Abstract
This study aimed to assess whether protective measures could reduce the health risks of air pollution in mice living in the chambers situated at a suburban site in Beijing. The living chambers of mice were divided into four groups: male mice with and without the high-efficiency particulate air (HEPA) filter (male group A and group B), as well as female mice with and without the HEPA filter (female group A and group B). The experiment was carried out from 1 December 2017 to 31 May 2018. Parameters of respiratory function during periods of clean air and air pollution were determined for all groups to evaluate the role of the indoor air filter (i.e., HEPA) in protection against respiratory health risks in mice. Significant differences in minute volumes were observed in male and female groups with versus without the HEPA. Additionally, respiratory health parameters including respiratory rate, duration of breaking, expiratory time, and relaxation time exhibited differences in female groups with HEPA versus without HEPA. Levels of inflammatory factors in the lungs were measured for all groups after 6months of exposure. Greater mean levels of IL-6 and TNF-α were found in the male groups without HEPA than in those with HEPA. Higher average concentrations of IL-6, T-AOC, SOD, GSH-Px, LDH, TNF-α, and TGF-β1 were found in the female group without HEPA than those without HEPA. Our study has proved the effective protection provided by indoor air filters (i.e., HEPA filters) in reducing respiratory health risks associated with PM2.5.
1. Introduction
Several studies have documented evidence that ambient fine particles (<2.5 microns in diameter, PM2.5) lead to increases in the incidence of mortality [1,2,3,4,5]. For example, an epidemiologic study estimated that ~4.2 million premature deaths resulted from exposure to ambient PM2.5 globally in 2015 [1]. The incidences of mortality associated with exposure to air pollution were not distributed evenly geographically. More than 90% of premature deaths due to exposure to air pollution were observed in developing countries of South-East Asia and the West Pacific Regions, where air pollution is high [1]. Ambient PM2.5 can enter the lungs and then result in cardiopulmonary diseases by triggering systemic inflammation [3,6,7]. Some studies have found that the increases in levels of inflammatory cytokines (e.g., IL-6, IL-8, and TNF-α) in bronchial fluid and circulating blood were linked directly with exposure to ambient PM2.5 [3,8,9]. In California, significant associations were found between blood inflammation cytokines in older adults and the mass concentration of urban PM2.5 [7]. In Shanghai, the levels of systemic inflammation cytokines in chronic obstructive pulmonary disease (COPD) patients were related to the concentrations of PM2.5 [10].
Mitigation of ambient PM2.5 concentration can facilitate public health without affecting economic development [11]. Reductions in anthropogenic emission sources such as mobile sources, biomass burning, coal combustion, etc., have proved to be effective mitigation measures in improving air quality regionally and globally [12,13,14]. Additionally, personal protective equipment for air pollution could also reduce the health risks of individuals from exposure to air pollution [15,16,17,18]. Barkjohn et al. [15] showed residential indoor air filtration could reduce the ambient levels of PM2.5 and O3 in homes in Shanghai. Meanwhile, the symptoms of airway resistance and inflammation in children with asthma were relieved with improvements in air quality with the aid of an indoor air filtration system [16].
Although significant advances in the role of indoor air filtration in reducing air pollution and protecting public health have been achieved [15,16], some points need to be further clarified. Experiments on the effects of indoor air filtration in reducing the respiratory health risks of human subjects exposed to long-term air pollution are difficult to perform due to the limits of many objective factors [17,18,19]. This study aims to investigate the role of indoor air filtration on the differences between the respiratory health risks of groups of different genders after long-term exposure to air pollution, by using an animal experiment with mice. This study designed an exposure system of ambient PM2.5 for mice equipped with a HEPA air filter and used this exposure system to accomplish the aimof the study. This work presents the essential information on the role of indoor air filtration in protecting respiratory health for the independent groups.
