Abstract
Due to increasing concerns about global warming and energy crisis, intensive efforts have been made to explore renewable and clean energy sources. Single-atom metals and two-dimensional (2D) nanomaterials have attracted extensive attention in the fields of energy and environment because of their unique electronic structures and excellent properties. In this review, we summarize the state-of-art progress on the single-atom metal supported at 2D MoS2 (single-atom metal/2D MoS2) for electrochemical CO2 reduction and water splitting. First, we introduce the advantages of single-atom metal/2D MoS2 catalysts in the fields of electrocatalytic CO2 reduction and water splitting, followed by the strategies for improving electrocatalytic performances of single-atom metal/2D MoS2 hybrid nanomaterials and the typical preparation methods. Furthermore, we discuss the important applications of the nanocomposites in electrocatalytic CO2 reduction and water splitting via some typical examples, particularly focusing on their synthesis routes, modification approaches, and physiochemical mechanisms for improving their electrocatalytic performances. Finally, our perspectives on the key challenges and future directions of exploring high-performance metal single-atom catalysts are presented based on recent achievements in the development of single-atom metal/2D MoS2 hybrid nanomaterials.
1. Introduction
Due to the contamination and global warming problems, it is necessary to search for alternative environmentally friendly energy sources and decrease the concentration of CO2 in the atmosphere [1,2,3]. The utilization of nonprecious metal electrocatalysts for water splitting and CO2 fixation for producing high-value-added fuels or chemicals may be the ultimate solution for sustainable and clean hydrogen energy, tackling the challenges posed by rising CO2 levels, and realizing a closed carbon cycle [4]. Proper electrocatalysts are extremely useful for improving the reaction rate of CO2 conversion to its reduced oxidation states, CO and formate/formic acid. From the perspective of reactivity, CO2 is chemically inert, and the initial bond energy of C=O is 806 kJ mol–1, so the conversion of CO2 into various products is a difficult problem in thermodynamics. Therefore, to make CO2 conversion happen at a reasonable rate, the potential energy necessarily needs to be much greater than the thermodynamic values. Once the reaction is started, CO2 can be converted into a mixture of products, mainly including carbon monoxide (CO), methane (CH4), ethylene (CH2CH2), methanol (CH3OH), ethanol (C2H5OH) formic acid (HCOOH), and acetate (CH3COOH). In addition, hydrogen is a by-product produced at a potential close to the potential for CO2 reduction [5,6,7,8,9]. However, the electrocatalysts for the CO2 reduction reaction (CO2RR) and water splitting face some problems, such as low product selectivity, poor faradaic efficiency (FE), and/or hard experimental conditions (in acidic media) [10,11]. In particular, the catalytic activity and selectivity of non-precious metal catalysts are generally much lower than those of noble metal catalysts [12]. The activity of catalysts is not only related to the composition and structure but also to the dimension or size. The most direct effect of the decrease in dimension or size is the change in the number of active sites. Compared to conventional nanoparticles, single-atom catalysts (SACs) have the advantages of unique electronic structure, strong metal-support interactions (SMSIs), and plenty of accessible active sites and thus can significantly improve catalytic activity and selectivity [13,14,15]. Therefore, SACs have shown prominent activity in various electrochemistry processes, including CO2RR [16,17], oxygen evolution reaction (OER) [18,19], and hydrogen evolution reaction (HER) [20,21]. However, since the surface energy increases with the decrease in particle size, the single atoms tend to aggregate into clusters or nanoparticles [22,23], which leads to degraded functions. Hence, it is indispensable to anchor the isolated atoms onto the appropriate supports to build stable configurations with atomic distribution [24,25]. On one hand, proper supports can serve as stabilizing functions via metal-support interactions [26]. The strong metal–support interactions can effectively tune the electronic structure of SACs to improve the electrocatalytic activity and selectivity [27,28,29]. Two-dimensional materials are recognized as ideal supports for SACs and as preferable alternatives for catalysts due to their unique electronic properties, high specific surface area, and substantial number of active sites [30,31,32,33]. For example, Mn supported on 2D VTe2 exhibits excellent catalytic performance for both HER and OER [34]. Recent advances have demonstrated that 2D materials can improve the performance of SACs and the inhomogeneity of active sites [35,36,37].
Two-dimensional molybdenum disulfide (MoS2), as the representative of transition metal dichalcogenides (TMDCs), has attracted much attention for HER, OER, and CO2RR due to its unique structure and easy functionalization [38,39]. In addition, owing to the high earth abundance, low price, and high HER catalytic activity, 2D MoS2 is regarded as a promising alternative for noble metals for water splitting [40]. In addition to water splitting, 2D MoS2 has a great potential application in CO2RR because Mo-exposed edges can enhance the chemisorption of the reactants and thus improve the electrochemical catalysis with a low overpotential of CO2RR (about ~54 mV) and high selectivity (the reduction product is only CO) [39,41]. However, the electrocatalytic performance of pristine 2D MoS2 is still not satisfactory mainly due to the lack of active sites at its basal plane and low conductivity. For example, MoS2 edges show poor oxygen evolution reaction (OER) activity [42], and the pristine basal plane of MoS2 is inert to the electrochemical reduction of CO2 [43]. Therefore, the successful combination of 2D MoS2 and single-atom metal can not only minimize the drawbacks and maximize the advantages of the individual components; more importantly, in addition to the desired performances, some novel functions may be generated for enhancing electrocatalytic CO2RR and overall water splitting.
Recently, single-atom metal/2D MoS2 hybrid nanomaterials have been booming in heterogeneous electrocatalysis, due to well-defined located metal centers, unique metal–support interaction, and identical coordination environment. However, there have been only a few studies on the systematic summarization of the nanomaterials for electrocatalytic CO2RR and overall water splitting. Thus, to fill this gap, a comprehensive review focusing on such a topic is extremely necessary and significant. In this review, we summarize the state-of-art progress on single-atom metal nanomaterials modified 2D MoS2 for electrocatalytic CO2RR and water splitting. We start briefly with a discussion of the unique advantages of single-atom metal/2D MoS2 hybrid nanomaterials for electrocatalytic CO2RR and water splitting. Furthermore, the synthetic methods for single-atom metal/2D MoS2 hybrid nanomaterials are summarized, including the one-pot chemical method, electrochemical process, and polyoxometalate template-based synthetic strategy. After that, we highlight the significant applications of single-atom metal/2D MoS2 hybrid nanomaterials in electrocatalytic CO2RR and water splitting with the discussions about some typical examples, particularly focusing on physiochemical mechanisms for improving their electrocatalytic activity. Finally, on the basis of previous studies, we provide our perspectives on the key challenges and future directions in utilizing single-atom metal/2D MoS2 hybrid nanomaterials for CO2RR and water splitting. We hope that this review can provide new insights for the further development and practical application of single-atom catalysts and 2D materials.
2. Synthesis Methods of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
The catalytic performances are determined by concrete improvements of synthetic methodologies [44]. The most widely employed approaches for SACs are pyrolysis, atomic layer deposition (ALD) method, physical vapor deposition (PVD), wet-chemistry strategy, and electronic deposition [45,46,47]. However, it is still hard to manipulate atoms in a highly accurate way for the control synthesis of theoretically designed SACs due to ultrahigh surface free energy. The synthesis methodologies for 2D MoS2 can be divided into two categories: top-down and bottom-up methods. The former mainly includes chemical vapor deposition (CVD) and solvothermal or hydrothermal methods [48,49,50,51,52]; the latter includes mechanical exfoliation, chemical or electrochemical exfoliation methods, and liquid-phase exfoliation [53]. However, the number of successful cases for single-atom metal/2D MoS2 hybrid nanomaterials is still limited compared to the synthesis methods for single-atom metal modified 3D supports, SACs preparation, and/or 2D materials. The methods for single-atom metal/2D MoS2 hybrid nanomaterials are derived from the approaches for SAC@3D supports, such as pyrolysis and coprecipitation. Considering that the common synthetic methods of SACs or 2D materials have been discussed in depth in previous reviews, this review focuses on some novel methods for single-atom metal/2D MoS2 hybrid nanomaterials, including the one-pot chemical method, the electrochemical process, and the polyoxometalate template-based synthetic strategy (Figure 1).
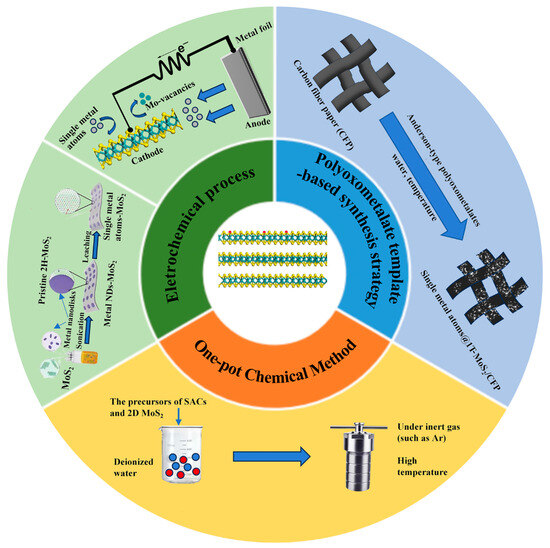
Figure 1.
