Assessment of the Air Cleaning Performance and Humidity and Temperature Control by Five Evergreen Woody Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Plant Species
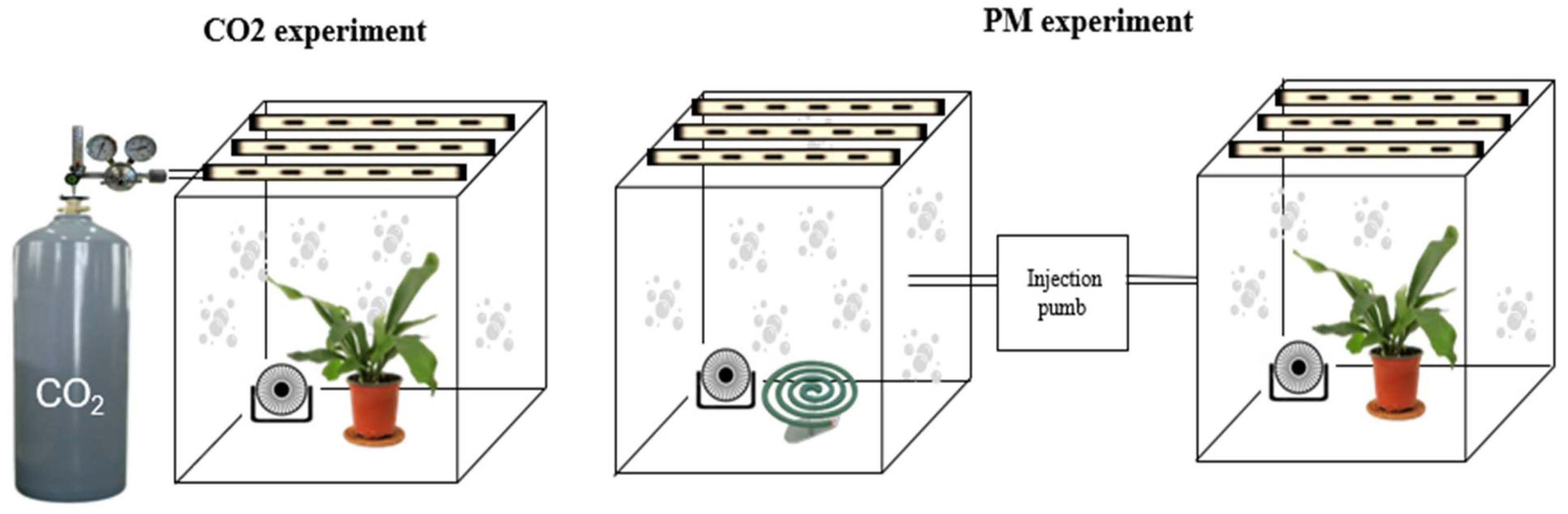
2.2. The PM and CO2 Reduction Study
2.3. Clean Air Delivery Rate (CADR) Analysis
3. Statistical Analysis
4. Results and Discussion
4.1. The Effect of Reducing PM of Plants
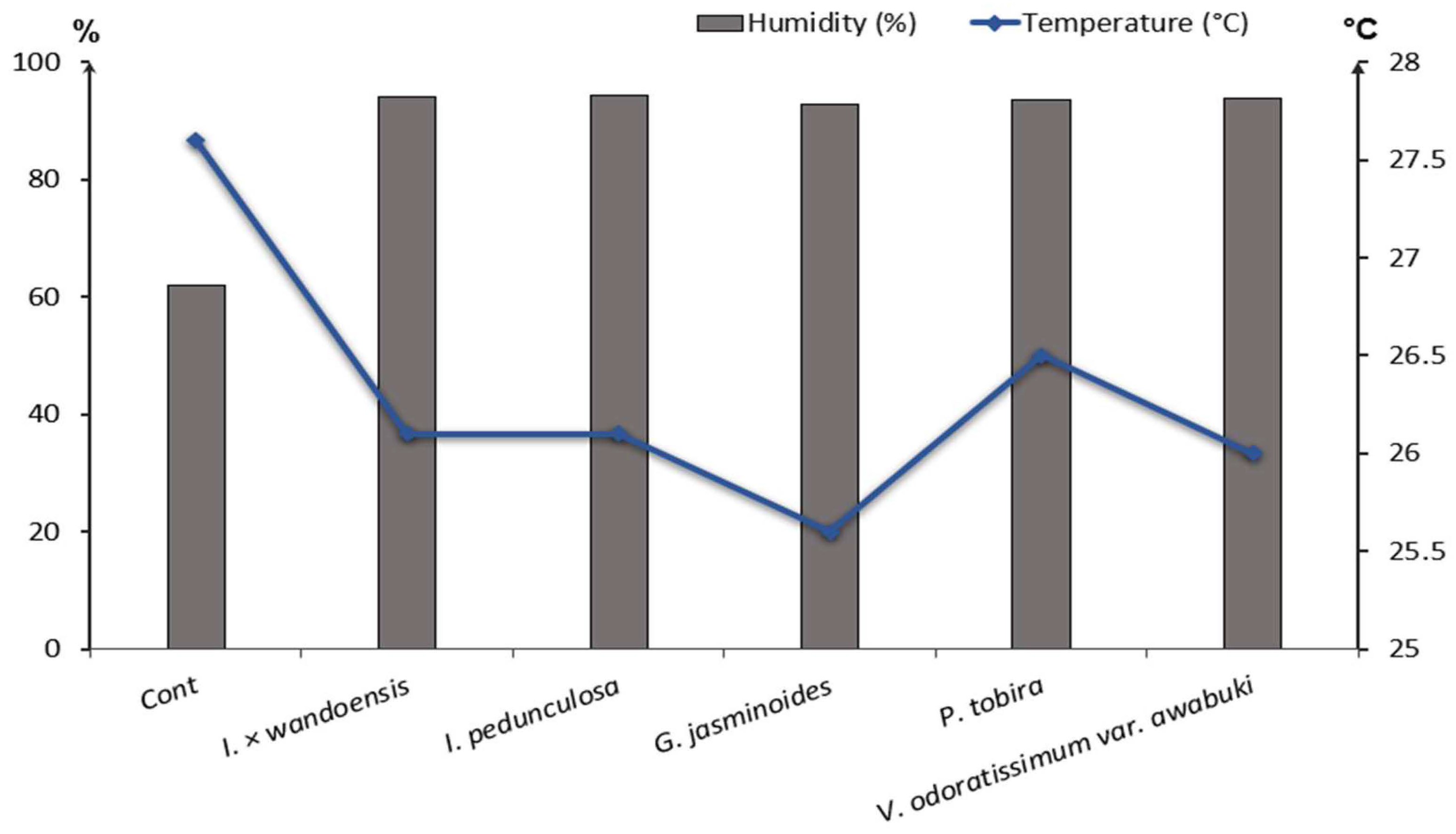
4.2. The Effectiveness of Plants at Reducing CO2 and Controlling Temperature and Humidity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nehr, S.; Duarte, R.M.B.O.; Almeida, A.S.; Baus, L.; Bergmann, K.C. Assessing the relevance of allergenic pollen in indoor environments-current knowledge base and research needs. Allergo J. Int. 2023, 32, 167–175. [Google Scholar] [CrossRef]
- Gubb, C.; Blanusa, T.; Griffiths, A.; Pfrang, C. Potted plants can remove the pollutant nitrogen dioxide indoors. Air Qual. Atmos. Health 2022, 15, 479–490. [Google Scholar] [CrossRef]
- Sowa, J.; Hendiger, J.; Maziejuk, M.; Sikora, T.; Osuchowski, Ł.; Kamińska, H. Potted plants as active and passive biofilters improving indoor air quality. IOP Conf. Ser. Earth Environ. Sci. 2019, 290, 012150. [Google Scholar] [CrossRef]
- Niza, I.L.; de Souza, M.P.; da Luz, I.M.; Broday, E.E. Sick building syndrome and its impacts on health, well-being and productivity: A systematic literature review. Indoor Built Environ. 2023. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Q. The basic roles of indoor plants in human health and comfort. Environ. Sci. Pollut. Res. Int. 2018, 25, 36087–36101. [Google Scholar] [CrossRef] [PubMed]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor exposure to selected air pollutants in the home environment: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef] [PubMed]
- Wargocki, P.; Sundell, J.; Bischof, W.; Brundrett, G.; Fanger, P.O.; Gyntelberg, F.; Hanssen, S.O.; Harrison, P.; Pickering, A.; Seppänen, O.; et al. Ventilation and health in non-industrial indoor environments: Report from a European multidisciplinary scientific consensus meeting (EUROVEN). Indoor Air 2002, 12, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Armijos-Moya, T.; de Visser, P.; Ottelé, M.; van den Dobbelsteen, A.; Bluyssen, P.M. Air cleaning performance of two species of potted plants and different substrates. Appl. Sci. 2022, 12, 248. [Google Scholar] [CrossRef]
