Abstract
PM2.5 and PM>2.5 samples were collected in Cotonou (Benin) using high volume cascade impaction air samplers. The samplings were based on continuous collection over twelve days. Physical and chemical characteristics of samples were determined by size distribution (laser granulometry), specific surface areas (BET method), inorganic elements (ICP-MS), water-soluble ions (IC), CHNS analysis and organic compounds (GC-MS). Average concentrations of air particulate matter were 180.9 µg/m3 and 94.5 µg/m3 in PM2.5 and PM>2.5, respectively. The higher water-soluble ions recorded were Ca2+,SO42−,NO3−, Na+ and Cl− for both PM. Moreover, concentrations were almost two-fold higher for PM2.5 compared to PM>2.5, with 10.7 µg/m3 of total metals found in PM2.5 versus 5.6 µg/m3 in PM>2.5. Both PM samples under study presented similar repartition of elements considering their percentages. Results suggested that PM>2.5 samples contain agglomerates of fine particles. Identification tools of major pollution source as inorganic elements, paraffins, fatty acids ratios and PAHs ratios indicated that PM under study originated from traffic exhaust.
1. Introduction
Air pollution particulate matter (PM) is a complex mixture of metals, salts, organic chemicals and biological materials [1,2,3]. It is a serious environmental health risk factor for humans [4,5]. Environmental toxicology studies used chemical and physical characterization of PM (source, size, mass, surface area, organic composition, metals, etc.) to assess adverse health effects linked to particles [6,7,8,9,10,11,12,13]. These studies have shown that particle exposure could cause many diseases in both developed and developing countries [14,15,16]. However, the underlying mechanisms of action generating adverse health effects are still unclear. Airborne particulate matter, especially the fraction of particles with aerodynamic dynameters below 2.5 μm (fine particles PM2.5) had large surface areas and adsorbed compounds such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), and many other organic compounds into their surface various. Their small diameters allow them to reach the alveolar compartment of lungs, where they are able to cause adverse effects on health [17,18,19,20,21]. It was shown that these particles cause more toxic effects than coarse particles [22].
Many studies have shown the influence of particle characteristics on biological responses that may cause many diseases [8,23,24]. The particles come from natural and anthropogenic sources such as industries, traffic, heating, etc. [25,26]. In a recent study, Yusuf et al. [27] found that transport activities are the major sources that emit heavy pollutants found in the environment. An urban area with mainly exhaust fumes is a strong source of PM, especially during the rainy season [28,29]. In developing countries, there is little data concerning the physicochemical characterization of PM and assessment of their adverse health effects [20,21,22,23,24,25,26,27,28,29,30]. In Sub-Saharan Africa countries, little air quality data is available; it appears that having a better knowledge on the air pollution is important [31,32,33]. Over the past decade, the air quality of Cotonou, the economic capital of Benin, has worsened in the city due to the widespread use of motorbikes with that consume gasoline of doubtful quality as the main fuel source and the presence of old second-hand cars [29,32,34,35,36,37]. The city had more than 94,000 motorbikes and more than 350,000 old second-hand cars [34,35,36,37,38]. In such a situation, in order to examine the quality of ambient air, a pilot study has reported that ambient air in Cotonou contained lot of air pollutants, including volatile organic compounds (VOCs) the benzene and its derivatives and ultrafine particles (UFPs) associated with polycyclic aromatic hydrocarbons (PAHs) at high concentrations [39,40].
In this study, samples of ambient particulate matter PM2.5 and fraction of particles with aerodynamic dynameters above 2.5 μm (PM>2.5) were collected in Cotonou where the road traffic is remarkably dense. The main objective was to determine physical and chemical characteristics of particles in order to choose specific physicochemical parameters for selection of possible tracers of urban activities.
2. Materials and Methods (See the Schematic Diagram, Figure S1)
2.1. Site Description and PM Sampling
Two PM (PM>2.5 and PM2.5) samples were collected in Cotonou, Benin (Figure 1) and samplings were based on continuous collection from 16 November 2010 to 8 December 2010. Samples were obtained from St Michel (6°22′2.3″ N/2°25′46.4″ E), a district which records dense road traffic. The sampling site (under urban-related emission sources) has SSW/SW as the prevailing wind direction. Other meteorological conditions were an average temperature of 31.4 °C, 72% of relative humidity and 3.0 m/s average wind speed during the period. Both types of particles were collected using high volume cascade impaction air samplers (Staplex, New York, NY, USA), with the collection method described by Billet et al. [41]. Briefly, the impactor’s plates were mounted without any filter and backup filter to maintain a constant aspiration flow rate (i.e., 80 m3/h). Two identical impacting systems have been used alternatively and were changed every three days. Samples were pooled after each collection. Sampling pumps were placed to approximately 50 m of the crossroad and about 1.5 m above the ground. ASECNA (Agence pour la Sécurité de la Navigation Aérienne en Afrique et à Madagascar) of the international airport Bernardin GANTIN supplied meteorological data (wind direction and speed, temperature and relative humidity). After PM sampling, impacting plates were dried under a laminar flow bench. Then, PM were removed from plates and divided into two size fractions: particles with aerodynamic dynameters above 2.5 μm (coarse particles PM>2.5) and particles with aerodynamic dynameters below 2.5 μm (fine particles PM2.5). The two types of particles were kept in cleaned vials and immediately stored at 4 °C. An amount of 1 g of PM>2.5 and more than 2 g of PM2.5 were collected. Thereafter, samples were convoyed for analysis in Dunkerque, France.
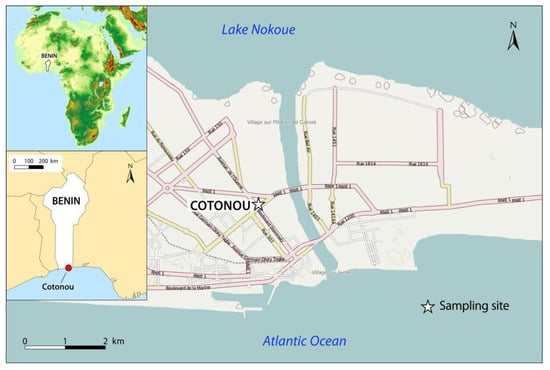
Figure 1.
Map of urban sampling site in Cotonou, Benin.
2.2. Experimental Analysis
2.2.1. PM Physical Characterization
PM Size Distribution
Each sample was prepared with 20 mg of particle in 50 mL of distilled water and well mixed. Samples suspensions were ultrasonically dispersed before measurement. PM size distribution was studied by using laser granulometry. The laser granulometer (Beckman Coulter LS 13 320 MW with Universal Liquid Module) measures particle size distributions over the range from 0.017 to 2000 µm of sample suspensions, based on polarization intensity differential scattering (PIDS). In all measurements, beam obscuration was kept within the limits of 8% to 12%. Cumulative frequency and relative frequency size distribution were used for analysis of the results.
Specific Surface Area
About 50 mg of each PM were placed in a small Pyrex U-shaped cell and analyzed by using a Quantasorb® Surface Area Analyzer (Quantachrome Corporation, New York, NY, USA), which is based on Brunauer–Emmett–Teller (BET) theory. Particle samples were outgassed at 200 °C for 1 h. Nitrogen was used as a calibration gas and helium as an inert carrier gas with a rate of 70% versus 30% nitrogen. Thereafter, volumes of pure nitrogen gas were adsorbed to their surface at −196 °C. The amount of nitrogen adsorbed at various partial pressures was used to calculate the sample’s surface area.
2.2.2. Inorganic Elements and Water-Soluble Ions
Both PM were mineralized (about 20 mg) using HNO3 and HClO4 (ratio 1:2) by microwave digestion (MARS XPRESS, CEM Corporation) (the experimental conditions were 1600 W of power, 180 °C and 20 min of run time). Thereafter, the solutions were filtered by Whatman cellulose filters, diluted up to 25 mL with ultrapure water and analyzed by inductively coupled plasma-mass spectrometry (ICP-MS, Varian® 820 MS). Calibration solutions were prepared in a 1% HNO3 matrix using a commercial 10 ppm multi-element standard (SCP33MS, SCP Science, Baie-d’Urfé, QC, Canada). The calibration range was from 0 to 50 ppb.
About 20 mg of particles collected was leached in 10 mL ultrapure water by ultrasonic agitation for 30 min. Extract solutions were filtered on cellulose acetate filter 0.45 µm. This step was repeated six times on the same aliquot to recover 95% of soluble ions and then 25 µL were injected. Soluble anions concentrations were determined by chemically suppressed ion chromatography (carbonate 1mM/bicarbonate 3.5 mM eluents and AG14A/AS14A columns, 1.2 mL/min) using a Dionex DX 100 ion chromatograph. Soluble cations concentrations were determined by chemically suppressed ion chromatography (methane sulfonic acid 20 mM eluents and CG12A/CS12A columns, 1 mL/min) using a Dionex ICS 900 ion chromatograph. The calibration ranges of soluble anions and soluble cations were from 0.5 to 100 ppm. Chromatographic data were analyzed by Chromeleon® software (Thermo Fisher Scientific, Waltham, MA, USA).
