Development and Validation of a Method for the Simultaneous Quantification of 21 Microbial Volatile Organic Compounds in Ambient and Exhaled Air by Thermal Desorption and Gas Chromatography–Mass Spectrometry
Abstract
:1. Introduction
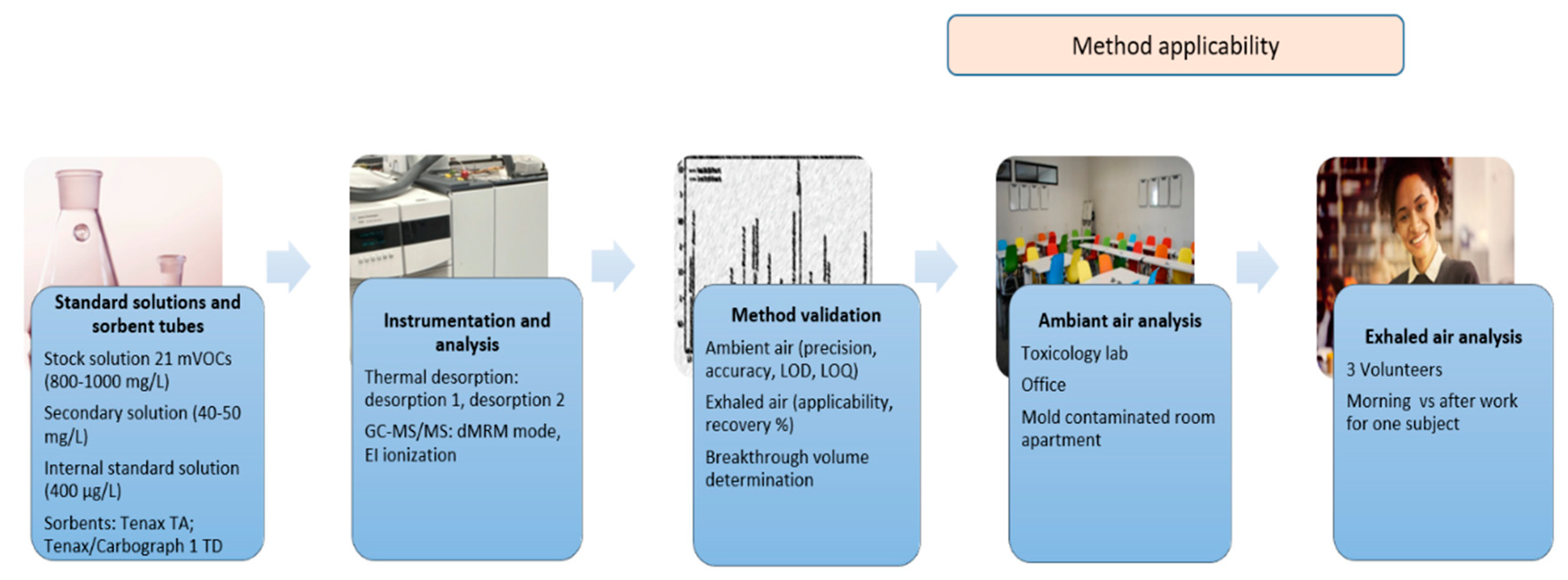
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Standard Solutions and Sorbent Tubes
2.3. Instrumentation, Desorption, and GC-MS/MS Analysis
2.4. Breakthrough Volume
2.5. Method Validation
2.6. Method Application
3. Results and Discussion
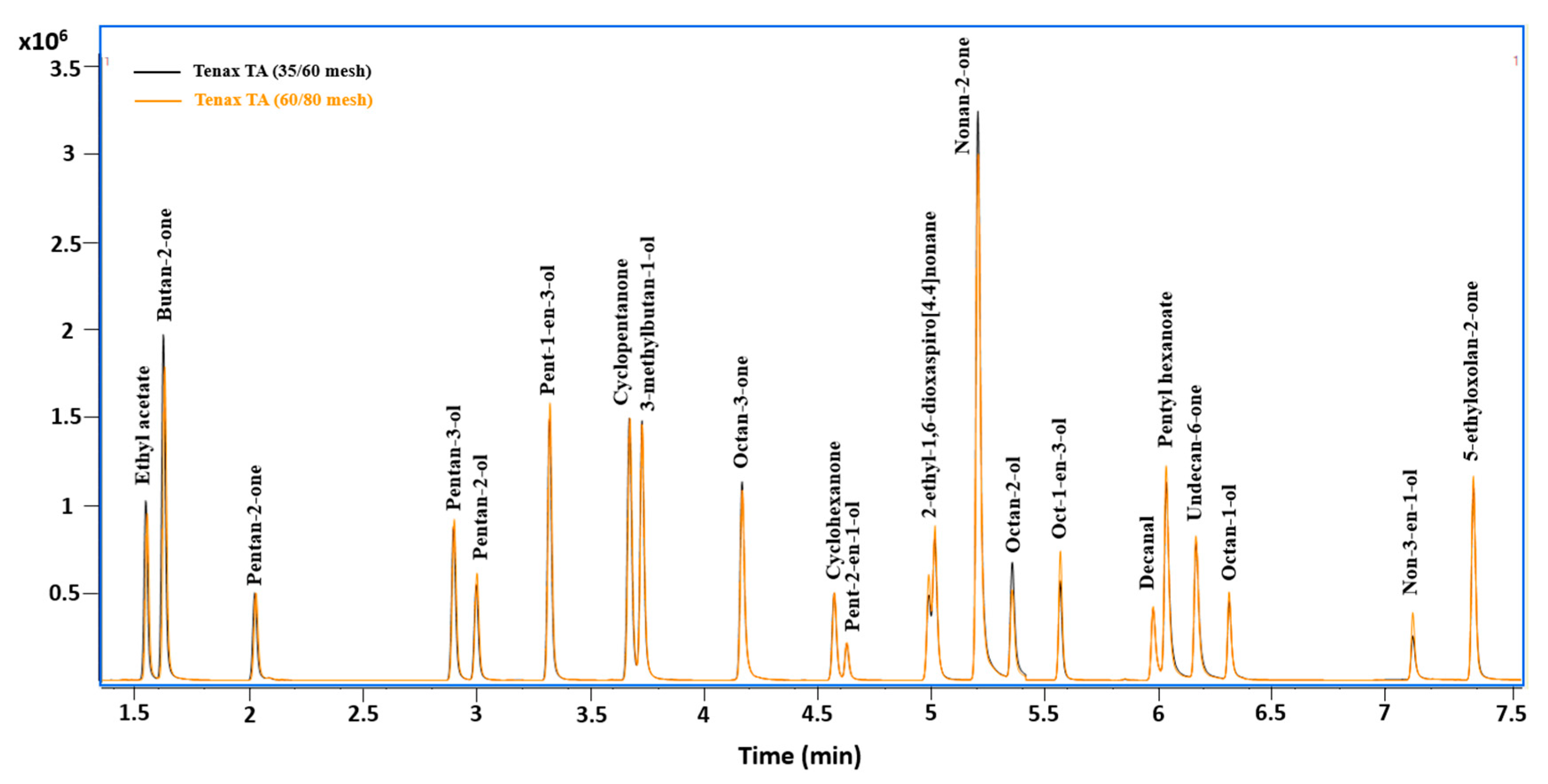
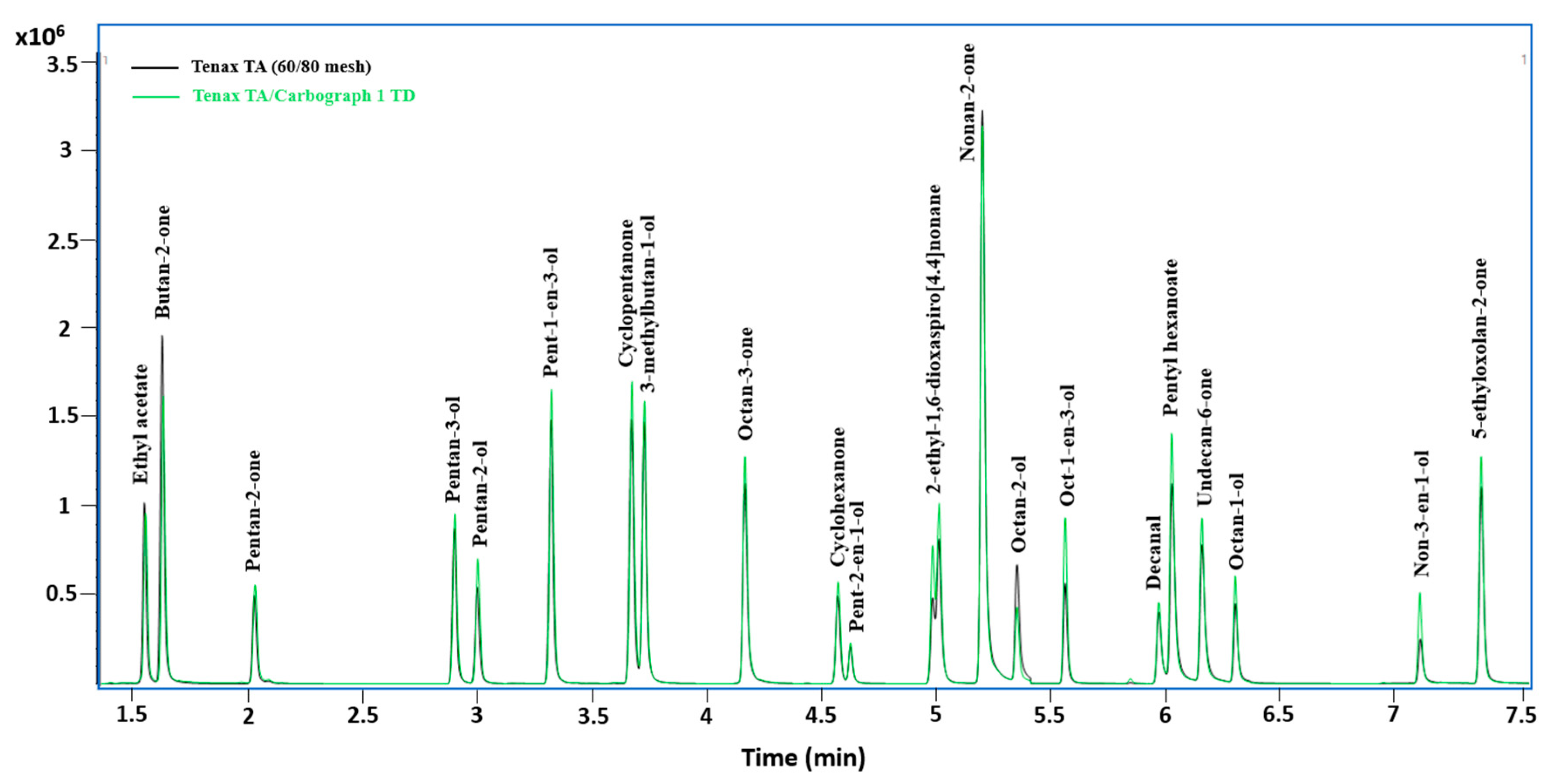
3.1. Sorbent Selection
3.2. Breakthrough Volume
3.3. Method Validation
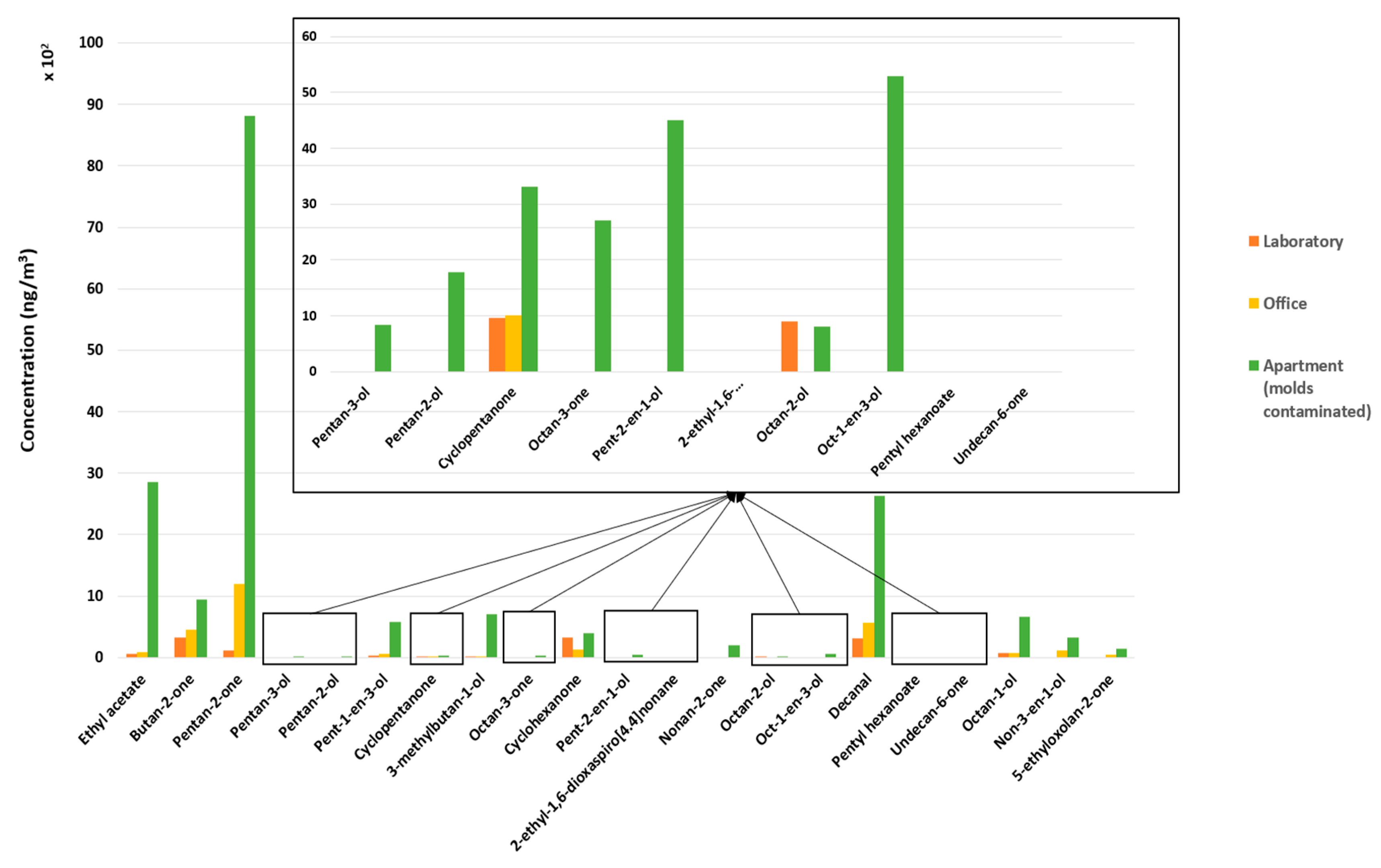
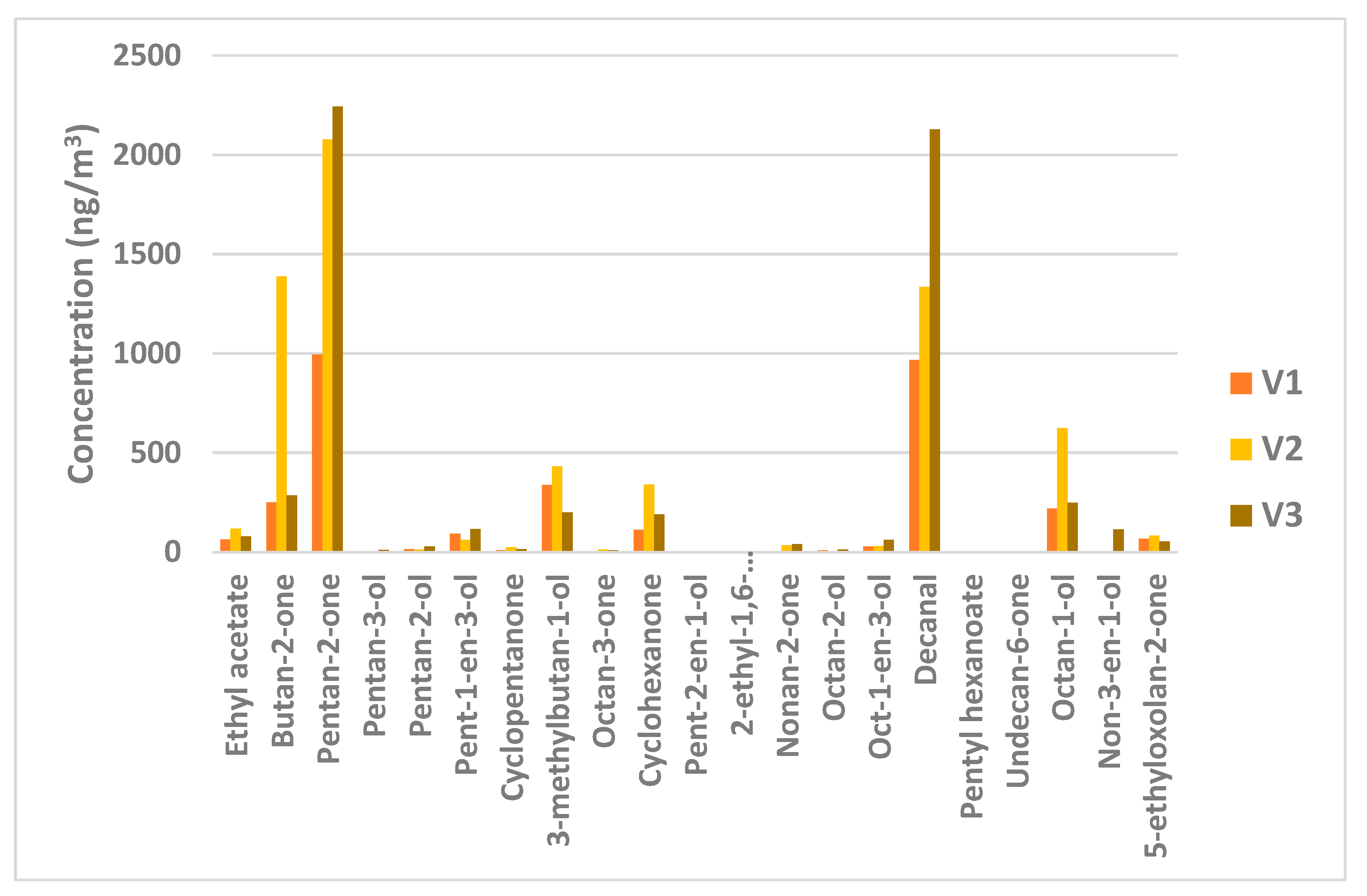
