Spatiotemporal Variability of the Lake Tana Water Quality Derived from the MODIS-Based Forel–Ule Index: The Roles of Hydrometeorological and Surface Processes
Abstract
1. Introduction
2. Study Area and Datasets
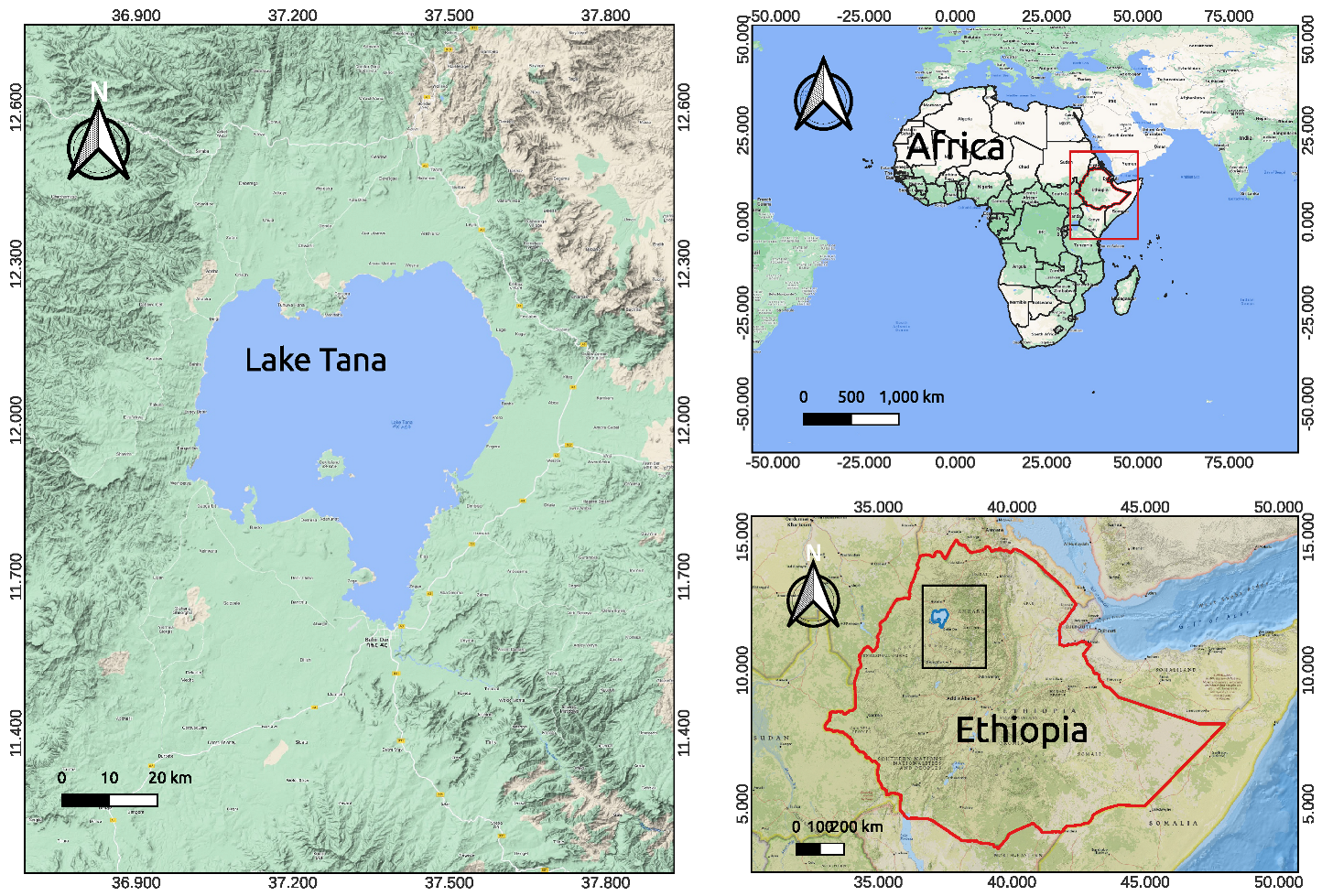
2.1. Description of Study Area
2.2. Datasets
2.2.1. MODIS Water Surface Reflectance Dataset
2.2.2. Diversity II Dataset
2.2.3. Reanalysis and Observational Datasets
2.2.4. Land-Use–Land-Cover Change Datasets
3. Methods
3.1. Correction for Spectral Reflectance of Day Light and Sky Light in MOD09A1 Reflectance
3.2. FUI Formulation
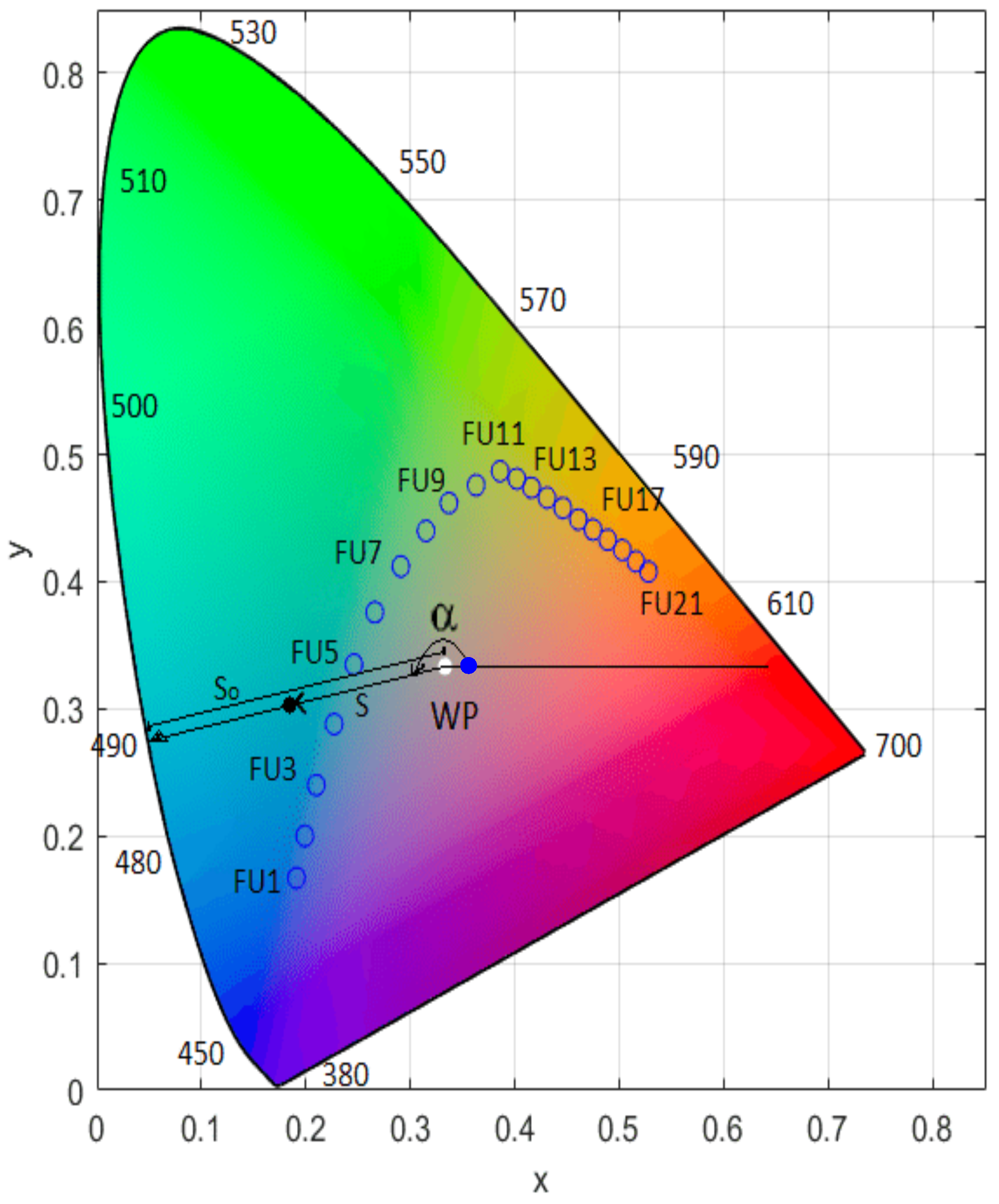
3.3. FUI-Based Trophic State Assessment of Lake Tana Water
3.4. Statistics
3.4.1. Correlation
3.4.2. Categorical Statistics
3.5. Composite Analysis for Establishing Qualitative Causal Linkage between FUI and Environmental Factors
4. Results and Discussion
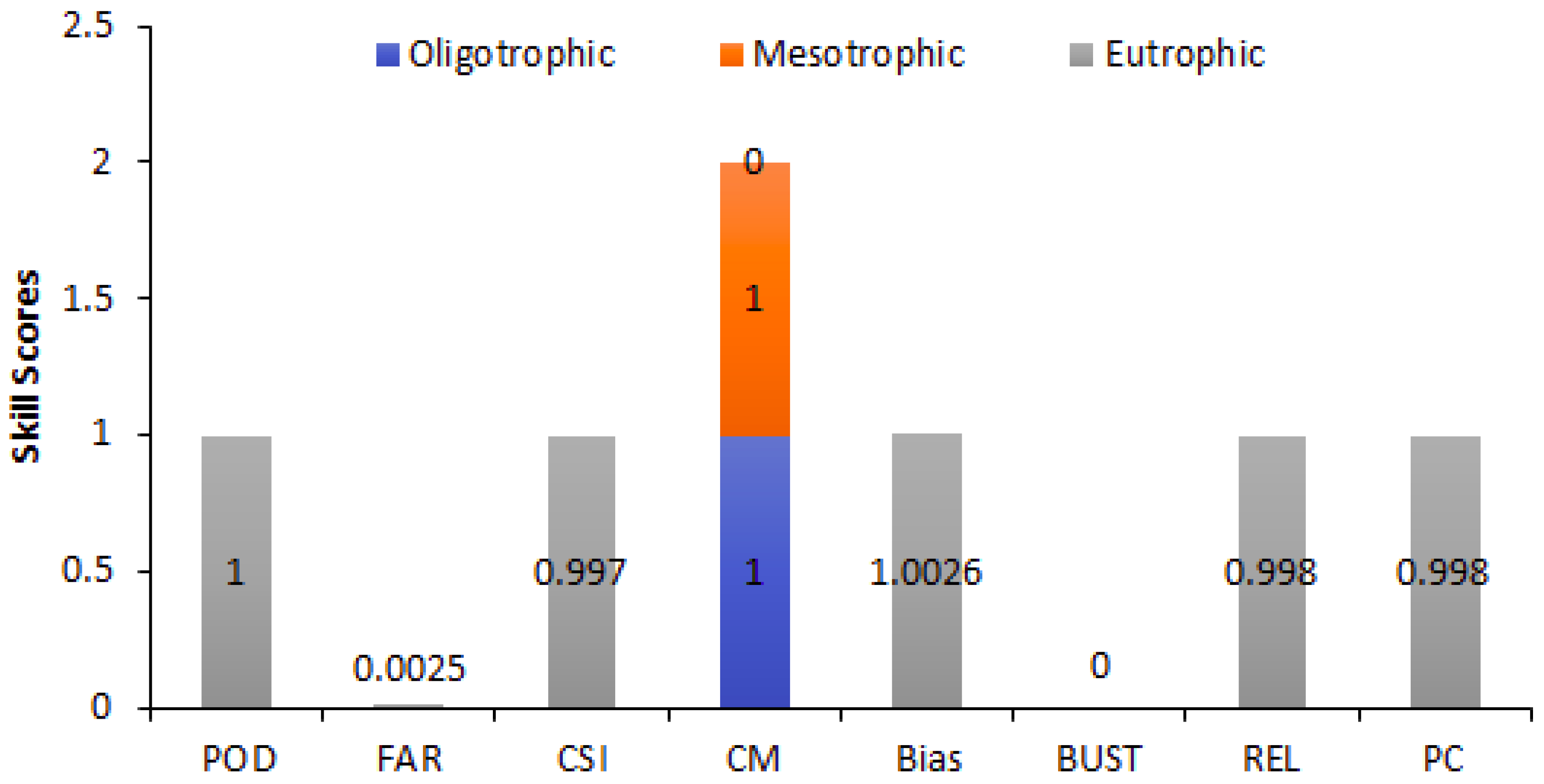
4.1. Performance of FUI as a Trophic State Indicator Based on Categorical Statistical Metrics and Pearson Correlation
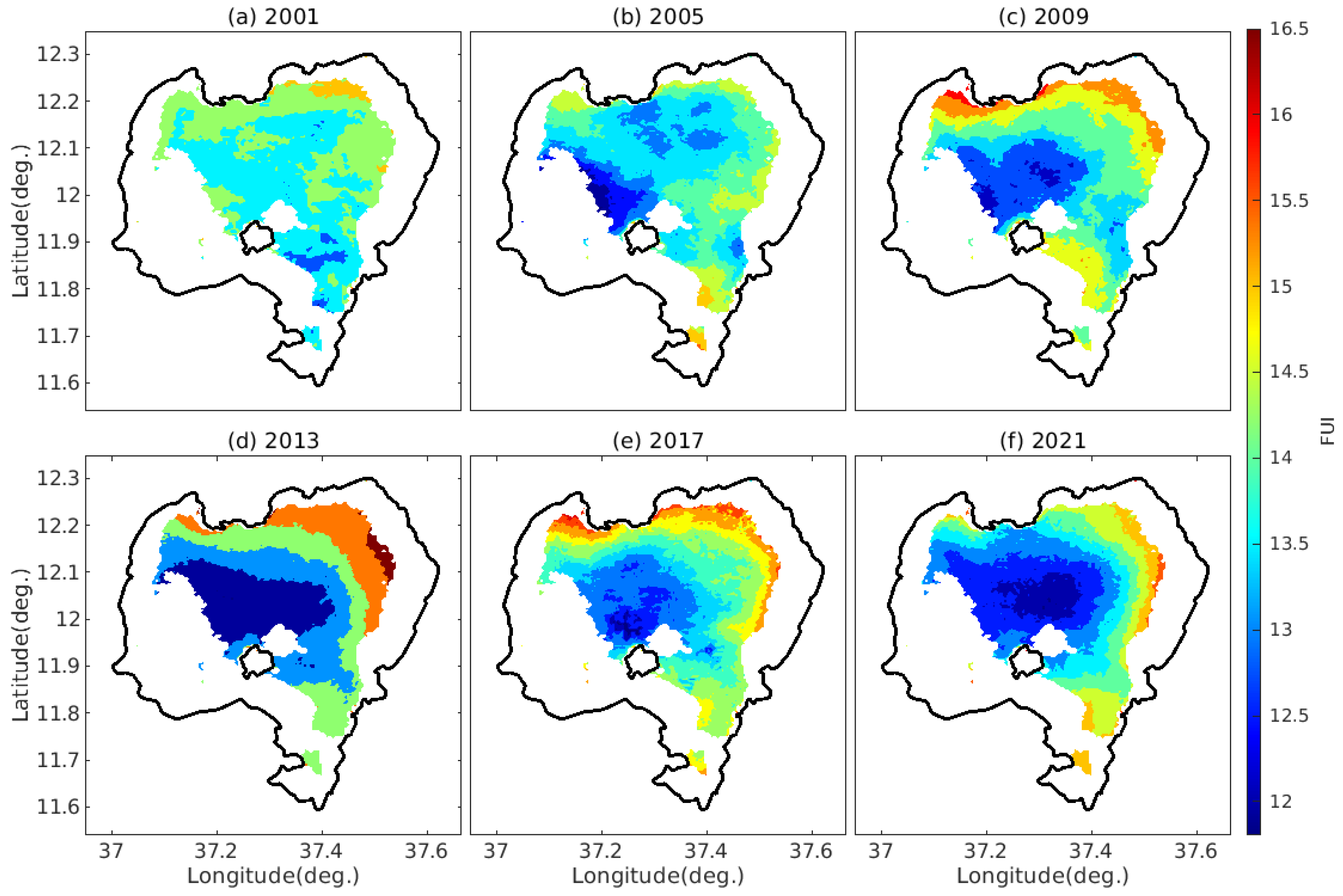
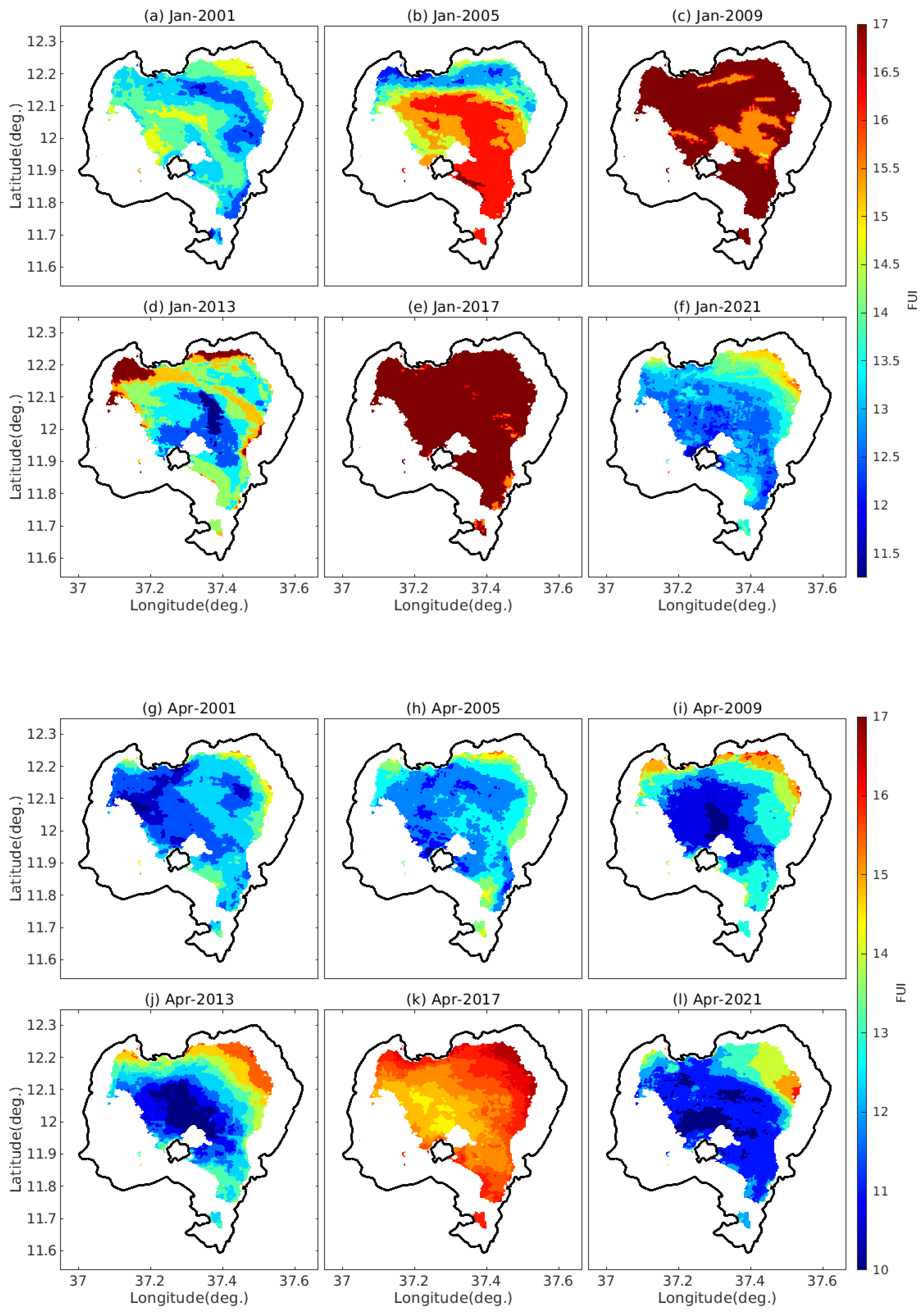
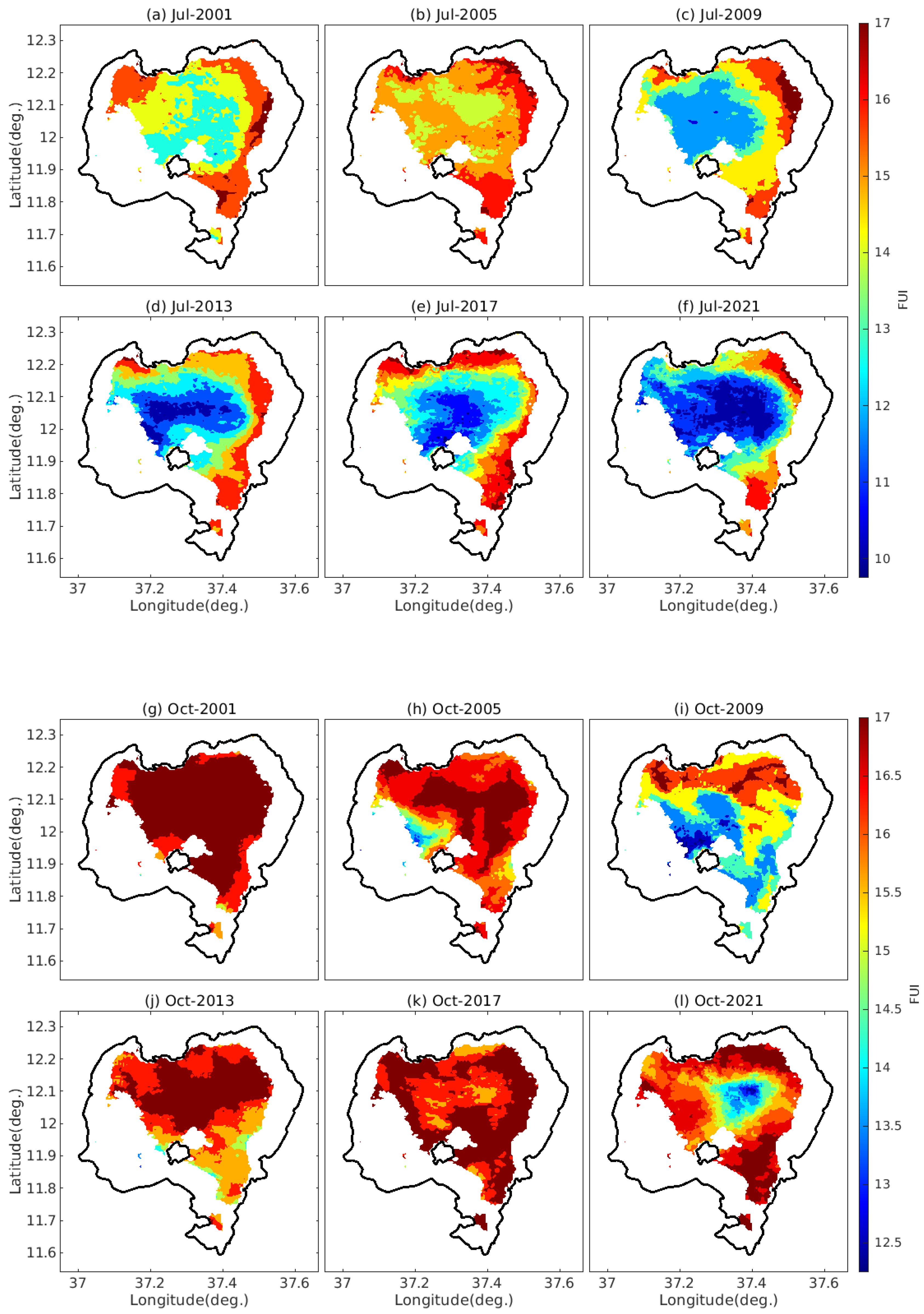
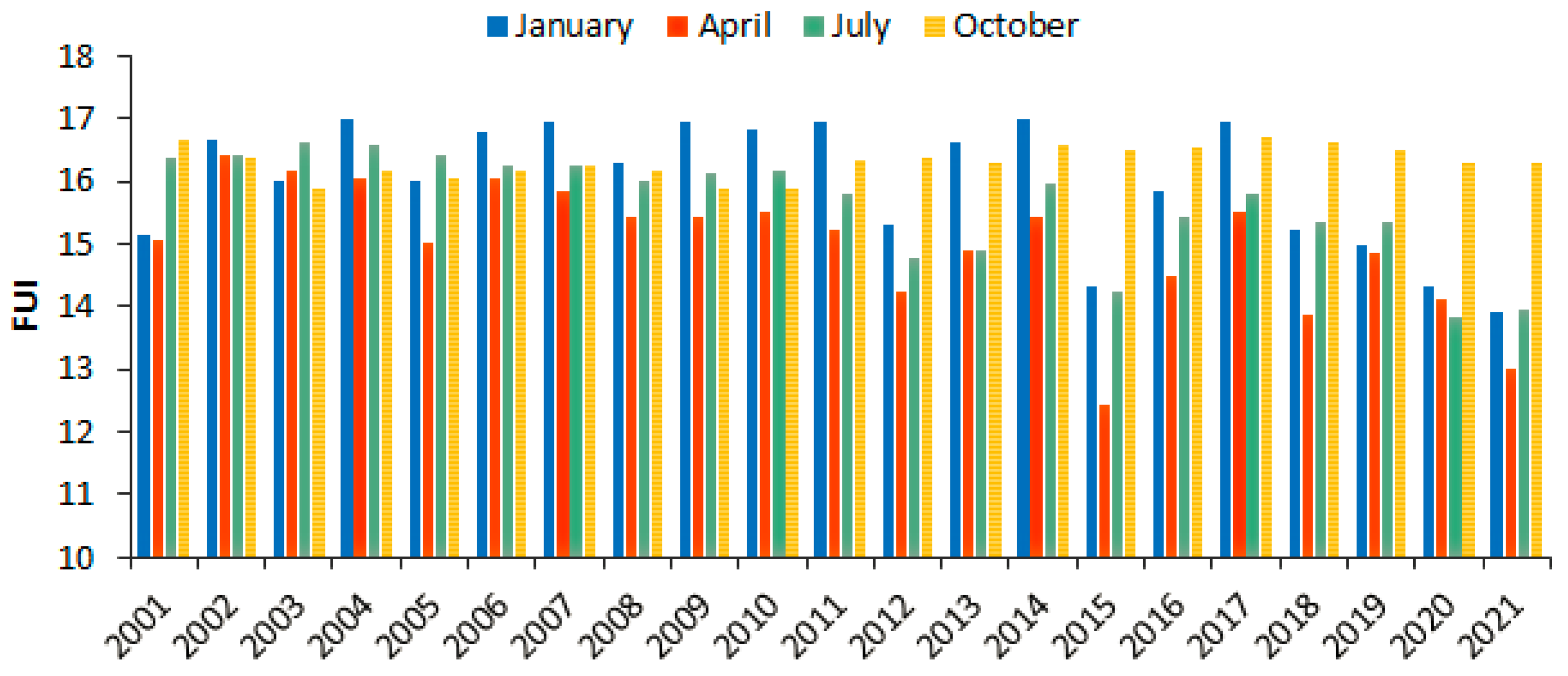
4.2. The Spatiotemporal Variability of FUI of Lake Tana’s Water
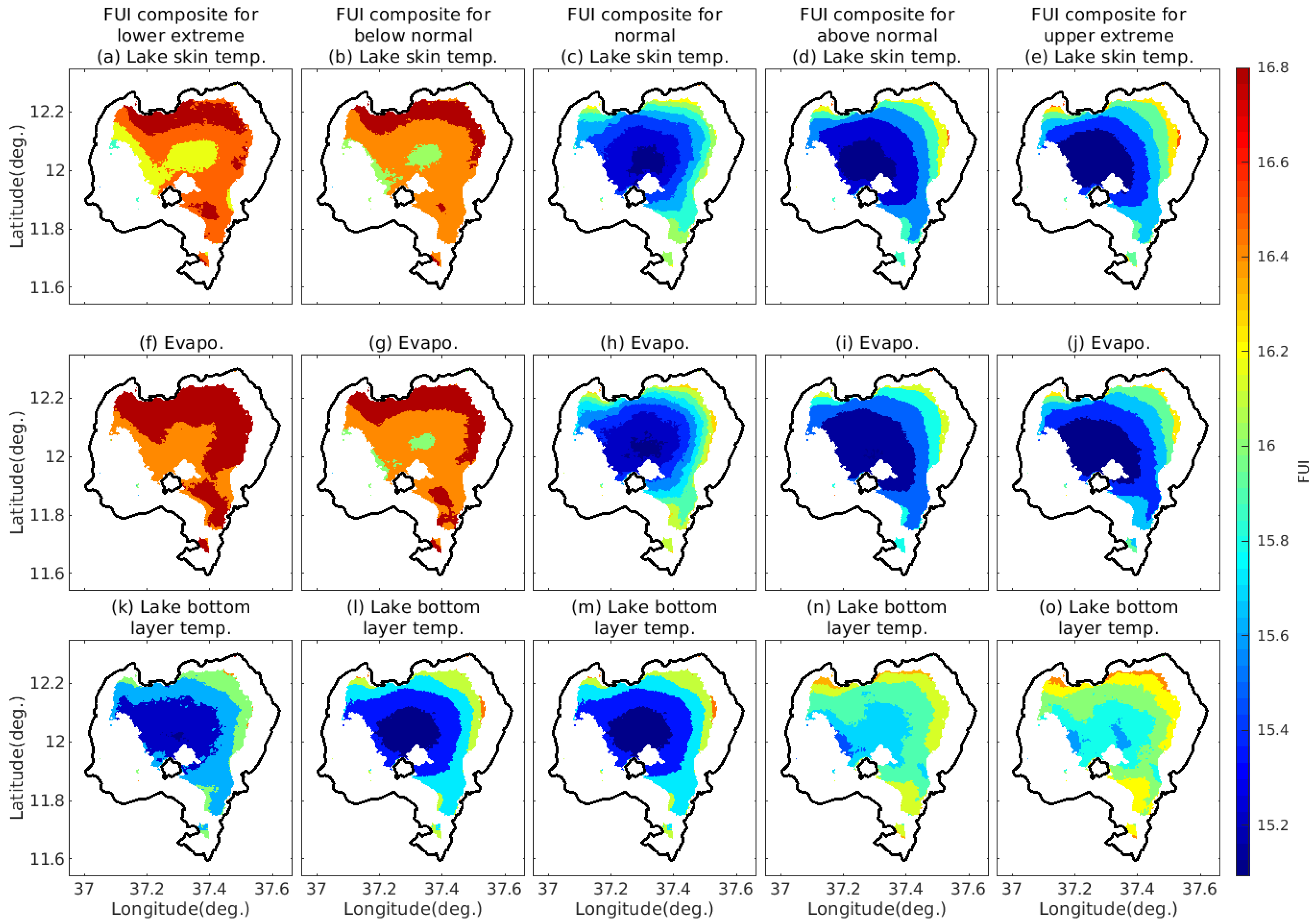
4.3. FUI Composites over Lake Tana under Different Directions of Changes of the Deriving Factors
4.3.1. FUI Composites under Different Ranges of Temperature and Evaporation
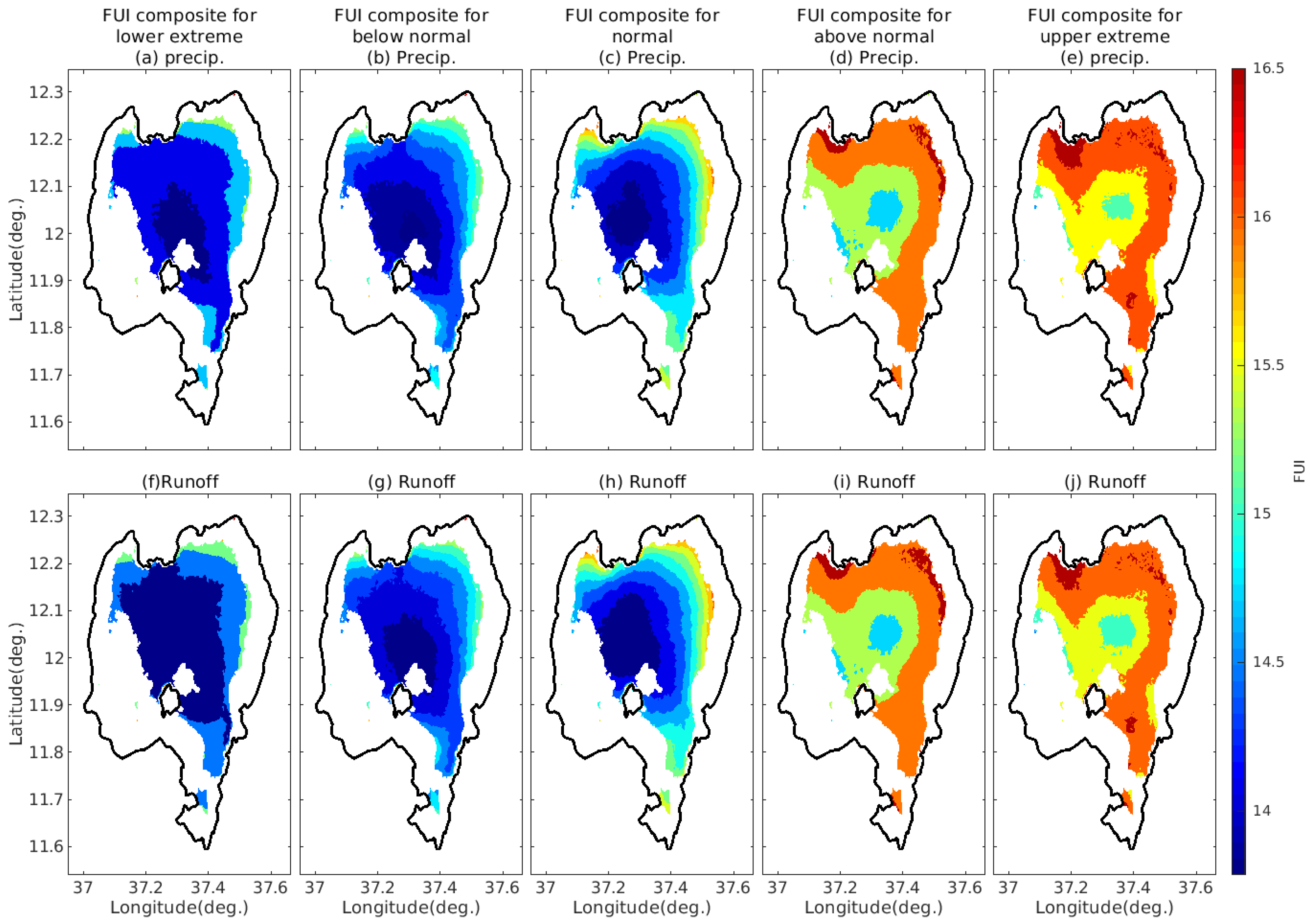
4.3.2. FUI Composites under Different Ranges of Precipitation and Surface Runoff
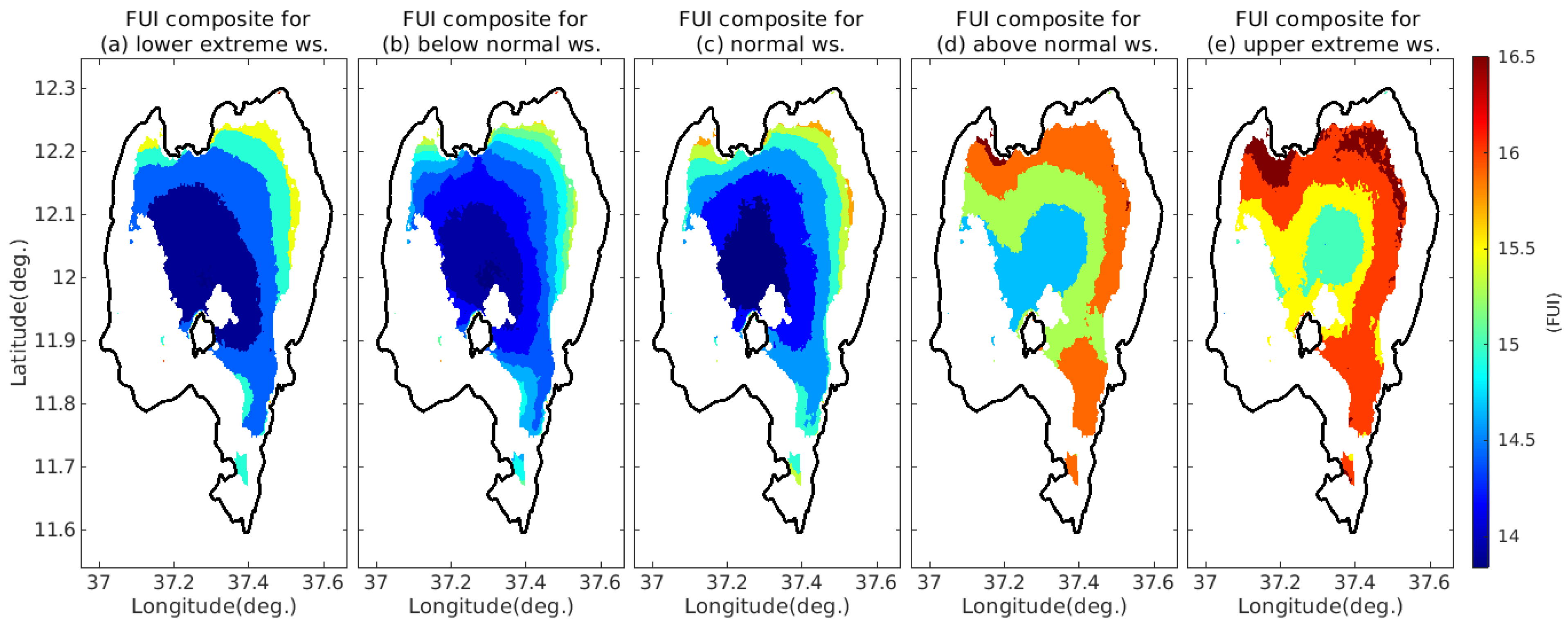
4.3.3. FUI Composites under Different Wind Speed Ranges
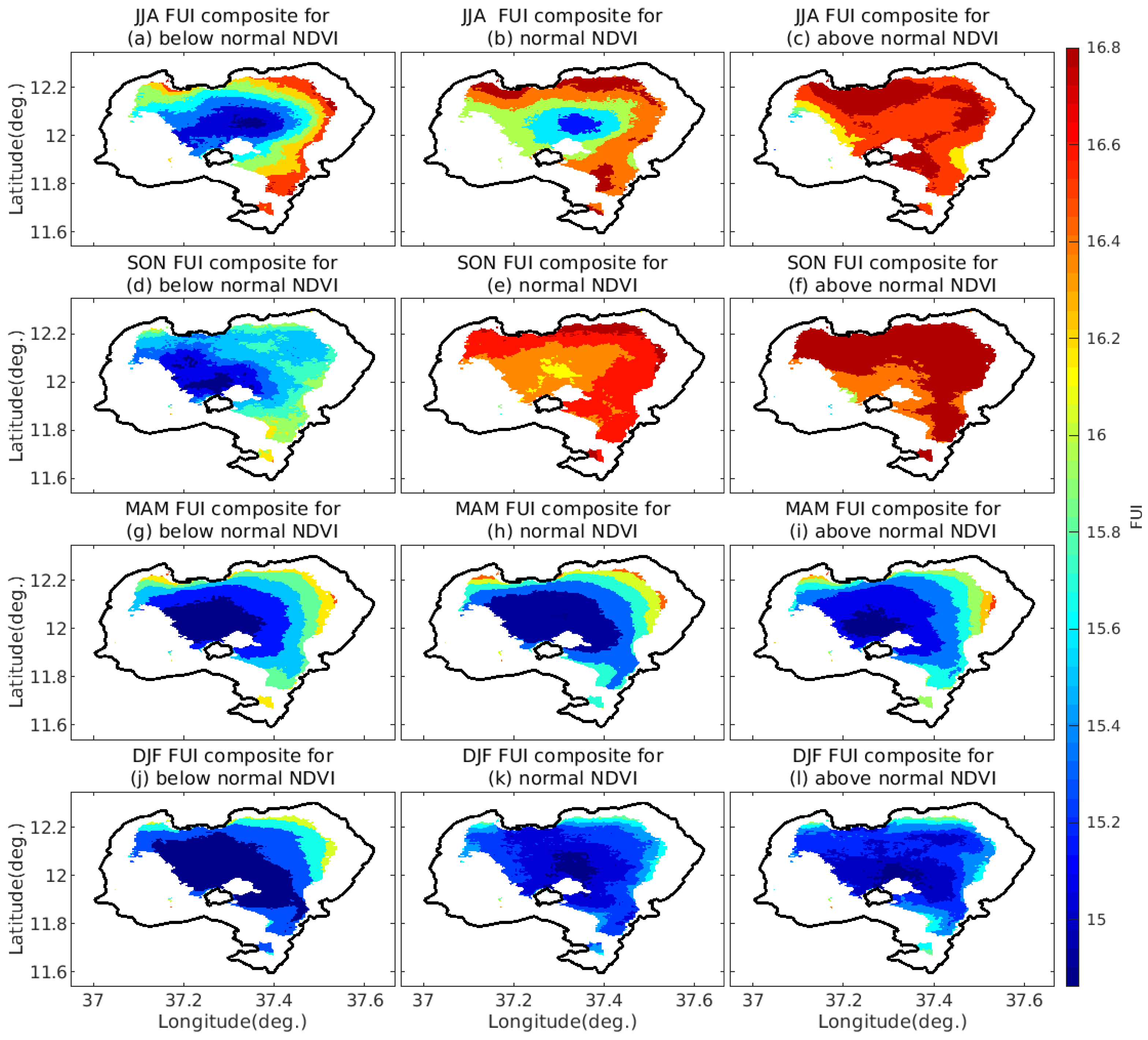
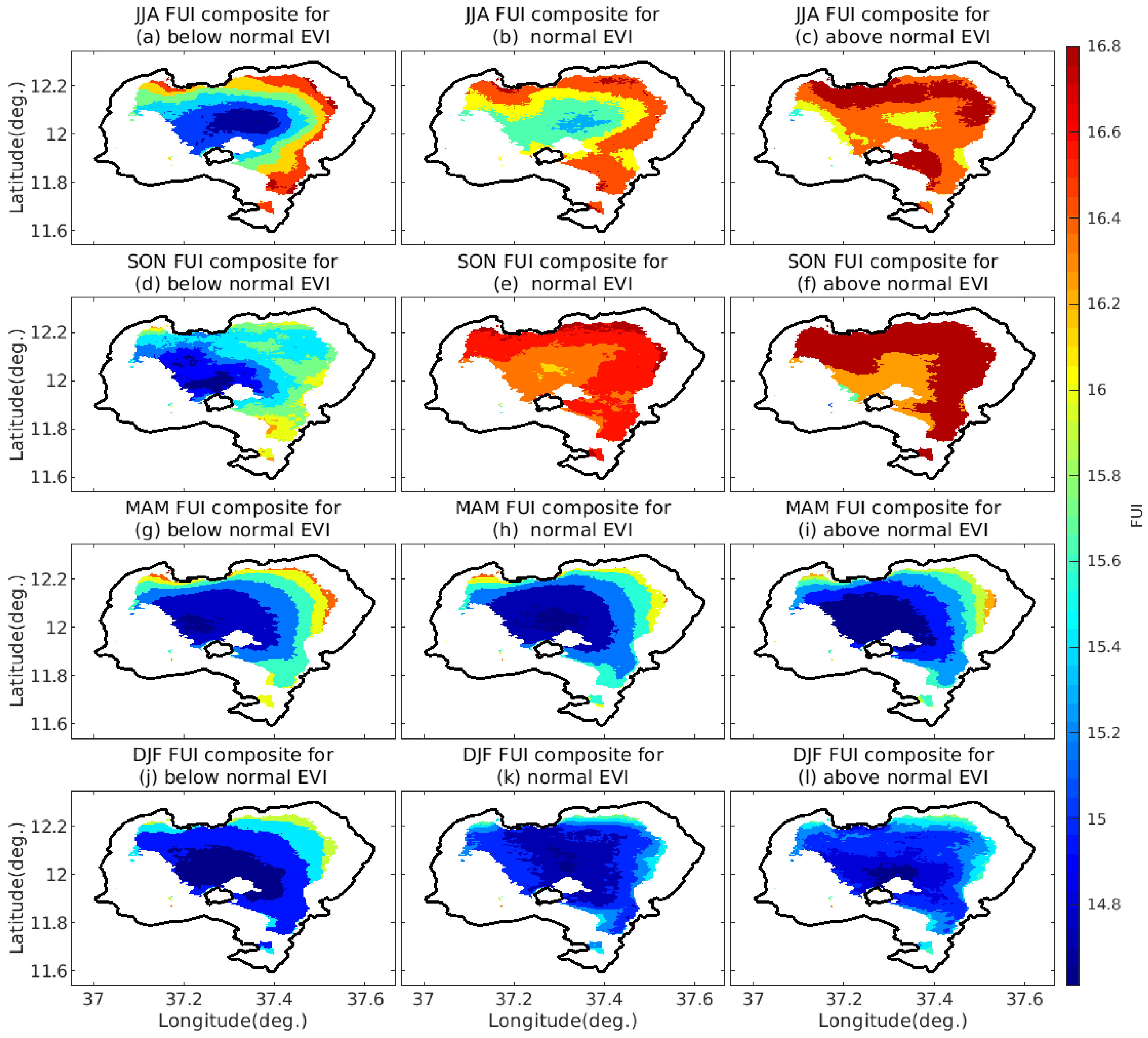
4.3.4. FUI Composites under Different Ranges of NDVI and EVI
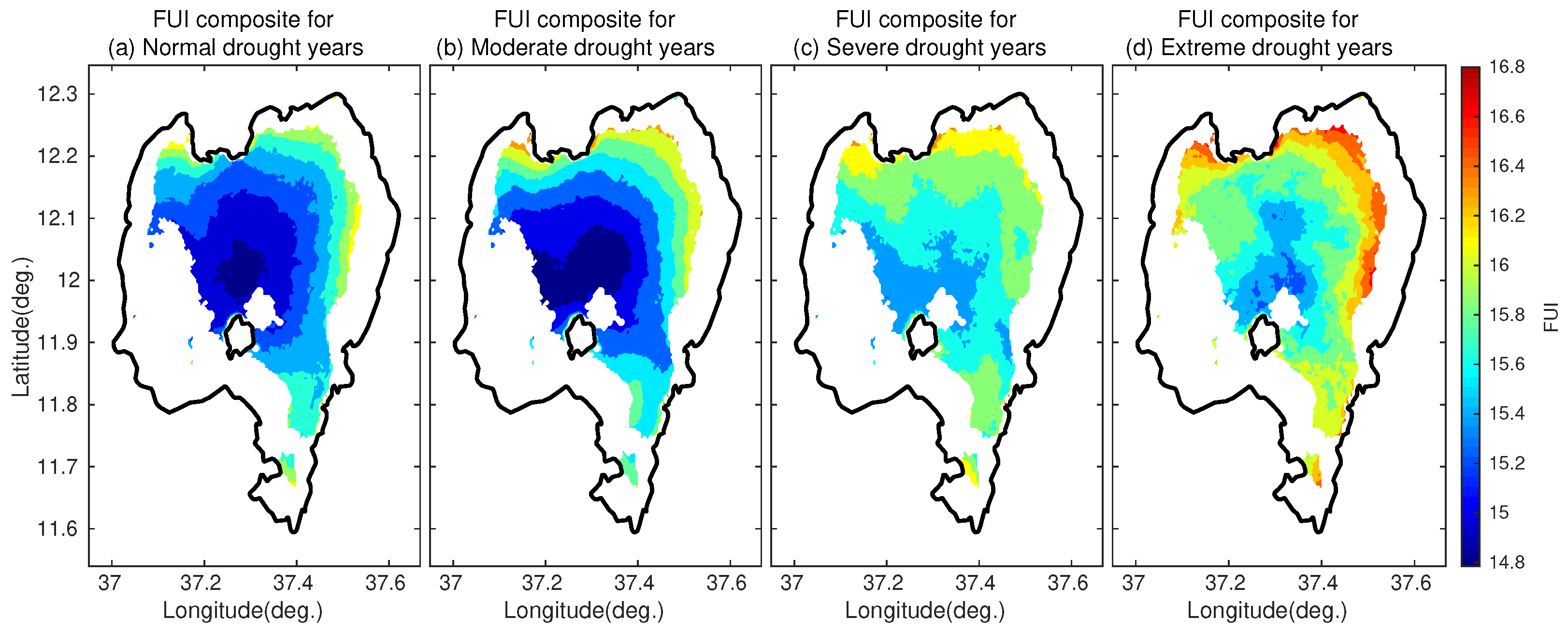
4.3.5. FUI Composites under Different Classes of Drought Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BUST | proportion of errors |
| Chl | chlorophyll a |
| CDOM | colored dissolved organic matter |
| CS | Copernicus Climate Change Service |
| CRU | Climatic Research Unit |
