Abstract
Background: The current COVID-19 pandemic has demonstrated the enormous importance of maintaining good hygienic conditions in everyday indoor environments for the prevention of infectious diseases. This includes sanitization methods capable of significantly reducing the microbial load in the air and on surfaces. However, in line with the ecological transition, alternative systems for environmental sanitization with reduced environmental impact are urgently needed. The photocatalytic reaction using UV-C light-emitting diode (UV-C LED) lamps with short wavelengths, especially in the range of 200–280 nanometers (nm), can significantly reduce the microbial load, safeguarding the environment thanks to reduced energy consumption. The objective of this review is to describe the latest innovations in the use of UV-C LED technology in the sanitization of indoor environments, reporting the fundamental principles on which its activity relies. Methods: Two databases (PubMed, Web of Science), were searched, following PRISMA guidelines. Results: A total of 1348 publications were identified, of which 379 were assessed in detail and, of these, 16 were included in the review. Conclusions: This literature review highlighted that UV-C LEDs irradiation represents a valid, eco-sustainable sanitization method that could be exploited as an alternative to chemical compounds to contain indoor microbiological pollution in living and working environments.
1. Introduction
In recent years, even more so due to the epidemiological emergency linked to the COVID-19 pandemic, various national and international organizations have turned their attention to air and surfaces’ hygienic features in indoor environments, two of the factors with the greatest impact on human health [1]. As reported by the World Health Organization (WHO), indoor air pollution, such as that represented by dampness and molds, chemicals, and other biological agents, is a major cause of morbidity and mortality worldwide [2]. These chemicals and biological agents may increase the risk of developing non-specific respiratory and neurologic symptoms, allergies, asthma, and lung cancer. The quality of indoor air is also important because people spend about 90% of their life in confined environments [3]. In particular, Indoor Air Quality (IAQ) is fundamental in those places where there are vulnerable people due to their health status or age such as in day-care centers, hospitals, schools, retirement homes, and other special environments. A reduction in IAQ is essentially due to an increase in the microbial load present in the air and on surfaces in indoor environments, which can originate from various sources, including people present and their activities, overcrowding, poor air changes, air conditioning, and poor maintenance ventilation systems [4]. It should be emphasized that the total microbial load represents only one of the many factors that affect indoor air quality, such as volatile organic compounds (VOCs), chemical contaminants or the concentration of substances such as carbon dioxide (CO2) or carbon monoxide (CO), but only microbial air quality will be discussed in this review. Thus, to preserve high levels of IAQ, air, and surfaces sanitization methods that use sanitizing chemicals, such as ozone, peroxygen compounds, or bleach are currently used [5,6,7]. Such products and compounds, besides being appreciated above all for their high effectiveness in eliminating microbial contaminants, are somewhat feared for their ability to generate by-products today mostly classified as toxic [8] and/or carcinogenic by the International Agency for Research on Cancer (IARC) Foundation. It is necessary to consider that UV-C rays react with chemical bonds within the molecular structure of particular compounds, such as volatile organic compounds (VOCs) present in indoor environments and facilitate their physical fragmentation. Besides the benefit deriving from the degradation of these VOCs determined by UV-C disinfection technologies, it should be underlined that UV-C action on these molecules leads to the formation of undesired degradation by-products, such as formaldehyde, with known toxicity [9]. Nevertheless, these formaldehyde levels were well below the US EPA health advisory of 1 mg/L for a lifetime exposure for a 70 Kg adult [10]. Another significant disadvantage in the use of chemical products is their reduced eco-sustainability and their high environmental impact, a risk factor as significant for human health as for the balance of ecosystems.
Filters are also used in air sanitization through which it’s possible to retain the coarser atmospheric particulate particles and a large part of the airborne microorganisms. However, the filters have limits of use regarding the need for frequent replacement by trained personnel and their ability to become a survival or growth substrate for bacteria and molds [11].
Therefore, it appears necessary to identify and use methods for sanitizing indoor environments with high efficiency but with a reduced environmental impact, in line with the ecological transition. Huge potential for use in this context can be identified in the use of UV irradiation.
The most useful way to characterize UV is by considering the irradiance value. Conceptually, irradiance represents the amount of UV energy that impacts a particular surface, and it is one of the factors that determine if the UV intensity is insufficient, sufficient, or excessive for a particular application. UV irradiance varies with lamp output power, efficiency, and distance to the surface [12]. Moreover, UV irradiance is useful to calculate the UV dose (D), which is the product of UV irradiance and specific exposure time on a given surface or microorganism [13].
UV sanitization technology is based on exposure to UV radiations of wavelength wandering around the range of 100–400 nm, that can be further subdivided into four main categories: long-wave ultraviolet UV-A (315–400 nm), medium-wave ultraviolet UV-B (280–315 nm), short-wave ultraviolet UV-C (200–280 nm), and vacuum ultraviolet V-UV (100–200 nm) [12] (Figure 1).
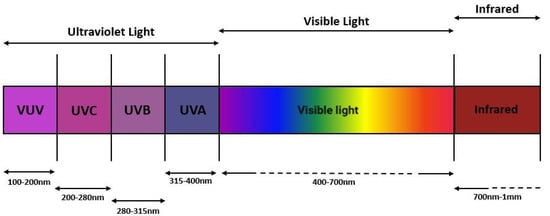
Figure 1.
Electromagnetic spectrum where different types of electromagnetic radiation are depicted: V-UV (100–200 nm); UV-C (200–280 nm); UV-B (280–315 nm); UV-A (315–400 nm); visible spectrum (400–700 nm); infrared spectrum (700 nm–1 mm).
