Hydrogenation of Carbon Dioxide to Value-Added Liquid Fuels and Aromatics over Fe-Based Catalysts Based on the Fischer–Tropsch Synthesis Route
Abstract
:1. Introduction
2. Direct Hydrogenation of CO2 to Liquid Fuels over Fe-Based Catalysts
2.1. Strategies for Improving the Catalytic Performance of Fe-Based Catalysts
2.1.1. Alkali Promoters
2.1.2. Secondary Metals
- (1)
- Cu Promoter
- (2)
- Co Promoter
- (3)
- Zn Promoter
2.1.3. Catalyst Supports
2.1.4. Preparation Methods
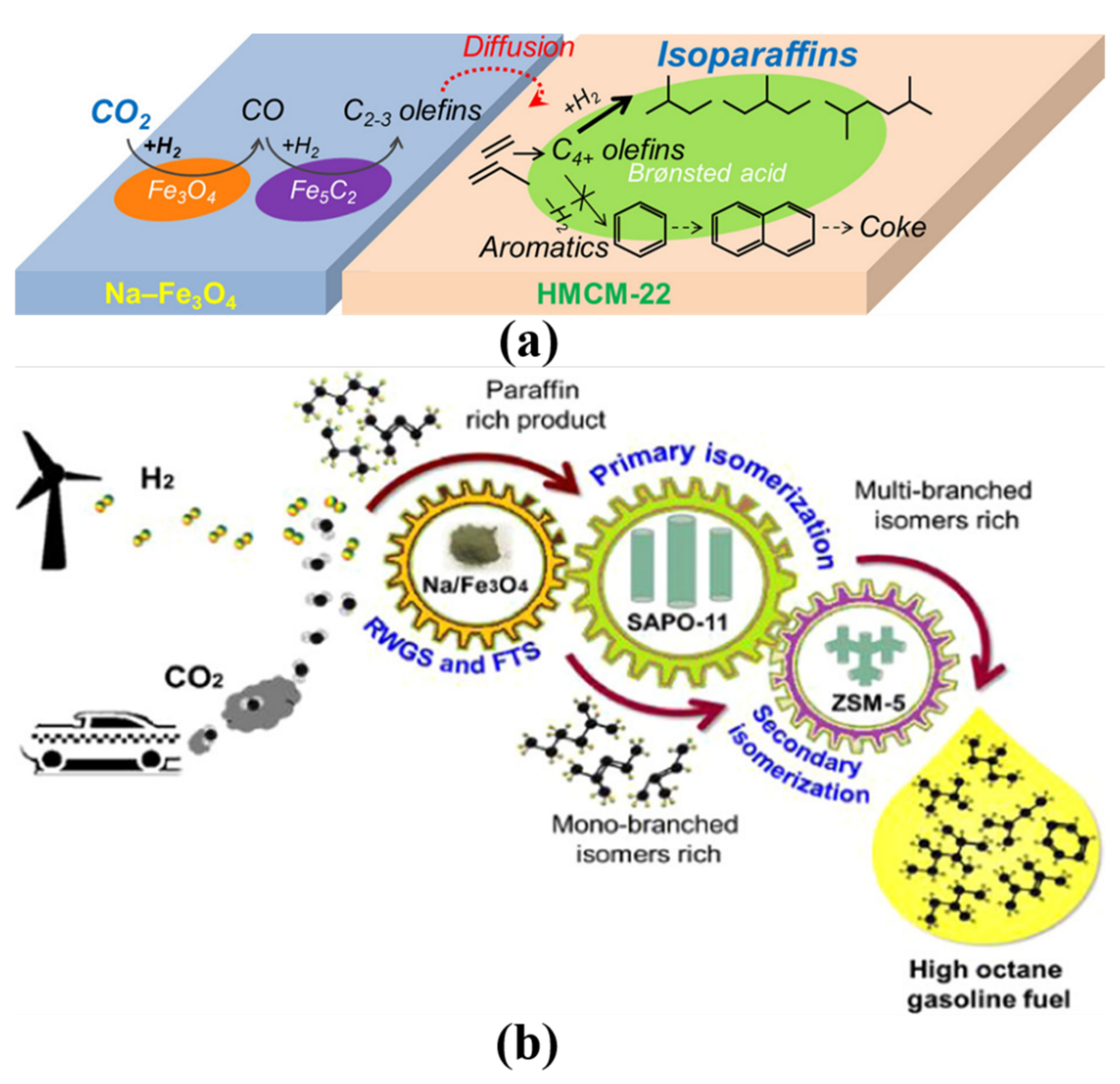
2.2. Tandem Catalysts Combined with Fe-Based Active Components and Zeolites
2.3. Effects of Reaction Conditions on Catalytic Performance
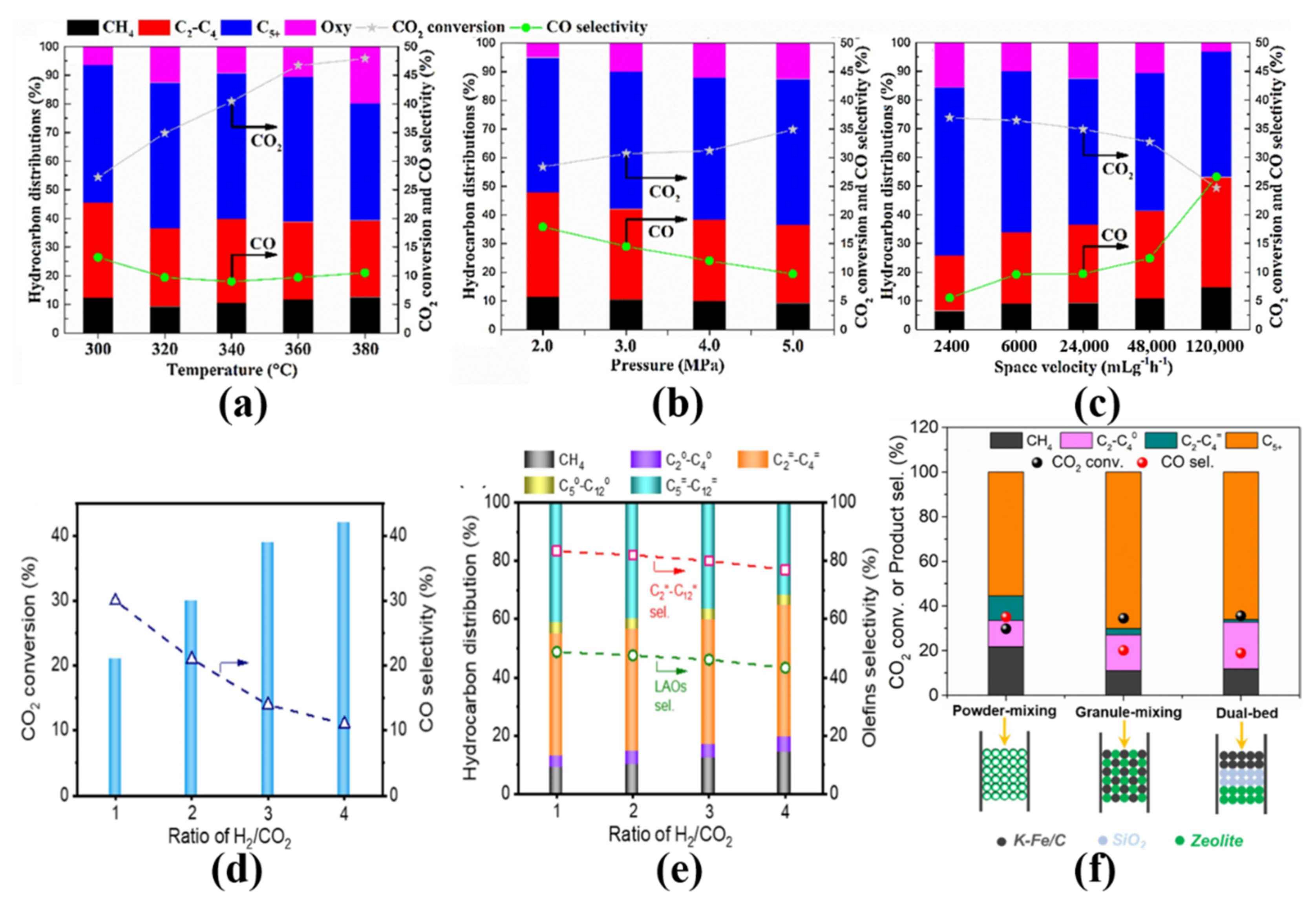
2.3.1. Reaction Temperature
2.3.2. Reaction Pressure
2.3.3. Space Velocity
2.3.4. H2/CO2 Ratio
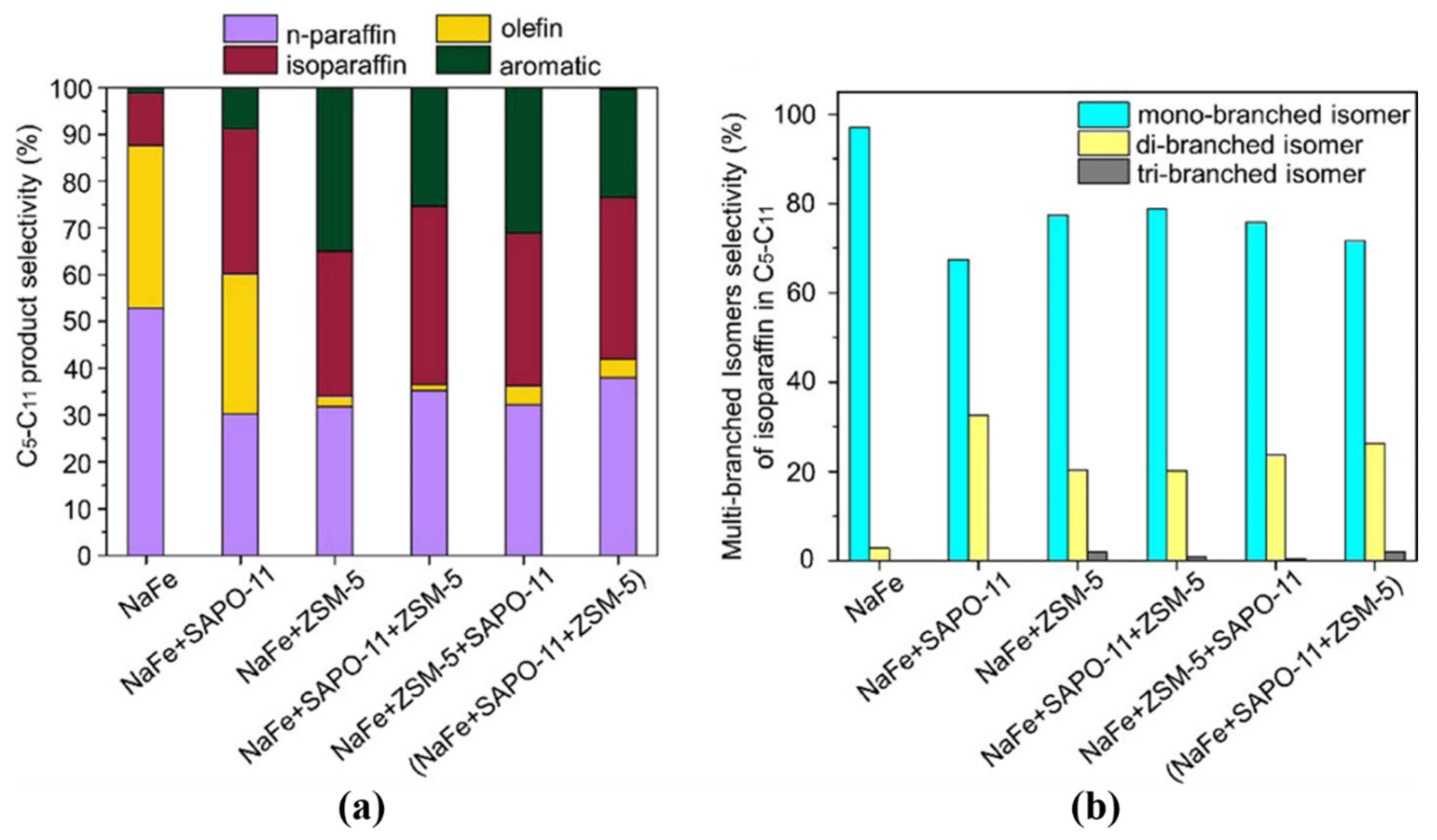
2.3.5. Mixing Mode of Different Active Components
3. Hydrogenation of CO2 to Aromatics over FeOx/Zeolite Tandem Catalysts
3.1. Strategies for Optimizing Product Distribution of Fe-Based Active Components
3.1.1. Metal Promoters
3.1.2. Catalyst Supports
3.1.3. Preparation Methods
3.2. Rational Modification of Zeolites