2. Experimental
2.1. Exposure System of Ambient PM2.5 for Mouse
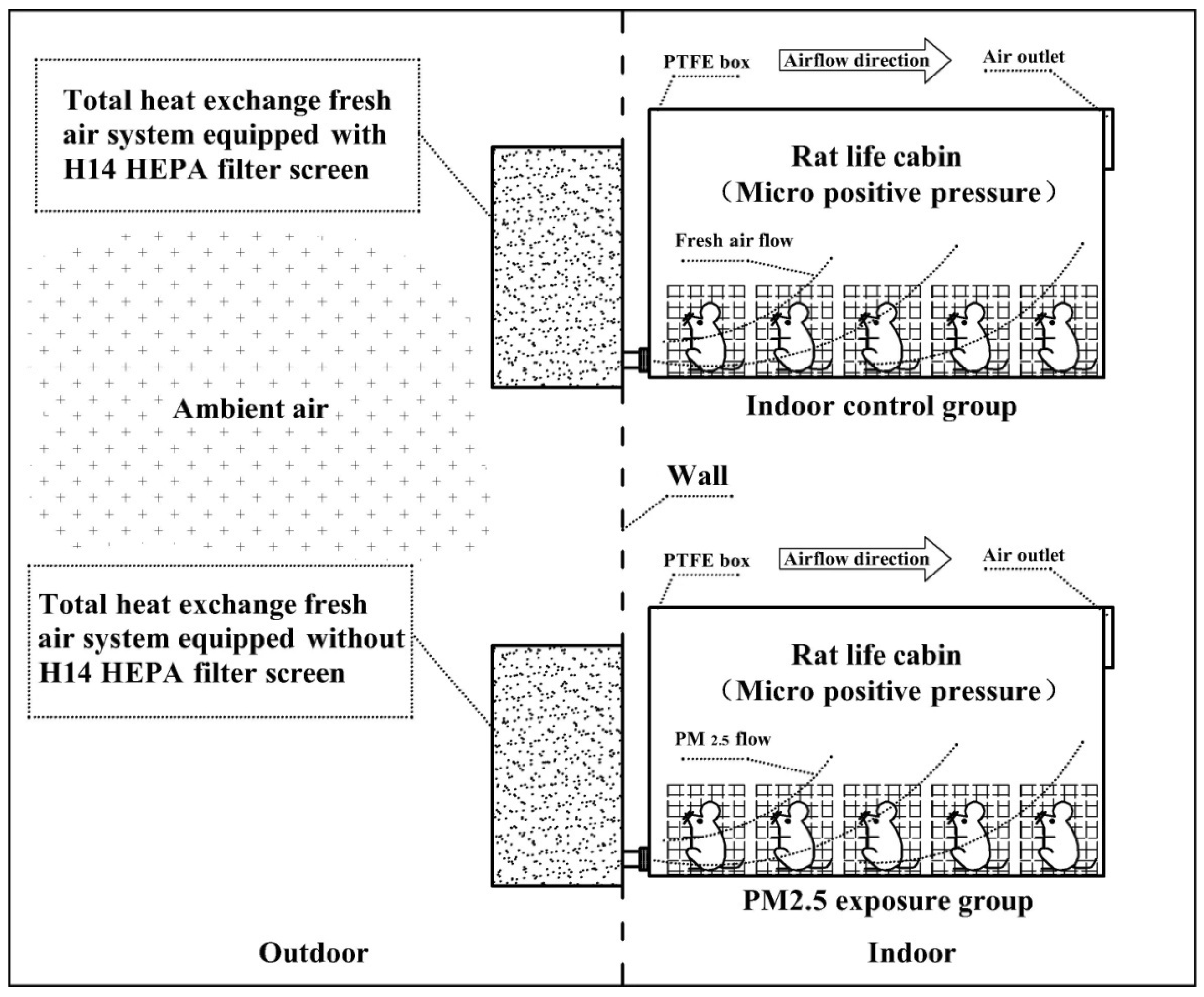
Figure 1 presents the diagrammatic sketch of the exposure system of ambient PM2.5 for the mouse. The system includes two separate sections that are for the control group and the exposure group, respectively. For the control group, the Swiss white mouse (purchased from Beijing Wei-Tong-Li-Hua Experimental Animal Technology Co., Ltd., Beijing, China.) is located in the living chambers maintained at a constant temperature (25 °C). The living chamber is made of Teflon (polytetrafluoroethylene) material and equipped with two ports. The port of exhaust gas with a diameter of 90 MMS is installed on the upper right surface of the living chamber. The other part of the air inlet is located on the upper left surface of the living chamber. The whole living chamber has kept the airtightness to ensure that the atmospheric pressure in the living chamber is slightly higher than ambient air. The port of the air inlet in the living chamber is connected with a fresh air system equipped with an H14 HEPA filter screen before absorbing ambient air. The flow rate was kept at 100 L min−1. HEPA filters are a type of pleated mechanical air filter, which are designed to control the ambient particles entering a clean area by filtration. Theoretically, HEPA filters could remove 99% of airborne particles with a size of 0.3microns (µm). HEPA filters are made up of a mat of very dense fibers that can trap smaller particles from the incoming air. There are several grades of HEPA filters, based on their ‘efficiency ratings’. One of the most commonly used HEPA filters is the H14 filter, which is designed to remove 99.99% of particles with a size of 0.3 microns (µm) from the air [15,16,17]. The airflow for the whole system is kept at 100 L min−1. A low-cost sensor PMS5003 (Plantower, Beijing, China) is used in the living chamber for monitoring levels of PM2.5. The sensor PMS5003 is an optical particle counter, which could use small fans to draw the particles of the interrogating laser beam for estimating the relative levels of PM2.5 quietly without affecting the behavior of the mouse. The PMS5003could measure the mass concentration of PM2.5 up to ~100 μg m−3 with 10% accuracy by converting the particle number concentration in an air volume of 0.1 L with a flow rate of 1 mL min−1 in small boxes with dimensions of 48 × 37 × 12 mm [20]. The PM2.5 results measured by PMS5003 were observed to agree well with those determined by the low-cost sensor OPC-N2 (Alphasense, London, UK) in a field study in the Czech Republic [20]. The detection limit of the sensor PMS5003 is estimated to be ~10 μg m−3 in a previous study [20]. In the exposure group, the Swiss white mice are situated in living chambers similar to those in the control group. The slight differences in the living chamber are those connected with a fresh air system equipped without an H14 HEPA filter screen before absorbing ambient air. The ambient air was pumped into living chambers with a flow rate of 100 L min−1 (Figure 1).
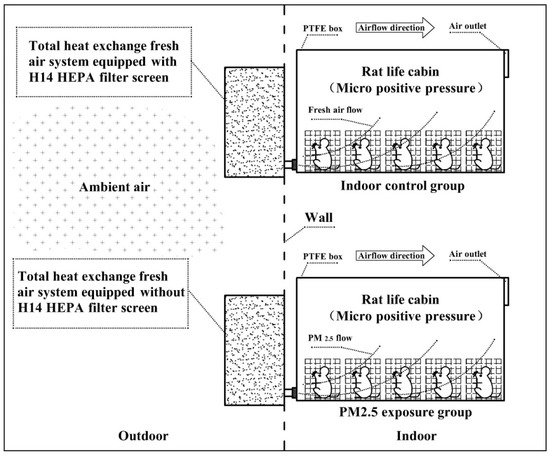
Figure 1.
The diagrammatic sketch of the experiments. Control group: Ambient air was filtered through the HEPA filter screen with a removal rate of PM2.5 higher than 99% and flowed into an indoor room where the living chambers of mice were located (n = 5). Exposure group: Ambient air was filtered through the impact cutter of PM2.5 and flowed into an indoor room where the living chambers of mice were located (n = 5). PTFE, Polytetrafluoroethylene.
2.2. Sampling Site
The exposure system experiments were carried out in the open area in Milu Park Ecological Research Center (Figure S1, 39.78 N, 116.47 E) [21]. The Milu Park is situated in a suburban area in southern Beijing and is surrounded by artificial wetlands with plenty of trees [22]. Thermo Scientific Tapered Element Oscillating Microbalance (TEOM) 1405-Ambient Particulate Monitor with Filter Dynamics Measurement System (FDMS) is used for monitoring hourly and daily mass concentration of PM2.5 in Milu Park from 1 December 2017 to 31 May 2018, which covers spring and winter seasons completely [22].
