module
2.2. Experimental Greenhouse
2.3. Carbon Dioxide Supply in the Greenhouse
2.4. Data Collection and Analysis Methods
3. Results and Discussion
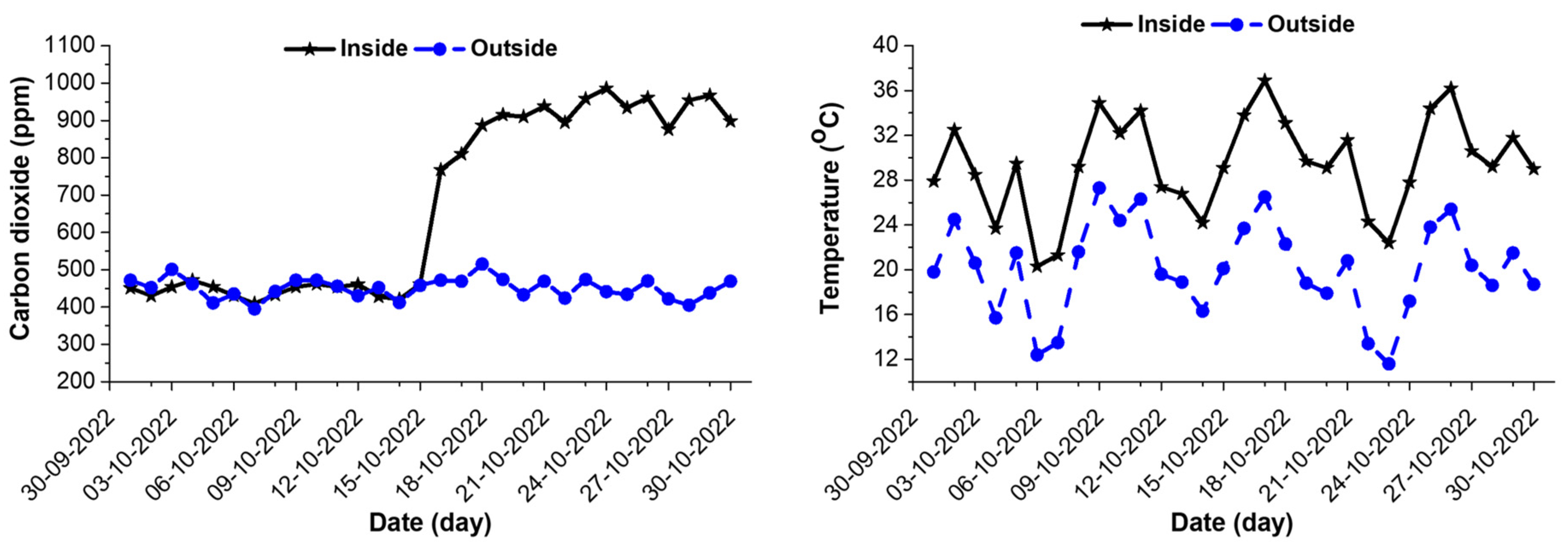
3.1. Variation of CO2 and Air Temperature inside the Greenhouse and Outside Environment
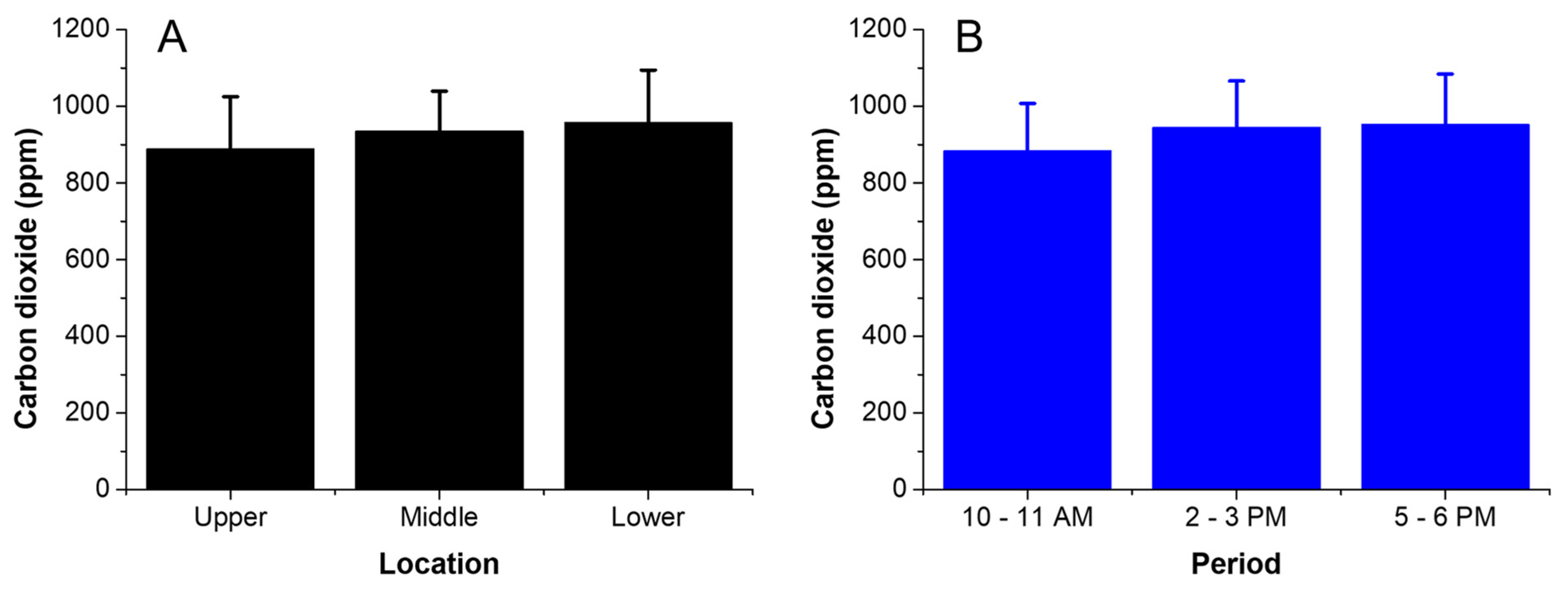
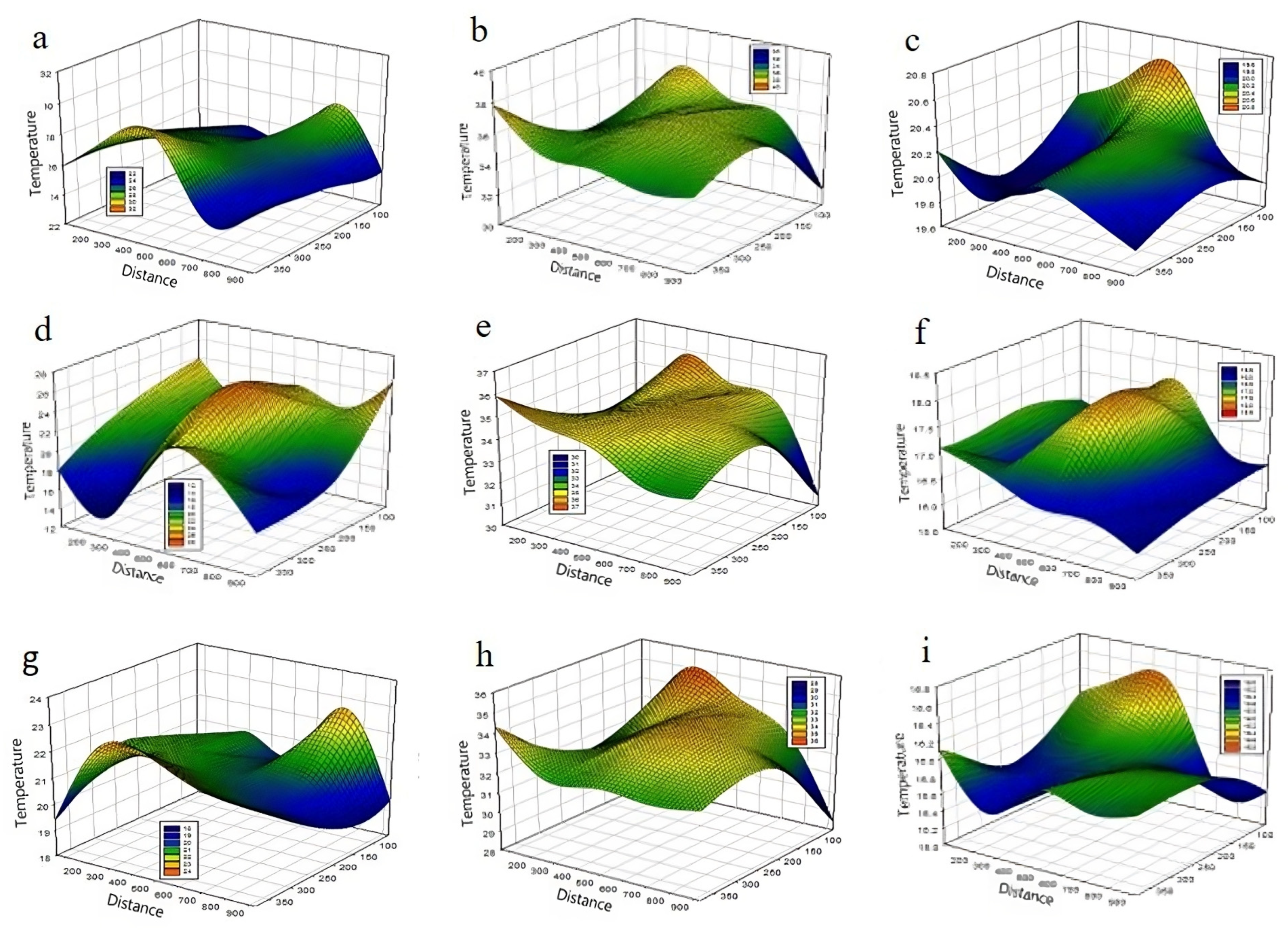
3.2. Vertical Distribution of CO2 and Temperature inside the Greenhouse in CS Conditions
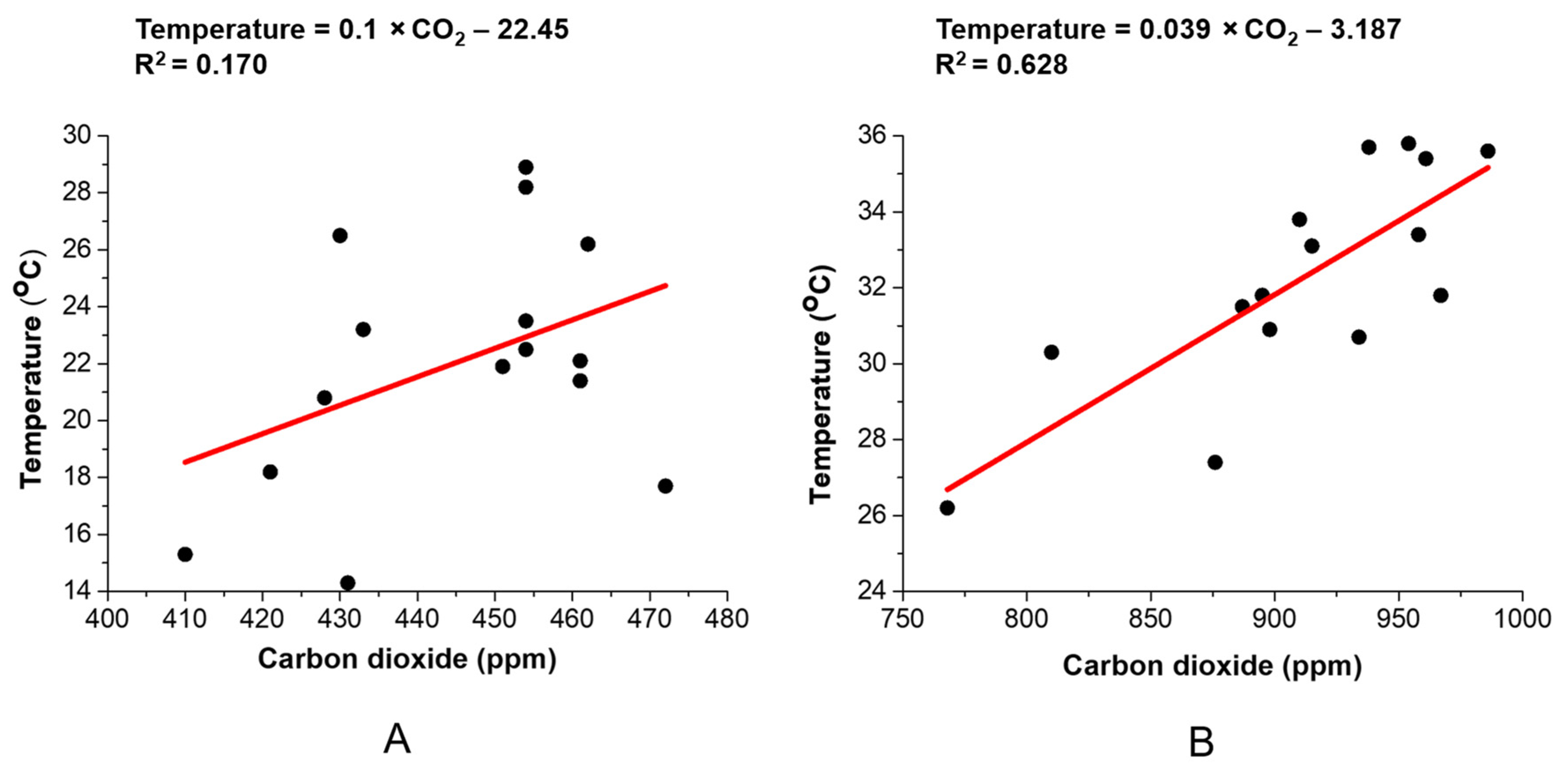
3.3. Relationship between CO2 and Temperature Variation inside the Greenhouse
3.4. Implications and Limitations of HF Applications in the Enhancement of CO2 and Temperature in Greenhouse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danyagri, G.; Dang, Q.-L. Effects of Elevated [CO2] and Low Soil Moisture on the Physiological Responses of Mountain Maple (Acer spicatum L.) Seedlings to Light. PLoS ONE 2013, 8, e76586. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Leite, F.F.G.D.; Adeyemi, M.A.; Sarker, A.J.; Cambareri, G.S.; Faverin, C.; Tieri, M.P.; Castillo-Zacarías, C.; Melchor-Martínez, E.M.; Iqbal, H.M.N. A Paradigm Shift to CO2 Sequestration to Manage Global Warming–With the Emphasis on Developing Countries. Sci. Total Environ. 2021, 790, 148169. [Google Scholar] [CrossRef] [PubMed]
- Xu, M. The Optimal Atmospheric CO2 Concentration for the Growth of Winter Wheat (Triticum aestivum). J. Plant Physiol. 2015, 184, 89–97. [Google Scholar] [CrossRef]
- Allan, R.P.; Hawkins, E.; Bellouin, N.; Collins, B. IPCC, 2021: Summary for Policymakers; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Smith, K.R.; Jerrett, M.; Anderson, H.R.; Burnett, R.T.; Stone, V.; Derwent, R.; Atkinson, R.W.; Cohen, A.; Shonkoff, S.B.; Krewski, D. Health and Climate Change 5 Public Health Benefits of Strategies to Reduce Greenhouse-Gas Emissions: Health Implications of Short-Lived Greenhouse Pollutants. Lancet 2009, 374, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations—Coalbeds and Deep Saline Aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Herzog, A.; Benson, S.M.; Hidy, G.M.; Gunter, W.D.; Penkala, S.J.; White, C.M. Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations. J. Air Waste Manag. Assoc. 2003, 53, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Fang, M.; Zhang, W.; Zhong, W.; Luo, Z.; Cen, K. Comparative Analysis of CO2 Separation from Flue Gas by Membrane Gas Absorption Technology and Chemical Absorption Technology in China. Energy Convers. Manag. 2008, 49, 3188–3197. [Google Scholar] [CrossRef]
