1. Introduction
For many types of scientific measurements, the standard practice is to collect samples on filters. Common examples include air pollution measurements such as PM2.5 and PM10 (airborne particulate matter aerodynamically smaller than 2.5 and 10 microns) in which air is pulled through a filter at a constant rate with instrumentation such as an MiniVol Sampler (AirMetrics, Springfield, OR, USA). Air pollution filter samples are commonly used worldwide for scientific research purposes [
1] as well as for day to day monitoring. Additional applications of filtration include studies of water turbidity [
2] and the measurement of light-absorbing particles in snow and ice [
3]. For turbidity and snow and ice samples, a known quantity of water is filtered, and the filters are preserved for further analysis of impurities.
The LAHM instrument was initially developed to determine light-absorbing particles on snow [
4]. An earlier version of the instrument was used to quantify light-absorbing particles on glaciers in Peru [
4]. In that study, the LAHM instrument results were shown to be well correlated to black carbon as measured by the Single Particle Soot Photometer (SP2: Droplet Measurement Technologies, Longmont, CO, USA) [
5]. Later studies showed that this was not typically the case when non-SP2-sized particles or non-soot particles were present [
6]. Further studies suggested that the LAHM instrument could determine the absorption cross section of the full particle load on the filter. These results were useful for calculations of the spectral reflectance of snow when compared to observations [
6]. The LAHM instrument directly measures light absorption rather than mass for the total population of particles on the filter, which facilitates the study of the impacts of light-absorbing particles on snow.
The analysis of filter samples is often conducted by gravimetric means, by weighing the filter before and after sample collection. Beyond mass, often the optical characteristics of particles collected on filters can be of interest. Optical techniques such as measuring light attenuation through a sample filter and comparing the attenuation to that of a clean filter are frequently utilized [
7,
8] and are the basis of operation for the Maggee science sootscan transmissometer and the Particle Soot Absorption Photometer (PSAP: Berkeley, CA, USA), used in the Global Atmospheric Watch (GAW) program. Light attenuation by particles on filters can be interpreted to estimate black carbon (BC) concentration on filters, as well as attributing the absorption between BC and other materials such as dust and brown carbon, as is conducted by [
9] for snow-borne impurities. A frequently used system for light-absorbing particles in snow is the Integrating Sphere integrating Sandwich spectrometer (ISSW: University of Washington, Washington, WA, USA) [
9]. The ISSW measures the spectra of light after it passes through a filter that is loaded with particles. The spectral information assists in distinguishing different species. More sophisticated instruments such as the Single Particle Soot Photometer (SP2) have been developed for the measurement of airborne BC and have been adapted for the measurement of snow-borne BC [
5].
For some applications, knowledge of the absorption cross section of filter-borne particles is sufficient without a complete understanding of the particle makeup. For applications such as light-absorbing particles in and on snow, the absorption of light by particles is the fundamental value necessary to estimate the radiative forcing caused by the particles. Source attribution requires knowledge of the exact quantity of the emission product of the source. Speciation of light-absorbing particles is not critical for understanding the impacts on snow (e.g., melting).
The convention for reporting light-absorbing particles on snow is to report the mass. In the case of the SP2, this is the mass of rBC (refractory Black Carbon), essentially, the portion of black carbon that is detected by the SP2. The LAHM instrument as well as the ISSW reports in units of eBC (effective Black Carbon), basically, the mass of black carbon that absorbs an equivalent amount of light as the particles on the filter. Thus, eBC is essentially a measure of the absorption cross section of the particles.
While instrumentation for the measurement of light-absorbing particles can be expensive, the basic measurement of the absorption of light is relatively straight forward. High time resolution and particle by particle information can be useful for some applications, but bulk measurements can be scientifically valuable and can be studied with lower cost techniques. For reasons of instrument cost, portability, and accuracy, we have developed the Light Absorption Heating Method (LAHM) instrument to characterize the absorption of particles on filters. The LAHM instrument can be used to analyze filters such as 47 mm diameter filters often used for PM2.5 studies as well as 25 mm filters used for waterborne and snow-borne particle studies. The LAHM instrument takes advantage of the fact that light-absorbing particles increase in temperature upon the absorption of visible light. A filter loaded with a sample is placed in the instrument in a thermally isolated location and exposed to visible light. A noncontact infrared thermometer measures the resulting temperature change of the filter. With appropriate calibration, the temperature increase of the filter can be directly related to the absorption cross section of the particles on the filter, which can be converted to particle mass with knowledge of the mass absorption cross section (MAC).