2.3. Bodyweight
The whole exposure experiment was conducted for 6 months from 1 December 2017 to 31 May 2018. The Swiss white mice used for the control group and exposure group in this study were 7 weeks old with the same body weight. In each group (i.e., control group and exposure group), there were four males and females, respectively. The measurements in body weight for each group were conducted every two weeks from 1 December 2017 to 31 May 2018, using an electronic balance with an accuracy of 0.1 mg (Sartorius, Goettingen, Germany). During this period, the feeding management included intakes of food and sterilized water as well as culture management and ventilation interval for the control group was the same as those in the exposure group.
2.4. Respiratory Function Parameters
Two individual sampling periods (15–18 March 2018 and 31 March–2 April 2018) were selected to examine the differences in respiratory function parameters of Swiss white mice between the control group and the exposure group in responses to the exposures to different levels of ambient PM2.5 mass concentrations. The hourly and daily mass concentrations of PM2.5 from 15 to 18 March 2018 were higher than 75 μg m−3, which is the 24 h value limit of PM2.5 recommended by Ambient Air Quality Standards in China (GB 3095-2012) [21,23]. While the hourly and daily mass levels of PM2.5 were found to be lower than 75 μg m−3 during the sampling period from 31 March to 2 April 2018. The respiratory function parameters include respiratory rate, tidal inhaled volume, minute volume, index of constriction, expiratory pause time, peak expiratory time, estimated peak inspiratory flow, estimated peak expiratory flow, inspiratory time, expiratory time, expiratory flow at 50%, expired volume, relaxation time, duration of breaking, duration of pause before inspiration, and rejection index for Swiss white mouse in the control group and the exposure group were estimated using whole-body plethysmography system (DSI Buxco, Wilmington, NC, USA) [24,25]. The measurements of the respiratory function parameters could be performed on the Swiss white mouse with a clear mind and unbound condition to eliminate the results interfered with by the stress response to the mouse by the trained staff [24]. The measurement was carried out at five parallel cavities at a time in a group of five mice in a large chamber of the whole-body plethysmography system. After the mice were placed gently in cavities, the upper cover of the cavity needs to be closed and kept for ~3 min until the mice were quiet.
Then, the whole-body plethysmography system was prepared to measure respiratory function parameters using a measurement program automatically. The whole process of measurements using a whole-body plethysmography system could last ~6 min for a group of four mice. Four groups, including male ones in the control group (Male Group A, n = 5), female ones in the control group (Female Group A, n = 5), male ones in the exposure group (Male Group B, n = 5), and female ones in the exposure group (Female Group B, n = 5), were adopted in the measurements of the respiratory function parameters. For each group, the respiratory function parameters of the mice were measured 80 times for the two individual sampling periods (15–18 March 2018 and 31 March–2 April 2018), respectively. In total, about 1200 measurements of respiratory function parameters were carried out for male and female mice in both the control and exposure groups for two individual sampling periods (e.g., 15–18 March 2018 and 31 March–2 April 2018), respectively.
The system pressure signal from the plethysmography was calibrated before each recording by injecting 50 mL of air into the system with a calibrated syringe in the large chamber of the whole-body plethysmography system. The independent calibration of each measurement was carried out automatically with the standard integral settings of the “Integral Channel Calculation module” of the whole-body plethysmography system [24,25]. The calculation module collects all data points and resets each cycle whereby the integral is reset each time the source signal passes from zero to a positive value.
2.5. Measurement of Cytokine in Lung Tissue
After 6-months of exposure, male and female mice in the control group and exposure group were all sacrificed by the trained staff. The lung tissues from all the mice were mixed with immobilizing solution containing 40% formaldehyde, dehydrated in a rank, and embedded with paraffin following standard histological techniques. The samples of the lung muscles were cut into slices (~0.5 μm) and stained with hematoxylin and eosin. Then, the slices of lung tissue with hematoxylin and eosin were observed and analyzed using an electron microscope (TE2000U, Nikon, Kyoto, Japan).
Additionally, the levels of biological indicators in lung tissue including Interleukin-6 (IL-6), total antibody oxidation capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), lactate dehydrogenase (LDH), tumor necrosis factor-α (TNF-α) and transforming growth factor-β1(TGF-β1) for male and female mice in the control and exposure groups were determined. The level of cytokines including IL-6, TNF-α, and TGF-β1 was measured using enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Measurements of T-AOC and GSH-Pxwere performed using a colorimetry kit (Jiancheng Corp, Nanjing, China) following the manufacturer’s instructions. Concentrations of SOD and LDH were determined with the immunohistochemistry method using a superoxide dismutaseassay Kit (Jiancheng Corp, Nanjing, China) and lactate dehydrogenase assay kit (Jiancheng Corp, Nanjing, China) complying with the manufacturer’s instructions, respectively. Each measurement of inflammatory indicators was carried out three times by the same trained staff. The repeatability expressed as a relative standard deviation lower than 5% was accepted.
2.6. Statistical Analysis
A normal distribution test is performed for all collected data on biological indicators. Then, biological data are presented as the means ± SDs for at least three independent mice experiments exposed to ambient PM2.5 samples of different sampling periods. Student’s t-test is used to compare the differences in biological data in response to the exposures of ambient PM2.5 samples of the control and exposure groups across different sampling periods. ANOVA analysis is carried out to examine the total differences in body weights across four groups. Furthermore, Student’s t-test is used to compare the differences in biological data in response to the exposures of ambient PM2.5 samples of the control and exposure groups over different sampling periods. All statistical analyses were carried out using SPSS V20.0 at the significant level of 0.05.