- Zheng, L. Oxy-Fuel Combustion for Power Generation and Carbon Dioxide (CO2) Capture; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0857090984. [Google Scholar]
- Styring, P.; Jansen, D.; De Coninick, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011; ISBN 0957258801. [Google Scholar]
- Ma, X.; Zhang, X.; Tian, D. Farmland Degradation Caused by Radial Diffusion of CO2 Leakage from Carbon Capture and Storage. J. Clean. Prod. 2020, 255, 120059. [Google Scholar] [CrossRef]
- Tcvetkov, P. Public Perception of Carbon Capture and Storage: A State-of-the-Art Overview. Heliyon 2019, 5, e02845. [Google Scholar] [CrossRef]
- Arning, K.; Offermann-van Heek, J.; Linzenich, A.; Kätelhön, A.; Sternberg, A.; Bardow, A.; Ziefle, M. Same or Different? Insights on Public Perception and Acceptance of Carbon Capture and Storage or Utilization in Germany. Energy Policy 2019, 125, 235–249. [Google Scholar] [CrossRef]
- Vinca, A.; Rottoli, M.; Marangoni, G.; Tavoni, M. The Role of Carbon Capture and Storage Electricity in Attaining 1.5 and 2 C. Int. J. Greenh. Gas Control 2018, 78, 148–159. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, J.; Xie, X.; Luo, X.; Su, T.; Ji, H. CO2 Reforming of CH4 to Syngas over Nickel-Based Catalysts. Environ. Chem. Lett. 2020, 18, 997–1017. [Google Scholar] [CrossRef]
- Harper, A.B.; Powell, T.; Cox, P.M.; House, J.; Huntingford, C.; Lenton, T.M.; Sitch, S.; Burke, E.; Chadburn, S.E.; Collins, W.J. Land-Use Emissions Play a Critical Role in Land-Based Mitigation for Paris Climate Targets. Nat. Commun. 2018, 9, 2938. [Google Scholar] [CrossRef] [PubMed]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of Biochar Application to the Environment and Economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. The Production and Application of Carbon Nanomaterials from High Alkali Silicate Herbaceous Biomass. Sci. Rep. 2020, 10, 2563. [Google Scholar] [CrossRef] [PubMed]
- Fuss, S.; Lamb, W.F.; Callaghan, M.W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; de Oliveira Garcia, W.; Hartmann, J.; Khanna, T. Negative Emissions—Part 2: Costs, Potentials and Side Effects. Environ. Res. Lett. 2018, 13, 63002. [Google Scholar] [CrossRef]
- Villa, J.A.; Bernal, B. Carbon Sequestration in Wetlands, from Science to Practice: An Overview of the Biogeochemical Process, Measurement Methods, and Policy Framework. Ecol. Eng. 2018, 114, 115–128. [Google Scholar] [CrossRef]
