Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model
Abstract
1. Introduction
2. Model Description
2.1. OpenSMOKE++
2.2. SAMM
2.3. Numerical Experiments
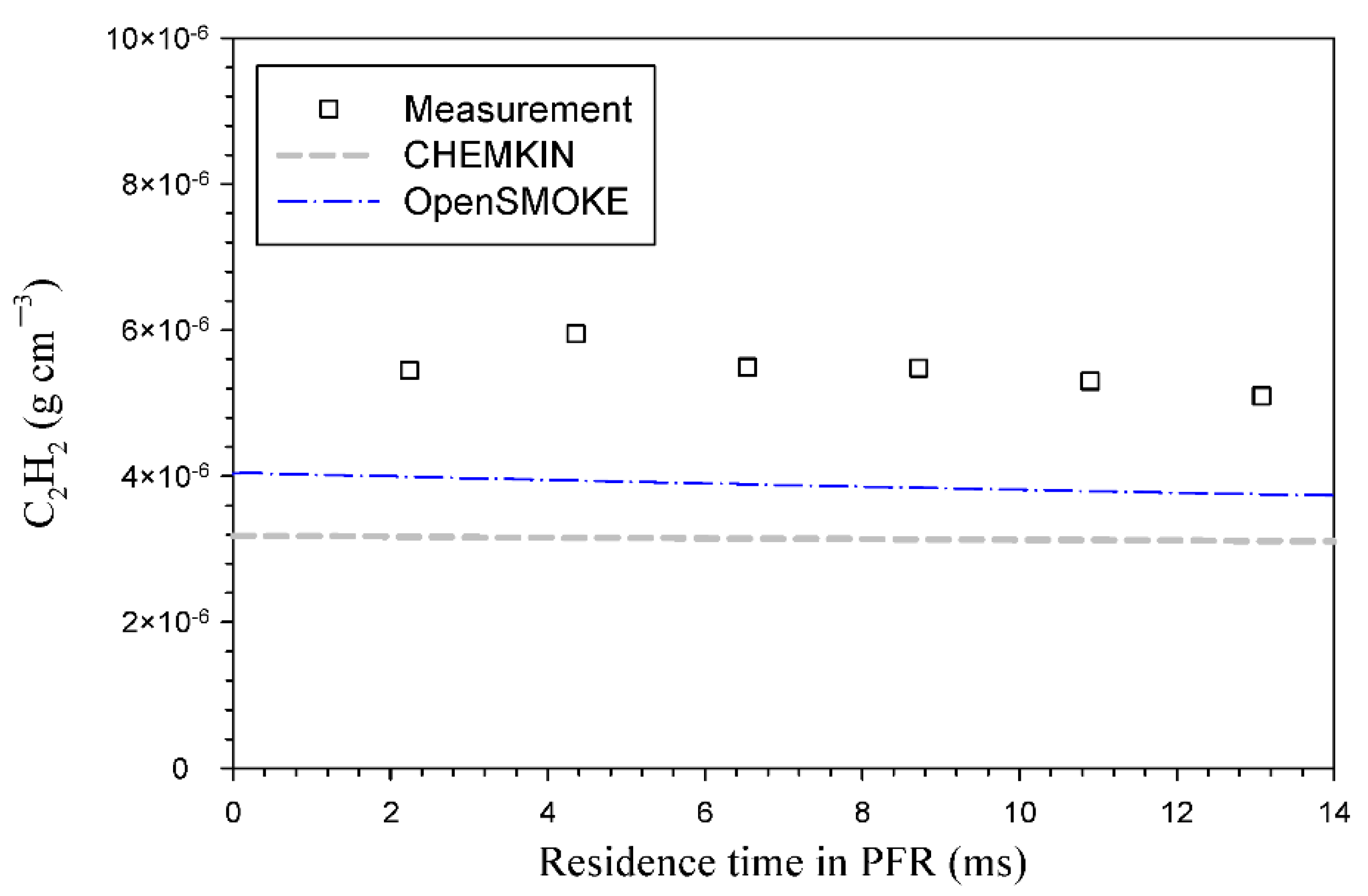
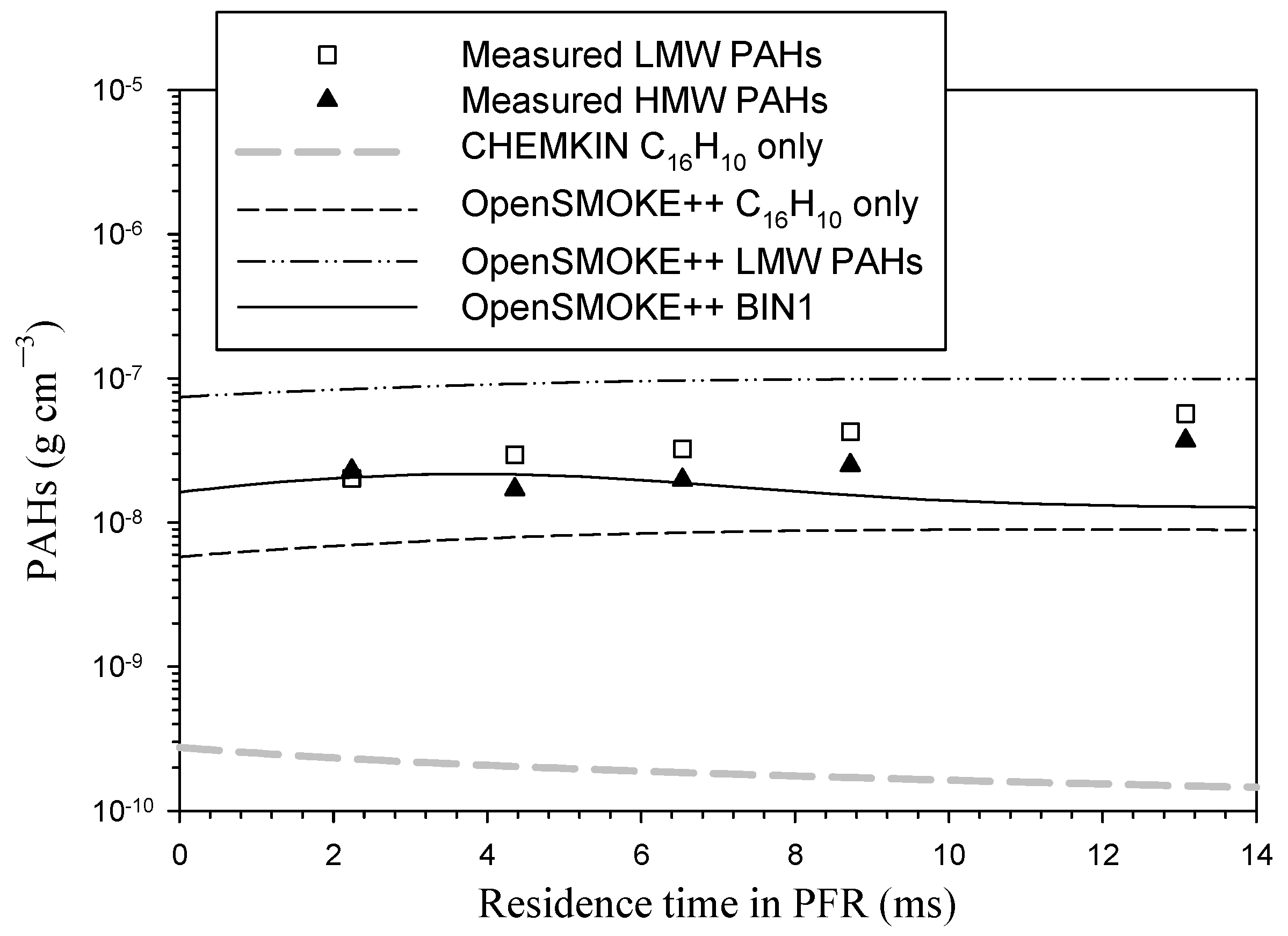
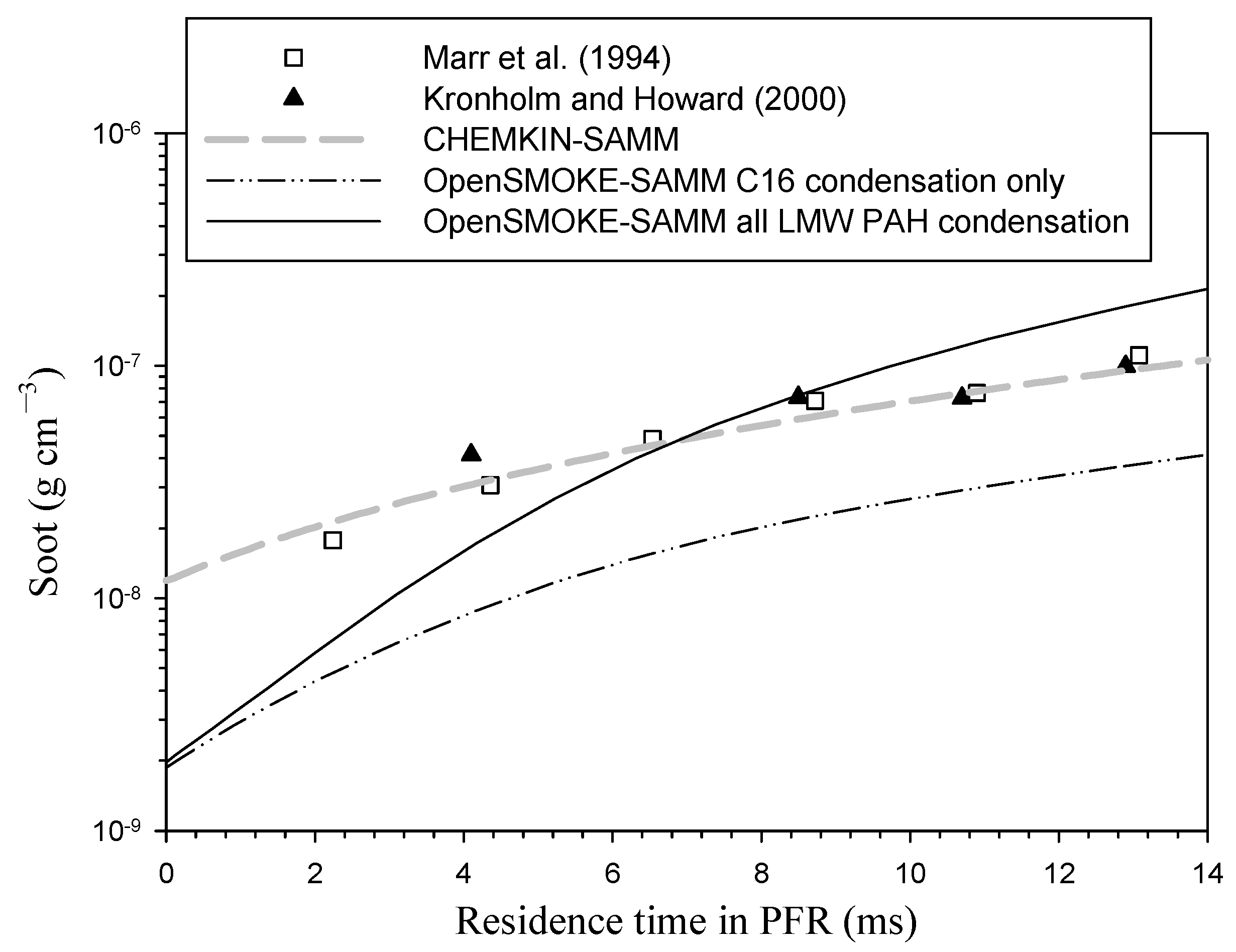
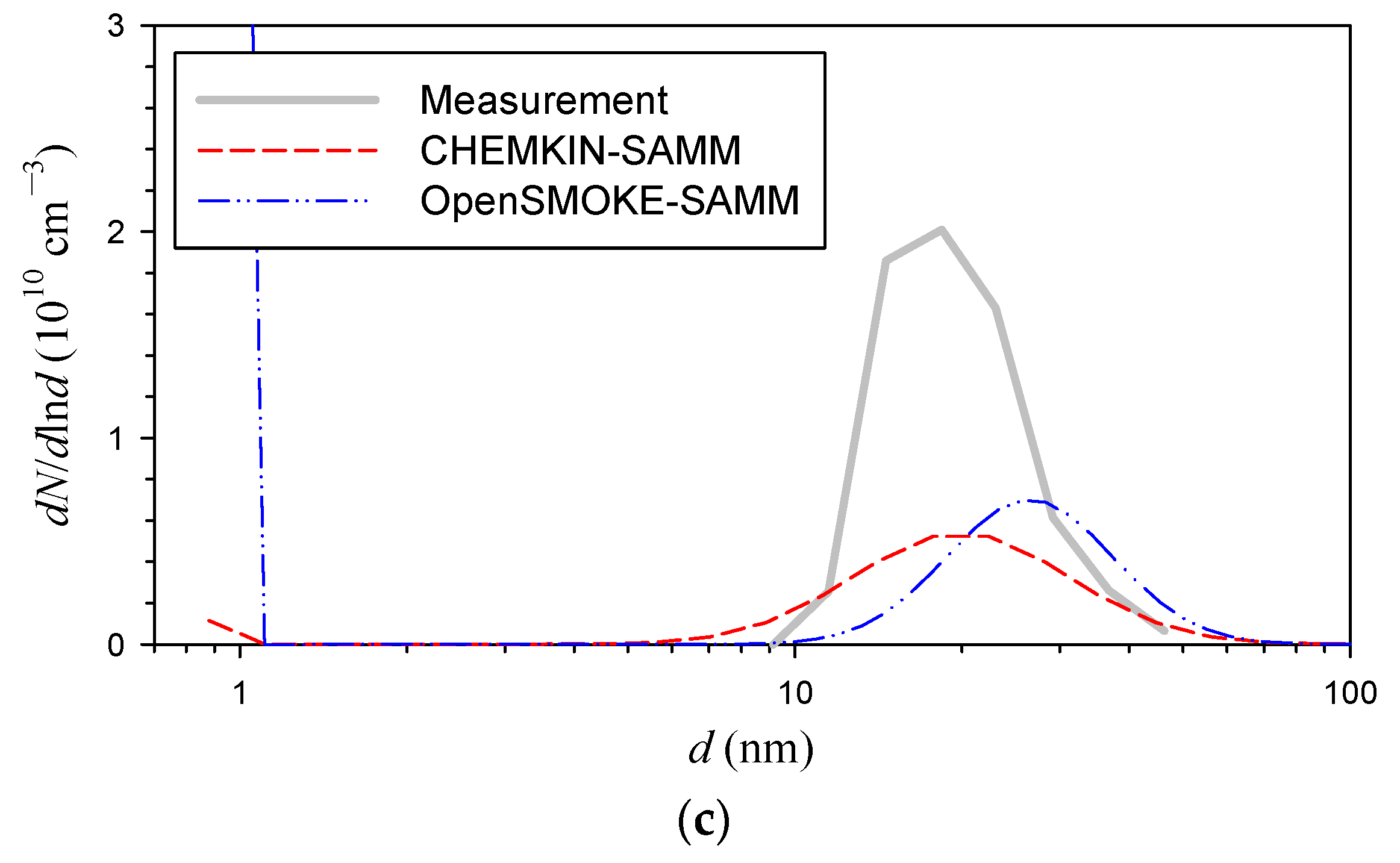
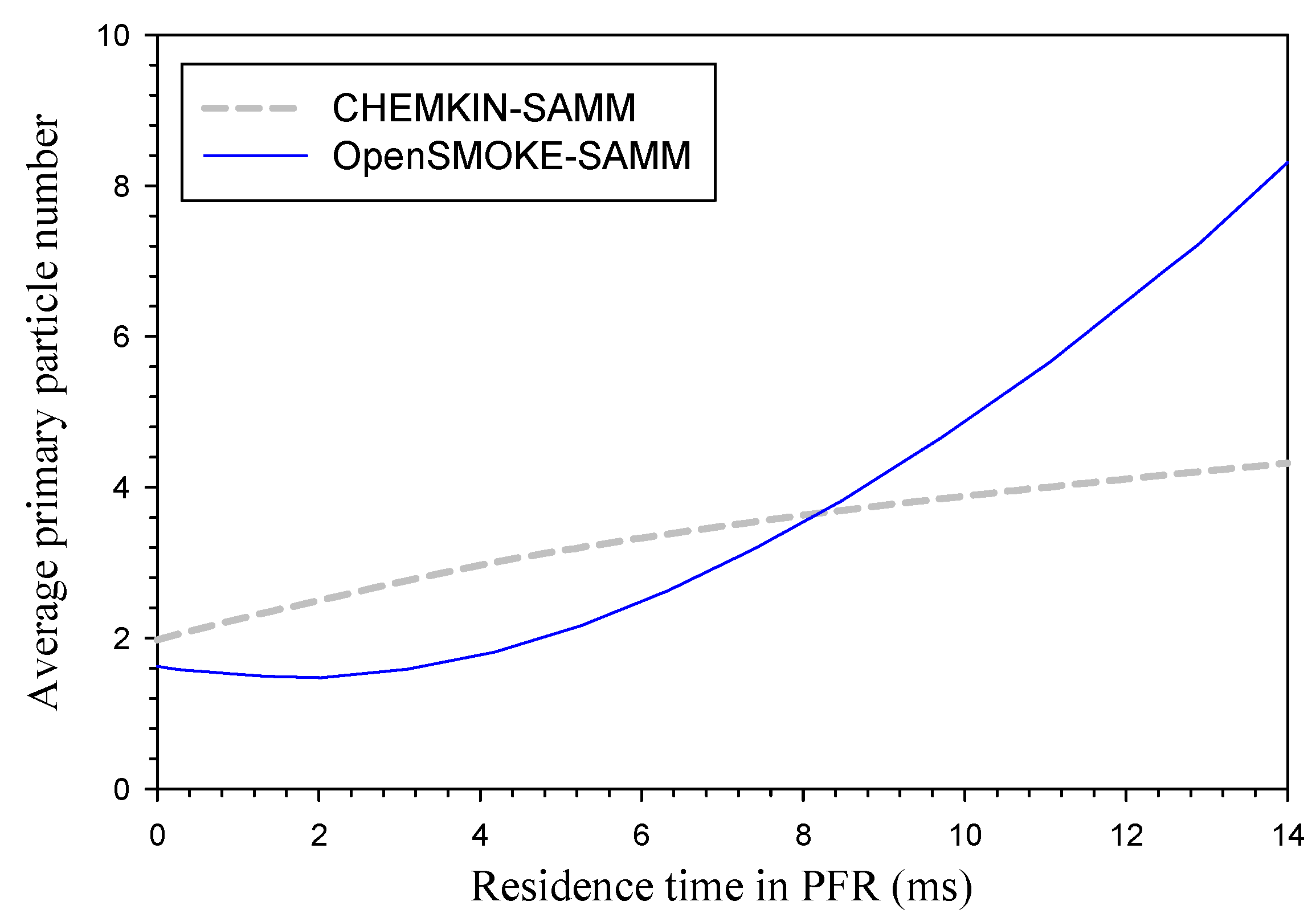
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef]
- Park, S.H.; Gong, S.L.; Bouchet, V.S.; Gong, W.; Makar, P.A.; Moran, M.D.; Stroud, C.A.; Zhang, J. Effects of black carbon aging on air quality predictions and direct radiative forcing estimation. Tellus B 2011, 63, 1026–1039. [Google Scholar] [CrossRef]
- Zhang, R.; Kook, S. Influence of fuel injection timing and pressure on in-flame soot particles in an automotive-size diesel engine. Environ. Sci. Technol. 2014, 48, 8243–8250. [Google Scholar] [CrossRef]
- Aubagnac-Karkar, D.; Michel, J.; Colin, O.; Vervisch-Kljakic, P.E.; Darabiha, N. Sectional soot model coupled to tabulated chemistry for Diesel RANS simulations. Combust. Flame 2015, 162, 3081–3099. [Google Scholar] [CrossRef]
- Michelsen, H.A. Probing soot formation, chemical and physical evolution, and oxidation: A review of in situ diagnostic techniques and needs. Proc. Combust. Inst. 2017, 36, 717–735. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, W.; Yu, W. A progress review of practical soot modelling development in diesel engine combustion. J. Traffic Transp. Eng. 2020, 7, 269–281. [Google Scholar] [CrossRef]
- Wang, L.; Haworth, D.C.; Turns, S.R.; Modest, M.F. Interactions among soot, thermal radiation, and NOx emissions in oxygen-enriched turbulent nonpremixed flames: A computational fluid dynamics modeling study. Combust. Flame 2005, 141, 170–179. [Google Scholar] [CrossRef]