2.2.3. Element Composition
Element concentrations (carbon, nitrogen, hydrogen and sulphur) were detected by using CHNS/O Elemental Analysers (FlashEA 1112 equipped to 2 injectors MAS200R, Thermo Fisher Scientific). Carbon is converted by oxidation to carbon dioxide; hydrogen to water; nitrogen to nitrogen gas/oxides of nitrogen; and sulphur to sulphur dioxide. CO2, H2O, N2 and SO2 obtained after oxidation and reduction of gases [42,43] were separated by a GC packed column (PTFE, 2 m, 6 × 5 mm), kept at 70 °C and detected by a thermo-conductivity detector. A quartz reactor was used to determine elements and kept at 950 °C with 140 mL/min of He flow as a carrier gas. For oxidation of the samples, a pressure of 250 kPa (He) and 220 kPa (O2) were required. Cycle run time, sampling delay and oxygen injection end were 600, 25, 17 s, respectively. PM samples were previously introduced in tin capsules and packed carefully. Sulphanilamide (Thermo Fisher Scientific) was used as the standard of calibration. After first analysis, each sample was incinerated at 450 °C in order to remove organic carbon and then analyzed again to determine mineral carbon. The amounts of gases in column output were proportional to the carbon, hydrogen, nitrogen and sulfur contained in the samples.
2.2.4. Organic Compounds
The volatile organic compounds (VOCs) analytical method was based on the protocol described by Caplain et al. [44]. Samples were previously trapped in glass tubes and VOCs were desorbed by thermal treatment at 220 °C using GC/MS (Combi Injector/Desorber module-EM640 Brüker). Desorption time was 3 min to desorb VOCs and compounds from C10 to C15. These conditions prevented the desorption of the semi-volatile compounds subsequently extracted with solvents.
After thermal desorption, the two particle samples were extracted by soxhlet extraction with 100 mL of dichloromethane for 16 h to determine polycyclic aromatic hydrocarbons (PAHs), paraffins and carboxylic acids. The extracts were concentrated under nitrogen flux. An amount of 1 µL of each sample was injected in a gas chromatograph (VARIAN3800) coupled to a mass spectrometer (VARIAN1200 TQ). The capillary column is a factor four VF-5 ms (30 m × 0.25 mm × 0.25 µm) and used helium as the carrier gas. The total time for this analysis was 60 min; 40 °C for 5 min; 5 °C/min up to 310 °C; and 310 °C for 3 min. The parameters of the mass detector were an impact electronic ion current = 70 eV and a source temperature of 280 °C. The samples were analyzed between 40 and 350 mass units. Compounds were identified by comparing retention times of chromatographic peaks from samples with those from standard mixtures and by comparing mass spectra with those contained in NIST and/or WILEY libraries.
3. Results and Discussion
3.1. PM Physical Characteristics
In this study, the St Michel district (sampling site) recorded SSW as the dominant wind direction. During the sampling period, average concentrations of air particulate matter were 180.9 µg/m3 for PM2.5 and 94.5 µg/m3 for PM>2.5. In Lagos (located in Nigeria, which is near to Benin), Adeleke et al. [45] reported extremely high levels of up to 272.8 μg/m3 for PM2.5. Our PM2.5 and PM>2.5 concentrations were higher than those previously reported in Dakar (Senegal): 85 µg/m3 and 57 µg/m3, respectively [46]. Figure 2 shows the size distribution by cumulative frequency (%) and relative frequency (%). The total PM numbers with geometric diameter ≤ 2.5 µm were identical (97.5%) in both PM. The highest particle numbers detected in size classes were also identical (55.2%) for 0.5–1.0 µm. For the 0.33–0.5 µm class, proportions of 24.0% and 22.5% were found for PM2.5 and PM>2.5, respectively.
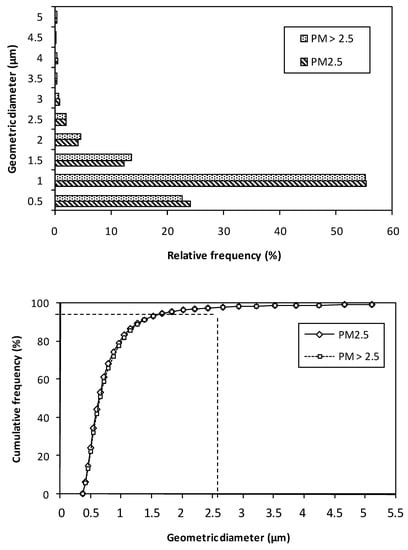
Figure 2.
Size distribution by cumulative frequency (%) and relative frequency (%) of PM2.5 and PM>2.5 collected in an urban site, Cotonou.
Studies conducted in West Africa have also shown a significant level of PM2.5 fraction in ambient air of urban city. However, distributions found in this work were markedly higher than those recorded in Abidjan (88.3% of fine particle number, [20] and Dakar (94.7% of fine particle number, [9]). Other countries have found PM concentrations markedly lower than those recorded in this work (Table 1).

Table 1.
Concentrations of fine and coarse particles and inorganic water-soluble ions measured in different continents.
In a nutshell, concentrations recorded in African countries, particularly Benin, were high and exceeded the PM concentrations of air quality guidelines revised on September, 2021 by the World Health Organization [59]. These guidelines promulgated that the highest level of PM2.5 should be 15 µg/m3 (24 h average concentration) and 5 µg/m3 (annual average). For PM10, the concentrations are 15 and 45 µg/m3 for annual and 24 h average concentration, respectively.
On the other hand, the studied particulate matters had higher specific surface areas ranging from 10 m2/g to 12 m2/g for PM2.5 and PM>2.5, respectively. Dieme et al. [9] and Kouassi et al. [20] had found similar values in urban cities (12 m2/g and 9 m2/g in Dakar and Abidjan, respectively). A recent study undertaken in Dakar found a specific surface area value of 10 m2/g for PM2.5 and 7 m2/g for PM>2.5 [46]. In reference materials, SRM1649a (mainly of coarse particles) and CRM8 (mainly of fine or ultrafine particles), values of specific surface areas were 35 m2/g for fine particles and 2 m2/g for coarse particles [60]. The high values of specific surface area recorded in this study were likely to be associated with carbonaceous particulate emissions from combustion processes, such as vehicular emissions. Similar results in PM2.5 and PM>2.5 suggest that PM>2.5 contain aggregates and/or agglomerates of fine particles within it. Particles having high specific surface areas are known to absorb chemical compounds such as volatile organic compounds and polycyclic aromatic hydrocarbons [41,61].
3.2. Inorganic Elements and Water-Soluble Ions
Water-soluble ions concentrations are presented in Figure 3. Ionic concentrations in PM2.5 were higher than those in PM>2.5. The most abundant compounds were Ca2+ (2.5 µg/m3/39.2% for PM2.5 versus 1.1 µg/m3/37.7% for PM>2.5), SO42− (1.4 µg/m3/21.5% versus 0.5 µg/m3/16.6%) and NO3− (0.8 µg/m3/13.0% versus 0.2 µg/m3/5.8%). Na+ and Cl− have nearly the same concentration values (0.6 µg/m3 for PM2.5 versus 0.5 µg/m3 for PM>2.5). Na+ and Cl− also presented similar percentages (9.8% and 9.7% for PM2.5, respectively, versus 15.8% and 17.9% for PM>2.5). Total water-soluble ions concentrations of PM2.5 were 6.3 µg/m3 versus 3.0 µg/m3 for PM>2.5.
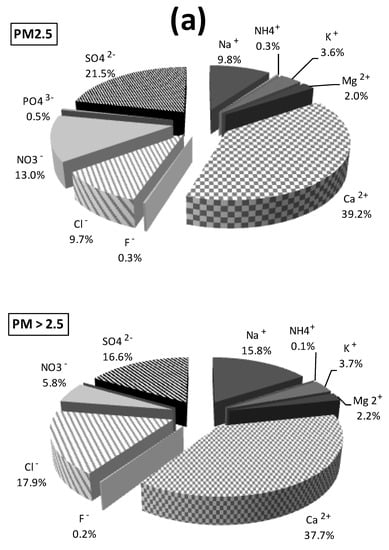
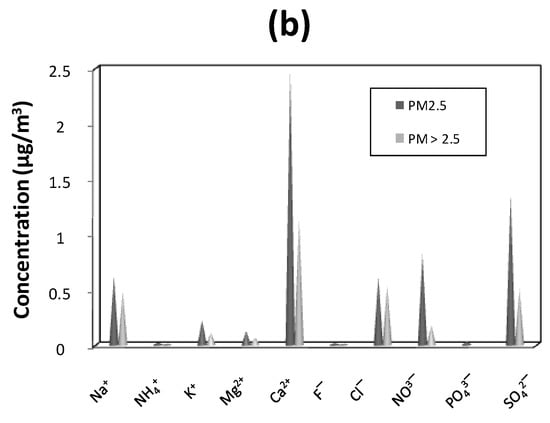
Figure 3.