3.2.1. Main Influencing Factors of Zeolites
- (1)
- Type of Zeolite
- (2)
- BAS of Zeolites
- (3)
- Preparation Methods
3.2.2. Strategies for Regulating the Pore Structure and Surface Acidity
- (1)
- Alkali Treatments
- (2)
- Modification of HZSM-5
- (3)
- SiO2 Coating
3.3. Effect of the Reaction Conditions on the Catalytic Performance
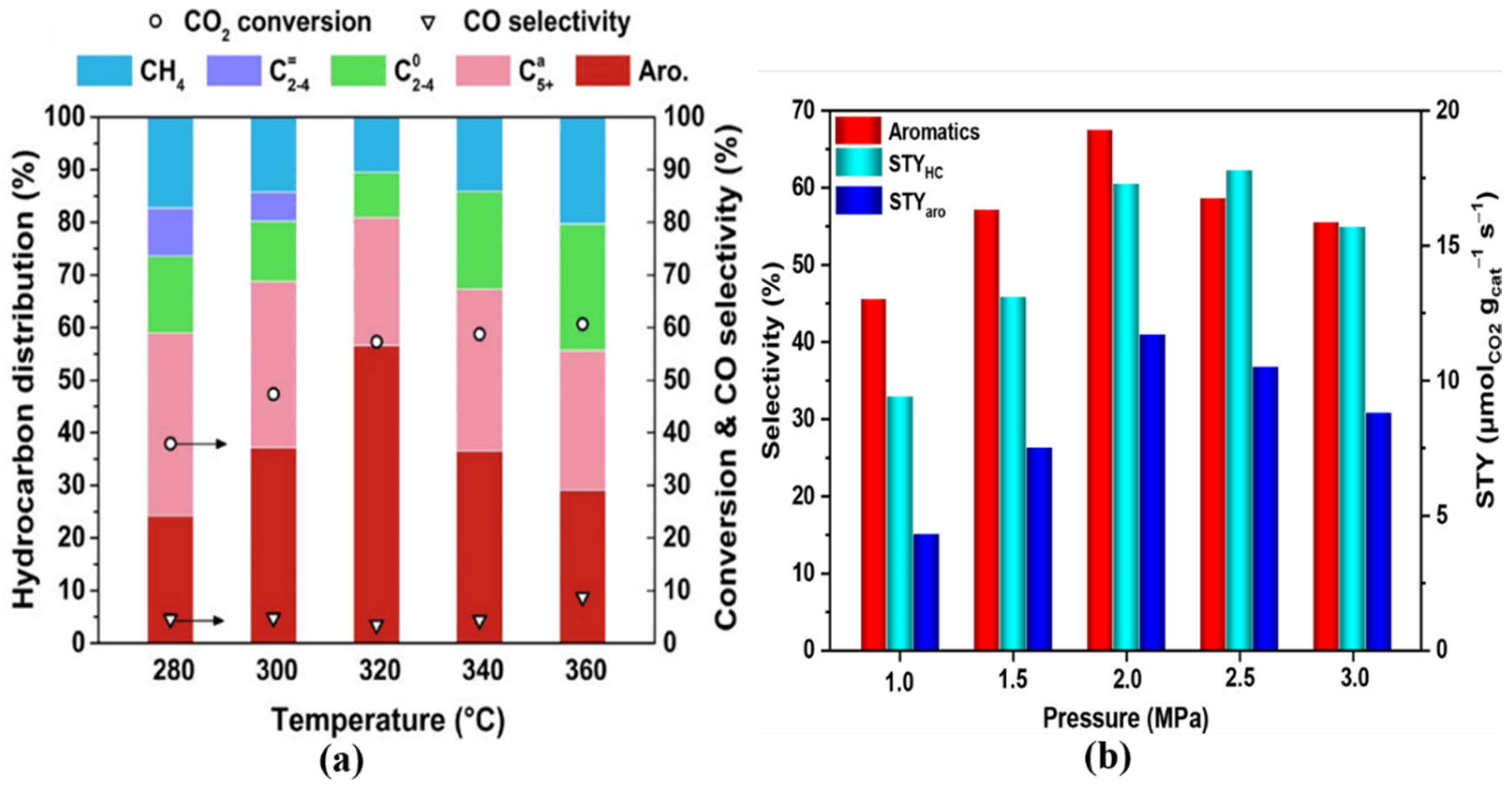
3.3.1. Reaction Temperature
3.3.2. Reaction Pressure
3.3.3. Space Velocity
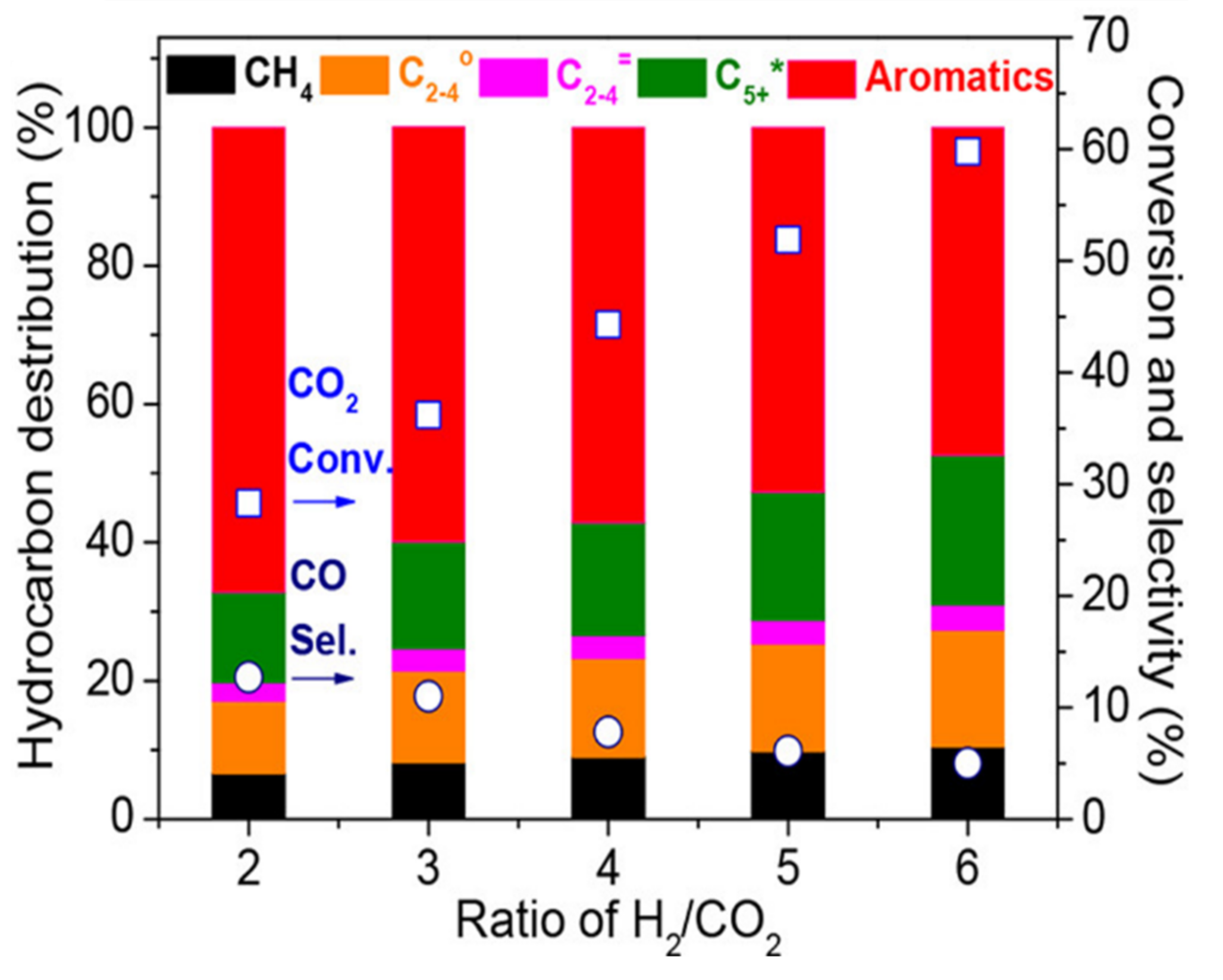
3.3.4. H2/CO2 Ratio
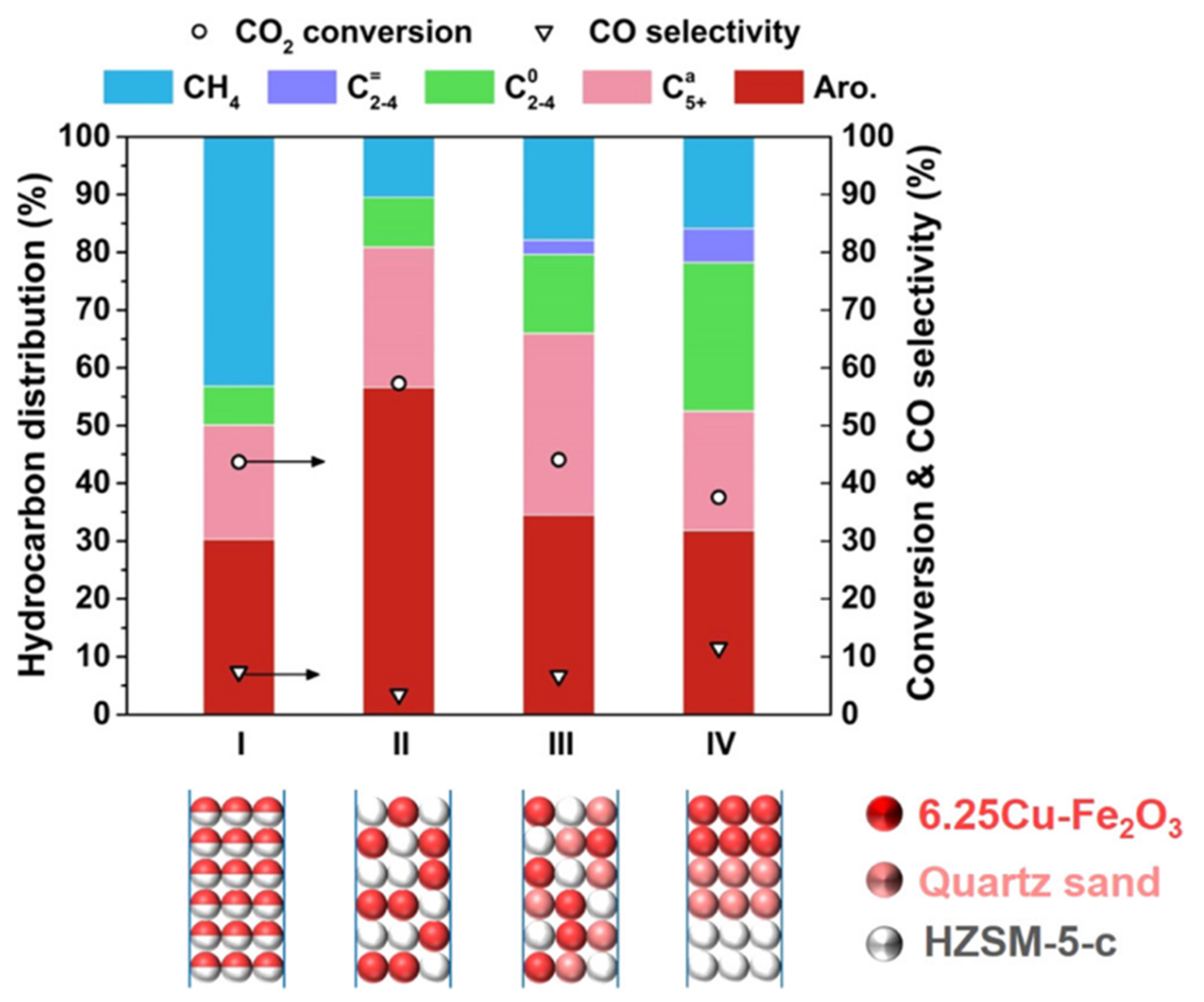
3.3.5. Mass Ratio and Mixing Mode of Different Active Components
4. Reaction Mechanism
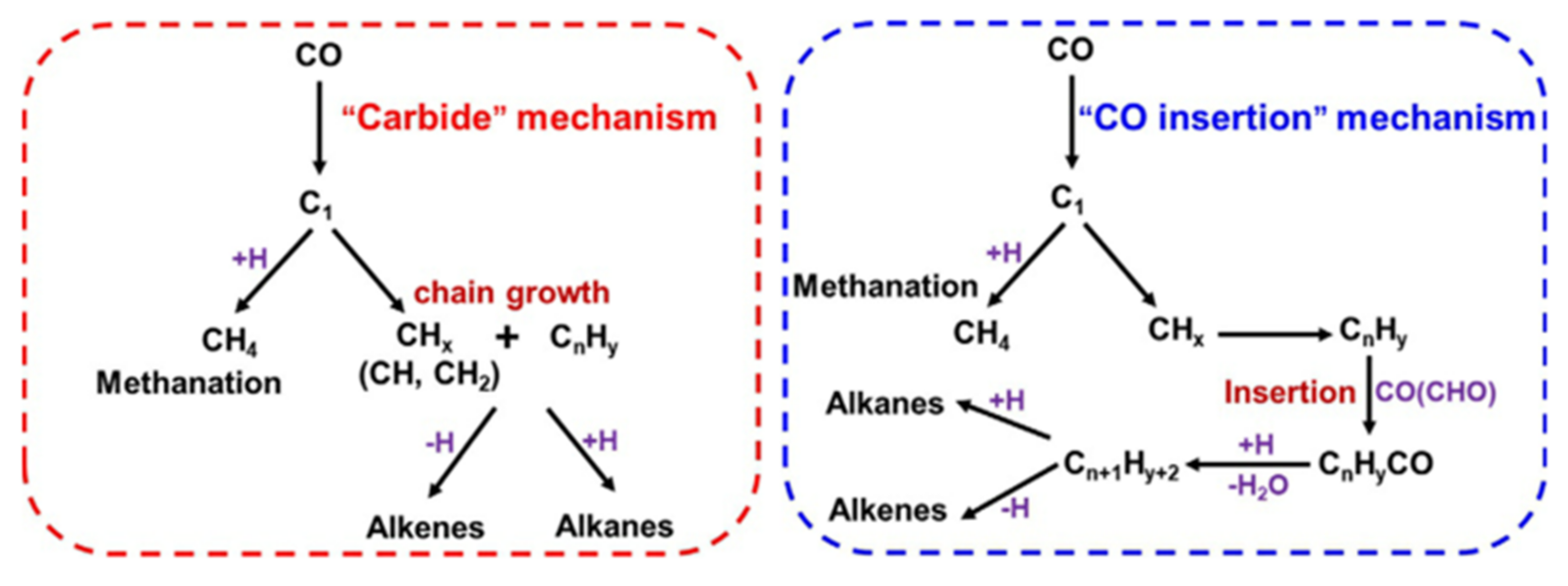
4.1. Mechanistic Study of the Hydrogenation of CO2 to Liquid Fuels
4.2. Mechanistic Study of the Hydrogenation of CO2 to Aromatics
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IEA. World CO2 emissions from fuel combustion by region, 1971–2018. In CO₂ Emissions from Fuel Combustion; IEA: Paris, France, 2020; Available online: https://www.iea.org/data-and-statistics/charts/world-CO2-emissions-from-fuel-combustion-by-region-1971-2018 (accessed on 14 March 2022).
