Temporal Variation and Potential Sources of Water-Soluble Inorganic Ions in PM2.5 in Two Sites of Mexico City
Abstract
:1. Introduction
2. Materials and Methods
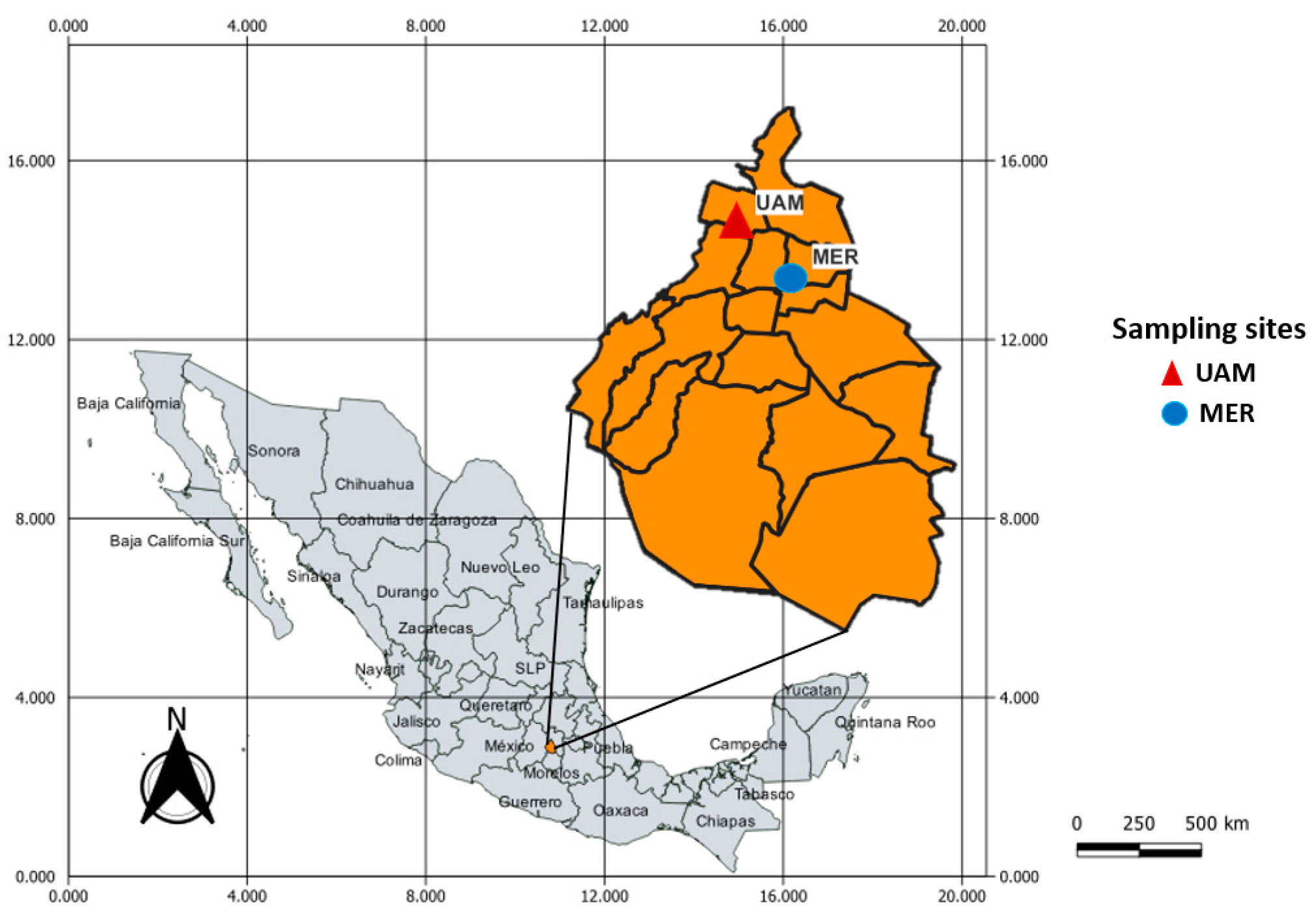
2.1. Sampling Sites
2.2. Identification and Quantification of Water Soluble Inorganic Ions
2.3. Measurement of pH and Conductivity
2.4. Ion Balance and PM2.5 Acidity
2.5. Conversion Flow Rate SOR and NOR