- Lucchini, T.; D’Errico, G.; Onorati, A.; Bonandrini, G.; Venturoli, L.; Di Gioia, R. Development and application of a computational fluid dynamics methodology to predict fuel–air mixing and sources of soot formation in gasoline direct injection engines. Int. J. Engine Res. 2014, 15, 581–596. [Google Scholar] [CrossRef]
- Tan, J.Y.; Bonatesta, F.; Ng, H.K.; Gan, S. Developments in computational fluid dynamics modelling of gasoline direct injection engine combustion and soot emission with chemical kinetic modelling. Appl. Therm. Eng. 2016, 107, 936–959. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. Toward accommodating realistic fuel chemistry in large-scale computations. Prog. Energy Combust. Sci. 2009, 35, 192–215. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Pitz, W.J.; Herbinet, O.; Curran, H.J.; Silke, E.J. A comprehensive detailed chemical kinetic reaction mechanism for combustion of n-alkane hydrocarbons from n-octane to n-hexadecane. Combust. Flame 2009, 156, 181–199. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Naik, C.V.; Herbinet, O.; Pitz, W.J.; Mehl, M.; Sarathy, S.M.; Curran, H.J. Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels. Combust. Flame 2011, 158, 742–755. [Google Scholar] [CrossRef]
- Ranzi, E.L.I.S.E.O.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.P.; Law, C.K. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, H.; Liu, F.; Smallwood, G.J.; Thomson, M.J. Implementation of an advanced fixed sectional aerosol dynamics model with soot aggregate formation in a laminar methane/air coflow diffusion flame. Combust. Theory Model. 2008, 12, 621–641. [Google Scholar] [CrossRef]
- Roy, S.P.; Arias, P.G.; Lecoustre, V.R.; Haworth, D.C.; Im, H.G.; Trouvé, A. Development of high fidelity soot aerosol dynamics models using method of moments with interpolative closure. Aerosol Sci. Technol. 2014, 48, 379–391. [Google Scholar] [CrossRef]