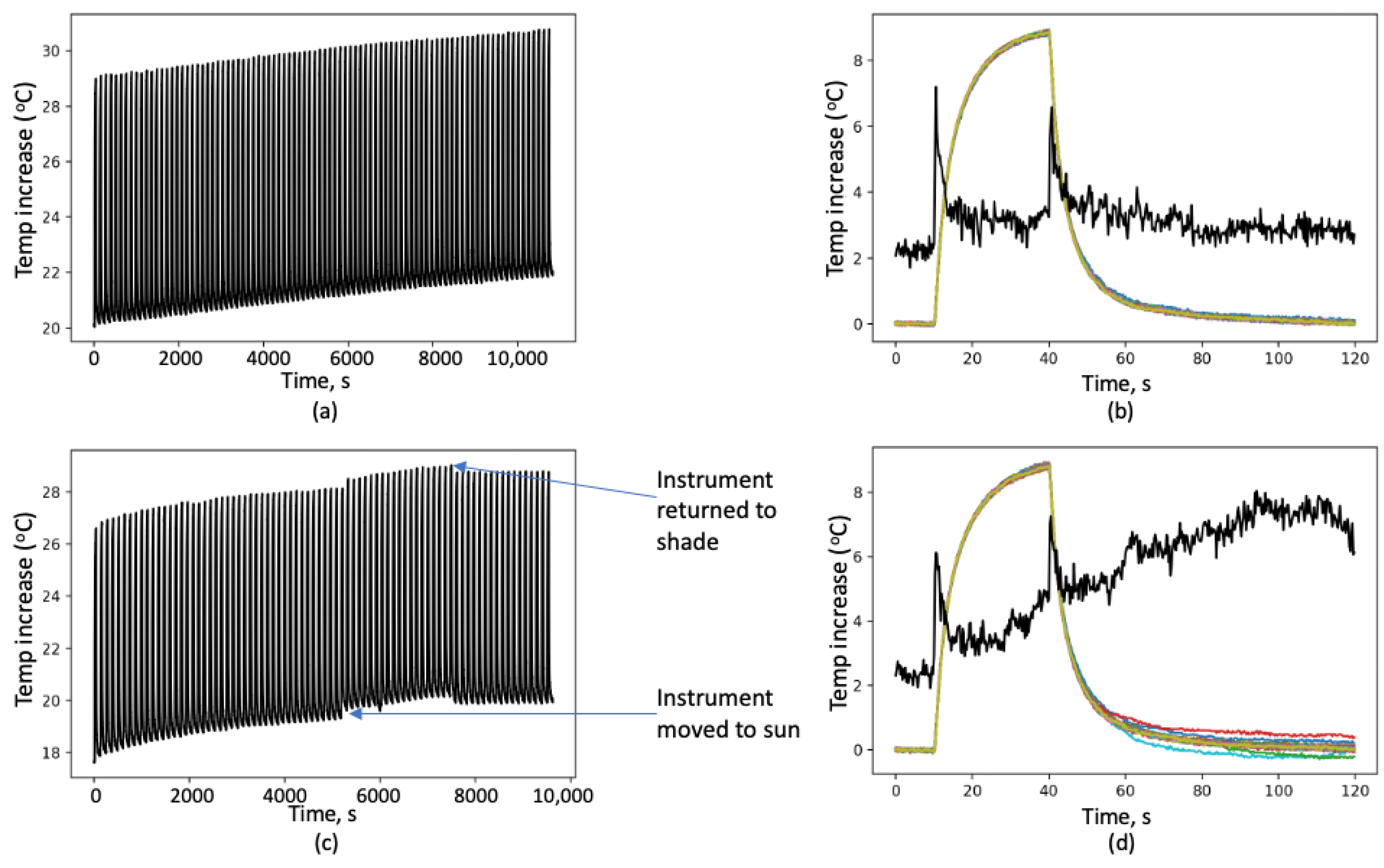
The technique is straightforward, does not involve costly equipment, and can be used to detect light absorption accurately across filter loadings spanning two orders of magnitude. The instrument has been refined from an earlier version [
4] to the point where it now produces repeatable results, with the uncertainty having been reduced by a factor of ten even under unstable laboratory conditions such as full sun exposure. The instrument version described in this publication was developed with ease of use and stability in mind. The previous version [
4] had an uncertainty of about 10% each time the same filter was analyzed. The current version reduced the uncertainty by a factor of 10. This improvement was achieved by improving several components of the system. This started with better isolating the filter from the surroundings during the measurement. The previous version had a more open format, and airflow in the laboratory could easily impact the measurements. Reproducibility of filter placement was improved in the current version by placing the light, filter, and infrared thermometer in line rather than having 45° angles between these components. In the current version, a microcontroller controls the light source and data collection; whereas, in the previous version, the operator operated the light manually. The better standardization of the initiation of the lighting phase of the measurement significantly reduces uncertainty as well.
