Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction
Abstract
:1. Introduction
2. The Global Cement Industry
Cutting Carbon Dioxide Emission through Different Technologies
3. Materials and Methods
3.1. Metakaolin as a Pozzolan in Concrete Production
3.2. Metakaolin as a Geopolymer in Concrete Production
3.3. Durability Test on Metakaolin-Based Geopolymer Concrete
3.4. Chemical Composition Test and Microstructural Analysis of Kaolin and Metakaolin
3.5. Statistical Analysis
4. Results and Discussion
4.1. Chemical Composition of Kaolin and Metakaolin
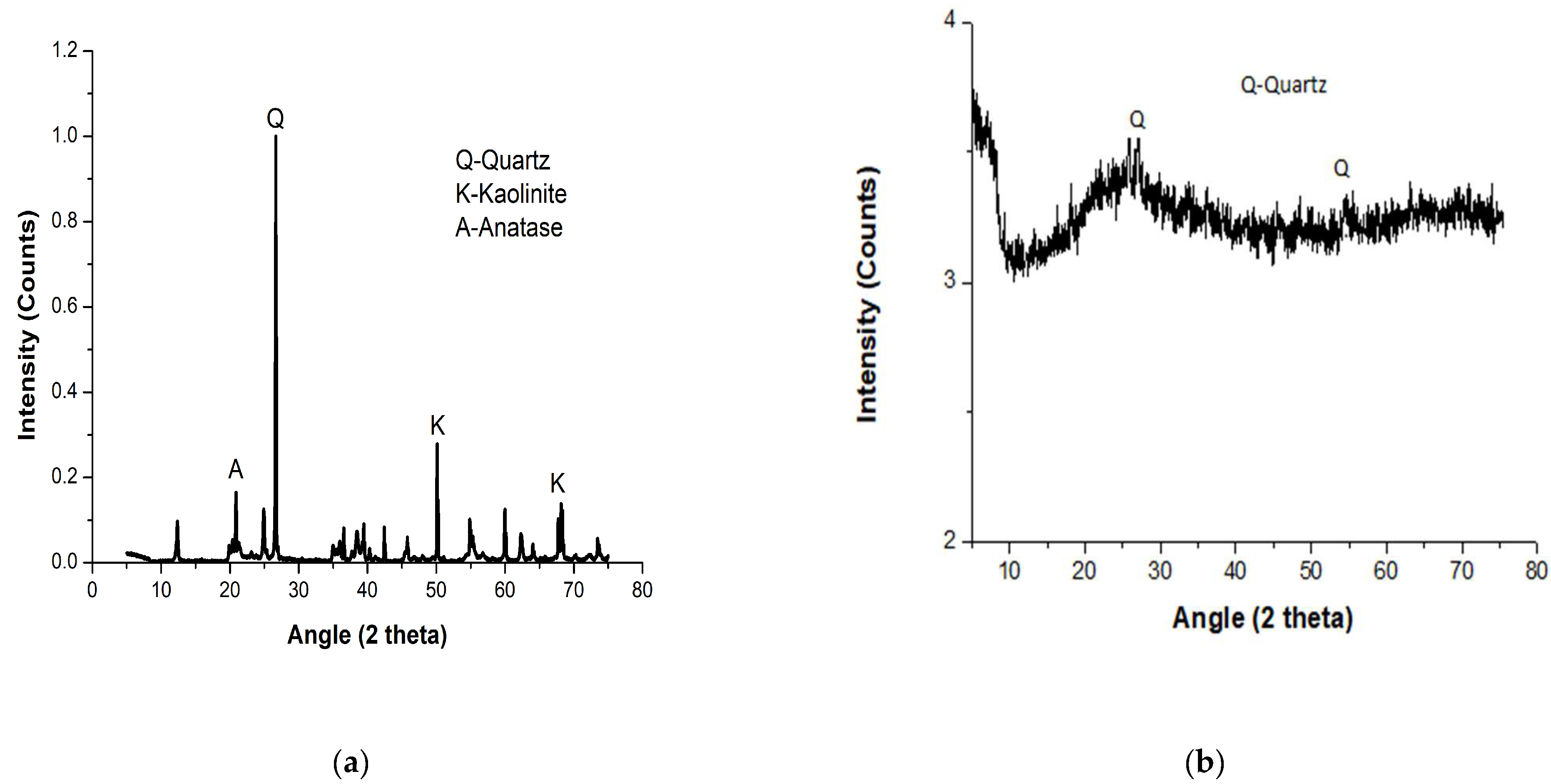
4.2. Microstructural Analysis of Ogun Samples of Kaolin and Metakaolin
4.3. Crystallographic Phases of Ogun Samples of Kaolin and Metakaolin
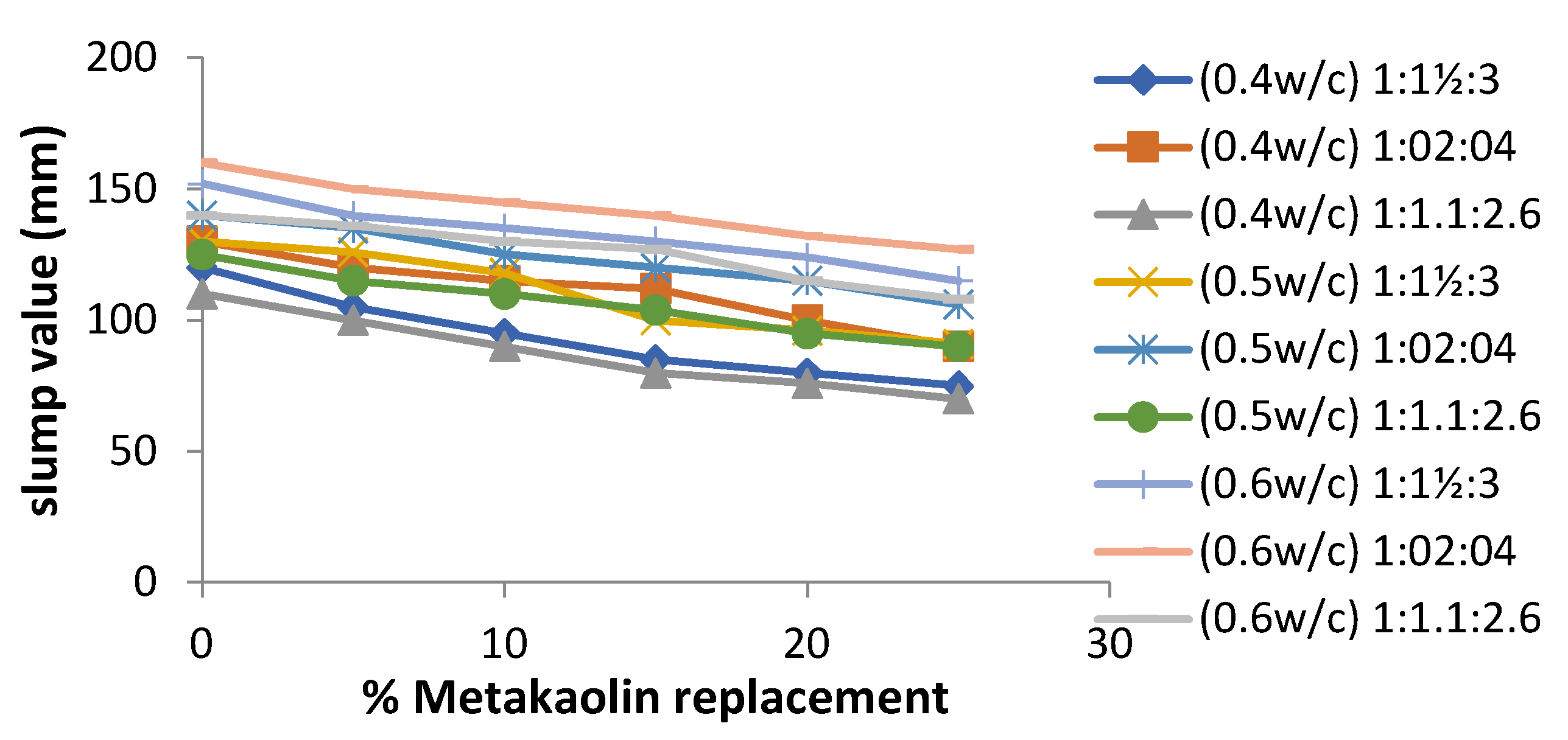
4.4. Workability of Cement-Metakaolin Concrete
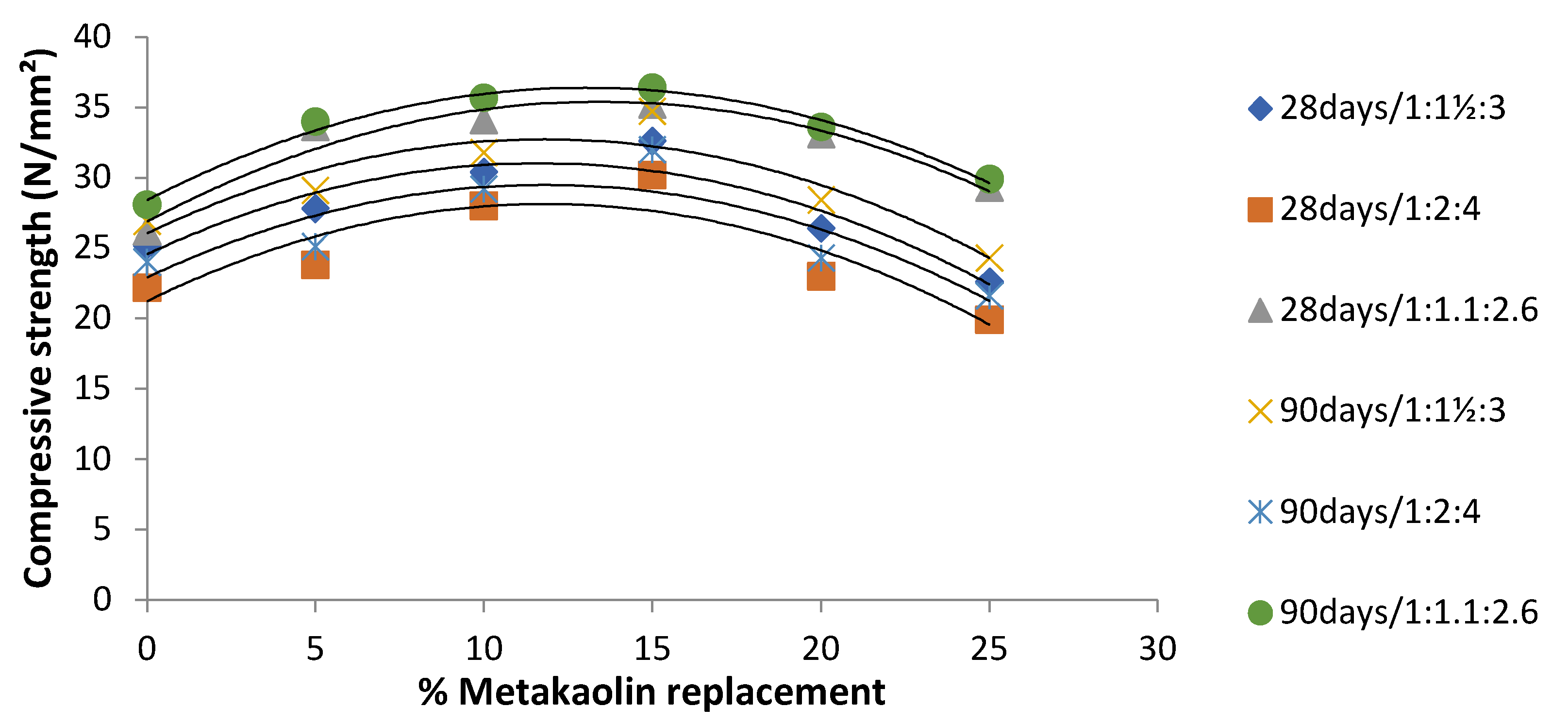
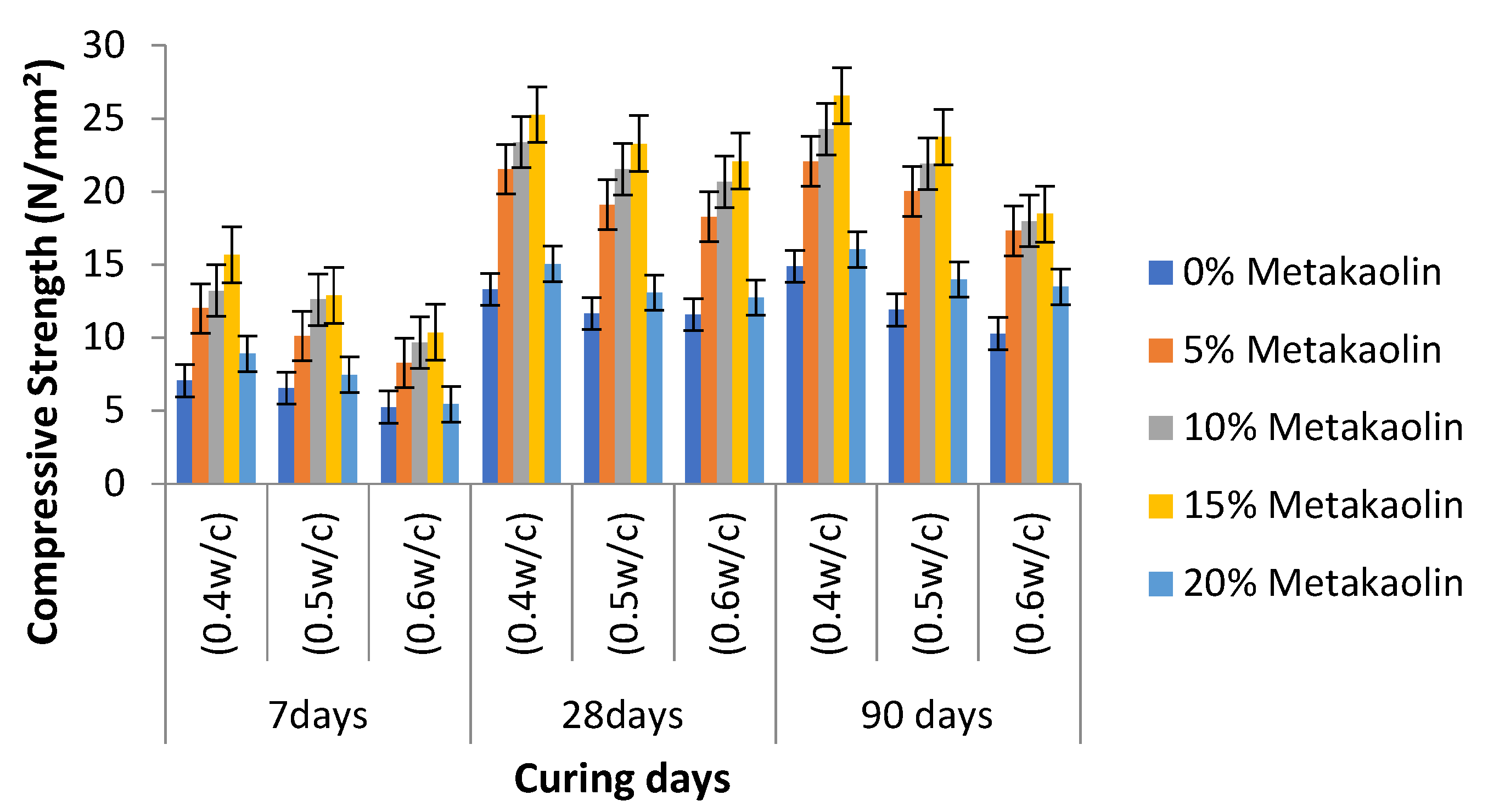
4.5. Compressive Strength of Cement-Metakaolin Concrete
4.6. Compressive Strength of Cement-Metakaolin Mortar
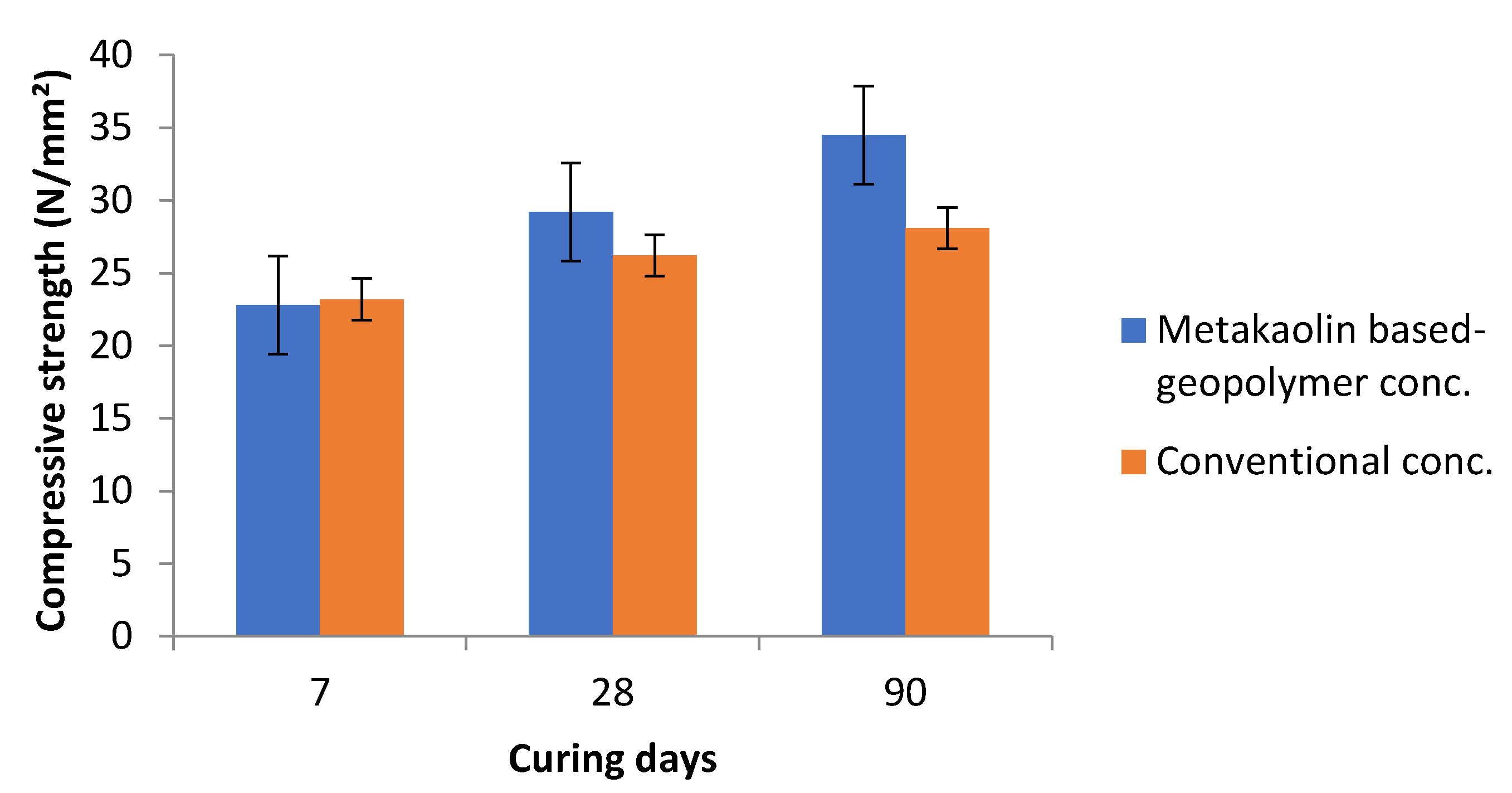
4.7. Compressive Strength of Metakaolin as a Geopolymer in Concrete Production
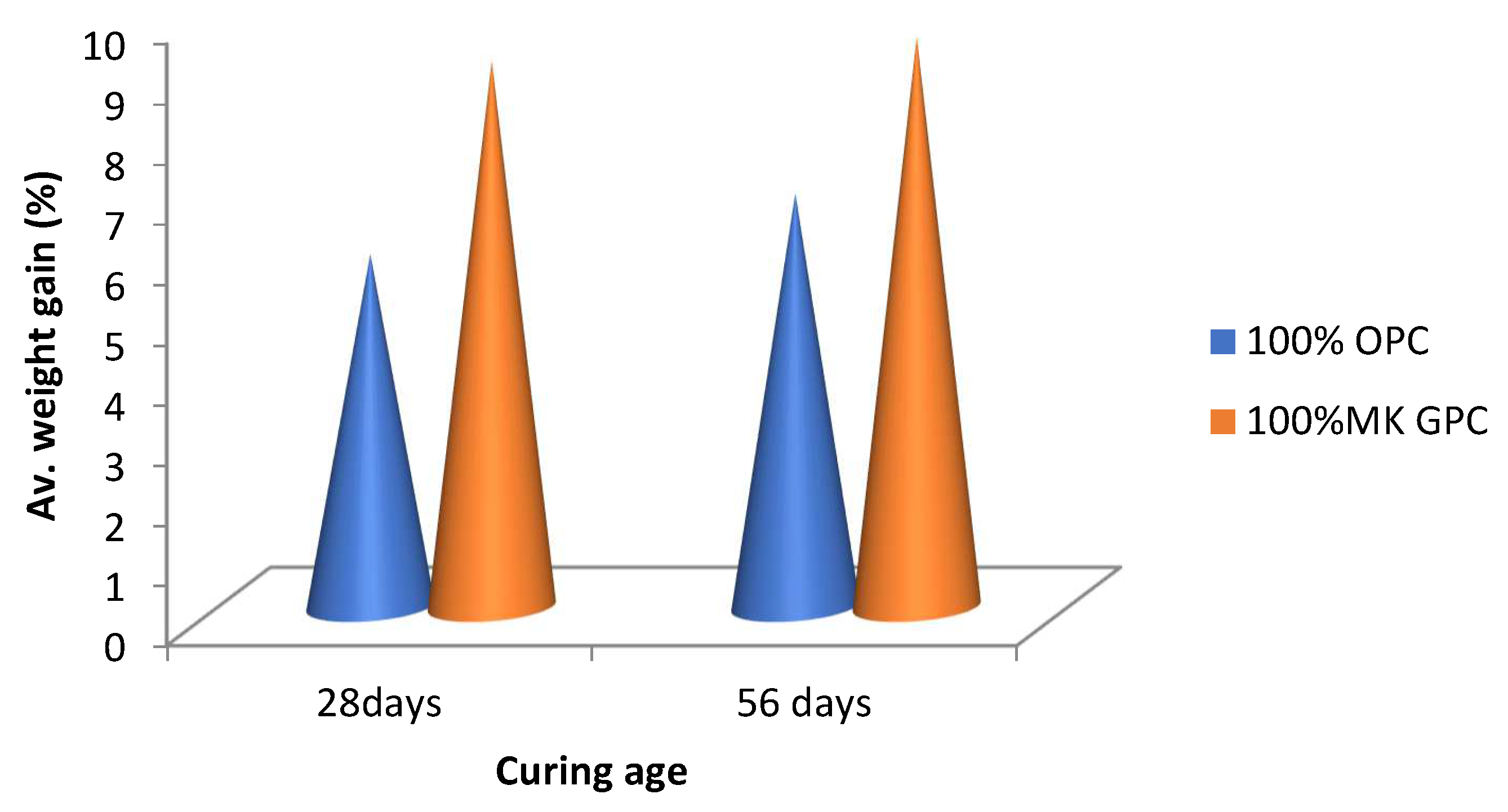
4.8. Water Absorption Capacity of Metakaolin-Based Geopolymer Concrete