- Rigopoulos, S. Modelling of soot aerosol dynamics in turbulent flow. Flow Turbul. Combust. 2019, 103, 565–604. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H. Detailed mechanism and modeling of soot particle formation. In Soot Formation in Combustion: Mechanisms and Models; Bockhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 165–192. [Google Scholar]
- Wick, A.; Nguyen, T.; Laurent, F.; Fox, R.O.; Pitsch, H. Modeling soot oxidation with the Extended Quadrature Method of Moments. Proc. Combust. Inst. 2017, 36, 789–797. [Google Scholar] [CrossRef]
- Salenbauch, S.; Hasse, C.; Vanni, M.; Marchisio, D.L. A numerically robust method of moments with number density function reconstruction and its application to soot formation, growth and oxidation. J. Aerosol Sci. 2019, 128, 34–49. [Google Scholar] [CrossRef]
- Colket, M.B.; Hall, R.J. Successes and uncertainties in modeling soot formation in laminar, premixed flames. In Soot formation in Combustion: Mechanisms and Models; Bockhorn, H., Ed.; Springer: Berlin, Germany, 1994; pp. 442–470. [Google Scholar]
- Pope, C.J.; Howard, J.B. Simultaneous particle and molecule modeling (SPAMM): An approach for combining sectional aerosol equations and elementary gas-phase reactions. Aerosol Sci. Technol. 1997, 27, 73–94. [Google Scholar] [CrossRef]
- Park, S.H.; Rogak, S.N.; Bushe, W.K.; Wen, J.Z.; Thomson, M.J. An aerosol model to predict size and structure of soot particles. Combust. Theory Model. 2005, 9, 499–513. [Google Scholar] [CrossRef]
- Blacha, T.; Di Domenico, M.; Gerlinger, P.; Aigner, M. Soot predictions in premixed and non-premixed laminar flames using a sectional approach for PAHs and soot. Combust. Flame 2012, 159, 181–193. [Google Scholar] [CrossRef]
- Goodson, M.; Kraft, M. An efficient stochastic algorithm for simulating nano-particle dynamics. J. Comput. Phys. 2002, 183, 210–232. [Google Scholar] [CrossRef]