3. Results
3.1. Indoor and Outdoor PM2.5 Mass
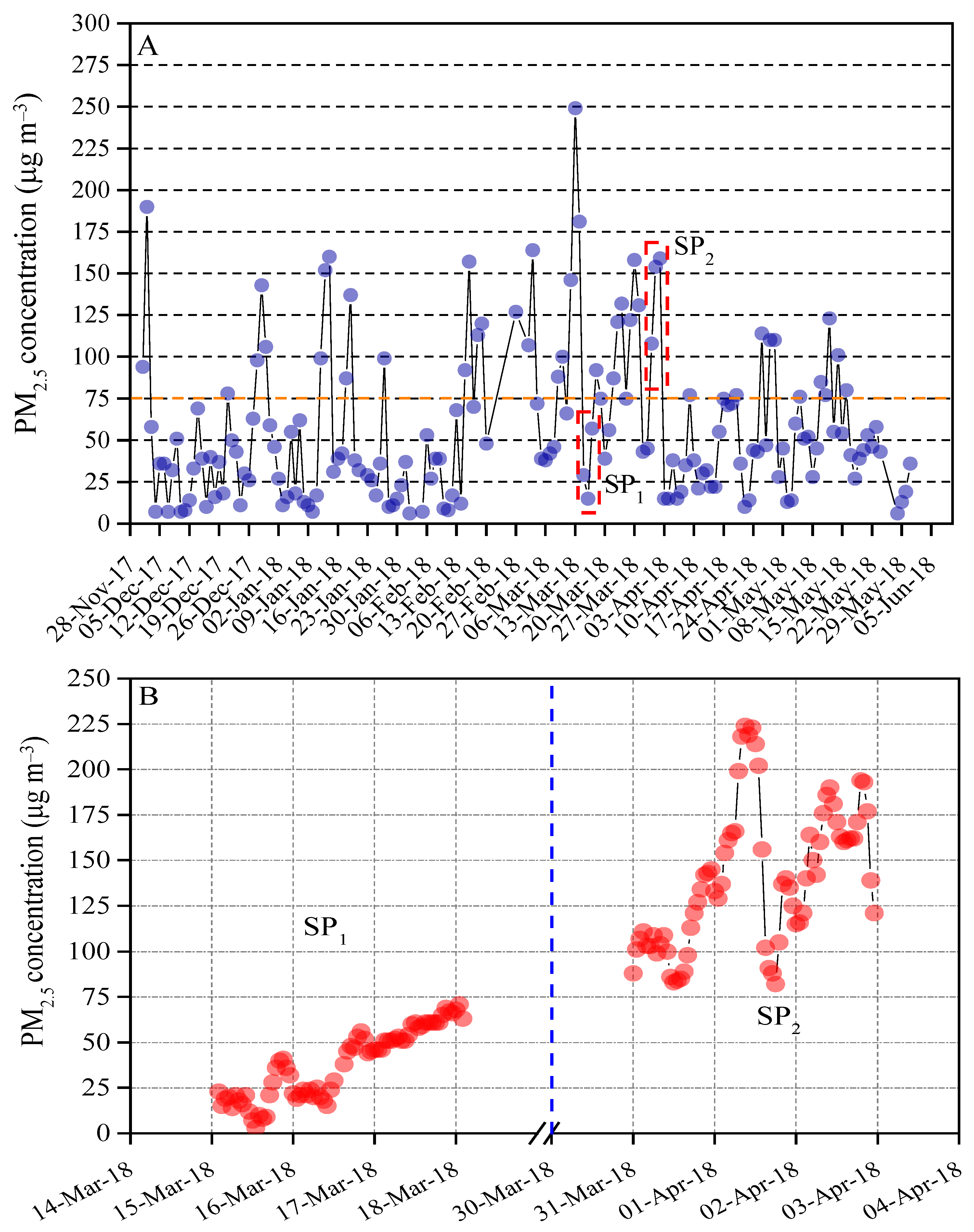
The daily PM2.5 mass varied from5 to 250 μg m−3 with an average concentration of 58 μg m−3. Of all 168 valid Partisol mass, 27% exceeded the limits of China’s ambient air quality standard (75 μg m−3) [22], while 59 daily PM2.5 mass was found to be lower than 35 μg m−3 and 64 daily PM2.5 mass were observed to be within the range of 35–75 μg m−3 (Figure 2A). Two-time periods (i.e., clean period and polluted period) with consecutive data points were selected for examining the effects of the indoor air filter on reductions in PM2.5-associated health risks of respiratory function in the mouse. The hourly mass concentration of PM2.5 from 15 to 18 March 2018 (SP1) was lower than 75 μg m−3 and thus considered to be a clean period. In contrast, the hourly mass concentration from 31 March to 2 April 2018 (SP2) was greater than 75 μg m−3 and chosen as an air polluted period (Figure 2B). For the exposure group, the indoor PM2.5 mass concentration of the living chamber of the mice approximately equals to ambient outdoor PM2.5 mass recorded by the online TEOM-FDMS system because the air inlet of the living chamber for mice connects to outdoor air directly. In the control group, the low-cost sensor PMS5003 was employed to record the indoor PM2.5 mass concentration. With the HEPA filter screen, the indoor PM2.5 mass concentration of the control group varied from the detection limits of low-cost sensor PMS5003 (~10 μg m−3) [20].
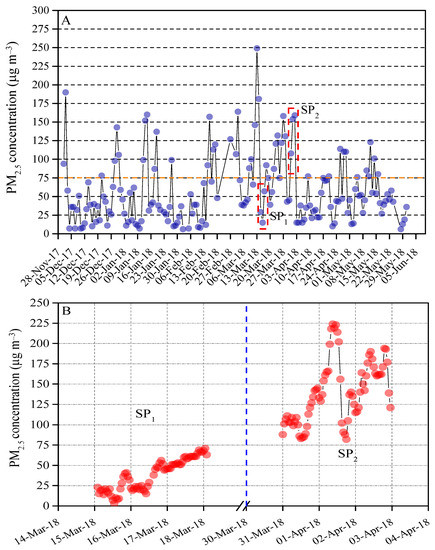
Figure 2.
(A) Daily mass concentration of PM2.5 from 1 December 2017 to 31 May 2018. 75 μg m−3 is the 24 h value limit of PM2.5 recommended by Ambient Air Quality Standards in China (GB 3095-2012). (B) Hourly mass concentration of PM2.5 from 15 to 18 March 2018 (SP1) and 31 March to 2 April 2018 (SP2), respectively.