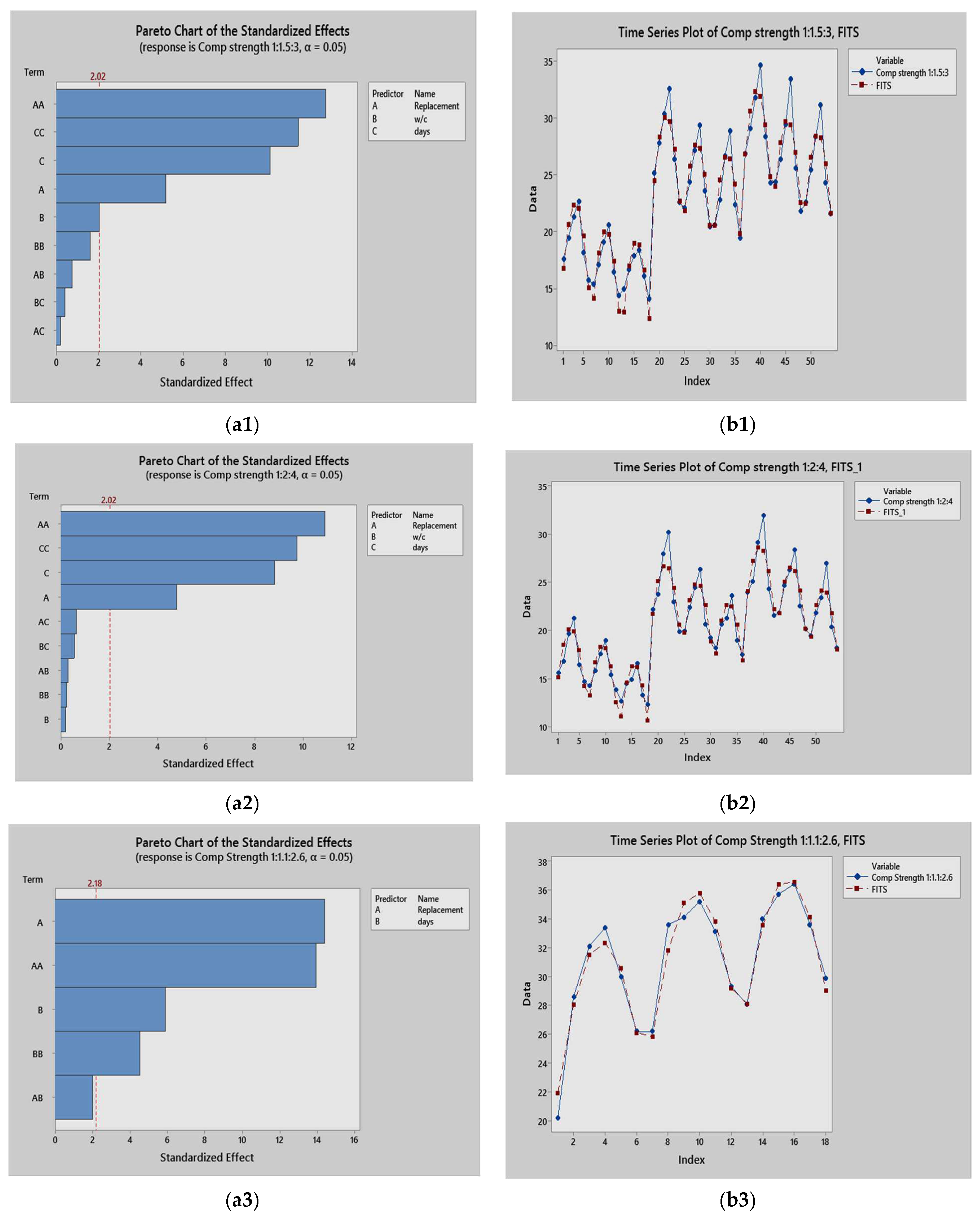
4.9. Statistical Analysis
5. Conclusions
- Kaolinite was present in crystalline form in the sample, as revealed by the XRD pattern and SEM image. The undissolved quartz present in the metakaolin was responsible for the enhanced compressive strength exhibited by the metakaolin mixes. The chemical composition test also showed that the study metakaolin has good pozzolanic and geopolymer characteristics due to its high concentrations and cumulative percentages of silica and alumina (93.39%).
- Metakaolin has a high capacity for absorption, as seen in the workability test; thus, the use of a superplasticizer is required, as additional water affects the strength of the concrete mix.
- Metakaolin admixed specimens showed greater strength responses than the OPC (control) specimens in cement-metakaolin mortar, as well as in cement-metakaolin concrete. For compressive strengths at all mix ratios, the optimum performance was seen at 15 wt.% of metakaolin substitution. This could be explained by the fact that metakaolin admixed mixes include more calcium silicate hydrates (CSH), a strength enhancer. In all combinations, compressive strengths increased when the water–cement ratio was decreased.