Proportion in % (a) and concentrations in µg/m3 (b) of water-soluble ions in the PM2.5 and PM>2.5 samples collected in an urban area of Cotonou (Benin).
The most abundant water-soluble ions concentrations recorded were Ca2+, SO42−, NO3−, Na+ and Cl− for both types of PM. Na+, Cl− and Mg2+ revealed sea salt influences [17,62]. While the high concentration of Ca2+ could come from natural erosion and/or dust from the cement industry, which is located approximately 1 km from the sampling site. Cazier et al. [17] found a similar result concerning amounts of CaCO3 which are significant. Sulphates and nitrates clearly revealed anthropogenic activities [8,9,17,54,62]. In West Africa, values of sulphate concentration are often high. Sulphates present in the studied particulate matter could come from natural sources such as the surface of the Atlantic Ocean due to the proximity of the West African countries of the sea [63,64]. With regards to nitrate concentrations found in PM2.5, they were also similar to those recently published [8,17,50,54]. Moreover, this nitrate value was lower than Dakar’s nitrate concentration (Table 1). The PM>2.5 nitrate value was smaller than those reported in Japan (1.0 µg/m3/10.6%, [54]). However, the nitrate value observed in this work indicated an anthropogenic contribution. Nitrates in PM come primarily from NOX (NO and NO2) and are mostly of anthropogenic origins. They come from high temperature combustion processes and their gas–particle conversion in the atmosphere forms stable nitrates in the particulate state [65].
Otherwise, concentrations were almost two-fold higher in PM2.5 than in PM>2.5 with total metals of 10.78 µg/m3 found in PM2.5 versus 5.68 µg/m3 in PM>2.5 (Table 2). Among the main elements (Al, Fe, Mg, Mn, Na, Ti and Zn) found in particles, Al and Fe represent the highest concentrations in the two types of samples. Medium studied elements Ba, Cr, Cu, Pb and Sr showed concentrations ranging from 0.01 to 0.04 µg/m3 in PM2.5 and from 0.005 to 0.02 µg/m3 for PM>2.5. Concentrations of other elements were relatively low (0.0005–0.001 µg/m3) in analyzed samples. Both PM samples under study presented similar repartition of elements as considering their percentage.

Table 2.
Inorganic elements of air pollution particulate matter collected.
In literature, inorganic elements were usually associated with natural environment (e.g., Na, Mg, Ca, Ti) and anthropogenic origin (e.g., Fe, Al, Mn, Ba, Cr, Zn, Pb, Cu, Cd) [8,9]. Moreover, Cr, As, Ni, Pb, Zn, Cu, V and Cd are carcinogenic, Cd and As are potentially mutagenic and Pb and Hg are foetal toxic [66,67]. The main sources of atmospheric heavy metals are natural emissions (marine spray and Saharan dust), heavy fuel oil combustion, non-exhaust road traffic and industrial metallurgical processes [68,69,70]. Different researchers have attributed different groups of heavy metals to natural sources. Ni, Mn and Co concentrations were attributed to natural sources [71]. Kelepertzis [72] attributed Ni, Cr, Co and Fe to natural origin. In this paper, the main concentrations of Al, Fe, Mg, Mn, Na, Ti and Zn could come mainly from anthropogenic sources. Vehicular emissions, waste disposal, sewage and industrial emissions are known to be important sources of urban atmospheric heavy metals [73,74,75]. Inorganic elements in Cotonou were high during the sampling period. High concentrations of Fe and Al were often measured in African countries when sites were submitted to soil dust resuspension from arid regions [70,75]. Dieme et al. [9], Kouassi et al. [20] and Weinstein et al. [50] had shown similar results in Abidjan, Dakar and Conakry, respectively. According to regulatory standards set by the European Parliament and the Council of the European Union [76], lead levels in the two types of particles were higher than those detected by Dieme et al. [9]. Other results around the world are presented in Table 3. Metal concentrations of PM2.5 fraction are twice higher than those of PM>2.5. Lee et al. [77] made a similar observation. This was likely because heavy metals are more easily accumulated in PM2.5 than in PM>2.5. Metals as Cr and Cu are known to be derived from non-exhaust emissions (brake wear) [78]. The present study showed that Cu and Cr concentrations are more than twice as high in PM2.5 as in PM>2.5. Wang et al. [79] had found similar concentrations of Cu for PM2.5 in China, but their Cr values were three times higher than our results. In addition, Zn is associated with tire wear [80]. Zn concentrations were 145 ng/m3 and 71 ng/m3 for PM2.5 and PM>2.5, respectively. These Zn concentrations in PM2.5 were higher than those found in Ethiopia (3 ng/m3), Senegal (2 ng/m3) and Greece (4 ng/m3), which are countries that have similar values of Zn [9,48,55]. However, other studies carried out in China showed Zn concentrations of up to 465 ng/m3 and 590 ng/m3 for PM2.5 and PM10, respectively [79]. On the other hand, Benin adopted unleaded gasoline, so the main sources of heavy metals of two PM samples from Cotonou are probably influenced by road traffic and notably non-exhaust emissions and/or cement industry emissions [81,82].

Table 3.
Concentration of heavy metals (ng/m3) in the ambient air of urban areas in different countries around the world.
3.3. Element Composition
Total carbon was the highest rate of elements in the two analyzed aerosols (Table 4). Total carbon was found to 22.97% in PM2.5 versus 14.09% for PM>2.5. Percentage of hydrogen were higher than nitrogen and sulfur in the two samples.

Table 4.
Element composition of two aerosols collected.
The total carbon in PM is the sum of OC and EC; the EC levels are generally lower than the OC values [31]. In urban area, the contribution of TC in PM2.5 was 40% in Ashwaina, Ghana [85], 59% in Dar es Salaam, Tanzania [86] and is 23% in the present study. The reported EC for PM2.5 (7.6%) and PM>2.5 (9.0%) are quite similar. The EC/TC ratio provides insight to the sources of the carbonaceous aerosol including biomass combustion and traffic [31]. EC/TC ratios between 0.1 and 0.2 have been associated with biomass-burning activities in Morogoro, Tanzania [86]. These results are similar to values found in India [87]. In Ashaiman, Ghana, this ratio was 0.21 [85]. Our results are far above those observed in Ghana and Tanzania. In this paper, the OC level was 21% and 13% for PM2.5 and PM>2.5, respectively. In Dar es Salaam, Tanzania reported OC percentages of 36% for PM2.5 and 24% for PM10; these values are attributed to traffic emissions [86].
In Eastern Spain, organic carbon (OC) + elemental carbon (EC) were higher than those of this study. These percentages were 32% and 22% for PM2.5 and PM10, respectively [62]. In Yokohama (Japan), OC + EC can reach 28% and 16% in PM2.5 and PM10, respectively [54]. A recent study in Delhi (India) reported a value of 22% of OC + EC [53]. The ratio of (OC + EC) PM2.5/(OC + EC) PM10 were similar in Spain, Japan and Benin. Results of study conducted by Aldabe et al. [84] in Spain were contrary to those in the present work; OC + EC concentrations were rather abundant in PM10 compared to PM2.5. In accordance with Rodriguez et al. [62], OC + EC associated with phosphate and ozone should be due to the influence of bio-mass burning. In the present study, phosphate was practically absent and lead, nitrate, OC + EC, nickel were relatively high. Thus, the two PM could mainly come from vehicle exhaust. Moreover, both PM presented similar repartition of elements as compared to the total carbon. This confirms the same source of PM found in metal analysis. The uppermost concentrations of organic carbon were often linked with organic compounds.
3.4. Organic Compounds
In this study, the volatile organic compounds (VOCs) detected in analyzed samples were benzene and its derivatives. VOCs concentrations were more concentrated (seven times) in PM2.5 (8.9 ng/m3) than in PM>2.5 (1.2 ng/m3) (Table 5). However, these concentrations were lower in PM2.5 than those reported by Dieme et al. [9] and Dergham et al. [8] in the Dakar urban site (37.4 ng/m3) and Dunkerque (51.3 ng/m3), respectively. The toluene/benzene (T/B) ratio found in PM2.5 samplings was 3.9 and 1.4 in PM>2.5. According to the literature, the T/B ratio ranges from 1.5 to 4.0 in urban areas indicated traffic emissions [88,89,90,91].

