Assessment of Wet Inorganic Nitrogen Deposition in an Oil Palm Plantation-Forest Matrix Environment in Borneo
Abstract
1. Introduction
2. Materials and Methods
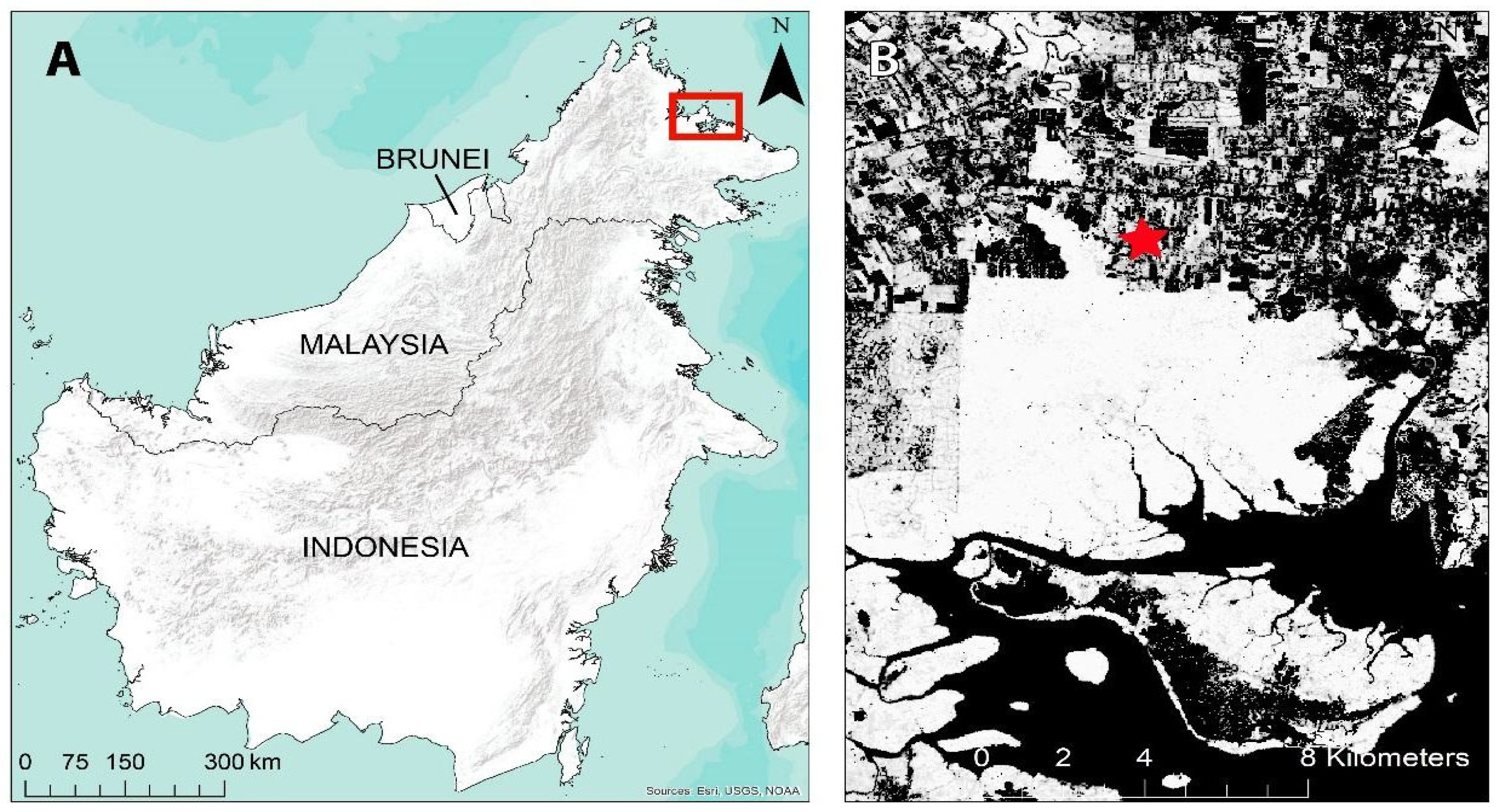
2.1. Study Area
2.2. Sampling and Analysis
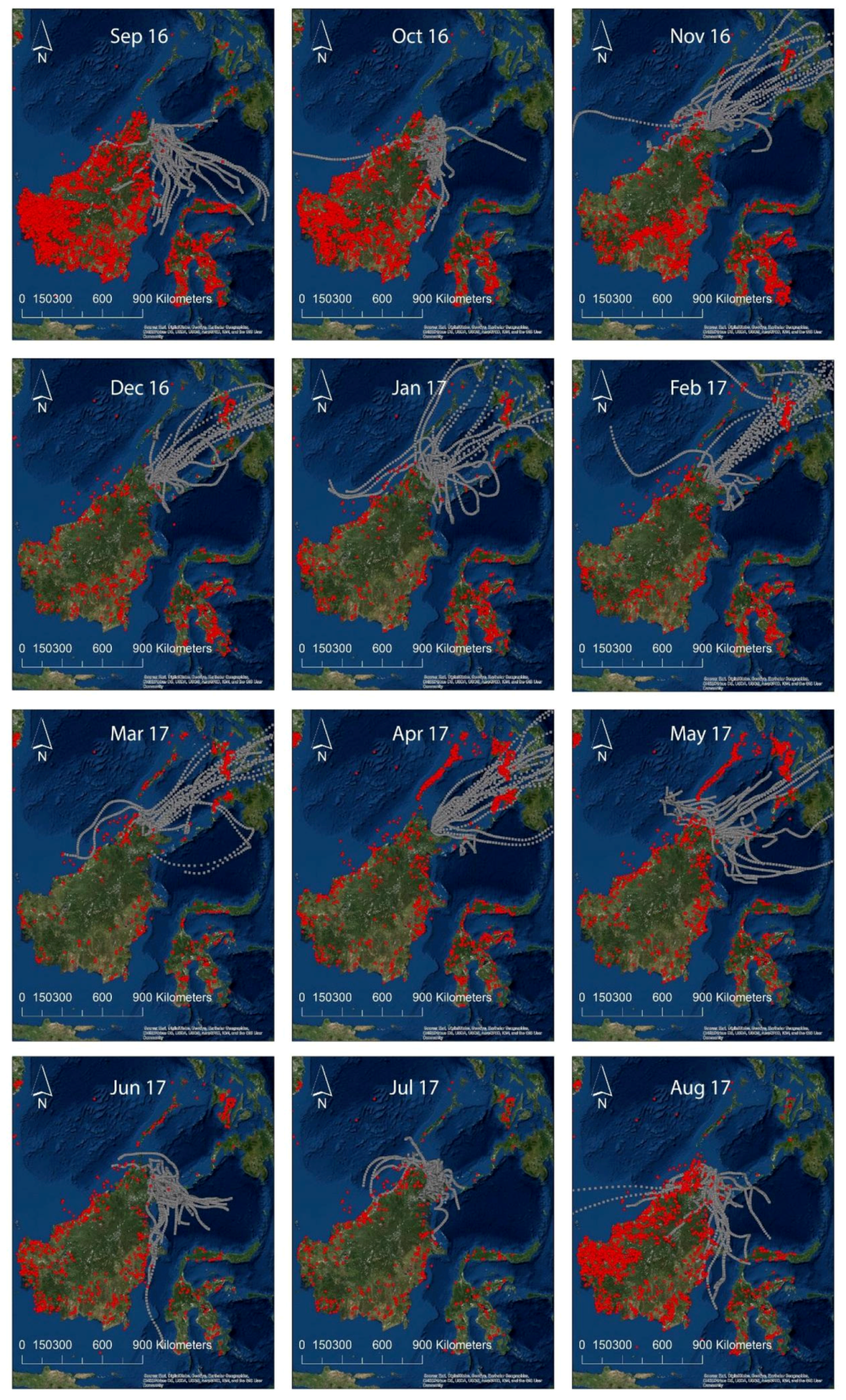
2.3. Wind Trajectories, Fires, Land Use and the Correlation with N Deposition
3. Results
4. Discussion
| Site | NH4+-N | NO3−-N | pH | Rainfall | Reference | |
|---|---|---|---|---|---|---|
| kg ha−1 year−1 | mm year−1 | |||||
| Forest | Forest type | |||||
| Sepilok (East Malaysia) | Lowland | 6.7 | 0.7 | 5.4 | 4637 | This study |
| Danum (East Malaysia) | Lowland | 0.7 | 0.4 | 5.1 | 2996 | Vet et al., 2014 [7] |
| Pasoh (West Malaysia) | Lowland | 2.3 | 3.9 | 5.8 | 2381 | Manokaran 1980 [40] |
| Berembun (West Malaysia) | Montane | 1.4 | 1.6 | 5.9 | 1979 | Yusop and Nik 1989 [42] |
| Tanah Rata (West Malaysia) | Montane | 1.4 | 2.1 | 4.9 | 2879 | Vet et al., 2014 [7] |
| City | ||||||
| Singapore | 11.5 | 13.4 | - | 2554 | Karthikeyan et al., 2009 [51] | |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha, H.F.V.; Andersen, K.M.; Lugli, L.F.; Santana, F.D.; Aleixo, I.F.; Moraes, A.M.; Garcia, S.; Di Ponzio, R.; Mendoza, E.O.; Brum, B.; et al. Direct evidence for phosphorus limitation on Amazon forest productivity. Nature 2022, 608, 558–562. [Google Scholar] [CrossRef]
- Benner, J.W.; Vitousek, P.M.; Ostertag, R. Nutrient cycling and nutrient limitation in tropical montane cloud forests. In Tropical Montane Cloud Forests: Science for Conservation and Management; Bruijnzeel, L., Scatena, F., Hamilton, L., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 90–100. [Google Scholar]