Several common synthesis methods of single-atom metal/2D MoS2 hybrid nanomaterials.
2.1. One-Pot Chemical Method
The principle of this method is directly to mix the precursors of SACs and 2D MoS2 for the following reactions under inert gas (e.g., Ar). The typical processes are described as follows: firstly, all precursors (e.g., (NH4)6Mo7O24·4H2O, H2PtCl6, and CS2) are dissolved in a certain amount of deionized water to form a homogeneous solution [20]; then, the resulting solution is transferred to a Teflon-lined stainless autoclave under Ar and maintained at a high temperature for a certain reaction time.
The one-pot method has the advantages of simple operation and saving synthetic costs. In addition, the catalysts prepared by this method can have sulfur vacancies and the doping features of metal atoms at the same time [54]. Until now, Cu@1T-MoS2, Ni@1T-MoS2, Fe@1T-MoS2, and Co@1T-MoS2 have been easily prepared through this method [55].
2.2. Electrochemical Process
The electrochemical process is used to synthesize SAC-modified 2D MoS2 via electrochemical etching of big-size metal precursor. Taking the single-atom cobalt (Co) array modified 2D MoS2 as an example (Figure 2), firstly 2D MoS2 and Co nanodisks (NDs) are synthesized using standard solvothermal procedures and standard air-free procedures, respectively. Then, the combination of Co NDs and 2D MoS2 is realized via an assembly process. Finally, single-atom Co array covalently bound onto distorted 1T-MoS2 nanosheets (denoted as SA Co-D 1T-MoS2) via Co-S bonds can be synthesized through electrochemical cyclic voltammetry (CV) leaching of Co nanodisks (NDs). In addition to electrochemical CV leaching, the electrochemical deposition can be used to synthesize the nanocomposites of SACs modified 2D MoS2, such as Pt, Cu, Sn, and Pd anchored on the 2D MoS2, because these single metal atoms (from metal ions in the electrolyte solution) can be introduced onto the MoS2 monolayer driven by applying the bias potential [56].
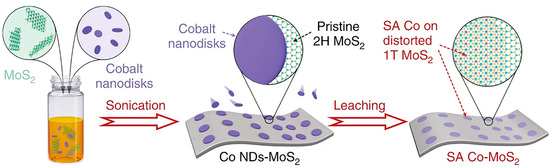
Figure 2.
Schematic illustration of synthetic method for Co@1T-MoS2. Reproduced with permission from Ref. [57]. Copyright 2019 Springer Nature.
2.3. Polyoxometalate Template-Based Synthetic Strategy
Highly purified and stable metallic 1T-MoS2 can be obtained via a hydrothermal method which introduces organic sulfur sources into (NH4)6Mo7O24·4H2O (denoted as Mo7). Here, Mo7 is a precursor that is a butterfly-shaped metal oxide cluster (Figure 3), and it belongs to the β-isomer of Anderson-type polyoxometalates (POMs). In addition, its unique structure makes it possible to tune the chemical environment of 1T-MoS2 with various metal atoms. Using Anderson-type polyoxometalates ([XH6Mo6O24]n−) as precursors, atomically designing metal doping sites onto metallic 1T-MoS2 can be achieved. [XH6Mo6O24]n− is denoted as XMo6 (X = FeIII, CoIII, n = 3; X = NiII, n = 4) [53].
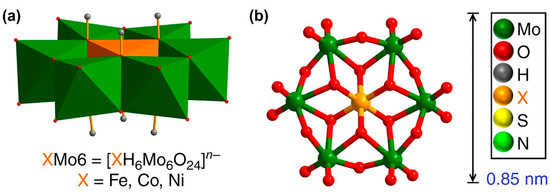
Figure 3.
Structure of POM precursors. (a) Polyhedral representation of the XMo6 precursors. (b) Ball and stick representation of the XMo6 precursors. Reproduced with permission from Ref. [58]. Copyright 2019 Springer Nature.
3. Applications of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
3.1. Electrochemical CO2 Reduction
Electrocatalytic CO2RR includes three steps, namely, the chemisorption of CO2 on the surface of electrocatalysts, the transfer of high-energy electrons and protons between two elements to break C=O bonds, and the desorption of products from the surface of the electrocatalysts [59]. For the hybrid system composed of single-atom metals and 2D MoS2, CO2RR more frequently occurred on metal, and the reaction paths or products are strongly dependent on the components of metal. These reaction products or paths include three types: (1) the reduction of CO2 to CO (e.g., on Au or Ag), (2) the reduction of CO2 to formic acid (e.g., on Sn and Pb), and (3) the reduction of CO or carbon–oxygen compounds to hydrocarbons or alcohols (e.g., on Cu, Fe, and Mn).
3.1.1. Noble Metal Modified 2D MoS2
Noble metal single-atom catalysts (NMSACs) have the advantages of high intrinsic activity, selectivity, and durability for electrocatalytic CO2RR due to their unique electronic structure [60]. On the other hand, in addition to acting as catalytic centers, monodispersed single noble metal atoms can also activate inert in-plane S-atoms and lead to improved electrochemical activity of 2D MoS2 [61]. Compared to the bulk MoS2, 2D MoS2 doped with a single-atom noble metal has the advantage of tunable electronic structures and spatial versatilities, exhibiting excellent performances in electrocatalytic CO2RR [62]. In addition, these nanocomposites in experimental studies are usually prepared via standard electrochemical methods, which can accurately adjust the concentration of anchored single atoms by deposition time and anode voltage [63], and limiting potentials are usually used to evaluate the electrocatalytic activity of CO2RR [16,64]. Up to now, there are few experimental studies on single-atom metal/2D MoS2 hybrid nanomaterials for electrocatalytic CO2RR; in contrast, a large number of studies are focused on density functional theory (DFT) calculations for evaluating the electroactivity of single-atom metal/2D MoS2 hybrid catalysts. In the hybrid electrocatalysts, these single atoms in DFT calculations are mainly concentrated in Pt, Pd, Au, Ag, and Ru and have an optimal loading amount for avoiding adverse effects on their performance. For example, single Pt atoms can introduce deep gap states, which leads to a decrease in Fermi level upshifting and strong binding with *HCOO [63]. The Ru doping can improve the H2 adsorption and dissociation, avoiding the competing HER [65]. Moreover, because key intermediate *HCOO plays an important role in the reaction pathway and potential determining steps (PDS) [66], the binding energy () of *HCOO on 2D MoS2 or single-atom metal is identified as the effective reactivity descriptor for theoretical calculations. Binding energy () has been calculated using the following expression,
where is the total DFT energy for the complex of intermediates (e.g., *HCOO) adsorbed on a catalyst surface (e.g., 2D MoS2 or single metal atom), is the DFT energy for the intermediates, and is the DFT energy for a substrate. The promising dopants should enhance the CO2 and *HCOO adsorption and meanwhile can weaken the CO adsorption. In addition, the binding strength can be described by the energy of the center of the d states relative to the Fermi level according to the d-band center model. In general, the high d-band center in energy relative to the Fermi level indicates strong CO adsorption. Chemical doping with heteroatoms or surface decorating is one of the promising approaches to modulating the electronic structure of 2D MoS2 for CO2RR. Proper dopants can enhance CO2 and COOH adsorption and simultaneously weaken CO adsorption. Mao et al. investigated 29 kinds of single-atom metals (including Ag, Au, and other transition metal (TM) atoms) doped 2D MoS2 with different doping concentrations and positions for electrochemical CO2RR via high-throughput density functional theory (DFT) calculations with the PBE functional by using the projector augmented wave (PAW) method (Figure 4a) to understand the relationship between the doping elements and the catalytic performance [67]. Of note, the calculated energies for the adsorption, desorption, or dissociation of the intermediates (such as COOH* and CHO*) on the active sites of doped 2D MoS2 may be suffered from the “basis set superposition error” (BSSE). In principle, the use of moderately diffuse basis functions for the valence set and crude approximations for the core orbitals will lead to the severe BSSE. For single noble metal atom doping, the binding energies of CO2 and COOH on the Pt- and Pd-doped MoS2 edge are greatly larger than that of the TM-doped MoS2 (Figure 4b,c) or pristine MoS2; meanwhile, the binding energies of CO on Pt- and Pd-doped MoS2 are much smaller than other TM-doped MoS2, except for Ni-doped MoS2. The stronger adsorption of CO2 and COOH with MoS2 edge can activate the reduction reaction, while the weaker adsorption of CO with MoS2 can accelerate the rate-limiting step of CO desorption.
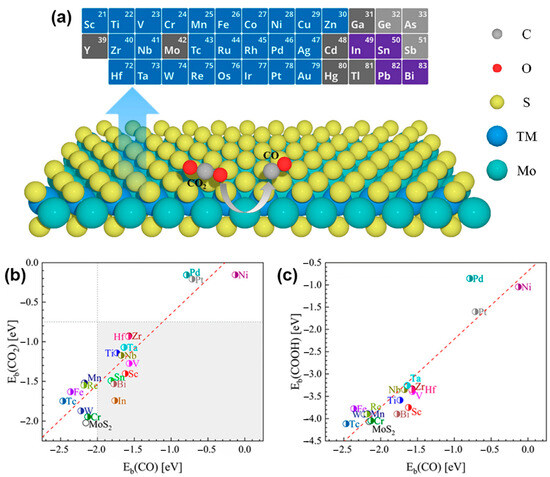
Figure 4.