- In geopolymer concrete, compressive strengths of metakaolin-based geopolymer concrete at 28 days curing showed an 11.5% increase in strength over the conventional (OPC) concrete.
- Metakaolin-based geopolymer concrete specimens exhibited high water absorption capacities, with a 55.78% and 37.85% increase over the conventional (OPC) concrete at 28 and 90 days curing, respectively.
- Results from the ANOVA test showed that the percentage replacement with metakaolin, curing time, and the calcining temperature all have significant effects on the compressive strength of the studied concrete specimens. The percentage replacement with metakaolin was significant, and had the greatest effect on the compressive strength. The empirical models developed in the study are useful for predicting the compressive strength.
- The use of low calcium-based materials, such metakaolin, in concrete production reduces manufacturing temperature and fuel consumption, which then leads to reduction in carbon emissions. When compared to conventional concrete, the inclusion of metakaolin in concrete production as a pozzolan or a geopolymer is projected to create roughly 70% and 100% less carbon dioxide [64].
- According to the results of the compressive strengths obtained from the experiments in this study, metakaolin-based geopolymer concretes are excellent for use in precast structural components and concrete goods, including power poles, sleepers for railroads, and interlocking blocks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alhorr, Y.; Eliskandarani, E.; Elsarrag, E. Approaches to reducing carbon dioxide emissions in the built environment: Low carbon cities. Int. J. Sustain. Built Environ. 2014, 3, 167–178. [Google Scholar] [CrossRef]
- International Energy Agency. Global Energy Review: CO2 Emissions in 2021. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 12 May 2022).
- Teixeira, E.R.; Mateus, R.; Camões, A.; Branco, F.G. Quality and durability properties and life-cycle assessment of high volume biomass fly ash mortar. Constr. Build. Mater. 2020, 197, 195–207. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Comans, R.N.J. Technical and environmental performance of lower carbon footprint cement mortars containing biomass fly ash as a secondary cementitious material. Resour. Conserv. Recycl. 2018, 134, 25–33. [Google Scholar] [CrossRef]
- Cumulative CO2 Emissions. Supplemental Data of Global Carbon Project 2021 (1.0), Our World in Data Based on the Global Carbon Project 2021. Available online: https://ourworldindata.org/co2-emissions (accessed on 14 May 2022).