- Moran, J.A.; Barker, M.G.; Moran, A.J.; Becker, P.; Ross, S.M. A comparison of the soil water, nutrient status, and litterfall characteristics of tropical heath and mixed-dipterocarp forest sites in Brunei. Biotropica 2000, 32, 2–13. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Kapos, V.; Franco, W. Nitrogen and phosphorus fertilization effects on Venezuelan montane forest trunk growth and litterfall. Ecology 1992, 73, 78–86. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.J.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Dentener, F.J.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20, GB4003. [Google Scholar] [CrossRef]
- Bobbink, R.; Loran, C.; Tomassen, H. (Eds.) Review and Revision of Empirical Critical Loads of Nitrogen for Europe; Umweltbundesamt/German Environment Agency: Dessau-Roßlau, Germany, 2022. [Google Scholar]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Zhou, G.; Fang, Y. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2018, 28, 398–413. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Hicks, W.K.; Cinderby, S.; Kuylenstierna, J.C.I.; Stock, W.D.; Dentener, F.J.; Giller, K.E.; Austin, A.T.; Lefroy, R.D.B.; Gimeno, B.S.; et al. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob. Chang. Biol. 2006, 12, 470–476. [Google Scholar] [CrossRef]
- Jones, D.S. ASEAN and transboundary haze pollution in Southeast Asia. Asia Eur. J. 2006, 4, 431–446. [Google Scholar] [CrossRef]
- Barber, C.V.; Schweithelm, J. Trial by Fire: Forest Fire and Forestry Policy in Indonesia’s Era of Crisis and Reform; World Resources Institute: Washington, DC, USA, 2000. [Google Scholar]