(a) Twenty-nine kinds of metal-doping elements in MoS2 as the calculation models for electrochemical CO2RR. TM atoms as dopants are from the elements in the periodic table of elements labeled by the blue color. (b) Binding energies of CO2 vs. CO on the metal-doped MoS2 edge. The gray rectangular area indicates the obvious decrease in CO binding energies. (c) Binding energies of COOH vs. CO on the metal-doped MoS2 edge. Reproduced with permission from Ref. [67]. Copyright 2020 American Chemical Society.
Atomic-level electroplating can accurately control the doping level and anchoring sites to maximize the activity and stability of the catalyst [63]. Xuan et al. developed an electroplating method to synthesize monodispersed noble metal atoms on 2D MoS2 [63]. To be specific, authors demonstrated a voltage gauged electrochemical deposition method to deposit single-atom Pt, Au, and Pd on 2D MoS2. The surface atomic doping levels for Pt, Au, and Pd can reach 1.1, 7.0, and 14 at.%, respectively, and the doping positions were accurately located on Mo- and S-vacancies. The doping Pt, Mo, and S atoms are homogeneously distributed on the MoS2 flake (Figure 5a–d). After doping single-atom metal on 2D MoS2, the electrochemical reactivity of the catalyst is driven higher than that of the pristine MoS2 flakes (Figure 5e). In addition, monodisperse precious metal atoms can exhibit improved saturated CO tolerance (Figure 5f) and provide an extra pathway for CO2RR. The overpotential of Pt-2D MoS2 at 10 mA cm2 only changes 9 mV after CO poisoning (Figure 5f), indicating that Pt-2D MoS2 is almost immune to CO poisoning. Thus, due to this CO tolerance, Pt-2D MoS2 electrocatalyst has more potential applications in electrocatalytic CO2RR than Au-2D MoS2 and Ag-2D MoS2. The enhanced electrochemical performance is attributed to stabilized Pt (II) atoms with fewer free electrons to coordinate with CO than Pt0 [68]. It is believed that these findings are beneficial to understanding the non-noble single-atom doping for fine-tuned electrochemical windows.
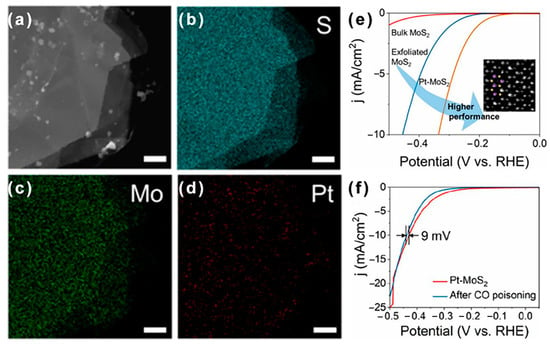
Figure 5.
(a–d) HAADF-STEM image and corresponding EDX mapping images of Pt-MoS2. (e) Single-atom electroplating-process-related polarization curves from bulk MoS2, exfoliated MoS2/GP, and Pt-MoS2 electrode. (f) CO tolerance test of Pt-2H-MoS2 catalyst in CO saturated 0.5 M H2SO4. Reproduced with permission from Ref. [63]. Copyright 2019 American Chemical Society.
3.1.2. Non-Noble Metal Modified 2D MoS2
Exploring MoS2-based non-noble-metal SACs for electrocatalytic CO2RR is expected to solve the problems of high overpotential, low Faraday efficiency, and unsatisfactory selectivity in CO2RR [69]. The geometric, electronic, spin states of the active center and magnetic states of transition metal SACs can be fine-tuned to determine the catalytic behavior and activity, which can allow SACs be precisely designed with 2D MoS2 as a support to improve electronic conductivity and increase the exposed active sites for electrocatalytic CO2RR [70]. Moreover, the TM-atom doping can significantly modify the binding energies of intermediates (e.g., *COOH) on 2D MoS2 edges, and thus regulate the electrocatalytic performance for CO2RR [71]. Remarkably, in addition to the ability to change the binding energies of key intermediates, non-noble-metal single-atom catalysts also have high CO formation turnover frequency (TOF), which makes it possible for their electrocatalytic performance to surpass that of NMSACs. For example, it has been proven that Nb-doped 2D MoS2 shows CO formation TOF two orders of magnitude higher than Ag-doped 2D MoS2 at an overpotential range of 100–650 mV [72].
Yu et al. used the first principles quantum theory to explore the stability of SACs with the non-noble 3d-series of metal single atoms (e.g., Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) supported on 2D MoS2 [73]. Among them, the Ni@MoS2 catalyst exhibits the most stability, which has been proved by the analysis results including electron localization function (ELF), density of states (DOS), and band structure. Spin-polarized projected DOS (PDOS) shows that Ni 3d orbitals play a critical role in bonding with the MoS2 surface; the orbital hybridization (obvious overlapping with DOS) is responsible for Ni–S bonding interaction. The electrons from Ni 4s/4p states transfer to S and Mo, and consequently, such process enhance the structural stability of SACs because of the ionic interaction between Niδ+ and Sδ−. The improvement in electrocatalytic performance for CO2RR is attributed to the changes of the spin densities and charge density difference by Ni doping and thus can improve the electrocatalytic performance for CO2RR. To supplement the theoretical and experimental gaps in the single-atom metal catalysts on 2D MoS2 toward CO2RR, Ren et al. explored single atoms (Fe, Co, Ni, Cu) supported on 2D MoS2 for their CO2RR performance via the combination of DFT calculations and computational hydrogen electrode (CHE) model [74]. The limiting potentials of Fe@MoS2, Co@MoS2, and Ni@MoS2 are determined as −0.39 V, −0.24 V, and −0.45 V, respectively, for CH4 production. The binding energy of *HCOO can be regarded as an effective descriptor for screening potential single-atom catalysts for CO2RR [66].
The endothermic and rate-limiting CO desorption step largely limits the electrocatalytic CO2RR performance of 2D MoS2 [75]. Nb@2D MoS2 had a high CO formation TOF and an extremely low onset overpotential (31 mV) for electrocatalytic CO2RR because low Nb doping concentrations (<~5%) can reduce the binding energies between intermediates and MoS2 edge atoms [72]. Energy-dispersive spectroscopy (EDS) mapping (Figure 6a) demonstrates that the distribution of Nb is homogeneous in the vertically aligned (VA)-Mo0.95Nb0.05S2 structure. The changes in doping percentage will affect the electrocatalytic performance of Nb@2D-MoS2 catalyst. When the doping percentage is 5%, Nb@2D MoS2 catalyst (VA-Mo0.95Nb0.05S2) exhibits the highest current density (Figure 6b) and the lowest overpotential (Figure 6c) among the samples. The VA-Mo0.95Nb0.05S2 catalyst has the best electrocatalytic performance for CO2RR, far exceeding that of the pristine MoS2 or Ta@2D MoS2 catalyst, because Nb doping can reduce the bonding strength between Mo edge and CO, and Nb@2D MoS2 catalyst can result in a faster turnover for CO desorption than pristine MoS2. However, a higher Nb doping percentage will bring about poor electron-transfer properties for CO2RR. VA-Mo0.95Nb0.05S2 catalyst generates a tunable mixture of CO and H2 ranging from 12% to 82% of CO formation at the range of studied potentials −0.16 to −0.8 V (Figure 6d). At the low overpotential range of 0–150 mV, the overall electrocatalytic performance of VA-Mo0.95Nb0.05S2 catalyst is one order of magnitude higher than that of the pristine VA-MoS2 [76]. The electrocatalytic activity of VA-Mo0.95Nb0.05S2 catalyst is also 2 orders of magnitude better than Ag-NP catalyst over the entire range of overpotentials (Figure 6e). The enhancement of electrocatalytic performance is due to the change of electronic properties of edge atoms by Nb doping.
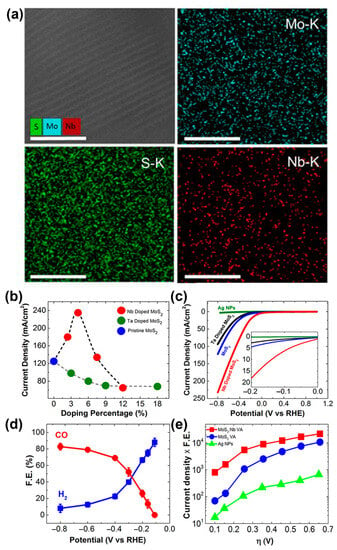
Figure 6.
(a) EDS maps Mo–K series, Nb–K series, and S–K series measured from the same region of the MoS2: Nb sample. (b) Current density as a function of dopant percentage for Nb-doped and Ta-doped MoS2 samples. (c) CV curves for Ag NPs, VA-MoS2, VA-Mo0.97Ta0.03S2, and VA-Mo0.95Nb0.05S2 in CO2 environment. (d) CO and H2 Faradaic efficiency (FE%) at different applied potentials for VA-Mo0.95Nb0.05S2. (e) CO formation partial current density for Ag nanoparticles, VA-MoS2, and VA-Mo0.95Nb0.05S2. Reproduced with permission from Ref. [72]. Copyright 2017 American Chemical Society.