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [Green Version]
- Rogelj, J.; den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, G. Carbon Dioxide Hydrogenation into Higher Hydrocarbons and Oxygenates: Thermodynamic and Kinetic Bounds and Progress with Heterogeneous and Homogeneous Catalysis. ChemSusChem 2017, 10, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 2010, 3, 884–890. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products-A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel Heterogeneous Catalysts for CO2 Hydrogenation to Liquid Fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Janke, C.; Duyar, M.S.; Hoskins, M.; Farrauto, R. Catalytic and adsorption studies for the hydrogenation of CO2 to methane. Appl. Catal. B Environ. 2014, 152–153, 184–191. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Zhong, Z.; Teo, J.; Gofer, Y.; Gedanken, A. Methanation of Carbon Dioxide on Ni Catalysts on Mesoporous ZrO2 Doped with Rare Earth Oxides. Catal. Lett. 2009, 130, 455–462. [Google Scholar] [CrossRef]
- Zhong, H.; Yao, G.; Cui, X.; Yan, P.; Wang, X.; Jin, F. Selective conversion of carbon dioxide into methane with a 98% yield on an in situ formed Ni nanoparticle catalyst in water. Chem. Eng. J. 2019, 357, 421–427. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Zhu, J.; Song, C.; Guo, X. Direct Transformation of Carbon Dioxide to Value-Added Hydrocarbons by Physical Mixtures of Fe5C2 and K-Modified Al2O3. Ind. Eng. Chem. Res. 2018, 57, 9120–9126. [Google Scholar] [CrossRef]
- Xu, Z.; McNamara, N.D.; Neumann, G.T.; Schneider, W.F.; Hicks, J.C. Catalytic Hydrogenation of CO2 to Formic Acid with Silica-Tethered Iridium Catalysts. ChemCatChem 2013, 5, 1769–1771. [Google Scholar] [CrossRef]
- Hao, C.; Wang, S.; Li, M.; Kang, L.; Ma, X. Hydrogenation of CO2 to formic acid on supported ruthenium catalysts. Catal. Today 2011, 160, 184–190. [Google Scholar] [CrossRef]
- Mori, K.; Taga, T.; Yamashita, H. Isolated Single-Atomic Ru Catalyst Bound on a Layered Double Hydroxide for Hydrogenation of CO2 to Formic Acid. ACS Catal. 2017, 7, 3147–3151. [Google Scholar] [CrossRef]
- Nie, X.; Li, W.; Jiang, X.; Guo, X.; Song, C. Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons. Adv. Catal. 2019, 65, 121–233. [Google Scholar]
- Nezam, I.; Zhou, W.; Gusmão, G.S.; Realff, M.J.; Wang, Y.; Medford, A.J.; Jones, C.W. Direct aromatization of CO2 via combined CO2 hydrogenation and zeolite-based acid catalysis. J. CO2 Util. 2021, 45, 101405. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Martinez-Espin, J.S.; Mortén, M.; Janssens, T.V.W.; Svelle, S.; Beato, P.; Olsbye, U. New insights into catalyst deactivation and product distribution of zeolites in the methanol-to-hydrocarbons (MTH) reaction with methanol and dimethyl ether feeds. Catal. Sci. Technol. 2017, 7, 2700–2716. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Han, Y.; Ge, Q.; Sun, J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem. Soc. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kangvansura, P.; Chew, L.M.; Saengsui, W.; Santawaja, P.; Poo-arporn, Y.; Muhler, M.; Schulz, H.; Worayingyong, A. Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N-functionalized carbon nanotubes. Catal. Today 2016, 275, 59–65. [Google Scholar] [CrossRef]
- Chew, L.M.; Kangvansura, P.; Ruland, H.; Schulte, H.J.; Somsen, C.; Xia, W.; Eggeler, G.; Worayingyong, A.; Muhler, M. Effect of nitrogen doping on the reducibility, activity and selectivity of carbon nanotube-supported iron catalysts applied in CO2 hydrogenation. Appl. Catal. A Gen. 2014, 482, 163–170. [Google Scholar] [CrossRef]
- Cui, M.; Qian, Q.; Zhang, J.; Wang, Y.; Asare Bediako, B.B.; Liu, H.; Han, B. Liquid fuel synthesis via CO2 hydrogenation by coupling homogeneous and heterogeneous catalysis. Chem 2021, 7, 726–737. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; Petrus van Bavel, A.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct conversion of CO2 to a jet fuel over CoFe alloy catalysts. Innovation 2021, 2, 100170. [Google Scholar] [CrossRef]
- Jo, H.; Khan, M.K.; Irshad, M.; Arshad, M.W.; Kim, S.K.; Kim, J. Unraveling the role of cobalt in the direct conversion of CO2 to high-yield liquid fuels and lube base oil. Appl. Catal. B Environ. 2022, 305, 121041. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, H.; Gao, P.; Chen, X.; Liu, H.; Zhong, L.; Wang, H.; Wei, W.; Sun, Y. Effect of alkali metals on the performance of CoCu/TiO2 catalysts for CO2 hydrogenation to long-chain hydrocarbons. Chin. J. Catal. 2018, 39, 1294–1302. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, H.; Gao, P.; Li, X.; Zhong, L.; Wang, H.; Liu, H.; Wei, W.; Sun, Y. Direct conversion of CO2 to long-chain hydrocarbon fuels over K-promoted CoCu/TiO2 catalysts. Catal. Today 2018, 311, 65–73. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Isaeva, V.I.; Tkachenko, O.P.; Chernyshev, V.V.; Kustov, L.M. Conversion of CO2 into liquid hydrocarbons in the presence of a Co-containing catalyst based on the microporous metal-organic framework MIL-53(Al). Fuel Processing Technol. 2018, 176, 101–106. [Google Scholar] [CrossRef]
- He, Z.; Cui, M.; Qian, Q.; Zhang, J.; Han, B. Synthesis of liquid fuel via direct hydrogenation of CO2. Proc. Natl. Acad. Sci. USA 2019, 116, 12654–12659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calizzi, M.; Mutschler, R.; Patelli, N.; Migliori, A.; Zhao, K.; Pasquini, L.; Zuettel, A. CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts. Nanomaterials 2020, 10, 1360. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, S.-J.; Kang, S.-H.; Lee, Y.-J.; Jun, K.-W.; Rhee, Y.-W. Effect of Cu content on the bifunctional Fischer–Tropsch Fe–Cu–K/ZSM5 catalyst. J. Ind. Eng. Chem. 2009, 15, 798–802. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Han, S.J.; Park, H.-G.; Lee, H.; An, K.; Jun, K.-W.; Kim, S.K. Atomically Alloyed Fe–Co Catalyst Derived from a N-Coordinated Co Single-Atom Structure for CO2 Hydrogenation. ACS Catal. 2021, 11, 2267–2278. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Bimetallic Fe–Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 2013, 3–4, 102–106. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, H.; Yu, G.; He, S.; Kang, J.; Liu, Z.; Cheng, K.; Zhang, Q.; Wang, Y. Selective hydrogenation of CO2 and CO into olefins over Sodium- and Zinc-Promoted iron carbide catalysts. J. Catal. 2021, 395, 350–361. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Tang, X.; Yin, H.; Kang, J.; Zhang, Q.; Wang, Y. Zn and Na promoted Fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105. [Google Scholar] [CrossRef]
- Choi, P.H.; Jun, K.-W.; Lee, S.-J.; Choi, M.-J.; Lee, K.-W. Hydrogenation of carbon dioxide over alumina supported Fe-K catalysts. Catal. Lett. 1996, 40, 115–118. [Google Scholar] [CrossRef]
- Albrecht, M.; Rodemerck, U.; Schneider, M.; Broering, M.; Baabe, D.; Kondratenko, E.V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal. B Environ. 2017, 204, 119–126. [Google Scholar] [CrossRef]
- Geng, S.; Jiang, F.; Xu, Y.; Liu, X. Iron-based Fischer-Tropsch synthesis for the efficient conversion of carbon dioxide into isoparaffins. ChemCatChem 2016, 8, 1303–1307. [Google Scholar] [CrossRef]