3.4. Method Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pietrogrande, M.C.; Casari, L.; Demaria, G.; Russo, M. Indoor Air Quality in Domestic Environments during Periods Close to Italian COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 4060. [Google Scholar] [CrossRef] [PubMed]
- Shaddick, G.; Salter, J.M.; Peuch, V.H.; Ruggeri, G.; Thomas, M.L.; Mudu, P.; Tarasova, O.; Baklanov, A.; Gumy, S. Global Air Quality: An Inter-Disciplinary Approach to Exposure Assessment for Burden of Disease Analyses. Atmosphere 2021, 12, 48. [Google Scholar] [CrossRef]
- Settimo, G.; Manigrasso, M.; Avino, P. Indoor Air Quality: A Focus on the European Legislation and State-of-the-Art Research in Italy. Atmosphere 2020, 11, 370. [Google Scholar] [CrossRef]
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review. Sci. Total Environ. 2017, 586, 610–622. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2020; Special Report; Health Effects Institute: Boston, MA, USA, 2020.
- Zar, H.J.; Ferkol, T.W. The global burden of respiratory disease-Impact on child health. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef]
- Tischer, C.G.; Heinrich, J. Exposure assessment of residential mould, fungi and microbial components in relation to children’s health: Achievements and challenges. Int. J. Hyg. Environ. Health 2013, 216, 109–114. [Google Scholar] [CrossRef]
- Hurraß, J.; Heinzow, B.; Aurbach, U.; Bergmann, K.-C.; Bufe, A.; Buzina, W.; Cornely, O.A.; Engelhart, S.; Fischer, G.; Gabrio, T.; et al. Medical diagnostics for indoor mold exposure. Int. J. Hyg. Environ. Health 2017, 220, 305–328. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Eric Gershwin, M. Mold and Human Health: A Reality Check. Clin. Rev. Allergy Immunol. 2017, 52, 305–322. [Google Scholar] [CrossRef]
- Reboux, G.; Bellanger, A.-P.; Roussel, S.; Grenouillet, F.; Millon, L. Moulds in dwellings: Health risks and involved species. Rev. Des Mal. Respir. 2010, 27, 169–179. [Google Scholar] [CrossRef]
- D’Halewyn, M.A.; Leclerc, J.M.; King, N.; Bélanger, M.; Legris, M.; Frenette, Y. Les risques à la santé associés à la présence de moisissures en milieu intérieur: Rapport scientifique. Inst. Natl. De St. Publique Québec 2002, 15–27. Available online: https://www.inspq.qc.ca/pdf/publications/126_RisquesMoisissuresMilieuInterieur.pdf (accessed on 22 August 2022).