- Jomehzadeh, F.; Nejat, P.; Calautit, J.K.; Yusof, M.B.M.; Zaki, S.A.; Hughes, B.R.; Yazid, M.N.A.W.M. A review on windcatcher for passive cooling and natural ventilation in buildings, part 1: Indoor air quality and thermal comfort assessment. Renew. Sustain. Energy Rev. 2017, 70, 736–756. [Google Scholar] [CrossRef]
- Liu, F.; Yan, L.; Meng, X.; Zhang, C. A review on indoor green plants employed to improve indoor environment. J. Build. Eng. 2022, 53, 104542. [Google Scholar] [CrossRef]
- Torpy, F.; Zavattaro, M.; Irga, P. Green wall technology for the phytoremediation of indoor air: A system for the reduction of high CO2 concentrations. Air Qual. Atmos. Health 2017, 10, 575–585. [Google Scholar] [CrossRef]
- Bandehali, S.; Miri, T.; Onyeaka, H.; Kumar, P. Current state of indoor air phytoremediation using potted plants and green walls. Atmosphere 2021, 12, 473. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco, A.; Egea, G. Influence of an active living wall on indoor temperature and humidity conditions. Ecol. Eng. 2016, 90, 120–124. [Google Scholar] [CrossRef]
- Cummings, B.E.; Waring, M.S. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Corada, K.; Woodward, H.; Alaraj, H.; Collins, C.M.; de Nazelle, A. A systematic review of the leaf traits considered to contribute to removal of airborne particulate matter pollution in urban areas. Environ. Pollut. 2021, 269, 116104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, D.Y.; Park, S.H.; Woo, S.Y.; Nie, H.; Kim, S.H. Particulate matter (PM) adsorption and leaf characteristics of ornamental sweet potato (Ipomoea batatas L.) cultivars and two common indoor plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre). Horticulturae 2022, 8, 26. [Google Scholar] [CrossRef]
- Popek, R.; Fornal-Pieniak, B.; Chyliński, F.; Pawełkowicz, M.; Bobrowicz, J.; Chrzanowska, D.; Piechota, N.; Przybysz, A. not only trees matter—Traffic-related PM accumulation by vegetation of urban forests. Sustainability 2022, 14, 2973. [Google Scholar] [CrossRef]
- Ter, S.; Chettri, M.K.; Shakya, K. Air pollution tolerance index of some tree species of Pashupati and Budhanilkantha area, Kathmandu. Amrit Res. J. 2020, 1, 20–28. [Google Scholar] [CrossRef]
- Gubb, C.; Blanusa, T.; Griffiths, A.; Pfrang, C. Interaction between plant species and substrate type in the removal of CO2 indoors. Air Qual. Atmos. Health 2019, 12, 1197–1206. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, H.; Li, J.; Xing, J.; Xu, N.; Zou, H. Water use efficiency and photosynthesis of Calamagrostis angustifolia leaves under drought stress through CO2 concentration increase. J. Plant Interact. 2022, 17, 60–74. [Google Scholar] [CrossRef]