UV-A represents the most abundant portion of ultraviolet rays that reach the earth’s surface, in fact only 5% is absorbed by the ozone layer. On the other hand, UV-B and UV-C are absorbed, correspondingly, for 95% and 100% in the upper part of the stratosphere. UV-A is mainly used in light therapy and 3D printing, but it is harmful to human health. Indeed, it can reach the dermis and cause lipid peroxidation, oxidative stress, and cellular apoptosis through the generation of reactive oxygen species (ROS) leading to premature aging and damage of many cellular components such as proteins or lipids [14,15]. Unlike UV-A, that exerts its harmful effects on cellular components through the generation of oxidative stress, UV-B, and even more UV-C rays, damage skin cells mostly by their direct radiant effect on DNA. V-UV has an even stronger ionizing power than UVC light and can generate high concentration reactive species such as ozone and OH radicals [16] but a wavelength in the range of 100–200 nm is absorbed by the air thus not being able to travel far in the air. Regarding the production of ozone, it must be emphasized that, as stated by the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER), UV-C can generate ozone if wavelengths below 240 nm are used [17]. The wavelength range of UV-B (only under 300 nm) and UV-C coincides with the absorption spectra of DNA, RNA, and proteins. In particular, they can damage DNA, causing strand breaks and possibly leading to mutation and neoplastic transformation [18]. Despite harmful effects to human health, this capability can be important in air and surface sanitization through the elimination of many microorganisms, especially using UV-C rays.
1.1. UV-C Absorption and Cellular DNA Damage
As is well-known from the literature, UV-C rays have an intense germicidal activity. As shown in Figure 2, this effect is made possible thanks to the enormous cell damage, the predominant nature of which involves cyclobutane pyrimidine dimers (CPD), which UV-C rays, at an optimal wavelength around 265 nm [17,19], induce into microorganism DNA/RNA. The genetic damage from exposure to UV-C rays is carried out indifferently at the nucleic acids that make up the genome of eukaryotic cells, viruses and prokaryotic microorganisms. Generally, the dimerization caused by exposure to UV-C occurs between two consecutive thymine residues, causing the collapse of Hydrogen bonds between Thymine and Adenine, forming a new Thymine–Thymine dimer [20,21].
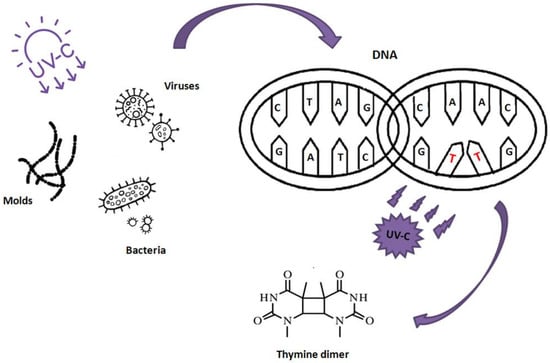
Figure 2.
Genetic damage by UV-C rays.
In other cases, also depending on the type of nucleic acid, different dimers may be formed, such as: Cytosine–Cytosine dimer, Cytosine–Thymine dimer, Uracil–Uracil dimer, Uracil–Thymine dimer and Uracil–Cytosine dimer [22]. As described in the study by Black et al. [23], upon exposure of DNA to wavelengths approaching its maximum absorption, the CPD may be formed by the covalent q4 interaction of two adjacent pyrimidines in the same polynucleotide chain. This results from the saturation of the respective 5, 6 double bonds and from the subsequent formation of a delimited 4-cyclobutyl ring that connects the two pyrimidines of the nitrogenous base with the adjacent one. The replication of the genome is inhibited, as they hinder the activity of the enzyme DNA polymerase causing a slowdown in cell replication and cell death. It should be noted that some microbial forms are able to repair this genetic damage, including some UV-resistant Mycobacterium avium [24] or numerous gram-positive bacteria, known to be resistant to UV radiation. This property is likely due to their high genomic content of G + C, as well as the reduced frequency of thymine dimers, and involves a reduction in the number of dimers formed after exposure to UV-C, with consequent reduced cell damage [25].
1.2. UV-C LEDs: Environmentally Friendly and Efficient
Nowadays, traditional UV-C radiation sources use low-pressure (less than 1 atm) mercury lamps to produce shorter-wavelength UV radiation which is combined with different filters to produce bands with numerous light-emitting wavelengths. Despite their high sanitization efficiency, mercury contained within them is hazardous and is easily absorbed by the skin or the respiratory tract, thus accumulating in the body, often with a fatal toxic effect.
Therefore, the United Nations Environment Programme (UNEP) with the Minamata Convention on Mercury in 2013 [26], a multilateral environmental agreement addressing specific human activities which are contributing to widespread mercury pollution, officially announced a total ban on the production of mercury-containing products after 2020. This also means that it is necessary to search for new solutions to replace mercury lamps as ultraviolet light sources with a valid alternative technology, that can be found in UV-C LEDs lamps.