Table 5.
Organic compounds in the two air pollution PM samples.
Polycyclic aromatic hydrocarbons (PAHs) concentrations were far more concentrated in PM2.5 than in PM>2.5. Reche et al. [92] and Li et al. [93] have found a similar result in Barcelona (Italy) and Beijing (China), respectively. The total PAHs levels in this work were lower than those found in Europe [94], Germany [95] and France [8]. However, our PAHs concentrations were higher than those reported by Pindado et al. [96] (in Spain). The quantity of benzo[a]pyrene, a leader of PAHs and classified as a carcinogenic B2 pollutant by the EPA, was smaller in PM>2.5 and greater in PM2.5 than those of recent studies performed in Spain [92,97]. Generally speaking, most of the PAHs have anthropogenic origins (vehicle exhaust, residential heating and power plants) and are usually distinguished by their emission sources using diagnostic ratios [93,98,99,100]. According to PAHs ratios in Table 6, the two types of PM are presented same ratio. BaP/(BaP + Chr), Flu/(Flu + Pyr), BaP/BghiP, BaA/(BaA + Chr) and ∑COMB/∑PAHs were 0.5, 0.3, 0.6, 0.4 and 0.9, respectively, indicating that PAHs adsorbed on PM can come from gasoline, diesel emissions, incomplete combustion and mainly road traffic emissions.

Table 6.
Characteristic ratios of PAHs used for source identification.
Traffic emissions usually produce long chain n-alkanes. Concentrations of these compounds were higher in PM2.5 than in PM>2.5 (Figure 4). However, PM>2.5 were more concentrated in heavy paraffins (C29 to C36), with the exception of C33.
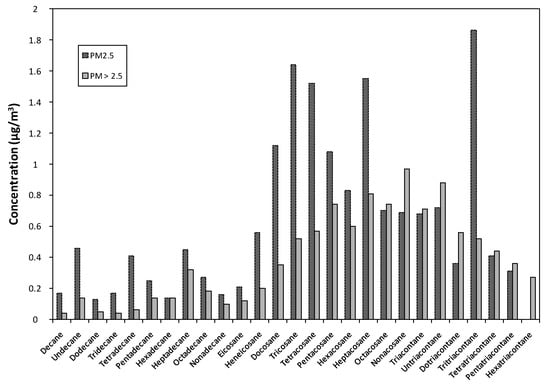
Figure 4.
Paraffins concentrations in the two air particulate matter.
In this work, total paraffins were 16.8 ng/m3 and 10.6 ng/m3 for PM2.5 and PM>2.5, respectively. For PM2.5, paraffin concentration was higher than those found at Dakar (Senegal) [9], Augsburg (Germany) [95] and Chapinerı’a (Spain) [96]. Tefera et al. [33] showed 60.01 ± 16.91 ng/m−3 for concentrations of C28-31 n-alkanes. These values can reach concentrations fifty times higher in Beijing city (China) [93]. Indeed, C13-C19 indicated microbiota and diesel origin. On the other side, C20-C37 were attributed to fossil fuel rubbish and plant waxes [17,95,108]. In accordance with Kotianová et al. [109], n-alkanes up to C20 and C25 are used as sources in gasoline powered vehicles and heavy duty diesel trucks, respectively. This study showed that paraffins in PM samples were mainly resulting from gasoline and diesel emissions, thus traffic emissions. These observations are in agreement with results obtained in the PAHs study.
Otherwise, saturated fatty acids indicate the presence of agricultural activity. Therefore, Figure 5 reports significant saturated carboxylic acids in studied PM samples. Hexadecanoic (palmitic) acid (C16) and octadecanoic (stearic) acid (C18) were most abundant. A similar remark was published by Li et al. [93]. The concentrations were 120.45 µg/m3 and 31.89 µg/m3 in PM2.5 for C16 and C18, respectively. The PM>2.5 had two times lower C16 (52.83 µg/m3) and C18 (13.94 µg/m3) than PM2.5. The total fatty acids were 330.76 µg/m3 for PM2.5 and 196.31 µg/m3 for PM>2.5.
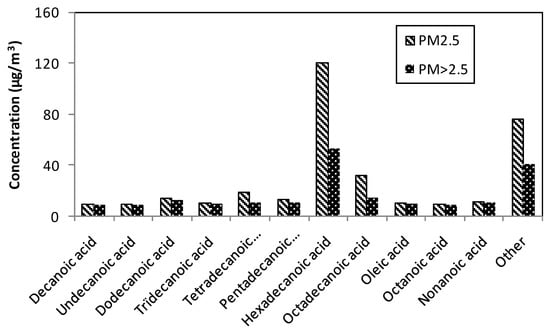
Figure 5.
Carboxylic acids with a long aliphatic tail of two air pollution particulate matter.
According to Oliveira et al. [110], fatty acids with even-numbered carbons of less than 20 carbons are considered to be derived from microbial activities and cooking meat. The ratio of oleic acid/stearic acid is a tool to identify aerosol aging [93]. Rogge et al. [111] suggested that when ratio of stearic acid/palmitic acid < 0.25, it originates from wood smoke and foliar vegetation combustion; between 0.25 and 0.5, it stems from car and diesel truck emissions; and is road dust when ranging from 0.5 to 1.0. In the present work, stearic acid/palmitic acid ratios were 0.26 in the two analyzed PM. Oleic acid/stearic acid ratios were 0.32 and 0.7 in PM2.5 and PM>2.5, respectively. Li et al. [93] reported a similar result in PM2.5 according to the collection period in Beijing. In light of these results, fatty acids concentrations indicated mostly vehicle emissions and rejoined conclusions of previous analyses.
4. Conclusions
Physicochemical characterization of air pollution particulate matters PM2.5 and PM>2.5 collected in Cotonou revealed astounding identical granulometry profile and specific surface areas between particles samples. This suggests that particle PM>2.5 samples contain agglomerates of fine particles. However, concentrations of different inorganic and organic compounds were higher in PM2.5 than in PM>2.5 every time. Tools to identify sources were used to detect pollutants origin. Inorganic elements, PAHs, paraffins and fatty acids levels studies clearly indicated that both PM came from natural and/or anthropogenic emission sources, particularly, vehicle exhaust influence and traffic emissions. These compounds have adverse health effects, and it is necessary to complete research with toxicological studies in order to establish a monitoring plan of air quality in African countries. Moreover, the results of this characterization study of airborne particulate matter can be used as relevant data for establishing air quality management policies. Policy makers should focus on anthropogenic sources with a high impact on PM concentration to achieve a better air quality in highly populated cities of Benin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos14020201/s1, Figure S1. Schematic diagram of the whole study on Cotonou’s air pollution impact on human’s health.