The LAHM instrument can be calibrated for known substances, and its sensitivity can span two orders of magnitude (e.g., from less than 1 µg to 50 µg of BC on a filter can be quantified to a high degree of certainty). Here, we describe the LAHM instrument from a theoretical perspective as well as the current instrument, which is being used by 10 research groups worldwide studying light-absorbing particles on glaciers. The LAHM instrument has been used to quantify light-absorbing particles extracted from snow and is being used in studies of aerosol absorption in the PM2.5 and PM10 size ranges. In
Section 2, the instrument is described in detail.
Section 3 describes the calibration, stability, and uncertainty of the instrument. In
Section 4, the results of two intercomparison studies are presented demonstrating the utility of the instrument for measuring the absorption cross section of airborne aerosols. In
Section 5, the work is summarized.
2. Instrument Description
The principle of operation of the LAHM instrument is that the temperature response of a substance to impinging light can be directly related to the absorption cross section of the substance. As suggested by the name of the LAHM technique, the thermodynamic state of particles on a filter are perturbed by increasing the quantity of light impinging on the substance leading to an increase in the temperature as the substance re-equilibrates with its surroundings. The increase in absorbed energy is accompanied by additional infrared emission as the filter temperature increases.
Basic thermodynamic considerations can be used to understand the response of the LAHM instrument. When the light is switched on, a portion of that energy is absorbed by the particles on the filter leading to an increase in the temperature of the filter. Once the filter is out of equilibrium with its surroundings, it begins to radiate more as indicated by its brightness temperature as well as directly transmitting energy by contact with air in order to return to equilibrium with its surroundings. A broadband visible wavelength range LED lightbulb is used to illuminate the filter, and an infrared detector measures the brightness temperature of the filter over time. The LAHM instrument works by monitoring the infrared brightness temperature after the LED is switched on and the filter nears a new equilibrium temperature.
2.1. Physical Design
The specific instrument description presented here is for an instrument designed to determine particle properties of particles on 25 mm filters.
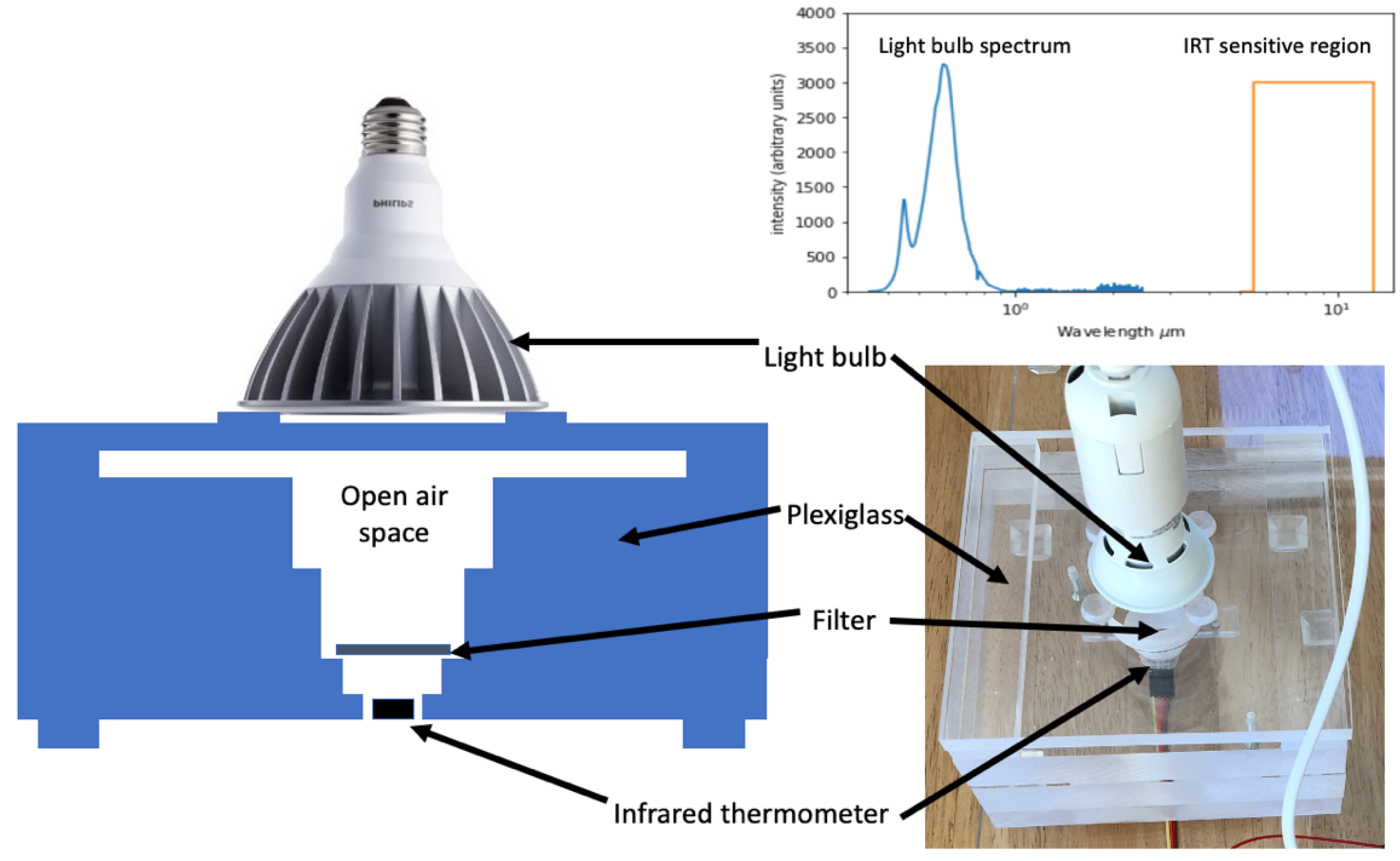