- Vigneshwaran, A.; Fayaz, K. Experimental study on the behaviour of self-compacting cured concrete using chemical admixture and metakaolin. In Proceedings of the International Conference on Emerging Trends in Engineering, Science and Sustainable Technology (ICETSST-2017), Erode, India, 17–18 February 2017; pp. 18–25. [Google Scholar]
- Khan, M.N.N.; Jamil, M.; Karim, M.R.; Zain, M.F.M.; Kaish, A.B.M.A. Filler effect of pozzolanic materials on the strength and microstructure development of mortar. KSCE J. Civ. Eng. 2017, 21, 274–284. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on flyash characteristics-towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.J.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003; pp. 234–236. [Google Scholar]
- Batis, G.; Pantazopoulou, P.; Tsivilis, S.; Badogiannis, E. The effect of metakaolin on the corrosion behavior of cement mortars. Cem. Concr. Compos. 2005, 27, 125–130. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Freire, A.L.; José, H.J.; Moreira, R.D.F.P.M. Potential applications for geopolymers in carbon capture and storage. Int. J. Greenh. Gas Control. 2022, 118, 103687. [Google Scholar] [CrossRef]
- Nazari, A.; Bagheri, A.; Sanjayan, J.G.; Dao, M.; Mallawa, C.; Zannis, P. Thermal shock reactions of Ordinary Portland cement and geopolymer concrete: Microstructural and mechanical investigation. Constr. Build. Mater. 2019, 196, 492–498. [Google Scholar] [CrossRef]
- Kathirvel, P.; Kaliyaperumal, S.R.M. Influence of recycled concrete aggregates on the flexural properties of reinforced alkali activated slag concrete. Constr. Build. Mater. 2016, 102, 51–58. [Google Scholar] [CrossRef]
- Zabihi, S.M.; Tavakoli, H.; Mohseni, E. Engineering and microstructural properties of fiber-reinforced rice husk–ash based geopolymer concrete. J. Mater. Civ Eng. 2018, 30, 04018183. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.; Binhussain, M.; Luqman, M.; Nizar, I.K. Effect of curing profile on kaolin-based geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef]
- Amin, M.; Elsakhawy, Y.; Abu el-hassan, K.; Abdelsalam, B.A. Behavior evaluation of sustainable high strength geopolymer concrete based on fly ash, metakaolin, and slag. Case Stud. Constr. Mater. 2022, 16, e00976. [Google Scholar] [CrossRef]
- Abiodun, Y.O.; Sadiq, O.M.; Adeosun, S.O.; Oyekan, G.L. Mineralogical Properties of Kaolin and Metakaolin from Selected Areas in Nigeria and Its Application to Concrete Production. West Indian J. Eng. 2019, 42, 57–64. Available online: https://sta.uwi.edu/eng/wije/vol4201_jul2019/documents/M0719019v42n1p576YOAbiodunJuly2019.pdf (accessed on 18 June 2022).
- Ilic, B.R.; Mitrovic, A.A.; Milicic, L.R. Thermal treatment of kaolin clay. Hem. Ind. 2010, 64, 351–356. [Google Scholar] [CrossRef]
- Biljana, R.; Alexandra, A.; Ljiljana, R. Thermal Treatment of Kaolin Clay to Obtain Metakaolin; Institute for Testing of Materials: Belgrade, Serbia, 2010; Volume 64, pp. 351–356. Available online: http://www.doiserbia.nb.rs/img/doi/0367-598x/2010/0367-598X1000014I.pdf (accessed on 21 June 2022).
- U.S. Geological Survey. Mineral Commodity Summaries 2020: U.S. Geological Survey, 2020. Available online: https://doi.org/10.3133/mcs2020 (accessed on 12 May 2022). [CrossRef]
- Yunsheng, Z.; Wei, S. Semi-empirical AM1 calculations on 6-memebered alumino-silicate rings model: Implications for dissolution process of metakaoline in alkaline solutions. J. Mater. Sci. 2007, 42, 3015–3023. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L.; Yantao, J. Study of polycondensation process of metakaolin-based geopolymeric cement using semi-empirical AM1 calculations. Adv. Cem. Res. 2009, 21, 67–73. [Google Scholar] [CrossRef]
- Gambo, S.; Ibrahim, K.; Aliyu, A.; Ibrahim, G.; Abdulsalam, H. Performance of metakaolin based concrete at elevated temperature. Niger. J. Technol. 2020, 9, 732–737. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Mohammed, A.A. Using of metakaolin to produce colored geopolymer concrete. In Proceedings of the 3rd International Conference in Physical Science and Advanced Materials (PAM 2021), Istanbul, Turkey, 24–28 September 2021. J. Phys. Conf. Ser. 2021, 2114, 012018. [Google Scholar] [CrossRef]
- Abdelli, K.; Tahlaiti, M.; Belarbi, R.; Oudjit, M.N. Influence of the pozzolanic reactivity of the blast furnace slag and metakaolin on mortars. Energy Procedia 2017, 139, 224–229. [Google Scholar] [CrossRef]
- Adjei, S.; Elkatatny, S.; Ayranci, K. Effect of Elevated Temperatures on the Microstructure of Metakaolin—Based Geopolymer. ACS Omega 2022, 7, 10268–10276. [Google Scholar] [CrossRef]
- Energy Council. Efficient Use of Energy Utilizing High Technology: An Assessment of Energy Use in Industry and Buildings; World Energy Council: London, UK, 1995. [Google Scholar]
- Cahn, D.; Greer, W.; Mikols, E.; Moir, R. Atmospheric CO2 and the US cement industry. World Cement 1997, 28, 64–68. [Google Scholar]
- Canada Portland Cement Association. Cement, Concrete and Climate Change, 1993 CSCE/CPCA Structural Concrete Conference 19–21 May 1993; Toronto Hilton International: Toronto, ON, Canada, 1993. [Google Scholar]
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Flyash/kaolin based Geopolymer Concretes and their mechanical properties. Const. Build. Mater. 2015, 98, 739–744. [Google Scholar] [CrossRef]
- Market Analysis Report. Limestone Market Size, Share & Trends Analysis Report by End-use. Advanced Materials 2018. Available online: https://www.grandviewresearch.com/industry-analysis/limestone-market (accessed on 2 June 2022).
- BDA. Brick Industry Sustainability. Available online: http://www.brick.org.uk/resources/brick-industry/sustainability/2010 (accessed on 17 June 2022).
- Worrell, E.; Galitsky, C. Energy Efficiency Improvement and Cost Saving Opportunities for Cement Making; Ernest Orlando Lawrence Berkeley National Laboratory, University of California: Berkeley, CA, USA, 2008. Available online: https://www.osti.gov/servlets/purl/927882 (accessed on 8 June 2022).
- Andrew, R.M.; Peters, G.P. The Global Carbon Project’s Fossil CO2 Emissions Dataset Zenodo 2021. Available online: https://doi.org/10.5281/zenodo.5569235 (accessed on 12 June 2022). [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The Potential for Carbon Capture. BioScience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Lundgren, T.; Marklund, P.O. Economics of Biofuels: An Overview. In Encyclopedia of Energy, Natural Resource, and Environmental Economics; Shogren, J.F., Ed.; Elsevier: Waltham, MA, USA, 2013; pp. 184–187. [Google Scholar] [CrossRef]
- Medeiros, D.L.; Sales, E.A.; Kiperstok, A. Energy production from microalgae biomass: Carbon footprint and energy balance. J. Clean. Prod. Integr. Clean. Prod. Into Sustain. Strateg. 2015, 96, 493–500. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae biofuel potentials (Review). Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar] [CrossRef]
- Olofsson, M.; Lindehoff, E.; Frick, B.; Svensson, F.; Legrand, C. Baltic Sea microalgae transform cement flue gas into valuable biomass. Algal Res. 2015, 11, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Portillo, E.; Alonso-Farinas, B.; Vega, F.; Cano, M.; Navarrete, B. Alternatives for oxygen-selective membrane systems and their integration into the oxy-fuel combustion process: A review. Sep. Purif. Technol. 2019, 229, 115708. [Google Scholar] [CrossRef]
- Eveloy, V. Hybridization of solid oxide electrolysis-based power-to-methane with oxyfuel combustion and carbon dioxide utilization for energy storage. Renew. Sustain. Energy Rev. 2019, 108, 550–571. [Google Scholar] [CrossRef]
- Cau, G.; Tola, V.; Ferrara, F.; Porcu, A.; Pettinau, A. CO2 -free coal-fired power generation by partial oxy-fuel and post-combustion CO2 capture: Techno-economic analysis. Fuel 2018, 214, 423–435. [Google Scholar] [CrossRef]
- Shaddix, C.R.; Molina, A. Particle imaging of ignition and devolatilization of pulverized coal during oxy-fuel combustion. Proc. Combust. Inst. 2009, 32, 2091–2098. [Google Scholar] [CrossRef]
- Andersen, J.; Rasmussen, C.L.; Giselsson, T.; Glarborg, P. Global combustion mechanisms for use in CFD modeling under oxy-fuel conditions. Energy Fuel 2009, 23, 1379–1389. [Google Scholar] [CrossRef]
- Tran, K.Q.; Trinh, T.N.; Bach, Q.V. Development of a biomass torrefaction process integrated with oxy-fuel combustion. Bioresour. Technol. 2016, 199, 408–413. [Google Scholar] [CrossRef]
- Niu, S.B.; Chen, M.Q.; Li, Y.; Xue, F. Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel 2016, 178, 129–138. [Google Scholar] [CrossRef]
- Huang, X.; Ai, N.; Li, L.; Jiang, Q.; Wang, Q.; Ren, J.; Wang, J. Simulation of CO2 Capture Process in Flue Gas from Oxy-Fuel Combustion Plant and Effects of Properties of Absorbent. Separations 2022, 9, 95. [Google Scholar] [CrossRef]
- BS 12390; Part 3: Testing Hardened Concrete: Compressive Strength of Test Specimens. British Standard Code of Practice, British Standards Institution: London, UK, 2009.