- Hyvönen, S.; Lohi, J.; Tuuminen, T. Moist and Mold Exposure is Associated with High Prevalence of Neurological Symptoms and MCS in a Finnish Hospital Workers Cohort. Saf. Health Work 2020, 11, 173–177. [Google Scholar] [CrossRef]
- Harding, C.F.; Pytte, C.L.; Page, K.G.; Ryberg, K.J.; Normand, E.; Remigio, G.J.; DeStefano, R.A.; Morris, D.B.; Voronina, J.; Lopez, A.; et al. Mold inhalation causes innate immune activation, neural, cognitive and emotional dysfunction. Brain Behav. Immun. 2020, 87, 218–228. [Google Scholar] [CrossRef]
- Mensah-Attipoe, J.; Täubel, M. Analysis Approaches for Fungi in Indoor Environmental Assessments. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Gomes, A., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 109–127. ISBN 978-3-319-61688-9. [Google Scholar]
- Shorter, C.; Täubel, M.; Pierse, N.; Douwes, J.; Howden-Chapman, P.; Hyvärinen, A.; Crane, J. Objective assessment of domestic mold contamination using quantitative PCR. J. Allergy Clin. Immunol. 2016, 137, 622–624. [Google Scholar] [CrossRef]
- Oneț, A.; Ilieș, D.C.; Ilieș, A.; Herman, V.; Burta, L.; Marcu, F.; Buhas, R.; Baias, Ș.; Oneț, C.; Ilieș, M.; et al. Indoor Air Quality Assessement and Its Perception. Case Study Historic Wooden Church, Romania. Rom. Biotechnol Lett. 2020, 25, 1547–1553. [Google Scholar] [CrossRef]
- Alborch, L.; Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. Temperature and incubation time effects on growth and ochratoxin A production by Aspergillus sclerotioniger and Aspergillus lacticoffeatus on culture media. Lett. Appl. Microbiol. 2011, 52, 208–212. [Google Scholar] [CrossRef]
- Mateo, J.; Mateo, R.; Jiménez, M. Accumulation of type A trichothecenes in maize, wheat and rice by Fusarium sporotrichioides isolates under diverse culture conditions. Int. J. Food Microbiol. 2002, 72, 115–123. [Google Scholar] [CrossRef]
- Cahagnier, B.; Melcion, D.; Richard-Molard, D. Growth of Fusarium moniliforme and its biosynthesis of fumonisin B1 on maize grain as a function of different water activities. Lett. Appl. Microbiol. 1995, 20, 247–251. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial Volatile Organic Compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Genet. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Brodhagen, M.; Tsitsigiannis, D.I.; Hornung, E.; Goebel, C.; Feussner, I.; Keller, N.P. Reciprocal oxylipin-mediated cross-talk in the Aspergillus-seed pathosystem. Mol. Microbiol. 2007, 67, 378–391. [Google Scholar] [CrossRef]
- Splivallo, R.; Valdez, N.; Kirchhoff, N.; Ona, M.C.; Schmidt, J.-P.; Feussner, I.; Karlovsky, P. Intraspecific genotypic variability determines concentrations of key truffle volatiles. New Phytol. 2012, 194, 823–835. [Google Scholar] [CrossRef]
- Wu, S.; Hayati, S.K.; Kim, E.; de la Mata, A.P.; Harynuk, J.J.; Wang, C.; Zhao, R. Henry’s Law Constants and Indoor Partitioning of Microbial Volatile Organic Compounds. Environ. Sci. Technol. 2022, 56, 7143–7152. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef]
- Wilkins, K.; Larsen, K. Variation of volatile organic compound patterns of mold species from damp buildings. Chemosphere 1995, 31, 3225–3236. [Google Scholar] [CrossRef]
- Elke, K.; Begerow, J.; Oppermann, H.; Kråmer, U.; Jermann, E.; Dunemann, L. Determination of selected microbial volatile organic compounds by diffusive sampling and dual-column capillary GC-FID-a new feasible approach for the detection of an exposure to indoor mould fungi? J. Environ. Monit. 1999, 1, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alcega, S.; Nasir, Z.A.; Ferguson, R.; Whitby, C.; Dumbrell, A.J.; Colbeck, I.; Gomes, D.; Tyrrel, S.; Coulon, F. Fingerprinting outdoor air environment using microbial volatile organic compounds (MVOCs)—A review. TrAC Trends Anal. Chem. 2017, 86, 75–83. [Google Scholar] [CrossRef]
- Kuske, M.; Romain, A.-C.; Nicolas, J. Microbial volatile organic compounds as indicators of fungi. Can an electronic nose detect fungi in indoor environments? Build. Environ. 2005, 40, 824–831. [Google Scholar] [CrossRef]
- Garcia-Alcega, S.; Nasar, Z.A.; Ferguson, R.; Noël, C.; Cravo-Laureau, C.; Whitby, C.; Dumbrell, A.J.; Colbeck, I.; Tyrrel, S.; Coulon, F. Can chemical and molecular biomarkers help discriminate between industrial, rural and urban environments? Sci. Total Environ. 2018, 631–632, 1059–1069. [Google Scholar] [CrossRef]
- Kim, J.L.; Elfman, L.; Mi, Y.; Wieslander, G.; Smedje, G.; Norbäck, D. Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools? associations with asthma and respiratory symptoms in pupils. Indoor Air 2006, 17, 153–163. [Google Scholar] [CrossRef]