- Balthasar, M.; Kraft, M. A stochastic approach to calculate the particle size distribution function of soot particles in laminar premixed flames. Combust. Flame 2003, 133, 289–298. [Google Scholar] [CrossRef]
- Singh, J.; Balthasar, M.; Kraft, M.; Wagner, W. Stochastic modeling of soot particle size and age distributions in laminar premixed flames. Proc. Combust. Inst. 2005, 30, 1457–1465. [Google Scholar] [CrossRef]
- Kim, M.Y.; Park, S.H. A numerical aerosol model Fractal Aggregate Moment Model (FAMM) to simulate simultaneous nucleation, coagulation, surface growth, and sintering of fractal aggregates. Aerosol Sci. Technol. 2019, 53, 493–507. [Google Scholar] [CrossRef]
- Park, S.H. Bi-modal moment model for predicting the formation and growth of soot aggregate particles. Particul. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Miller, J.A. Chemkin-II: A Fortran Chemical Kinetics Package for the Analysis of Gas-Phase Chemical Kinetics; Sandia National Lab. (SNL-CA): Livermore, CA, USA, 1989; Report No.: SAND-89-8009. [Google Scholar]
- Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2 hydrocarbons. Combust. Flame 2000, 121, 122–136. [Google Scholar] [CrossRef]
- Eberle, C.; Gerlinger, P.; Geigle, K.P.; Aigner, M. Numerical investigation of transient soot evolution processes in an aero-engine model combustor. Combust. Sci. Technol. 2015, 187, 1841–1866. [Google Scholar] [CrossRef]
- Yuen, A.C.Y.; Yeoh, G.H.; Timchenko, V.; Cheung, S.C.P.; Barber, T.J. Importance of detailed chemical kinetics on combustion and soot modelling of ventilated and under-ventilated fires in compartment. Int. J. Heat Mass Transfer. 2016, 96, 171–188. [Google Scholar] [CrossRef]
- Akridis, P.; Rigopoulos, S. Modelling of soot formation in laminar diffusion flames using a comprehensive CFD-PBE model with detailed gas-phase chemistry. Combust. Theory Model. 2017, 21, 35–48. [Google Scholar] [CrossRef][Green Version]