For the TM atoms as dopants in MoS2, the introduction of V, Zr, and Hf into MoS2 can significantly promote the desorption of CO from the MoS2 edge, thus achieving the optimal performance for electrocatalytic CO2 reduction, because the TM doping can significantly modulate the binding energies of the CO2 reduction species. For example, Mao et al. screened out three dopants (i.g., V, Zr, or Hf) in 2D MoS2 via high-throughput DFT calculations for outstanding electrochemical performance with the PBE functional by using the projector-augmented wave (PAW) method (Figure 7a) [67]. The dopants can not only activate the O–C–O bond by enhancing the binding energy of CO2 but also allow CO to desorb more easily by reducing the binding energies of CO. Compared to pristine 2D MoS2, the binding energies of CO are significantly decreased by 0.59, 0.59 and 0.58 eV for V-, Zr- and Hf-doped MoS2 edges, respectively. Moreover, PDOS shows that the higher the energy of the d-band center relative to the Fermi level, the stronger the CO adsorption, which is consistent with the d-band center theory. Specifically, by doping Zr and Hf into 2D MoS2, the binding energies of CO are reduced by 0.59 and 0.58 eV, respectively, so the center of the d-band shifts to Fermi level by 0.22 eV relative to the pristine MoS2. In addition, the dopant position is extremely important to the catalytic activity for CO2RR, but relatively, the doping concentration is insignificant. Such a conclusion has already been proven by the research work of Nørskov et al. via DFT calculations regarding the electrocatalytic CO2 reduction on the MoS2 edge with the Mo edge replaced by TM atoms. As shown in Figure 7b, the closer the doping position is located to the active sites of the Mo edge, the more obvious the doping effect is because single-atom metals can affect the local electronic structures of 2D MoS2. This work developed an effective method to improve the electrocatalytic activity of 2D MoS2 by doping near the active molybdenum at low doping concentrations. In addition, other studies about single-atom-metal-modified 2D MoS2 (as shown in Table 1) also prove the importance of the intermediate *HCOO in the reaction pathway and potential determining steps.
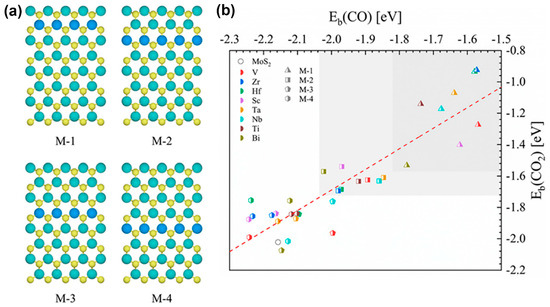
Figure 7.
(a) TM-doped MoS2 structures with different positions of dopants. The green, blue, and yellow balls represent Mo, TM, and S atoms, respectively. (b) Binding energies of CO2 vs. CO for the M-x (x = 1, 2, 3, 4) MoS2. The x indicates the row next to the Mo edge for TM doping. Reproduced with permission from Ref. [67]. Copyright 2020 American Chemical Society.

Table 1.
Summary of various single-atom metals supported at 2D MoS2 for electrochemical CO2 reduction.
3.2. Electrochemical Water Splitting
In a conventional water electrolyzer, HER reaction occurs at the cathode and H2 is separated out, while OER reaction occurs at the anode and O2 is separated out (Figure 8a) [78,79,80]. Under standard conditions, a thermodynamic potential of 1.23 V is required to drive electrochemical water splitting (Figure 8b) for HER and OER. However, in real conditions, the input potential of water splitting in practical electrolyzers is much larger than 1.23 V. In general, a high-performance electrocatalyst for water splitting is still focused on noble-metal-based catalysts (e.g., Pt for HER and IrO2 or RuO2 for OER) (Table 2); however, it is necessary to develop noble metal-free electrocatalysts or decrease the loading amount of noble metals electrocatalysts because of the prohibitive cost and scarce reserve. For the reaction mechanisms (e.g., the Volmer–Heyrovsky mechanism) or paths for HER used in calculation models, there are three elementary steps regarding interactions between the water dissociates and a reactive hydrogen intermediate (absorbed hydrogen on the catalyst surface, Had), including the Volmer step followed by either the Heyrovsky step (H2O + Had + e– ↔ H2 + OH−) or the Tafel recombination step (2Had ↔ H2). The detailed mechanism or reaction path is dependent on the components of metal.

Figure 8.
(a) Illustration of conventional water electrolyzers. (b) Water-splitting reactions under acidic and alkaline conditions.
3.2.1. Noble-Metal-Modified 2D MoS2
Single-atom noble metal catalysts (SACs) can not only decrease the loading amount but also significantly improve the number of catalytic active sites by reducing the size of materials to the atomic-scale range, which can fully take advantage of reaction sites in electrocatalysts. For the hybrid system of single-atom noble metal modified 2D MoS2, the noble metal doping can enhance the adsorption capacity of 2D MoS2 for gas molecules and metal ions [81]. The introduced noble metals can not only function as active centers for facilitating water adsorption and dissociation but also improve the electrical conductivity by modifying the electronic structure of 2D MoS2 [82]. In addition, noble metal single atoms are able to change the chemical bond between H and S/Mo atoms, which can enhance the intrinsic activity of each active center for electrocatalytic water splitting [83]. The in-plane S position adjacent to the doped noble atoms can produce new active sites for HER [84]. At present, Pt@2D MoS2 catalyst is still a hot spot in the field of single noble metal atoms/2D MoS2 hybrid nanomaterials for electrocatalytic water splitting, and this catalyst is usually prepared using the one-pot method, which is beneficial to the uniform distribution of Pt atoms in the 2D MoS2 plane. Pt doping can significantly reduce the activation energy of electrocatalytic water splitting. In addition to Pt atoms, noble metal atoms such as Ru, Pd, and Au are also applied to enhance the electrocatalytic water splitting performance of 2D MoS2. For example, the synergistic effect between S vacancies and Ru sites can improve the catalytic performance of active sites [85]. Doping Pd atoms can introduce abundant S vacancies and convert 2H MoS2 into stable 1T-MoS2, enhancing electrical transport [86]. In addition, doping with Au atoms can modify the band structure of the 2D MoS2, which is beneficial for electronic transfer [87]. All the improvements in electrocatalytic performance indicate that doping noble metal atoms can improve the electrocatalytic water splitting performance of 2D MoS2.
Compared toH2 evolution, the release of diatomic O2 is challenging because there are four electrons and four protons involved in the eventual formation of an O–O bond. In general, the elementary substances of Cu and Co are used for either HER or OER, but rarely for electrochemically overall water splitting. Xu et al. screened 28 metal single atoms supported on MoS2 edges as bifunctional electrocatalysts for overall water splitting by using DFT calculations [83]. Authors proposed a simple equation including the chemical environment and local structure of the active center, which can be used as a new structure descriptor to forecast the oxygen evolution reaction activities for MoS2-based SACs. Among these metal single atoms, the T1-vacancy (in which 37.5% of sulfur atoms are added to the initial Mo termination) modified using a Pt single atom exhibits the lowest theoretical overpotential for HER and OER, which is comparable to those of the noble metal group benchmark catalysts for overall water splitting. Compared with pure MoS2 terminations, the Pt single atom can increase the intrinsic activity of each active center by changing the chemical bond between H and S/Mo atoms. The high OER performance is attributed to the moderate d-band center of the single metal atom. By analyzing the electrical structure of S/Mo near the hydrogen, the improvement of intrinsic HER activity is attributed to the change in the bond between H and S/Mo.
Similarly, Bao et al. [20] and Zhang et al. [87] also reported that the electrocatalytic activity of Pt@2D-MoS2 catalyst in the HER process was greatly improved compared to pure MoS2. With (NH4)6Mo7O24, H2PtCl6 and CS2 as precursors, Pt@2D-MoS2 catalyst was synthesized through a one-pot chemical method, which realized the uniform distribution of Pt atoms in the 2D MoS2 nanosheets. The transmission electron microscopy (TEM) image (Figure 9a) shows that the structure of Pt@2D-MoS2 catalyst is flower-like 2D nanosheets. As shown in Figure 9b, single Pt atoms uniformly disperse on the entire 2D nanosheets and substitute the Mo atoms. Compared to blank glassy carbon (GC) electrode and bulk MoS2, the electrocatalytic activity of Pt@2D-MoS2 catalyst for HER is significantly improved after the Pt atom doping (Figure 9c). As shown in Tafel plots, differently from Pt/C electrocatalyst (32 mV dec−1), the value of Pt@2D-MoS2 catalyst (96 mV dec−1) is closer to that of pure few-layer MoS2 nanosheets (FL-MoS2) (98 mV dec−1), which indicates that the S atoms instead of the Pt atoms are the active sites of HER reaction (Figure 9d). Moreover, Pt@2D-MoS2 electrocatalyst exhibits show extremely stable performance (Figure 9e,f) because the current intensity and the potential values are nearly unchanged even after suffering from long-term CV sweeps (>1000 cycles). All these enhancements are attributed to the activation of the in-plane S position adjacent to the doped Pt atom and the change of H adsorption free energy (ΔGH) based on the DFT calculations because Pt doping leads to a decrease in band gap, improved electron transport properties, and reduced activation energy of water splitting.
