Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Experimental Design
2.3. Analysis and Calculation
3. Results
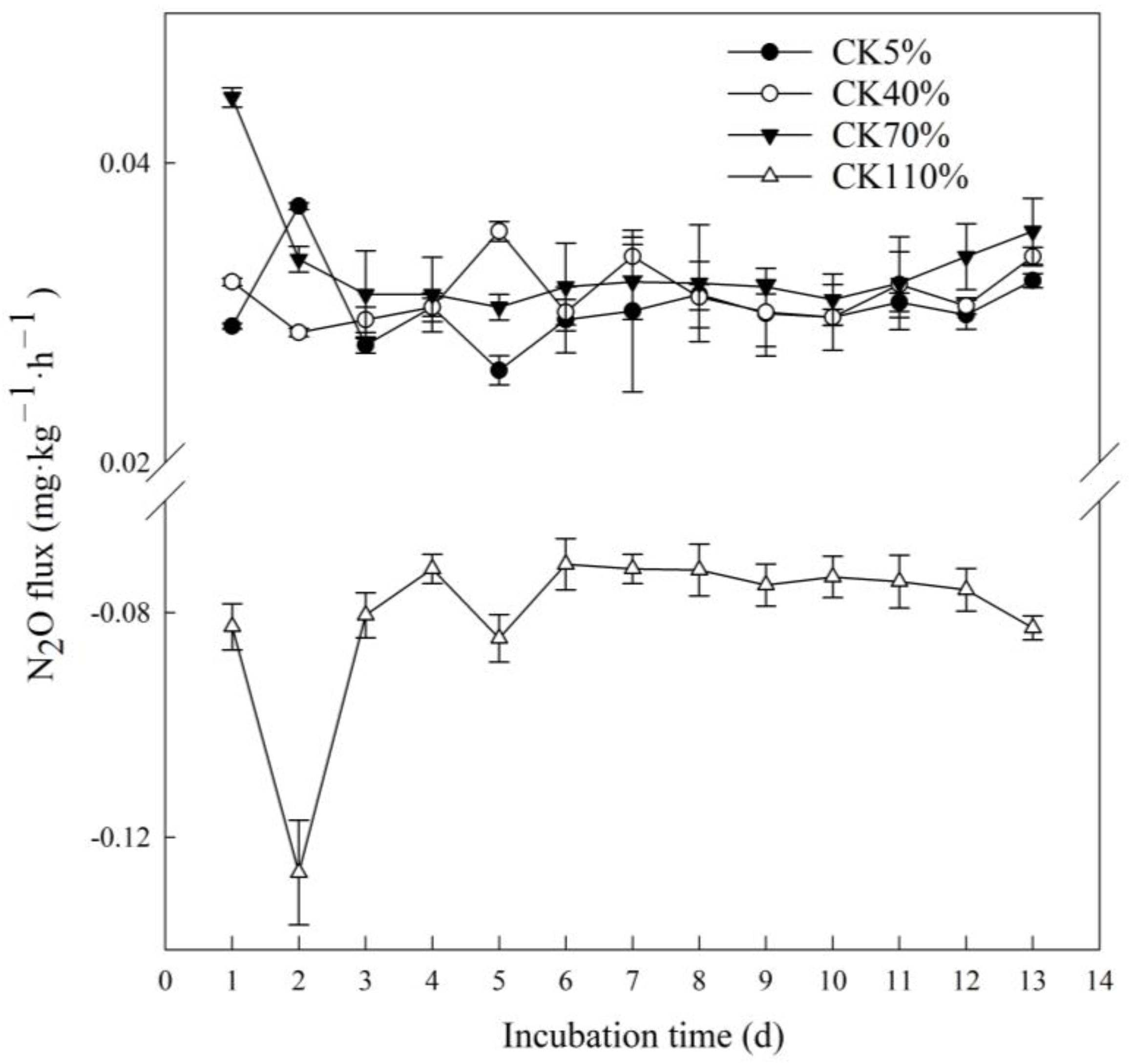
3.1. N2O Emission Varied with Soil Moisture
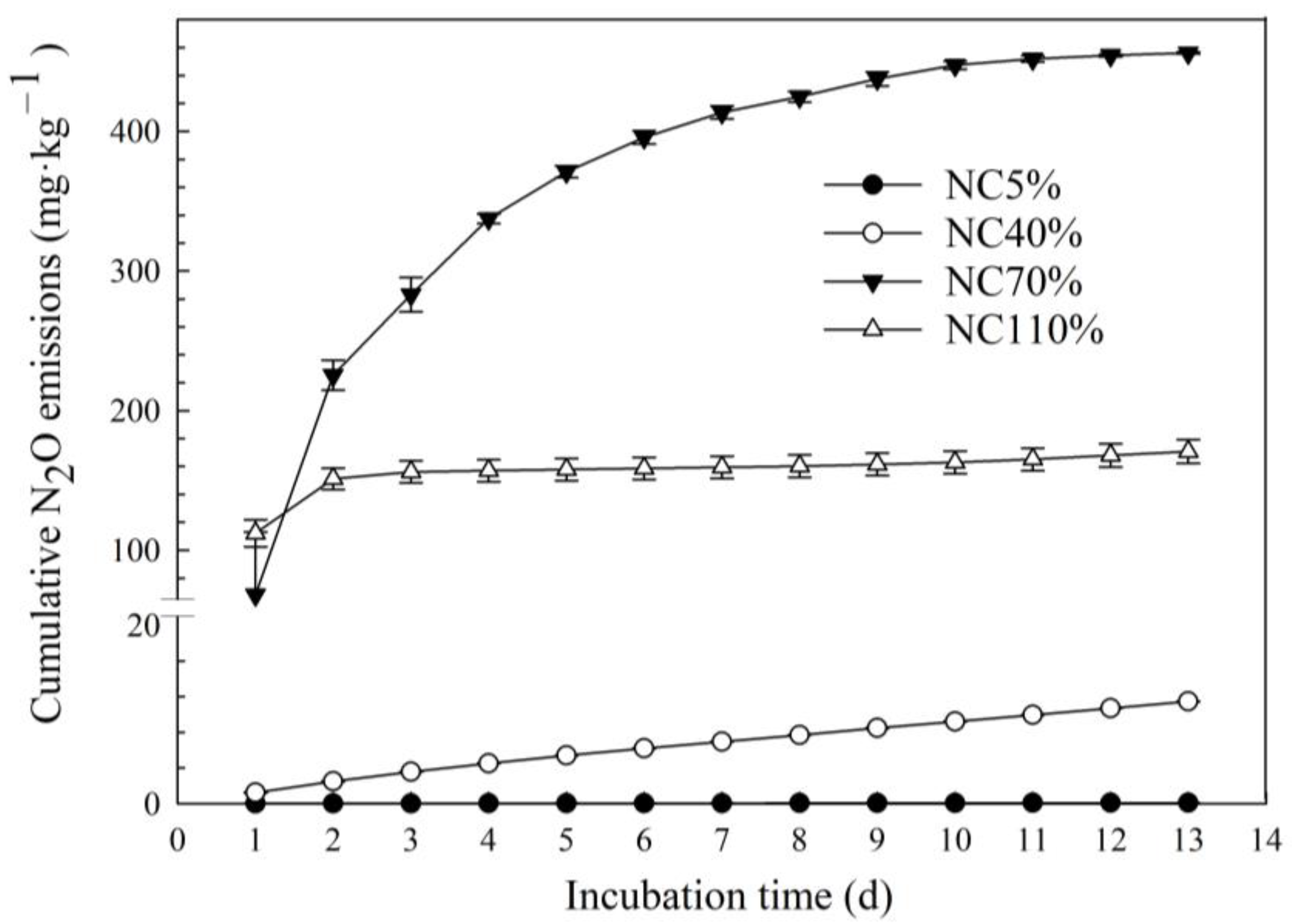
3.2. Nitrogen Forms
4. Discussion
4.1. Responses of N2O Emissions and Production Mechanisms to Soil Moisture
4.2. Estimation of the Contribution of Nitrogen Transformation to N2O Production
4.3. Impacts of Exogenous Carbon and Nitrogen on N2O Production and Emission from Soils with Different Moisture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Revell, L.E.; Bodeker, G.E.; Smale, D.; Lehmann, R.; Huck, P.E.; Williamson, B.E.; Rozanov, E.; Struthers, H. The effectiveness of N2O in depleting stratospheric ozone. Geophys. Res. Lett. 2012, 39, 99–112. [Google Scholar] [CrossRef]
- Tian, H.Q.; Xu, R.T.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B-Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.F.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Change Biol. 2018, 25, 640–659. [Google Scholar] [CrossRef] [Green Version]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Kool, D.M.; Wrage, N.; Zechmeister-Boltenstern, S.; Pfeffer, M.; Brus, D.; Oenema, O.; Van Groenigen, J.W. Nitrifier denitrification can be a source of N2O from soil: A revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 2010, 61, 759–772. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B-Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Colliver, B.B.; Stephenson, T. Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol. Adv. 2000, 18, 219–232. [Google Scholar] [CrossRef]
- Lawton, T.J.; Bowen, K.E.; Sayavedra-Soto, L.A.; Arp, D.J.; Rosenzweig, A.C. Characterization of a Nitrite Reductase Involved in Nitrifier Denitrification. J. Biol. Chem. 2013, 288, 25575–25583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Cardoso, A.D.; Quintana, B.G.; Janusckiewicz, E.R.; Brito, L.D.; Morgado, E.D.; Reis, R.A.; Ruggieri, A.C. N2O emissions from urine-treated tropical soil: Effects of soil moisture and compaction, urine composition, and dung addition. Catena 2017, 157, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Kuang, W.N.; Gao, X.P.; Tenuta, M.; Gui, D.W.; Zeng, F.J. Relationship between soil profile accumulation and surface emission of N2O: Effects of soil moisture and fertilizer nitrogen. Biol. Fertil. Soils 2019, 55, 97–107. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, S.Q.; Zhang, J.B.; Cai, Z.C. Effects of soil moisture on gross N transformations and N2O emission in acid subtropical forest soils. Biol. Fertil. Soils 2014, 50, 1099–1108. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109, D17302. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.F.; Winsley, T.; Ferrari, B.C.; Siciliano, S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Qin, H.L.; Xing, X.Y.; Tang, Y.F.; Zhu, B.L.; Wei, X.M.; Chen, X.B.; Liu, Y. Soil moisture and activity of nitrite- and nitrous oxide-reducing microbes enhanced nitrous oxide emissions in fallow paddy soils. Biol. Fertil. Soils 2020, 56, 53–67. [Google Scholar] [CrossRef]