- Lohman, D.J.; Bickford, D.; Sodhi, N.S. The burning issue. Science 2007, 316, 376. [Google Scholar] [CrossRef] [PubMed]
- Aiken, S.R. Runaway fires, smoke-haze pollution, and unnatural disasters in Indonesia. Geogr. Rev. 2004, 94, 55–79. [Google Scholar] [CrossRef]
- Andreae, M.O.; Browell, E.V.; Garstang, M.; Gregory, G.L.; Harriss, R.C.; Hill, G.F.; Jacob, D.J.; Pereira, M.C.; Sachse, G.W.; Setzer, A.W.; et al. Biomass-burning emissions and associated haze layers over Amazonia. J. Geophys. Res. 1988, 93, 1509–1527. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Andreae, M.O. Biomass burning in the tropics: Impact on atmospheric chemistry and biogeochemical cycles. Science 1990, 250, 1669–1678. [Google Scholar] [CrossRef]
- Lobert, J.M.; Schaffe, D.H.; Hao, W.M.; Crutzen, P.J. Importance of biomass burning in the atmospheric budgets of nitrogen-containing gases. Nature 1990, 346, 552–554. [Google Scholar] [CrossRef]
- Carrasco, L.R.; Larrosa, C.; Edwards, D.P. A double-edged sword for tropical forests. Science 2014, 346, 38–41. [Google Scholar] [CrossRef]
- Albanito, F.; Lebender, U.; Cornulier, T.; Sapkota, T.B.; Brentrup, F.; Stirling, C.; Hillier, J. Direct nitrous oxide emissions from tropical and sub-tropical agricultural systems—A review and modelling of emission factors. Sci. Rep. 2017, 7, 44235. [Google Scholar] [CrossRef]
- Abram, N.K.; Xofis, P.; Tzanopoulos, J.; MacMillan, D.C.; Ancrenaz, M.; Chung, R.; Peter, L.; Ong, R.C.; Lackman, I.; Goossens, B.; et al. Synergies for improving oil palm production and forest conservation in floodplain landscapes. PLoS ONE 2014, 9, e95388. [Google Scholar] [CrossRef]