3.2. Changes in Body Weights
The initial bodyweight of the male mouse for the control group and exposure group was ~37 g, which was higher than the body weight (~28 g) of the female mouse for the control group and exposure group (Figure S2). During 6-months of exposure, a steady increase in body weight was observed in all of the groups (male mouse with HEPA air filter system, male mouse without HEPA filter, the female mouse with HEPA air filter system, and female mouse without HEPA filter). The highest average body weight was found to be ~48 g (male mouse with HEPA air filter system), followed by ~42 g (male mouse without HEPA air filter system), ~35 g (female mouse with HEPA air filter system), and ~32 g (female mouse without HEPA air filter system). No significant differences in body weights were observed between mice with HEPA air filter systems and without HEPA air filter systems.
3.3. Respiratory Function
The detailed mean levels of respiratory function parameters for the mice in four groups were presented during the clean air period in Table S1. No significant differences were observed in mean levels of the respiratory function parameters including respiratory rate, tidal inhaled volume, minute volume, index of constriction, expiratory pause time, peak expiratory time, estimated peak inspiratory flow, estimated peak expiratory flow, inspiratory time, expiratory time, expiratory flow at 50% expired volume, relaxation time, duration of breaking, duration of pause before inspiration, and rejection index between the control group (mouse with HEPA filter) and exposure group (mouse without HEPA filter) during the air clean period (Table S1).
During the air polluted period, no significant differences in the respiratory function parameters including tidal inhaled volume, index of constriction, expiratory pause time, peak expiratory time, estimated peak inspiratory flow, estimated peak expiratory flow, inspiratory time, expiratory flow at 50% expired volume, duration of pause before inspiration and rejection index were found between the control group and the exposure group for both male and female mouse (Table 1). A higher mean level of respiratory rate (545.2 ± 23.6 BPM) and duration of breaking (18.3 ± 0.7%) was found in the female mice with HEPA filter than those (502.7 ± 19.3 BPM; 15.8 ± 1.3 %) in the female mice without HEPA filter. Meanwhile, lower levels of expiratory time and relaxation time were observed in the female mice with HEPA filter (0.07 ± 0.005 s; 0.04 ± 0.005 s) relative to those in the female mice without HEPA filter (0.09 ± 0.009 s; 0.06 ± 0.007 s). These findings indicate that female mice raise their respiratory performance to breathe in more air for maintaining the normal function of the lung during the air-polluted period. On the contrary, the mean levels of respiratory rate, expiratory time, relaxation time, and duration of breaking were observed to be 525.8 ± 21.6 BPM, 0.07 ± 0.008 s, 0.05 ± 0.004 s, and 17.5 ± 1.2% in the mice of the control group, which were similar to those in the mice of the exposure group (527.8 ± 29.9 BPM, 0.08 ± 0.008 s, 0.05 ± 0.007 s, and 17.2 ± 0.9 %). Greater mean levels in minute volume (i.e., the total amount of gas entering the lung per minute) were observed for both male mice (100.4 ± 15.8 mL min−1) and female mice (98.2 ± 6.1 mL min−1) without HEPA filter compared to those (83.5 ± 9.1 mL min−1; 91.3 ± 8.5 mL min−1) with HEPA filter. The results indicate that more air is interchanged in the lung without the protection of personal protective equipment.

Table 1.
The summary data (mean± standard deviation) on indicators of respiratory function in mice exposed to ambient air with and without HEPA filter from 31 March 31 to 2 April 2018 (air polluted period).
3.4. Inflammatory Responses
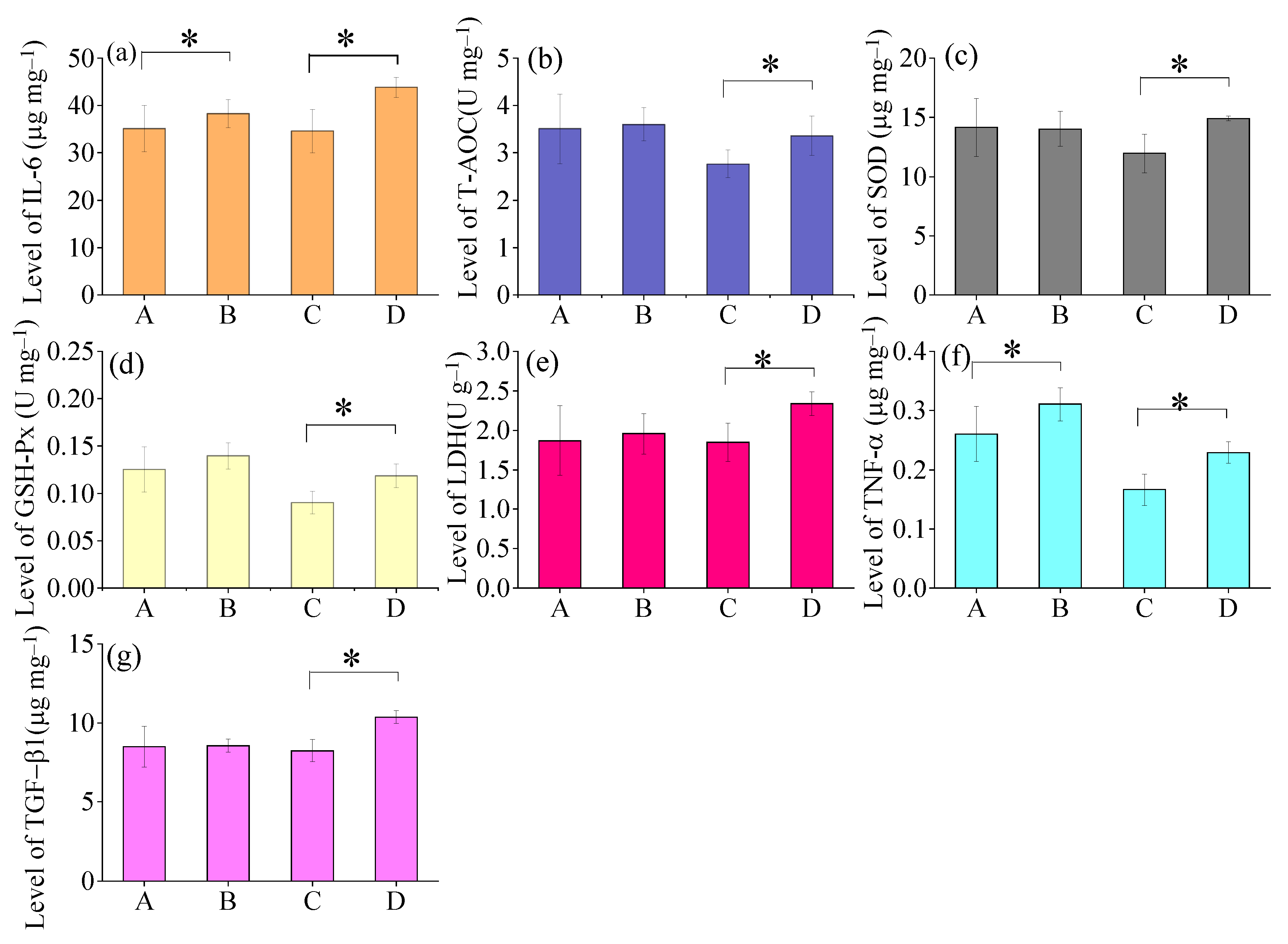