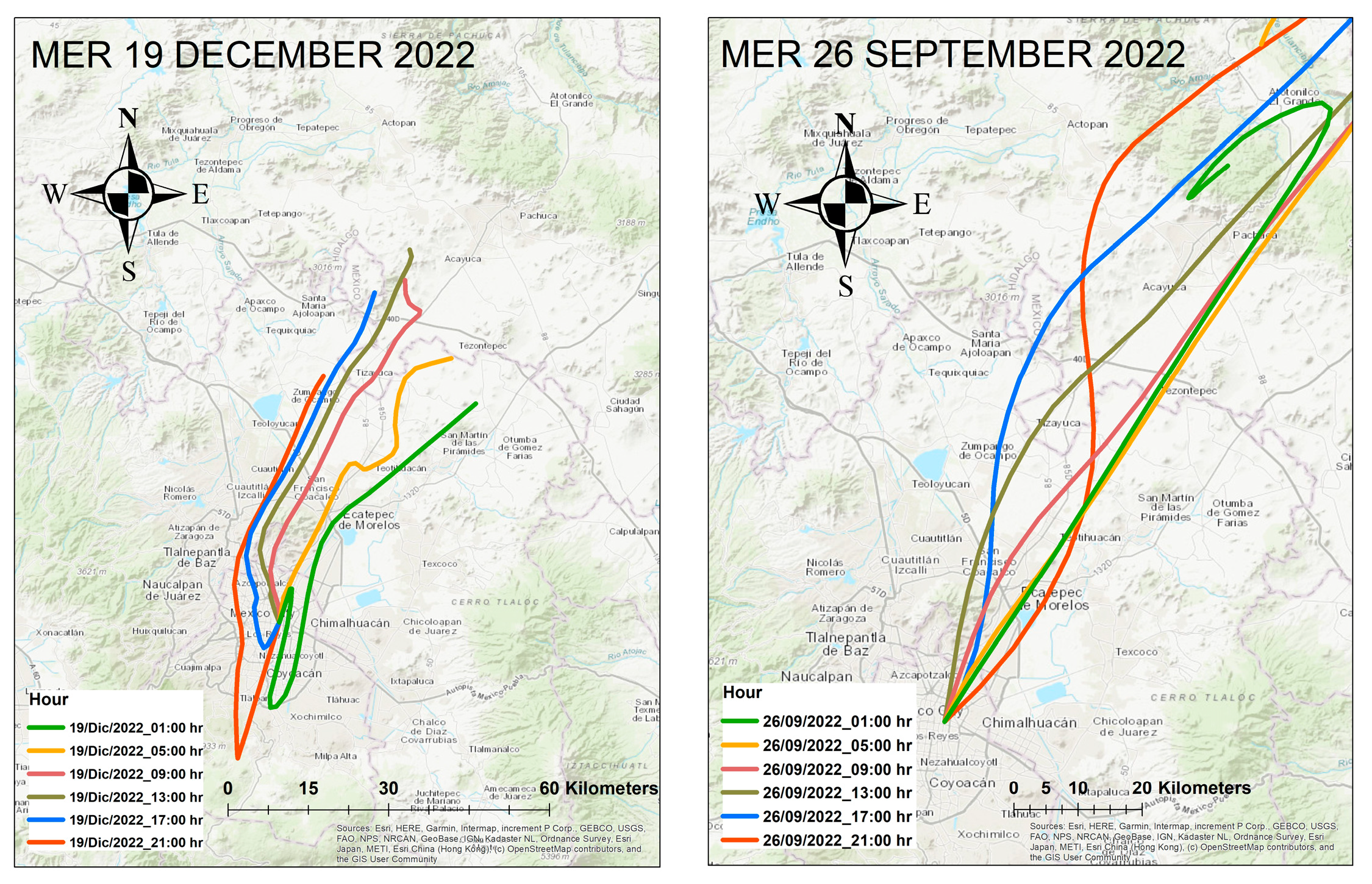
2.6. Aerosol Trajectories with HYSPLIT
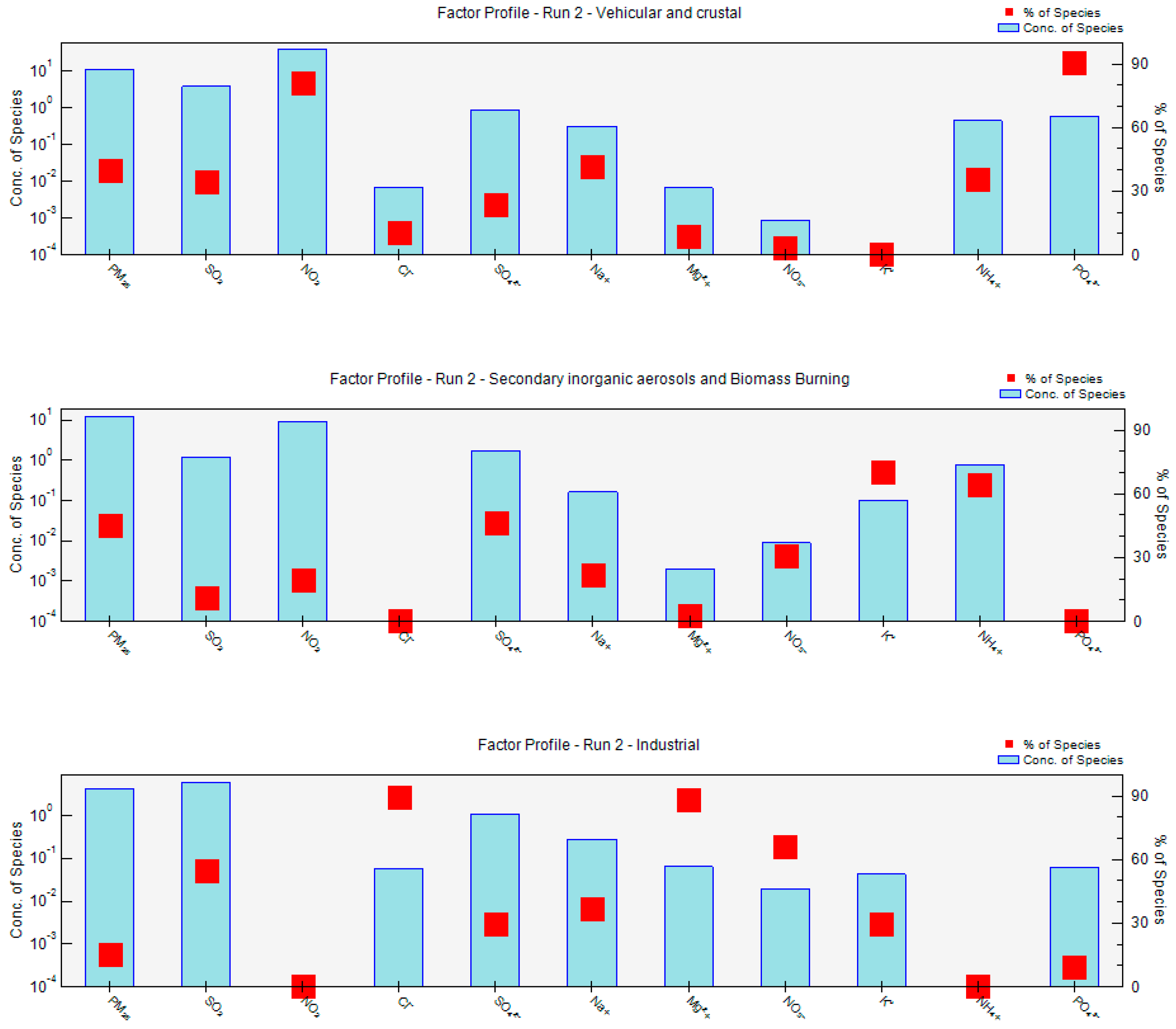
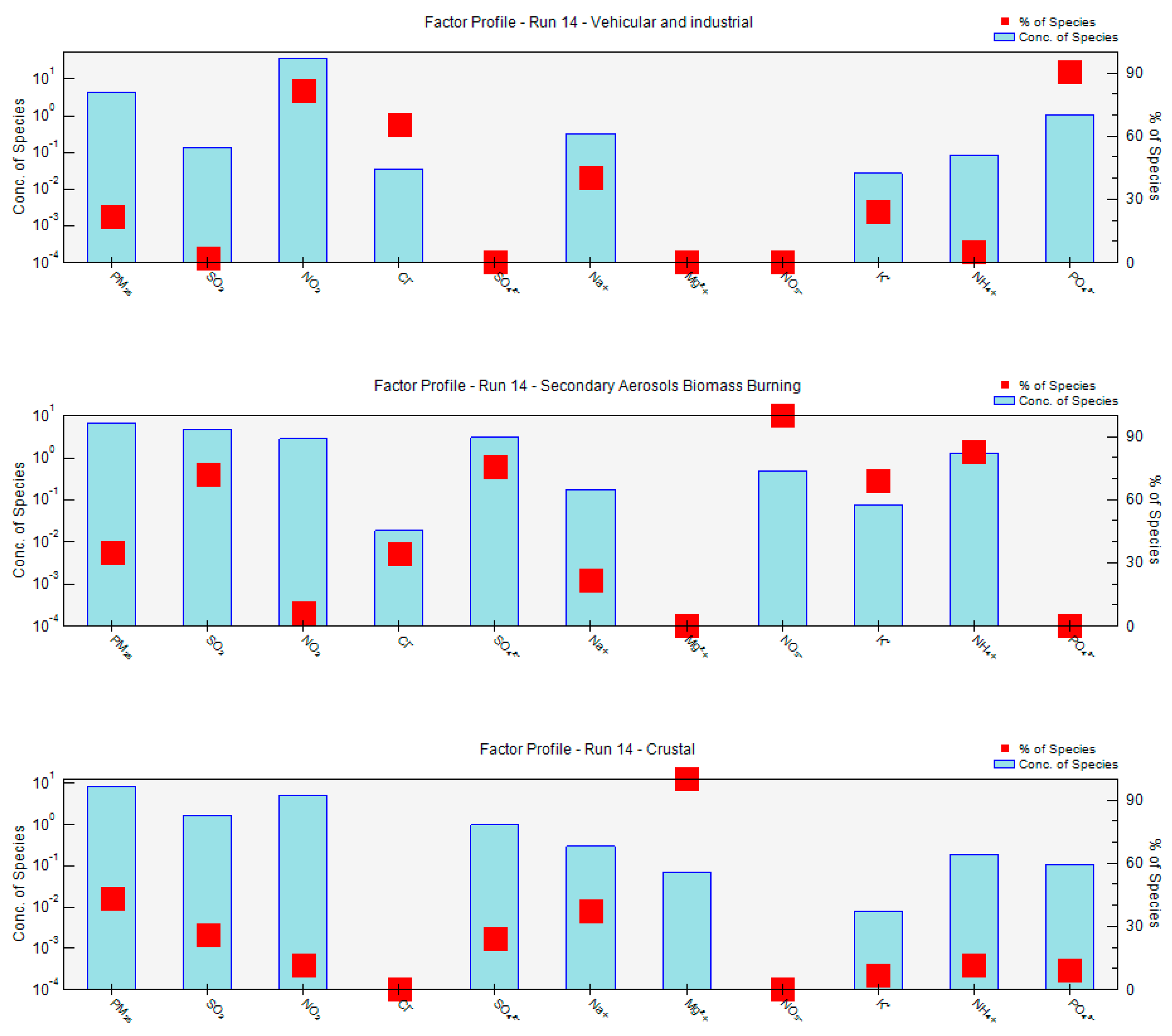
2.7. Receptor Model Analysis PMF
2.8. Quality Control and Quality Assurance
2.9. Statistical Analysis
3. Results and Discussion
3.1. PM2.5 and Water Soluble Inorganic Ion Concentrations
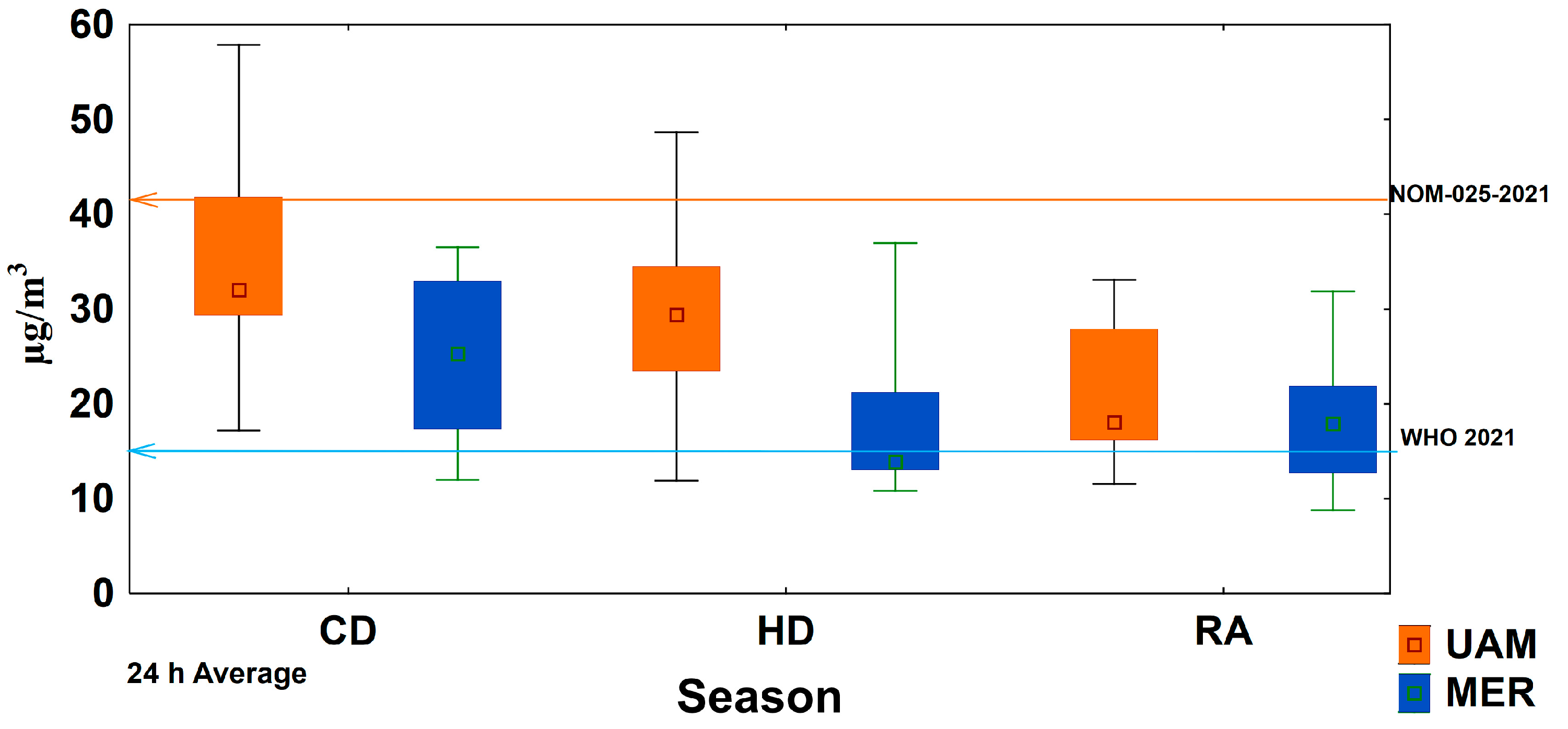
3.2. Ion Balance and Seasonal Acidity