| C.I.E | Commission on Illumination |
| CSI | critical success index |
| CM | categorical miss |
| CA | composite analysis |
| DJF | December, January, February |
| DO | dissolved oxygen |
| ESA | European Space Agency |
| ECMWF | European Center for Medium-Range Weather Forecasts |
| EVI | enhanced vegetation index |
| FUI | Forel–Ule index |
| FBI | Frequency bias index |
| FUME | Forel–Ule MERIS |
| FUB | Free University of Berlin |
| FAR | false alarm ratio |
| JJA | June, July, August |
| LULC | land-use–land-cover |
| MERIS | medium resolution imaging spectrometer |
| MODIS | moderate resolution imaging spectroradiometer |
| MSS | multi-spectral scanner |
| MAM | March, April, May, |
| NDVI | normalized difference vegetation index |
| NASA | National Aeronautics and Space Administration |
| NCEP | National Centers for Environmental Prediction |
| NCAR | National Center for Atmospheric Research |
| NPS | non-point source |
| OAC | optically active component |
| OECD | Organization for Economic Cooperation and Development |
| POD | probability of detection |
| PC | percent correct |
| R | remote-sensing reflectance |
| RGB | red, green, blue |
| REL | reliability |
| SDD | Secchi disk depth |
| SPEI | standardized precipitation evapotranspiration index |
| SON | September, October, November |
| TSI | trophic state index |
| TP | total phosphorus |
| TN | total nitrogen |
| TSM | total suspended matter |
References
- Kondratyev, K.Y.; Pozdnyakov, D.; Pettersson, L. Water quality remote sensing in the visible spectrum. Int. J. Remote Sens. 1998, 19, 957–979. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Zhang, F.; Shen, Q.; Li, J.; Yang, F. Remote Sensing-Based Analysis of Spatial and Temporal Water Colour Variations in Baiyangdian Lake after the Establishment of the Xiong’an New Area. Remote Sens. 2021, 13, 1729. [Google Scholar] [CrossRef]
- Gallie, E.A.; Murtha, P. A modification of chromaticity analysis to separate the effects of water quality variables. Remote Sens. Environ. 1993, 44, 47–65. [Google Scholar] [CrossRef]
- Sathyendranath, S. Remote Sensing of Ocean Colour in Coastal, and other Optically-Complex, Waters; International Ocean Colour Coordinating Group: Dartmouth, NS, Canada, 2000. [Google Scholar] [CrossRef]