- Dominici, L.; Fleck, R.; Gill, R.L.; Pettit, T.J.; Irga, P.J.; Comino, E.; Torpy, F.R. Analysis of lighting conditions of indoor living walls: Effects on CO2 removal. J. Build. Eng. 2021, 44, 102961. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, S.; Zhou, B.; Zhang, X.; Zhao, Z. Plant stomatal conductance determined transpiration and photosynthesis both contribute to the enhanced negative air ion (NAI). Ecol. Indic. 2021, 130, 108114. [Google Scholar] [CrossRef]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Quantification of the traffic-generated particulate matter capture by plant species in a living wall and evaluation of the important leaf characteristics. Sci. Total Environ. 2018, 635, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.; Odsuren, U.; Kim, S.Y.; Park, B.J. Particulate matter accumulation and leaf traits of ten woody species growing with different air pollution conditions in Cheongju city, South Korea. Atmosphere 2022, 13, 1351. [Google Scholar] [CrossRef]
- Moya, T.A.; van den Dobbelsteen, A.; Ottelé, M.; Bluyssen, P.M. A review of green systems within the indoor environment. Indoor Built Environ. 2019, 28, 298–309. [Google Scholar] [CrossRef]
- Räsänen, J.V.; Holopainen, T.; Joutsensaari, J.; Ndam, C.; Pasanen, P.; Rinnan, Å.; Kivimäenpää, M. Effects of species-specific leaf characteristics and reduced water availability on fine particle capture efficiency of trees. Environ. Pollut. 2013, 183, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Popek, R.; Stankiewicz-Kosyl, M.; Zhu, C.Y.; Małecka-Przybysz, M.; Maulidyawati, T.; Mikowska, K.; Deluga, D.; Griżuk, K.; Sokalski-Wieczorek, J.; et al. Where trees cannot grow–Particulate matter accumulation by urban meadows. Sci. Total Environ. 2021, 785, 147310. [Google Scholar] [CrossRef]
- Sgrigna, G.; Baldacchini, C.; Dreveck, S.; Cheng, Z.; Calfapietra, C. Relationships between air particulate matter capture efficiency and leaf traits in twelve tree species from an Italian urban-industrial environment. Sci. Total Environ. 2020, 718, 137310. [Google Scholar] [CrossRef]
- Yadav, R.; Pandey, P. Assessment of air pollution tolerance index (APTI) and anticipated performance index (API) of roadside plants for the development of greenbelt in urban area of Bathinda city, Punjab, India. Bull. Environ. Contam. Toxicol. 2020, 105, 906–914. [Google Scholar] [CrossRef]
- Jang, B.K.; Park, K.; Lee, S.Y.; Lee, H.; Yeon, S.H.; Ji, B.; Lee, C.H.; Cho, J.S. Screening of particulate matter reduction ability of 21 indigenous Korean evergreen species for indoor use. Int. J. Environ. Res. Public Health 2021, 18, 9803. [Google Scholar] [CrossRef]