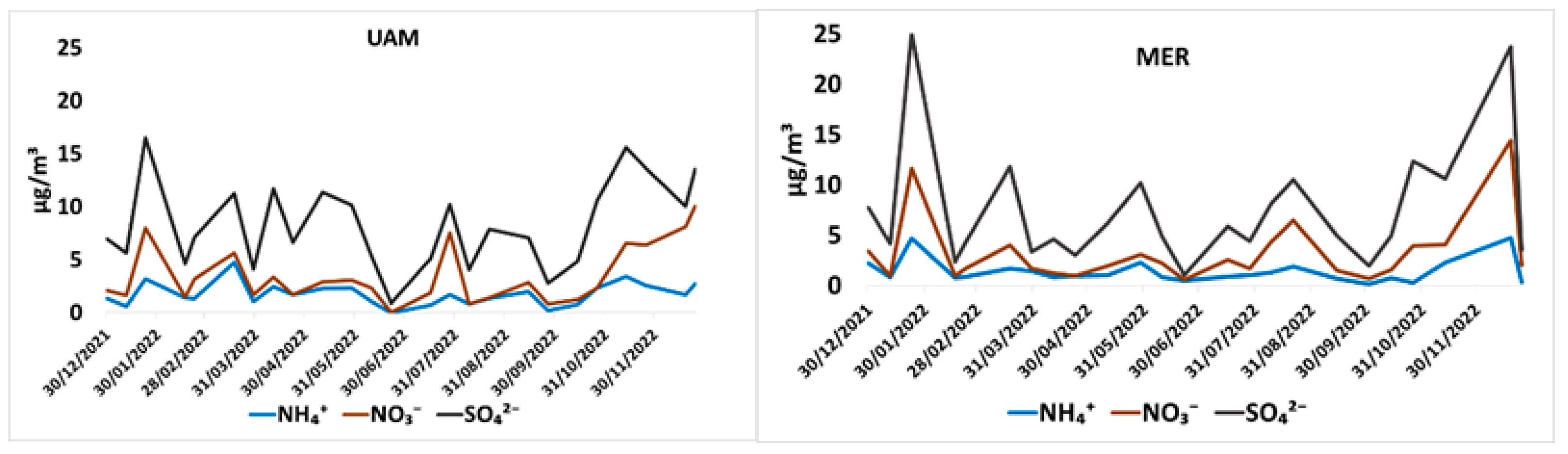
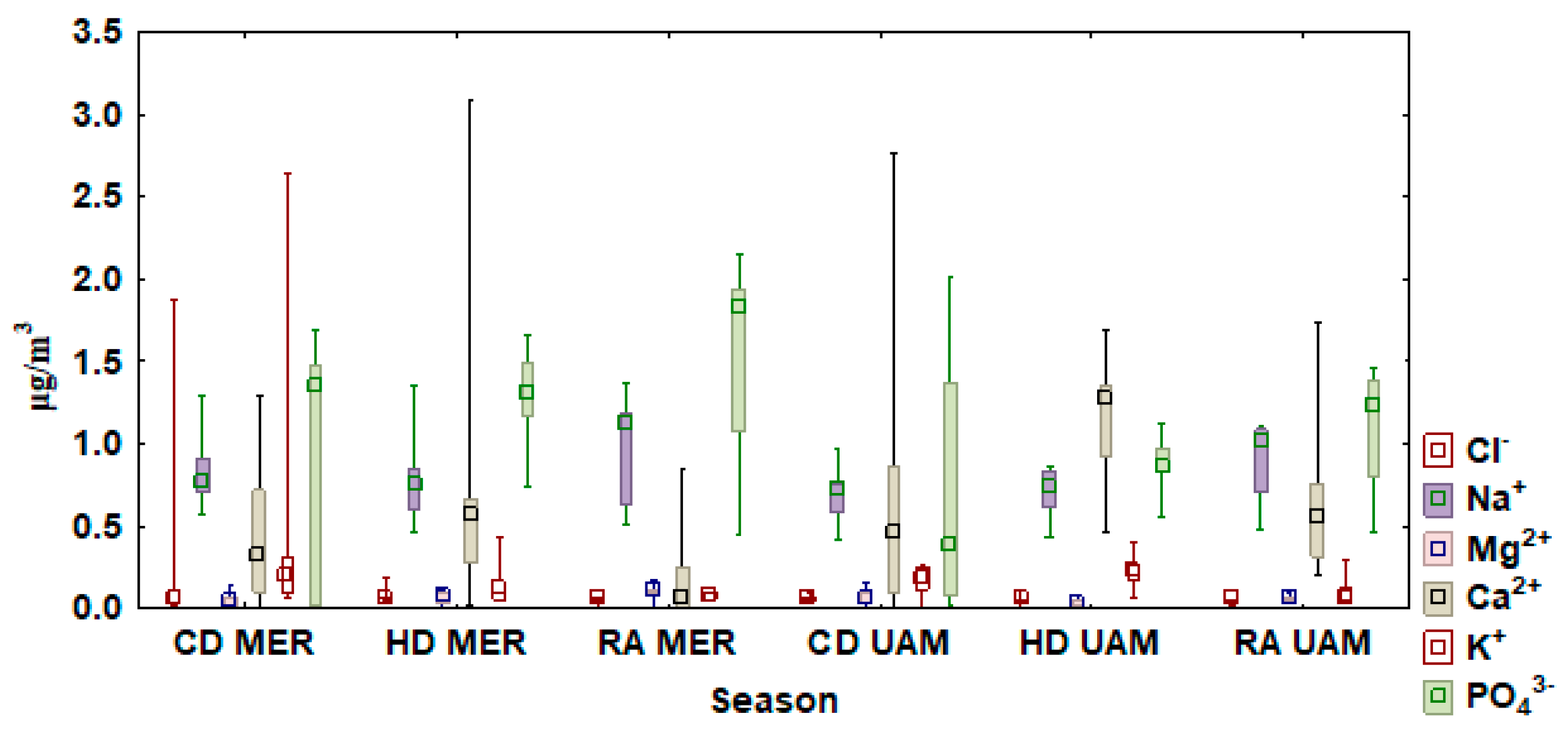
3.3. Water Soluble Ion Concentrations
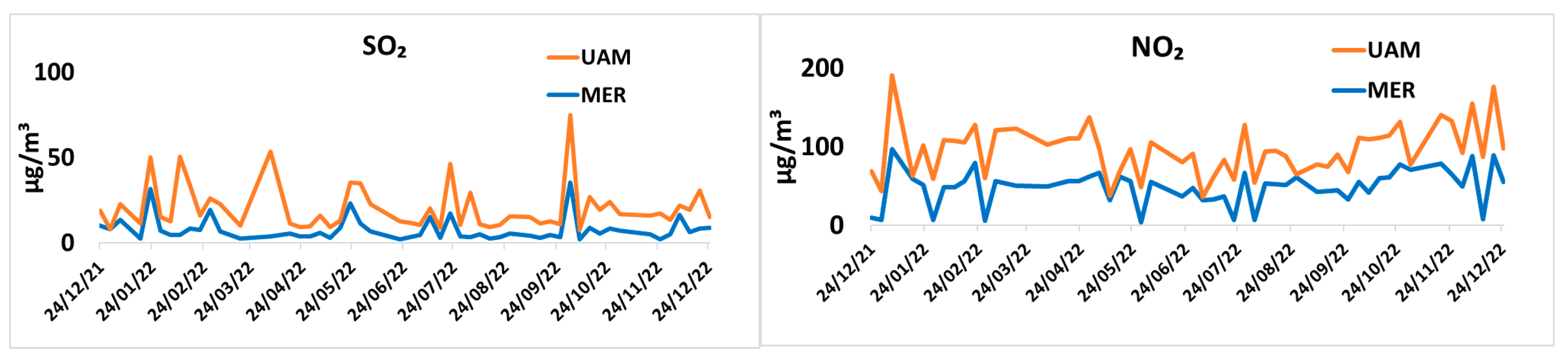
3.4. Water Soluble Inorganic Ion Concentrations
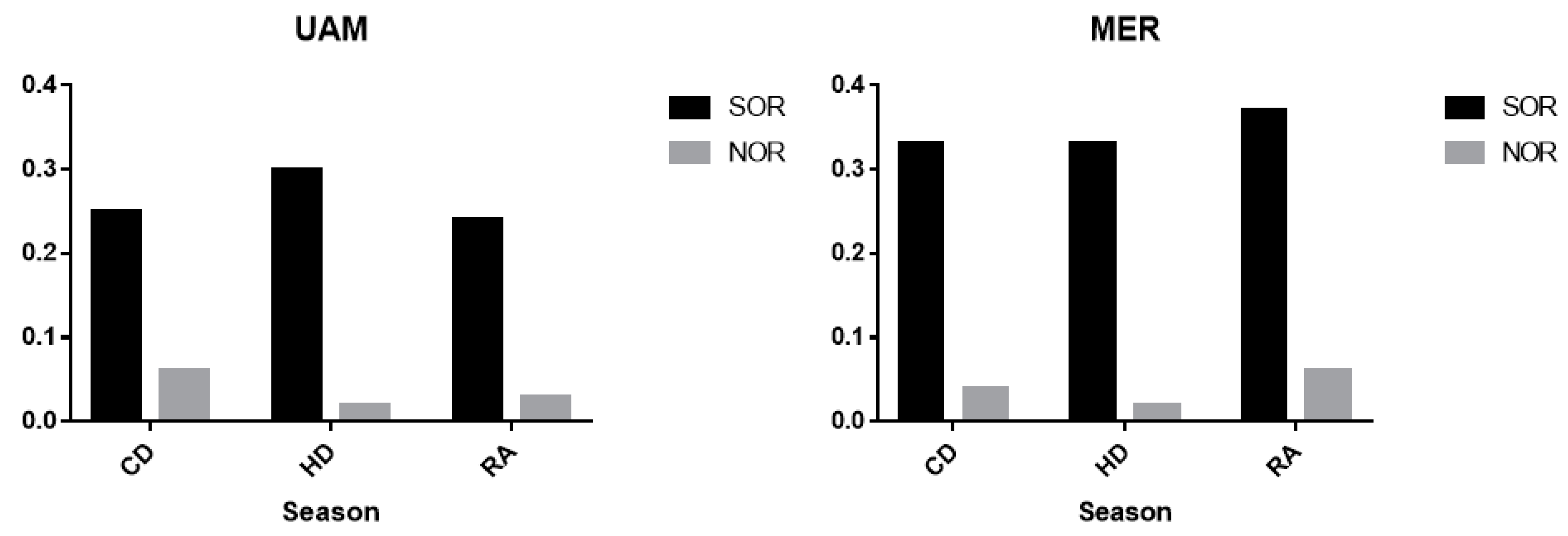
3.5. Sulfur Oxidation Rate and Nitrogen Oxidation Rate
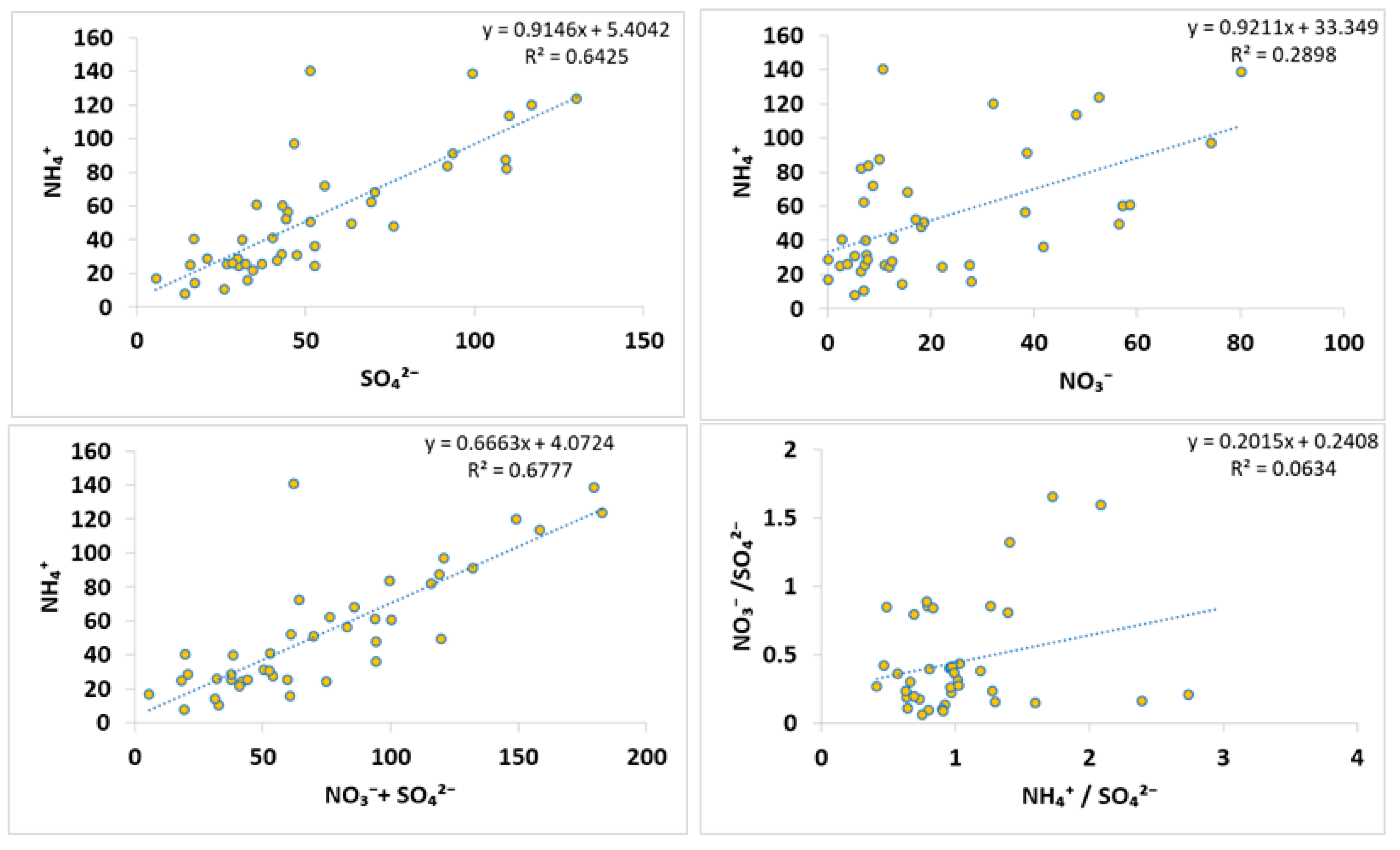
3.6. Molar Ratios
3.7. Meteorological Information
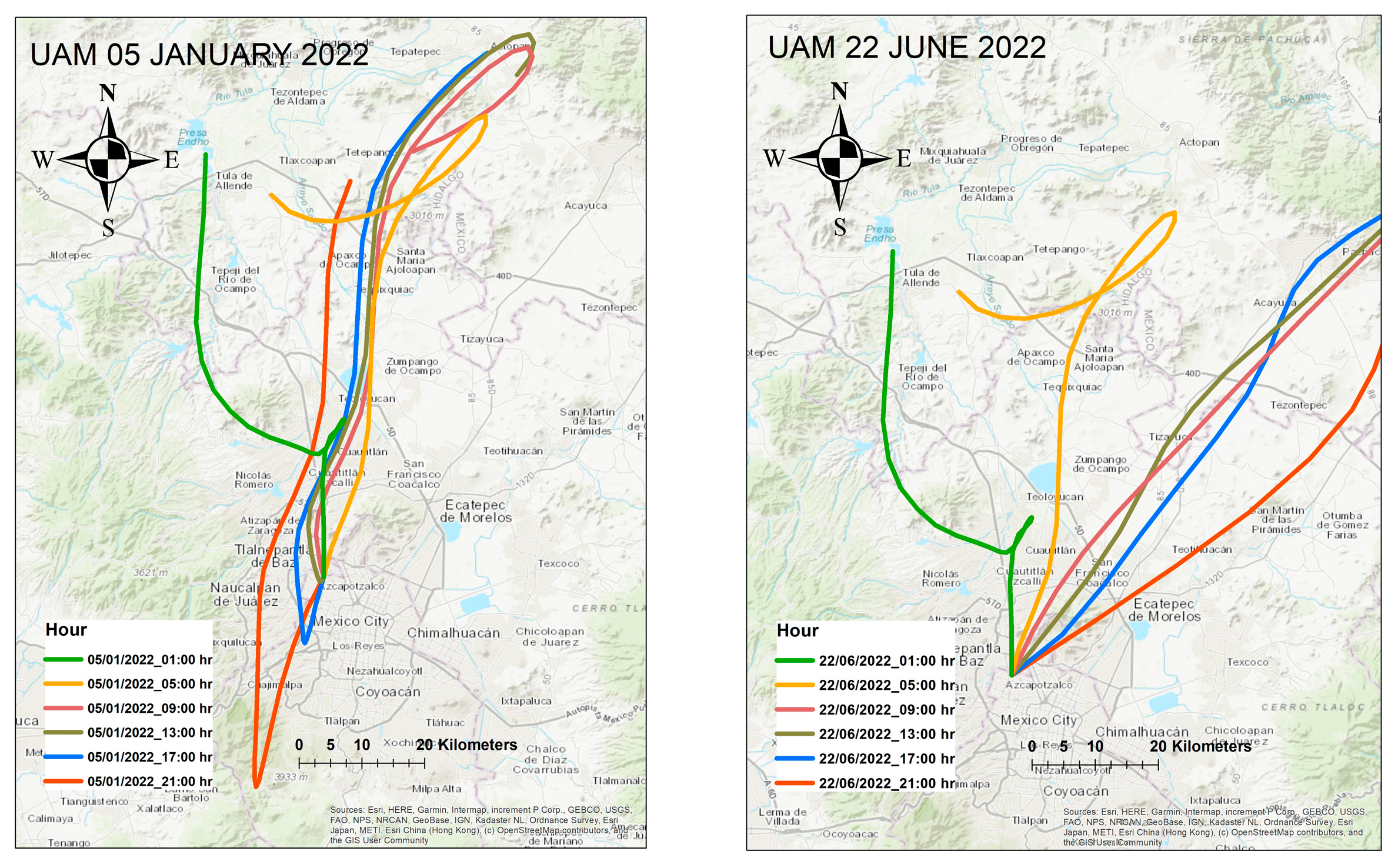