As shown in Figure 3, the mean levels of IL-6 and TNF-α were observed to be higher in Male Group B (IL-6: 38.3 ± 2.9 μg mg−1; TNF-α: 0.31 ± 0.02 μg mg−1) than Male Group A (IL-6: 35.1 ± 3.2 μg mg−1; TNF-α: 0.26 ± 0.04 μg mg−1) after 6-month exposure of air pollution. Moreover, all the measured biological indicators including IL-6, T-AOC, SOD, GSH-Px, LDH, TNF-α, and TGF-β1 exhibited greater levels in Female Group B (IL-6: 43.8 ± 2.1 μg mg−1; T-AOC: 3.4 ± 0.4 U mg−1; SOD: 14.9 ± 0.2 μg mg−1; GSH-Px: 0.11 ± 0.01 U mg−1; LDH: 2.33 ± 0.05 U g−1; TNF-α: 0.22 ± 0.02 μg mg−1; TGF-β1: 10.4 ± 0.4 μg mg−1) compared to those with Female Group A(IL-6: 34.6 ±4.6 μg mg−1; T-AOC:2.8 ±0.3 U mg−1; SOD: 11.9 ± 1.6 μg mg−1;GSH-Px: 0.09 ± 0.01 U mg−1; LDH: 1.85 ± 0.24 U g−1; TNF-α: 0.17 ± 0.02 μg mg−1; TGF-β1:8.2 ± 0.7 μg mg−1) after 6-month exposure of air pollution.
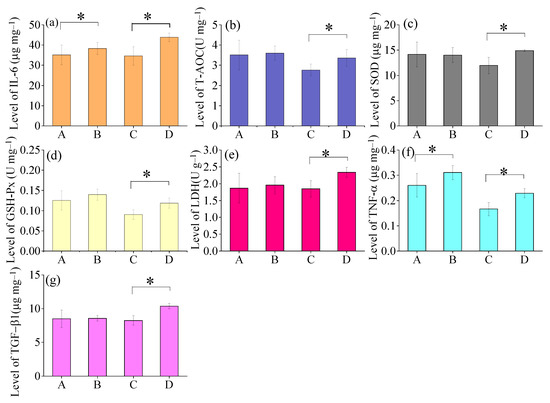
Figure 3.
Changes in levels of inflammatory factors of mice lungs among different groups after 6months of exposure to ambient air: A. Male Group A (n = 5), B. Male Group B (n = 5), C. Female Group A (n = 5), D. Female Group B (n = 5). (a) IL-6, (b) T-AOC, (c) SOD, (d) GSH-Px, (e) LDH, (f) TNF-α, and (g) TGF-β1 * Means within two groups are significantly different at p < 0.05 (Student’s t-test).
Pathological sections of the lungs for mice in the four groups after 6-months of exposure to ambient air were performed (Figure S3). Pulmonary fibrosis was observed in both male mice groups and female groups without HEPA filter (Figure S3B,D), while minor changes in the lungs were found in male mice groups and female groups with HEPA filter (Figure S3A,C).
4. Discussion
This study was carried out to illustrate the role of HEPA in the protection of respiratory risk associated with ambient air pollution via an animal experiment with mice. By conducting the control experiments within the same gender, the study was capable of showing differences in respiratory risk associated with ambient air pollution in male and female mice with and without the aid of protection of HEPA filter.
Huang et al. [26] showed that elevated air pollution is associated with a higher level of body mass index and a greater risk of obesity in a systematic review and meta-analysis. Zhang et al. [27] found long-term exposure to air pollution was associated with increased body weight in a large population of Chinese children and adolescents (~45,000). However, no significant changes in body weights were found across male and female groups with and without HEPA filters. Our results indicate that the HEPA air filter system can effectively reduce the impact of air pollutants on body weights to a certain extent.
We observed greater performance of respiratory function in male and female groups with HEPA filters than in those groups without HEPA filters during the air pollution period and after a long-term period (e.g., 6-months), supporting that HEPA could alleviate the respiratory risks due to the exposures to air pollution [28,29]. These observed findings were also supported by the results of biological indicators. The degrees of inflammatory responses were found to be severe in the mice without HEPA filters relative to those without HEPA filters. Wenke et al. [30] found that air filters composed of either a prefilter or a secondary fiberglass filter could reduce bioaerosols and the risk of introducing airborne transmitted pathogens to pig facilities. Chen et al. [31] illustrated that air filters (i.e., HEPA) could decrease acute pulmonary inflammation in mice due to the exposure. Haghani et al. [32] presented the findings that greater inflammatory responses were observed in adult mice brains with exposure to ambient particulate matter (PM) relative to the filtered ambient PM. Our results are consistent with prior findings conducted among children with asthma living in homes in Shanghai with indoor air filters [15,16]. The symptoms of airway resistance and inflammation were reduced in response to the improvements in air quality with the protection of an indoor air filtration system [15,16]. Another study conducted by Tamana and their colleagues [33] demonstrated that improvements in cardiometabolic health in children with a mean age of 23.8 months in Mongolia were found when the exposure to air pollution in their mothers was reduced through a HEPA filter during the gestation period.