- Wang, J.; You, Z.; Zhang, Q.; Deng, W.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Xie, T.; Wang, J.; Ding, F.; Zhang, A.; Li, W.; Guo, X.; Song, C. CO2 hydrogenation to hydrocarbons over alumina-supported iron catalyst: Effect of support pore size. J. CO2 Util. 2017, 19, 202–208. [Google Scholar] [CrossRef]
- Boreriboon, N.; Jiang, X.; Song, C.; Prasassarakich, P. Higher Hydrocarbons Synthesis from CO2 Hydrogenation Over K- and La-Promoted Fe-Cu/TiO2 Catalysts. Top. Catal. 2018, 61, 1551–1562. [Google Scholar] [CrossRef]
- Sai Prasad, P.S.; Bae, J.W.; Jun, K.-W.; Lee, K.-W. Fischer–Tropsch Synthesis by Carbon Dioxide Hydrogenation on Fe-Based Catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Han, S.J.; Min, J.E.; Park, H.-G.; Jun, K.-W.; Kim, S.K. Mechanistic insights into Cu and K promoted Fe-catalyzed production of liquid hydrocarbons via CO2 hydrogenation. J. CO2 Util. 2019, 34, 522–532. [Google Scholar] [CrossRef]
- Khan, M.K.; Butolia, P.; Jo, H.; Irshad, M.; Han, D.; Nam, K.-W.; Kim, J. Selective Conversion of Carbon Dioxide into Liquid Hydrocarbons and Long-Chain α-Olefins over Fe-Amorphous AlOx Bifunctional Catalysts. ACS Catal. 2020, 10, 10325–10338. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu-Fe Interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Wang, X.; Song, C. Fe-Cu Bimetallic Catalysts for Selective CO2 Hydrogenation to Olefin-Rich C2+ Hydrocarbons. Ind. Eng. Chem. Res. 2018, 57, 4535–4542. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Ning, W.; Li, B.; Wang, B.; Yang, X.; Jin, Y. Enhanced Production of C5+ Hydrocarbons from CO2 Hydrogenation by the Synergistic Effects of Pd and K on γ-Fe2O3 Catalyst. Catal. Lett. 2019, 149, 431–440. [Google Scholar] [CrossRef]
- Ma, D.; Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. 2020, 132, 21920–21928. [Google Scholar]
- Liang, B.; Sun, T.; Ma, J.; Duan, H.; Li, L.; Yang, X.; Zhang, Y.; Su, X.; Huang, Y.; Zhang, T. Mn decorated Na/Fe catalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2019, 9, 456–464. [Google Scholar] [CrossRef]
- Yao, B.; Xiao, T.; Makgae, O.A.; Jie, X.; Gonzalez-Cortes, S.; Guan, S.; Kirkland, A.I.; Dilworth, J.R.; Al-Megren, H.A.; Alshihri, S.M.; et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 6395. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Gao, W.; Chen, F.; Wu, X.; Wang, K.; He, Y.; Zhang, P.; Yang, G.; Tsubaki, N. Multi-Promoters Regulated Iron Catalyst with Well-Matching Reverse Water-Gas Shift and Chain Propagation for Boosting CO2 Hydrogenation. J. CO2 Util. 2021, 52, 101700. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, B.; Geng, S.; Xu, Y.; Liu, X. Hydrogenation of CO2 into hydrocarbons: Enhanced catalytic activity over Fe-based Fischer-Tropsch catalysts. Catal. Sci. Technol. 2018, 8, 4097–4107. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.-N.; Choi, S.H.; Jang, J.-H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective Production of Aromatics Directly from Carbon Dioxide Hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Jiang, X.; Zhang, A.; Song, C.; Guo, X. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Liu, M.; Hu, S.; Ding, F.; Song, C.; Guo, X. Fe-MOF-derived highly active catalysts for carbon dioxide hydrogenation to valuable hydrocarbons. J. CO2 Util. 2017, 21, 100–107. [Google Scholar] [CrossRef]
- Kosol, R.; Guo, L.; Kodama, N.; Zhang, P.; Reubroycharoen, P.; Vitidsant, T.; Taguchi, A.; Abe, T.; Chen, J.; Yang, G.; et al. Iron catalysts supported on nitrogen functionalized carbon for improved CO2 hydrogenation performance. Catal. Commun. 2021, 149, 106216. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ji, X.; Wei, J.; Wen, Z.; Yao, R.; Xu, H.; Ge, Q. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 11. [Google Scholar] [CrossRef]
- Wang, S.; Wu, T.; Lin, J.; Ji, Y.; Yan, S.; Pei, Y.; Xie, S.; Zong, B.; Qiao, M. Iron–Potassium on Single-Walled Carbon Nanotubes as Efficient Catalyst for CO2 Hydrogenation to Heavy Olefins. ACS Catal. 2020, 10, 6389–6401. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Maksimov, S.V.; Maslakov, K.I.; Chernavskii, P.A.; Pokusaeva, Y.A.; Koklin, A.E.; Bogdan, V.I.; Savilov, S.V. Sintered Fe/CNT framework catalysts for CO2 hydrogenation into hydrocarbons. Carbon 2020, 168, 475–484. [Google Scholar] [CrossRef]
- Williamson, D.L.; Herdes, C.; Torrente-Murciano, L.; Jones, M.D.; Mattia, D. N-Doped Fe@CNT for Combined RWGS/FT CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 7395–7402. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Zhang, C.; Han, S.J.; Park, H.-G.; Kim, Y.T.; Yang, S.; Jun, K.-W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [Green Version]
- Noreen, A.; Li, M.; Fu, Y.; Amoo, C.C.; Wang, J.; Maturura, E.; Du, C.; Yang, R.; Xing, C.; Sun, J. One-Pass Hydrogenation of CO2 to Multibranched Isoparaffins over Bifunctional Zeolite-Based Catalysts. ACS Catal. 2020, 10, 14186–14194. [Google Scholar] [CrossRef]
- Guo, L.; Sun, S.; Li, J.; Gao, W.; Zhao, H.; Zhang, B.; He, Y.; Zhang, P.; Yang, G.; Tsubaki, N. Boosting liquid hydrocarbons selectivity from CO2 hydrogenation by facilely tailoring surface acid properties of zeolite via a modified Fischer-Tropsch synthesis. Fuel 2021, 306, 121684. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wen, Z.; Fang, C.; Ge, Q.; Xu, H. New insights into the effect of sodium on Fe3O4- based nanocatalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2016, 6, 4786–4793. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Infantes-Molina, A.; Lietti, L.; Forzatti, P.; Iaquaniello, G.; Palo, E.; Picutti, B.; Brignoli, F. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. Appl. Catal. B Environ. 2017, 200, 530–542. [Google Scholar] [CrossRef]
- Boreriboon, N.; Jiang, X.; Song, C.; Prasassarakich, P. Fe-based bimetallic catalysts supported on TiO2 for selective CO2 hydrogenation to hydrocarbons. J. CO2 Util. 2018, 25, 330–337. [Google Scholar] [CrossRef]
- Dry, M.E.; Shingles, T.; Boshoff, L.J.; Oosthuizen, G.J. Heats of chemisorption on promoted iron surfaces and the role of alkali in Fischer-Tropsch synthesis. J. Catal. 1969, 15, 190–199. [Google Scholar] [CrossRef]
- Douglass, G.; Miller, M.M. A study of the effects of potassium addition to supported iron catalysts in the Fischer-Tropsch reaction. J. Phys. Chem. B 1988, 92, 6081–6085. [Google Scholar]
- Bukur, D.B.; Mukesh, D.; Patel, S.A. Promoter effects on precipitated iron catalysts for Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 1990, 29, 194–204. [Google Scholar] [CrossRef]
- Al-Dossary, M.; Ismail, A.A.; Fierro, J.L.G.; Bouzid, H.; Al-Sayari, S.A. Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction. Appl. Catal. B Environ. 2015, 165, 651–660. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Koeken, A.C.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.-W.; Xu, Y.-Y.; Bai, L.; Li, Y.-W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Y.; Liu, R.; Li, X.; Ullah, N.; Li, Z. CO2 hydrogenation to C5+ hydrocarbons over K-promoted Fe/CNT catalyst: Effect of potassium on structure–activity relationship. Appl. Organomet. Chem. 2021, 35, e6253. [Google Scholar] [CrossRef]