3.8. Hysplit Backward Trajectories
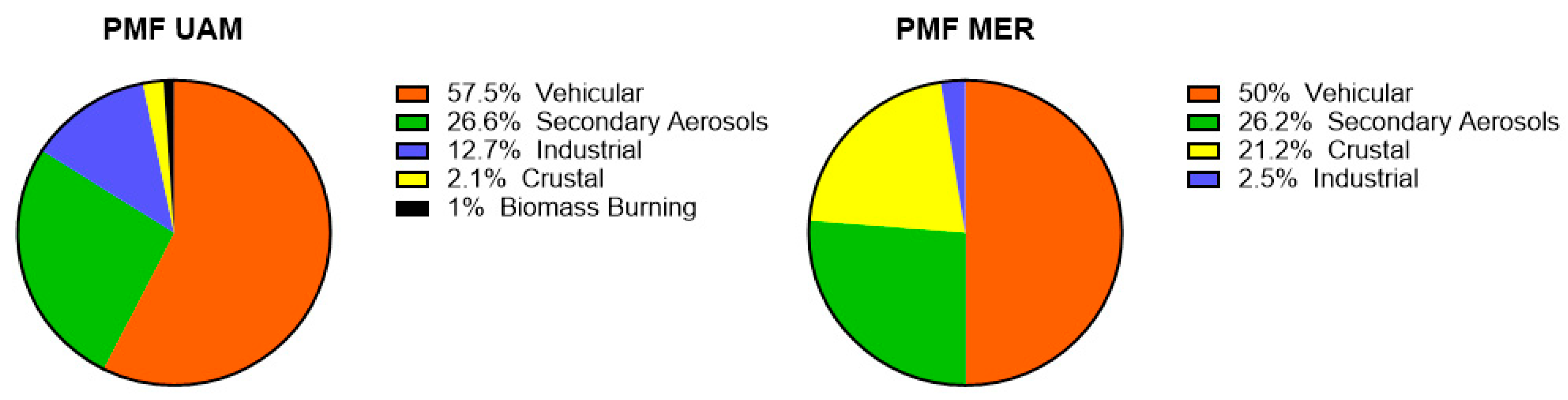
3.9. Positive Matrix Factorization Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Global Guidelines on Air Quality: Suspended Particles (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Summary [WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide 2021. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 15 October 2023).