- Balthasar, M.; Mauss, F.; Pfitzner, M.; Mack, A. Implementation and validation of a new soot model and application to aeroengine combustors. J. Eng. Gas Turbines Power 2002, 124, 66–74. [Google Scholar] [CrossRef]
- Zucca, A.; Marchisio, D.L.; Barresi, A.A.; Fox, R.O. Implementation of the population balance equation in CFD codes for modelling soot formation in turbulent flames. Chem. Eng. Sci. 2006, 61, 87–95. [Google Scholar] [CrossRef]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A tensorial approach to computational continuum mechanics using object-oriented techniques. Comput. Phys. 1998, 12, 620–631. [Google Scholar] [CrossRef]
- Jasak, H.; Jemcov, A.; Tukovic, Z. OpenFOAM: A C++ library for complex physics simulations. In International Workshop on Coupled Methods in Numerical Dynamics; IUC Dubrovnik Croatia: Dubrovnik, Croatia, 2007. [Google Scholar]
- Atoof, H.; Emami, M.D. Numerical simulation of laminar premixed CH4/air flame by flamelet-generated manifolds: A sensitivity analysis on the effects of progress variables. J. Taiwan Inst. Chem. Eng. 2016, 60, 287–293. [Google Scholar] [CrossRef]
- Jiang, X.; Chan, T.L. Lagrangian particle tracking with new weighted fraction Monte Carlo method for studying the soot particle size distributions in premixed flames. Int. J. Numer. Methods Heat Fluid Flow 2022, 32, 1961–1998. [Google Scholar] [CrossRef]
- Dworkin, S.B.; Zhang, Q.; Thomson, M.J.; Slavinskaya, N.A.; Riedel, U. Application of an enhanced PAH growth model to soot formation in a laminar coflow ethylene/air diffusion flame. Combust. Flame 2011, 158, 1682–1695. [Google Scholar] [CrossRef]
- Messig, D.; Hunger, F.; Keller, J.; Hasse, C. Evaluation of radiation modeling approaches for non-premixed flamelets considering a laminar methane air flame. Combust. Flame 2013, 160, 251–264. [Google Scholar] [CrossRef]