- Gallant, K.; Withey, P.; Risk, D.; van Kooten, G.C.; Spafford, L. Measurement and Economic Valuation of Carbon Sequestration in Nova Scotian Wetlands. Ecol. Econ. 2020, 171, 106619. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Wong, S.; Yuan, H.; Gunter, W.D.; Zhou, Z.; Feng, X. CO2 Separation for Enhanced Coalbed Methane Recovery. In Proceedings of the International Symposium on Ecomaterials and Ecoprocesses 2003: The 42. Annual Conference of Metallurgists of CIM in Conjunction with the 33. Annual Hydrometallurgical Meeting of the Metallurgical Society of CIM, 2003 TMS Fall Extraction, Vancouver, BC, Canada, 24–27 August 2003. [Google Scholar]
- Flesher, J.R. Pebax® Polyether Block Amide—A New Family of Engineering Thermoplastic Elastomers. In High Performance Polymers: Their Origin and Development; Springer: Berlin/Heidelberg, Germany, 1986; pp. 401–408. [Google Scholar]
- Hasse, D.; Kulkarni, S.; Sanders, E.; Corson, E.; Tranier, J.-P. CO2 Capture by Sub-Ambient Membrane Operation. Energy Procedia 2013, 37, 993–1003. [Google Scholar] [CrossRef]
- Uragami, T.; Naito, Y.; Sugihara, M. Studies on Synthesis and Permeability of Special Polymer Membranes. Polym. Bull. 1981, 4, 617–622. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Modeling Water Flux in Forward Osmosis: Implications for Improved Membrane Design. AIChE J. 2007, 53, 1736–1744. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled Effects of Internal Concentration Polarization and Fouling on Flux Behavior of Forward Osmosis Membranes during Humic Acid Filtration. J. Memb. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Liu, L.; Chakma, A.; Feng, X. Preparation of Hollow Fiber Poly(Ether Block Amide)/Polysulfone Composite Membranes for Separation of Carbon Dioxide from Nitrogen. Chem. Eng. J. 2004, 105, 43–51. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, X.; Tang, C.Y.; Fu, Q.S.; Nie, S. Effect of Draw Solution Concentration and Operating Conditions on Forward Osmosis and Pressure Retarded Osmosis Performance in a Spiral Wound Module. J. Memb. Sci. 2010, 348, 298–309. [Google Scholar] [CrossRef]
- Mallevialle, J.; Odendaal, P.E.; Wiesner, M.R. Water Treatment Membrane Processes; American Water Works Association: Austin, TX, USA, 1996; ISBN 0070015597. [Google Scholar]