- Maya, A.; Del Pilar, M.; Posada, P.I.M.; Nowak, D.J.; Hoehn, R.E. Remoción de contaminantes atmosféricos por el bosque urbano en el valle de Aburrá. Colomb. For. 2019, 22, 5–16. [Google Scholar] [CrossRef]
- Correa, M.A.; Franco, S.A.; Gómez, L.M.; Aguiar, D.; Colorado, H.A. Characterization Methods of Ions and Metals in Partic-ulate Matter Pollutants on PM2.5 and PM10 Samples from Several Emission Sources. Sustainability 2023, 15, 4402. [Google Scholar] [CrossRef]
- Wang, D.; Qu, W.J.; Cao, G.L.; Zhang, X.Y.; Che, H.Z. (Analysis of water-soluble species in emission particulate from regional stalk burning and their emission factors). China Powder Sci. Technol. 2008, 29, 305–309. [Google Scholar]
- Parrish, D.D.; Kuster, W.C.; Shao, M.; Yokouchi, Y.; Kondo, Y.; Goldan, P.D.; de Gouw, J.A.; Koike, M.; Shirai, T. Comparison of air pollutant emissions among mega-cities. Atmos. Environ. 2009, 43, 6435–6441. [Google Scholar] [CrossRef]
- Mugica, V.; Ortiz, E.; Molina, L.; De Vizcaya-Ruiz, A.; Nebot, A.; Quintana, R.; Aguilar, J.; Alcántara, E. PM Composition and Source Reconciliation in Mexico City. Atmos. Environ. 2009, 43, 5068–5074. [Google Scholar]
- Molina, L.; Molina, M.J. (Eds.) Air Quality in the Mexico Megacity: An Integrated Assessment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; Volume 2. [Google Scholar]
- WHO. Air Quality Deteriorating in Many of the World’s Cities. Available online: https://www.who.int/news/item/07-05-2014-air-quality-deteriorating-in-many-of-the-world-s-cities (accessed on 30 September 2023).
- IPCC, Climate Change. The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 996. [Google Scholar]
- Zhang, L.; Vet, R.; Wiebe, A.; Mihele, C.; Sukloff, B.; Chan, E.; Moran, M.D.; Iqbal, S. Characterization of the size-segregated water-soluble inorganic ions at eight Canadian rural sites. Atmos. Meas. Tech. 2008, 8, 7133–7151. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.J.; Tie, X.X.; Shen, Z.X.; Liu, S.X.; Ding, H.; Li, W.T. Water-soluble ions in atmospheric aerosols measured in Xi’an, China: Seasonal variations and sources. Atmos. Res. 2011, 102, 110–119. [Google Scholar] [CrossRef]
- Salam, A.; Assaduzzaman; Hossain, M.N.; Alam Siddiki, A.K.M.N. Water Soluble Ionic Species in the Atmospheric Fine Particulate Matters (PM2.5) in a Southeast Asian Mega City (Dhaka, Bangladesh). Open J. Air Pollut. 2015, 4, 99–108. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Błaszczak, B.; Szopa, S.; Klejnowski, K.; Sówka, I.; Zwoździak, A.; Jabłońska, M.; Mathews, B. PM2.5 in the central part of Upper Silesia, Poland: Concentrations, elemental composition, and mobility of components. Environ. Monit. Assess. 2013, 185, 581–601. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Y.; Hu, B.; Wen, T.; Xin, J.; Li, X.; Wang, Y. Size-resolved aerosol water-soluble ions during the summer and winter seasons in Beijing: Formation mechanisms of secondary inorganic aerosols. Chemosphere 2017, 183, 119–131. [Google Scholar] [CrossRef]
- Farren, N.J.; Dunmore, R.E.; Mead, M.I.; Nadzir, M.S.M.; Abu Samah, A.; Phang, S.-M.; Bandy, B.J.; Sturges, W.T.; Hamilton, J.F. Chemical characterisation of water-soluble ions in atmospheric particulate matter on the east coast of Peninsular Malaysia. Atmos. Meas. Tech. 2019, 19, 1537–1553. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Han, L.; Cheng, S.; Zhuang, G.; Ning, H.; Wang, H.; Wei, W.; Zhao, X. The changes and long-range transport of PM2.5 in Beijing in the past decade. Atmos. Environ. 2015, 110, 186–195. [Google Scholar] [CrossRef]
- Berner, E.K. Acid Rain and Acid Deposition; Salem Press Encyclopedia of Science; Grey House Publishing: Amenia, NY, USA, 2013. [Google Scholar]
- Zhang, Y.; Tang, A.; Wang, C.; Ma, X.; Li, Y.; Xu, W.; Xia, X.; Zheng, A.; Li, W.; Fang, Z.; et al. PM2.5 and water-soluble inorganic ion concentrations decreased faster in urban than rural areas in China. J. Environ. Sci. 2022, 122, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Jiang, N.; Wang, S.-B.; Duan, S.-G.; Zahng, R.-Q. Seasonal Characteristics and Source Analysis of Water-Soluble Ions in PM2.5 of Anyang City. Huan Jing Ke Xue 2020, 41, 75–81. [Google Scholar]
- Salam, A.; Bauer, H.; Kassin, K.; Ullah, S.M.; Puxbaum, H. Aerosol Chemical Characteristics of a MegaCity in Southeast Asia (Dhaka, Bangladesh). Atmos. Environ. 2003, 37, 2517–2528. [Google Scholar] [CrossRef]
- Rolph, G.D.; Ngan, F.; Draxler, R.R. Modeling the fallout from stabilized nuclear clouds using the HYSPLIT atmospheric dis-persion model. J. Environ. Radioact. 2014, 136, 41–55.5. [Google Scholar] [CrossRef]
- Cesari, D.; Donateo, A.; Conte, M.; Merico, E.; Giangreco, A.; Giangreco, F.; Contini, D. An inter-comparison of PM2.5 at urban and urban background sites: Chemical characterization and source apportionment. Atmos. Res. 2016, 174, 106–119. [Google Scholar] [CrossRef]
- Belis, C.; Karagulian, F.; Amato, F.; Almeida, M.; Artaxo, P.; Beddows, D.; Bernardoni, V.; Bove, M.; Carbone, S.; Cesari, D.; et al. A new methodology to assess the performance and uncertainty of source apportionment models II: The results of two European intercomparison exercises. Atmos. Environ. 2015, 123, 240–250. [Google Scholar] [CrossRef]
- Meng, C.; Wang, L.; Zhang, F.; Wei, Z.; Ma, S.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Yin, L.; Niu, Z.; Chen, X.; Chen, J.; Zhang, F.; Xu, L. Characteristics of water-soluble inorganic ions in PM2.5 and PM2.5–10 in the coastal urban agglomeration along the Western Taiwan Strait Region, China. Environ. Sci. Pollut. Res. 2014, 21, 5141–5156. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Fujita, E.M.; Watson, J.G.; Lu, Z.; Lawson, D.R.; Ashbaugh, L.L. Evaluation of filter-based aerosol measurements during the 1987 Southern California Air Quality Study. Environ. Monit. Assess. 1994, 30, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yuan, C.-S.; Huang, H.-C.; Lee, C.-L.; Wu, S.-P.; Tong, C. Inter-comparison of seasonal variation, chemical characteristics, and source identification of atmospheric fine particles on both sides of the Taiwan strait. Sci. Rep. 2016, 6, 22956. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, S.; Harrison, R.M.; Song, C.; Wei, L.; Zhang, Q.; Sun, Y.; Lei, L.; Zhang, C.; Yao, X.; et al. An interlaboratory comparison of aerosol inorganic ion measurements by ion chromatography: Implications for aerosol pH estimate. Atmos. Meas. Tech. 2020, 13, 6325–6341. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Yan, Q.; Wu, F.; Cheng, Y.; Zheng, S.; Wu, J.; Yang, G.; Zheng, M.; Tang, L.; et al. The impacts of pollution control measures on PM2.5 reduction: Insights of chemical composition, source variation and health risk. Atmos. Environ. 2018, 197, 103–117. [Google Scholar] [CrossRef]
- Hernández-Moreno, A.; Trujillo-Páez, F.I.; Mugica-Álvarez, V. Quantification of primary PM2.5 Mass Exchange in three Mexican Megalopolis Metropolitan Areas. Urban Clim. 2023, 51, 101608. [Google Scholar] [CrossRef]
- Nan, J.; Ke, W.; Xue, Y.; Fangcheng, S.; Shasha, Y.; Qiang, L.; Ruiqin, Z. Chemical Characteristics and Source Apportionment by Two Receptor Models of Size-segregated Aerosols in an Emerging Megacity in China. Aerosol Air Qual. 2018, 18, 1375–1390. [Google Scholar]
- Wang, Y.Q. MeteoInfo: GIS software for meteorological data visualization and analysis. Meteorol. Appl. 2014, 21, 360–368. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, H.; Yi, A.; Zhang, Z.; Zheng, N.; Fang, X.; Xiao, H. Characterization and source analysis of water–soluble ions in PM2.5 at a background site in Central China. Atmos. Res. 2020, 239, 104881. [Google Scholar] [CrossRef]
- Hernández-López, A.E.; del Campo, J.M.M.; Mugica-Álvarez, V.; Hernández-Valle, B.L.; Mejía-Ponce, L.V.; Pineda-Santamaría, J.C.; Reynoso-Cruces, S.; Mendoza-Flores, J.A.; Rozanes-Valenzuela, D. A study of PM2.5 elemental composition in southwest mexico city and development of receptor models with positive matrix factorization. Rev. Int. De Contam. Ambient. 2020, 37, 67–88. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, J.; Arimoto, R.; Han, Y.; Zhu, C.; Tian, J.; Liu, S. Chemical Characteristics of Fine Particles (PM1) from Xi’an, China. Aerosol Sci. Technol. 2010, 44, 461–472. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, N.; Yu, X.; Dong, Z.; Duan, S.; Zhang, L.; Zhang, R. Sources and spatial distribution of PM2.5-bound polycyclic aromatic hydrocarbons in Zhengzhou. Atmos. Res. 2016, 216, 65–75. [Google Scholar] [CrossRef]
- Jang, E.; Alam, M.S.; Harrison, R.M. Source apportionment of polycyclic aromatic hydrocarbons in urban air using positive matrix factorization and spatial distribution analysis. Atmos. Environ. 2013, 79, 271–285. [Google Scholar] [CrossRef]
- Allan, M.A. Manual for the GAW Precipitation Chemistry Program: Guidelines, Data Quality Objectives and Standard Operating Procedures; World Meteorological Organization: Geneva, Switzerland, 2004.