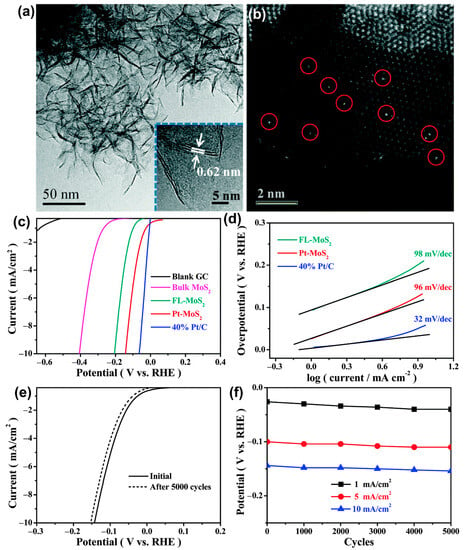
Figure 9.
(a) TEM image and (b) HAADF-STEM images of Pt-MoS2. (c) HER polarization curves for Pt–MoS2 in comparison with blank GC electrode, bulk MoS2, FL-MoS2, and 40% Pt/C. (d) Tafel plots for FL-MoS2, Pt–MoS2, and 40% Pt/C, respectively. € Durability measurement of Pt–MoS2. (f) Potential values recorded initially and after every 1000 CVs from the polarization curves of durability measurements for Pt–MoS2 at 1 mA cm−2, 5 mA cm−2, and 10 mA cm−2, respectively. Reproduced with permission from Ref. [20]. Copyright 2015 RSC publishing.
3.2.2. Non-Noble Metal Modified 2D MoS2
2D MoS2 nanosheets exhibit excellent electrocatalytic performance for hydrogen evolution, especially the 2D MoS2 with 1T-metallic phase with superior HER catalytic activity to the 2H semiconducting phase. Although anchoring single-atom noble metal onto 2D MoS2 can enhance the electrocatalytic activity for overall water splitting, developing non-precious metals with comparable electrocatalysis activity with that of noble metal is still the best strategy for saving costs [88]. TM-doping can not only modify the electronic structure of 2D MoS2 but also induce lattice distortion and effectively increase the exchange current density. In essence, the doping enlarges the surface active areas and improves electrical conductivity [42,89,90]. Therefore, coupling of 2D MoS2 with TM atoms with excellent OER activities can realize the improvement of the overall water splitting performance [91,92,93,94].
Wang et al. developed a direct one-pot hydrothermal method to synthesize pure 1T-MoS2 with flower-like morphology and high stability at a low temperature of 200 °C, and TMs (Ni, Co, Fe) were further doped via the one-pot method to improve the HER activity and OER activity of 1T-MoS2 in alkaline media [55]. As shown in Figure 10a, the changes of ΔGH as the descriptor were used to evaluate the HER activity of TMs (Ni, Co, Fe)-doped 1T-MoS2 for water splitting. The ideal ΔGH should be close to 0 eV because a large positive ΔGH is not kinetically favored for the adsorption of hydrogen on active sites of the catalyst, and a large negative ΔGH indicates difficult spontaneous release of adsorbed hydrogen. Based on this principle, pristine 1T-MoS2 has a strong adsorption effect on H2O (Figure 10a), which is adverse to the dissociation and electrocatalytic processes. In contrast, doping Fe, Co, and Ni atoms can change the electronic structure of MoS2, which can decrease its adsorption of H2O and increase the possibility of H2O dissociation. In particular, Ni-doped 1T-MoS2 electrocatalyst exhibits the best performance for HER, proved by its the lowest overpotential of 1.19 V. In addition, consistent with XPS results, the charge difference density of Ni@1T-MoS2 indicates that Ni plays a role in losing electrons, and Mo acquires electrons (Figure 10b). In addition, the Mo 3d spectrum exhibits a significant increase in electron density because the S-3p orbital can strongly hybridize with the Ni-3d orbital. Moreover, the doping of the Ni atom can enhance the metallic property of the 2D MoS2 based on the analysis of its TDOS; therefore, Ni@2D-MoS2 catalyst has the highest charge density at the Fermi surface (Figure 10c), which can accelerate electron exchange. As shown in Figure 10d, DFT-optimized geometries of alkaline HER on Ni@1T-MoS2 catalyst show that the active site for water splitting is the Ni atom. Furthermore, as the molar ratio of Mo/Ni reaches ~6.7, the hybrid electrocatalyst can achieve the best electrocatalytic performance of η10 of 112 mV for HER and 224 mV for OER. The overall water-splitting reactivity is attributed to single-atomic Ni, which changes the electronic structure of 2D 1T-MoS2.
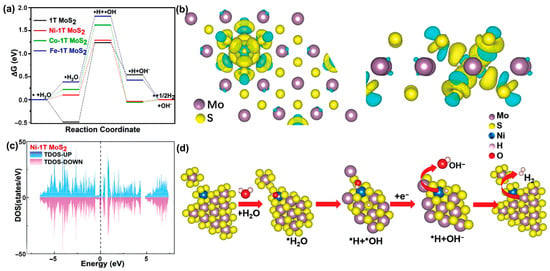
Figure 10.
(a) Gibbs free energy diagrams for the H2 production pathway on the 1T-MoS2 and TM-doped 1T-MoS2. (b) Charge difference density of Ni@1T-MoS2. (c) DOS of Ni@1T-MoS2. (d) DFT-optimized geometries of alkaline HER on Ni@1T-MoS2. Reproduced with permission from Ref. [55]. Copyright 2022 John Wiley and Sons.
Similarly, Zheng et al. prepared several selenium-doped MoS2 nanofoams (Se-MoS2-NF) with different Se contents based on a one-pot solvothermal method [93]. Se-doped MoS2 nanofoam has abundant spherical cavities (Figure 11a), which is beneficial for the mass transportation of reactants and increasing the number of active sites. Figure 11b shows the homogeneous distribution of Mo, S, and Se elements in Se-doped MoS2 nanofoam. In addition, Se-MoS2-NF has richer edges than both MoS2 nanofoam (MoS2-NF) and few-layer MoS2 (MoS2-FL) (Figure 11c), which means that the electrocatalytic HER performance of Se-MoS2-NF can be significantly improved. HER polarization curves show that Se-MoS2-NF catalyst exhibits better HER activity than those of bulk MoS2, MoS2-FL, and MoS2-NF (Figure 11d). Moreover, 9.1% Se-doped in 2D MoS2 can obtain the highest HER activity with a large current density of 1000 mA cm−2 and a much lower overpotential of 382 mV than that of commercial Pt/C catalyst (Figure 11e). The introduction of Se confined in the surface of 2D MoS2 can enable activation of the basal plane, stabilization of the edges, and optimization of the hydrogen adsorption activity.
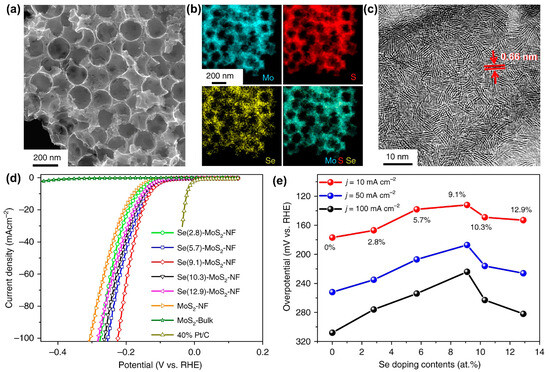
Figure 11.
(a) SEM image, (b) EDX mappings, and (c) HRTEM image of the Se-MoS2-NF. (d) HER polarization curves for the Se-MoS2-NF with different Se deposited contents. (e) Changes of overpotentials depending on the Se deposited content at different current densities. Reproduced with permission from Ref. [93]. Copyright 2020 Springer Nature.
Qiao et al. proposed a simple, inexpensive way for modifying MoS2 nanosheets on Cu nanorods to design MoS2/Cu structure as a multifunctional catalyst [77]. The electrocatalyst shows markedly improved performances for both HER and OER and great durability in alkaline media. The facial and scalable synthetic effect between Cu nanorods and MoS2 nanosheets leads to great electrocatalytic performance. This study provides a possible strategy for developing electrocatalysts with desired catalytic performance and stability through simple and large-scale manufacturing techniques. Similarly, Zhu et al. explored the electrocatalytic water-splitting activity of Cu@2H-MoS2 and Co@2H-MoS2 via DFT calculations [94]. It is found that anchoring single-atom Cu or Co onto 2D 2H-MoS2 can change the surface charge distribution and electronic band structure of 2H-MoS2. In addition, the imbalance of charge distribution will bring about electron transfer. To be specific, the charge distribution of pristine MoS2 should be 0.15 e for the Mo atom and −0.08 e for the S atom, but the Mo atom in Cu@2H-MoS2 acquires 0.05 e, while the Mo atom in Co@2H-MoS2 decreases by 0.12 e. The electron injection is beneficial for destabilizing the 2H-MoS2 and promoting the phase transition of 2H-MoS2 → 1T-MoS2. Moreover, by doping single-atom Cu or Co, inert in-plane S-atoms of 2D 2H-MoS2 can be activated, thus significantly improving the electrochemical activity of overall water splitting. Relatively, the Co@2H-MoS2 catalyst shows better HER performance with smaller Gibbs free energy than hydrogen adsorption (ΔGH = 0.08 eV), and the Cu@2H-MoS2 catalyst exhibits a lower energy barrier and a reduced overpotential of 1.25 eV for OER.