- Davidovits, J. Handbook of Geopolymer Chemistry and Applications; Geopolymer Institute Press: Saint-Quentin, France, 2008; Volume 2, pp. 277–278. [Google Scholar]
- BS 1881; Part 122: Testing Fresh Concrete: Method for Determination of Water Absorption. British Standard Code of Practice, British Standards Institution: London, UK, 1983.
- Abiodun, Y.O.; Sadiq, O.M.; Adeosun, S.O. Microstructural, mechanical and pozzolanic characteristics of metakaolin-based geopolymer. Geol. Geophys. Environ. 2020, 46, 57–69. [Google Scholar] [CrossRef]
- Ding, J.T.; Li, Z.J. Effects of metakaolin and silica fume on properties of concrete. ACI Mater. J. 2022, 99, 393–398. [Google Scholar]
- Lenka, S.; Panda, K.C. Effect of metakaolin on the properties of conventional and self compacting concrete. Adv. Concr. Constr. 2017, 5, 31–48. [Google Scholar] [CrossRef]
- Kostuch, J.A.; Walter, G.V.; Jones, T.R. High performance concretes containing metakaolin—A review. In Proceedings of the International Conference—Concrete, Dundee, Scotland, UK, 7–9 September 2000; Volume 2, pp. 1799–1811. [Google Scholar]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali-metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Rangan, V.B. Fly Ash-Based Geopolymer Concrete. In Proceedings of the International Workshop on Geopolymer Cement and Concrete, Villa Clara, Cuba, 23–30 June 2019; Allied Publishers Private Limited: Mumbai, India, 2010; pp. 68–106. [Google Scholar]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Shekarchi, M.; Bonakdar, A.; Bakhshi, M.; Mirdamadi, A.; Mobasher, B. Transport properties in metakaolin blended concrete. Constr. Build. Mater. 2010, 24, 2217–2223. [Google Scholar] [CrossRef]
- Ababneh, A.; Matalkah, F.; Aqel, R. Synthesis of kaolin-based alkali-activated cement: Carbon footprint, cost and energy assessment. J. Mater. Res. Technol. 2020, 9, 8367–8378. [Google Scholar] [CrossRef]
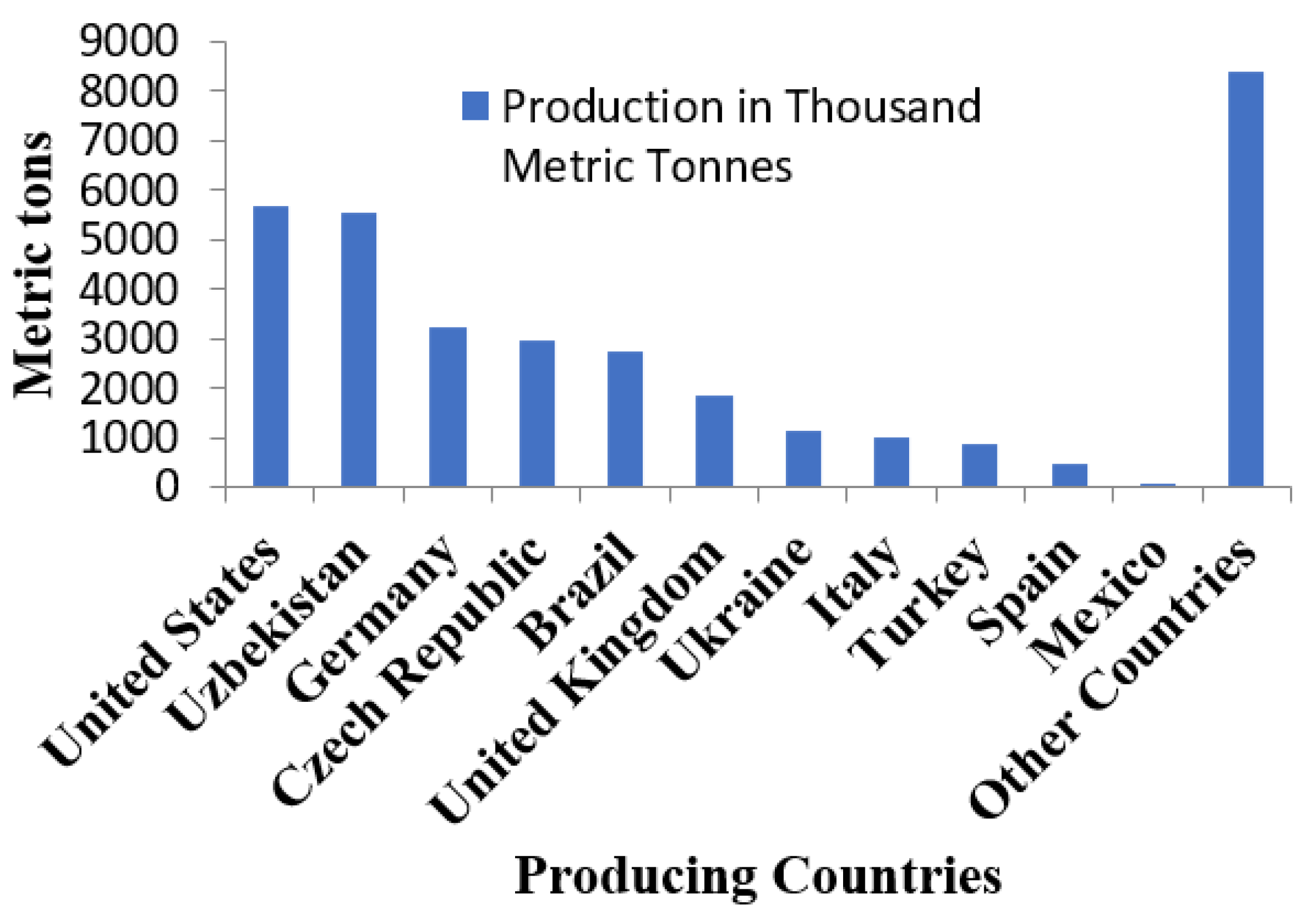
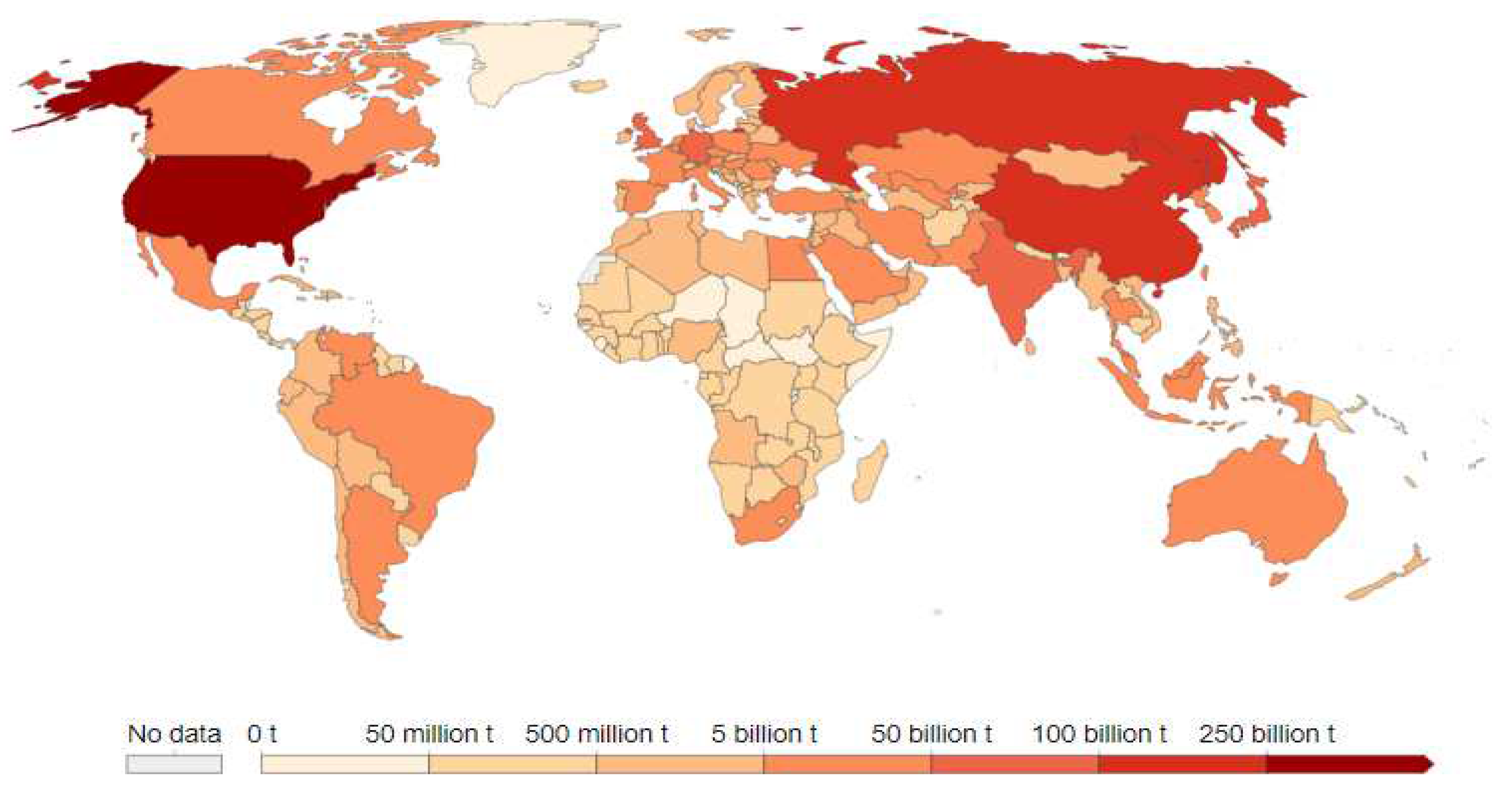


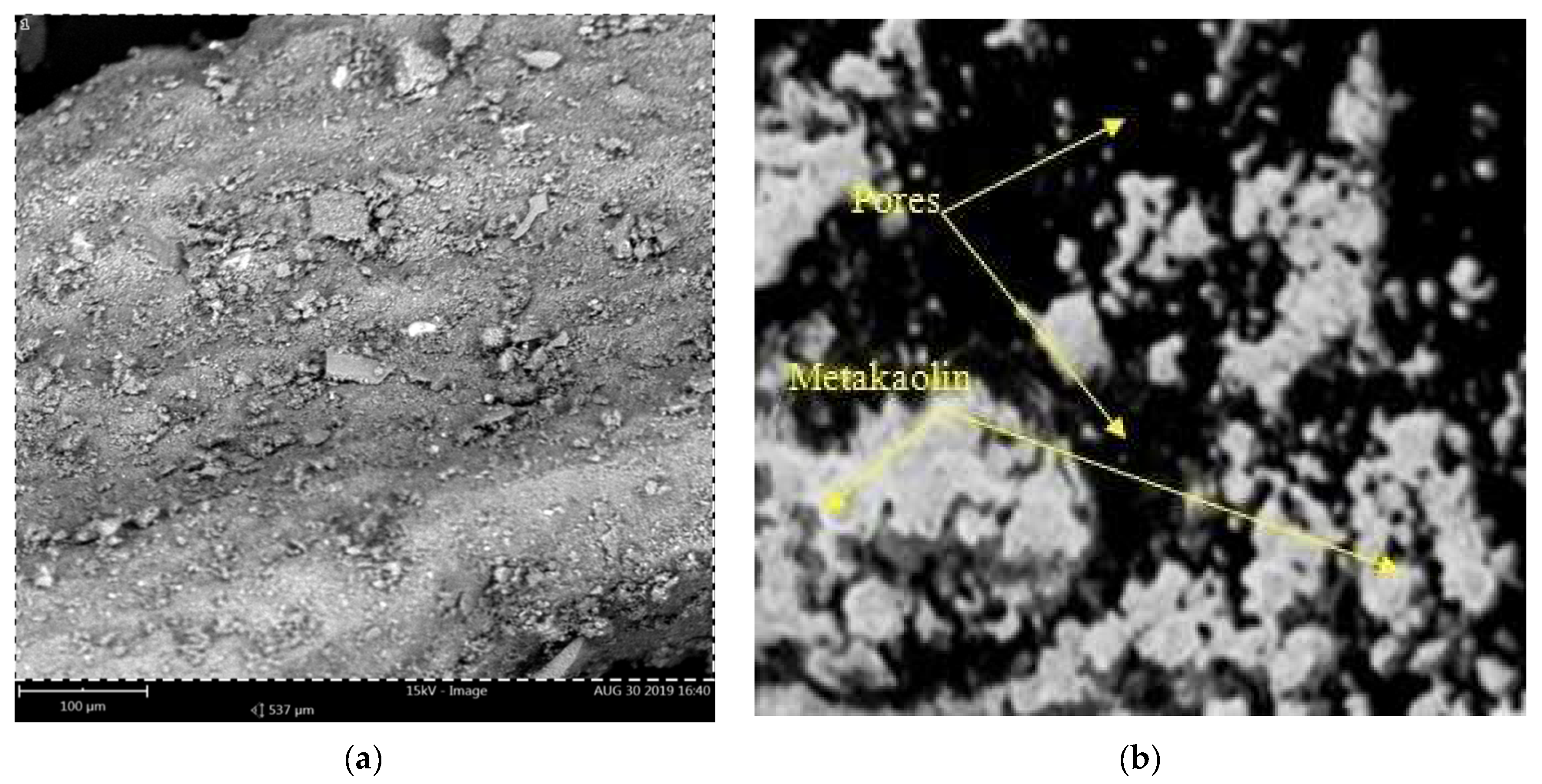
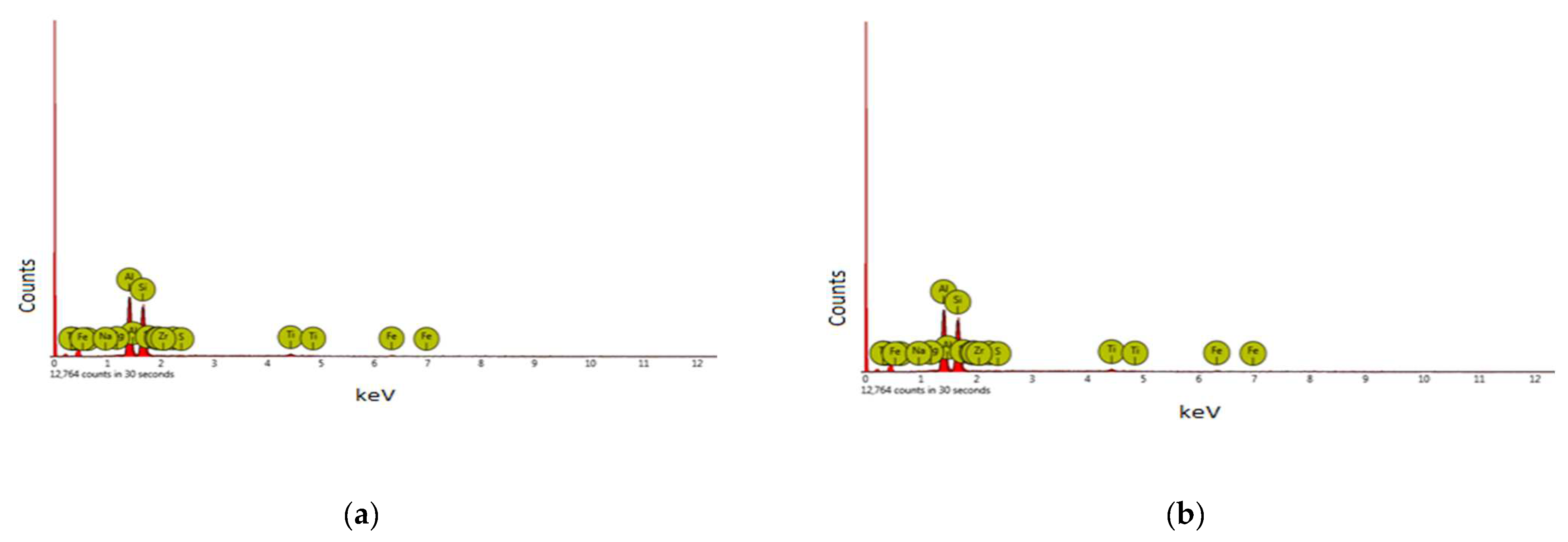