- Tian, S.; Pan, Y.; Wang, Y. Ion balance and acidity of size-segregated particles during haze episodes in urban Beijing. Atmos. Res. 2018, 201, 159–167. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, K.; Xu, G. Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China. Atmosphere 2023, 14, 135. [Google Scholar] [CrossRef]
- Geng, N.; Wang, J.; Xu, Y. PM2.5 in an industrial district of Zhengzhou, China: Chemical composition and source 309 appor-tionment. Particuology 2013, 11, 99–109. [Google Scholar] [CrossRef]
- Budhavant, K.B.; Rao, P.S.P.; Safai, P.D.; Gawhane, R.D.; Raju, M.P. Chemistry of rainwater and aerosols over Bay of Bengal during CTCZ program. J. Atmos. Chem. 2010, 65, 171–183. [Google Scholar] [CrossRef]
- Xiong, C.; Yu, S.; Chen, X.; Li, Z.; Zhang, Y.; Li, M.; Liu, W.; Li, P.; Seinfeld, J.H. Dominant Contributions of Secondary Aerosols and Vehicle Emissions to Water-Soluble Inorganic Ions of PM2.5 in an Urban Site in the Metropolitan Hangzhou, China. Atmosphere 2021, 12, 1529. [Google Scholar] [CrossRef]
- Sitaras, I.E.; Siskos, P.A. The role of primary and secondary air pollutants in atmospheric pollution: Athens urban area as a case study. Environ. Chem. Lett. 2008, 6, 59–69. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.; Shi, A.; Huang, Y.; Yan, J.; Nie, T.; Yan, X.; Li, X. Secondary inorganic aerosols formation during haze episodes at an urban site in Beijing, China. Atmos. Environ. 2018, 177, 275–282. [Google Scholar] [CrossRef]
- Yao, X.; Chan, C.K.; Fang, M.; Cadle, S.; Chan, T.; Mulawa, P.; He, K.; Ye, B. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 2002, 36, 4223–4234. [Google Scholar] [CrossRef]
- Suzuki, I.; Hayashi, K.; Igarashi, Y.; Takahashi, H.; Sawa, Y.; Ogura, N.; Akagi, T.; Dokiya, Y. Seasonal variation of water-soluble ion species in the atmospheric aerosols at the summit of Mt. Fuji. Atmos. Environ. 2008, 42, 8027–8035. [Google Scholar] [CrossRef]
- IBC-CSIC. Estudio de Evaluación Preliminar de Niveles de Hidrocarburos Aromáticos Policiclicos (PAHs) en Aire Ambiente en la Comunidad Autónoma de Aragon Durante el Periodo Estival. DGA-Dpto. Medio Ambiente. 2009, 17–35. Available online: http://docplayer.es/148271981-Estudio-de-evaluacion-preliminar-de-niveles-de-hidrocarburos-aromaticos-policiclicos.html (accessed on 10 October 2023).
- Alvarado, A.S.; Luján, P.M.; Bomblat, C. Modelación de las emisiones del parque automotor en la ciudad de Cochabam-ba-Bolivia. Acta Nova 2004, 2, 475–492. [Google Scholar]
- Liu, T.; Mu, L.; Li, X.; Li, Y.; Liu, Z.; Jiang, X.; Feng, C.; Zheng, L. Characteristics and source apportionment of water-soluble inorganic ions in atmospheric particles in Lvliang, China. Environ. Geochem. Health 2023, 45, 4203–4217. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.-Y.; Yi, S.-M.; Heo, J. Source apportionment of PM2.5 using positive matrix factorization (PMF) at a rural site in Korea. J. Environ. Manag. 2018, 214, 325–334. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Chen, K.; Zhang, X.; Kuang, X.; Jiang, H.; Wang, H.; Zhen, Z.; He, C. Characteristics and sources of WSI in North China Plain: A simultaneous measurement at the summit and foot of Mount Tai. J. Environ. Sci. 2020, 92, 264–277. [Google Scholar] [CrossRef]