- Pan, D.; Ma, R. Several key problems of lake water quality remote sensing. J. Lake Sci. 2008, 20, 139–144. [Google Scholar] [CrossRef]
- Prieur, L.; Sathyendranath, S. An optical classification of coastal and oceanic waters based on the specific spectral absorption curves of phytoplankton pigments, dissolved organic matter, and other particulate materials 1. Limnol. Oceanogr. 1981, 26, 671–689. [Google Scholar] [CrossRef]
- Lesht, B.M.; Barbiero, R.P.; Warren, G.J. Satellite ocean color algorithms: A review of applications to the Great Lakes. J. Great Lakes Res. 2012, 38, 49–60. [Google Scholar] [CrossRef]
- Yang, W.; Matsushita, B.; Chen, J.; Fukushima, T. Estimating constituent concentrations in case II waters from MERIS satellite data by semi-analytical model optimizing and look-up tables. Remote Sens. Environ. 2011, 115, 1247–1259. [Google Scholar] [CrossRef]
- Ma, R.; Pan, D.; Duan, H.; Song, Q. Absorption and scattering properties of water body in Taihu Lake, China: Backscattering. Int. J. Remote Sens. 2009, 30, 2321–2335. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Shen, Q.; Zhang, B.; Zhang, F.; Lu, Z. MODIS-based radiometric color extraction and classification of inland water with the Forel–Ule scale: A case study of Lake Taihu. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 8, 907–918. [Google Scholar] [CrossRef]
- Chen, J.; Quan, W.; Zhang, M.; Cui, T. A simple atmospheric correction algorithm for MODIS in shallow turbid waters: A case study in Taihu Lake. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 6, 1825–1833. [Google Scholar] [CrossRef]
- Wang, M.; Shi, W. The NIR-SWIR combined atmospheric correction approach for MODIS ocean color data processing. Opt. Express 2007, 15, 15722–15733. [Google Scholar] [CrossRef] [PubMed]
- Barysheva, L. On the issue of intercorrespondence of color scales used in limnology. Remote Monit. Large Lakes 1987, 60–65. [Google Scholar]