Our findings are not without limitations. Although we achieved the observations on respiratory performance and inflammatory response of mice associated with long-term ambient air pollution with the protection of HEPA filters, our results were limited by the measurement site. The experiments were carried out at a suburban site in Beijing where the highest daily level of PM2.5 is found to be lower than 250 μg m−3. Prior studies indicated that the health burdens associated with exposure to air pollution are distributed geographically unevenly and observed to be higher in countries with increasing levels of air pollution [1,34]. It is speculated that the findings on the protection of respiratory risk via a HEPA filter could vary slightly with enhanced levels of PM2.5 in areas compared to this study [30,31,32,33]. The notable strength of this study suggests the effective protection of HEPA filtersin reducing PM2.5 associated with respiratory health risks for both male and female groups through the innovative experimental design.
5. Conclusions
Our study indicates that mice in groups without HEPA filters exchange more air through the lungs than groups with HEPA filters during a high air pollution period, resulting in greater respiratory health risks. The differences in levels of the respiratory function parameters between the control group and exposure group in the air-polluted period within the same gender revealed that HEPA can reduce respiratory health risks resulting from ambient air pollution. Additionally, significant differences in the levels of inflammatory factors in mice lungs between groups with and without HEPA filters after 6months of exposure to ambient air were observed. These findings indicate that HEPA filters can decrease the inflammatory response due to exposure to air pollution in mice. This study provides field data on the effective protection of HEPA filters in reducingPM2.5-associated respiratory health risks.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos13071005/s1, Figure S1. The location of sampling site (Milu Park, 39.78 N, 116.47 E); Figure S2. Changes in weights of mice with HEPA air filter system and without HEPA air filter system were collected from 1 December 2017 to 31 May 2018, respectively. The data on mice weight for each group were obtained every two weeks from 1 December 2017 to 31 May 2018; Figure S3. Pathological section of the lung for mice in different groups after 6-months exposure to ambient air. A. Male Group A, B. Male Group B, C. Female Group A, D. Female Group B; Table S1. The summary data (mean ± standard deviation) on indicators of respiratory function in mice exposed to ambient air with and without HEPA filter from 15–18 March 2018 (clean period). Figures S1–S3 and Table S1 describe details of the locations of the sampling site, results of changes in weights of mice with the HEPA air filter system and without the HEPA air filter system, as well as the data on indicators of respiratory function in mice exposed to ambient air with and without the HEPA filter.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Z.Y., Q.L., Y.L., Q.G., Y.S., Z.C. and Z.Z. The first draft of the manuscript was written by Q.L. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Nature Science Foundation of China (41475133).
Institutional Review Board Statement
The mice included in the present study were recruited from Beijing Vital River Laboratory Animal Technology Co., Ltd. The animal study protocol was approved by the ethical committee of Beijing Milu Ecological Research Center (approval ID number, 2018-006) and was carried out following the current Chinese legislation on animal protection.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors upon requirement without reservation.
Acknowledgments
We would like to appreciate the comments from anonymous reviewers for improving the quality of the manuscript.
Conflicts of Interest
There are no interest of conflict to declare.
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L. Assessment of the health impacts of particulate matter characteristics. Res. Rep. Health Eff. Inst. 2012, 161, 5–38. [Google Scholar]
- Becher, R.; Bucht, A.; Øvrevik, J.; Hongslo, J.K.; Dahlman, H.J.; Samuelsen, J.T.; Schwarze, P.E. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban sir and mineral particles. Inhal. Toxicol. 2007, 19, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Baumgartner, J.; Schauer, J.J. Source apportionment of fine-particle, water-soluble organic nitrogen and its association with the inflammatory potential of lung epithelial cells. Environ. Sci. Technol. 2019, 53, 9845–9854. [Google Scholar] [CrossRef]
- Liu, Q.; Baumgartner, J.; Zhang, Y.; Schauer, J.J. Source apportionment of Beijing air pollution during a severe winter haze event and associated pro-inflammatory responses in lung epithelial cells. Atmos. Environ. 2016, 126, 28–35. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Q.; Liu, Y.; Qi, X.; Wang, X. Cell cycle arrest of human bronchial epithelial cells modulated by differences in chemical components of particulate matter. RSC Adv. 2021, 11, 10582–10591. [Google Scholar] [CrossRef]
- Liu, Q.; Baumgartner, J.; Zhang, Y.; Liu, Y.; Sun, Y.; Zhang, M. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ. Sci. Technol. 2014, 48, 12920–12929. [Google Scholar] [CrossRef]
- Liu, C.; Cai, J.; Qiao, L.; Wang, H.; Xu, W.; Li, H.; Zhao, Z.; Chen, R.; Kan, H. The acute effects of fine particulate matter constituents on blood inflammation and coagulation. Environ. Sci. Technol. 2017, 51, 8128–8137. [Google Scholar] [CrossRef]