- Ryan, T.J.; Beaucham, C. Dominant microbial volatile organic compounds in 23 US homes. Chemosphere 2013, 90, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M. Biological Monitoring versus Air Monitoring Strategies in Assessing Environmental–Occupational Exposure. J. Environ. Monit. 2012, 14, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of microbial volatile organic compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef]
- Schuchardt, S.; Kruse, H. Quantitative volatile metabolite profiling of common indoor fungi: Relevancy for indoor air analysis. J. Basic Microbiol. 2009, 49, 350–362. [Google Scholar] [CrossRef]
- Claeson, A.-S.; Sandström, M.; Sunesson, A.-L. Volatile organic compounds (VOCs) emitted from materials collected from buildings affected by microorganisms. J. Environ. Monit. 2007, 9, 240–245. [Google Scholar] [CrossRef]
- El Aroussi, B.; Marchand, G.; Aubin, S.; Bouchard, M.; Haddad, S. Utilisation des Composés Organiques Volatils Microbiens Comme Biomarqueurs de L’exposition Aux Moisissures en Milieu de Travail—Étude de Faisabilité; Rapports Scientifiques: (R-1037); Institut de Recherche Robert-Sauvé en Santé et en Sécurité de Travail (IRSST): Montréal, QC, Canada, 2018. [Google Scholar]
- Schuchardt, S.; Strube, A. Microbial volatile organic compounds in moldy interiors: A long-term climate chamber study. J. Basic Microbiol. 2012, 53, 532–538. [Google Scholar] [CrossRef]
- Piecková, E.; Jesenská, Z. Microscopic Fungi in Dwellings and Their Health Implications in Humans. Ann. Agric. Environ. Med. AAEM 1999, 6, 1–11. [Google Scholar] [PubMed]
- Peyret, T.; Poulin, P.; Krishnan, K. A unified algorithm for predicting partition coefficients for PBPK modeling of drugs and environmental chemicals. Toxicol. Appl. Pharmacol. 2010, 249, 197–207. [Google Scholar] [CrossRef]
- Tabbal, S.; El Aroussi, B.; Bouchard, M.; Marchand, G.; Haddad, S. A new headspace solid-phase microextraction coupled with gas chromatography-tandem mass spectrometry method for the simultaneous quantification of 21 microbial volatile organic compounds in urine and blood. Chemosphere 2022, 296, 133901. [Google Scholar] [CrossRef]
- Wazeerud-Din, I.J.; Silva, L.K.; Smith, M.M.; Newman, C.A.; Blount, B.C.; De Jesús, V.R. Quantification of seven microbial volatile organic compounds in human serum by solid-phase microextraction gas chromatography-tandem mass spectrometry. Chemosphere 2021, 266, 128970. [Google Scholar] [CrossRef]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.F.; Luo, W.; Isabelle, L.M.; Bender, D.A.; Baker, R.J. Determination of a Wide Range of Volatile Organic Compounds in Ambient Air Using Multisorbent Adsorption/Thermal Desorption and Gas Chromatography/Mass Spectrometry. Anal. Chem. 1998, 70, 5213–5221. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Batterman, S. Performance evaluation of a sorbent tube sampling method using short path thermal desorption for volatile organic compounds. J. Environ. Monit. 2000, 2, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ribes, A.; Carrera, G.; Gallego, E.; Roca, X.; Berenguer, M.J.; Guardino, X. Development and validation of a method for air-quality and nuisance odors monitoring of volatile organic compounds using multi-sorbent adsorption and gas chromatography/mass spectrometry thermal desorption system. J. Chromatogr. A 2007, 1140, 44–55. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tubes and GC–MS/olfactometry. Sensors Actuators B Chem. 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Rodríguez-Navas, C.; Forteza, R.; Cerdà, V. Use of Thermal Desorption–Gas Chromatography–Mass Spectrometry (TD–GC–MS) on Identification of Odorant Emission Focus by Volatile Organic Compounds Characterisation. Chemosphere 2012, 89, 1426–1436. [Google Scholar] [CrossRef]
- Schieweck, A.; Gunschera, J.; Varol, D.; Salthammer, T. Analytical procedure for the determination of very volatile organic compounds (C3–C6) in indoor air. Anal. Bioanal. Chem. 2018, 410, 3171–3183. [Google Scholar] [CrossRef]
- Haworth, J.J.; Pitcher, C.K.; Ferrandino, G.; Hobson, A.R.; Pappan, K.L.; Lawson, J.L.D. Breathing new life into clinical testing and diagnostics: Perspectives on volatile biomarkers from breath. Crit. Rev. Clin. Lab. Sci. 2022, 59, 353–372. [Google Scholar] [CrossRef]
- U.S.EPA. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Method TO-14; Center for Environmental Research Information, Office of Research and Development: Cincinnati, OH, USA, 1999.
- U.S.EPA. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Method TO-17; Center for Environmental Research Information, Office of Research and Development: Cincinnati, OH, USA, 1999.