| Processes/Materials | Alternative Technology | Reduction in CO2 Emissions |
|---|---|---|
| Raw materials | Calcium carbide slag Steel slag, calcareous oil shale | 374 kg of CO2 per ton of clinker 60 kg of CO2 per ton of clinker |
| Cement production | Fluidized bed kiln | 20–30 kg of CO2 per ton of product |
| Emerging supplementary cementitious materials | Geopolymer cement | 300 kg of CO2 per ton of product |
| Industrial recycling | CO2 from cement process converted into high-energy algae biomass | In order to create 1 ton of dry algae biomass, 1800 kg of CO2 will be used. |
| Fuel technologies | Oxygen enrichment and oxy-fuel | 404–676 kg of CO2 per ton of cement |
| Post-combustion carbon capture | absorption | 690–725 kg of CO2 per ton of clinker |
| Chemical Oxides | Kaolin | Metakaolin |
|---|---|---|
| SiO2 | 48.50 | 53.49 |
| Al2O3 | 32.75 | 39.90 |
| Fe2O3 | 4.28 | 0.52 |
| CaO | 1.08 | 0.12 |
| MgO | 0.49 | 0.21 |
| Na2O | 0.16 | 0.11 |
| K2O | 1.48 | 0.53 |
| SO3 | - | 0.01 |
| LOI | 9.26 | 4.51 |
| Peaks at 2θ | Compound Name | Crystal System | Formula | Reference Code |
|---|---|---|---|---|
| 19.3814 | Anatase | Tetragonal | Ti4O8 | 96-900-8216 |
| 27.0007 | Quartz | Hexagonal | Si3O6 | 96-901-3322 |
| 50.2894; 68.5130 | Kaolinite | Anorthic | Al2Si2O9H4 | 96-900-9235 |
| Mix 1:1½:3 | ||||||
|---|---|---|---|---|---|---|
| df | SS | MS | F | Significance F | p-value | |
| Regression | 6 | 1420.058 | 236.6763 | 110.5078 | 5.33 × 10−26 | |
| % Replacement | 2 | 422.34 | 211.17 | 98.5986 | 0.0000 | |
| Curing days | 2 | 400.438 | 200.19 | 93.4718 | 0.0001 | |
| Water–cement ratio | 2 | 597.28 | 298.64 | 139.440 | 0.0001 | |
| Residual | 47 | 100.6606 | 2.141715 | |||
| Total | 53 | 1520.719 | ||||
| Mix 1:2:4 | ||||||
| Regression | 6 | 1079.384 | 179.8974 | 83.99413 | 1.99 × 10−23 | 0.0000 |
| % Replacement | 2 | 425.98 | 212.99 | 99.4450 | 0.0004 | |
| Curing days | 2 | 326.98 | 163.49 | 76.3340 | 0.0001 | |
| Water–cement ratio | 2 | 326.424 | 163.212 | 76.2037 | 0.0001 | |
| Residual | 47 | 100.6639 | 2.141785 | |||
| Total | 53 | 1180.048 | ||||
| Mix 1:1.1:2.6 | ||||||