- Benchrif, A.; Guinot, B.; Bounakhla, M.; Cachier, H.; Damnati, B.; Baghdad, B. Aerosols in Northern Morocco: Input pathways and their chemical fingerprint. Atmos. Environ. 2018, 174, 140–147. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.-C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Shie, R.-H.; Zhu, J.-J.; Hsu, C.-Y. A hybrid methodology to quantitatively identify inorganic aerosol of PM2.5 source contribution. J. Hazard. Mater. 2022, 428, 128173. [Google Scholar] [CrossRef] [PubMed]
- He, J. Pollution haven hypothesis and environmental impacts of foreign direct investment: The case of industrial emission of sulfur dioxide (SO2) in Chinese provinces. Ecol. Econ. 2006, 60, 228–245. [Google Scholar] [CrossRef]
- Bi, X.; Dai, Q.; Wu, J.; Zhang, Q.; Zhang, W.; Luo, R.; Cheng, Y.; Zhang, J.; Wang, L.; Yu, Z.; et al. Characteristics of the main primary source profiles of particulate matter across China from 1987 to 2017. Atmos. Meas. Tech. 2019, 19, 3223–3243. [Google Scholar] [CrossRef]
- Dai, L.; Wang, L.; Li, L.; Liang, T.; Zhang, Y.; Ma, C.; Xing, B. Multivariate geostatistical analysis and source identification of heavy metals in the sediment of Poyang Lake in China. Sci. Total. Environ. 2018, 621, 1433–1444. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Wang, H.; Zhao, Z.; Ma, S.; Ye, Z. Characteristics and Oxidative Potential of Ambient PM2.5 in the Yangtze River Delta Region: Pollution Level and Source Apportionment. Atmosphere 2023, 14, 425. [Google Scholar] [CrossRef]
- Ding, L.; Fang, X.; Dong, H.; Zhao, W. Characteristics of PM2.5 water-soluble ions and source identification during springtime 2017 in Wuhan. Ekoloji 2019, 28, 505–515. [Google Scholar]
- Gong, J.; Tao, M.; Liu, Q.; Ding, C.; Li, P.; Schauer, J.J. Sources of Aerosol Acidity at a Suburban Site of Nanjing and Their Associations with Chlorophyll Depletion. ACS Earth Space Chem. 2021, 5, 3437–3447. [Google Scholar] [CrossRef]
- Cheng, B.; Ma, Y.; Li, H.; Feng, F.; Zhang, Y.; Qin, P. Water-soluble ions and source apportionment of PM2.5 depending on synoptic weather patterns in an urban environment in spring dust season. Sci. Rep. 2022, 12, 21953. [Google Scholar] [CrossRef] [PubMed]
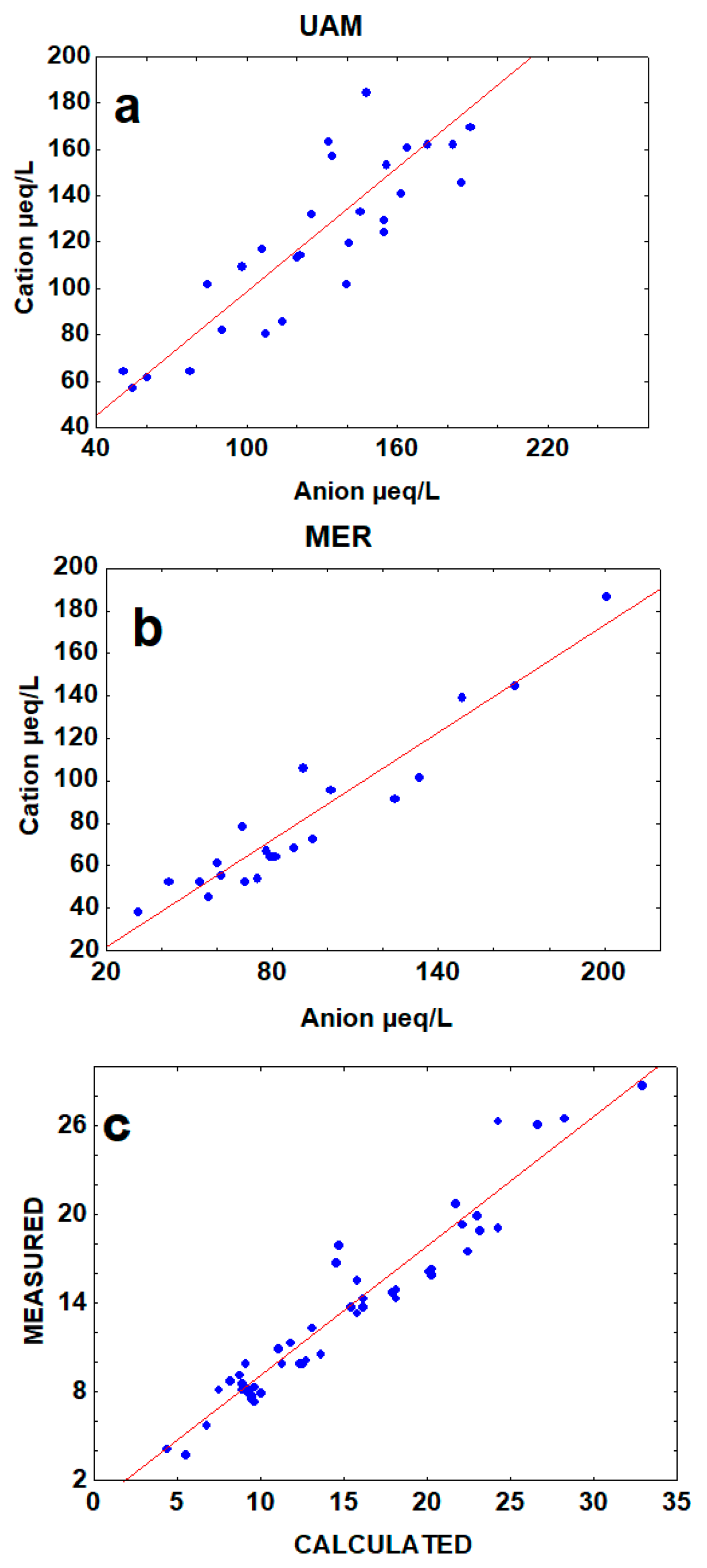
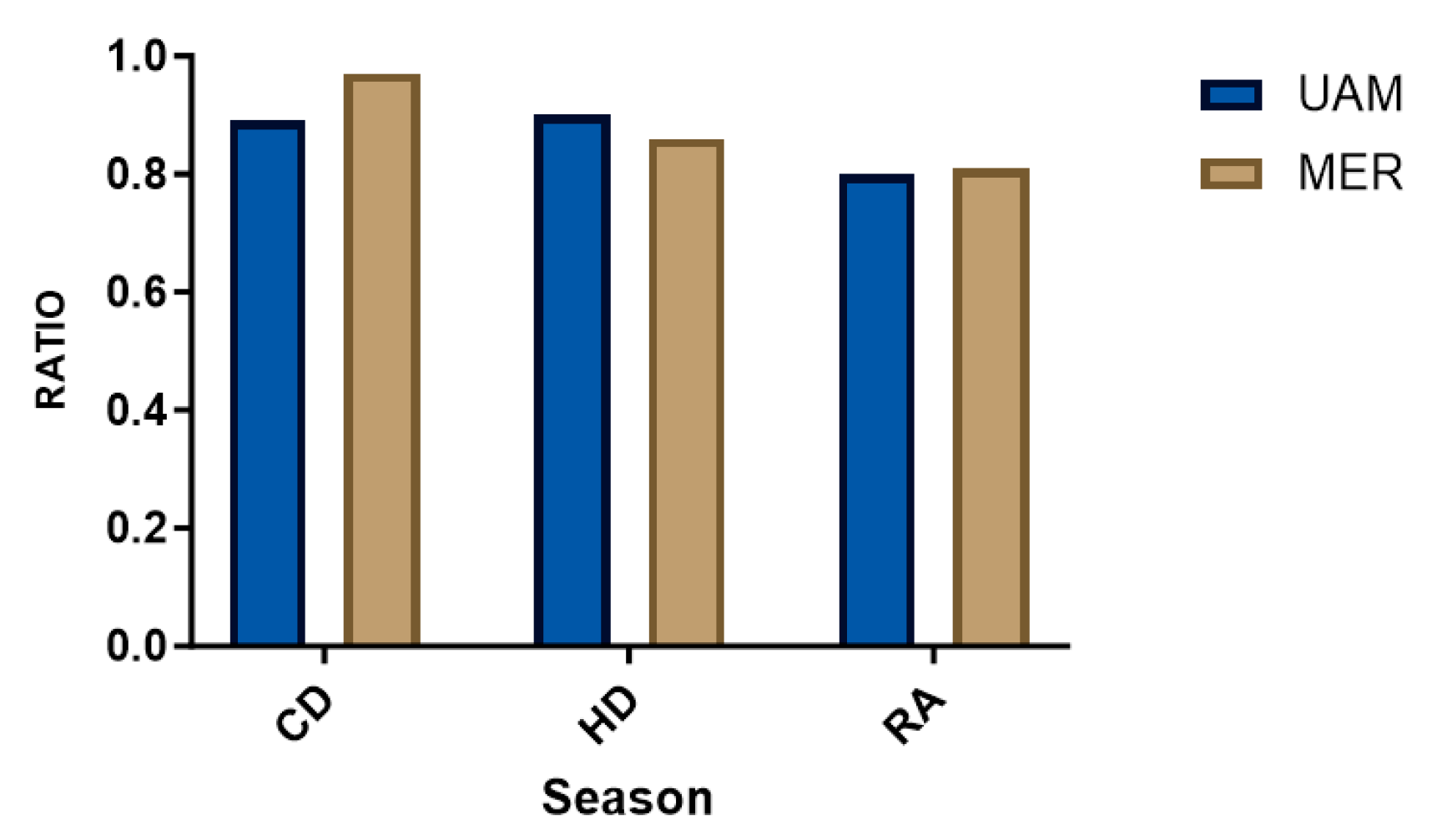
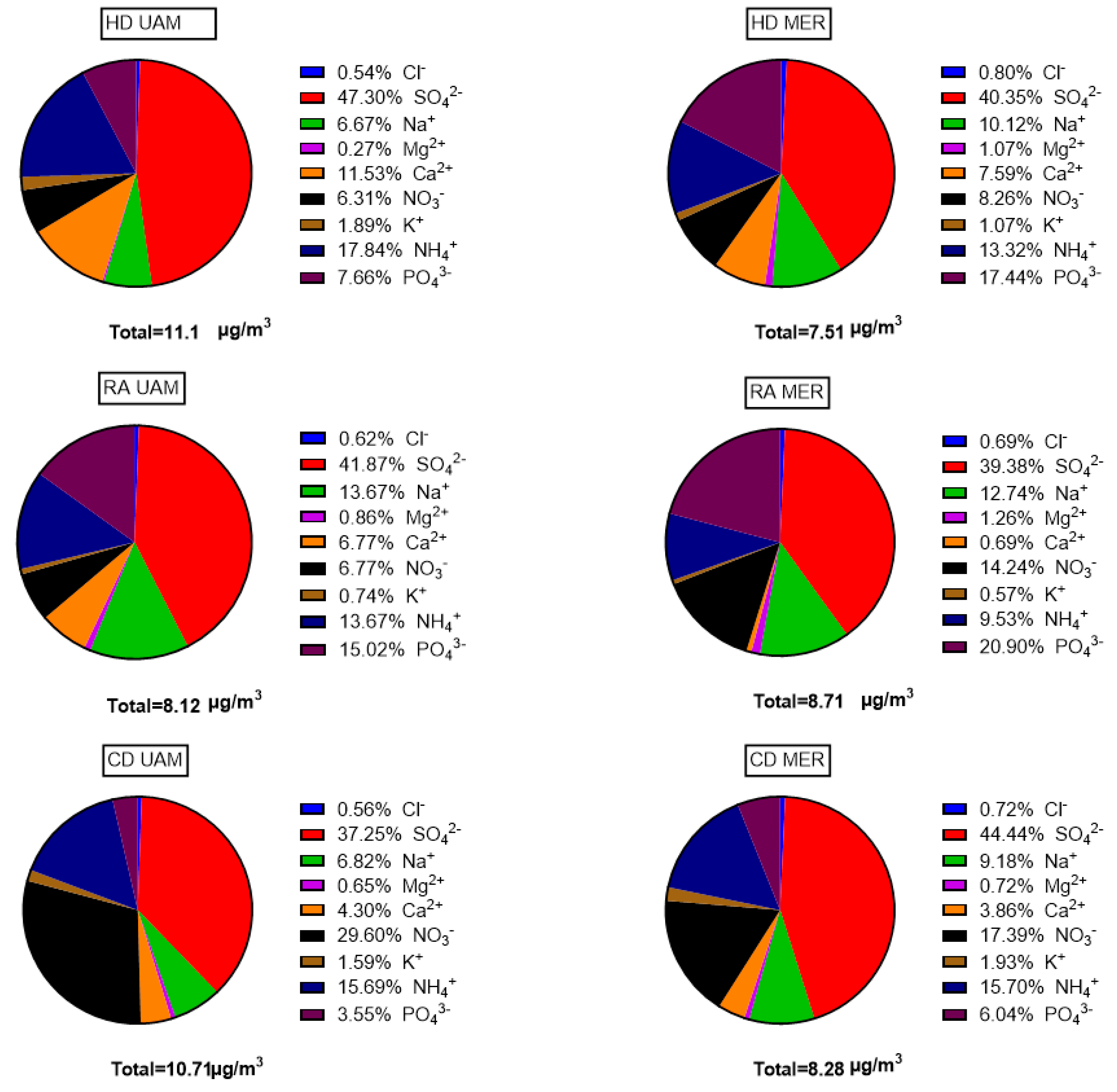
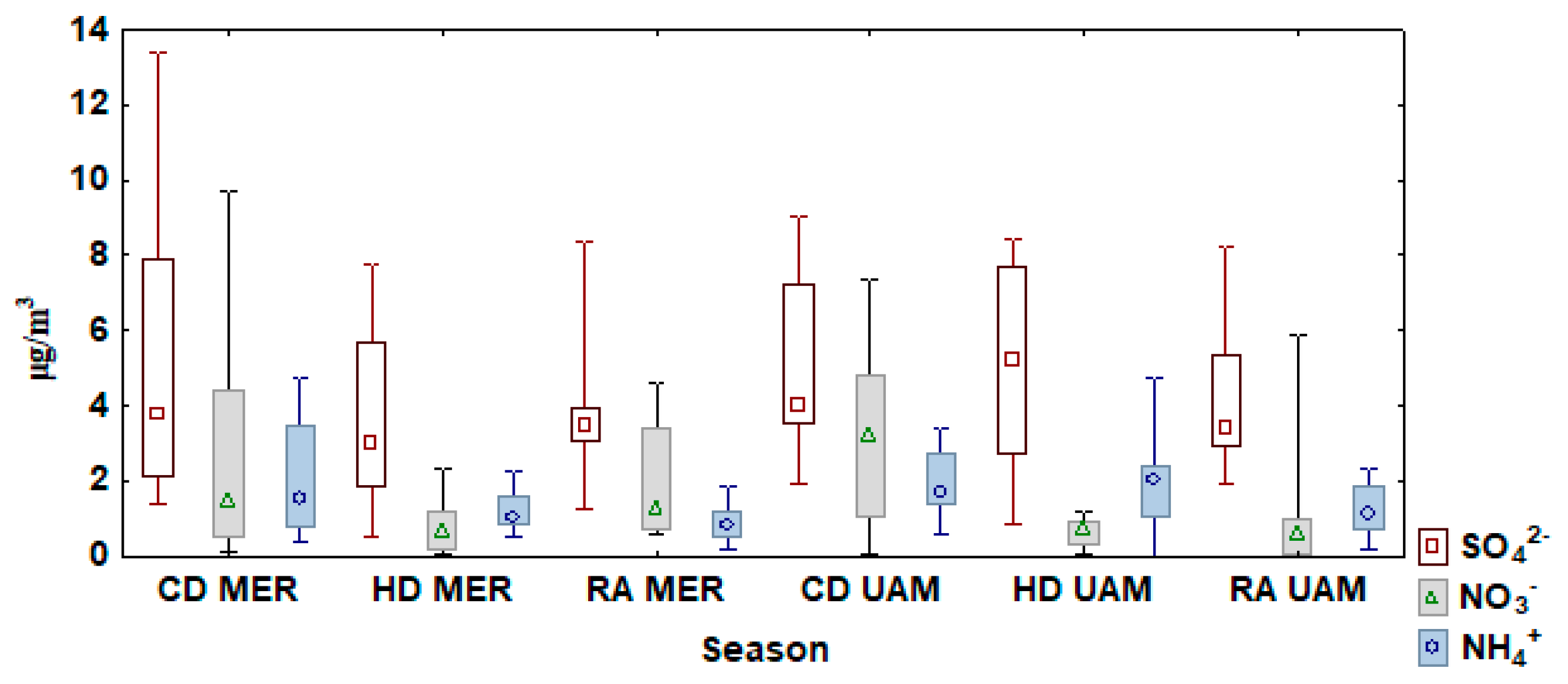
| SITE/SPECIES | N * | Mean | Median | Min | Max | SD | VC |
|---|---|---|---|---|---|---|---|
| UAM | |||||||
| PM2.5 | 61 | 28.4 | 28.5 | 11.2 | 62.3 | 11.2 | 39.4 |
| SO2 | 54 | 12.7 | 8.8 | 3.9 | 49.6 | 9.9 | 78.1 |
| NO2 | 55 | 46.4 | 48.3 | 3.8 | 93.9 | 20.5 | 44.2 |
| Cl− | 23 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 45.5 |
| SO42− | 25 | 4.8 | 4.0 | 0.9 | 9.0 | 2.4 | 50.6 |
| Na+ | 25 | 0.8 | 0.8 | 0.4 | 1.1 | 0.2 | 29.1 |
| Mɡ2+ | 25 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 63.3 |
| Ca2+ | 25 | 0.9 | 0.7 | 0.0 | 2.8 | 0.7 | 76.5 |
| NO3− | 25 | 1.7 | 0.8 | 0.0 | 7.4 | 2.2 | 125.8 |
| K+ | 21 | 0.2 | 0.2 | 0.0 | 0.4 | 0.1 | 61.8 |
| NH4+ | 25 | 1.8 | 1.7 | 0.0 | 4.7 | 1.1 | 61.4 |
| PO43− | 23 | 0.9 | 0.9 | 0.0 | 2.0 | 0.5 | 58.4 |
| MER | |||||||
| PM2.5 | 61 | 20.7 | 18.8 | 8.8 | 41.1 | 8.4 | 40.4 |
| SO2 | 54 | 7.8 | 5.5 | 2.1 | 35.2 | 6.8 | 87.1 |
| NO2 | 57 | 46.1 | 51.5 | 3.4 | 97.1 | 24.0 | 52.1 |
| Cl− | 23 | 0.1 | 0.1 | 0.0 | 1.9 | 0.4 | 263.6 |
| SO42− | 24 | 4.3 | 3.4 | 0.5 | 13.4 | 3.0 | 71.4 |
| Na+ | 23 | 0.9 | 0.8 | 0.5 | 1.4 | 0.3 | 31.4 |
| Mɡ2+ | 24 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 62.9 |
| Ca2+ | 24 | 0.5 | 0.3 | 0.0 | 3.1 | 0.7 | 141.6 |
| NO3− | 24 | 1.9 | 0.9 | 0.0 | 9.7 | 2.3 | 126.3 |
| K+ | 24 | 0.2 | 0.1 | 0.0 | 2.6 | 0.5 | 240.6 |