- Ashton, P.S. Conservation of Borneo biodiversity: Do small lowland parks have a role, or are big inland sanctuaries sufficient? Brunei as an example. Biodivers. Conserv. 2010, 19, 343–356. [Google Scholar] [CrossRef]
- Sellan, G.; Brearley, F.Q.; Nilus, R.; Titin, J.; Majalap, N. Differences in soil properties among contrasting soil types in Northern Borneo. J. Trop. For. Sci. 2021, 33, 191–202. [Google Scholar] [CrossRef]
- Fox, J.E.D. A Handbook to Kabili-Sepilok Forest Reserve. Sabah Forest Record No. 9; Borneo Literature Bureau: Kuching, Malaysia, 1973. [Google Scholar]
- Nilus, R. Effect of Edaphic Variation on Forest Structure, Dynamics and Regeneration in a Lowland Tropical Rainforest in Borneo. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2003. [Google Scholar]
- EPA Quality Assurance Handbook for Air Pollution Measurement Systems: Volume V. Manual for Precipitation Measuremnt Systems Part 1. Quality Assurance Manual; EPA: Washington, DC, USA, 1983.
- Hansen, M.C.; Potapov, P.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Draxler, R.R.; Rolph, G.D. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model Access via NOAA ARL READY Website (College Park, MD:NOAAAir Resources Laboratory). Available online: http://arl.noaa.gov/HYSPLIT.php (accessed on 22 December 2019).
- Gaveau, D.L.A.; Sheil, D.; Husnayaen; Salim, M.A.; Arjasakusuma, S.; Ancrenaz, M.; Pacheco, P.; Meijaard, E. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Sci. Rep. 2016, 6, 32017. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System. Version 3.8-Zanzibar. Open Source Geospatial Foundation Project. 2019. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/referencespapers.aspx?referenceid=2631129 (accessed on 22 October 2022).