- Liu, Q.; Baumgartner, J.; de Foy, B.; Schauer, J.J. A global perspective on national climate mitigation priorities in the context of air pollution and sustainable development. City Environ. Interact. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Lai, A.M.; Clark, S.; Carter, E.; Shan, M.; Ni, K.; Yang, X.; Baumgartner, J.; Schauer, J.J. Impacts of stove/fuel use and outdoor air pollution on chemical composition of household particulate matter. Indoor Air 2020, 30, 294–305. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, N.; Huang, J.; Zang, Z.; Guan, X.; Ma, X.; Luo, Y.; Li, J.; Zhang, X.; Zhang, Y. Estimations of indirect and direct anthropogenic dust emission at the global scale. Atmos. Environ. 2019, 200, 50–60. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, H.; Hao, J. Global anthropogenic atmospheric emission inventory of twelve typical hazardous trace elements, 1995–2012. Atmos. Environ. 2020, 220, 117061. [Google Scholar] [CrossRef]
- Barkjohn, K.K.; Norris, C.; Cui, X.; Fang, L.; Zheng, T.; Schauer, J.J.; Li, Z.; Zhang, Y.; Black, M.; Zhang, J.; et al. Real-time measurements of PM2.5 and ozone to assess the effectiveness of residential indoor air filtration in Shanghai homes. Indoor Air 2021, 31, 74–87. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Teng, Y.; Cui, X.; Barkjohn, K.K.; Norris, C.; Fang, L.; Lin, L.; Wang, Q.; Zhou, X.; et al. Associations of personal exposure to air pollutants with airway mechanics in children with asthma. Environ. Int. 2020, 138, 105647. [Google Scholar] [CrossRef]
- Waring, M.S.; Siegel, J.A.; Corsi, R.L. Ultrafine particle removal and generation by portable air cleaners. Atmos. Environ. 2008, 42, 5003–5014. [Google Scholar] [CrossRef]
- Isiugo, K.; Jandarov, R.; Cox, J.; Chillrud, S.; Grinshpun, S.A.; Hyttinen, M.; Yermakov, M.; Wang, J.; Ross, J.; Reponen, T. Predicting indoor concentrations of black carbon in residential environments. Atmos. Environ. 2019, 201, 223–230. [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Jung, D.-Y.; Shin, Y.-J.; Lee, K.-S.; Lee, S.-Y.; Ahn, K.; Kim, K.W.; Shin, Y.H.; Suh, D.I.; Hong, S.-J.; et al. Association of ambient air pollution with depressive and anxiety symptoms in pregnant women: A prospective cohort study. Int. J. Hyg. Environ. Health 2021, 237, 113823. [Google Scholar] [CrossRef]
- Bauerová, P.; Šindeláˇrová, A.; Rychlík, Š.; Novák, Z.; Keder, J. Low-cost air quality sensors: One-year field comparative measurement of different gas sensors and particle counters with reference monitors at tusimice observatory. Atmosphere 2020, 11, 492. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Liu, Q. Estimations of ambient fine particle and ozone level at a suburban site of Beijing in winter. Environ. Res. Commun. 2021, 3, 081008. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Liu, Q.; Qi, X.; Qu, J.; Zhang, S.; Wang, X.; Jia, K.; Zhu, M. Study on chemical components and sources of PM2.5 during heavy air pollution periods at a suburban site in Beijing of China. Atmos. Pollut. Res. 2021, 12, 188–199. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Feng, T.; Hao, G.; Yang, S.; Wang, N.; Yan, L.; Pang, Y.; Niu, Y.; Zhang, R. Ambient air pollution exposed during preantral-antral follicle transition stage was sensitive to associate with clinical pregnancy for women receiving IVF. Environ. Pollut. 2020, 265, 114973. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Shanmuganathan, D.; Jayarajan, P.; Abraham, R.; Kancharla, B. Comparison of whole body and head out plethysmography using respiratory stimulant and depressant in conscious rats. J. Pharmacol. Tox. Met. 2012, 65, 37–43. [Google Scholar] [CrossRef]
- Fares, R.; Bory, C.; Valgalier, C.; Baudet, S. Implementation of DSI FinePointe® whole body plethysmography method in freely moving rats. J. Pharmacol. Tox. Met. 2019, 99, 106595. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, X.; Huang, J.; Lu, X.; Liu, F.; Gu, D. Ambient air pollution and body weight status in adults: A systematic review and meta-analysis. Environ. Pollut. 2020, 265, 114999. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, B.; Chen, G.; Song, Y.; Li, S.; Yang, Z.; Dong, Y.; Wang, Z.; Ma, J.; Guo, Y. Ambient air pollution and obesity in school-aged children and adolescents: A multicenter study in China. Sci. Total Environ. 2021, 771, 144583. [Google Scholar] [CrossRef]
- Mousavi, E.S.; Godri Pollitt, K.J.; Sherman, J.; Martinello, R.A. Performance analysis of portable HEPA filters and temporary plastic anterooms on the spread of surrogate coronavirus. Build. Environ. 2020, 183, 107186. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lu, M.-C.; Yang, S.-F.; Bien, M.-Y.; Chen, Y.-F.; Li, Y.-T. Respiratory care for the critical patients with 2019 novel coronavirus. Respir. Med. 2021, 186, 106516. [Google Scholar] [CrossRef]
- Wenke, C.; Pospiech, J.; Reutter, T.; Truyen, U.; Speck, S. Efficiency of different air filter types for pig facilities at laboratory scale. PLoS ONE 2017, 12, e0186558. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Chen, Y.Y.; Lin, W.; Chien, C.W.; Chen, C.H.; Wen, Y.C.; Hsiao, T.C.; Chuang, H.C. Effects of human umbilical cord-derived mesenchymal stem cells on the acute cigarette smoke-induced pulmonary inflammation model. Front. Physiol. 2020, 11, 962. [Google Scholar] [CrossRef]
- Haghani, A.; Johnson, R.; Safi, N.; Zhang, H.; Thorwald, M.; Mousavi, A.; Woodward, N.C.; Shirmohammadi, F.; Coussa, V.; Wise, J.P.; et al. Toxicity of urban air pollution particulate matter in developing and adult mouse brain: Comparison of total and filter-eluted nanoparticles. Environ. Int. 2020, 136, 105510. [Google Scholar] [CrossRef]
- Tamana, S.K.; Gombojav, E.; Kanlic, A.; Banzrai, C.; Batsukh, S.; Enkhtuya, E.; Boldbaatar, B.; Lanphear, B.P.; Lear, S.A.; McCandless, L.C.; et al. Portable HEPA filter air cleaner use during pregnancy and children’s body mass index at two years of age: The UGAAR randomized controlled trial. Environ. Int. 2021, 56, 106728. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Tang, Z.; Hood, R.B.; Ford, J.; Schwartz, J.D.; Jones, D.P.; Laden, F.; Liang, D. Periconception air pollution, metabolomic biomarkers, and fertility among women undergoing assisted reproduction. Environ. Int. 2021, 155, 106666. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).