Both types of lamp are valid for disinfection purposes, but it is necessary to underline the differences between them and above all the disinfection yield. Until the last decade, mercury vapor UV lamps have been used preferentially given their high power. However, it should be noted that the latest generation UV-C LED lamps retain the advantages of traditional mercury UV lamps compared to chemical disinfection methods, overcoming a series of disadvantages that have always characterized mercury UV lamps. In fact, mercury UV lamps have several limitations, including low activity at refrigeration temperatures, a long warm-up time, a high risk of mercury exposure, and can only emit light at 254 nm [27,28]. UV-C LEDs can overcome these limitations, which is why they are becoming so popular. In addition to UV-C LEDs, as an alternative to low-pressure mercury vapor lamps, other types of UV lamps have spread, such as the excimer technology (pulsed xenon lamps, krypton-chloride excimer lamps). This technology shares some advantages with UV-C LEDs, such as they are mercury-free, have a longer lifetime, and don’t need a warm-up time. However, the use of excimer lamps, such as pulsed xenon lamps, in a continuous air disinfection system is limited due to the pulsatile nature of these lamps, and low efficiency [16,17]. Compared to traditional lamps, UV-C LEDs have many different characteristics: they are mercury-free and contain only tiny elements of metals held within a crystalline structure that cannot leach in case of breakage or disposal, don’t produce ozone, have high-power-density and advanced controls allow for a much smaller footprint, suffer minimal damage from repeated cycles, don’t require warm-up time for maximum intensity output, and can emit different wavelengths. These advantages, coupled with virtually instant starts and tunable wavelengths, offer great flexibility in UV-C LEDs ballast design [29]. Furthermore, it should also be considered that, compared to UV mercury lamps, UV-C LEDs have smaller dimensions, making them more versatile and adaptable to many applications. Moreover, LED emission is concentrated in one unique direction with an angle of about 120°–150°, making it easier for perfect collimation of the emission beam directly to a precise and lossless target. These highly focused radiation patterns allow for more options for orientation and, therefore, a unique ballast design, compared to traditional UV lamps [30]. In addition, several studies demonstrated that UV-C LEDs have much higher inactivating efficacy against different types of bacteria, such as Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes, than traditional mercury UV lamps at the same dosages with intensity adjustment [27,31,32].
Finally, yet importantly, costs are certainly a very relevant aspect because represent a major concern for people trying to install UV-C LED lamps. UV-C mercury lamps may offer a lower initial cost than LED lights, but the total cost of ownership will be higher (i.e., cost per Watt can be up to 100 times higher). Moreover, the UVC LEDs turn on as the air passes through which facilitates a longer lifeline and lower running costs. This is compared to mercury lamps which cannot be turned on and off and so degrade at a quicker rate. As reported in Table 1, one aspect to be considered with the same importance is the eco-friendly characteristic of UV LED lamps compared to those with mercury vapor; this lies in their duration, estimated at around 9000/15,000 h of activity against [33] almost three times of the life of mercury lamps, as well as in the reduced energy consumption giving both economic and environmental advantages such as reduced production of metal waste. Indeed, the LEDs can reach the maximum power almost instantaneously at switching on, without any “warm-up” time [34]. Moreover, energy saving is also due to the ability of UV-C LEDs to convert 90% of electricity into UV-C rays, as opposed to traditional UV lamps where the percentage of electricity converted into UV is around 40% and the remaining energy is converted into light and heat. In fact, the UV-C LEDs produce light rays with a specific wavelength (275 ± 5 nm) starting from small amounts of electricity (in the order of a few mWs) with low thermal resistance.

Table 1.
Advantages and differences of UV-C LEDs compared to low-pressure mercury vapor UV lamps.
Sanitization using UV-C LEDs technology is an ecological physical disinfection method that, unlike chemical chemicals, is highly effective against most of the microorganisms present in the environment, not creating forms of microbial resistance. The absence of mercury, in addition to increasing the safety of use of the LEDs, represents an enormous advantage for the environment since the risk of environmental contamination by mercury is also eliminated, thus not requiring any special treatment during recycling. However, it must be emphasized that the various components of LEDs, such as aluminum, steel, and copper, are about 95% recyclable. Another very important feature of LEDs, which involves a reduced environmental impact, is their reduced energy consumption, estimated at around 90% [38]. Reduced consumption essentially depends on the reduced heat dispersion of the LEDs. In fact, traditional UV lamps disperse part of the energy in the form of heat when they are crossed by an electric current [39], reducing their efficiency. Conversely, UV-C LEDs can convert 90% of electricity into UV-C rays and their generation occurs immediately after switching on [34], further reducing energy consumption. Furthermore, it is estimated that UV-C LED lamps offer 50% lower CO2 emissions, and that replacing mercury lamps with UV LED lamps can lead to 67 tons of CO2 reduction per year in the atmosphere. In addition to the emission of CO2, the emission of other greenhouse gases, such as ozone, would also be greatly reduced since the UV-C LEDs do not produce ozone unlike traditional mercury vapor lamps. An aspect that is still little considered today when it comes to alternative technologies is their origin. In fact, considering the traditional UV lamps, they are almost entirely produced in China, thus increasing the environmental impact linked to the emissions related to their transport. Unlike UV lamps, LEDs are produced in several countries including European Union countries, thus reducing transport distances and emissions.
2. Methods
2.1. Scope and Definitions
A systematic literature search of the databases was carried out, following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [40]. A narrative approach was adopted to provide a descriptive summary of the studies, aiming to present the latest innovations in the use of UV-C LEDs technology in the sanitization of air and surfaces in indoor environments [41,42], avoiding a simple comparison of results. To write this review, a systematic search and narrative review method were adopted involving four main steps: first, a systematic search process and application of inclusion and exclusion criteria; second, data extraction and synthesis of results; third, an analysis of key findings by narrative review; and fourth, a quality appraisal procedure that included all studies. The systematic literature search was performed using two databases (PubMed, Web of Science), to search for relevant literature, on 15 July 2022. Our review focused primarily on studies of indoor air and surface disinfection using ultraviolet germicidal inactivation by UV-C LEDs. We have limited our attention to studies where only UV-C rays were used. It should be noted that in the first part of this review we also considered articles where traditional UV lamps were used, but this only to compare them to LEDs.