Figure 1 shows a schematic diagram of the instrument as well as a photo. With simple configuration changes, 47 mm filters can be accommodated. The instrument is composed of several plexiglass sheets 15 × 15 cm in size. Thick clear sheets of plexiglass are used because they do not change temperature rapidly, and stray light leaves the system rather than being reflected back to the filter, where it could increase uncertainty. The bottom sheet is 2 cm thick and has a 23 mm diameter hole that penetrates 1.5 cm into the sheet. The remaining 0.5 cm is drilled to the diameter of an infrared thermometer (IRT) module (MLX90614, Melexis Microelectronics, leper, Belgium, approximately 0.8 cm diameter). The datasheet for the IRT module states that its sensitivity range is from 5.5 to 14 microns. The IRT module is secured on bottom of the sheet with space for the thermometer electronics. For analysis, a 25 mm filter is placed over the 23 mm hole; this placement positions most of the filter underside into the field of view of the IRT. Two additional 2.5 cm thick sheets with increasingly larger diameter holes are placed on top of the first sheet for analysis. The additional sheets enable direct transmission of light to the filter. To prevent the accumulation of warmed air above the filter, a gap is created using two 2.5 × 15 cm bars 0.5 cm thick and a final 15 × 15 sheet 0.5 cm thick again. An LED light (Philips 7W LED 50W replacement indoor flood, Philips Electronics, Amsterdam, The Netherlands) is then placed onto four small rubber bumpers on top of the top sheet. The LED light spectrum ranges from 400 to 800 nm and peaks at 600 nm (see
Figure 1 inset for the LED spectrum compared to the IRT sensitivity region. The rubber bumpers create another small air gap thus further reducing the possibility of the transfer of heat directly from the bulb to the instrument (although the bulb temperature change is only 1–2 °C even after several hours of operation). The final configuration was determined through experimentation in an effort to reduce the heat exchange between the components of the instrument. The IRT is monitored with an Arduino microcontroller that also controls the light through a relay system.
2.2. Data System
An Arduino program has been written to operate the system through five illumination and cooling cycles for each sample measurement. Taking the average temperature increase over 3–5 cycles substantially reduces the instrument uncertainty, while additional cycles do not appreciably improve the results. The first four illumination periods were 30 s long, and the final was 120 s, all followed by 90 s cooling periods. Unless otherwise stated, temperature profiles presented in this publication are the average of the first four heating cycles with ambient temperature removed by averaging the ten seconds before the initial heating cycle began. The final 120 s cycle can be used to estimate the actual mass of extremely heavily loaded filters, which has a very high uncertainty and is, therefore, not described here. During operation, the temperature data are saved at 4 Hz, and the resulting data are saved for later final analysis. The instrument has been mainly used with 25 mm filters, investigating light-absorbing particles extracted from glaciers.