- Mølgaard, B.; Koivisto, A.J.; Hussein, T.; Hämeri, K. A new clean air delivery rate test applied to five portable indoor air cleaners. Aerosol Sci. Technol. 2014, 48, 409–417. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor air quality: A review of cleaning technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Han, K.T.; Ruan, L.W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, F.; Wang, Y.; Yu, Y.; Wang, Z.; Liu, X.; Ding, K. Assisted deposition of PM2.5 from indoor air by ornamental potted plants. Sustainability 2019, 11, 2546. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Gong, J.; Yang, B.; Zhang, Z.; Wang, B.; Zhu, C.; Shi, J.; Yue, K. Comparison of the suitability of plant species for greenbelt construction based on particulate matter capture capacity, air pollution tolerance index, and antioxidant system. Environ. Pollut. 2020, 263, 114615. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Chen, Q. Potential of thirteen urban greening plants to capture particulate matter on leaf surfaces across three levels of ambient atmospheric pollution. Int. J. Environ. Res. Public Health 2019, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Meng, H.; Zhang, T. How does leaf surface micromorphology of different trees impact their ability to capture particulate matter? Forests 2018, 9, 681. [Google Scholar] [CrossRef]
- Kong, L.; Yu, H.; Chen, M.; Piao, Z.; Dang, J.; Sui, Y. Effects of particle matters on plant: A review. Phyton 2019, 88, 367–378. [Google Scholar] [CrossRef]
- Sengupta, S.; Sengupta, S.; Chanda, C.K.; Saha, H. Modeling the effect of relative humidity and precipitation on photovoltaic dust accumulation processes. IEEE J. Photovolt. 2021, 11, 1069–1077. [Google Scholar] [CrossRef]
- Kim, J.J.; Hann, T.; Lee, S.J. Effect of flow and humidity on indoor deposition of particulate matter. Environ. Pollut. 2019, 255, 113263. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in situ pilot-scale phytoremediation of airborne VOCs and particulate matter with an active green wall. Air Qual. Atmos. Health 2019, 12, 33–44. [Google Scholar] [CrossRef]
- Panyametheekul, S.; Rattanapun, T.; Ongwandee, M. Ability of artificial and live houseplants to capture indoor particulate matter. Indoor Built Environ. 2018, 27, 121–128. [Google Scholar] [CrossRef]
- Das, M.; Das, M.; Mukherjee, A. Air pollution tolerance index (APTI) used for assessing air quality to alleviate climate change: A review. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 45–54. [Google Scholar]