2.2. Data Source and Search Strategy
We searched two databases: PubMed, Web of Science. The search strategy combined the terms UV-C with the following terms: DISINFECTIONS, or AIR DISINFECTION, or SURFACES DISINFECTION, or INDOOR ENVIRONMENTS to select original publications focused on the use of UV-C LEDs to disinfect air and surfaces in indoor environments, increasing IAQ in there. The articles identified during the search, using the combinations of terms previously mentioned, were examined to remove duplicates. After removal of duplicates, titles and abstracts of the articles were screened and the publications that didn’t comply with the inclusion criteria were removed. The full text for eligible studies was then sourced. The selection procedure is reported in Figure 3.
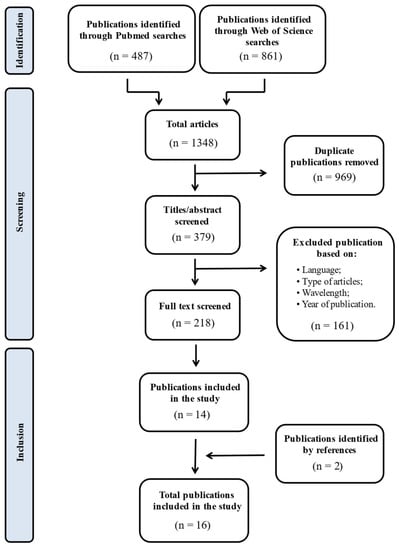
Figure 3.
Selection procedure to find eligible publications.
At the end of the bibliographic search, a total of 1348 studies were identified. From the total of studies identified with the keywords, 969 duplicates between the two databases were removed using Endnote X7 program. After removing duplicates, 161 studies were excluded by title and abstract based on inclusion and exclusion criteria. Only 218 studies found to be suitable, according to the inclusion and exclusion criteria, were then evaluated reading the full text. Through this procedure, we found 14 eligible articles and added 2 articles found by reference screening. At the end, 16 articles were included in the review.
2.3. Inclusion Criteria
In this review, articles were included if published in English. Moreover, studies were included according to the following criteria:
- Should present reliable methodology and information enough regarding the UV-C;
- Should be focused on the UV-C LEDs efficacy;
- Must focus on sanitizing indoor air and surfaces with UV-C LEDs;
- Ozone must not be used in combination with UV-C LEDs;
- The articles must be published in the previous five years;
- The articles must be available in English.
The inclusion criteria have been shown in Table 2.

Table 2.
Summary of the inclusion and exclusion criteria.
2.4. Exclusion Criteria
As reported in Table 2, exclusion criteria were reviews, meta-analyses, dissertations, conference papers, commentaries and editorials, and other publications other than journal articles. Moreover, studies were excluded if they have used UV wavelengths other than UV-C and if they haven’t used only LEDs to disinfect air and surfaces in indoor environments.
3. Application of UV-C LEDs in Air Sanitization
As defined by WHO, low air quality is a significant risk factor for human health [43]. Exposure to indoor air pollutants can lead to a wide range of adverse health outcomes in both children and adults, from respiratory illnesses to cancer to eye problems. In particular, children that live in polluted confined environments have an increased risk of experiencing asthma [44,45] and respiratory infections. Air disinfection through UV-C lamps is accomplished via several methods, such as irradiating the upper-room air only (ideal for use in occupied rooms) or irradiating the full room (ideal for complete air sanitization but in a room without people) [46]. As reported by Kim and Kang [47], optimal results in air sanitizing were obtained using UV-C irradiation; they investigated the possibility of inactivating viral (MS2, Qβ, ϕX174), bacterial (Escherichia coli O157: H7; Salmonella enterica serovar Typhimurium, Listeria monocytogenes, Staphylococcus aureus), and fungal (Aspergillus flavus, Alternaria japonica) aerosols in a chamber-type air disinfection system (30 by 30 by 30 cm) by using a UV-C LEDs system placed inside the chamber. The UV-C LED arrays used in this study were composed of sixteen UV-C LED package chips arrayed linearly at 11 mm from each other and a 40 mm gap on both sides to connect it to a cooling panel. After the nebulization step, the air containing microbial particles was irradiated with the UVC LED array for up to 10 min for viruses, 1 min for bacteria, and 5 min for fungi with the fan still running. A 2.5-log reduction was achieved at a dosage of 1.5 mJ/cm2 for E. coli O157:H7, 3- to 4.5-log reductions at 4.6 mJ/cm2 for S. Typhimurium, L. monocytogenes, and S. aureus., 4.7 and 4.9-log reductions within 46 mJ/cm2 for MS2 and Qβ viruses, and 4 log reductions within 4.6 mJ/cm2 for ϕX174 virus. Unlike ϕX174 virus and bacteria mentioned above, A. flavus and A. japonica required an irradiation dosage approximately 5 times higher (about 23 mJ/cm2) to achieve 4 log reductions, because eukaryotic cells show greater resistance to UV-C irradiation.
An innovative UV-C LEDs technology, for air sanitization was also tested by Nunayon, et al. [48]. In this work, the researcher tested and compared, in a full-size experimental chamber, a novel upper room UV-C LEDs device with a conventional mercury vapor lamp system. The test chamber operated at a constant temperature (T, °C) and relative humidity (RH, %) of 24 °C and 55%, respectively. The results showed that the conventional mercury vapor lamp system’s bactericidal effect was not significantly higher than the peak reduction reported for the UV-C LEDs system. Despite this, considering not only the abatement rate, but also the various technical-economic advantages of LEDs, the researchers concluded by stating that the UV-C LEDs system has a high potential to be used as a safe and effective irradiated light source to inactivate indoor airborne pathogens. Subsequently, the same team of researchers compared the antibacterial efficacy of the tested UV-C LEDs system by both holding it in a stationary position and rotating it. The sanitizing effectiveness of the UV-C LEDs system in poorly mixed ventilation conditions has been shown to improve by 22.36–49.86% by rotating the UV-C LED system to sweep across the entire room compared to the direct stationary mode of irradiation operation of the UV-C LEDs system [49].