- Bukata, R.P.; Jerome, J.H.; Kondratyev, K.Y.; Pozdnyakov, D.V.; Kotykhov, A.A. Modelling the radiometric color of inland waters: Implications to a) remote sensing and b) limnological color scales. J. Great Lakes Res. 1997, 23, 254–269. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of variations in ocean color 1. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Smith, R.C.; Baker, K.S. Optical properties of the clearest natural waters (200–800 nm). Appl. Opt. 1981, 20, 177–184. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, B.; Spyrakos, E.; Tyler, A.N.; Shen, Q.; Zhang, F.; Kuster, T.; Lehmann, M.K.; Wu, Y.; et al. Trophic state assessment of global inland waters using a MODIS-derived Forel–Ule index. Remote Sens. Environ. 2018, 217, 444–460. [Google Scholar] [CrossRef]
- Wernand, M.; Hommersom, A.; van der Woerd, H.J. MERIS-based ocean colour classification with the discrete Forel–Ule scale. Ocean Sci. 2013, 9, 477–487. [Google Scholar] [CrossRef]
- Woerd, H.J.; Wernand, M.R. True colour classification of natural waters with medium-spectral resolution satellites: SeaWiFS, MODIS, MERIS and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef]
- Van der Woerd, H.J.; Wernand, M.R. Hue-angle product for low to medium spatial resolution optical satellite sensors. Remote Sens. 2018, 10, 180. [Google Scholar] [CrossRef]
- Alföldi, T.; Munday, J., Jr. Water quality analysis by digital chromaticity mapping of Landsat data. Can. J. Remote Sens. 1978, 4, 108–126. [Google Scholar] [CrossRef]
- Jerome, J.H. Colours of natural waters: 1. Factors controlling the dominant wavelength. Northwest Sci. 1994, 68, 43–52. [Google Scholar]
- Wernand, M.R. Poseidon’s Paintbox: Historical Archives of Ocean Colour in Global-Change Perspective. Ph.D. Thesis, University Utrecht, Utrecht, The Netherlands, 2011. [Google Scholar]
- Wang, S.; Li, J.; Zhang, W.; Cao, C.; Zhang, F.; Shen, Q.; Zhang, X.; Zhang, B. A dataset of remote-sensed Forel–Ule Index for global inland waters during 2000–2018. Sci. Data 2021, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valjarević, A.; Popovici, C.; Djekić, T.; Morar, C.; Filipović, D.; Lukić, T. Long-term monitoring of high optical imagery of the stratospheric clouds and their properties new approaches and conclusions. Egypt. J. Remote Sens. Space Sci. 2022, 25, 1037–1043. [Google Scholar] [CrossRef]