- Tang, Y.; Fellin, P.; Otson, R. Evaluation of a Transportable Gas Chromatograph for Monitoring of Indoor Airborne Volatile Organic Compounds with a Gas Sampling Valve or a Concentrator. Indoor Built Environ. 1995, 4, 27–36. [Google Scholar] [CrossRef]
- Ras, M.R.; Borrull, F.; Marcé, R.M. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. TrAC Trends Anal. Chem. 2009, 28, 347–361. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Malpartida, M.G. Breath Biopsy and Discovery of Exclusive Volatile Organic Compounds for Diagnosis of Infectious Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 564194. [Google Scholar] [CrossRef]
- Bortoli, M.; Knoppel, H.; Pecchio, E.; Schauenburg, H.; Vissers, H. Comparison of Tenax and Carbotrap for VOC Sampling in Indoor Air. Indoor Air 1992, 2, 216–224. [Google Scholar] [CrossRef]
- Kozicki, M.; Wiejak, A.; Piasecki, M.; Abram, A. Identification of MVOCs Produced by Coniophora puteana and Poria placenta Growing on WPC Boards by Using Subtraction Mass Spectra. Int. J. Environ. Res. Public Health 2019, 16, E2499. [Google Scholar] [CrossRef] [PubMed]
- Woolfenden, E. Monitoring VOCs in Air Using Sorbent Tubes Followed by Thermal Desorption-Capillary GC Analysis: Summary of Data and Practical Guidelines. J. Air Waste Manag. Assoc. 1997, 47, 20–36. [Google Scholar] [CrossRef]
- Patil, S.; Lonkar, S. Thermal Desorption—Gas Chromatography for the Determination of Benzene, Aniline, Nitrobenzene and Chlorobenzene in Workplace Air. J. Chromatogr. A 1992, 600, 344–351. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef]
- Khan, M.F.; Sahani, M.; Nadzir, M.S.M.; Yik, L.C.; Hoque, H.M.S.; Hamid, H.H.A.; Wahab, M.I.A.; Munna, F.T.; Amin, N.; Misran, H.; et al. Volatile Organic Compound Analysis by Sorbent Tube-Thermal Desorption-Gas Chromatography: A Review. Int. J. Eng. Technol. 2018, 7, 165–175. [Google Scholar] [CrossRef]
- Maceira, A.; Vallecillos, L.; Borrull, F.; Marcé, R.M. New approach to resolve the humidity problem in VOC determination in outdoor air samples using solid adsorbent tubes followed by TD-GC–MS. Sci. Total Environ. 2017, 599-600, 1718–1727. [Google Scholar] [CrossRef]
- Wu, C.-H.; Feng, C.-T.; Lo, Y.-S.; Lin, T.-Y.; Lo, J.-G. Determination of volatile organic compounds in workplace air by multisorbent adsorption/thermal desorption-GC/MS. Chemosphere 2004, 56, 71–80. [Google Scholar] [CrossRef]
- SIST EN. 16516:2018+A1:2020—Construction Products: Assessment of Release of Dangerous Substances—Determination of Emissions into Indoor Air. Available online: https://standards.iteh.ai/catalog/standards/sist/fe1939d9-7a49-4f83-b4f2-38e7c4758d1c/sist-en-16516-2018a1-2020 (accessed on 10 August 2022).
- Wang, B.; Zhao, Y.; Lan, Z.; Yao, Y.; Wang, L.; Sun, H. Sampling methods of emerging organic contaminants in indoor air. Trends Environ. Anal. Chem. 2016, 12, 13–22. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA); Office of Regulatory Affairs (ORA). Methods, method verification and validation. In Laboratory Manual Volume II, ORA-LAB.5.4.5; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/73920/download (accessed on 7 June 2021).
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminśki, E.; Libbey, L.M.; Stawicki, S.; Wasowicz, E. Identification of the Predominant Volatile Compounds Produced by Aspergillus flavus. Appl. Microbiol. 1972, 24, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Korpia, A.; Pasanen, A.-L.; Viitanenb, H. Volatile metabolites of Serpula lacrymans, Coniophora puteana, Poria placenta, Stachybotrys chartarum and Chaetomium globosum. Build. Environ. 1998, 34, 205–211. [Google Scholar] [CrossRef]
- Règlement sur la Santé et la Sécurité du Travail (RSST) du Québec. Loi Sur la Santé et la Sécurité du Travail. 2022. chapitre S-2.1, a. 223. Available online: https://www.legisquebec.gouv.qc.ca/fr/pdf/rc/S-2.1,%20R.%2013.pdf (accessed on 8 August 2022).

| Parameter | Retained Conditions |
|---|---|
| GC | |
| Column | DB-wax (30 m × 0.32 mm, 0.5 µm) |
| Carrier gas | Ultra-high purity He (99.999%) |
| Carrier gas flow | 1.8 mL/min |
| Oven program | Initial temperature: 65 °C 3 °C/min to 70 °C 20 °C/min to 220 °C Total run time: 9.17 min |
| MS/MS | |
| Mode | dMRM (dynamic multiple reaction monitoring) |
| Ionization source | EI, 70 eV |
| Transfer line temperature | 245 °C |
| MS source temperature | 230 °C |
| Emission current | 35 µA |