- Falbo, L.; Martinelli, M.; Visconti, C.G.; Lietti, L.; Forzatti, P.; Bassano, C.; Deiana, P. Effects of Zn and Mn Promotion in Fe-Based Catalysts Used for COx Hydrogenation to Long-Chain Hydrocarbons. Ind. Eng. Chem. Res. 2017, 56, 13147–13157. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Zeng, Y.; Kosol, R.; Cui, Y.; Kodama, N.; Guo, X.; Prasert, R.; Tharapong, V.; Liu, G.; et al. Heteroatom doped iron-based catalysts prepared by urea self-combustion method for efficient CO2 hydrogenation. Fuel 2020, 276, 118102. [Google Scholar] [CrossRef]
- Numpilai, T.; Chanlek, N.; Poo-Arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Chareonpanich, M.; Kongkachuichay, P.; Yigit, N.; Rupprechter, G.; et al. Tuning Interactions of Surface-adsorbed Species over Fe−Co/K−Al2O3 Catalyst by Different K Contents: Selective CO2 Hydrogenation to Light Olefins. ChemCatChem 2020, 12, 3306–3320. [Google Scholar] [CrossRef]
- Gong, W.; Ye, R.-P.; Ding, J.; Wang, T.; Shi, X.; Russell, C.K.; Tang, J.; Eddings, E.G.; Zhang, Y.; Fan, M. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission. Appl. Catal. B Environ. 2020, 278, 119302. [Google Scholar] [CrossRef]
- Velu, S.; Gangwal, S.K. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption. Solid State Ion. 2006, 177, 803–811. [Google Scholar] [CrossRef]
- Smeds, S.; Salmi, T.; Lindfors, L.P.; Krause, O. Chemisorption and TPD studies of hydrogen on Ni/Al2O3. Appl. Catal. A Gen. 1996, 144, 177–194. [Google Scholar] [CrossRef]
- Willauer, H.D.; Ananth, R.; Olsen, M.T.; Drab, D.M.; Hardy, D.R.; Williams, F.W. Modeling and kinetic analysis of CO2 hydrogenation using a Mn and K-promoted Fe catalyst in a fixed-bed reactor. J. CO2 Util. 2013, 3–4, 56–64. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, A.; Liu, M.; Zuo, Y.; Li, K.; Guo, X.; Song, C. CO2 Hydrogenation to Hydrocarbons over Iron-based Catalyst: Effects of Physicochemical Properties of Al2O3 Supports. Ind. Eng. Chem. Res. 2014, 53, 17563–17569. [Google Scholar] [CrossRef]
- Shi, Y.; Hou, S.; Qiu, X.; Zhao, B. MOFs-Based Catalysts Supported Chemical Conversion of CO2. In Metal-Organic Framework; Springer: Cham, Switzerland, 2020; pp. 373–426. [Google Scholar]
- Cui, W.G.; Zhang, G.Y.; Hu, T.L.; Bu, X.H. Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4. Coord. Chem. Rev. 2019, 387, 79–120. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Imbert, F.E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl. Catal. A Gen. 2013, 452, 117–131. [Google Scholar] [CrossRef]
- Purohit, R.D.; Sharma, B.P.; Pillai, K.T.; Tyagi, A.K. Ultrafine ceria powders via glycine-nitrate combustion. Mater. Res. Bull. 2001, 36, 2711–2721. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, X.; Hu, B.; Zhang, J.; Hao, Q.; Chen, H.; Guo, X.; Ma, X. Hydrogenation of CO2 to Aromatics over Fe–K/Alkaline Al2O3 and P/ZSM-5 Tandem Catalysts. Ind. Eng. Chem. Res. 2020, 59, 19194–19202. [Google Scholar] [CrossRef]
- Liang, J.; Guo, L.; Gao, W.; Wang, C.; Guo, X.; He, Y.; Yang, G.; Tsubaki, N. Direct Conversion of CO2 to Aromatics over K–Zn–Fe/ZSM-5 Catalysts via a Fischer–Tropsch Synthesis Pathway. Ind. Eng. Chem. Res. 2022, 61, 10336–10346. [Google Scholar] [CrossRef]
- Abelló, S.; Montané, D. Exploring Iron-based Multifunctional Catalysts for Fischer–Tropsch Synthesis: A Review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef]
- Chen, J.; Liang, T.; Li, J.; Wang, S.; Qin, Z.; Wang, P.; Huang, L.; Fan, W.; Wang, J. Regulation of Framework Aluminum Siting and Acid Distribution in H-MCM-22 by Boron Incorporation and Its Effect on the Catalytic Performance in Methanol to Hydrocarbons. ACS Catal. 2016, 6, 2299–2313. [Google Scholar] [CrossRef]
- Bjørgen, M.; Akyalcin, S.; Olsbye, U.; Benard, S.; Kolboe, S.; Svelle, S. Methanol to hydrocarbons over large cavity zeolites: Toward a unified description of catalyst deactivation and the reaction mechanism. J. Catal. 2010, 275, 170–180. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Wen, Z.; Ji, X.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Catalytic Hydrogenation of CO2 to Isoparaffins over Fe-Based Multifunctional Catalysts. ACS Catal. 2018, 8, 9958–9967. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Maitlis, P.M.; Zanotti, V. The role of electrophilic species in the Fischer–Tropsch reaction. Chem. Commun. 2009, 13, 1619–1634. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Liu, B.; Wang, T.; Zheng, J.; Li, W.; Liu, D.; Liu, X. Selective production of aromatics from CO2. Catal. Sci. Technol. 2019, 9, 593–610. [Google Scholar] [CrossRef]
- Jiang, J.; Wen, C.; Tian, Z.; Wang, Y.; Zhai, Y.; Chen, L.; Li, Y.; Liu, Q.; Wang, C.; Ma, L. Manganese-Promoted Fe3O4 Microsphere for Efficient Conversion of CO2 to Light Olefins. Ind. Eng. Chem. Res. 2020, 59, 2155–2162. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gascon, J. Metal Organic Framework-Derived Iron Catalysts for the Direct Hydrogenation of CO2 to Short Chain Olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Lyons, T.W.; Guironnet, D.; Findlater, M.; Brookhart, M. Synthesis of p-Xylene from Ethylene. J. Am. Chem. Soc. 2012, 134, 15708–15711. [Google Scholar] [CrossRef]
- Shen, X.Q.; Kang, J.C.; Niu, W.; Wang, M.H.; Zhang, Q.H.; Wang, Y. Impact of hierarchical pore structure on the catalytic performances of MFI zeolites modified by ZnO for the conversion of methanol to aromatics. Catal. Sci. Technol. 2017, 7, 3598–3612. [Google Scholar] [CrossRef]
- Niziolek, A.M.; Onel, O.; Floudas, C.A. Production of benzene, toluene, and xylenes from natural gas via methanol: Process synthesis and global optimization. AIChE J. 2016, 62, 1531–1556. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, D.; Zhang, T.; Huang, Y. Direct conversion of syngas to aromatics: A review of recent studies. Chin. J. Catal. 2020, 41, 561–573. [Google Scholar] [CrossRef]
- Wen, C.; Jiang, J.; Cai, C.; Tian, Z.; Xu, X.; Wu, J.; Wang, C.; Ma, L. Single-Step Selective Conversion of Carbon Dioxide to Aromatics over Na-Fe3O4/Hierarchical HZSM-5 Zeolite Catalyst. Energy Fuels 2020, 34, 11282–11289. [Google Scholar] [CrossRef]
- Wang, S.W.; Wu, T.J.; Lin, J.; Tian, J.; Ji, Y.S.; Pei, Y.; Yan, S.R.; Qiao, M.H.; Xu, H.L.; Zong, B.N. FeK on 3D Graphene-Zeolite Tandem Catalyst with High Efficiency and Versatility in Direct CO2 Conversion to Aromatics. ACS Sustain. Chem. Eng. 2019, 7, 17825–17833. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B Environ. 2021, 283, 119648. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Yan, P.; Nawaz, M.A.; Liu, D. High Conversion to Aromatics via CO2-FT over a CO-Reduced Cu-Fe2O3 Catalyst Integrated with HZSM-5. ACS Catal. 2020, 10, 11268–11279. [Google Scholar] [CrossRef]
- Sibi, M.G.; Khan, M.K.; Verma, D.; Yoon, W.; Kim, J. High-yield synthesis of BTEX over Na-FeAlOx/Zn-HZSM-5@SiO2 by direct CO2 conversion and identification of surface intermediates. Appl. Catal. B Environ. 2022, 301, 120813. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Wu, Q.; Wang, C.; Guo, X.; He, Y.; Zhang, P.; Yang, G.; Liu, G.; Wu, J.; et al. Capsule-like zeolite catalyst fabricated by solvent-free strategy for para-Xylene formation from CO2 hydrogenation. Appl. Catal. B Environ. 2022, 303, 120906. [Google Scholar] [CrossRef]
- Ramirez, A.; Chowdhury, A.D.; Dokania, A.; Cnudde, P.; Caglapn, M.; Yarulina, I.; Abou-Hamad, E.; Gevers, L.; Ould-Chikh, S.; De Wispelaere, K.; et al. Effect of Zeolite Topology and Reactor Configuration on the Direct Conversion of CO2 to Light Olefins and Aromatics. ACS Catal. 2019, 9, 6320–6334. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Hong, S.Y.; Dong, H.C.; Yang, J.I.; Jung, H.; Ji, C.P. A new synthesis of carbon encapsulated Fe5C2 nanoparticles for high-temperature Fischer–Tropsch synthesis. Nanoscale 2015, 7, 16616–16620. [Google Scholar] [CrossRef]