- Shang, F.Z.; Ren, S.M.; Yang, P.L.; Chi, Y.B.; Xue, Y.D. Effects of Different Irrigation Water Types, N Fertilizer Types, and Soil Moisture Contents on N2O Emissions and N Fertilizer Transformations in Soils. Water Air Soil Pollut. 2016, 227, 225. [Google Scholar] [CrossRef]
- Well, R.; Kurganova, I.; de Gerenyu, V.L.; Flessa, H. Isotopomer signatures of soil-emitted N2O under different moisture conditions-A microcosm study with arable loess soil. Soil Biol. Biochem. 2006, 38, 2923–2933. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Szymańska, M.; Stępień, W.; Rutkowska, B.; Szulc, W. Soil N2O emissions under conventional tillage conditions and from forest soil. Soil Tillage Res. 2019, 190, 86–91. [Google Scholar] [CrossRef]
- Cai, Y.J.; Ding, W.X.; Luo, J.F. Nitrous oxide emissions from Chinese maize-wheat rotation systems: A 3-year field measurement. Atmos. Environ. 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Porre, R.J.; van Groenigen, J.W.; De Deyn, G.B.; de Goede, R.G.M.; Lubbers, I.M. Exploring the relationship between soil mesofauna, soil structure and N2O emissions. Soil Biol. Biochem. 2016, 96, 55–64. [Google Scholar] [CrossRef]
- Flint, M.K.; Martin, J.B.; Summerall, T.I.; Barry-Sosa, A.; Christner, B.C. Nitrous oxide processing in carbonate karst aquifers. J. Hydrol. 2021, 594, 125936. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Wang, Z.; Han, G.; Li, J.; Wang, B.; Wang, F.; Bai, L. Nitrous oxide (N2O) emissions from a mesotrophic reservoir on the Wujiang River, southwest China. Acta Geochim. 2017, 36, 667–679. [Google Scholar] [CrossRef]
- Liu, X.L.; Liu, C.Q.; Li, S.L.; Wang, F.S.; Wang, B.L.; Wang, Z.L. Spatiotemporal variations of nitrous oxide (N2O) emissions from two reservoirs in SW China. Atmos. Environ. 2011, 45, 5458–5468. [Google Scholar] [CrossRef]
- Wang, L.; Sheng, R.; Yang, H.C.; Wang, Q.; Zhang, W.Z.; Hou, H.J.; Wu, J.S.; Wei, W.X. Stimulatory effect of exogenous nitrate on soil denitrifiers and denitrifying activities in submerged paddy soil. Geoderma 2017, 286, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.H.; Guo, H.R.; Huang, H.; Ma, T.Y.; Song, W.; Chen, C.J.; Liu, X.Y. Atmospheric nitrogen deposition and its responses to anthropogenic emissions in a global hotspot region. Atmos. Res. 2021, 248, 105137. [Google Scholar] [CrossRef]