Author Contributions
Conceptualization, F.B.C., F.C., D.C. and A.V.; methodology, F.B.C., D.D., P.G., A.D., A.V. and. F.C.; software, F.B.C.; validation, F.C., A.V. and D.C.; formal analysis, F.B.C. and A.V.; investigation, F.B.C., D.D., P.G., A.D., F.C., D.C. and A.V.; resources, F.B.C. and A.V.; data curation, F.B.C. and A.V.; writing—original draft preparation, F.B.C. and A.V.; writing—review and editing, F.C., A.V. and D.C.; visualization, F.B.C., F.C., A.V. and D.C.; supervision, F.C., A.V. and D.C.; project administration, L.A.-F., F.A., A.S., F.C., A.V. and D.C.; funding acquisition, L.A.-F., F.A. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study benefited grants from the Service de Coopération et d’Action Culturelle de l’Ambassade de France in Benin (Grant number 779212C).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The Unité de Chimie Environnementale et Interactions sur le Vivant (UCEIV) and Centre Commun de Mesures (CCM), participate in the Climibio project, which is financed by the Communauté Urbaine de Dunkerque, the Région Nord-Pas de Calais, the Ministère de l’Enseignement Supérieur et de la Recherche, the National Center for Scientific Research in France (CNRS) and European Regional Development Fund (ERDF). We would like to thank Denis Marin who drew the map of the study area.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baulig, A.; Singh, S.; Marchand, A.; Schins, R.; Barouki, R.; Garlatti, M.; Marano, F.; Baeza-Squiban, A. Role of Paris PM2.5 Components in the pro-Inflammatory Response Induced in Airway Epithelial Cells. Toxicology 2009, 261, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Osornio-Vargas, A.R.; Serrano, J.; Rojas-Bracho, L.; Miranda, J.; García-Cuellar, C.; Reyna, M.A.; Flores, G.; Zuk, M.; Quintero, M.; Vázquez, I.; et al. In Vitro Biological Effects of Airborne PM2.5 and PM10 from a Semi-Desert City on the Mexico–US Border. Chemosphere 2011, 83, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Gualtieri, M.; Ferrero, L.; Porto, C.L.; Udisti, R.; Bolzacchini, E.; Camatini, M. Seasonal Variations in Chemical Composition and in Vitro Biological Effects of Fine PM from Milan. Chemosphere 2010, 78, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J. Will the Circle Be Unbroken: A History of the U.S. National Ambient Air Quality Standards. J. Air Waste Manag. Assoc. 2007, 57, 652–697. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Krewski, D.; Jerrett, M.; Shi, Y.; Calle, E.E.; Thun, M.J. Cardiovascular Mortality and Exposure to Airborne Fine Particulate Matter and Cigarette Smoke: Shape of the Exposure-Response Relationship. Circulation 2009, 120, 941–948. [Google Scholar] [CrossRef]
- Bachwenkizi, J.; Liu, C.; Meng, X.; Zhang, L.; Wang, W.; van Donkelaar, A.; Martin, R.V.; Hammer, M.S.; Chen, R.; Kan, H. Fine Particulate Matter Constituents and Infant Mortality in Africa: A Multicountry Study. Environ. Int. 2021, 156, 106739. [Google Scholar] [CrossRef]
- Bachwenkizi, J.; Liu, C.; Meng, X.; Zhang, L.; Wang, W.; van Donkelaar, A.; Martin, R.V.; Hammer, M.S.; Chen, R.; Kan, H. Maternal Exposure to Fine Particulate Matter and Preterm Birth and Low Birth Weight in Africa. Environ. Int. 2022, 160, 107053. [Google Scholar] [CrossRef]
- Dergham, M.; Lepers, C.; Verdin, A.; Billet, S.; Cazier, F.; Courcot, D.; Shirali, P.; Garçon, G. Prooxidant and Proinflammatory Potency of Air Pollution Particulate Matter (PM2.5–0.3) Produced in Rural, Urban, or Industrial Surroundings in Human Bronchial Epithelial Cells (BEAS-2B). Chem. Res. Toxicol. 2012, 25, 904–919. [Google Scholar] [CrossRef]
- Dieme, D.; Cabral-Ndior, M.; Garçon, G.; Verdin, A.; Billet, S.; Cazier, F.; Courcot, D.; Diouf, A.; Shirali, P. Relationship between Physicochemical Characterization and Toxicity of Fine Particulate Matter (PM2.5) Collected in Dakar City (Senegal). Environ. Res. 2012, 113, 1–13. [Google Scholar] [CrossRef]
- Kalisa, E.; Kuuire, V.; Adams, M. Children’s Exposure to Indoor and Outdoor Black Carbon and Particulate Matter Air Pollution at School in Rwanda, Central-East Africa. Environ. Adv. 2023, 11, 100334. [Google Scholar] [CrossRef]
- Lala, M.A.; Onwunzo, C.S.; Adesina, O.A.; Sonibare, J.A. Particulate Matters Pollution in Selected Areas of Nigeria: Spatial Analysis and Risk Assessment. Case Stud. Chem. Environ. Eng. 2023, 7, 100288. [Google Scholar] [CrossRef]
- Vanker, A.; Barnett, W.; Chartier, R.; MacGinty, R.; Zar, H.J. Personal Monitoring of Fine Particulate Matter (PM2.5) Exposure in Mothers and Young Children in a South African Birth Cohort Study—A Pilot Study. Atmos. Environ. 2023, 294, 119513. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshioka, Y.; Fujimura, M.; Kayamuro, H.; Yamashita, K.; Higashisaka, K.; Nakanishi, R.; Morishita, Y.; Nabeshi, H.; Yamashita, T.; et al. Urban Aerosols Induce Pro-Inflammatory Cytokine Production in Macrophages and Cause Airway Inflammation in Vivo. Biol. Pharm. Bull. 2010, 33, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, H.; Xu, Q.; Chen, B.; Kan, H. Fine Particulate Matter Constituents and Cardiopulmonary Mortality in a Heavily Polluted Chinese City. Environ. Health Perspect. 2012, 120, 373–378. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, Source and Chemical Composition as Determinants of Toxicity Attributable to Ambient Particulate Matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Y.; Wu, S.; Deng, F.; Liu, Y.; Yao, W. The Relationship between Particulate Matter (PM 10) and Hospitalizations and Mortality Of Chronic Obstructive Pulmonary Disease: A Meta-Analysis. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 307–315. [Google Scholar] [CrossRef]
- Cazier, F.; Dewaele, D.; Delbende, A.; Nouali, H.; Garçon, G.; Verdin, A.; Courcot, D.; Bouhsina, S.; Shirali, P. Sampling Analysis and Characterization of Particles in the Atmosphere of Rural, Urban and Industrial Areas. Procedia Environ. Sci. 2011, 4, 218–227. [Google Scholar] [CrossRef]
- Delfino, R.J.; Gillen, D.L.; Tjoa, T.; Staimer, N.; Polidori, A.; Arhami, M.; Sioutas, C.; Longhurst, J. Electrocardiographic ST-Segment Depression and Exposure to Traffic-Related Aerosols in Elderly Subjects with Coronary Artery Disease. Environ. Health Perspect. 2011, 119, 196–202. [Google Scholar] [CrossRef]
- Kocbach, A.; Li, Y.; Yttri, K.E.; Cassee, F.R.; Schwarze, P.E.; Namork, E. Physicochemical Characterisation of Combustion Particles from Vehicle Exhaust and Residential Wood Smoke. Part. Fibre Toxicol. 2006, 3, 1. [Google Scholar] [CrossRef]
- Kouassi, K.S.; Billet, S.; Garçon, G.; Verdin, A.; Diouf, A.; Cazier, F.; Djaman, J.; Courcot, D.; Shirali, P. Oxidative Damage Induced in A549 Cells by Physically and Chemically Characterized Air Particulate Matter (PM2.5) Collected in Abidjan, Côte d’Ivoire. J. Appl. Toxicol. 2010, 30, 310–320. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Sørensen, M.; Loft, S.; Overvad, K.; Tjønneland, A. Lung Cancer Incidence and Long-Term Exposure to Air Pollution from Traffic. Environ. Health Perspect. 2011, 119, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Senlin, L.; Zhenkun, Y.; Xiaohui, C.; Minghong, W.; Guoying, S.; Jiamo, F.; Paul, D. The Relationship between Physicochemical Characterization and the Potential Toxicity of Fine Particulates (PM2.5) in Shanghai Atmosphere. Atmos. Environ. 2008, 42, 7205–7214. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.R.; Park, Y.J.; Lee, S.Y.; Chung, K.H. Organic Extracts of Urban Air Pollution Particulate Matter (PM2.5)-Induced Genotoxicity and Oxidative Stress in Human Lung Bronchial Epithelial Cells (BEAS-2B Cells). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Anderson, H.R.; Bremner, S.A.; Marston, L.; Seemungal, T.A.; Strachan, D.P.; Wedzicha, J.A. Outdoor Air Pollution and Respiratory Health in Patients with COPD. Thorax 2011, 66, 591–596. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Ampah, J.D. Evaluation of Biodiesel on Speciated PM2.5, Organic Compound, Ultrafine Particle and Gaseous Emissions from a Low-Speed EPA Tier II Marine Diesel Engine Coupled with DPF, DEP and SCR Filter at Various Loads. Energy 2022, 239, 121837. [Google Scholar] [CrossRef]
- Varenik, A.V. The Characteristics of PM2.5 and PM10 and Elemental Carbon Air Pollution in Sevastopol, Crimean Peninsula. Appl. Sci. 2022, 12, 7758. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L. Effect of Low Bioethanol Fraction on Emissions, Performance, and Combustion Behavior in a Modernized Electronic Fuel Injection Engine. Biomass Conv. Bioref. 2021, 11, 885–893. [Google Scholar] [CrossRef]
- Akinwumiju, A.S.; Ajisafe, T.; Adelodun, A.A. Airborne Particulate Matter Pollution in Akure Metro City, Southwestern Nigeria, West Africa: Attribution and Meteorological Influence. J. Geovis. Spat. Anal. 2021, 5, 11. [Google Scholar] [CrossRef]