- Zender, C.S.; Krolewski, A.G.; Tosca, M.G.; Randerson, J.T. Tropical biomass burning smoke plume size, shape, reflectance, and age based on 2001–2009 MISR imagery of Borneo. Atmos. Chem. Phys. 2012, 12, 3437–3454. [Google Scholar] [CrossRef]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.N.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cindereby, S.; Davidson, E.; Dentener, F.J.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Yamashita, N.; Sase, H.; Kobayashi, R.; Leong, K.; Hanapi, J.M.; Uchiyama, S.; Urban, S.; Toh, Y.Y.; Muhamad, M.; Gidiman, J.; et al. Atmospheric deposition versus rock weathering in the control of streamwater chemistry in a tropical rain-forest catchment in Malaysian Borneo. J. Trop. Ecol. 2014, 30, 481–492. [Google Scholar] [CrossRef]
- Manokaran, N. The nutrient contents of precipitation, through fall and stem flow in a lowland tropical rain forest in Peninsular Malaysia. Malay. For. 1980, 34, 266–289. [Google Scholar]
- Okuda, T.; Suzuki, M.; Adachi, N.; Yoshida, K.; Niiyama, K.; Noor, N.S.; Hussein, N.A.; Manokaran, N.; Hashim, M. Logging history and its impact on forest structure and species composition in the Pasoh Forest Reserve—Implications for the sustainable management of natural resources and landscapes. In Pasoh: Ecology of a Lowland Rain Forest in Southeast Asia; Okuda, T., Manokaran, N., Matsumoto, Y., Niiyama, K., Thomas, S.C., Ashton, P.S., Eds.; Springer: Tokyo, Japan, 2003; pp. 15–34. [Google Scholar]
- Yusop, Z.; Nik, A.R. Rainfall chemistry and nutrient loading in a peninsular Malaysia forest site. J. Trop. For. Sci. 1989, 1, 201–214. [Google Scholar]
- Sellan, G. Ecological Responses of a Bornean Heath Forest (Kerangas) to Experimental Lime and Nitrogen. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2019. [Google Scholar]
- Sellan, G.; Thompson, J.; Majalap, N.; Robert, R.; Brearley, F.Q. Impact of soil nitrogen availability and pH on tropical heath forest organic matter decomposition and decomposer activity. Pedobiologia J. Soil Ecol. 2020, 80, 150645. [Google Scholar] [CrossRef]