- Wang, R.; Shi, L.; Tang, C.Y.; Chou, S.; Qiu, C.; Fane, A.G. Characterization of Novel Forward Osmosis Hollow Fiber Membranes. J. Memb. Sci. 2010, 355, 158–167. [Google Scholar] [CrossRef]
- Kreulen, H.; Smolders, C.A.; Versteeg, G.F.; van Swaaij, W.P.M. Microporous Hollow Fibre Membrane Modules as Gas-Liquid Contactors Part 2. Mass Transfer with Chemical Reaction. J. Memb. Sci. 1993, 78, 217–238. [Google Scholar] [CrossRef]
- Xue, J.; Schulz, A.; Wang, H.; Feldhoff, A. The Phase Stability of the Ruddlesden-Popper Type Oxide (Pr0.9La0.1)2.0Ni0.74Cu0.21Ga0.05O4+δ in an Oxidizing Environment. J. Membr. Sci. 2016, 497, 357–364. [Google Scholar] [CrossRef]
- Agrahari, G.K.; Verma, N.; Bhattacharya, P.K. Application of Hollow Fiber Membrane Contactor for the Removal of Carbon Dioxide from Water under Liquid–Liquid Extraction Mode. J. Memb. Sci. 2011, 375, 323–333. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Julian, H.; Wenten, I.G. Polysulfone Membranes for CO2/CH4 Separation: State of the Art. IOSR J. Eng. 2012, 2, 484–495. [Google Scholar] [CrossRef]
- Mohamad, M.B.; Fong, Y.Y.; Shariff, A. Gas Separation of Carbon Dioxide from Methane Using Polysulfone Membrane Incorporated with Zeolite-T. Procedia Eng. 2016, 148, 621–629. [Google Scholar] [CrossRef]
- Won, W.; Feng, X.; Lawless, D. Pervaporation with Chitosan Membranes: Separation of Dimethyl Carbonate/Methanol/Water Mixtures. J. Memb. Sci. 2002, 209, 493–508. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of Polymeric Hollow Fibers as Sustainable Technologies: Past, Present, and Future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Harasimowicz, M.; Orluk, P.; Zakrzewska-Trznadel, G.; Chmielewski, A. Application of Polyimide Membranes for Biogas Purification and Enrichment. J. Hazard. Mater. 2007, 144, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C. Environmental Sustainability of Greenhouse Covering Materials. Sustainability 2019, 11, 6129. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ala’a, H.; García-Peñas, A.; Mola, G.T.; Si, C.; Stadler, F.J. Bio-Inspired and Biomaterials-Based Hybrid Photocatalysts for Environmental Detoxification: A Review. Chem. Eng. J. 2020, 382, 122937. [Google Scholar]
- La Mantia, F.P. Closed-Loop Recycling. A Case Study of Films for Greenhouses. Polym. Degrad. Stab. 2010, 95, 285–288. [Google Scholar] [CrossRef]