| Quad temperature | Q1: 150 °C Q2: 150 °C |
| Compounds | Range of Linearity (ng/m3) | R2 | LOD (ng/m3) | LOQ (ng/m3) | R% (Min) | R% (Max) | Repeatability CV% (Min) | Repeatability CV% (Max) | Intermediate Precision CV% (Min) | Intermediate Precision CV% (Max) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethyl acetate | 30–8070 | 0.999 | 26 | 78.78 | 90 | 102 | 0.69 | 0.56 | 0.52 | 0.59 |
| Butan-2-one | 120–14,250 | 0.999 | 75.30 | 228.19 | 96 | 95 | 0.22 | 0.54 | 0.21 | 0.47 |
| Pentan-2-one | 120–13,890 | 0.999 | 72.04 | 218.31 | 96 | 96 | 0.14 | 0.25 | 0.11 | 0.37 |
| Pentan-3-ol | 4–1860 | 0.999 | 2.09 | 6.34 | 87 | 107 | 0.16 | 0.27 | 1.14 | 0.34 |
| Pentan-2-ol | 4–1790 | 0.999 | 1.76 | 5.32 | 80 | 107 | 0.16 | 0.23 | 0.49 | 0.44 |
| Pent-1-en-3-ol | 8–2010 | 0.999 | 6.58 | 19.93 | 110 | 104 | 0.23 | 0.19 | 0.55 | 0.48 |
| Cyclopentanone | 4–2090 | 0.999 | 2.16 | 6.54 | 94 | 107 | 0.18 | 0.14 | 0.58 | 0.34 |
| 3-methylbutan-1-ol | 4–1750 | 0.999 | 3.04 | 9.22 | 90 | 108 | 0.07 | 0.52 | 0.86 | 0.42 |
| Octan-3-one | 4–1760 | 0.999 | 3.34 | 10.12 | 107 | 105 | 0.25 | 0.48 | 0.56 | 0.84 |
| Cyclohexanone | 30–8160 | 0.999 | 11.52 | 34.90 | 92 | 105 | 0.27 | 0.29 | 0.71 | 0.55 |
| Pent-2-en-1-ol | 30–6990 | 0.999 | 25.11 | 76.08 | 104 | 104 | 0.60 | 0.38 | 2.04 | 0.48 |
| 2-ethyl-1,6-dioxaspiro [4.4] nonane | 30–7880 | 0.999 | 26.43 | 80.08 | 84 | 110 | 0.32 | 0.32 | 0.85 | 0.76 |
| Nonan-2-one | 30–6950 | 0.999 | 22.65 | 68.65 | 92 | 110 | 0.43 | 0.20 | 0.40 | 0.28 |
| Octan-2-ol | 7–1770 | 0.999 | 2.40 | 7.26 | 80 | 118 | 1.16 | 0.13 | 0.25 | 0.23 |
| Oct-1-en-3-ol | 7–1770 | 0.999 | 4.98 | 15.11 | 113 | 113 | 0.71 | 0.23 | 0.61 | 0.48 |
| Decanal | 120–13,800 | 0.999 | 108.37 | 328.39 | 80 | 102 | 4.35 | 0.16 | 0.61 | 0.22 |
| Pentyl hexanoate | 120–14,490 | 0.999 | 55.71 | 168.83 | 93 | 106 | 0.21 | 0.21 | 0.37 | 0.35 |
| Undecan-6-one | 30–6970 | 0.999 | 25.07 | 75.97 | 95 | 105 | 0.16 | 0.62 | 0.41 | 0.47 |
| Octan-1-ol | 30–6960 | 0.999 | 10.53 | 31.90 | 92 | 108 | 0.21 | 0.27 | 0.39 | 0.54 |
| Non-3-en-1-ol | 120–13,880 | 0.999 | 70.95 | 214.99 | 82 | 94 | 0.60 | 0.71 | 1.38 | 0.58 |
| 5-ethyloxolan-2-one | 40–8820 | 0.999 | 9.59 | 29.06 | 87 | 110 | 0.27 | 0.22 | 0.29 | 0.25 |
| Compounds | R% (Min) | R% (Max) |
|---|---|---|
| Ethyl acetate | 118 | 118 |
| Butan-2-one | 103 | 119 |
| Pentan-2-one | 107 | 105 |
| Pentan-3-ol | 97 | 93 |
| Pentan-2-ol | 102 | 93 |
| Pent-1-en-3-ol | 103 | 83 |
| Cyclopentanone | 108 | 117 |
| 3-methylbutan-1-ol | 84 | 96 |
| Octan-3-one | 104 | 117 |
| Cyclohexanone | 107 | 118 |
| Pent-2-en-1-ol | 87 | 100 |
| 2-ethyl-1,6-dioxaspiro [4.4] nonane | 106 | 109 |
| Nonan-2-one | 111 | 115 |
| Octan-2-ol | 102 | 102 |
| Oct-1-en-3-ol | 100 | 99 |
| Decanal | 85 | 93 |
| Pentyl hexanoate | 94 | 102 |
| Undecan-6-one | 105 | 109 |
| Octan-1-ol | 96 | 107 |
| Non-3-en-1-ol | 90 | 110 |
| 5-ethyloxolan-2-one | 101 | 117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabbal, S.; El Aroussi, B.; Bouchard, M.; Marchand, G.; Haddad, S. Development and Validation of a Method for the Simultaneous Quantification of 21 Microbial Volatile Organic Compounds in Ambient and Exhaled Air by Thermal Desorption and Gas Chromatography–Mass Spectrometry. Atmosphere 2022, 13, 1432. https://doi.org/10.3390/atmos13091432
Tabbal S, El Aroussi B, Bouchard M, Marchand G, Haddad S. Development and Validation of a Method for the Simultaneous Quantification of 21 Microbial Volatile Organic Compounds in Ambient and Exhaled Air by Thermal Desorption and Gas Chromatography–Mass Spectrometry. Atmosphere. 2022; 13(9):1432. https://doi.org/10.3390/atmos13091432
Chicago/Turabian StyleTabbal, Sarah, Badr El Aroussi, Michèle Bouchard, Geneviève Marchand, and Sami Haddad. 2022. "Development and Validation of a Method for the Simultaneous Quantification of 21 Microbial Volatile Organic Compounds in Ambient and Exhaled Air by Thermal Desorption and Gas Chromatography–Mass Spectrometry" Atmosphere 13, no. 9: 1432. https://doi.org/10.3390/atmos13091432
APA StyleTabbal, S., El Aroussi, B., Bouchard, M., Marchand, G., & Haddad, S. (2022). Development and Validation of a Method for the Simultaneous Quantification of 21 Microbial Volatile Organic Compounds in Ambient and Exhaled Air by Thermal Desorption and Gas Chromatography–Mass Spectrometry. Atmosphere, 13(9), 1432. https://doi.org/10.3390/atmos13091432