An aspect to consider is the size of the room because the UV-C rays, especially if the UV-C LEDs are installed on the ceiling, will not be able to sanitize the air present on the opposite side of the room, thus reducing the effectiveness of air sanitization. For this reason, a valid solution, tested and confirmed in many studies, is to insert these UV-C LEDs inside ventilation ducts where the area crossed by the air is considerably reduced, thus making possible homogeneous and optimal irradiation of the air which will then be introduced into the confined environment.
Anyway, the type, survival and spreading of airborne microorganisms, which can then settle on the surfaces, are tightly linked to physical favorable environmental parameters, too, such as relative humidity and temperature [50].
Integration of UV-C LEDs in a Continuous Sanitation Air (CSA) System
As previously mentioned, to provide better IAQ, UV-C LEDs are installed in an air duct of CSA systems [51] that can be then installed in Heating, Ventilation, and Air Conditioning (HVAC) systems of public builds or public transportations. One of the main advantages of the integration of UV-C LEDs in a CSA system is the possibility of continuously sanitizing the air in rooms where there are people, such as inside a train or a public office. These CSA systems are always equipped with High-Efficiency Particulate Air (HEPA) filters to retain the particulate (about 99.97% of airborne particles) and microorganisms present in the air, but often the only use of filters is unable to guarantee optimal IAQ levels, especially in highly crowded public environments. Another aspect to consider is that HEPA filters can become an optimal proliferation site for microorganisms, compromising air quality. In addition to negatively impacting IAQ, dirty filters with a high microbial load could represent a biological hazard for workers who must replace worn filters and maintenance of the system. Exactly for these reasons, the UV-C lamps are generally located, inside the air ducts, in a position to directly irradiate the HEPA filters surface. As confirmed in the study conducted by D’Orazio A. and D’Alessandro D. [52], direct irradiation of the filters by the UV-C rays allows increasing the effectiveness of air sanitization as well as the half-life of the HEPA filters themselves thanks to the reduction of the microbial load present in the filters. In addition to the combination of UV-C LEDs and HEPA filters, Baldelli G. et al. [51] conducted a study testing the air sanitization capacity of a CSA system, installed outside the passenger compartment of a train, inside the HVAC box. In this CSA system the UV-C LEDs were installed in addition to ISO Coarse 90% filter and an ionizer. In the train setting, the microclimatic parameters ranged as follows: 19–24 °C and 43–57% RH. The tested CSA device demonstrated high efficiency in microbial inactivation thanks to the synergy between UV-C LEDs, filters, and the ionizer. During the study, the researchers evaluated the efficiency of the entire CSA system, which demonstrated a microbial killing rate of 98.96 ± 0.6%. On the other hand, the ionizer or the ISO Coarse 90% filter alone led to lower sanitization efficiency (89.48 ± 0.08% and 88.69 ± 0.37%, respectively).
A problem that we may encounter in the use of these types of CSA is related to the possible formation of condensation along the aeraulic ducts, which can favor the proliferation of pathogenic microorganisms, including bacteria of the Legionella genus, which can be diffused in the air of the confined environment [53,54]. In this case, the integration of UV-C LEDs inside a CSA can solve this problem by sanitizing the duct, but it is essential to pay attention to the relative humidity and the temperature inside the aeraulic system.
4. Application of UV-C LEDs in Surfaces Disinfection
Just like air, surfaces also play an important role in the transmission of infections. Today, a search for “no-touch” technologies is underway, such as UV-C LED lamps, capable of disinfecting surfaces and materials from microorganisms. An aspect to consider is that the no-touch UV technology depends on the distance between the lamp and the surface to be disinfected. In fact, as stated by the inverse square law, doubling the distance between the lamp and the surface to be disinfected quadruples the time required for disinfection. Different studies [55,56] compared the surface sanitizing effectiveness between UV-C lamps and standard operating protocol (SOP), essentially based on the use of disinfectant chemicals. In the study of Liscynesky et al. [57], in rooms of patients with confirmed C. difficile infection, 32 out of 238 (13%) high-touch surfaces were positive after bleach disinfection and only 1 out of 238 (0.4%) was positive after UV-C treatment.
Furthermore, UV-C LEDs treatment was applied to inactivate Salmonella spp. deposited on chicken breast samples and common food contact surfaces such as stainless steel and high-density polyethylene obtaining a reduction of Salmonella spp. up to 99.999% at a 3.6 J/cm2 after 15 min [58], and to disinfect chicken transport crates resulting in a significant reduction in C. jejuni, Enterobacteriaceae, and total aerobic bacteria on these surfaces at 61.2 mJ/cm² after 3 min [59].
Germicidal irradiation with UV-C rays is also considered a promising method for viruses’ inactivation, as well as for bacteria and molds, on surfaces and materials of common use. In this case, it should be emphasized that the effectiveness of ultraviolet irradiation strongly depended on the type of viral nucleic acid, capsid structure of viruses, host cell repair mechanisms, and relative humidity [60]. In fact, to achieve a 90% viral reduction on a surface, the UV dose required for viruses with nucleic acid RNA and double-stranded DNA (dsRNA/dsDNA) is approximately 2 to 3 times higher than for viruses with nucleic acid single-stranded RNA/DNA (ssRNA and ssDNA). Another susceptibility factor to be considered, as previously mentioned, is relative humidity (optimal at 55%) since the water coating on the viral capsule could protect against UV-induced damage to DNA or RNA.