Qi et al. explored a top-down assembly/leaching method to realize Co array covalently anchored onto distorted 1T-MoS2 nanosheets (SA Co-D 1T-MoS2) [57]. As shown in Figure 12a–d, the atomic dispersion of Co atoms and the phase transformation of MoS2 are observed. Specifically, both the HAADF-STEM image (Figure 12b) and simulated pattern (Figure 12c, d) demonstrate the obvious interface between SA Co-D 1T-MoS2 and 2H MoS2. Single Co atoms are homogeneously distributed on top of Mo atoms on the MoS2 slab. The SA Co-D 1T-MoS2 electrocatalyst containing 3.54% Co (wt.%) shows an incredibly small onset overpotential for HER, even comparable with that of 10% Pt/C (Figure 12e). In addition, the Tafel slope of SA Co-D 1T-MoS2 (32 mV dec−1) is also comparable to that of Pt/C (30 mV dec−1), preceding those of non-Pt based electrocatalysts (Figure 12f). Moreover, the low Tafel slope exhibits that a Tafel-rate-determining-step mechanism, rather than the common Volmer reaction, is responsible for the electrocatalytic HER of SA Co-D 1T-MoS2 [95].
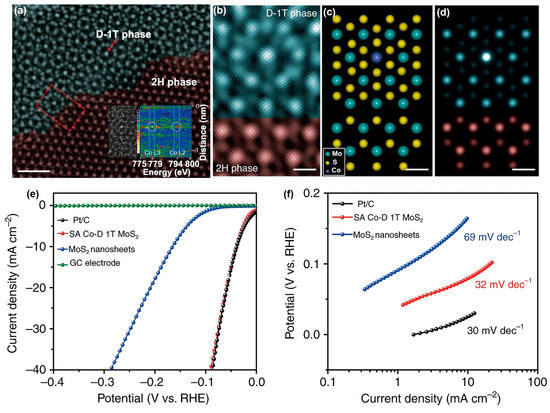
Figure 12.
(a) Aberration-corrected HAADF-STEM image of SA Co-D 1T-MoS2. (b) Enlarged HAADF-STEM image in the red square area of Figure 12a. (c) Theoretical model and (d) simulated STEM images. All scale bars are 2 Å. (e) Polarization curves and (f) Tafel plots of different catalysts. Reproduced with permission from Ref. [57]. Copyright 2019 Springer Nature.

Table 2.
Summary of typical single-atom metal/2D MoS2 hybrid nanomaterials for water splitting.
Table 2.
Summary of typical single-atom metal/2D MoS2 hybrid nanomaterials for water splitting.
| Catalyst | Electrolyte | η (mV)/Best Ratio (w.t.% or Concentration) | Tafel Slope (mV dec −1) | Stability Test | Ref. |
|---|---|---|---|---|---|
| Co/2D MoS2 | 0.5 M H2SO4 | 42/3.5% Co/1T-MoS2 | 32 | 10,000 CVs | [57] |
| Pt/2D MoS2 | 0.1 M H2SO4 | 60/1.5% Pt/MoS2 | 96 | 5000 CVs | [20] |
| Pd/2D MoS2 | 0.5 M H2SO4 | 89/1% Pd/1T-MoS2 | 62 | 5000 CVs | [86] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 98/Ni/MoS2 | 103 | 2000 CVs | [96] |
| Ru/2D MoS2 | 0.5 M H2SO4 | 114/46 μg cm−2 Ru/MoS2 | - | 10 h | [97] |
| Cu/2D MoS2 | 0.5 M H2SO4 | 131/1% Cu/MoS2 | 51 | 7 h | [54] |
| Fe, Co, Ni, Pd, Pt/2D MoS2 | 0.5 M H2SO4 | 140/2.7% Pd/1T-MoS2 | 57 | 1000 CVs | [98] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 174/1% Ni/MoS2 | 69 | 1000 CVs | [99] |
| Au, Pt, Pd/2D MoS2 | 0.5 M H2SO4 | 210/1.1% Pt/MoS2 | 104 | 5 h | [63] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 263/2.7% Ni/MoS2 | 81 | 1000 CVs | [100] |
| Ru/2D MoS2 | 1.0 M PBS | 125/46 μg cm−2 Ru/MoS2 | - | 10 h | [97] |
| Ru/2D MoS2 | 1.0 M KOH | 41/46 μg cm−2 Ru/MoS2 | 114 | 20 h | [97] |
| Ir/2D MoS2 | 1.0 M KOH | 44/Ir/1T-MoS2 | 32 | 9000 CVs | [101] |
| Ni/2D MoS2 | 1.0 M KOH | 110/Ni/MoS2 | 119 | 2000 CVs | [96] |
Note: overpotential value (η) and stability test were performed at the current density of 10 mA cm−2; CVs, cycles.
4. Challenges and Prospects
In this review, we have summarized the main advantages and features, synthesis methods, and applications of single-atom metal/2D-MoS2 hybrid nanomaterials for electrocatalytic CO2RR and water splitting. We highlight their synthesis routes, modification approaches, and physiochemical mechanisms for improving their electrocatalytic performances of single-atom metal/2D MoS2 hybrid nanomaterials in electrocatalytic CO2RR and water splitting with some typical examples.
Although research has considerably progressed in single-atom metal/2D-MoS2 hybrid catalysts for CO2RR and water splitting [64,102,103,104,105,106,107,108,109,110], certain challenges remain in the development of control synthesis methods for obtaining low-cost and high-performance electrocatalysts, solving the aggregation and stability of single-atom catalysts due to high surface energy [111,112], clarifying the reaction mechanisms of water or CO2 on the surfaces or interfaces of catalysts [113,114,115], understanding the influences of metal–support interactions and the number, type, and microenvironment of active sites on the catalysis reactions. These problems limit their practical applications in future.
Therefore, from a long-term view, more efforts in the future are focused on control synthesis, structure–property characterization, and reaction mechanisms. First, it is necessary to work hard on controllable preparation to ensure the dispersion of single metal atoms and the catalytic activity of hybrid catalysts [116] because the control, high-quality, and large-scale synthesis of single-atom catalysts is the precondition or bottleneck for deep understanding of the structure–property relationship and the reaction mechanism. For example, the non-noble metals of SACs such as Fe, Ni, and Cu nanoparticles are easily oxidized in the air [17]. Most previous research has focused more on the coordination environment or components of single atoms, but relatively, the structural control (e.g., crystal phases and structural defects on the basal planes or plane edges) of 2D materials is not paid enough attention. In addition to the electrode materials, the overall modulations from the perspective of the whole electrochemical rection devices, such as the mass and electron transfer of electrode/electrolyte interface, should be carefully considered. Second, research efforts in the future may be focused on the development and utilization of some in situ characterization methods (e.g., in situ Fourier transform infrared spectroscopy), analysis methods for the specific coordination environment of the single atoms (e.g., X-ray absorption near edge structure spectra), and theoretical predictions (e.g., DFT or machine learning) for a better understanding of the structure–property relationship [117,118,119,120,121], especially the relationship between the defects of these hybrid nanomaterials and their electrocatalytic activity. The DFT calculations can simulate the electrocatalytic reaction efficiency of single-atom metal/2D MoS2 hybrid nanomaterials and clarify the electron transfer mechanism between 2D MoS2 and single-atom metals [17]. Therefore, DFT calculations can act as a powerful tool for the prediction or screening of new electrocatalysts. Last but not least, except for the electrocatalyst itself, to accurately identify the clear catalytic mechanisms, fundamental research and optimized standard experimental systems of electrocatalytic water splitting and CO2RR are still required [14]. Combination of DFT and machine learning based on data-driven scientific research motivated by artificial intelligence has great potential in understanding electrocatalytic mechanisms at an atomic level. We hope this review can provide some valuable suggestions for the development of advanced electrocatalysts for the environment and energy fields.