- You, Z.; Deng, W.; Zhang, Q.; Wang, Y. Hydrogenation of carbon dioxide to light olefins over non-supported iron catalyst. Chin. J. Catal. 2013, 34, 956–963. [Google Scholar] [CrossRef]
- Ngantsoue-Hoc, W.; Zhang, Y.; O’Brien, R.J.; Luo, M.; Davis, B.H. Fischer−Tropsch synthesis: Activity and selectivity for Group I alkali promoted iron-based catalysts. Appl. Catal. A Gen. 2002, 236, 77–89. [Google Scholar] [CrossRef]
- Rongxian, B.; Yisheng, T.; Yizhuo, H. Study on the carbon dioxide hydrogenation to iso-alkanes over Fe–Zn–M/zeolite composite catalysts. Fuel Processing Technol. 2004, 86, 293–301. [Google Scholar] [CrossRef]
- Wu, T.J.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.W.; Xie, S.H.; Pei, Y.; Yan, S.R.; Qiao, M.H.; Xu, H.L.; et al. Porous Graphene-Confined Fe-K as Highly Efficient Catalyst for CO2 Direct Hydrogenation to Light Olefins. ACS Appl. Mater. Inter. 2018, 10, 23439–23443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D Honeycomb-Like Structured Graphene and Its High Efficiency as a Counter-Electrode Catalyst for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, M.; Chen, S.; Wang, X.; Zhou, Z.; Wu, Y.; Zhang, T.; Yang, G.; Han, Y.; Tan, Y. Hydrogenation of CO2 into aromatics over a ZnCrOx-zeolite composite catalyst. Chem. Commun. 2019, 55, 973–976. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Liu, X. Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites. Appl. Catal. A Gen. 2018, 552, 168–183. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Zhang, K.; Chen, G.; Yang, Y.; Liu, S.; Ding, C.; Meng, Y.; Liu, P. Hierarchical ZSM-5 zeolite synthesized via dry gel conversion-steam assisted crystallization process and its application in aromatization of methanol. Powder Technol. 2018, 328, 415–429. [Google Scholar] [CrossRef]
- Hirota, Y.; Murata, K.; Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Dry gel conversion synthesis of SAPO-34 nanocrystals. Mater. Chem. Phys. 2010, 123, 507–509. [Google Scholar] [CrossRef]
- Meng, Y.; Genuino, H.C.; Kuo, C.-H.; Huang, H.; Chen, S.-Y.; Zhang, L.; Rossi, A.; Suib, S.L. One-Step Hydrothermal Synthesis of Manganese-Containing MFI-Type Zeolite, Mn–ZSM-5, Characterization, and Catalytic Oxidation of Hydrocarbons. J. Am. Chem. Soc. 2013, 135, 8594–8605. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Zhang, K.; Feng, W.; Liu, S.; Ding, C.; Liu, P. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics. Microporous Mesoporous Mater. 2017, 247, 103–115. [Google Scholar] [CrossRef]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, W.; Rao, Y.; Li, Z.; Tsubaki, N.; Wu, M. Cation modulating electrocatalyst derived from bimetallic metal–organic frameworks for overall water splitting. J. Mater. Chem. A 2017, 5, 6170–6177. [Google Scholar] [CrossRef]
- Zhang, C.; Kwak, G.; Park, H.-G.; Jun, K.-W.; Lee, Y.-J.; Kang, S.C.; Kim, S. Light hydrocarbons to BTEX aromatics over hierarchical HZSM-5: Effects of alkali treatment on catalytic performance. Microporous Mesoporous Mater. 2019, 276, 292–301. [Google Scholar] [CrossRef]
- Zhang, C.; Kwak, G.; Lee, Y.-J.; Jun, K.-W.; Gao, R.; Park, H.-G.; Kim, S.; Min, J.-E.; Kang, S.C.; Guan, G. Light hydrocarbons to BTEX aromatics over Zn-modified hierarchical ZSM-5 combined with enhanced catalytic activity and stability. Microporous Mesoporous Mater. 2019, 284, 316–326. [Google Scholar] [CrossRef]
- Stepanov, A.G.; Arzumanov, S.S.; Gabrienko, A.A.; Parmon, V.N.; Ivanova, I.I.; Freude, D. Significant influence of Zn on activation of the C-H bonds of small alkanes by Bronsted acid sites of zeolite. Chemphyschem 2008, 9, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chu, Y.; Qi, G.; Wang, Q.; Gao, W.; Wang, X.; Li, S.; Xu, J.; Deng, F. Probing the active sites for methane activation on Ga/ZSM-5 zeolites with solid-state NMR spectroscopy. Chem. Commun. 2020, 56, 12029–12032. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgärtl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis–Brønsted Acid Pairs in Ga/H-ZSM-5 To Catalyze Dehydrogenation of Light Alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Saito, H.; Sekine, Y.; Hensen, E.J.M.; Kosinov, N. Different mechanisms of ethane aromatization over Mo/ZSM-5 and Ga/ZSM-5 catalysts. Catal. Today 2021, 369, 184–192. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-Xylene Selectivity in Making Aromatics from Methanol with a Surface-Modified Zn/P/ZSM-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Miao, D.; Ding, Y.; Yu, T.; Li, J.; Pan, X.; Bao, X. Selective Synthesis of Benzene, Toluene, and Xylenes from Syngas. ACS Catal. 2020, 10, 7389–7397. [Google Scholar] [CrossRef]
- Baertsch, C.D.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Permeation of Aromatic Hydrocarbon Vapors through Silicalite−Zeolite Membranes. J. Phys. Chem. 1996, 100, 7676–7679. [Google Scholar] [CrossRef]
- Zheng, S.; Heydenrych, H.R.; Jentys, A.; Lercher, J.A. Influence of Surface Modification on the Acid Site Distribution of HZSM-5. J. Phys. Chem. B 2002, 106, 9552–9558. [Google Scholar] [CrossRef]
- Gao, J.J.; Wang, Y.L.; Ping, Y.; Hu, D.C.; Xu, G.W.; Gu, F.N.; Su, F.B. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Moon, S.; Chae, H.-J.; Park, M.B. Oligomerization of light olefins over ZSM-5 and beta zeolite catalysts by modifying textural properties. Appl. Catal. A Gen. 2018, 553, 15–23. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Wang, J.; Liu, H.; Li, M.; Miao, S.; Li, C. Highly Selective Conversion of Carbon Dioxide to Aromatics over Tandem Catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef] [Green Version]
- Zecevic, J.; Vanbutsele, G.; de Jong, K.P.; Martens, J.A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 2015, 528, 245–248. [Google Scholar] [CrossRef]
- Cheng, K.; Gu, B.; Liu, X.L.; Kang, J.C.; Zhang, Q.H.; Wang, Y. Direct and Highly Selective Conversion of Synthesis Gas into Lower Olefins: Design of a Bifunctional Catalyst Combining Methanol Synthesis and Carbon-Carbon Coupling. Angew. Chem. 2016, 128, 4803–4806. [Google Scholar] [CrossRef]
- Li, M.Z.; Nawaz, M.A.; Song, G.Y.; Zaman, W.Q.; Liu, D.H. Influential Role of Elemental Migration in a Composite Iron-Zeolite Catalyst for the Synthesis of Aromatics from Syngas. Ind. Eng. Chem. Res. 2020, 59, 9043–9054. [Google Scholar] [CrossRef]
- Su, T.; Qin, Z.; Huang, G.; Ji, H.; Jiang, Y.; Chen, J. Density functional theory study on the interaction of CO2 with Fe3O4(111) surface. Appl. Surf. Sci. 2016, 378, 270–276. [Google Scholar] [CrossRef]
- Hakim, A.; Marliza, T.S.; Abu Tahari, N.M.; Wan Isahak, R.W.N.; Yusop, R.M.; Mohamed Hisham, W.M.; Yarmo, A.M. Studies on CO2 Adsorption and Desorption Properties from Various Types of Iron Oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888–7897. [Google Scholar] [CrossRef]