- Turner, D.A.; Williams, I.D.; Kemp, S. Greenhouse Gas Emission Factors for Recycling of Source-Segregated Waste Materials. Resour. Conserv. Recycl. 2015, 105, 186–197. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Papadopoulos, A.P.; Hao, X. Effects of Three Greenhouse Cover Materials on Tomato Growth, Productivity, and Energy Use. Sci. Hortic. 1997, 70, 165–178. [Google Scholar] [CrossRef]
- Miller, S.A. Natural Fiber Textile Reinforced Bio-Based Composites: Mechanical Properties, Creep, and Environmental Impacts. J. Clean. Prod. 2018, 198, 612–623. [Google Scholar] [CrossRef]
- Hao, X.; Papadopoulos, A.P. Effects of Supplemental Lighting and Cover Materials on Growth, Photosynthesis, Biomass Partitioning, Early Yield and Quality of Greenhouse Cucumber. Sci. Hortic. 1999, 80, 1–18. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Zhang, N.; Zhang, X.; Song, M.; Chen, Z.; Li, Z. Effects of the Gas/Particle Flow and Combustion Characteristics on Water-Wall Temperature and Energy Conversion in a Supercritical down-Fired Boiler at Different Secondary-Air Distributions. Energy 2022, 238, 121983. [Google Scholar] [CrossRef]
- Korea Meteorological Agency Weather Information in Jinju Location. Available online: https://www.weather.go.kr/w/index.do (accessed on 30 November 2022).
- Arulmozhi, E.; Basak, J.K.; Sihalath, T.; Park, J.; Kim, H.T.; Moon, B.E. Machine Learning-Based Microclimate Model for Indoor Air Temperature and Relative Humidity Prediction in a Swine Building. Animals 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Basak, J.K.; Eun, N.; Shihab, K.; Shahriar, A.; Paudel, B.; Eun, B. Applicability of Statistical and Machine Learning—Based Regression Algorithms in Modeling of Carbon Dioxide Emission in Experimental Pig Barns. Air Qual. Atmos. Health 2022, 15, 1899–1912. [Google Scholar] [CrossRef]
- Moon, T.W.; Jung, D.H.; Chang, S.H.; Son, J.E. Estimation of Greenhouse CO2 Concentration via an Artificial Neural Network That Uses Environmental Factors. Hortic. Environ. Biotechnol. 2018, 59, 45–50. [Google Scholar] [CrossRef]
- Kläring, H.-P.; Hauschild, C.; Heißner, A.; Bar-Yosef, B. Model-Based Control of CO2 Concentration in Greenhouses at Ambient Levels Increases Cucumber Yield. Agric. For. Meteorol. 2007, 143, 208–216. [Google Scholar] [CrossRef]
- Bennis, N.; Duplaix, J.; Enéa, G.; Haloua, M.; Youlal, H. Greenhouse Climate Modelling and Robust Control. Comput. Electron. Agric. 2008, 61, 96–107. [Google Scholar] [CrossRef]