Gidari et al. established the persistence of SARS-CoV-2 on inanimate surfaces and material during UV-C irradiation, noting that a smaller dose of UV-C (10.25–23.71 mJ/cm2) is enough to reduce the viral charge of >99.99% on surfaces. Similar results were obtained in many other laboratory studies [61,62]; moreover, some results indicated that a 99.9% inactivation can be reached after 1 min of treatment with a dose of 83.1 J/m2 [63]. Even 10 s of UV-C exposure strongly reduced viral loads [64] concluding that UV-C LEDs proved to be an effective and quick method for disinfecting SARS-CoV-2-contaminated surfaces. Similar killing rates were obtained in other studies against different types of viruses such as, for example, in the study by Mariita RM et al. [65] during which they used UV-C LEDs against Human norovirus (HuNoV) obtaining a 99.9% virus reduction with a UV-C dose of 22.5 mJ/cm−2 at a wavelength of 269 nm at 7 cm source–surface distance.
Therefore, the high susceptibility of bacteria and viruses to UV-C LEDs makes the latter a valid eco-sustainable alternative in the sanitization of indoor environments, as long as attention is paid to their position.
5. Results and Discussion
Nowadays, well known is the environmental pollution, due to human activity, and its enormous negative impact on the ecosystem and on human health. A high negative impact is exerted by high concentrations of pollutants in the air, which are also deposited on surfaces, present in indoor environments. A high concentration of pollutants, including chemical and/or biological pollutants, in the indoor air leads to a significant reduction in IAQ, at the same time increasing the negative impact on our health, and consequently causing an increase in the risk of respiratory diseases. There are several sanitizing methods used to guarantee optimal IAQ levels but many of these, despite being very effective in sanitizing environments, can be dangerous for our health [8]. A valid alternative in environmental sanitization is represented by LEDs tuned to a wavelength in the range of UV-C rays (200–280 nm). The application of LEDs and interest in them is growing sharply in the last 10 years, especially after the announcement of the total ban on the production of mercury-containing products after 2020 was declared during the Minamata conference [26]. In fact, UV-C LEDs don’t contain mercury, unlike traditional mercury lamps, and this entails easier disposal and above all a reduced environmental impact. Their reduced environmental impact also depends on the low energy consumption required by LEDs, and this makes them not only more eco-sustainable but also cheaper to run and 50–90 times more efficient, compared to mercury lamps [48,66]. To date, optimal sanitization results with UV-C LEDs have been obtained in the sanitization of air and surfaces, reaching inactivation rates up to 99.9% both against bacteria and viruses in indoor environments [58,61,62,63,65].
However, it must be considered that the position of the LEDs and their distance from the surface to be sanitized considerably affects the rate of microbial abatement. That is why the LEDs must be positioned in a position close to the affected surface to reduce the dispersion of UV-C rays, thus maintaining high sanitizing efficiency. For this reason, UV-C LEDs are often installed inside an air duct of a CSA system. Furthermore, UV-C LED-based CSA systems are often equipped with other components such as ionizers and filters to improve the sanitization efficiency. UV-C LEDs’ efficiency in air sanitization was demonstrated in several studies [47,48,49,51] in which the researchers assessed inactivation rates up to 98%, thus underlining the enormous potential of UV-C LEDs in indoor air sanitization. Equally, optimal inactivation rates were obtained in the surface sanitization with UV-C LEDs.
However, despite their high efficiency in microbial inactivation, it must be emphasized that overexposure to UV-C radiation can be very dangerous and harmful to the human body, causing burns, severe forms of photokeratitis, and inflammation of the cornea. Due to this, it is appropriate to install UV-C LEDs inside a CSA system to avoid direct exposure to UV-C rays, or in devices with motion sensor equipment, to guarantee efficient and healthy sanitization for users.
6. Conclusions
The principal aim of this review was to describe and underline the potential of UV-C LEDs in indoor environmental sanitization, reporting the fundamental principles on which relies their activity and the last innovations in their use. Our research identified 16 studies that talked about the UV-C LEDs’ efficiency and their application in air and surface sanitization, comparing them to traditional vapor mercury UV lamps. UV-C LEDs are smaller, mercury-free, ozone-free than traditional mercury vapor lamps, and present lower energy consumption. Therefore, thanks to the reduced energy consumption, the higher lifetime, and the absence of mercury, UV-C LEDs represent a valid eco-sustainable alternative for indoor environment sanitization, in line with the ecological transition. In conclusion, the use of UV-C LED lamps is particularly advantageous, both on a hygienic and ecological level, in replacement of the most common chemical sanitization methods and, in comparison, to common vapor mercury UV-C lamps in the sanitization of air and surfaces in confined environments.