- Kattel, S.P.; Liu, P.; Chen, J. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Pham, T.H.; Qi, Y.; Yang, J.; Duan, X.; Qian, G.; Zhou, X.; Chen, D.; Yuan, W. Insights into Hägg Iron-Carbide-Catalyzed Fischer–Tropsch Synthesis: Suppression of CH4 Formation and Enhancement of C–C Coupling on χ-Fe5C2 (510). ACS Catal. 2015, 5, 2203–2208. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.B.; Argyle, M.D.; Zhong, Q.; Wang, Y.J.; Russell, C.K.; Xu, Z.H.; Russell, A.G.; Li, Q.H.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Duan, X.; Qian, G.; Zhou, X.; Chen, D. CO Activation Pathways of Fischer–Tropsch Synthesis on χ-Fe5C2 (510): Direct versus Hydrogen-Assisted CO Dissociation. J. Phys. Chem. C 2014, 118, 10170–10176. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Janik, M.J.; Chen, Y.; Guo, X.; Song, C. Mechanistic Insight into C–C Coupling over Fe–Cu Bimetallic Catalysts in CO2 Hydrogenation. J. Phys. Chem. C 2017, 121, 13164–13174. [Google Scholar] [CrossRef]
- van Santen, R.A.; Markvoort, A.J.; Filot, I.A.W.; Ghouri, M.M.; Hensen, E.J.M. Mechanism and microkinetics of the Fischer-Tropsch reaction. Phys. Chem. Chem. Phys. 2013, 15, 17038–17063. [Google Scholar] [CrossRef] [Green Version]
- Helden, P.V.; Berg, J.A.V.D.; Petersen, M.A.; Rensburg, W.J.V.; Ciobc, I.M.; Loosdrecht, J.V.D. Computational investigation of the kinetics and mechanism of the initial steps of the Fischer–Tropsch synthesis on cobalt. Faraday Discuss. 2017, 197, 117–151. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, C.; Mao, Y.; Hu, P. Quantitative Determination of C–C Coupling Mechanisms and Detailed Analyses on the Activity and Selectivity for Fischer–Tropsch Synthesis on Co(0001): Microkinetic Modeling with Coverage Effects. ACS Catal. 2019, 9, 5957–5973. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, J.; Holmen, A.; Chen, D. Investigation of C1 + C1 Coupling Reactions in Cobalt-Catalyzed Fischer-Tropsch Synthesis by a Combined DFT and Kinetic Isotope Study. Catalysts 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Asiaee, A.; Benjamin, K.M. A density functional theory based elementary reaction mechanism for early steps of Fischer-Tropsch synthesis over cobalt catalyst. 1. Reaction kinetics. Mol. Catal. 2017, 436, 218–227. [Google Scholar] [CrossRef]
- Wang, B.; Liang, D.; Zhang, R.; Ling, L. Crystal Facet Dependence for the Selectivity of C2 Species over Co2C Catalysts in the Fischer–Tropsch Synthesis. J. Phys. Chem. C 2018, 122, 29249–29258. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhang, R.G.; Ling, L.X.; Wang, Q.; Wang, B.J.; Li, D.B. Insight into the preferred formation mechanism of long-chain hydrocarbons in Fischer-Tropsch synthesis on Hcp Co(10–11) surfaces from DFT and microkinetic modeling. Catal. Sci. Technol. 2017, 7, 3758–3776. [Google Scholar] [CrossRef]
- Batchu, R.; Galvita, V.V.; Alexopoulos, K.; Van der Borght, K.; Poelman, H.; Reyniers, M.-F.; Marin, G.B. Role of intermediates in reaction pathways from ethene to hydrocarbons over H-ZSM-5. Appl. Catal. A Gen. 2017, 538, 207–220. [Google Scholar] [CrossRef]
- Wang, X.; Shen, M.; Song, L.; Su, Y.; Wang, J. Surface basicity on bulk modified phosphorus alumina through different synthesis methods. Phys. Chem. Chem. Phys. 2011, 13, 15589–15596. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. J. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Turek, A.M.; Wachs, I.E.; DeCanio, E. Acidic properties of alumina-supported metal oxide catalysts: An infrared spectroscopy study. J. Phys. Chem. 1992, 96, 5000–5007. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Mechanism of the Catalytic Conversion of Methanol to Hydrocarbons. ACS Catal. 2013, 3, 18–31. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, W.; Kang, J.; He, S.; Shi, S.; Zhang, Q.; Pan, Y.; Wen, W.; Wang, Y. Bifunctional Catalysts for One-Step Conversion of Syngas into Aromatics with Excellent Selectivity and Stability. Chem 2017, 3, 334–347. [Google Scholar] [CrossRef]
- Tran, T.V.; Le-Phuc, N.; Nguyen, T.H.; Dang, T.T.; Ngo, P.T.; Nguyen, D.A. Application of NaA Membrane Reactor for Methanol Synthesis in CO2 Hydrogenation at Low Pressure. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
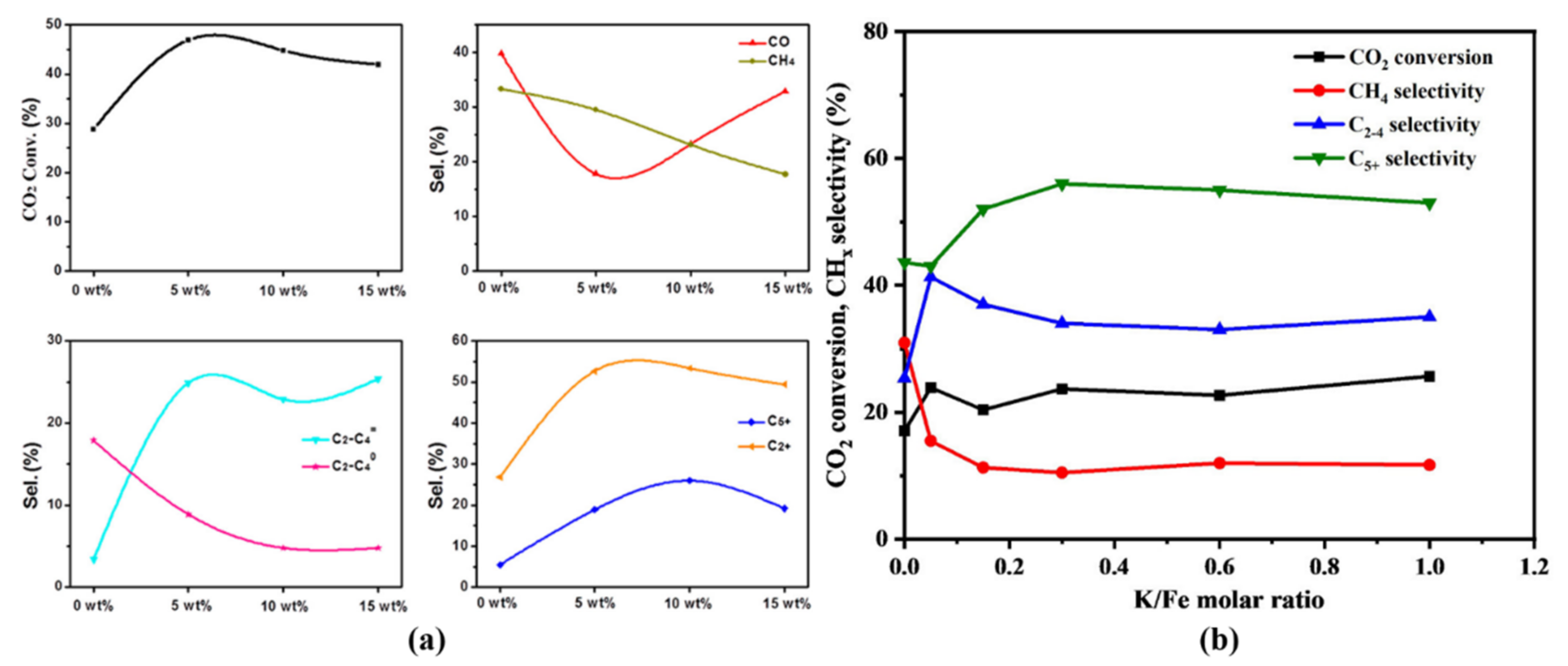

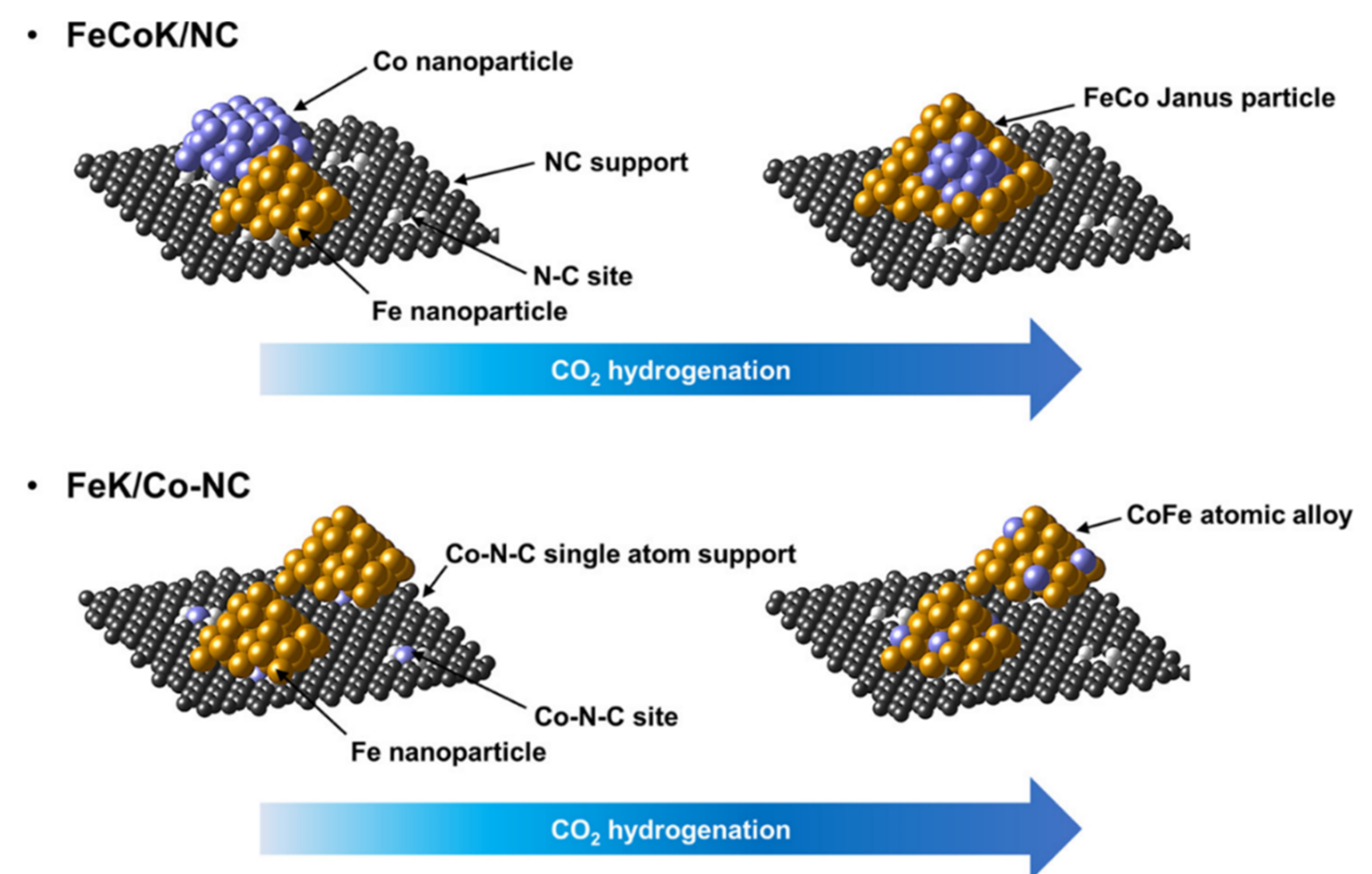
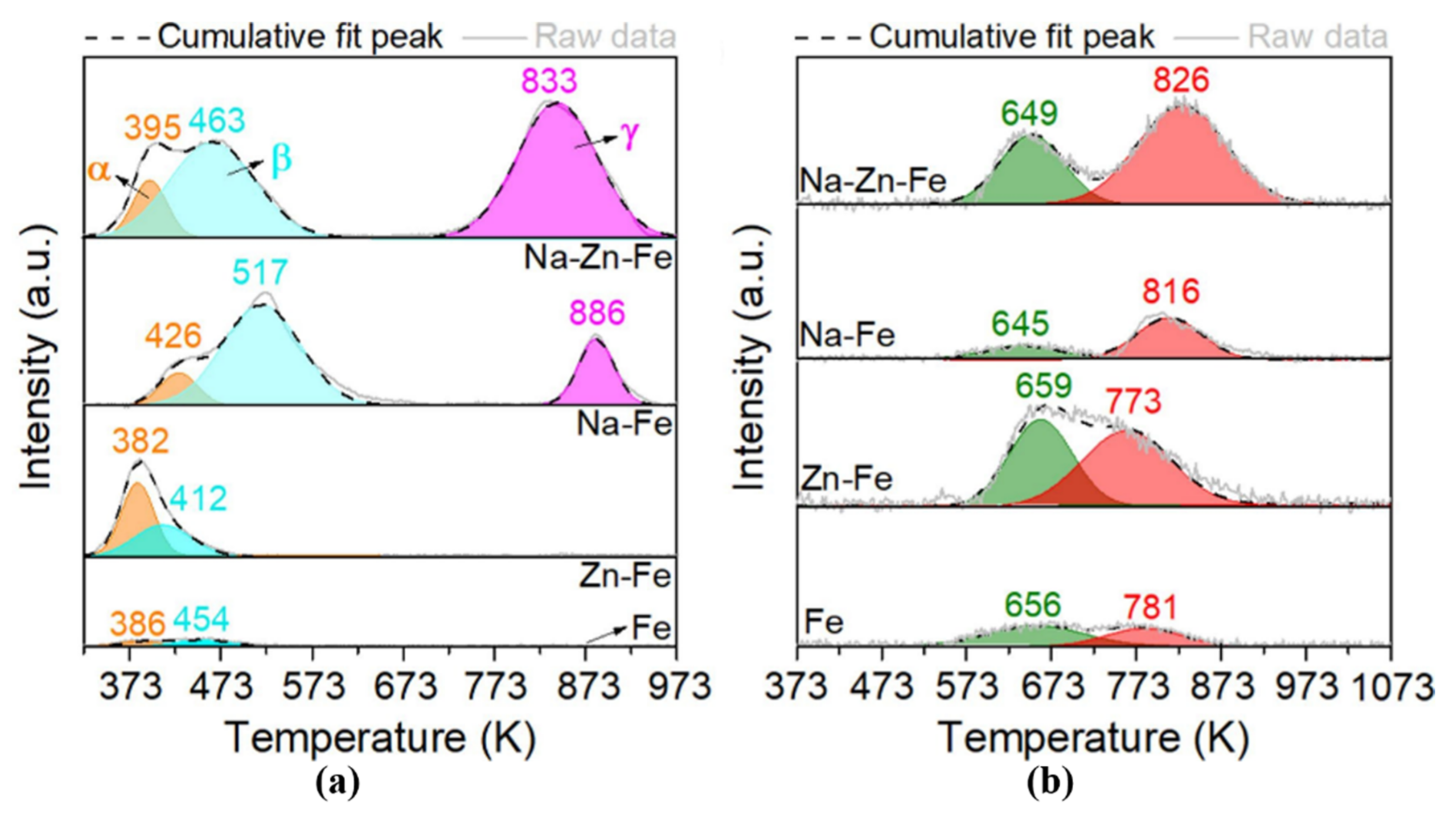
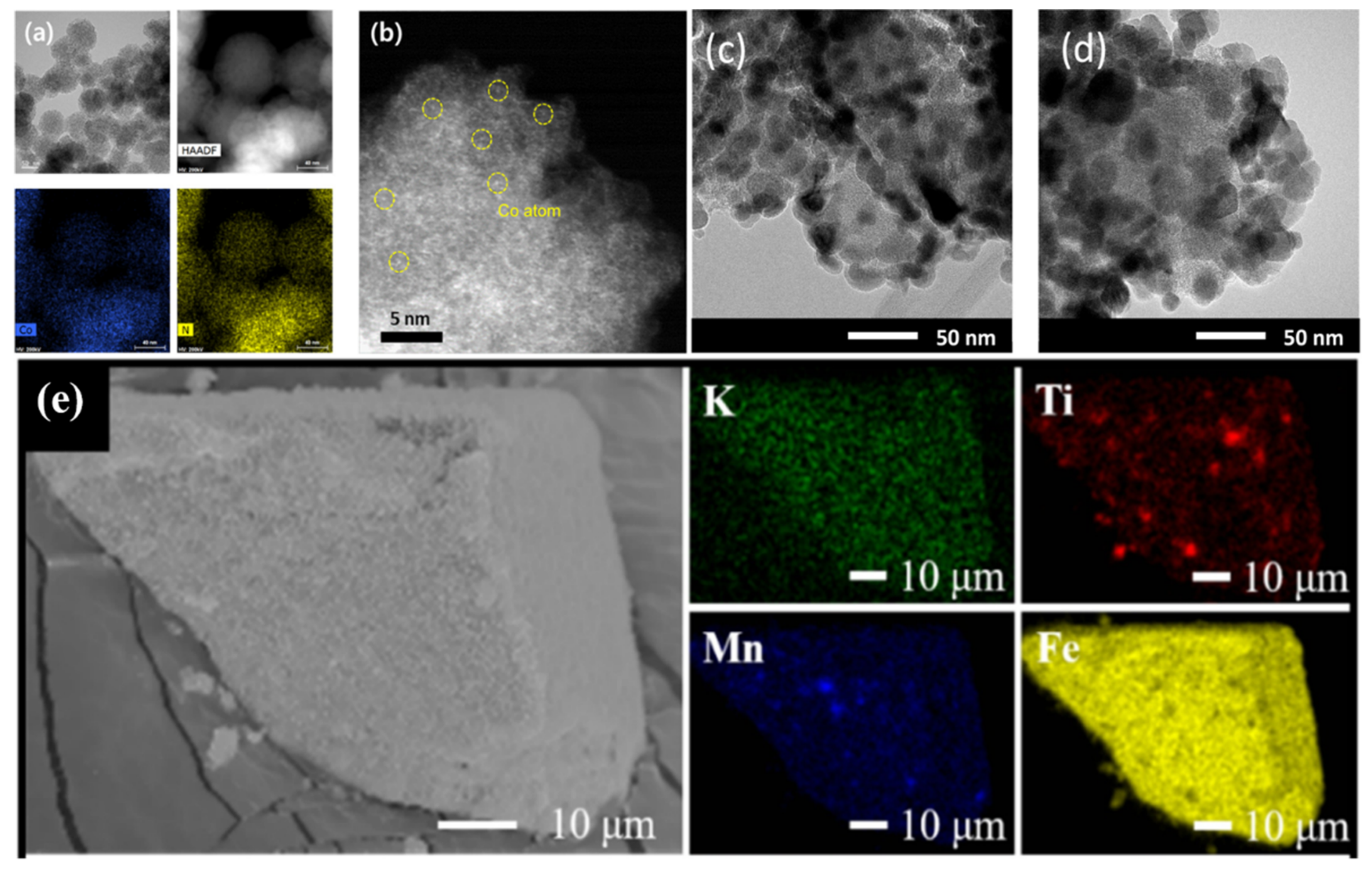
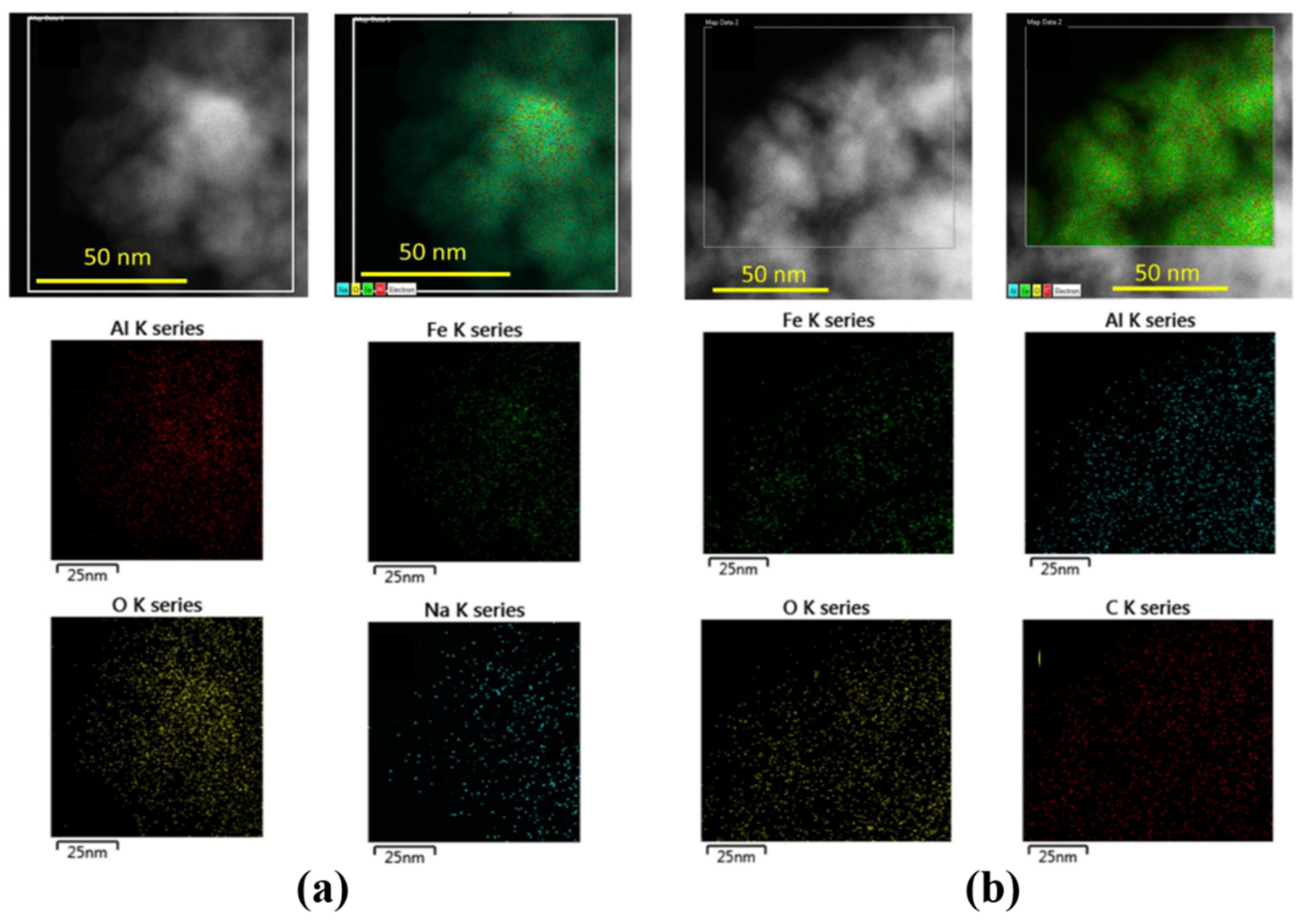
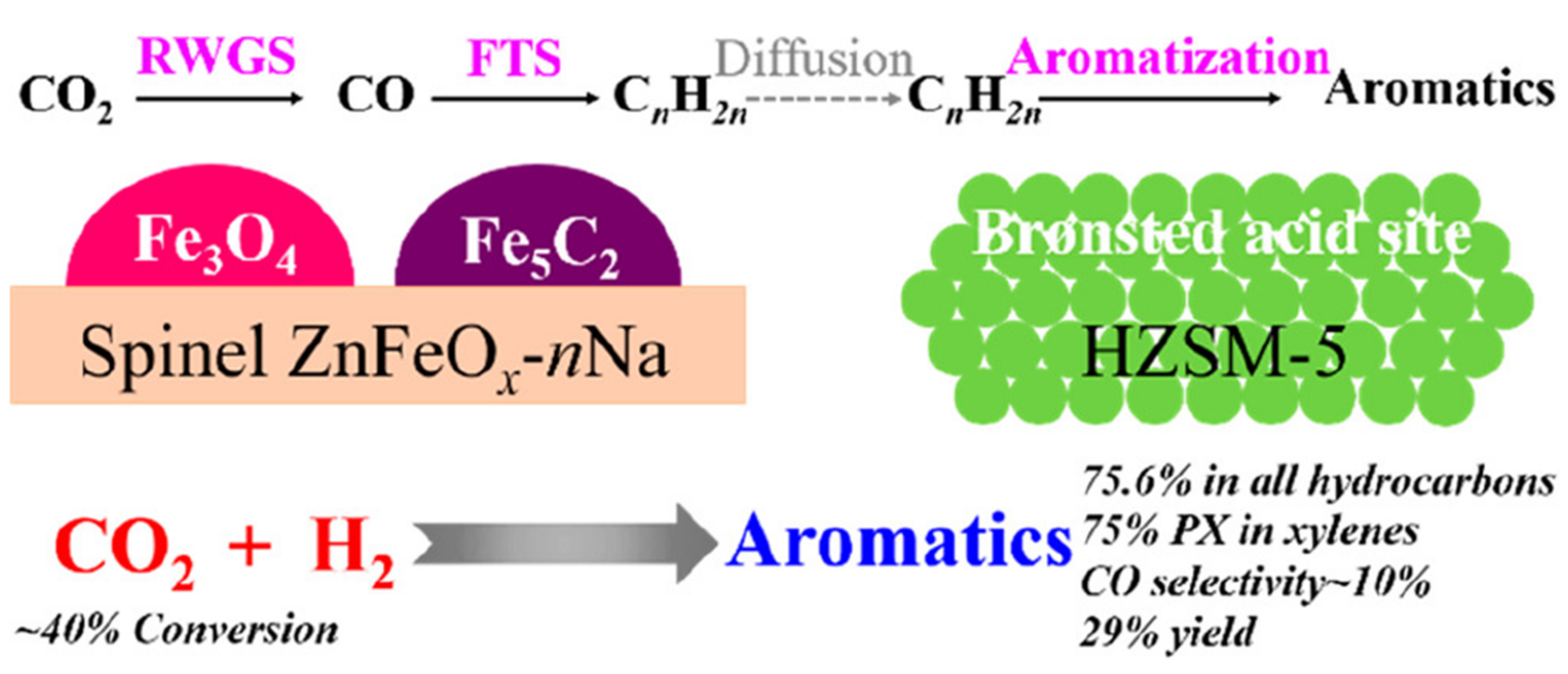

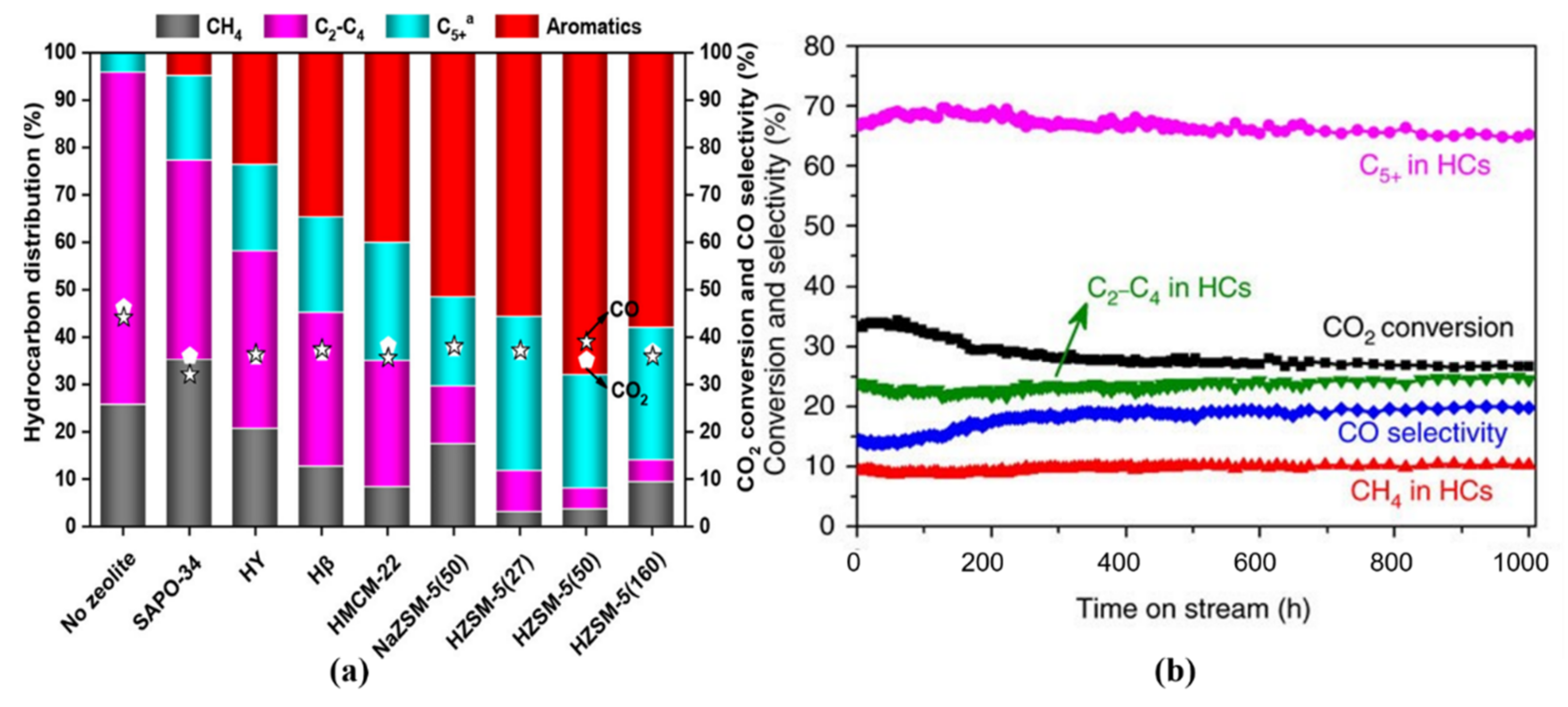

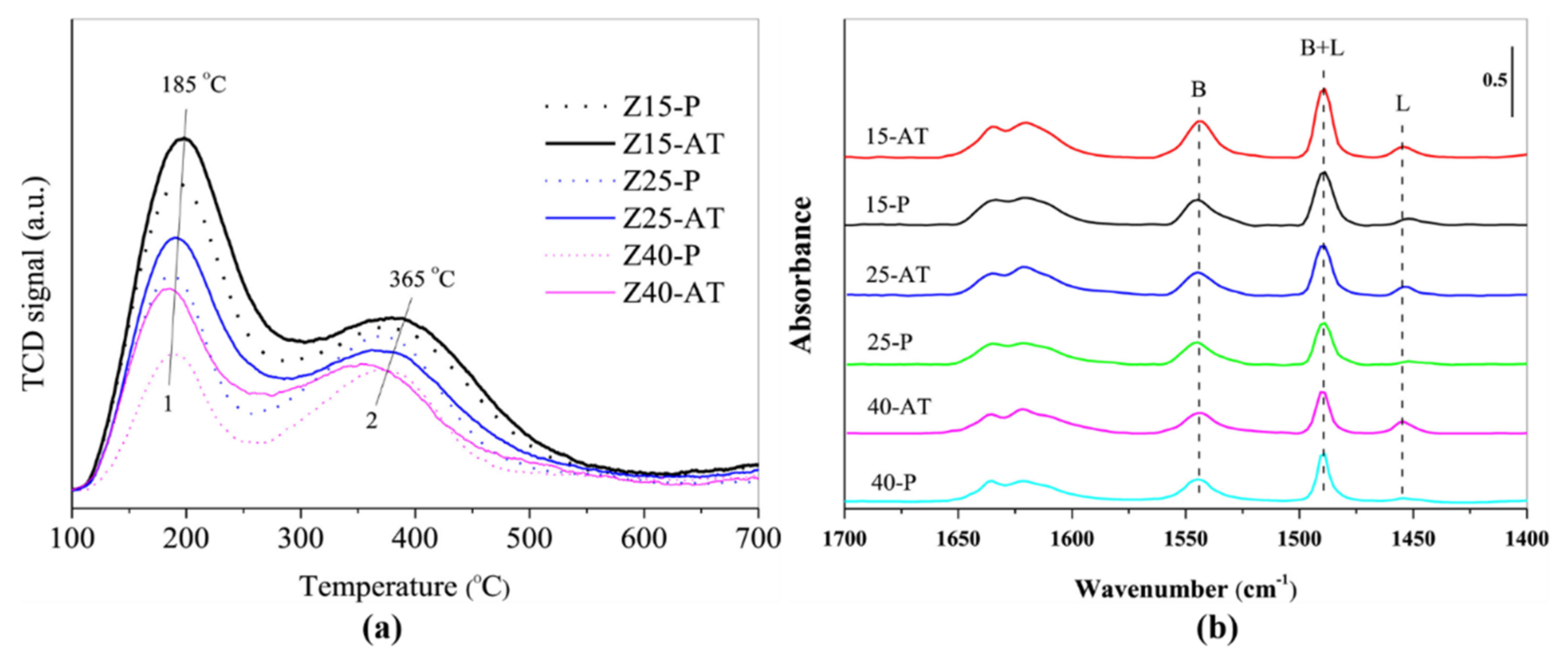
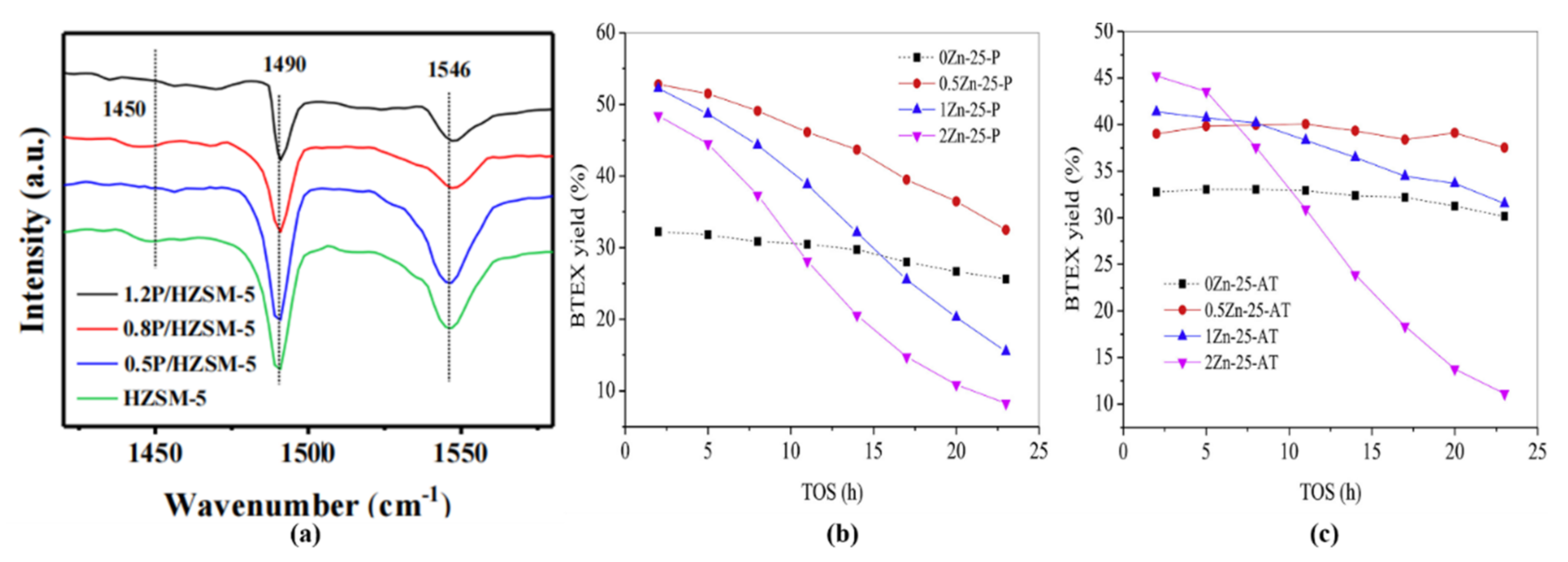




| Reaction Conditions | Hydrocarbon Distribution (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalysts | H2/CO2 Ratios | T (°C) | P (MPa) | GHSV/mL·g−1·h−1 | CO2 conv. (%) | CO Sel. (%) | CH4 | C2–4 | C2–40 a | C2–4= a | C5+ | O/P b | Ref. |
| Fe-based catalyst | ||||||||||||||
| 1 | Fe-K/alumina | 2.0 | 400 | N.A. | 1900 | 69.9 | 3.9 | 16.7 | 47.6 | N.A. | N.A. | 35.8 | N.A. g | [41] |
| 2 | Fe2O3-CT600 | 3.0 | 350 | 1.5 | 1140 | 40 | 15 | 14.1 | 43.5 | N.A. | N.A. | 42.4 | N.A. | [42] |
| 3 | Fe5C2-10K/a-Al2O3 | 3.0 | 320 | 3.0 | 3600 | 31.5 | 18.6 | 14.9 | 49.4 | 5.4 | 44.0 | 35.7 | 8.1 | [16] |
| 4 | 92.6Fe7.4K | 3.0 | 300 | 2.5 | 560 | 41.7 | 6.0 | 11.0 | 29.6 | 6.6 | 23.0 | 59.6 | 3.5 | [43] |
| 5 | K-Fe/ZrO2 | 3.0 | 300 | 2.0 | 1200 | 43.0 | 15.0 | 18.0 | 53.2 | N.A. | N.A. | 27.8 | N.A. | [44] |
| 6 | FeK/Al2O3 | 3.0 | 400 | 3.0 | 3600 | 42.5 | 23.9 | 30.0 | 40.5 | N.A. | N.A. | 29.6 | N.A. | [45] |
| 7 | Fe-Cu-K-La/TiO2 | 3.0 | 300 | 1.1 | 3600 | 23.2 | 33.0 | 19.4 | 37.3 | N.A. | N.A. | 43.3 c | N.A. | [46] |
| 8 | Fe-Cu-Al-K | 3.0 | 265 | 1.3 | 2240 f | 15.6 | 22.8 | 12.8 | 36.0 | N.A. | N.A. | 51.0 | N.A. | [47] |
| 9 | Fe-Cu-K-Al | 3.0 | 300 | 2.5 | 5000 | 35.7 | N.A. | N.A. | N.A. | N.A. | N.A. | 50.7 | N.A. | [48] |
| 10 | FeAlOx-5 | 3.0 | 330 | 3.5 | 4000 | 36.8 | 7.2 | 12.1 | 30.1 | N.A. | N.A. | 57.8 | 0.7 h | [49] |
| 11 | 10Fe3Cu1K/Al2O3 | 3.0 | 400 | 3.0 | 3600 | 41.7 | 26.5 | 37.8 | 43.4 | N.A. | N.A. | 17.8 | N.A. | [50] |
| 12 | Fe0.17Cu1K/Al2O3 | 3.0 | 300 | 1.1 | 3600 | 29.3 | 17.0 | 8.4 | 26.5 | N.A. | N.A. | 65.1 | N.A. | [51] |
| 13 | CuFeO2-6 e | 3.0 | 300 | 1.0 | 1800 | 17.3 | 31.7 | 2.6 | 31.0 | N.A. | N.A. | 66.3 | 7.3 | [52] |
| 14 | PdKFe2O3 | 3.0 | 235 | 1.6 | 6000 | 20.7 | 18.2 | 13.6 | 28.1 | N.A. | N.A. | 58.3 | N.A. | [53] |
| 15 | FeMnNa | 3.0 | 340 | 2.0 | 12,000 | 35.0 | 18.1 | 13.1 | 38.7 | N.A. | N.A. | 48.2 | 8.1 | [54] |
| 16 | 5Mn-Na/Fe | 3.0 | 320 | 3.0 | 2040 | 38.6 | 11.7 | 13.4 | 38.8 | 4.5 | 26.6 | 47.8 | 7.5 | [55] |
| 17 | Fe-Mn-K | 3.0 | 300 | 1.0 | 2400 | 38.2 | 5.6 | 10.4 | 27.7 | N.A. | N.A. | 61.7 | N.A. | [56] |
| 18 | K3/FeMn10Ti20 | 2.8 | 320 | 5.0 | 24,000 | 34.9 | 9.7 | 9.2 | 27.2 | 12.6 h | N.A. | 51.0 | 2.7 | [57] |
| 19 | 10Fe0.8K0.53Co | 3.0 | 300 | 2.5 | 560 | 54.6 | 2.0 | 19.3 | 32.8 | 7.9 | 24.9 | 48.0 | 3.2 | [58] |
| 20 | 10Fe0.8K0.53Ru | 3.0 | 300 | 2.5 | 560 | 47.1 | 3.1 | 16.9 | 28.0 | 7.6 | 20.3 | 55.1 | 2.7 | [58] |
| 21 | Na-ZnFe2O4 | 3.0 | 340 | 1.0 | 1800 | 34 | 11.7 | 8.6 | 28.1 | N.A. | N.A. | 51.6 | 11.3 | [59] |
| 22 | ZnFe2O4-nNa | 3.0 | 320 | 3.0 | 4000 | 38.4 | 11.2 | 11.0 | 39.3 | N.A. | N.A. | 49.7 | N.A. | [60] |
| 23 | Na-Zn-Fe | 3.0 | 340 | 2.5 | 15,000 | 39.0 | 14.0 | 12.0 | 47.7 | 4.7 | 43.0 | 40.5 | 9.8 | [40] |
| 24 | FeZn-NC | 3.0 | 320 | 3.0 | 7200 | 29.3 | 19.9 | 20.7 | 45.1 | 7.6 | 37.5 | 34.2 | 4.9 | [61] |
| 25 | FeZnK-NC | 3.0 | 320 | 3.0 | 7200 | 34.6 | 21.2 | 24.2 | 47.7 | 7.1 | 40.6 | 28.0 | 5.7 | [61] |
| 26 | N-K-600-0 | 3.0 | 400 | 3.0 | 3600 | 46.0 | 17.5 | 32.2 | 40.8 | 17.6 | 23.2 | 26.9 | 1.3 | [62] |