- Lee, I.-B.; Short, T.H. Verification of Computational Fluid Dynamic Temperature Simulations in a Full-Scale Naturally Ventilated Greenhouse. Trans. ASAE 2001, 44, 119. [Google Scholar] [CrossRef]
- Elanchezhian, A.; Basak, J.K.; Park, J.; Khan, F.; Okyere, F.G.; Lee, Y.; Bhujel, A.; Lee, D.; Sihalath, T.; Kim, T. Evaluating Different Models Used for Predicting the Indoor Microclimatic Parameters of a Greenhouse. Appl. Ecol. Environ. Res. 2020, 18, 2141–2161. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Li, D.; Miao, Z. Automatic Carbon Dioxide Enrichment Strategies in the Greenhouse: A Review. Biosyst. Eng. 2018, 171, 101–119. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, X.; Li, C.; Ye, Q. Effects of CO2 Enrichment on Photosynthesis and Growth in Gerbera Jamesonii. Sci. Hortic. 2014, 177, 77–84. [Google Scholar] [CrossRef]
- Critten, D.L. Optimization of CO2 Concentration in Greenhouses: A Modelling Analysis for the Lettuce Crop. J. Agric. Eng. Res. 1991, 48, 261–271. [Google Scholar] [CrossRef]
- Titus, J.E.; Wendlberger, S.J. Gauging Submersed Plant Response to CO2 Enrichment: Pot Size Matters. Aquat. Bot. 2016, 134, 82–86. [Google Scholar] [CrossRef]
- Jin, C.; Du, S.; Wang, Y.; Condon, J.; Lin, X.; Zhang, Y. Carbon Dioxide Enrichment by Composting in Greenhouses and Its Effect on Vegetable Production. J. Plant Nutr. Soil Sci. 2009, 172, 418–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of Carbon Dioxide Enrichment on Health-Promoting Compounds and Organoleptic Properties of Tomato Fruits Grown in Greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Seginer, I.; van Straten, G.; van Beveren, P.J.M. Day-to-Night Heat Storage in Greenhouses: 2 Sub-Optimal Solution for Realistic Weather. Biosyst. Eng. 2017, 161, 188–199. [Google Scholar] [CrossRef]
- Hao, F.; Shen, M.; He, Y.; Feng, L. Three Dimensional Steady Simulation of Microclimate Pattern inside Single Plastic Greenhouse Using Computational Fluid Dynamics. Nongye Jixie Xuebao = Trans. Chin. Soc. Agric. Mach. 2014, 45, 297–304. [Google Scholar]
- Kozai, T.; Kubota, C.; Takagaki, M.; Maruo, T. Greenhouse Environment Control Technologies for Improving the Sustainability of Food Production. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): 1107, Brisbane, Australia, 17 August 2014; pp. 1–14. [Google Scholar]