- Harris, E.; Joss, A.; Emmenegger, L.; Kipf, M.; Wolf, B.; Mohn, J.; Wunderlin, P. Isotopic evidence for nitrous oxide production pathways in a partial nitritation-anammox reactor. Water Res. 2015, 83, 258–270. [Google Scholar] [CrossRef]
- Opdyke, M.R.; Ostrom, N.E.; Ostrom, P.H. Evidence for the predominance of denitrification as a source of N2O in temperate agricultural soils based on isotopologue measurements. Glob. Biogeochem. Cycles 2009, 23, GB4018. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 1058. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Liu, M.; Van Zwieten, L.; Yang, X.; Yu, C.; Wang, H.; Song, Z. Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change, Southwest China. Agric. Ecosyst. Environ. 2020, 301, 107027. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Tracing Riverine Particulate Black Carbon Sources in Xijiang River Basin: Insight from Stable Isotopic Composition and Bayesian Mixing Model. Water Res. 2021, 194, 116932. [Google Scholar] [CrossRef] [PubMed]
- Jäger, N.; Stange, C.F.; Ludwig, B.; Flessa, H. Emission rates of N2O and CO2 from soils with different organic matter content from three long-term fertilization experiments-a laboratory study. Biol. Fertil. Soils 2011, 47, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Bergstermann, A.; Cárdenas, L.; Bol, R.; Gilliam, L.; Goulding, K.; Meijide, A.; Scholefield, D.; Vallejo, A.; Well, R. Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol. Biochem. 2011, 43, 240–250. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Ge, X.; Wu, Q.; Wang, Z.; Gao, S.; Wang, T. Sulfur isotope and stoichiometry–based source identification of major ions and risk assessment in Chishui River Basin, Southwest China. Water 2021, 13, 1231. [Google Scholar] [CrossRef]
- Han, R.; Wang, Z.; Shen, Y.; Wu, Q.; Liu, X.; Cao, C.; Gao, S.; Zhang, J. Anthropogenic Gd in urban river water: A case study in Guiyang, SW China. Elementa-Sci. Anthrop. 2021, 9, 00147. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Wang, Z.; Gao, S.; Jia, H.; Shen, Y. Distribution, water quality, and health risk assessment of trace elements in three streams during the wet season, Guiyang, Southwest China. Elementa-Sci. Anthrop. 2021, 9, 00133. [Google Scholar] [CrossRef]
- Liu, X.; Han, G.; Zeng, J.; Liu, J.; Li, X.; Boeckx, P. The effects of clean energy production and urbanization on sources and transformation processes of nitrate in a subtropical river system: Insights from the dual isotopes of nitrate and Bayesian model. J. Clean. Prod. 2021, 325, 129317. [Google Scholar] [CrossRef]