| NH4+ | 24 | 1.4 | 0.9 | 0.1 | 4.8 | 1.2 | 85.4 |
| PO43− | 23 | 1.3 | 1.4 | 0.0 | 2.2 | 0.6 | 47.8 |
| Season | Relative Humidity (%) | Temperature (°C) | Main Wind Direction | Wind Speed (m/s) | Wind Rose |
|---|---|---|---|---|---|
| UAM | 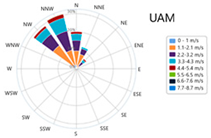 | ||||
| CD | 36 | 15 | Northeast | 5 | |
| HD | 55 | 26 | North | 6 | |
| RA | 70 | 17 | North | 4 | |
| 5 January 2022 | 55.5 | 11.6 | Northwest | 0.9 | |
| 22 June 2022 | 45 | 15 | Northeast | 5 | |
| MER | 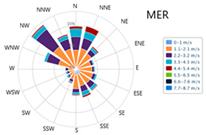 | ||||
| CD | 20 | 18 | North | 6 | |
| HD | 50 | 25 | Southwest | 5 | |
| RA | 80 | 15 | Northeast | 3.7 | |
| 19 December 2022 | 60 | 12 | Northeast | 5 | |
| 26 September 2022 | 50 | 22 | Northeast | 6 |
| Factor | Site | Source | Tracers |
|---|---|---|---|
| 1 | Mexico City | VE CR | NO2, NH4+, PO43−, Na+ |
| 2 | Mexico City | SA | SO42−, NH4+ |
| 3 | Mexico City | IN BB | Cl−, K+ |
| 1 | UAM | VE CR | NO2, NH4+, NO3−, PO43−, Mg2+, Na+ |
| 2 | UAM | SA BB | SO42−, NO3−, NH4+, K+ |
| 3 | UAM | IN | Cl−, SO2, NO3− |
| 1 | MER | VE IN | NO2, NH4+, Cl−, |
| 2 | MER | SA | SO42−, NO3−, NH4+, |
| 3 | MER | CR | Na+, Mg2+, PO43− |
| Parameter | Mexico City (This Study) | Tetuan Morocco [54] | Jiangsu China [60] | Wuhan China [61] | Nanjing China [62] | Lyliang China [51] | Lanzhou China [63] |
|---|---|---|---|---|---|---|---|
| µg/m3 | |||||||
| PM2.5 | 24 | 18 | 68.9 | 101.3 | 91.2 | 36.3 | 54.3 |
| ΣWSII | 10.7 | 6 | 32.4 | 59 | 44.9 | 29.1 | 13.2 |
| SO42− | 4.5 | 3 | 9.0 | 18.9 | 23.2 | 7.6 | 3.9 |
| NO3− | 1.8 | 1.1 | 10.5 | 13.7 | 16.9 | 8.8 | 3.3 |
| NH4+ | 1.6 | 1 | 6.4 | 24.9 | 5.3 | 7.3 | 1.3 |
| K+ | 0.2 | ND | 0.9 | 0.1 | 1.6 | ND | 0.4 |
| Cl− | 0.1 | ND | 1.7 | 0.8 | 2.1 | 4.7 | 2.6 |
| % | |||||||
| SNA/PM2.5 | 32.9 | 28.3 | 37.6 | 56.8 | 49.8 | 65.3 | 15.7 |
| Secondary Aerosols/PM2.5 | 26.4 | ND | 20 | 35 | 27 | 43 | 10.8 |
| Sources | VE, SA, IN, CR, BB | AM, RT, BB, FSEA, ASEA, ORF | VE, BB, SA, DU | RD, SEA, SN, SAM, CC, BB | DU, SS, INMS, BB | SA, BB, CC, DU, VE | DU, IN, BB, MES, SA, SES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Vázquez, F.; Sosa-Echevería, R.; Alarcón-Jiménez, A.L.; Figueroa-Lara, J.d.J.; Torres-Rodríguez, M.; Valle-Hernández, B.L.; Mugica-Álvarez, V. Temporal Variation and Potential Sources of Water-Soluble Inorganic Ions in PM2.5 in Two Sites of Mexico City. Atmosphere 2023, 14, 1585. https://doi.org/10.3390/atmos14101585
Millán-Vázquez F, Sosa-Echevería R, Alarcón-Jiménez AL, Figueroa-Lara JdJ, Torres-Rodríguez M, Valle-Hernández BL, Mugica-Álvarez V. Temporal Variation and Potential Sources of Water-Soluble Inorganic Ions in PM2.5 in Two Sites of Mexico City. Atmosphere. 2023; 14(10):1585. https://doi.org/10.3390/atmos14101585
Chicago/Turabian StyleMillán-Vázquez, Fernando, Rodolfo Sosa-Echevería, Ana Luisa Alarcón-Jiménez, José de Jesús Figueroa-Lara, Miguel Torres-Rodríguez, Brenda Liz Valle-Hernández, and Violeta Mugica-Álvarez. 2023. "Temporal Variation and Potential Sources of Water-Soluble Inorganic Ions in PM2.5 in Two Sites of Mexico City" Atmosphere 14, no. 10: 1585. https://doi.org/10.3390/atmos14101585
APA StyleMillán-Vázquez, F., Sosa-Echevería, R., Alarcón-Jiménez, A. L., Figueroa-Lara, J. d. J., Torres-Rodríguez, M., Valle-Hernández, B. L., & Mugica-Álvarez, V. (2023). Temporal Variation and Potential Sources of Water-Soluble Inorganic Ions in PM2.5 in Two Sites of Mexico City. Atmosphere, 14(10), 1585. https://doi.org/10.3390/atmos14101585