- Stanghellini, C.; Incrocci, L.; Gázquez, J.C.; Dimauro, B. Carbon Dioxide Concentration in Mediterranean Greenhouses: How Much Lost Production? In Proceedings of the International Symposium on High Technology for Greenhouse System Management: Greensys2007 801, Naples, Italy, 4–6 October 2007; pp. 1541–1550. [Google Scholar]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Solar Greenhouse an Option for Renewable and Sustainable Farming. Renew. Sustain. Energy Rev. 2011, 15, 3934–3945. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Hassan, S.G.; Li, D. Prediction of the Temperature in a Chinese Solar Greenhouse Based on LSSVM Optimized by Improved PSO. Comput. Electron. Agric. 2016, 122, 94–102. [Google Scholar] [CrossRef]
- Kuroyanagi, T.; Yasuba, K.; Higashide, T.; Iwasaki, Y.; Takaichi, M. Efficiency of Carbon Dioxide Enrichment in an Unventilated Greenhouse. Biosyst. Eng. 2014, 119, 58–68. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Zheng, L.; Zheng, C.; Wang, Y. Wireless Mid-Infrared Spectroscopy Sensor Network for Automatic Carbon Dioxide Fertilization in a Greenhouse Environment. Sensors 2016, 16, 1941. [Google Scholar] [CrossRef]
- Amir, R.; Teitel, M.; Shemer, L. CO2 Enrichment in a Fan-Ventilated Greenhouse under Different Ventilation Modes. In Proceedings of the International Conference on Sustainable Greenhouse Systems-Greensys2004 691, Leuven, Belgium, 12–16 September 2004; pp. 619–624. [Google Scholar]
- Zeng, S.; Hu, H.; Xu, L.; Li, G. Nonlinear Adaptive PID Control for Greenhouse Environment Based on RBF Network. Sensors 2012, 12, 5328–5348. [Google Scholar] [CrossRef] [PubMed]
- Basak, J.K.; Qasim, W.; Okyere, F.G.; Khan, F.; Lee, Y.J.; Park, J.; Kim, H.T. Regression Analysis to Estimate Morphology Parameters of Pepper Plant in a Controlled Greenhouse System. J. Biosyst. Eng. 2019, 44, 57–68. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Jansen, A.E. CO2 Separation with Polyolefin Membrane Contactors and Dedicated Absorption Liquids: Performances and Prospects. Sep. Purif. Technol. 2002, 27, 231–242. [Google Scholar] [CrossRef]
- Mohamed, S.J.; Jellings, A.J.; Fuller, M.P. Positive Effects of Elevated CO2 and Its Interaction with Nitrogen on Safflower Physiology and Growth. Agron. Sustain. Dev. 2013, 33, 497–505. [Google Scholar] [CrossRef]