- Liu, X.L.; Han, G.; Zeng, J.; Liu, M.; Li, X.Q.; Boeckx, P. Identifying the sources of nitrate contamination using a combined dual isotope, chemical and Bayesian model approach in a tropical agricultural river: Case study in the Mun River, Thailand. Sci. Total Environ. 2021, 760, 143938. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, C.Q.; Zhao, Y.; Li, Z. Changes of hydrochemical composition and heavy metals concentration in shallow groundwater from karst hilly areas in Guiyang region, China. J. Cent. South Univ. Technol. 2010, 17, 1216–1222. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.X.; Qin, Y.M.; He, Q.S.; Yi, Y.; Ji, Z.L. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2018, 649, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lang, M.; Zhu, S.X.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Greenhouse gas emissions are affected by land use type in two agroforestry systems: Results from an incubation experiment. Ecol. Res. 2020, 35, 1073–1086. [Google Scholar] [CrossRef]
- Wu, H.T.; Hao, X.H.; Xu, P.; Hu, J.L.; Jiang, M.D.; Shaaban, M.; Zhao, J.S.; Wu, Y.P.; Hu, R.G. CO2 and N2O emissions in response to dolomite application are moisture dependent in an acidic paddy soil. J. Soils Sediments 2020, 20, 3136–3147. [Google Scholar] [CrossRef]
- Wu, J.; Brookes, P.C. The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol. Biochem. 2005, 37, 507–515. [Google Scholar] [CrossRef]
- Ball, B.C. Soil structure and greenhouse gas emissions: A synthesis of 20 years of experimentation. Eur. J. Soil Sci. 2013, 64, 357–373. [Google Scholar] [CrossRef]
- Pareja-Sanchez, E.; Cantero-Martinez, C.; Alvaro-Fuentes, J.; Plaza-Bonilla, D. Impact of tillage and N fertilization rate on soil N2O emissions in irrigated maize in a Mediterranean agroecosystem. Agric. Ecosyst. Environ. 2020, 287, 106687. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.P.; Khalid, M.S.; Peng, Q.A.; Xu, X.Y.; Wu, L.; Younas, A.; Bashir, S.; Mo, Y.L.; Lin, S.; et al. Reduction in soil N2O emissions by pH manipulation and enhanced nosZ gene transcription under different water regimes. Environ. Pollut. 2018, 235, 625–631. [Google Scholar] [CrossRef]
- Rochette, P.; Tremblay, N.; Fallon, E.; Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Bertrand, N.; Parent, L.E. N2O emissions from an irrigated and non-irrigated organic soil in eastern Canada as influenced by N fertilizer addition. Eur. J. Soil Sci. 2010, 61, 186–196. [Google Scholar] [CrossRef]
- Qin, S.P.; Hu, C.S.; Clough, T.J.; Luo, J.F.; Oenema, O.; Zhou, S.G. Irrigation of DOC-rich liquid promotes potential denitrification rate and decreases N2O/(N2O+N2) product ratio in a 0–2 m soil profile. Soil Biol. Biochem. 2017, 106, 1–8. [Google Scholar] [CrossRef]
- Vilain, G.; Garnier, J.; Tallec, G.; Cellier, P. Effect of slope position and land use on nitrous oxide (N2O) emissions (Seine Basin, France). Agric. For. Meteorol. 2010, 150, 1192–1202. [Google Scholar] [CrossRef]