Author Contributions
Conceptualization, X.G.; writing—original draft preparation, J.W.; writing—review and editing, X.G., T.Z., Y.A., and P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52000059). Additionally, this study was funded by the Key Lab of Modern Optical Technologies of Jiangsu Province, Soochow University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.A.; Luo, M.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic carbon nitride composites with gold and ZIF-67 nanoparticles as visible-light-promoted catalysts for CO2 conversion and bisphenol A degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416. [Google Scholar] [CrossRef]
- Khan, I.; Kang, K.; Khan, A.; Jiyuan, G.; Khan, S.; Khan, S.; Basir, A.; Sadiq, S. Efficient CO2 conversion and organic pollutants degradation over Sm3+ doped and rutile TiO2 nanorods decorated-GdFeO3 nanorods. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Khan, I.; Luo, M.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green synthesis of SrO bridged LaFeO3/g-C3N4 nanocomposites for CO2 conversion and bisphenol A degradation with new insights into mechanism. Environ. Res. 2022, 207, 112650. [Google Scholar] [CrossRef]
- Liu, H.; Grasseschi, D.; Dodda, A.; Fujisawa, K.; Olson, D.; Kahn, E.; Zhang, F.; Zhang, T.; Lei, Y.; Branco, R.B.N. Spontaneous chemical functionalization via coordination of Au single atoms on monolayer MoS2. Sci. Adv. 2020, 6, eabc9308. [Google Scholar] [CrossRef]
- Shan, J.; Ye, C.; Jiang, Y.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 2022, 8, eabo0762. [Google Scholar] [CrossRef]
- Xu, J.; Lai, S.; Qi, D.; Hu, M.; Peng, X.; Liu, Y.; Liu, W.; Hu, G.; Xu, H.; Li, F. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021, 14, 1374–1381. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Lei, D.; Ye, R.; Zhao, H.; Wong, K.-Y. Transition metal dichalcogenide-based mixed-dimensional heterostructures for visible-light-driven photocatalysis: Dimensionality and interface engineering. Nano Res. 2021, 14, 2003–2022. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Sun, J.-F.; Wu, J.-T.; Xu, Q.-Q.; Zhou, D.; Yin, J.-Z. CO2 electrochemical reduction using single-atom catalysts. Preparation, characterization and anchoring strategies: A review. Environ. Chem. Lett. 2020, 18, 1593–1623. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3, 1800492. [Google Scholar] [CrossRef]
- Lee, W.H.; Ko, Y.-J.; Kim, J.-Y.; Min, B.K.; Hwang, Y.J.; Oh, H.-S. Single-atom catalysts for the oxygen evolution reaction: Recent developments and future perspectives. Chem. Commun. 2020, 56, 12687–12697. [Google Scholar] [CrossRef]
- Li, X.; Cui, P.; Zhong, W.; Li, J.; Wang, X.; Wang, Z.; Jiang, J. Graphitic carbon nitride supported single-atom catalysts for efficient oxygen evolution reaction. Chem. Commun. 2016, 52, 13233–13236. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- He, T.; Zhang, C.; Du, A. Single-atom supported on graphene grain boundary as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Eng. Sci. 2019, 194, 58–63. [Google Scholar] [CrossRef]
- Vancsó, P.; Popov, Z.I.; Pető, J.n.; Ollár, T.; Dobrik, G.; Pap, J.z.S.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Transition metal chalcogenide single layers as an active platform for single-atom catalysis. ACS Energy Lett. 2019, 4, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, K.; Lin, Y.; Song, L.; Liu, J.; Liu, Y.; Zhang, L.; Wu, Z.; Song, S.; Li, J. A single-atom manipulation approach for synthesis of atomically mixed nanoalloys as efficient catalysts. Angew. Chem. 2020, 132, 13670–13676. [Google Scholar] [CrossRef]
- Su, H.; Zhou, W.; Zhang, H.; Zhou, W.; Zhao, X.; Li, Y.; Liu, M.; Cheng, W.; Liu, Q. Dynamic evolution of solid–liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 2020, 142, 12306–12313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Guan, P.; Liu, C.; Huang, H.; Xue, F.; Dong, X.; Pennycook, S.J.; Chisholm, M.F. Catalytically active single-atom niobium in graphitic layers. Nat. Commun. 2013, 4, 1924. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, J.; Liu, J.; Bai, Y.; Su, X.; Wang, W.; Cao, Z.; Zhao, H.; Ao, Y.; Wang, P. Polyaniline Functionalization of Defective 1T-MoS2 Nanosheets for Improved Electron and Mass Transfer: Implications for Electrochemical Sensors. ACS Appl. Nano Mater. 2023, 6, 11725–11736. [Google Scholar] [CrossRef]
- Hou, C.C.; Zou, L.; Sun, L.; Zhang, K.; Liu, Z.; Li, Y.; Li, C.; Zou, R.; Yu, J.; Xu, Q. Single-atom iron catalysts on overhang-eave carbon cages for high-performance oxygen reduction reaction. Angew. Chem. 2020, 132, 7454–7459. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, L.; Yin, X.; Wang, Z.; Zhang, L.; Ma, H.; Ke, Y.; Du, Y.; Xi, S.; Wee, A.T. Enhanced electrocatalytic hydrogen evolution activity in single-atom Pt-decorated VS2 nanosheets. ACS Nano 2020, 14, 5600–5608. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Liu, X.; Xiao, W.; Luo, Z.; Wang, W.; Zhang, Y.; Liu, J.-C. Understanding the hydrogen evolution reaction activity of doped single-atom catalysts on two-dimensional GaPS4 by DFT and machine learning. J. Energy Chem. 2023, 81, 93–100. [Google Scholar] [CrossRef]
- Xiao, Z.; Gan, X.; Zhu, T.; Lei, D.; Zhao, H.; Wang, P. Activating the Basal Planes in 2H-MoTe2 Monolayers by Incorporating Single-Atom Dispersed N or P for Enhanced Electrocatalytic Overall Water Splitting. Adv. Sustain. Syst. 2022, 6, 2100515. [Google Scholar] [CrossRef]
- Gan, X.; Lei, D.; Wong, K.-Y. Two-dimensional layered nanomaterials for visible-light-driven photocatalytic water splitting. Mater. Today Energy 2018, 10, 352–367. [Google Scholar] [CrossRef]
- Pan, U.N.; Paudel, D.R.; Das, A.K.; Singh, T.I.; Kim, N.H.; Lee, J.H. Ni-nanoclusters hybridized 1T–Mn–VTe2 mesoporous nanosheets for ultra-low potential water splitting. Appl. Catal. B Environ. 2022, 301, 120780. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; Volume 2. [Google Scholar]
- Yang, J.; Mohmad, A.R.; Wang, Y.; Fullon, R.; Song, X.; Zhao, F.; Bozkurt, I.; Augustin, M.; Santos, E.J.; Shin, H.S. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 2019, 18, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, R.; Veselý, M.; Sofer, Z.k. Recent developments on the single atom supported at 2D materials beyond graphene as catalysts. ACS Catal. 2020, 10, 9634–9648. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy level engineering of MoS2 by transition-metal doping for accelerating hydrogen evolution reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Gan, X.; Lee, L.Y.S.; Wong, K.-y.; Lo, T.W.; Ho, K.H.; Lei, D.Y.; Zhao, H. 2H/1T phase transition of multilayer MoS2 by electrochemical incorporation of S vacancies. ACS Appl. Energy Mater. 2018, 1, 4754–4765. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Lei, D.; Wang, P. Improving electrocatalytic activity of 2H-MoS2 nanosheets obtained by liquid phase exfoliation: Covalent surface modification versus interlayer interaction. J. Catal. 2020, 391, 424–434. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Linghu, Y.; Tong, T.; Li, C.; Wu, C. The catalytic mechanism of CO2 electrochemical reduction over transition metal-modified 1T’-MoS2 monolayers. Appl. Surf. Sci. 2022, 590, 153001. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kuo, C.-T.; Kovarik, L.; Hoffman, A.S.; Boubnov, A.; Driscoll, D.M.; Morris, J.R.; Bare, S.R.; Karim, A.M. A versatile approach for quantification of surface site fractions using reaction kinetics: The case of CO oxidation on supported Ir single atoms and nanoparticles. J. Catal. 2019, 378, 121–130. [Google Scholar] [CrossRef]
- Repp, J.; Moresco, F.; Meyer, G.; Rieder, K.-H.; Hyldgaard, P.; Persson, M. Substrate mediated long-range oscillatory interaction between adatoms: Cu/Cu(111). Phys. Rev. Lett. 2000, 85, 2981. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef]
- Zhang, F.; Momeni, K.; AlSaud, M.A.; Azizi, A.; Hainey, M.F.; Redwing, J.M.; Chen, L.-Q.; Alem, N. Controlled synthesis of 2D transition metal dichalcogenides: From vertical to planar MoS2. 2D Mater. 2017, 4, 025029. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Quan, F.; Zhan, G.; Jia, F.; Ai, Z.; Zhang, L. Accelerated dinitrogen electroreduction to ammonia via interfacial polarization triggered by single-atom protrusions. Chem 2020, 6, 885–901. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. 2011, 123, 11289–11293. [Google Scholar] [CrossRef]
- Ji, L.; Yan, P.; Zhu, C.; Ma, C.; Wu, W.; Wei, C.; Shen, Y.; Chu, S.; Wang, J.; Du, Y. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 87–93. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, G.; Ke, X.; Chen, X.; Chen, X.; Wang, Y.; Huang, G.; Dong, J.; Chu, S.; Sui, M. Direct Synthesis of Stable 1T-MoS2 Doped with Ni Single Atoms for Water Splitting in Alkaline Media. Small 2022, 18, 2107238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2021, 122, 1273–1348. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced electrochemical hydrogen evolution. Nat. Commun. 2019, 10, 982. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Deng, T.; Zheng, W.; Zhang, W. Increasing the range of non-noble-metal single-atom catalysts. Chin. J. Catal. 2017, 38, 1489–1497. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Thomas, J.M.; Saghi, Z.; Gai, P.L. Can a single atom serve as the active site in some heterogeneous catalysts? Top. Catal. 2011, 54, 588–594. [Google Scholar] [CrossRef]