5. Conclusions
The Light Absorption Heating Method instrument fulfills a significant need in research and monitoring. For applications such as the measurement of light absorption by particles on snow, the LAHM instrument provides a method to quantify the absorption characteristics of the particle population making it much more straightforward to quantify the impact of the particle population. This compares to other techniques that measure particle concentrations, sizes, and composition, and use those particle characteristics to calculate the light absorption. For air pollution sampling, the LAHM instrument can be used to estimate the bulk absorption cross section of particles collected on filters. In this article, we have shown that the LAHM instrument is very stable, provides repeatable results over time, and that potential uncertainties can be quantified or minimized. The results are comparable to other techniques of measuring absorption; however, knowledge of the particle MAC is critical to determining exact mass of a specific particle type. Conversely, with knowledge of the exact mass, the LAHM instrument provides a unique method to directly measure the relative differences in MAC for different substances. Additionally, the LAHM instrument is made of low cost components making it cost effective for student projects, laboratory classes, as well as for research and monitoring by research groups with lower levels of financial support.
For optimal results, the filters should be lightly loaded. Absorption cross sections of more than half of the filter area can lead to substantial uncertainties. If the absorption cross section is too high, the likelihood of absorbing particles being hidden by other particles increases significantly.
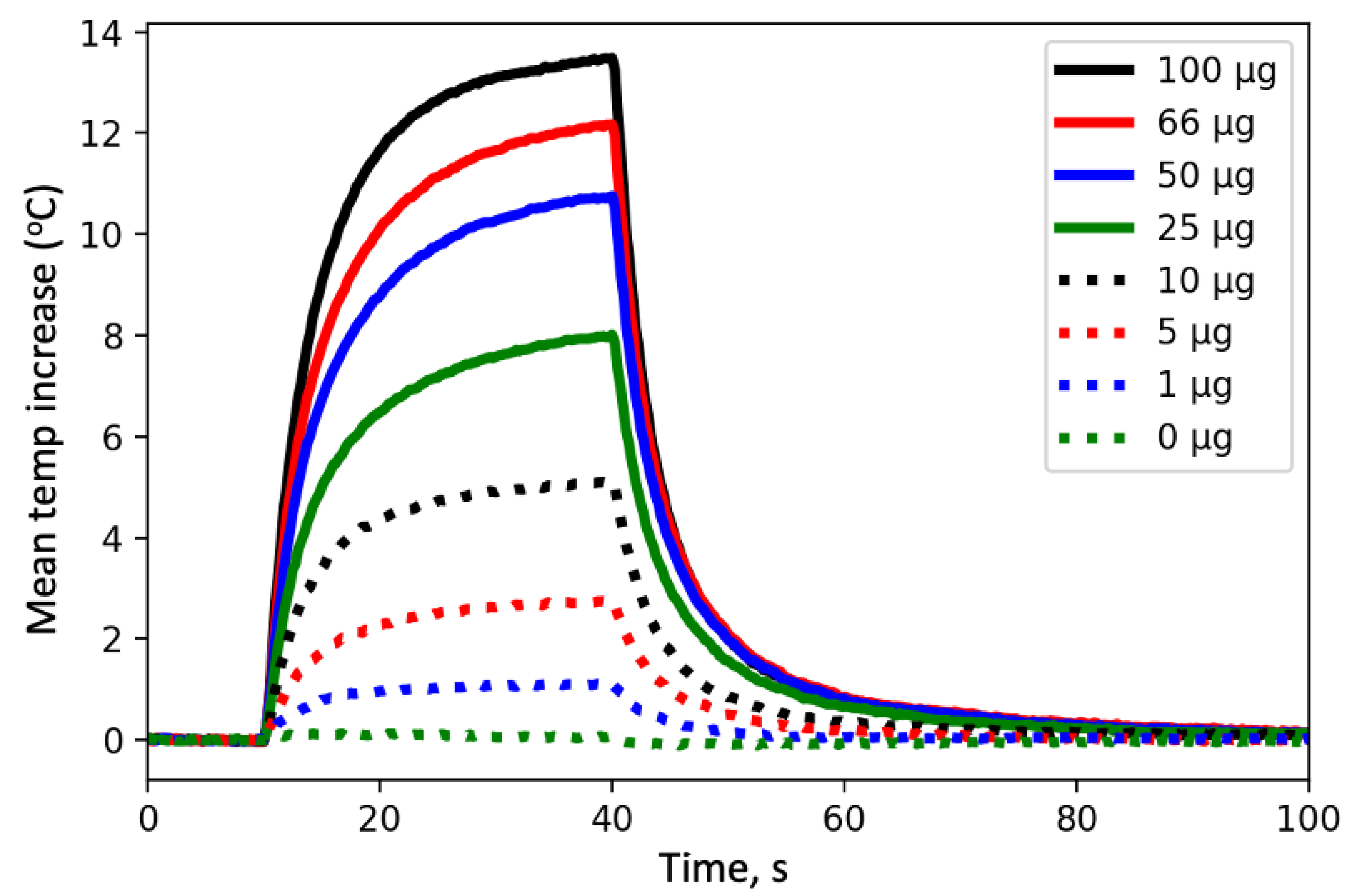
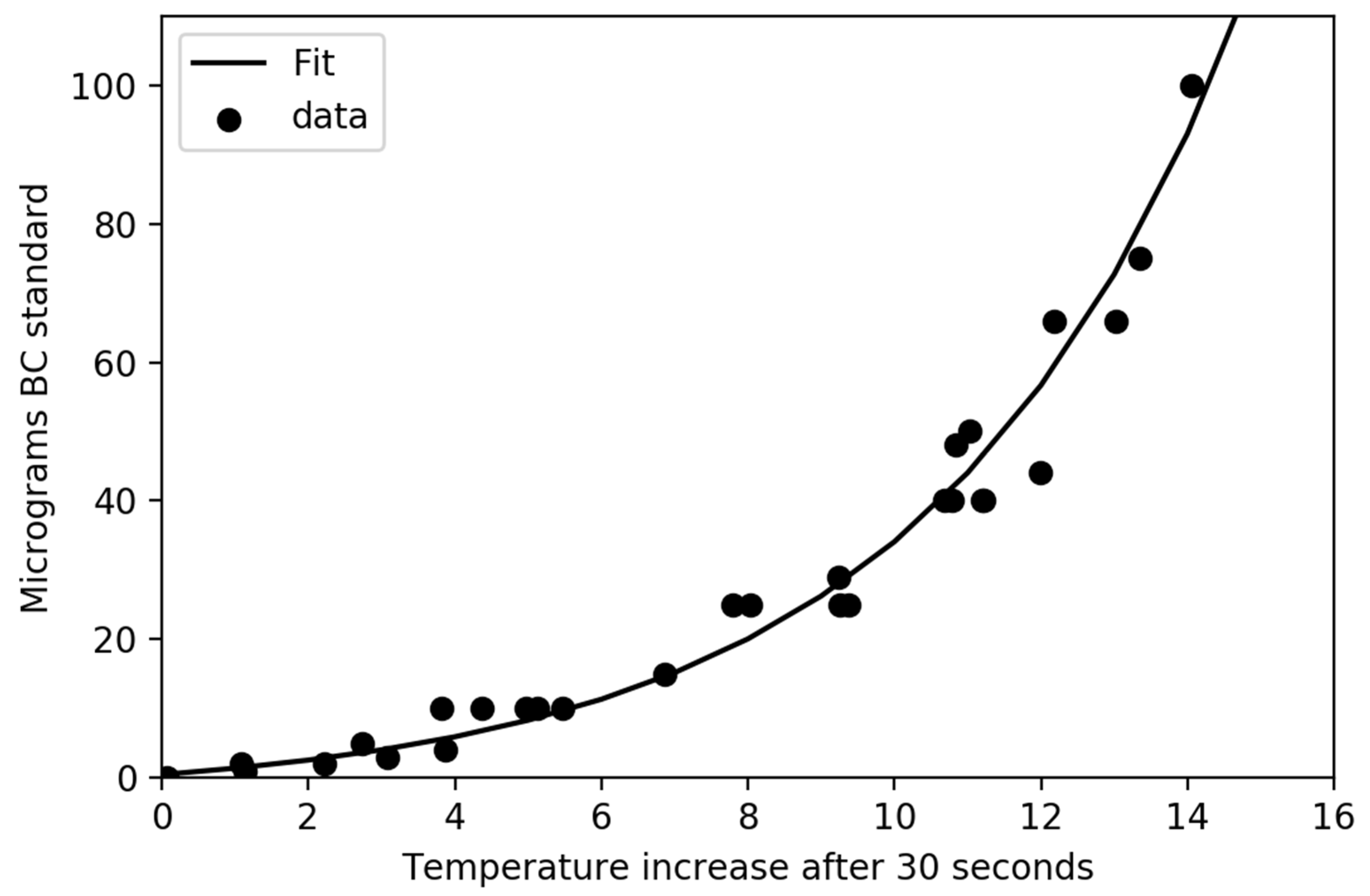
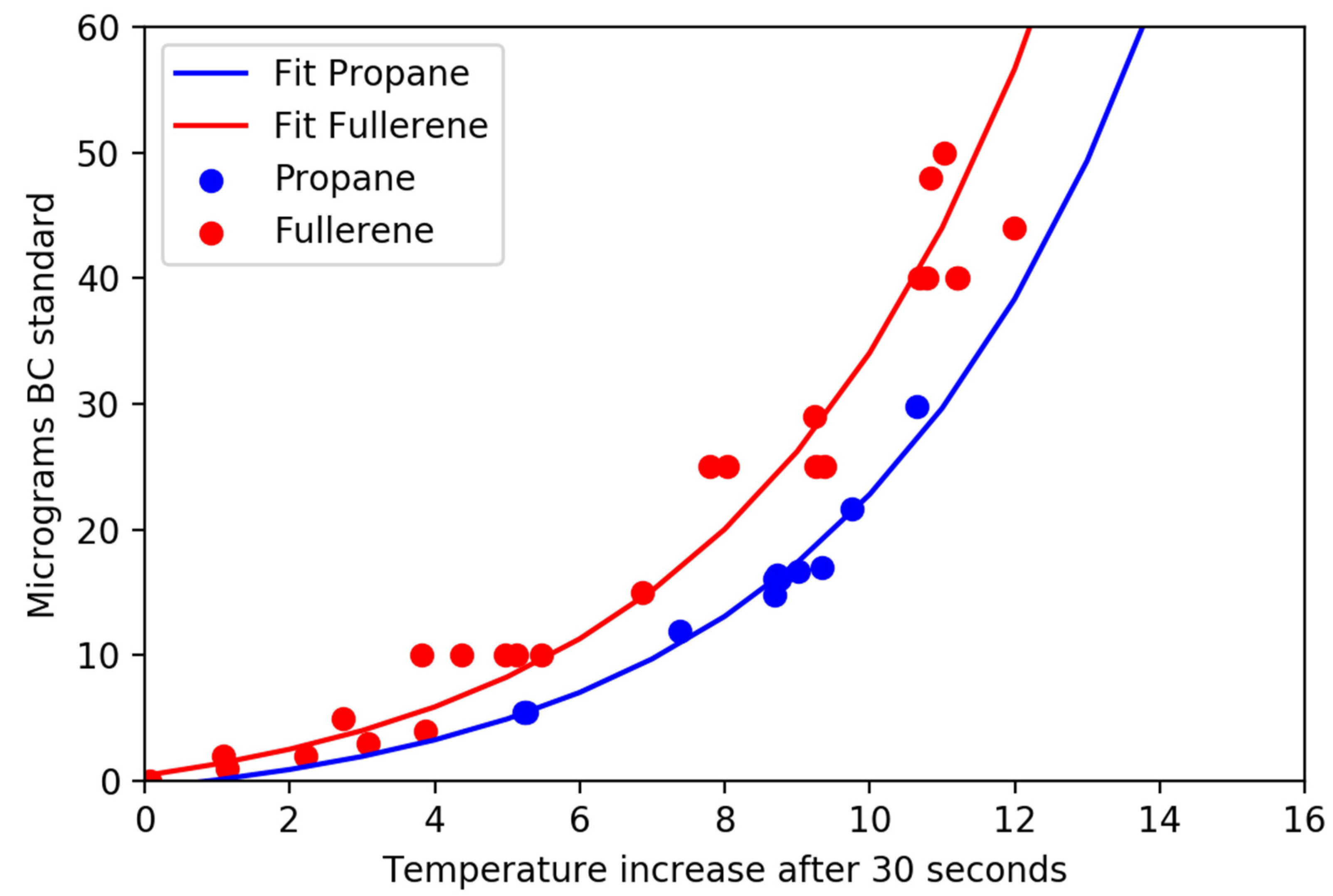
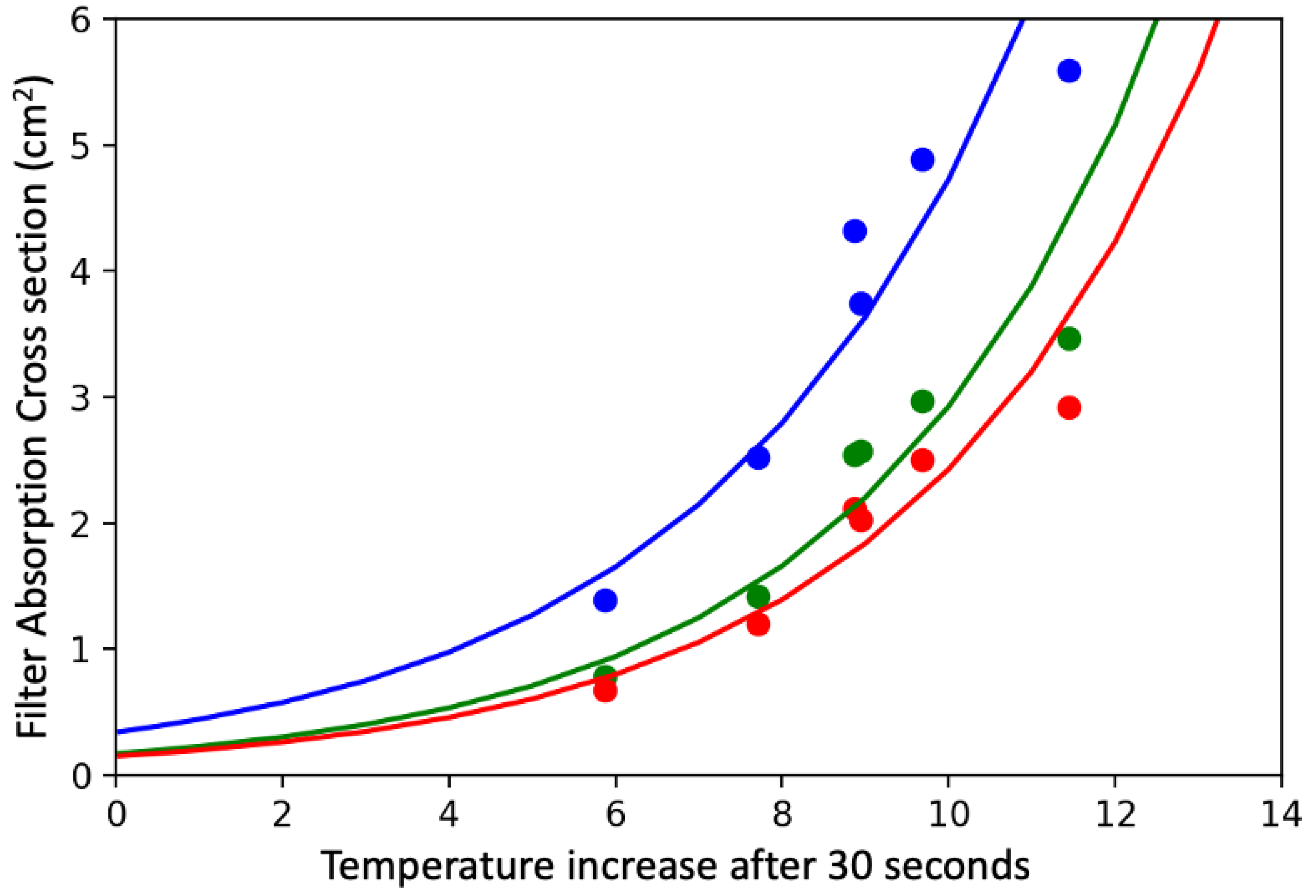
Figure 3 and
Figure 5 clearly demonstrate that small differences in mass can lead to large changes in temperature when the total mass loads are low; thus, the sensitivity is much better at low values of absorption. Data from overloaded filters (not shown) suggests that particles with different MAC values behave quite differently, likely due to scattering within the particles on the filter. This suggests an upper limit of approximately 25 micrograms of highly efficient absorbers (soot) on a 25 mm filter or up to 100 micrograms of soot on a 47 mm filter. While the results presented herein are for 25 mm filters, relationships for 47 mm filters are under development. Uncertainty estimates suggest that there is a potential 1% uncertainty in temperature change for a filter that warms 12 °C which leads to an uncertainty of 3% or less for light absorption.