- Muhammad, S.; Wuyts, K.; Samson, R. Immobilized atmospheric particulate matter on leaves of 96 urban plant species. Environ. Sci. Pollut. Res. 2020, 27, 36920–36938. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Urrintuya, O.; Kim, S.Y.; Yang, J.C.; Sung, J.W.; Park, B.J. Removal potential of particulate matter of 12 woody plant species for landscape planting. J. People Plants Environ. 2020, 23, 647–654. [Google Scholar] [CrossRef]
- Dela Cruz, M.; Christensen, J.H.; Thomsen, J.D.; Müller, R. Can ornamental potted plants remove volatile organic compounds from indoor air?—A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 13909–13928. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Awad, J. Improving the IAQ for learning efficiency with indoor plants in university classrooms in Ajman, United Arab Emirates. Buildings 2021, 11, 289. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H.; Del Pilar Hernandez, A. Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynth. Res. 1985, 7, 137–149. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Torpy, F.R.; Irga, P.J.; Burchett, M.D. Profiling indoor plants for the amelioration of high CO2 concentrations. Urban For. Urban Green. 2014, 13, 227–233. [Google Scholar] [CrossRef]
- Mulenga, C.; Clarke, C.; Meincken, M. Physiological and growth responses to pollutant-induced biochemical changes in plants: A review. Pollution 2020, 6, 827–848. [Google Scholar] [CrossRef]
- Chauhan, A. Photosynthetic pigment changes in some selected trees induced by automobile exhaust in Dehradun, Uttarakhand. New York Sci. J. 2010, 3, 45–51. [Google Scholar]
- Zheng, Y.; Li, F.; Hao, L.; Shedayi, A.A.; Guo, L.; Ma, C.; Huang, B.; Xu, M. The optimal CO2 concentrations for the growth of three perennial grass species. BMC Plant Biol. 2018, 18, 27. [Google Scholar] [CrossRef]
- Suhaimi, M.M.; Leman, A.M.; Afandi, A.; Hariri, A.; Idris, A.F.A.; Dzulkifli, S.N.M.; Gani, P. Effectiveness of indoor plant to reduce CO2 in indoor environment. MATEC Web Conf. 2017, 103, 05004. [Google Scholar] [CrossRef]
- Weerasinghe, N.H.; Silva, P.K.; Jayasinghe, R.R.; Abeyrathna, W.P.; John, G.K.P.; Halwatura, R.U. Reducing CO2 level in the indoor urban built environment: Analysing indoor plants under different light levels. Clean. Eng. Technol. 2023, 14, 100645. [Google Scholar] [CrossRef]

| Species | Plant Height (cm) | Leaf Area (cm−2) | Photosynthetic Rate (µmol CO2 m−2s−1) |
|---|---|---|---|
| Ilex × wandoensis | 41.66 ± 3.23 | 979.85 ± 235.46 | 3.20 ± 0.09 |