- Basak, J.K.; Qasim, W.; Khan, F.; Okyere, F.G.; Lee, Y.; Arulmozhi, E.; Park, J.; Cho, W.; Kim, H.T. Assessment of the Effect of Dimethyl Ether (DME) Combustion on Lettuce and Chinese Cabbage Growth in Greenhouse. J. Bio-Environ. Control 2019, 28, 293–301. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Lemonnier, P.; Wedow, J.M. The Influence of Rising Tropospheric Carbon Dioxide and Ozone on Plant Productivity. Plant Biol. 2020, 22, 5–11. [Google Scholar] [CrossRef]
- Basak, J.K.; Qasim, W.; Okyere, F.G.; Khan, F.; Lee, Y.J.; Park, J.; Kim, H.T. Mitigating Chilling Stress of Pepper Plant (‘Capsicum annuum’L.) Using Dimethyl Ether Combustion Gas in Controlled Greenhouse. Aust. J. Crop Sci. 2019, 13, 1992–2002. [Google Scholar] [CrossRef]
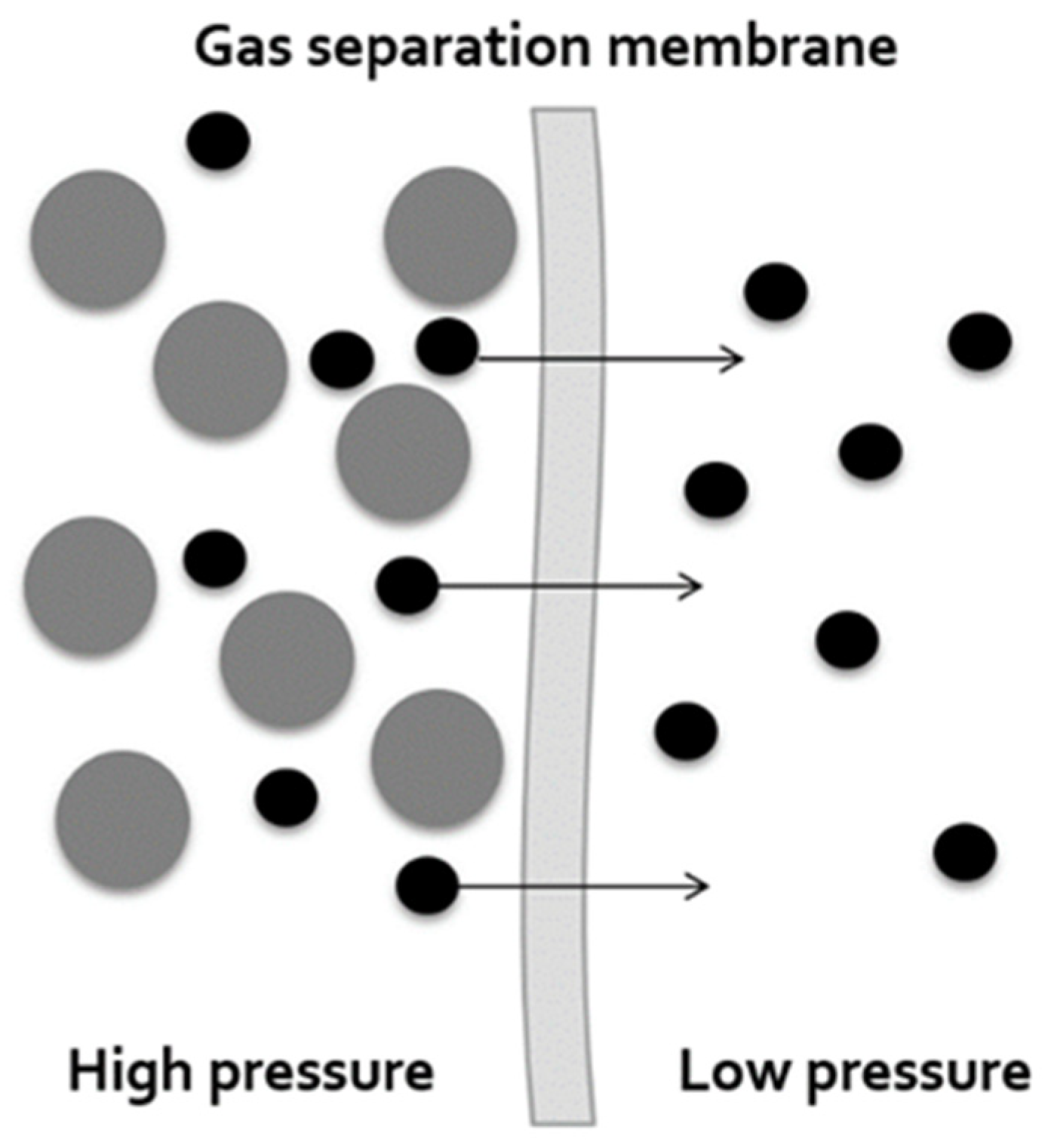


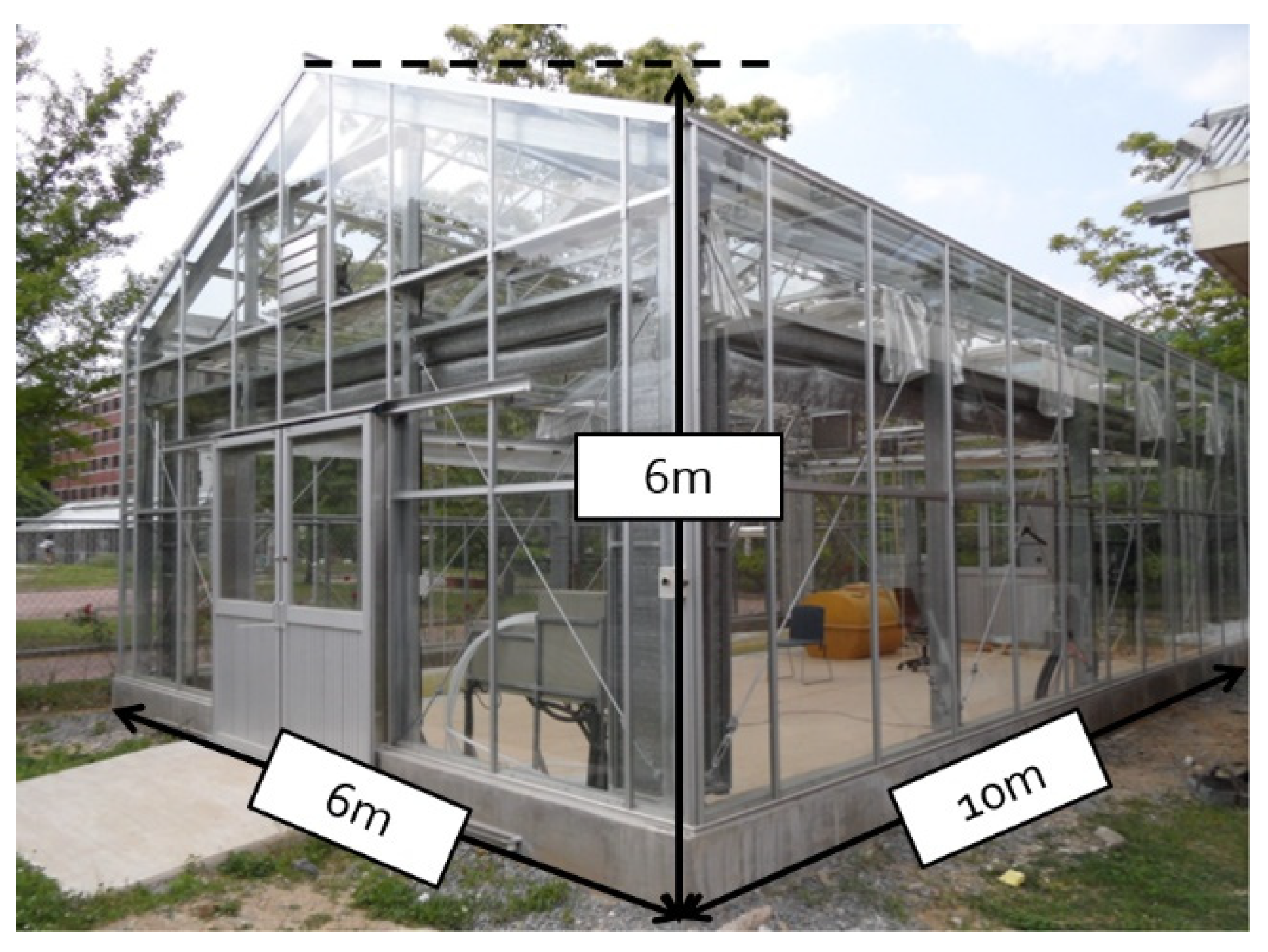
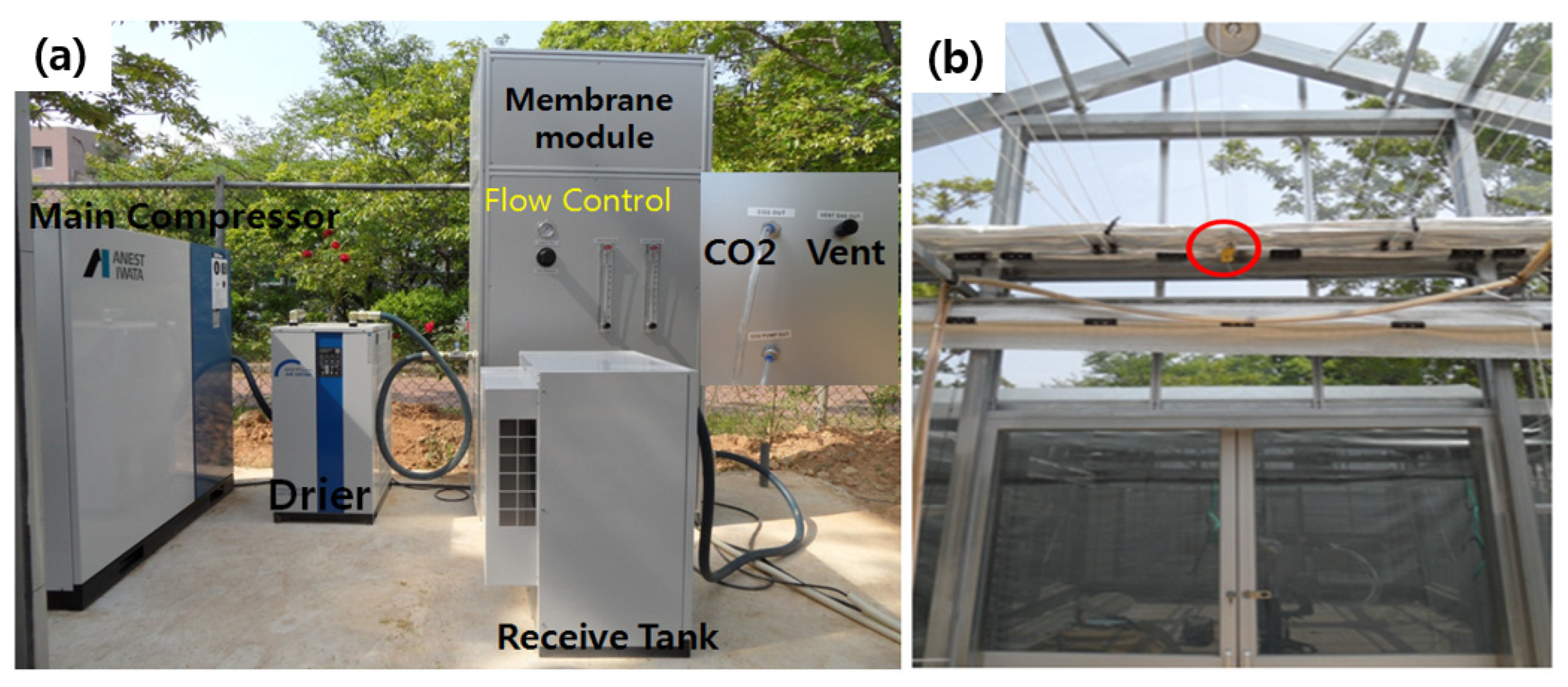



| Parameters | Specification |
|---|---|
| Size | 600 × 800 × 400 (W × H × D, mm) |
| Range of supply gas pressure | 0.40–1.0 MPa |
| Conditions of supply gas | Siloxane ≤ 0.1 ppm, H2S ≤ 3ppm |
| Range of operation pressure | 0.40–0.7 MPa |
| Range of operation temperature | 15~45 °C |
| Port of supply gas | 3/8″ Ferrel type |
| Port of methane recycling, CO2 exhaust | 6 mm tube type |
| Period | Location | CO2 Concentration (ppm) | Temperature (°C) |
|---|---|---|---|
| WCS | Outside | 448.13 ± 27.94 | 18.27 ± 4.40 |
| Inside | 445.07 ± 18.02 | 27.18 ± 4.37 | |
| CS | Outside | 453.93 ± 28.49 | 18.04 ± 4.13 |
| Inside | 910.47 ± 59.44 | 29.34 ± 4.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.E.; Basak, J.K.; Kim, H.T. Application of Hollow Fiber Membrane for the Separation of Carbon Dioxide from Atmospheric Air and Assessment of Its Distribution Pattern in a Greenhouse. Atmosphere 2023, 14, 299. https://doi.org/10.3390/atmos14020299
Kim NE, Basak JK, Kim HT. Application of Hollow Fiber Membrane for the Separation of Carbon Dioxide from Atmospheric Air and Assessment of Its Distribution Pattern in a Greenhouse. Atmosphere. 2023; 14(2):299. https://doi.org/10.3390/atmos14020299
Chicago/Turabian StyleKim, Na Eun, Jayanta Kumar Basak, and Hyeon Tae Kim. 2023. "Application of Hollow Fiber Membrane for the Separation of Carbon Dioxide from Atmospheric Air and Assessment of Its Distribution Pattern in a Greenhouse" Atmosphere 14, no. 2: 299. https://doi.org/10.3390/atmos14020299
APA StyleKim, N. E., Basak, J. K., & Kim, H. T. (2023). Application of Hollow Fiber Membrane for the Separation of Carbon Dioxide from Atmospheric Air and Assessment of Its Distribution Pattern in a Greenhouse. Atmosphere, 14(2), 299. https://doi.org/10.3390/atmos14020299