- Marzouk, O.A.; Huckaby, E.D. A comparative study of eight finite-rate chemistry kinetics for CO/H2 combustion. Eng. Appl. Comput. Fluid Mech. 2010, 4, 331–356. [Google Scholar]
- Gentile, G.; Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. A comprehensive CFD model for the biomass pyrolysis. Chem. Eng. Trans. 2015, 43, 445–450. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. OpenSMOKE++: An object-oriented framework for the numerical modeling of reactive systems with detailed kinetic mechanisms. Comput. Phys. Commun. 2015, 192, 237–264. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. A computational tool for the detailed kinetic modeling of laminar flames: Application to C2H4/CH4 coflow flames. Combust. Flame 2013, 160, 870–886. [Google Scholar] [CrossRef]
- Li, Z.; Malik, M.R.; Cuoci, A.; Parente, A. Edcsmoke: A new combustion solver for stiff chemistry based on OpenFOAM®. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017. [Google Scholar]
- Saggese, C.; Ferrario, S.; Camacho, J.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Wang, H.; Faravelli, T. Kinetic modeling of particle size distribution of soot in a premixed burner-stabilized stagnation ethylene flame. Combust. Flame 2015, 162, 3356–3369. [Google Scholar] [CrossRef]
- D’Alessio, A.; Barone, A.C.; Cau, R.; D’Anna, A.; Minutolo, P. Surface deposition and coagulation efficiency of combustion generated nanoparticles in the size range from 1 to 10 nm. Proc. Combust. Inst. 2005, 30, 2595–2603. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Chen, D.; Zainuddin, Z.; Yapp, E.; Akroyd, J.; Mosbach, S.; Kraft, M. A fully coupled simulation of PAH and soot growth with a population balance model. Proc. Combust. Inst. 2013, 34, 1827–1835. [Google Scholar] [CrossRef]
- Jeong, J.I.; Choi, M. A simple bimodal model for the evolution of non-spherical particles undergoing nucleation, coagulation and coalescence. J. Aerosol Sci. 2003, 34, 965–976. [Google Scholar] [CrossRef]