- Carroll, M.L.; DiMiceli, C.M.; Townshend, J.; Sohlberg, R.A.; Elders, A.; Devadiga, S.; Sayer, A.M.; Levy, R. Development of an operational land water mask for MODIS Collection 6, and influence on downstream data products. Int. J. Digit. Earth 2017, 10, 207–218. [Google Scholar] [CrossRef]
- Gezie, A.; Assefa, W.W.; Getnet, B.; Anteneh, W.; Dejen, E.; Mereta, S.T. Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Biol. Invas. 2018, 20, 2517–2534. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Kibret, A.A.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Dagnew, D.C.; Abebe, W.B.; Melesse, A.M. Potential of water hyacinth infestation on Lake Tana, Ethiopia: A prediction using a GIS-based multi-criteria technique. Water 2019, 11, 1921. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Melesse, A.M.; Tilahun, S.A.; Abate, M.; Dagnew, D.C. Water hyacinth: Review of its impacts on hydrology and ecosystem services—Lessons for management of Lake Tana. Extrem. Hydrol. Clim. Var. 2019, 237–251. [Google Scholar] [CrossRef]
- Enyew, B.G.; Assefa, W.W.; Gezie, A. Socioeconomic effects of water hyacinth (Echhornia Crassipes) in Lake Tana, North Western Ethiopia. PLoS ONE 2020, 15, e0237668. [Google Scholar] [CrossRef]
- Worqlul, A.W.; Ayana, E.K.; Dile, Y.T.; Moges, M.A.; Dersseh, M.G.; Tegegne, G.; Kibret, S. Spatiotemporal dynamics and environmental controlling factors of the Lake Tana water hyacinth in Ethiopia. Remote Sens. 2020, 12, 2706. [Google Scholar] [CrossRef]
- Goshu, G.; Koelmans, A.A. and de Klein, J.J.M. Water Quality of Lake Tana Basin, Upper Blue Nile, Ethiopia. A Review of Available Data. In Social and Ecological System Dynamics: Characteristics, Trends, and Integration in the Lake Tana Basin, Ethiopia; Stave, K., Goshu, G., Aynalem, S., Eds.; Springer: Berlin, Germany, 2017; pp. 127–141. [Google Scholar]
- Kassa, Y.; Mengistu, S.; Wondie, A.; Tibebe, D. Distribution of macrophytes in relation to physico-chemical characters in the south western littoral zone of Lake Tana, Ethiopia. Aquat. Bot. 2021, 170, 103351. [Google Scholar] [CrossRef]