- Xuan, N.; Chen, J.; Shi, J.; Yue, Y.; Zhuang, P.; Ba, K.; Sun, Y.; Shen, J.; Liu, Y.; Ge, B. Single-atom electroplating on two dimensional materials. Chem. Mater. 2018, 31, 429–435. [Google Scholar] [CrossRef]
- Meng, Z.; Fan, J.; Chen, A.; Xie, X. Functionalized MoS2 catalysts for CO2 capture and conversion: A review. Mater. Today Chem. 2023, 29, 101449. [Google Scholar] [CrossRef]
- Detz, H.; Butera, V. Insights into the mechanistic CO2 conversion to methanol on single Ru atom anchored on MoS2 monolayer. Mol. Catal. 2023, 535, 112878. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Qi, K.; Zhao, Z. Single atom supported on MoS2 as efficient electrocatalysts for the CO2 reduction reaction: A DFT study. Appl. Surf. Sci. 2022, 602, 154211. [Google Scholar] [CrossRef]
- Mao, X.; Wang, L.; Xu, Y.; Li, Y. Modulating the MoS2 edge structures by doping transition metals for electrocatalytic CO2 reduction. J. Phys. Chem. C 2020, 124, 10523–10529. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.-j.; Zhuang, L. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S. Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Zhong, W.; Li, Y.; Qiu, Y.; Zheng, L.; Jiang, J.; Jiang, H.-L. Regulating photocatalysis by spin-state manipulation of cobalt in covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 16723–16731. [Google Scholar] [CrossRef]
- Hong, X.; Chan, K.; Tsai, C.; Nørskov, J.K. How doped MoS2 breaks transition-metal scaling relations for CO2 electrochemical reduction. Acs Catal. 2016, 6, 4428–4437. [Google Scholar] [CrossRef]
- Abbasi, P.; Asadi, M.; Liu, C.; Sharifi-Asl, S.; Sayahpour, B.; Behranginia, A.; Zapol, P.; Shahbazian-Yassar, R.; Curtiss, L.A.; Salehi-Khojin, A. Tailoring the edge structure of molybdenum disulfide toward electrocatalytic reduction of carbon dioxide. ACS Nano 2017, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q. Theoretical studies of non-noble metal single-atom catalyst Ni1/MoS2: Electronic structure and electrocatalytic CO2 reduction. Sci. China Mater. 2022, 66, 1079–1088. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, D.; Yang, H.; Liu, X.; Luo, J. Dual-doping promotes the carbon dioxide electroreduction activity of MoS2 nanosheet array. ACS Appl. Energy Mater. 2021, 4, 7492–7496. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Jhong, H.-R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.; Masel, R.I. Nanoparticle silver catalysts that show enhanced activity for carbon dioxide electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ali, S.; Zada, A.; Ilyas, N.; Shaha, R.; Wang, Y.; Qiao, L. Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion. Mater. Des. 2021, 204, 109674. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Pham, V.P.; Yeom, G.Y. Recent advances in doping of molybdenum disulfide: Industrial applications and future prospects. Adv. Mater. 2016, 28, 9024–9059. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Z.; Ouyang, X. Adsorption of radionuclides on the monolayer MoS2. Mater. Res. Express 2018, 5, 045506. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Cheng, D. Design of high-performance MoS2 edge supported single-metal atom bifunctional catalysts for overall water splitting via a simple equation. Nanoscale 2019, 11, 20228–20237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, F.; Zhang, S.; Liang, Y.; Wang, R. Engineering MoS2 basal planes for hydrogen evolution via synergistic ruthenium doping and nanocarbon hybridization. Adv. Sci. 2019, 6, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Luo, M.; Liu, Z.; Peng, M.; Chen, D.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.; Tan, Y. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat. Commun. 2021, 12, 1687. [Google Scholar] [CrossRef]
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Zhao, Q.; Huang, M.; Ouyang, X. Electrocatalytic and photocatalytic performance of noble metal doped monolayer MoS2 in the hydrogen evolution reaction: A first principles study. Nano Mater. Sci. 2021, 3, 89–94. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, X.; Xu, Y.; Yan, Q.; Zhu, M.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Wang, G. Vertically oriented Ni-doped MoS2 nanosheets supported on hollow carbon microtubes for enhanced hydrogen evolution reaction and water splitting. Compos. Part B Eng. 2021, 224, 109229. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Yang, Y.; Luo, M.; Huang, Y.; Sun, Y.; Chao, Y.; Yang, W.; Yang, W. Lavender-like Ga-doped Pt3Co nanowires for highly stable and active electrocatalysis. ACS Catal. 2020, 10, 3018–3026. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Shang, H.; Jin, L.; Chen, C.; Wang, Y.; Yuan, M.; Du, Y. Ir-doped Pd nanosheet assemblies as bifunctional electrocatalysts for advanced hydrogen evolution reaction and liquid fuel electrocatalysis. Inorg. Chem. 2020, 59, 3321–3329. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Wan, H.; Ji, X.; Xu, K.; Zha, D.; Miao, L.; Jiang, J. Mutually beneficial Co3O4@MoS2 heterostructures as a highly efficient bifunctional catalyst for electrochemical overall water splitting. J. Mater. Chem. A 2018, 6, 2067–2072. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, L.; Gao, M.; Chen, X.; Zhou, W.; Ma, C.; Wu, L.; Zhu, J.; Meng, X.; Hu, J. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11, 3315. [Google Scholar] [CrossRef]
- Zhu, T.; Gan, X.; Xiao, Z.; Dai, S.; Xiao, H.; Zhang, S.; Dong, S.; Zhao, H.; Wang, P. Single-atom dispersed Cu or Co on 2H-MoS2 monolayer for improving electrocatalytic activity of overall water splitting. Surf. Interfaces 2021, 27, 101538. [Google Scholar] [CrossRef]
- Liang, H.-W.; Brüller, S.; Dong, R.; Zhang, J.; Feng, X.; Müllen, K. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. Single-atom ruthenium based catalyst for enhanced hydrogen evolution. Appl. Catal. B Environ. 2019, 249, 91–97. [Google Scholar] [CrossRef]
- Lau, T.H.; Wu, S.; Kato, R.; Wu, T.-S.; Kulhavy, J.; Mo, J.; Zheng, J.; Foord, J.S.; Soo, Y.-L.; Suenaga, K. Engineering monolayer 1T-MoS2 into a bifunctional electrocatalyst via sonochemical doping of isolated transition metal atoms. ACS Catal. 2019, 9, 7527–7534. [Google Scholar] [CrossRef]
- Luo, R.; Luo, M.; Wang, Z.; Liu, P.; Song, S.; Wang, X.; Chen, M. The atomic origin of nickel-doping-induced catalytic enhancement in MoS2 for electrochemical hydrogen production. Nanoscale 2019, 11, 7123–7128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28, 1807086. [Google Scholar] [CrossRef]
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2018, 4, 368–374. [Google Scholar] [CrossRef]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z. High phase-purity 1T′-MoS2-and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.-C.; Dai, H.; Wang, Q.; Yang, R.; Yu, H.; Liao, M.; Zhang, J.; Chen, W.; Wei, Z. Boundary activated hydrogen evolution reaction on monolayer MoS2. Nat. Commun. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Xue, H.; Sun, J.; Guo, N.; Song, T.; Sun, J.; Hao, Y.-R.; Wang, Q. Co-Construction of Sulfur Vacancies and Heterogeneous Interface into Ni3S2/MoS2 Catalysts to Achieve Highly Efficient Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2208037–2208043. [Google Scholar]
- Yang, H.; Ma, D.; Li, Y.; Zhao, Q.; Pan, F.; Zheng, S.; Lou, Z. Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chin. J. Struct. Chem. 2023, 42, 100031. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Z.; Lin, Z.; Shen, S.; Zhong, W. Fabricating Ru single atoms and clusters on CoP for boosted hydrogen evolution reaction. Chin. J. Struct. Chem. 2023, 42, 100035. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and direction toward high-performance MoS2 hydrogen evolution catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, T.; Xie, N.; Nie, G.; Zhang, H. Versatile Applications of Metal Single-Atom@2D Material Nanoplatforms. Adv. Sci. 2019, 6, 1901787. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, W.; Han, Y.; Xin, P.; Zheng, X.; Yan, W.; Dong, J.; Zhang, J.; Wang, D.; Li, Y. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res. 2021, 14, 2418–2423. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Guo, C.; Li, H.; Dong, J.; Guo, P.; Wang, D.; Li, Y.; Toste, F.D. A heterogeneous iridium single-atom-site catalyst for highly regioselective carbenoid O–H bond insertion. Nat. Catal. 2021, 4, 523–531. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.; Sautet, P. Introducing structural sensitivity into adsorption–energy scaling relations by means of coordination numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Koper, M.T.; Calle-Vallejo, F. Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 2014, 136, 15694–15701. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhao, Z.-J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma catalysis: Synergistic effects at the nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Song, T.T.; Zhou, J.; Wang, S.; Chi, D.; Shen, L.; Yang, M.; Feng, Y.P. High-throughput screening of transition metal single atom catalysts anchored on molybdenum disulfide for nitrogen fixation. Nano Energy 2020, 68, 104304. [Google Scholar] [CrossRef]
- Chen, B.W.; Xu, L.; Mavrikakis, M. Computational methods in heterogeneous catalysis. Chem. Rev. 2020, 121, 1007–1048. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).