- Matsoukas, T.; Friedlander, S.K. Dynamics of aerosol agglomerate formation. J. Colloid Interface Sci. 1991, 146, 495–506. [Google Scholar] [CrossRef]
- Zhang, H.R.; Violi, A.; Sarofim, A.F.; Frenklach, M. A simulation of soot formation using particle dynamics with one dimensional nucleation mode. In Proceedings of the Third Joint Meeting of the US Sections of the Combustion Institute, The central States Section, Chicago, IL, USA, 16–19 March 2003. [Google Scholar]
- Wen, J.Z.; Thomson, M.J.; Park, S.H.; Rogak, S.N.; Lightstone, M.F. Study of soot growth in a plug flow reactor using a moving sectional model. Proc. Combust. Inst. 2005, 30, 1477–1484. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Simultaneous nucleation, condensation, and coagulation in aerosol reactors. J. Colloid Interface Sci. 1988, 124, 416–427. [Google Scholar] [CrossRef]
- Rogak, S.N.; Flagan, R.C. Coagulation of aerosol agglomerates in the transition regime. J. Colloid Interface Sci. 1992, 151, 203–224. [Google Scholar] [CrossRef]
- Marr, J.A.; Giovane, L.M.; Longwell, J.P.; Howard, J.B.; Lafleur, A.L. Soot and tar production in a jet-stirred/plug-flow reactor system: High and low C2H2 concentration environments. Combust. Sci. Technol. 1994, 101, 301–309. [Google Scholar] [CrossRef]
- Kronholm, D.F.; Howard, J.B. Analysis of soot surface growth pathways using published plug-flow reactor data with new particle size distribution measurements and published premixed flame data. Proc. Combust. Inst. 2000, 28, 2555–2561. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kelesidis, G.A.; Goudeli, E.; Pratsinis, S.E. Flame synthesis of functional nanostructured materials and devices: Surface growth and aggregation. Proc. Combust. Inst. 2017, 36, 29–50. [Google Scholar] [CrossRef]