Author Contributions
Conceptualization, F.P., G.F.S., G.A., G.B. (Giulia Baldelli) and G.B. (Giorgio Brandi); Formal analysis and data curation, F.P., G.F.S., G.A., G.B. (Giulia Baldelli), M.P.A. and G. B. (Giorgio Brandi); Writing—review and editing, F.P. G.F.S., G.A., G.B. (Giulia Baldelli) and G.B. (Giorgio Brandi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, E.; Rosen, J. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Dales, R.; Liu, L.; Wheeler, A.J.; Gilbert, N.L. Quality of indoor residential air and health. CMAJ Can. Med. Assoc. J. 2008, 179, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Correia, G.; Rodrigues, L.; Gameiro da Silva, M.; Gonçalves, T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef] [PubMed]
- Moccia, G.; De Caro, F.; Pironti, C.; Boccia, G.; Capunzo, M.; Borrelli, A.; Motta, O. Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina 2020, 56, 578. [Google Scholar] [CrossRef]
- Block, S.S. Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Sharafi, S.M.; Ebrahimpour, K.; Nafez, A. Environmental disinfection against COVID-19 in different areas of health care facilities: A review. Rev. Environ. Health 2021, 36, 193–198. [Google Scholar] [CrossRef]
- Mitsuboshi, S.; Yamaguchi, R.; Uchida, H.; Kamoshida, S.; Hashi, H. Inappropriate use of ozone generators and their sales status: Questionnaire survey of healthcare providers and investigation of online sales. J. Hosp. Infect. 2021, 117, 1–3. [Google Scholar] [CrossRef]
- Farhanian, D.; Haghighat, F. Photocatalytic oxidation air cleaner: Identification and quantification of by-products. Build. Environ. 2014, 72, 34–43. [Google Scholar] [CrossRef]
- USEPA. Community Water Systems Survey, Volume I: Overview; United States Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Nazarenko, Y. Air filtration and SARS-CoV-2. Epidemiol. Health 2020, 42, e2020049. [Google Scholar] [CrossRef]
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2008. [Google Scholar]
- Method of Evaluating the UV Dose to Airborne Microorganisms Transiting In-Duct Ultraviolet Germicidal Irradiation Devices. Available online: https://www.iso.org/standard/67814.html (accessed on 16 August 2022).
- Cadet, J.; Douki, T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J. Investig. Dermatol. 2011, 131, 1005–1007. [Google Scholar] [CrossRef]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect. Dis. 2020, 20, 127. [Google Scholar] [CrossRef]
- Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). Opinion on Biological Effects of UV-C Radiation Relevant to Health with Particular Reference to UV-C Lamps; European Commission: Luxembourg, 2018. [Google Scholar]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Walker, C.M.; Ko, G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007, 41, 5460–5465. [Google Scholar] [CrossRef]
- Trivellin, N.; Piva, F.; Fiorimonte, D.; Buffolo, M.; De Santi, C.; Orlandi, V.T.; Dughiero, F.; Meneghesso, G.; Zanoni, E.; Meneghini, M. UV-based technologies for SARS-CoV2 inactivation: Status and perspectives. Electronics 2021, 10, 1703. [Google Scholar] [CrossRef]
- Black, H.S.; deGruijl, F.R.; Forbes, P.D.; Cleaver, J.E.; Ananthaswamy, H.N.; deFabo, E.C.; Ullrich, S.E.; Tyrrell, R.M. Photocarcinogenesis: An overview. J. Photochem. Photobiol. B Biol. 1997, 40, 29–47. [Google Scholar] [CrossRef]
- Schiavano, G.F.; De Santi, M.; Sisti, M.; Amagliani, G.; Brandi, G. Disinfection of Mycobacterium avium subspecies hominissuis in drinking tap water using ultraviolet germicidal irradiation. Environ. Technol. 2018, 39, 3221–3227. [Google Scholar] [CrossRef]
- Nocker, A.; Shah, M.; Dannenmann, B.; Schulze-Osthoff, K.; Wingender, J.; Probst, A.J. Assessment of UV-C-induced water disinfection by differential PCR-based quantification of bacterial DNA damage. J. Microbiol. Methods 2018, 149, 89–95. [Google Scholar] [CrossRef]
- Larson, H.J. The Minamata Convention on Mercury: Risk in perspective. Lancet 2014, 383, 198–199. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, D.K.; Kang, D.H. Using UVC Light-Emitting Diodes at Wavelengths of 266 to 279 Nanometers to Inactivate Foodborne Pathogens and Pasteurize Sliced Cheese. Appl. Environ. Microbiol. 2016, 82, 11–17. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Autin, O.; Goslan, E.H.; Hassard, F. Application of ultraviolet light-emitting diodes (UV-LED) to full-scale drinking-water disinfection. Water 2019, 11, 1894. [Google Scholar] [CrossRef]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.J.; Stuetz, R. Point-of-use water disinfection using ultraviolet and visible light-emitting diodes. Sci. Total Environ. 2016, 553, 626–635. [Google Scholar] [CrossRef]
- Murashita, S.; Kawamura, S.; Koseki, S. Inactivation of Nonpathogenic Escherichia coli, Escherichia coli O157:H7, Salmonella enterica Typhimurium, and Listeria monocytogenes in Ice Using a UVC Light-Emitting Diode. J. Food Prot. 2017, 80, 1198–1203. [Google Scholar] [CrossRef]
- Nyhan, L.; Przyjalgowski, M.; Lewis, L.; Begley, M.; Callanan, M. Investigating the Use of Ultraviolet Light Emitting Diodes (UV-LEDs) for the Inactivation of Bacteria in Powdered Food Ingredients. Foods 2021, 10, 797. [Google Scholar] [CrossRef]
- Sellera, F.P.; Sabino, C.P.; Cabral, F.V.; Ribeiro, M.S. A systematic scoping review of ultraviolet C (UVC) light systems for SARS-CoV-2 inactivation. J. Photochem. Photobiol. 2021, 8, 100068. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Bennie, J.; Hopkins, J. Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef]
- Purefize. Available online: https://www.purefize.com/about-uv-disinfection/uv-light-and-the-environment/ (accessed on 17 August 2022).