- Khalid, M.S.; Shaaban, M.; Hu, R.G. N2O, CH4, and CO2 Emissions from Continuous Flooded, Wet, and Flooded Converted to Wet Soils. J. Soil Sci. Plant Nutr. 2019, 19, 342–351. [Google Scholar] [CrossRef]
- Lan, T.; Han, Y.; Roelcke, M.; Nieder, R.; Cai, Z.C. Processes leading to N2O and NO emissions from two different Chinese soils under different soil moisture contents. Plant Soil 2013, 371, 611–627. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Maljanen, M.; Martikkala, M.; Koponen, H.; Virkajärvi, P.; Martikainen, P. Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil. Soil Biol. Biochem. 2007, 39, 914–920. [Google Scholar] [CrossRef]
- Chen, H.H.; Li, X.C.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Change Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Philippot, L.; Andert, J.; Jones, C.M.; Bru, D.; Hallin, S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob. Change Biol. 2011, 17, 1497–1504. [Google Scholar] [CrossRef]
- Krause, H.M.; Thonar, C.; Eschenbach, W.; Well, R.; Mäder, P.; Behrens, S.; Kappler, A.; Gattinger, A. Long term farming systems affect soils potential for N2O production and reduction processes under denitrifying conditions. Soil Biol. Biochem. 2017, 114, 31–41. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Z.L.; Zheng, X.H.; Ju, X.T.; Yao, Z.S.; Butterbach-Bahl, K.; Zhang, C.; Wei, H.H.; Huang, B.X. Using field-measured soil N2O fluxes and laboratory scale parameterization of N2O/(N2O+N2) ratios to quantify field-scale soil N2 emissions. Soil Biol. Biochem. 2020, 148, 107904. [Google Scholar] [CrossRef]
- Congreves, K.A.; Phan, T.; Farrell, R.E. A new look at an old concept: Using 15N2O isotopomers to understand the relationship between soil moisture and N2O production pathways. Soil 2019, 5, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.B.; Mäder, C.; Cai, Z.C. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 2015, 84, 199–209. [Google Scholar] [CrossRef]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle-could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Laughlin, R.J. Measurement of nitrous oxide and di-nitrogen emissions from agricultural soils. Nutr. Cycl. Agroecosyst. 1998, 52, 131–139. [Google Scholar] [CrossRef]
- Rassamee, V.; Sattayatewa, C.; Pagilla, K.; Chandran, K. Effect of Oxic and Anoxic Conditions on Nitrous Oxide Emissions from Nitrification and Denitrification Processes. Biotechnol. Bioeng. 2011, 108, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; MacDonald, J.D.; Pelster, D.E.; Bertrand, N. NH3 volatilization, soil concentration and soil pH following subsurface banding of urea at increasing rates. Can. J. Soil Sci. 2013, 93, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Compton, J.E.; Boone, R.D. Soil nitrogen transformations and the role of light fraction organic matter in forest soils. Soil Biol. Biochem. 