- Ayele, H.S.; Atlabachew, M. Review of characterization, factors, impacts, and solutions of Lake eutrophication: Lesson for Lake Tana, Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 14233–14252. [Google Scholar] [CrossRef] [PubMed]
- Eshete, G.D.; Asitatikie, A.N.; Almnewu, H.N.; Belew, A.Z. Analysis of the spatial and temporal variability of direct rainfall in Lake Tana, Ethiopia. Appl. Water Sci. 2022, 12. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Steenhuis, T.S.; Kibret, A.A.; Eneyew, B.M.; Kebedew, M.G.; Zimale, F.A.; Worqlul, A.W.; Moges, M.A.; Abebe, W.B.; Mhiret, D.A.; et al. Water Quality Characteristics of a Water Hyacinth Infested Tropical Highland Lake: Lake Tana, Ethiopia. Front. Water 2022, 4, 774710. [Google Scholar] [CrossRef]
- Mucheye, T.; Haro, S.; Papaspyrou, S.; Caballero, I. Water Quality and Water Hyacinth Monitoring with the Sentinel-2A/B Satellites in Lake Tana (Ethiopia). Remote Sens. 2022, 14, 4921. [Google Scholar] [CrossRef]
- Kebedew, M.G.; Kibret, A.A.; Tilahun, S.A.; Belete, M.A.; Zimale, F.A.; Steenhuis, T.S. The Relationship of Lake Morphometry and Phosphorus Dynamics of a Tropical Highland Lake: Lake Tana, Ethiopia. Water 2020, 12, 2243. [Google Scholar] [CrossRef]
- Kebedew, M.G.; Tilahun, S.A.; Zimale, F.A.; Steenhuis, T.S. Bottom Sediment Characteristics of a Tropical Lake: Lake Tana, Ethiopia. Hydrology 2020, 7, 18. [Google Scholar] [CrossRef]
- Vijverberg, J.; Sibbing, F.A.; Dejen, E. Lake Tana: Source of the blue nile. In The Nile; Springer: Berlin, Germany, 2009; pp. 163–192. [Google Scholar] [CrossRef]
- Mesfin, W.M. An Introductory Geography of Ethiopia; Berhanena Selam HSI Printing Press: Addis Ababa, Ethiopia, 1972. [Google Scholar]
- Kebede, S.; Travi, Y.; Alemayehu, T.; Marc, V. Impact of rainfall variation and water resources development on Lake Tana level and its blue Nile flow. J. Hydrol. 2006, 316, 233–247. [Google Scholar] [CrossRef]
- Minale, A.S. Water level fluctuations of Lake Tana and its implication on local communities livelihood, northwestern Ethiopia. Int. J. River Basin Manag. 2020, 18, 503–510. [Google Scholar] [CrossRef]
- Arsiso, B.K.; Tsidu, G.M. Impacts of land-use–land-cover change on land surface temperature over Lake Tana basin.