| Regression | 6 | 278.1837 | 46.36395 | 56.34497 | 1.27 × 10−7 | |
| % Replacement | 2 | 141.9877 | 70.99385 | 57.51806 | 0.0001 | |
| Curing days | 2 | 46.991 | 23.4955 | 19.0357 | 0.0001 | |
| Water–cement ratio | 2 | 89.205 | 44.6025 | 36.1362 | 0.0001 | |
| Residual | 13 | 16.04575 | 1.234288 | |||
| Total | 19 | 294.2294 | ||||
| Cement-Metakaolin mortar | ||||||
| Regression | 6 | 1403.341 | 233.8901 | 85.77639 | 1.49×10-20 | |
| % Replacement | 2 | 524.269 | 262.1345 | 93.1347 | 0.0000 | |
| Curing days | 2 | 277.707 | 138.8535 | 50.9228 | 0.0000 | |
| Water–cement ratio | 2 | 601.365 | 300.6825 | 110.272 | 0.0000 | |
| Residual | 38 | 103.6162 | 2.726743 | |||
| Total | 44 | 1506.957 | ||||
| Metakaolin-based geopolymer concrete | ||||||
| Regression | 4 | 134.8333 | 33.70833 | 187.2685 | 4.7424 × 10−2 | 0.0000 |
| % Replacement | 2 | 69.258 | 34.629 | 192.38 | 0.0052 | |
| Curing days | 1 | 17.3343 | 17.3343 | 96.301 | 0.01023 | |
| Water–cement ratio | 1 | 48.241 | 48.241 | 268.006 | 0.0201 | |
| Residual | 2 | 0.36 | 0.18 | |||
| Total | 6 | 135.1933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abiodun, Y.O.; Olanrewaju, O.A.; Gbenebor, O.P.; Ochulor, E.F.; Obasa, D.V.; Adeosun, S.O. Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction. Atmosphere 2022, 13, 1494. https://doi.org/10.3390/atmos13091494
Abiodun YO, Olanrewaju OA, Gbenebor OP, Ochulor EF, Obasa DV, Adeosun SO. Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction. Atmosphere. 2022; 13(9):1494. https://doi.org/10.3390/atmos13091494
Chicago/Turabian StyleAbiodun, Yetunde Oyebolaji, Oludolapo Akanni Olanrewaju, Oluwashina Philips Gbenebor, Ezenwanyi Fidelia Ochulor, Dumebi Victoria Obasa, and Samson Oluropo Adeosun. 2022. "Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction" Atmosphere 13, no. 9: 1494. https://doi.org/10.3390/atmos13091494
APA StyleAbiodun, Y. O., Olanrewaju, O. A., Gbenebor, O. P., Ochulor, E. F., Obasa, D. V., & Adeosun, S. O. (2022). Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction. Atmosphere, 13(9), 1494. https://doi.org/10.3390/atmos13091494