| Ilex pedunculosa | 40.80 ± 5.56 | 877.01 ± 103.71 | 1.40 ± 0.03 |
| Gardenia jasminoides | 43.12 ± 4.60 | 710.61 ± 126.52 | 1.95 ± 0.02 |
| Pittosporum tobira | 42.32 ± 2.08 | 443.89 ± 152.56 | 2.63 ± 0.04 |
| Viburnum odoratissimum var. awabuki | 38.98 ± 5.40 | 961.28 ± 168.71 | 3.03 ± 0.01 |
| Species | CADR (m3/h/Leaf Area) | ||
|---|---|---|---|
| PM1 | PM2.5 | PM10 | |
| Ilex × wandoensis | 1.02 ± 0.39 | 0.99 ± 0.38 | 0.85 ± 0.18 |
| Ilexpedunculosa | 3.01 ± 0.94 | 3.08 ± 0.96 | 3.04 ± 0.97 |
| Gardenia jasminoides | 1.26 ± 0.47 | 1.18 ± 0.45 | 1.04 ± 0.41 |
| Pittosporum tobira | 1.37 ± 0.20 | 1.26 ± 0.36 | 1.13 ± 0.46 |
| Viburnum odoratissimum var. awabuki | 0.68 ± 0.09 | 0.73 ± 0.10 | 0.67 ± 0.11 |
| Species | Time (h) | RH (%) | T (°C) | N(0) | N(t) | λe | λn | CADR (m3/h/Leaf Area) | ŋ (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 7 | 62.01 ± 1.23 | 27.6 ± 0.25 | 1848.97 | 1835.65 | 0.001 | - | 0.72 | |
| Ilex × wandoensis | 7 | 93.98 ± 2.58 | 26.1 ± 0.59 | 1800.37 | 701.28 | 0.135 | 0.87 ± 0.22 | 61.05 | |
| Ilexpedunculosa | 7 | 94.20 ± 3.09 | 26.1 ± 0.68 | 1801.09 | 805.68 | 0.115 | 0.83 ± 0.15 | 55.27 | |
| Gardenia jasminoides | 7 | 92.84 ± 3.93 | 25.6 ± 0.49 | 1889.65 | 1091.17 | 0.078 | 0.70 ± 0.13 | 42.26 | |
| Pittosporum tobira | 7 | 93.63 ± 1.20 | 26.5 ± 0.27 | 1800.73 | 1414.45 | 0.034 | 0.48 ± 0.27 | 21.45 | |
| Viburnum odoratissimum var. awabuki | 7 | 93.81 ± 3.20 | 26.0 ± 0.70 | 1789.57 | 592.20 | 0.158 | 1.04 ± 0.06 | 66.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, H.-T.; Park, J.; Lee, E.; Cho, W.; Kwon, H.; Park, B.-J. Assessment of the Air Cleaning Performance and Humidity and Temperature Control by Five Evergreen Woody Plants. Atmosphere 2023, 14, 1819. https://doi.org/10.3390/atmos14121819
Bui H-T, Park J, Lee E, Cho W, Kwon H, Park B-J. Assessment of the Air Cleaning Performance and Humidity and Temperature Control by Five Evergreen Woody Plants. Atmosphere. 2023; 14(12):1819. https://doi.org/10.3390/atmos14121819
Chicago/Turabian StyleBui, Huong-Thi, Jihye Park, Eunyoung Lee, Wonwoo Cho, Hyuckhwan Kwon, and Bong-Ju Park. 2023. "Assessment of the Air Cleaning Performance and Humidity and Temperature Control by Five Evergreen Woody Plants" Atmosphere 14, no. 12: 1819. https://doi.org/10.3390/atmos14121819
APA StyleBui, H.-T., Park, J., Lee, E., Cho, W., Kwon, H., & Park, B.-J. (2023). Assessment of the Air Cleaning Performance and Humidity and Temperature Control by Five Evergreen Woody Plants. Atmosphere, 14(12), 1819. https://doi.org/10.3390/atmos14121819