- Bires, Z.; Raj, S. Determinants of environmental conservation in Lake Tana Biosphere Reserve, Ethiopia. Heliyon 2019, 5, e01997. [Google Scholar] [CrossRef]
- Wondie, A.; Mengistou, S. Seasonal variability of secondary production of cladocerans and rotifers, and their trophic role in Lake Tana, Ethiopia, a large, turbid, tropical highland lake. Afr. J. Aquat. Sci. 2014, 39, 403–416. [Google Scholar] [CrossRef]
- Minale, A.S. Retrospective analysis of land cover and use dynamics in Gilgel Abbay Watershed by using GIS and remote sensing techniques, Northwestern Ethiopia. Int. J. Geosci. 2013, 4, 1003–1008. [Google Scholar] [CrossRef]
- Minale, A.S.; Rao, K.K. Hydrological dynamics and human impact on ecosystems of Lake Tana, northwestern Ethiopia. Ethiop. J. Environ. Stud. Manag. 2011, 4, 1. [Google Scholar] [CrossRef]
- Vermote, E.; Vermeulen, A. Atmospheric correction algorithm: Spectral reflectances (MOD09). ATBD Version 1999, 4, 1–107. [Google Scholar]
- Hou, X.; Feng, L.; Duan, H.; Chen, X.; Sun, D.; Shi, K. Fifteen-year monitoring of the turbidity dynamics in large lakes and reservoirs in the middle and lower basin of the Yangtze River, China. Remote Sens. Environ. 2017, 190, 107–121. [Google Scholar] [CrossRef]
- Klein, I.; Gessner, U.; Dietz, A.J.; Kuenzer, C. Global WaterPack–A 250 m resolution dataset revealing the daily dynamics of global inland water bodies. Remote Sens. Environ. 2017, 198, 345–362. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Wu, Y.; Zhang, B.; Chen, X.; Zhang, F.; Shen, Q.; Peng, D.; Tian, L. MODIS observations of water color of the largest 10 lakes in China between 2000 and 2012. Int. J. Digit. Earth 2016, 9, 788–805. [Google Scholar] [CrossRef]
- Wu, G.; Cui, L.; He, J.; Duan, H.; Fei, T.; Liu, Y. Comparison of MODIS-based models for retrieving suspended particulate matter concentrations in Poyang Lake, China. Int. J. Appl. Earth Obs. Geoinf. 2013, 24, 63–72. [Google Scholar] [CrossRef]
- Odermatt, D.; Danne, O.; Philipson, P.; Brockmann, C. Diversity II water quality parameters from ENVISAT (2002–2012): A new global information source for lakes. Earth Syst. Sci. Data 2018, 10, 1527–1549. [Google Scholar] [CrossRef]
- Carlson, R.E. A trophic state index for lakes 1. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, Y.; Dong, R. A Universal Fuzzy Logic Optical Water Type Scheme for the Global Oceans. Remote Sens. 2021, 13, 4018. [Google Scholar] [CrossRef]
- Mu noz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A state-of-the-art global reanalysis dataset for land applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Assamnew, A.D.; Tsidu, G.M. Assesing improvement in the fifth-generation ECMWF atmospheric reanalysis (ERA5) precipitation over East Africa. Int. J. Climatol. 2022, 43, 17–37. [Google Scholar] [CrossRef]
- Jarchow, C.J.; Didan, K.; Barreto-Mu noz, A.; Nagler, P.L.; Glenn, E.P. Application and comparison of the MODIS-derived enhanced vegetation index to VIIRS, landsat 5 TM and landsat 8 OLI platforms: A case study in the arid colorado river delta, Mexico. Sensors 2018, 18, 1546. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.J.; Tanré, D. Algorithm for remote sensing of tropospheric aerosol from MODIS. NASA MODIS Algorithm Theor. Basis Doc. Goddard Space Flight Cent. 1998, 85, 3–68. [Google Scholar]
- Vermote, E.; Kotchenova, S.; Ray, J. MODIS Surface Reflectance User’s Guide, Version 1.3. In MODIS Land Surface Reflectance Science Computing Facility; NASA: Washington, DC, USA, 2011. [Google Scholar]
- Shenglei, W.; Junsheng, L.; Bing, Z.; Qian, S.; Fangfang, Z.; Zhaoyi, L. A simple correction method for the MODIS surface reflectance product over typical inland waters in China. Int. J. Remote Sens. 2016, 37, 6076–6096. [Google Scholar] [CrossRef]
- Cie, C. Commission Internationale de l’Eclairage Proceedings, 1931; Cambridge University: Cambridge, CA, USA, 1932. [Google Scholar]
- Wang, S.; Li, J.; Zhang, B.; Lee, Z.; Spyrakos, E.; Feng, L.; Liu, C.; Zhao, H.; Wu, Y.; Zhu, L.; et al. Changes of water clarity in large lakes and reservoirs across China observed from long-term MODIS. Remote Sens. Environ. 2020, 247, 111949. [Google Scholar] [CrossRef]
- Wagh, P.; Sojan, J.M.; Babu, S.J.; Valsala, R.; Bhatia, S.; Srivastav, R. Indicative lake water quality assessment using remote sensing images-effect of COVID-19 lockdown. Water 2020, 13, 73. [Google Scholar] [CrossRef]
- White, E. Lake eutrophication in New Zealand—A comparison with other countries of the Organisation for Economic Co-operation and Development. N. Z. J. Mar. Freshw. Res. 1983, 17, 437–444. [Google Scholar] [CrossRef]
- Li, H.E.; Lee, J.H.W.; Cai, M. Nutrient load estimation methods for rivers. Int. J. Sediment Res. 2003, 18, 346. [Google Scholar]
- Kolo, B.; Ogugbuaja, V.; Dauda, M. Study on the level of sulphates, phosphates, and nitrates in water and aqueous sediments of Lake Chad Basin Area of Borno State, Nigeria. Cont. J. Water Air Soil Pollut. 2010, 1, 13–18. [Google Scholar]
- Mainstone, C.P.; Parr, W. Phosphorus in rivers—Ecology and management. Sci. Total Environ. 2002, 282, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Tibebe, D.; Kassa, Y.; Melaku, A.; Lakew, S. Investigation of spatio-temporal variations of selected water quality parameters and trophic status of Lake Tana for sustainable management, Ethiopia. Microchem. J. 2019, 148, 374–384. [Google Scholar] [CrossRef]
- Yang, X.e.; Wu, X.; Hao, H.l.; He, Z.l. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Beeton, A.M.; Edmondson, W.T. The eutrophication problem. J. Fish. Board Can. 1972, 29, 673–682. [Google Scholar] [CrossRef]
- Bigham Stephens, D.L.; Carlson, R.E.; Horsburgh, C.A.; Hoyer, M.V.; Bachmann, R.W.; Canfield, D.E., Jr. Regional distribution of Secchi disk transparency in waters of the United States. Lake Reserv. Manag. 2015, 31, 55–63. [Google Scholar] [CrossRef]
- Rodhe, W. Crystallization of eutrophication concepts in northern Europe. In Proceedings of the Symposium on Eutrophication: Causes, Consequences, Correctives; National Academy of Sciences: Washington, DC, USA, 1969. [Google Scholar] [CrossRef]
- Bryers, G.; Bowman, E. Protocol for Monitoring Trophic Levels of New Zealand Lakes and Reservoirs; Number 99/2, Lakes Consulting, Report; Ministry for the Environment: Wellington, New Zealand, 2000.