- Charlson, R.J.; Rodhe, H. Factors controlling the acidity of natural rainwater. Nature 1982, 295, 683–685. [Google Scholar] [CrossRef]
- Brinck, K.; Fischer, R.; Groeneveld, J.; Lehmann, S.; De Paula, M.D.; Putz, S.; Sexton, J.O.; Song, D.; Huth, A. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 2017, 8, 14855. [Google Scholar] [CrossRef] [PubMed]
- Giglio, L.; Csiszar, I.; Justice, C.O. Global distribution and seasonality of active fires as observed with the Terra and Aqua Moderate Resolution Imaging Spectroradiometer (MODIS) sensors. J. Geophys. Res. Biogeosci. 2006, 111, 02016. [Google Scholar] [CrossRef]
- Bauters, M.; Drake, T.W.; Verbeeck, H.; Bodé, S.; Hervé-Fernández, P.; Zito, P.; Podgorski, D.C.; Boyemba, F.; Makelele, I.; Cizungu Ntaboba, L.; et al. High fire-derived nitrogen deposition on central African forests. Proc. Natl. Acad. Sci. USA 2018, 115, 549–554. [Google Scholar] [CrossRef]
- Radojevic, M. Chemistry of forest fires and regional haze with emphasis on Southeast Asia. Pure Appl. Geophys. 2003, 160, 157–187. [Google Scholar] [CrossRef]
- Ayers, G.P.; Peng, L.C.; Fook, L.S.; Kong, C.W.; Gillett, R.W.; Manins, P.C. Atmospheric concentrations and deposition of oxidised sulfur and nitrogen species at Petaling Jaya, Malaysia, 1993–1998. Tellus Ser. B Chem. Phys. Meteorol. 2000, 52, 60–73. [Google Scholar] [CrossRef]
- Karthikeyan, S.; He, J.; Palani, S.; Balasubramanian, R.; Burger, D. Determination of total nitrogen in atmospheric wet and dry deposition samples. Talanta 2009, 77, 979–984. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Lee, D.S.; Asman, W.A.H.; Dentener, F.J.; Van Der Hoek, K.W.; Olivier, J.G.J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar] [CrossRef]
- Fowler, D.; Nemitz, E.; Misztal, P.; di Marco, C.; Skiba, U.M.; Ryder, J.; Helfter, C.; Neil Cape, J.; Owen, S.; Dorsey, J.; et al. Effects of land use on surface-atmosphere exchanges of trace gases and energy in Borneo: Comparing fluxes over oil palm plantations and a rainforest. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3196–3209. [Google Scholar] [CrossRef] [PubMed]
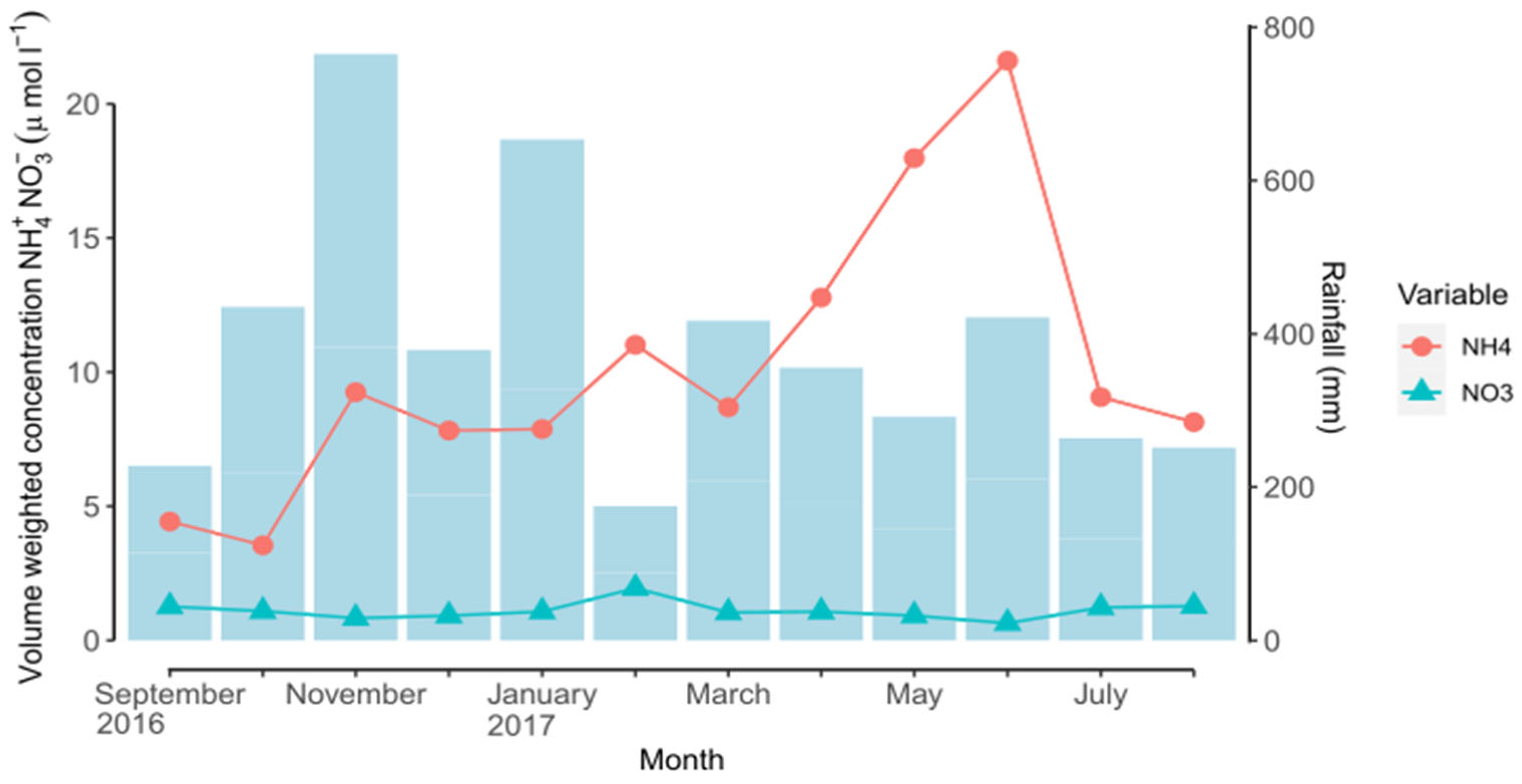