- Balthasar, M.; Frenklach, M. Detailed kinetic modeling of soot aggregate formation in laminar premixed flames. Combust. Flame 2005, 140, 130–145. [Google Scholar] [CrossRef]
- Morgan, N.; Kraft, M.; Balthasar, M.; Wong, D.; Frenklach, M.; Mitchell, P. Numerical simulations of soot aggregation in premixed laminar flames. Proc. Combust. Inst. 2007, 31, 693–700. [Google Scholar] [CrossRef]
- Veshkini, A.; Dworkin, S.B.; Thomson, M.J. Understanding soot particle size evolution in laminar ethylene/air diffusion flames using novel soot coalescence models. Combust. Theory Model. 2016, 20, 707–734. [Google Scholar] [CrossRef]
- Ono, K.; Dewa, K.; Matsukawa, Y.; Saito, Y.; Matsushita, Y.; Aoki, H.; Era, K.; Aoki, T.; Yamaguchi, T. Experimental evidence for the sintering of primary soot particles. J. Aerosol Sci. 2017, 105, 1–9. [Google Scholar] [CrossRef]
- Botero, M.L.; Eaves, N.; Dreyer, J.A.; Sheng, Y.; Akroyd, J.; Yang, W.; Kraft, M. Experimental and numerical study of the evolution of soot primary particles in a diffusion flame. Proc. Combust. Inst. 2019, 37, 2047–2055. [Google Scholar] [CrossRef]


| Mechanism | CHEMKIN-SAMM | OpenSMOKE-SAMM |
|---|---|---|
| Nucleation | Pyrene | BIN1 molecules |
| Condensation | Pyrene | LMW PAHs (C9 to C16) |
| Surface reaction | C2H2 | C2H2, C3H3, i-C4H3, i-C4H5, and C5H5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H. Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model. Atmosphere 2022, 13, 847. https://doi.org/10.3390/atmos13050847
Park SH. Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model. Atmosphere. 2022; 13(5):847. https://doi.org/10.3390/atmos13050847
Chicago/Turabian StylePark, Sung Hoon. 2022. "Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model" Atmosphere 13, no. 5: 847. https://doi.org/10.3390/atmos13050847
APA StylePark, S. H. (2022). Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model. Atmosphere, 13(5), 847. https://doi.org/10.3390/atmos13050847