- Claus, H. Ozone Generation by Ultraviolet Lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef] [PubMed]
- NLPIP. Lighting Answer. Available online: https://www.lrc.rpi.edu/programs/nlpip/lightinganswers/mwmhl/restriketimes.asp (accessed on 17 August 2022).
- Novruzova, A.H.E. New technologies in energy sector and automated energy accounting systems and their main factors of influence on ecology. Int. J. Tech. Phys. Probl. Eng. 2020, 42, 53–57. [Google Scholar]
- Belloli, M.; Cigarini, M.; Milesi, G.; Mutti, P.; Berni, E. Effectiveness of two UV-C light-emitting diodes (LED) systems in inactivating fungal conidia on polyethylene terephthalate. Innov. Food Sci. Emerg. Technol. 2022, 79, 103050. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Baiz, N.; Banerjee, S.; Rudnai, P.; Rive, S.; on behalf of the SINPHONIE Group. Indoor air quality and sources in schools and related health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2013, 16, 491–550. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. UVC LED Irradiation Effectively Inactivates Aerosolized Viruses, Bacteria, and Fungi in a Chamber-Type Air Disinfection System. Appl. Environ. Microbiol. 2018, 84, e00944-18. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Zhang, H.; Lai, A.C.K. Comparison of disinfection performance of UVC-LED and conventional upper-room UVGI systems. Indoor Air 2020, 30, 180–191. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Zhang, H.H.; Lai, A.C.K. A novel upper-room UVC-LED irradiation system for disinfection of indoor bioaerosols under different operating and airflow conditions. J. Hazard. Mater. 2020, 396, 122715. [Google Scholar] [CrossRef]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6 (Suppl. S6), S737–S746. [Google Scholar] [CrossRef] [Green Version]
- Baldelli, G.; Aliano, M.P.; Amagliani, G.; Magnani, M.; Brandi, G.; Pennino, C.; Schiavano, G.F. Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments. Int. J. Environ. Res. Public Health 2022, 19, 1559. [Google Scholar] [CrossRef]
- D’Orazio, A.; D’Alessandro, D. Air bio-contamination control in hospital environment by UV-C rays and HEPA filters in HVAC systems. Ann. Ig. Med. Prev. Comunita 2020, 32, 449–461. [Google Scholar]
- Prussin, A.J., II; Schwake, D.O.; Marr, L.C. Ten Questions Concerning the Aerosolization and Transmission of Legionella in the Built Environment. Build. Environ. 2017, 123, 684–695. [Google Scholar] [CrossRef]
- Al-Matawah, Q.; Al-Zenki, S.; Al-Azmi, A.; Al-Waalan, T.; Al-Salameen, F.; Hejji, A.B. Legionella detection and subgrouping in water air-conditioning cooling tower systems in Kuwait. Environ. Sci. Pollut. Res. Int. 2015, 22, 10235–10241. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.M.; Totaro, M.; Baggiani, A.; Privitera, G.P. Evaluation of an Ultraviolet C (UVC) Light-Emitting Device for Disinfection of High Touch Surfaces in Hospital Critical Areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef]
- Andersen, B.M.; Bånrud, H.; Bøe, E.; Bjordal, O.; Drangsholt, F. Comparison of UV C light and chemicals for disinfection of surfaces in hospital isolation units. Infect. Control Hosp. Epidemiol. 2006, 27, 729–734. [Google Scholar] [CrossRef]
- Liscynesky, C.; Hines, L.P.; Smyer, J.; Hanrahan, M.; Orellana, R.C.; Mangino, J.E. The Effect of Ultraviolet Light on Clostridium difficile Spore Recovery Versus Bleach Alone. Infect. Control Hosp. Epidemiol. 2017, 38, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Fernandez, M.; Montoya, B.; Schmidt, M.; Thompson, J. UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods 2021, 10, 1459. [Google Scholar] [CrossRef]
- Moazzami, M.; Fernström, L.-L.; Hansson, I. Reducing Campylobacter jejuni, Enterobacteriaceae and total aerobic bacteria on transport crates for chickens by irradiation with 265-nm ultraviolet light (UV–C LED). Food Control. 2021, 119, 107424. [Google Scholar] [CrossRef]
- Tseng, C.C.; Li, C.S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 Survival on Surfaces and the Effect of UV-C Light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Della Camera, A.; Ferraro, P.; Amodeo, D.; Corazza, A.; Nante, N.; Cevenini, G. An Emerging Innovative UV Disinfection Technology (Part II): Virucide Activity on SARS-CoV-2. Int. J. Environ. Res. Public Health 2021, 18, 3873. [Google Scholar] [CrossRef] [PubMed]
- Trivellin, N.; Buffolo, M.; Onelia, F.; Pizzolato, A.; Barbato, M.; Orlandi, V.T.; Del Vecchio, C.; Dughiero, F.; Zanoni, E.; Meneghesso, G.; et al. Inactivating SARS-CoV-2 Using 275 nm UV-C LEDs through a Spherical Irradiation Box: Design, Characterization and Validation. Materials 2021, 14, 2315. [Google Scholar] [CrossRef]
- Bormann, M.; Alt, M.; Schipper, L.; van de Sand, L.; Otte, M.; Meister, T.L.; Dittmer, U.; Witzke, O.; Steinmann, E.; Krawczyk, A. Disinfection of SARS-CoV-2 Contaminated Surfaces of Personal Items with UVC-LED Disinfection Boxes. Viruses 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Mariita, R.M.; Wilson Miller, A.C.; Randive, R.V. Evaluation of the virucidal efficacy of Klaran UVC LEDs against surface-dried norovirus. Access Microbiol. 2022, 4, 000323. [Google Scholar] [CrossRef]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).