- Nducol, N.; Tchuente Siaka, Y.F.; Younui Yakum-Ntaw, S.; Saidou; Dika Manga, J.; Vardamides, J.C.; Hamadou, Y.A.; Simo, A. Ambient Air Particle Mass Concentrations in the Urban Area of the Capital City of Yaoundé (Cameroon, Central Africa): Monthly and Seasonal Behaviour. Int. J. Environ. Anal. Chem. 2021, 101, 2909–2925. [Google Scholar] [CrossRef]
- Diouf, A.; Garçon, G.; Diop, Y.; Ndiaye, B.; Thiaw, C.; Fall, M.; Kane-Barry, O.; Ba, D.; Haguenoer, J.M.; Shirali, P. Environmental Lead Exposure and Its Relationship to Traffic Density among Senegalese Children: A Cross-Sectional Study. Hum. Exp. Toxicol. 2006, 25, 637–644. [Google Scholar] [CrossRef]
- Agbo, K.E.; Walgraeve, C.; Eze, J.I.; Ugwoke, P.E.; Ukoha, P.O.; Van Langenhove, H. A Review on Ambient and Indoor Air Pollution Status in Africa. Atmos. Pollut. Res. 2021, 12, 243–260. [Google Scholar] [CrossRef]
- Petkova, E.P.; Jack, D.W.; Volavka-Close, N.H.; Kinney, P.L. Particulate Matter Pollution in African Cities. Air Qual. Atmos. Health 2013, 6, 603–614. [Google Scholar] [CrossRef]
- Tefera, W.; Kumie, A.; Berhane, K.; Gilliland, F.; Lai, A.; Sricharoenvech, P.; Patz, J.; Samet, J.; Schauer, J.J. Source Apportionment of Fine Organic Particulate Matter (PM2.5) in Central Addis Ababa, Ethiopia. Int. J. Environ. Res. Public Health 2021, 18, 11608. [Google Scholar] [CrossRef]
- Avogbe, P.H.; Ayi-Fanou, L.; Cachon, B.; Chabi, N.; Debende, A.; Dewaele, D.; Aissi, F.; Cazier, F.; Sanni, A. Hematological Changes among Beninese Motor-Bike Taxi Drivers Exposed to Benzene by Urban Air Pollution. Afr. J. Environ. Sci. Technol. 2011, 5, 464–472. [Google Scholar] [CrossRef]
- Cachon, B.; Ayi-Fanou, L.; Cazier, F.; Genevray, P.; Adéoti, K.; Dewaele, D.; Debende, A.; Aissi, F.; Sanni, A. Analysis of Gasoline Used by Motorbike-Taxi Drivers in Cotonou. Environ. Pollut. 2013, 2, 39. [Google Scholar] [CrossRef]
- Kounouhewa, B.; Koto N’Gobi, G.; Houngue, H.; Müller, L.; Wirtz, M.; Yurtsever-Kneer, S.; Fink, H.; Kneer, A.; Barbe, S. Cotonou’s next Breath: Particulate Matter Monitoring and Capturing. Sci. Afr. 2020, 8, e00367. [Google Scholar] [CrossRef]
- da Silva Leite, A.; Léon, J.-F.; Macouin, M.; Rousse, S.; da Trindade, R.I.F.; Proietti, A.; Drigo, L.; Antonio, P.Y.J.; Akpo, A.B.; Yoboué, V.; et al. PM2.5 Magnetic Properties in Relation to Urban Combustion Sources in Southern West Africa. Atmosphere 2021, 12, 496. [Google Scholar] [CrossRef]
- Kèlomé, N.C.; Lévêque, J.; Andreux, F.; Milloux, M.-J.; Oyédé, L.-M. C4 Plant Isotopic Composition (δ13C) Evidence for Urban CO2 Pollution in the City of Cotonou, Benin (West Africa). Sci. Total Environ. 2006, 366, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Avogbe, P.H.; Ayi-Fanou, L.; Autrup, H.; Loft, S.; Fayomi, B.; Sanni, A.; Vinzents, P.; Møller, P. Ultrafine Particulate Matter and High-Level Benzene Urban Air Pollution in Relation to Oxidative DNA Damage. Carcinogenesis 2005, 26, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Ayi Fanou, L.; Mobio, T.A.; Creppy, E.E.; Fayomi, B.; Fustoni, S.; Møller, P.; Kyrtopoulos, S.; Georgiades, P.; Loft, S.; Sanni, A.; et al. Survey of Air Pollution in Cotonou, Benin—Air Monitoring and Biomarkers. Sci. Total Environ. 2006, 358, 85–96. [Google Scholar] [CrossRef]
- Billet, S.; Garçon, G.; Dagher, Z.; Verdin, A.; Ledoux, F.; Cazier, F.; Courcot, D.; Aboukais, A.; Shirali, P. Ambient Particulate Matter (PM2.5): Physicochemical Characterization and Metabolic Activation of the Organic Fraction in Human Lung Epithelial Cells (A549). Environ. Res. 2007, 105, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Baneschi, I.; Dallai, L.; Giazzi, G.; Guidi, M.; Krotz, L. A Method for the Definition of the Carbon Oxidation Number in the Gases Dissolved in Waters and the Redox Variations Using an Elemental Analyser (FlashEA 1112). Preliminary Data from a Stratified Lake. J. Geochem. Explor. 2013, 124, 14–21. [Google Scholar] [CrossRef]
- Eksperiandova, L.P.; Fedorov, O.I.; Stepanenko, N.A. Estimation of Metrological Characteristics of the Element Analyzer EuroVector EA-3000 and Its Potential in the Single-Reactor CHNS Mode. Microchem. J. 2011, 99, 235–238. [Google Scholar] [CrossRef]
- Caplain, I.; Cazier, F.; Nouali, H.; Mercier, A.; Déchaux, J.-C.; Nollet, V.; Joumard, R.; André, J.-M.; Vidon, R. Emissions of Unregulated Pollutants from European Gasoline and Diesel Passenger Cars. Atmos. Environ. 2006, 40, 5954–5966. [Google Scholar] [CrossRef]
- Adeleke, M.A.; Bamgbose, J.T.; Oguntoke, O.; Itua, E.O.; Bamgbose, O. Assessment of Health Impacts of Vehicular Pollution on Occupationally Exposed People in Lagos Metropolis, Nigeria. Trace Elem. Electrolytes 2011, 28, 128–133. [Google Scholar] [CrossRef]
- Ndong Ba, A.; Cazier, F.; Verdin, A.; Garcon, G.; Cabral, M.; Courcot, L.; Diouf, A.; Courcot, D.; Gualtieri, M.; Fall, M. Physico-Chemical Characterization and in Vitro Inflammatory and Oxidative Potency of Atmospheric Particles Collected in Dakar City’s (Senegal). Environ. Pollut. 2019, 245, 568–581. [Google Scholar] [CrossRef]
- Benchrif, A.; Tahri, M.; Guinot, B.; Chakir, E.M.; Zahry, F.; Bagdhad, B.; Bounakhla, M.; Cachier, H.; Costabile, F. Aerosols in Northern Morocco-2: Chemical Characterization and PMF Source Apportionment of Ambient PM2.5. Atmosphere 2022, 13, 1701. [Google Scholar] [CrossRef]
- Tefera, W.; Kumie, A.; Berhane, K.; Gilliland, F.; Lai, A.; Sricharoenvech, P.; Samet, J.; Patz, J.; Schauer, J.J. Chemical Characterization and Seasonality of Ambient Particles (PM2.5) in the City Centre of Addis Ababa. Int. J. Environ. Res. Public Health 2020, 17, 6998. [Google Scholar] [CrossRef]
- Kinney, P.L.; Gichuru, M.G.; Volavka-Close, N.; Ngo, N.; Ndiba, P.K.; Law, A.; Gachanja, A.; Gaita, S.M.; Chillrud, S.N.; Sclar, E. Traffic Impacts on PM2.5 Air Quality in Nairobi, Kenya. Environ. Sci. Policy 2011, 14, 369–378. [Google Scholar] [CrossRef]
- Weinstein, J.P.; Hedges, S.R.; Kimbrough, S. Characterization and Aerosol Mass Balance of PM2.5 and PM10 Collected in Conakry, Guinea during the 2004 Harmattan Period. Chemosphere 2010, 78, 980–988. [Google Scholar] [CrossRef]
- Dionisio, K.L.; Rooney, M.S.; Arku, R.E.; Friedman, A.B.; Hughes, A.F.; Vallarino, J.; Agyei-Mensah, S.; Spengler, J.D.; Ezzati, M. Within-Neighborhood Patterns and Sources of Particle Pollution: Mobile Monitoring and Geographic Information System Analysis in Four Communities in Accra, Ghana. Environ. Health Perspect. 2010, 118, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-P.; Cai, M.-J.; Xu, C.; Zhang, N.; Zhou, J.-B.; Yan, J.-P.; Schwab, J.J.; Yuan, C.-S. Chemical Nature of PM2.5 and PM10 in the Coastal Urban Xiamen, China: Insights into the Impacts of Shipping Emissions and Health Risk. Atmos. Environ. 2020, 227, 117383. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mandal, T.K. Chemical Composition of Fine Mode Particulate Matter (PM2.5) in an Urban Area of Delhi, India and Its Source Apportionment. Urban Clim. 2017, 21, 106–122. [Google Scholar] [CrossRef]
- Khan, M.F.; Shirasuna, Y.; Hirano, K.; Masunaga, S. Characterization of PM2.5, PM2.5–10 and PM> 10 in Ambient Air, Yokohama, Japan. Atmos. Res. 2010, 96, 159–172. [Google Scholar] [CrossRef]
- Diapouli, E.; Manousakas, M.; Vratolis, S.; Vasilatou, V.; Maggos, T.; Saraga, D.; Grigoratos, T.; Argyropoulos, G.; Voutsa, D.; Samara, C.; et al. Evolution of Air Pollution Source Contributions over One Decade, Derived by PM10 and PM2.5 Source Apportionment in Two Metropolitan Urban Areas in Greece. Atmos. Environ. 2017, 164, 416–430. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Vultaggio, M.; Di Carlo, I. ATR-FTIR Spectral Analysis and Soluble Components of PM10 And PM2.5 Particulate Matter over the Urban Area of Palermo (Italy) during Normal Days and Saharan Events. Int. J. Environ. Res. Public Health 2019, 16, 2507. [Google Scholar] [CrossRef]
- Sillanpää, M.; Hillamo, R.; Saarikoski, S.; Frey, A.; Pennanen, A.; Makkonen, U.; Spolnik, Z.; Van Grieken, R.; Braniš, M.; Brunekreef, B.; et al. Chemical Composition and Mass Closure of Particulate Matter at Six Urban Sites in Europe. Atmos. Environ. 2006, 40 (Suppl. 2), 212–223. [Google Scholar] [CrossRef]
- Franzin, B.T.; Guizellini, F.C.; Hojo, O.; Pastre, I.A.; de Marchi, M.R.R.; Silva, H.F.; Fertonani, F.L.; Oliveira, C.M. Chemical and Morpho-Structural Characterization of Atmospheric Aerosol (PM10 and PM2.5) in a City of São Paulo State, Brazil. Environ. Sci. Pollut. Res. Int. 2021, 28, 59486–59498. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://www.who.int/publications-detail-redirect/9789240034228 (accessed on 27 December 2022).