2002, 34, 933–943. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Cuhel, J.; Simek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the Effect of Soil pH on N2O and N2 Emissions and Denitrifier Community Size and Activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.Z.; Miambi, E.; Robert, A.; Bernoux, M.; Brauman, A. Xylophagous termites: A potential sink for atmospheric nitrous oxide. Eur. J. Soil Biol. 2012, 53, 121–125. [Google Scholar] [CrossRef]
- Wu, D.M.; Dong, W.X.; Oenema, O.; Wang, Y.Y.; Trebs, I.; Hu, C.S. N2O consumption by low-nitrogen soil and its regulation by water and oxygen. Soil Biol. Biochem. 2013, 60, 165–172. [Google Scholar] [CrossRef]
- Schlesinger, W.H. An estimate of the global sink for nitrous oxide in soils. Glob. Change Biol. 2013, 19, 2929–2931. [Google Scholar] [CrossRef]
- Audet, J.; Hoffmann, C.C.; Andersen, P.M.; Baattrup-Pedersen, A.; Johansen, J.R.; Larsen, S.E.; Kjaergaard, C.; Elsgaard, L. Nitrous oxide fluxes in undisturbed riparian wetlands located in agricultural catchments: Emission, uptake and controlling factors. Soil Biol. Biochem. 2014, 68, 291–299. [Google Scholar] [CrossRef]



| pH | DTN (mg L−1) | DOC (mg L−1) | DON (mg L−1) | (mg L−1) | (mg L−1) | Bulk Density (g cm−3) |
|---|---|---|---|---|---|---|
| 7.36 ± 0.26 | 1.95 ± 0.07 | 13.92 ± 1.64 | 0.28 ± 0.07 | 0.18 ± 0.01 | 0.49 ± 0.04 | 1.2 ± 0.09 |
| Experimental Condition | Soil Moisture (WFPS) | C and N Addition | Temperature (°C) | Number of Replicates | Soil Sample Collection |
|---|---|---|---|---|---|
| CK5% | 5% | × | 25 | 3 | × |
| CK40% | 40% | × | 25 | 3 | × |
| CK70% | 70% | × | 25 | 3 | × |
| CK110% | 110% | × | 25 | 3 | × |
| NC5% | 5% | √ | 25 | 11 | √ |
| NC40% | 40% | √ | 25 | 11 | √ |
| NC70% | 70% | √ | 25 | 11 | √ |
| NC110% | 110% | √ | 25 | 11 | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Liu, X.; Qin, C.; Xing, W.; Gu, Y.; Wang, X.; Bai, L.; Li, J. Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China. Atmosphere 2022, 13, 1200. https://doi.org/10.3390/atmos13081200
Wei L, Liu X, Qin C, Xing W, Gu Y, Wang X, Bai L, Li J. Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China. Atmosphere. 2022; 13(8):1200. https://doi.org/10.3390/atmos13081200
Chicago/Turabian StyleWei, Lai, Xiaolong Liu, Caiqing Qin, Wencong Xing, Yongbo Gu, Xiaoxia Wang, Li Bai, and Jun Li. 2022. "Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China" Atmosphere 13, no. 8: 1200. https://doi.org/10.3390/atmos13081200
APA StyleWei, L., Liu, X., Qin, C., Xing, W., Gu, Y., Wang, X., Bai, L., & Li, J. (2022). Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China. Atmosphere, 13(8), 1200. https://doi.org/10.3390/atmos13081200