| Date | pH | NH4+-N | NO3−-N | |
|---|---|---|---|---|
| 2016 | September | 5.37 | 0.35 | 0.08 |
| October | 5.17 | 0.21 | 0.07 | |
| November | 5.38 | 0.99 | 0.09 | |
| December | 5.30 | 0.41 | 0.05 | |
| 2017 | January | 5.44 | 0.72 | 0.09 |
| February | 5.45 | 0.27 | 0.05 | |
| March | 5.34 | 0.51 | 0.06 | |
| April | 5.44 | 0.64 | 0.05 | |
| May | 5.46 | 0.73 | 0.04 | |
| June | 5.39 | 1.28 | 0.04 | |
| July | 5.32 | 0.33 | 0.04 | |
| August | 5.47 | 0.29 | 0.04 | |
| Total | - | 6.74 | 0.71 | |
| NH4+ kg ha−1 | NO3− kg ha−1 | NH4+ µmol L−1 | NO3− µmol L−1 | Rainwater pH | Rainfall (mm) | |
|---|---|---|---|---|---|---|
| MFC | −0.300.34 | 0.270.42 | −0.220.48 | 0.250.38 | 0.040.90 | −0.370.24 |
| MLUC | −0.040.90 | 0.210.35 | −0.090.78 | −0.300.51 | −0.190.55 | −0.310.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellan, G.; Majalap, N.; Thompson, J.; Dise, N.B.; Field, C.D.; Pappalardo, S.E.; Codato, D.; Robert, R.; Brearley, F.Q. Assessment of Wet Inorganic Nitrogen Deposition in an Oil Palm Plantation-Forest Matrix Environment in Borneo. Atmosphere 2023, 14, 297. https://doi.org/10.3390/atmos14020297
Sellan G, Majalap N, Thompson J, Dise NB, Field CD, Pappalardo SE, Codato D, Robert R, Brearley FQ. Assessment of Wet Inorganic Nitrogen Deposition in an Oil Palm Plantation-Forest Matrix Environment in Borneo. Atmosphere. 2023; 14(2):297. https://doi.org/10.3390/atmos14020297
Chicago/Turabian StyleSellan, Giacomo, Noreen Majalap, Jill Thompson, Nancy B. Dise, Chris D. Field, Salvatore E. Pappalardo, Daniele Codato, Rolando Robert, and Francis Q. Brearley. 2023. "Assessment of Wet Inorganic Nitrogen Deposition in an Oil Palm Plantation-Forest Matrix Environment in Borneo" Atmosphere 14, no. 2: 297. https://doi.org/10.3390/atmos14020297
APA StyleSellan, G., Majalap, N., Thompson, J., Dise, N. B., Field, C. D., Pappalardo, S. E., Codato, D., Robert, R., & Brearley, F. Q. (2023). Assessment of Wet Inorganic Nitrogen Deposition in an Oil Palm Plantation-Forest Matrix Environment in Borneo. Atmosphere, 14(2), 297. https://doi.org/10.3390/atmos14020297