- Okuda, T. Measurement of the Specific Surface Area and Particle Size Distribution of Atmospheric Aerosol Reference Materials. Atmos. Environ. 2013, 75, 1–5. [Google Scholar] [CrossRef]
- Courcot, D.; Laversin, H.; Ledoux, F.; Cazier, F.; Matta, J.; Cousin, R.; Aboukais, A. Composition and Textural Properties of Soot and Study of Their Oxidative Elimination by Catalytic Process. Int. J. Environ. Pollut. 2009, 39, 253–263. [Google Scholar] [CrossRef]
- Rodríguez, S.; Querol, X.; Alastuey, A.; Viana, M.-M.; Alarcón, M.; Mantilla, E.; Ruiz, C.R. Comparative PM10–PM2.5 Source Contribution Study at Rural, Urban and Industrial Sites during PM Episodes in Eastern Spain. Sci. Total Environ. 2004, 328, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.E.; Im, U.; Mezuman, K.; Gao, C.Y. Desert Dust, Industrialization, and Agricultural Fires: Health Impacts of Outdoor Air Pollution in Africa. J. Geophys. Res. Atmos. 2019, 124, 4104–4120. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Smith, S.J.; Easter, R.; Ma, P.-L.; Qian, Y.; Yu, H.; Li, C.; Rasch, P.J. Global Source Attribution of Sulfate Concentration and Direct and Indirect Radiative Forcing. Atmos. Chem. Phys. 2017, 17, 8903–8922. [Google Scholar] [CrossRef]
- Fan, W.; Chen, T.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D. A Review of Secondary Organic Aerosols Formation Focusing on Organosulfates and Organic Nitrates. J. Hazard. Mater. 2022, 430, 128406. [Google Scholar] [CrossRef]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The Characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Cheng, S. Heavy Metal Pollution in China: Origin, Pattern and Control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Cheung, K.; Daher, N.; Kam, W.; Shafer, M.M.; Ning, Z.; Schauer, J.J.; Sioutas, C. Spatial and Temporal Variation of Chemical Composition and Mass Closure of Ambient Coarse Particulate Matter (PM10–2.5) in the Los Angeles Area. Atmos. Environ. 2011, 45, 2651–2662. [Google Scholar] [CrossRef]
- Fang, G.-C.; Huang, Y.-L.; Huang, J.-H. Study of Atmospheric Metallic Elements Pollution in Asia during 2000–2007. J. Hazard. Mater. 2010, 180, 115–121. [Google Scholar] [CrossRef]
- Talbi, A.; Kerchich, Y.; Kerbachi, R.; Boughedaoui, M. Assessment of Annual Air Pollution Levels with PM1, PM2.5, PM10 and Associated Heavy Metals in Algiers, Algeria. Environ. Pollut. 2018, 232, 252–263. [Google Scholar] [CrossRef]
- Ali, M.H.; Mustafa, A.-R.A.; El-Sheikh, A.A. Geochemistry and Spatial Distribution of Selected Heavy Metals in Surface Soil of Sohag, Egypt: A Multivariate Statistical and GIS Approach. Environ. Earth Sci. 2016, 75, 1257. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of Heavy Metals in Agricultural Soils of Mediterranean: Insights from Argolida Basin, Peloponnese, Greece. Geoderma 2014, 221–222, 82–90. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy Metals from Non-Exhaust Vehicle Emissions in Urban and Motorway Road Dusts. Environ. Monit. Assess 2016, 188, 369. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Tan, J. Atmospheric Heavy Metals and Arsenic in China: Situation, Sources and Control Policies. Atmos. Environ. 2013, 74, 93–101. [Google Scholar] [CrossRef]
- Jin, Y.; O’Connor, D.; Ok, Y.S.; Tsang, D.C.W.; Liu, A.; Hou, D. Assessment of Sources of Heavy Metals in Soil and Dust at Children’s Playgrounds in Beijing Using GIS and Multivariate Statistical Analysis. Environ. Int. 2019, 124, 320–328. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council of the European Union. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:152:0001:0044:FR:PDF (accessed on 28 April 2013).