| 27 | FeK/Co-NC | 3.0 | 300 | 2.5 | N.A. | 51.7 | 21.6 | N.A. | N.A. | N.A. | N.A. | 42.4 | N.A. | [36] |
| 28 | FeK/C-1EDA | 3.0 | 300 | 1.0 | N.A. | 20.1 | 31.7 | 17.2 | 43.3 | 37.7 | 5.6 | 39.5 | 1.2 | [63] |
| 29 | Fe/C-bio | 3.0 | 320 | 1.0 | 2240 e | 30.5 | 23.2 | 11.8 | 24.4 | N.A. | N.A. | 63.8 | N.A. | [64] |
| 30 | FeK/SWNTs | 3.0 | 340 | 2.0 | 9000 | 52.7 | 9.6 | 13.5 | 31.1 | N.A. | N.A. | 55.4 | N.A. | [65] |
| 31 | Fe/CNT | 2.0 | 350 | 8.5 | 14,400 | 22.0 | 18.0 | 42.7 | 42.7 | N.A. | N.A. | 14.6 | N.A. | [66] |
| 32 | Na-Fe/CNTs | 3.0 | 370 | 1.5 | 1200 | 48.0 | 24.8 | 27.5 | 52.5 | N.A. | N.A. | 19.9 | N.A. | [67] |
| 33 | FeK/MPC | 3.0 | 300 | 2.5 | 2000 | 50.6 | 8.2 | 16.8 | 34.7 | N.A. | N.A. | 48.5 | N.A. | [68] |
| Non Fe-based catalyst | ||||||||||||||
| 1 | Na-CoCu/TiO2 | 3.0 | 250 | 5.0 | 3000 | 18.4 | 30.2 | 26.1 | 31.8 | N.A. | N.A. | 42.1 | N.A. | [30] |
| 2 | 2.5K-CoCu/TiO2 | 3.0 | 250 | 5.0 | 3000 | 13.0 | 35.1 | 34.1 | 30.8 | N.A. | N.A. | 35.1 | 0.3 | [31] |
| 3 | 10%Co/MIL-53(Al) | 3.0 | 260 | 3.0 | 1000 | 25.3 | 26.6 | 35.1 | 29.8 | N.A. | N.A. | 35.0 | N.A. | [32] |
| 4 | Co6/MnOx | 1.0 | 200 | 8.0 | N.A. | 15.3 | 0.4 | N.A. | 46.6 d | N.A. | N.A. | 53.4 | N.A. | [33] |
| 5 | CMO-10 | 3.0 | 270 | 4.0 | 4000 | 64.3 | 0.2 | 44.2 | 22.9 | N.A. | N.A. | 32.9 | N.A. | [29] |
| 6 | Co-Fe-0.81Na | 3.0 | 240 | 3.0 | 5500 | 10.2 | 5.2 | 17.8 | 9.4 | N.A. | N.A. | 72.9 | N.A. | [28] |
| Entry | Catalysts | Reaction Conditions | Hydrocarbon Distribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2/CO2 Ratios | T (°C) | P (MPa) | GHSV/mL·g−1·h−1 | CO2 Conv. (%) | CO Select. (%) | Oxy c | CH4 | C2–4 | C5+ | Ref. | |||
| Multifunctional Fe-based catalyst with zeolite | |||||||||||||
| 1 | FeAlOx-5/HZSM-5 | 3.0 | 330 | 3.5 | 4000 | 36.8 | 16.0 | N.A. | 10.0 | 20.0 | 70.0 | N.A. | [49] |
| 2 | Na-Fe3O4/HZSM-5 | 3.0 | 320 | 3.0 | 4000 | 22.0 | 20.1 | N.A. | 4.0 | 16.6 | 79.4 a | N.A. | [69] |
| 3 | Na-Fe3O4/HZSM-5(160) | 3.0 | 320 | 3.0 | 4000 | 33.6 | 14.2 | N.A. | 7.9 | 18.4 | 73.7 b | N.A. | [69] |
| 4 | 92.6Fe7.4K/HZSM-5 | 3.0 | 300 | 2.5 | 560 | 43.9 | 6.1 | N.A. | 9.5 | 10.8 d | N.A. | I-C4-6/C4-6 69.7 | [43] |
| 5 | NaFe+SAPO-11+ZSM-5 | 3.0 | 350 | 3.0 | 4800 | 31.2 | 13.20 | 0.7 | 9.2 | 18.3 | 72.5 | I-C5+/C5+ 38.2 | [70] |
| 6 | K-Fe/C-KZSM-5 | 2.5 | 320 | 2.0 | 4800 | 34.5 | 18.8 | N.A. | 11.0 | 18.9 | 70.1 | N.A. | [71] |
| Entry | Reaction Conditions | Hydrocarbon Distribution (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalysts | H2/CO2 Ratios | T (°C) | P (MPa) | GHSV/mL·g−1·h−1 | CO2 Conv. (%) | CO Sel. (%) | CH4 | C2–4 | C2–40 a | C2–4= a | C5+ | O/P b | Ref. | |
| Co-precipitation Methods | ||||||||||||||
| 1 | Fe-Cu-K-Al | 3.0 | 300 | 2.5 | 5000 | 35.7 | N.A. | N.A. | N.A. | N.A. | N.A. | 50.7 | N.A. | [48] |
| 2 | FeAlOx-5 | 3.0 | 330 | 3.5 | 4000 | 36.8 | 7.2 | 12.1 | 30.1 | N.A | N.A | 57.8 | N.A | [49] |
| 3 | CMO-10 | 3.0 | 270 | 4.0 | 4000 | 64.3 | 0.2 | 44.2 | 22.9 | N.A. | N.A. | 32.9 | N.A. | [29] |
| 4 | Co-Fe-0.81Na | 3.0 | 240 | 3.0 | 5500 | 10.2 | 5.2 | 17.8 | 9.4 | N.A. | N.A. | 72.9 | N.A. | [28] |
| Incipient Wetness Impregnation (IWI) Methods | ||||||||||||||
| 5 | Fe0.17Cu1K/Al2O3 | 3.0 | 300 | 1.1 | 3600 | 29.3 | 17.0 | 8.4 | 26.5 | N.A. | N.A. | 65.1 | N.A. | [51] |
| 6 | 10Fe0.8K0.53Co | 3.0 | 300 | 2.5 | 560 | 54.6 | 2.0 | 19.3 | 32.8 | 7.9 | 24.9 | 48.0 | 3.2 | [58] |
| 7 | FeK/Co-NC | 3.0 | 300 | 2.5 | N.A. | 51.7 | 21.6 | N.A. | N.A. | N.A. | N.A. | 42.4 | N.A. | [36] |
| 8 | FeK/MPC | 3.0 | 300 | 2.5 | 2000 | 50.6 | 8.2 | 16.8 | 34.7 | N.A. | N.A. | 48.5 | N.A. | [68] |
| Template-assisted Synthesis Methods | ||||||||||||||
| 9 | Fe2O3-CT600 | 3.0 | 350 | 1.5 | 1140 | 40.0 | 15.0 | 14.1 | 43.5 | N.A. | N.A. | 42.4 | N.A. | [42] |
| Sol–Gel Methods | ||||||||||||||
| 10 | Na-Zn-Fe | 3.0 | 340 | 2.5 | 15,000 | 39.0 | 14.0 | 12.0 | 47.7 | 4.7 | 43.0 | 40.5 | 9.8 | [40] |
| Hydrothermal Synthesis Methods (HSM) | ||||||||||||||
| 11 | CuFeO2-6 c | 3.0 | 300 | 1.0 | 1800 | 17.3 | 31.7 | 2.6 | 31.0 | N.A. | N.A. | 66.3 | 7.3 | [52] |
| 12 | Na-ZnFe2O4 | 3.0 | 340 | 1.0 | 1800 | 34.0 | 11.7 | 8.6 | 28.1 | N.A. | N.A. | 51.6 | 11.3 | [59] |
| Organic Combustion Methods (OCM) | ||||||||||||||
| 13 | Fe-Mn-K | 3.0 | 300 | 1.0 | 2400 | 38.2 | 5.6 | 10.4 | 27.7 | N.A. | N.A. | 61.7 | N.A. | [56] |
| Entry | Catalysts | Reaction Conditions | CO2 Conv. (%) | CO Select. (%) | Hydrocarbon Distribution (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2/CO2 ratios | T (°C) | P (MPa) | GHSV /mL·g−1·h−1 | CH4 | C2–4 a | Aro. b | C5+ c | Yield | Ref. | ||||
| 1 | Na/Fe-HZSM-5 | 3.0 | 300 | 1.0 | 4800 | 21.8 | 40.9 | 14.7 | 25.5 | 54.7 | 5.1 | 22.4 | [104] |
| 2 | 1Na-Fe/HZSM-5 | 3.0 | 340 | 3.0 | 4000 | 32.3 | 16.6 | 5.6 | 19.9 | 63.5 | 11.2 | 20.4 | [111] |
| 3 | K-3Fe/Zn/HZSM-5(21) | 3.0 | 320 | 3.0 | 7200 | 43.6 | 11.5 | 13.6 | 23.3 | 25.4 | 37.7 | 11.1 | [96] |
| 4 | 15Fe-10K/Al2O3&P-HZSM-5 | 1.0 | 400 | 3.0 | 6000 | 36.4 | 10.2 | 10.8 | 39.6 | 39.5 | 10.1 | 14.4 | [95] |
| 5 | FeK1.5/HSG|HZSM-5(50) | 3.0 | 340 | 2.0 | 26,000 | 35.0 | 39.0 | 3.5 | 4.4 | 68.0 | 24.0 | 23.8 | [112] |
| 6 | ZnFeOx-4.25Na/HZSM-5 | 3.0 | 320 | 3.0 | 4000 | 36.2 | 6.9 | 11.1 | 16.5 | 60.0 | 15.6 | 21.8 | [60] |
| 7 | Na-Fe@C/H-ZSM-5 | 3.0 | 320 | 3.0 | 6000 | 33.3 | 13.3 | 4.8 | 10.4 | 50.2 | 34.6 | 16.8 | [113] |
| 8 | Na-Fe3O4/HZSM-5 | 2.0 | 320 | 3.0 | 4000 | 27.7 | 16.0 | 5.9 | 29.6 | 44.5 | 20.0 | 12.3 | [114] |
| 9 | 6.25Cu-Fe2O3/HZSM-5-pt | 3.0 | 320 | 3.0 | 1000 | 55.4 | 4.41 | 12.5 | 9.4 | 61.9 | 15.4 | 34.3 | [115] |
| 10 | 6.25Cu-Fe2O3/HZSM-5-dg | 3.0 | 320 | 3.0 | 1000 | 49.7 | 6.1 | 20.8 | 10.5 | 44.9 | 22.4 | 22.3 | [115] |
| 11 | 6.25Cu-Fe2O3/HZSM-5-hy | 3.0 | 320 | 3.0 | 1000 | 54.5 | 4.45 | 12.3 | 10.0 | 55.2 | 21.5 | 30.1 | [115] |
| 12 | Na–FeAlOx/Zn-HZSM-5(12.5)@SiO2 | 3.0 | 370 | 3.0 | 4000 | 45.2 | 15.3 | 13.8 | 26.2 | 38.7 | 21.3 | 17.5 | [116] |
| 13 | 2.83Na-FeMn/HZSM-5(105) | 3.0 | 320 | 3.0 | 4000 | 27.0 | 21.9 | 9.0 | 28.4 | 36.5 | 26.1 | 9.9 | [117] |
| 14 | Fe2O3@KO2/ZSM-5 | 3.0. | 375 | 3.0 | 5000 | 47.4 | 13.7 | 14.9 | 45.9 | 23.9 | 15.3 | 11.3 | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Hu, K.; Gao, R.; Zhang, L.; Wang, L.; Zhang, C. Hydrogenation of Carbon Dioxide to Value-Added Liquid Fuels and Aromatics over Fe-Based Catalysts Based on the Fischer–Tropsch Synthesis Route. Atmosphere 2022, 13, 1238. https://doi.org/10.3390/atmos13081238
Wang Q, Hu K, Gao R, Zhang L, Wang L, Zhang C. Hydrogenation of Carbon Dioxide to Value-Added Liquid Fuels and Aromatics over Fe-Based Catalysts Based on the Fischer–Tropsch Synthesis Route. Atmosphere. 2022; 13(8):1238. https://doi.org/10.3390/atmos13081238
Chicago/Turabian StyleWang, Qiang, Kehao Hu, Ruxing Gao, Leiyu Zhang, Lei Wang, and Chundong Zhang. 2022. "Hydrogenation of Carbon Dioxide to Value-Added Liquid Fuels and Aromatics over Fe-Based Catalysts Based on the Fischer–Tropsch Synthesis Route" Atmosphere 13, no. 8: 1238. https://doi.org/10.3390/atmos13081238
APA StyleWang, Q., Hu, K., Gao, R., Zhang, L., Wang, L., & Zhang, C. (2022). Hydrogenation of Carbon Dioxide to Value-Added Liquid Fuels and Aromatics over Fe-Based Catalysts Based on the Fischer–Tropsch Synthesis Route. Atmosphere, 13(8), 1238. https://doi.org/10.3390/atmos13081238