- Forsberg, C. Eutrophication parameters and trophic state indices in 30 Swedish waste-receiving lakes. Arch. Fur Hydrobiol. 1980, 89, 189–207. [Google Scholar] [CrossRef]
- Carlson, R.E. Expanding the trophic state concept to identify non-nutrient limited lakes and reservoirs. Enhancing States’S Lake Manag. Programs 1991, 59–71. [Google Scholar]
- Joniak, T.; Nagengast, B.; Kuczyńska-Kippen, N. Can popular systems of trophic classification be used for small water bodies? Oceanol. Hydrobiol. Stud. 2009, 38, 145–151. [Google Scholar] [CrossRef]
- Sheela, A.; Letha, J.; Joseph, S.; Ramachandran, K. Trophic state index of a lake system using IRS (P6-LISS III) satellite imagery. Environ. Monit. Assess. 2011, 177, 575–592. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, A.; Tian, Q. Weighted composite analysis and its application: An example using ENSO and geopotential height. Atmos. Sci. Lett. 2017, 18, 435–440. [Google Scholar] [CrossRef]
- Chree, C. III. Some phenomena of sunspots and of terrestrial magnetism at Kew Observatory. Philos. Trans. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1913, 212, 75–116. [Google Scholar] [CrossRef]
- Chree, C. VI. Some phenomena of sunspots and of terrestrial magnetism.—Part II. Philos. Trans. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1914, 213, 245–277. [Google Scholar] [CrossRef]
- Kosaka, Y.; Nakamura, H. Mechanisms of meridional teleconnection observed between a summer monsoon system and a subtropical anticyclone. Part I: The Pacific–Japan pattern. J. Clim. 2010, 23, 5085–5108. [Google Scholar] [CrossRef]
- Fogt, R.L.; Bromwich, D.H.; Hines, K.M. Understanding the SAM influence on the South Pacific ENSO teleconnection. Clim. Dyn. 2011, 36, 1555–1576. [Google Scholar] [CrossRef]
- Laken, B.A.; Pallé, E.; Čalogović, J.; Dunne, E.M. A cosmic ray-climate link and cloud observations. J. Space Weather. Space Clim. 2012, 2, A18. [Google Scholar] [CrossRef]
- Vollenweider, R.; Kerekes, J. Eutrophication of Waters. Monitoring, Assessment and Control; Organization for Economic Co-Operation and Development (OECD): Paris, France, 1982; Volume 156. [Google Scholar]
- Barki, D.N.; Singa, P. Assessment of trophic state of lakes in terms of Carlson’s trophic state index. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 14297–14302. [Google Scholar]
- Peperzak, L. Climate change and harmful algal blooms in the North Sea. Acta Oecol. 2003, 24, S139–S144. [Google Scholar] [CrossRef]
- Chung, E.G.; Bombardelli, F.A.; Schladow, S.G. Modeling linkages between sediment resuspension and water quality in a shallow, eutrophic, wind-exposed lake. Ecol. Model. 2009, 220, 1251–1265. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Valdemarsen, T.; Quintana, C.O.; Flindt, M.R.; Kristensen, E. Organic N and P in eutrophic fjord sediments–rates of mineralization and consequences for internal nutrient loading. Biogeosciences 2015, 12, 1765–1779. [Google Scholar] [CrossRef]
- Trolle, D.; Hamilton, D.P.; Pilditch, C.A.; Duggan, I.C.; Jeppesen, E. Predicting the effects of climate change on trophic status of three morphologically varying lakes: Implications for lake restoration and management. Environ. Model. Softw. 2011, 26, 354–370. [Google Scholar] [CrossRef]
- Feng, W.; Zhan, J.; Deng, X. Influencing factors of lake eutrophication in China–A case study in 22 lakes in China. Ecol. Environ. Sci. 2012, 21, 94–100. [Google Scholar]
- Joehnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Su, Z.; Lin, C.; Ma, R.; Luo, J.; Liang, Q. Effect of land use change on lake water quality in different buffer zones. Appl. Ecol. Environ. Res. 2015, 13, 489–503. [Google Scholar]
- Oh, J.; Sankarasubramanian, A. Interannual hydroclimatic variability and its influence on winter nutrient loadings over the Southeast United States. Hydrol. Earth Syst. Sci. 2012, 16, 2285–2298. [Google Scholar] [CrossRef]
- Zoppini, A.; Ademollo, N.; Bensi, M.; Berto, D.; Bongiorni, L.; Campanelli, A.; Casentini, B.; Patrolecco, L.; Amalfitano, S. Impact of a river flood on marine water quality and planktonic microbial communities. Estuar. Coast. Shelf Sci. 2019, 224, 62–72. [Google Scholar] [CrossRef]
- Ascott, M.; Lapworth, D.; Gooddy, D.; Sage, R.; Karapanos, I. Impacts of extreme flooding on riverbank filtration water quality. Sci. Total Environ. 2016, 554, 89–101. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Hounshell, A.G.; Luettich, R.A.; Rossignol, K.L.; Osburn, C.L.; Bales, J. Recent increase in catastrophic tropical cyclone flooding in coastal North Carolina, USA: Long-term observations suggest a regime shift. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Hutchins, M.; Harding, G.; Jarvie, H.; Marsh, T.; Bowes, M.; Loewenthal, M. Intense summer floods may induce prolonged increases in benthic respiration rates of more than one year leading to low river dissolved oxygen. J. Hydrol. 2020, 8, 100056. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Wilby, R.L.; Battarbee, R.W.; Kernan, M.; Wade, A.J. A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. 2009, 54, 101–123. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 202216. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W. Climate change impacts on the hydrological cycle. Ecohydrol. Hydrobiol. 2008, 8, 195–203. [Google Scholar] [CrossRef]
- Braga, F.; Zaggia, L.; Bellafiore, D.; Bresciani, M.; Giardino, C.; Lorenzetti, G.; Maicu, F.; Manzo, C.; Riminucci, F.; Ravaioli, M.; et al. Mapping turbidity patterns in the Po river prodelta using multi-temporal Landsat 8 imagery. Estuar. Coast. Shelf Sci. 2017, 198, 555–567. [Google Scholar] [CrossRef]
- Joshi, I.D.; D’Sa, E.J.; Osburn, C.L.; Bianchi, T.S. Turbidity in Apalachicola Bay, Florida from Landsat 5 TM and field data: Seasonal patterns and response to extreme events. Remote Sens. 2017, 9, 367. [Google Scholar] [CrossRef]
- Du, X.; Hendy, I.; Schimmelmann, A. A 9000-year flood history for Southern California: A revised stratigraphy of varved sediments in Santa Barbara Basin. Mar. Geol. 2018, 397, 29–42. [Google Scholar] [CrossRef]
- George, G.; Hurley, M.; Hewitt, D. The impact of climate change on the physical characteristics of the larger lakes in the English Lake District. Freshw. Biol. 2007, 52, 1647–1666. [Google Scholar] [CrossRef]
- Rui, X.; Zhi, C.; Yun, Z. Impact assessment of climate change on algal blooms by a parametric modeling study in Han River. J. Resour. Ecol. 2012, 3, 209–219. [Google Scholar] [CrossRef]
- Moore, M.T.; Pierce, J.R.; Farris, J.L. Water-quality analysis of an intensively used on-farm storage reservoir in the northeast Arkansas Delta. Arch. Environ. Contam. Toxicol. 2015, 69, 89–94. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, J.D.; Paz, J.O.; Tagert, M.L.M. Seasonal water quality changes in on-farm water storage systems in a south-central US agricultural watershed. Agric. Water Manag. 2017, 187, 131–139. [Google Scholar] [CrossRef]
- Ahearn, D.S.; Sheibley, R.W.; Dahlgren, R.A.; Anderson, M.; Johnson, J.; Tate, K.W. land-use–land-cover influence on water quality in the last free-flowing river draining the western Sierra Nevada, California. J. Hydrol. 2005, 313, 234–247. [Google Scholar] [CrossRef]
- Salvetti, R.; Acutis, M.; Azzellino, A.; Carpani, M.; Giupponi, C.; Parati, P.; Vale, M.; Vismara, R. Modelling the point and non-point nitrogen loads to the Venice Lagoon (Italy): The application of water quality models to the Dese-Zero basin. Desalination 2008, 226, 81–88. [Google Scholar] [CrossRef]
- Ouyang, W.; Wang, X.; Hao, F.; Srinivasan, R. Temporal-spatial dynamics of vegetation variation on non-point source nutrient pollution. Ecol. Model. 2009, 220, 2702–2713. [Google Scholar] [CrossRef]
- Foote, K.J.; Joy, M.K.; Death, R.G. New Zealand dairy farming: Milking our environment for all its worth. Environ. Manag. 2015, 56, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Puertas, J. Determination of COD, BOD, and suspended solids loads during combined sewer overflow (CSO) events in some combined catchments in Spain. Ecol. Eng. 2005, 24, 199–217. [Google Scholar] [CrossRef]
- Giri, S.; Qiu, Z. Understanding the relationship of land uses and water quality in twenty first century: A review. J. Environ. Manag. 2016, 173, 41–48. [Google Scholar] [CrossRef]
- Lintern, A.; Webb, J.; Ryu, D.; Liu, S.; Bende-Michl, U.; Waters, D.; Leahy, P.; Wilson, P.; Western, A. Key factors influencing differences in stream water quality across space. Wiley Interdiscip. Rev. Water 2018, 5, e1260. [Google Scholar] [CrossRef]
- Gámez, T.E.; Benton, L.; Manning, S.R. Observations of two reservoirs during a drought in central Texas, USA: Strategies for detecting harmful algal blooms. Ecol. Indic. 2019, 104, 588–593. [Google Scholar] [CrossRef]
- Jones, E.; van Vliet, M.T. Drought impacts on river salinity in the southern US: Implications for water scarcity. Sci. Total Environ. 2018, 644, 844–853. [Google Scholar] [CrossRef]
- Momblanch, A.; Paredes-Arquiola, J.; Munné, A.; Manzano, A.; Arnau, J.; Andreu, J. Managing water quality under drought conditions in the Llobregat River Basin. Sci. Total Environ. 2015, 503, 300–318. [Google Scholar] [CrossRef]
- Gibson, P.B.; Waliser, D.E.; Guan, B.; DeFlorio, M.J.; Ralph, F.M.; Swain, D.L. Ridging associated with drought across the western and southwestern United States: Characteristics, trends, and predictability sources. J. Clim. 2020, 33, 2485–2508. [Google Scholar] [CrossRef]
- Mosley, L.M. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Palmer, T.A.; Montagna, P.A. Impacts of droughts and low flows on estuarine water quality and benthic fauna. Hydrobiologia 2015, 753, 111–129. [Google Scholar] [CrossRef]

| FUI | Range | FUI | Range | FUI | Range |
|---|---|---|---|---|---|
| 1 | 8 | 15 | |||
| 2 | 9 | 16 | |||
| 3 | 10 | 17 | |||
| 4 | 11 | 18 | |||
| 5 | 12 | 19 | |||
| 6 | 13 | 20 | |||
| 7 | 14 | 21 |
| FUI Range | Water Clarity | Trophic State |
|---|---|---|
| FUI | very clear | oligotrophic |
| FUI | moderately clear | mesotrophic |
| 10 and (645 nm) | moderately clear | mesotrophic |
| 10 and (645 nm) | turbid | eutrophic |
| FUI | |||||
|---|---|---|---|---|---|
| Oligotrophic | Mesotrophic | Eutrophic | Total | ||
| Oligotrophic | A | B | C | D | |
| MERIS | Mesotrophic | E | F | G | H |
| TSI | Eutrophic | I | J | K | L |
| Total | M | N | O | P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abegaz, N.T.; Tsidu, G.M.; Arsiso, B.K. Spatiotemporal Variability of the Lake Tana Water Quality Derived from the MODIS-Based Forel–Ule Index: The Roles of Hydrometeorological and Surface Processes. Atmosphere 2023, 14, 289. https://doi.org/10.3390/atmos14020289
Abegaz NT, Tsidu GM, Arsiso BK. Spatiotemporal Variability of the Lake Tana Water Quality Derived from the MODIS-Based Forel–Ule Index: The Roles of Hydrometeorological and Surface Processes. Atmosphere. 2023; 14(2):289. https://doi.org/10.3390/atmos14020289
Chicago/Turabian StyleAbegaz, Nuredin Teshome, Gizaw Mengistu Tsidu, and Bisrat Kifle Arsiso. 2023. "Spatiotemporal Variability of the Lake Tana Water Quality Derived from the MODIS-Based Forel–Ule Index: The Roles of Hydrometeorological and Surface Processes" Atmosphere 14, no. 2: 289. https://doi.org/10.3390/atmos14020289
APA StyleAbegaz, N. T., Tsidu, G. M., & Arsiso, B. K. (2023). Spatiotemporal Variability of the Lake Tana Water Quality Derived from the MODIS-Based Forel–Ule Index: The Roles of Hydrometeorological and Surface Processes. Atmosphere, 14(2), 289. https://doi.org/10.3390/atmos14020289