- Lee, P.-K.; Youm, S.-J.; Jo, H.Y. Heavy Metal Concentrations and Contamination Levels from Asian Dust and Identification of Sources: A Case-Study. Chemosphere 2013, 91, 1018–1025. [Google Scholar] [CrossRef]
- Gietl, J.K.; Lawrence, R.; Thorpe, A.J.; Harrison, R.M. Identification of Brake Wear Particles and Derivation of a Quantitative Tracer for Brake Dust at a Major Road. Atmos. Environ. 2010, 44, 141–146. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Chen, Y.; Chen, Z.; Xu, S. Contamination Characteristics and Possible Sources of PM10 and PM2.5 in Different Functional Areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- Klöckner, P.; Reemtsma, T.; Eisentraut, P.; Braun, U.; Ruhl, A.S.; Wagner, S. Tire and Road Wear Particles in Road Environment—Quantification and Assessment of Particle Dynamics by Zn Determination after Density Separation. Chemosphere 2019, 222, 714–721. [Google Scholar] [CrossRef]
- Choplin, A. Cementing Africa: Cement Flows and City-Making along the West African Corridor (Accra, Lomé, Cotonou, Lagos). Urban Stud. 2020, 57, 1977–1993. [Google Scholar] [CrossRef]
- Soussia, T.; Guedenon, P.; Lawani, R.; Gbaguidi, C.D.; Edorh, P.A. Assessment of Cement Dust Deposit in a Cement Factory in Cotonou (Benin). J. Environ. Prot. 2015, 06, 675. [Google Scholar] [CrossRef]
- Contini, D.; Belosi, F.; Gambaro, A.; Cesari, D.; Stortini, A.M.; Bove, M.C. Comparison of PM10 Concentrations and Metal Content in Three Different Sites of the Venice Lagoon: An Analysis of Possible Aerosol Sources. J. Environ. Sci. 2012, 24, 1954–1965. [Google Scholar] [CrossRef]
- Aldabe, J.; Elustondo, D.; Santamaría, C.; Lasheras, E.; Pandolfi, M.; Alastuey, A.; Querol, X.; Santamaría, J.M. Chemical Characterisation and Source Apportionment of PM2.5 and PM10 at Rural, Urban and Traffic Sites in Navarra (North of Spain). Atmos. Res. 2011, 102, 191–205. [Google Scholar] [CrossRef]
- Ofosu, F.G.; Hopke, P.K.; Aboh, I.J.K.; Bamford, S.A. Characterization of Fine Particulate Sources at Ashaiman in Greater Accra, Ghana. Atmos. Pollut. Res. 2012, 3, 301–310. [Google Scholar] [CrossRef]
- Mkoma, S.L.; Chi, X.; Maenhaut, W. Characteristics of Carbonaceous Aerosols in Ambient PM10 and PM2.5 Particles in Dar Es Salaam, Tanzania. Sci. Total Environ. 2010, 408, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Engling, G.; Zhang, Y.-N.; Chan, C.-Y.; Sang, X.-F.; Lin, M.; Ho, K.-F.; Li, Y.-S.; Lin, C.-Y.; Lee, J.J. Characterization and Sources of Aerosol Particles over the Southeastern Tibetan Plateau during the Southeast Asia Biomass-Burning Season. Tellus B Chem. Phys. Meteorol. 2011, 63, 117–128. [Google Scholar] [CrossRef]
- Buczynska, A.J.; Krata, A.; Stranger, M.; Locateli Godoi, A.F.; Kontozova-Deutsch, V.; Bencs, L.; Naveau, I.; Roekens, E.; Van Grieken, R. Atmospheric BTEX-Concentrations in an Area with Intensive Street Traffic. Atmos. Environ. 2009, 43, 311–318. [Google Scholar] [CrossRef]
- Liu, J.; Mu, Y.; Zhang, Y.; Zhang, Z.; Wang, X.; Liu, Y.; Sun, Z. Atmospheric Levels of BTEX Compounds during the 2008 Olympic Games in the Urban Area of Beijing. Sci. Total Environ. 2009, 408, 109–116. [Google Scholar] [CrossRef]
- Miller, L.; Xu, X.; Grgicak-Mannion, A.; Brook, J.; Wheeler, A. Multi-Season, Multi-Year Concentrations and Correlations amongst the BTEX Group of VOCs in an Urbanized Industrial City. Atmos. Environ. 2012, 61, 305–315. [Google Scholar] [CrossRef]
- Miller, L.; Lemke, L.D.; Xu, X.; Molaroni, S.M.; You, H.; Wheeler, A.J.; Booza, J.; Grgicak-Mannion, A.; Krajenta, R.; Graniero, P.; et al. Intra-Urban Correlation and Spatial Variability of Air Toxics across an International Airshed in Detroit, Michigan (USA) and Windsor, Ontario (Canada). Atmos. Environ. 2010, 44, 1162–1174. [Google Scholar] [CrossRef]
- Reche, C.; Moreno, T.; Amato, F.; Viana, M.; van Drooge, B.L.; Chuang, H.-C.; Bérubé, K.; Jones, T.; Alastuey, A.; Querol, X. A Multidisciplinary Approach to Characterise Exposure Risk and Toxicological Effects of PM10 and PM2.5 Samples in Urban Environments. Ecotoxicol. Environ. Saf. 2012, 78, 327–335. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Guo, X.; Wang, Y. Seasonal Variation and Source Apportionment of Organic and Inorganic Compounds in PM2.5 and PM10 Particulates in Beijing, China. J. Environ. Sci. 2013, 25, 741–750. [Google Scholar] [CrossRef]
- van Drooge, B.L.; Ballesta, P.P. Seasonal and Daily Source Apportionment of Polycyclic Aromatic Hydrocarbon Concentrations in PM10 in a Semirural European Area. Environ. Sci. Technol. 2009, 43, 7310–7316. [Google Scholar] [CrossRef] [PubMed]
- Pietrogrande, M.C.; Abbaszade, G.; Schnelle-Kreis, J.; Bacco, D.; Mercuriali, M.; Zimmermann, R. Seasonal Variation and Source Estimation of Organic Compounds in Urban Aerosol of Augsburg, Germany. Environ. Pollut. 2011, 159, 1861–1868. [Google Scholar] [CrossRef]
- Pindado, O.; Pérez, R.M.; García, S.; Sánchez, M.; Galán, P.; Fernández, M. Characterization and Sources Assignation of PM2.5 Organic Aerosol in a Rural Area of Spain. Atmos. Environ. 2009, 43, 2796–2803. [Google Scholar] [CrossRef]
- Callén, M.S.; de la Cruz, M.T.; López, J.M.; Mastral, A.M. PAH in Airborne Particulate Matter.: Carcinogenic Character of PM10 Samples and Assessment of the Energy Generation Impact. Fuel Process. Technol. 2011, 92, 176–182. [Google Scholar] [CrossRef]
- Dvorská, A.; Lammel, G.; Klánová, J. Use of Diagnostic Ratios for Studying Source Apportionment and Reactivity of Ambient Polycyclic Aromatic Hydrocarbons over Central Europe. Atmos. Environ. 2011, 45, 420–427. [Google Scholar] [CrossRef]
- Ladji, R.; Yassaa, N.; Balducci, C.; Cecinato, A.; Meklati, B.Y. Distribution of the Solvent-Extractable Organic Compounds in Fine (PM1) and Coarse (PM1–10) Particles in Urban, Industrial and Forest Atmospheres of Northern Algeria. Sci. Total Environ. 2009, 408, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J. PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Terzi, E.; Cai, Q.-Y. On the Use of PAH Molecular Diagnostic Ratios in Sewage Sludge for the Understanding of the PAH Sources. Is This Use Appropriate? Chemosphere 2007, 69, 1337–1339. [Google Scholar] [CrossRef]
- Cazier, F.; Genevray, P.; Dewaele, D.; Nouali, H.; Verdin, A.; Ledoux, F.; Hachimi, A.; Courcot, L.; Billet, S.; Bouhsina, S.; et al. Characterisation and Seasonal Variations of Particles in the Atmosphere of Rural, Urban and Industrial Areas: Organic Compounds. J. Environ. Sci. 2016, 44, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Landkocz, Y.; Ledoux, F.; André, V.; Cazier, F.; Genevray, P.; Dewaele, D.; Martin, P.J.; Lepers, C.; Verdin, A.; Courcot, L.; et al. Fine and Ultrafine Atmospheric Particulate Matter at a Multi-Influenced Urban Site: Physicochemical Characterization, Mutagenicity and Cytotoxicity. Environ. Pollut. 2017, 221, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Ledoux, F.; Farhat, M.; Kfoury, A.; Courcot, D.; Afif, C. PM2.5 Characterization of Primary and Secondary Organic Aerosols in Two Urban-Industrial Areas in the East Mediterranean. J. Environ. Sci. 2021, 101, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, M.; Çabuk, H. Gas–Particle Partitioning and Seasonal Variation of Polycyclic Aromatic Hydrocarbons in the Atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef]
- Ravindra, K.; Bencs, L.; Wauters, E.; de Hoog, J.; Deutsch, F.; Roekens, E.; Bleux, N.; Berghmans, P.; Van Grieken, R. Seasonal and Site-Specific Variation in Vapour and Aerosol Phase PAHs over Flanders (Belgium) and Their Relation with Anthropogenic Activities. Atmos. Environ. 2006, 40, 771–785. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K.; Pavuluri, C.M.; Swaminathan, T. Molecular Characterization of Urban Organic Aerosol in Tropical India: Contributions of Biomass/Biofuel Burning, Plastic Burning, and Fossil Fuel Combustion. Atmos. Chem. Phys. Disc. 2009, 9, 21669–21716. [Google Scholar] [CrossRef]
- Kotianová, P.; Puxbaum, H.; Bauer, H.; Caseiro, A.; Marr, I.L.; Čík, G. Temporal Patterns of N-Alkanes at Traffic Exposed and Suburban Sites in Vienna. Atmos. Environ. 2008, 42, 2993–3005. [Google Scholar] [CrossRef]
- Oliveira, C.; Pio, C.; Alves, C.; Evtyugina, M.; Santos, P.; Gonçalves, V.; Nunes, T.; Silvestre, A.J.D.; Palmgren, F.; Wåhlin, P.; et al. Seasonal Distribution of Polar Organic Compounds in the Urban Atmosphere of Two Large Cities from the North and South of Europe. Atmos. Environ. 2007, 41, 5555–5570. [Google Scholar] [CrossRef]
- Rogge, W.F.; Medeiros, P.M.; Simoneit, B.R.T. Organic Marker Compounds for Surface Soil and Fugitive Dust from Open Lot Dairies and Cattle Feedlots. Atmos. Environ. 2006, 40, 27–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).