Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review
Abstract
:1. Introduction
2. MOFs as Catalysts in NOx SCR
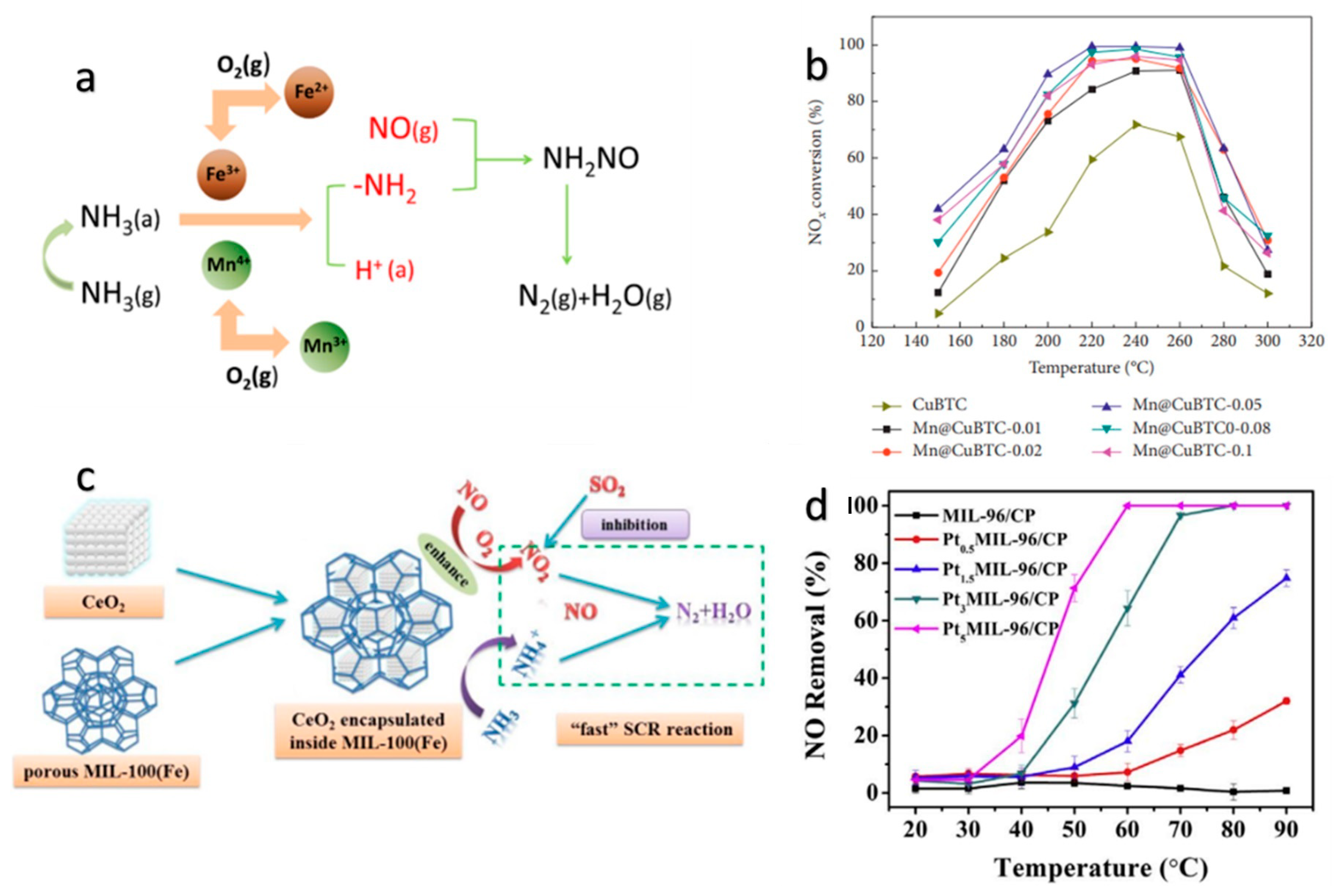
2.1. Monometallic MOFs as Catalysts for NOx SCR
2.2. Bimetallic MOFs as NOx SCR Catalysts
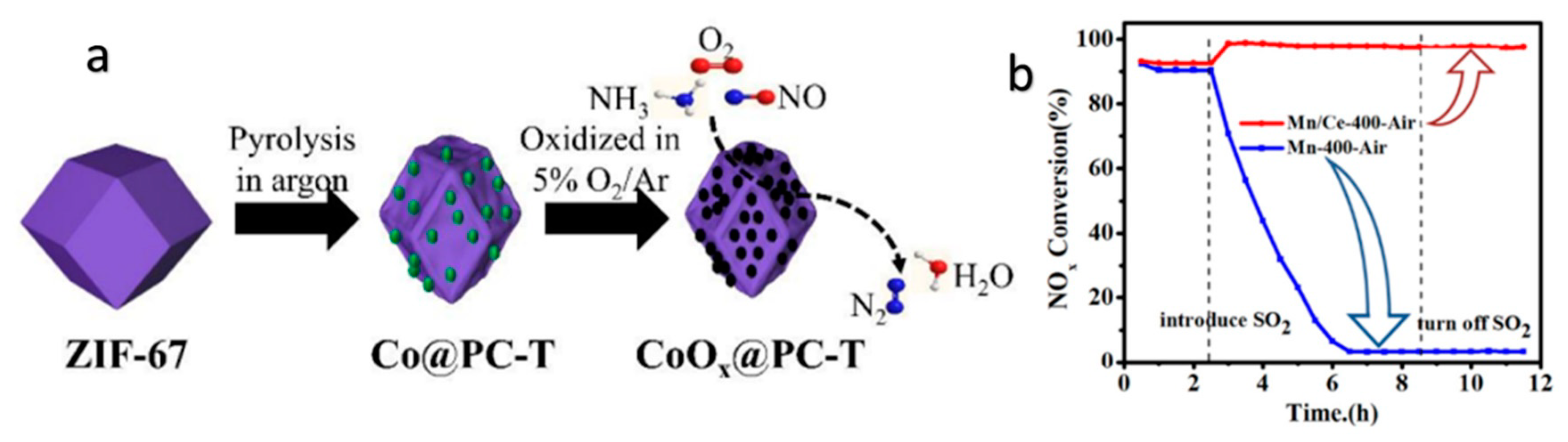
2.3. MOF-Derived NOx SCR Catalyst
3. Reaction Mechanism of MOF Catalysts in NOx SCR
4. Conclusion and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Lee, J.M. Conventional and new materials for selective catalytic reduction (SCR) of NOx. ChemCatChem 2018, 10, 1499–1511. [Google Scholar] [CrossRef]
- Yan, G.; Tao, L.; Tao, L.; Cheng, K.; Hongming, X.U. Performance of V2O5-WO3-MoO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar]
- Komatsu, T.; Tomokuni, K.; Yamada, I. Outstanding low temperature HC-SCR of NOx over platinum-group catalysts supported on mesoporous materials expecting diesel-auto emission regulation. Catal. Today 2006, 116, 244–249. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Na, J.; Hossain, M.S.A.; Yan, X.; Zhang, H.; Amin, M.A.; Qi, J.; Yamauchi, Y.; Li, J. Macroscopic MOF architectures: Effective strategies for practical application in water treatment. Small 2021, 18, 2104387. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Shahrak, M.N.; Ehsani, A.; Kiaei, Z.; Torkzaban, H.; Ershadi, M.; Eshkalak, S.K.; Haddadi-Asl, V.; Chinnappan, A. A review on the field patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors. Coord. Chem. Rev. 2020, 422, 213441. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.-L.; Xu, Q. Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NO x with NH3. ACS Appl. Mater. Interfaces 2016, 8, 26817–26826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Q.; Dong, X.; Wang, Q.; Niu, Y.; Chen, Y.; Jiang, H. Enhancing the water resistance of Mn-MOF-74 by modification in low temperature NH3-SCR. Catalysts 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Gu, K.; Huang, X.; Chen, Y. A DFT study on the effect of oxygen vacancies and H2O in Mn-MOF-74 on SCR reactions. Phys. Chem. Chem. Phys. 2019, 21, 19226–19233. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, J.; Wang, C.; Li, Y.; Chen, Y.; Zhang, M. Effect of cosolvent and temperature on the structures and properties of Cu-MOF-74 in low-temperature NH3-SCR. Ind. Eng. Chem. Res. 2017, 56, 3542–3550. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Wang, C.; Chen, Y.; Zhang, M. Selective catalytic reduction of NOx with NH3 on Cu-BTC-derived catalysts: Influence of modulation and thermal treatment. Catal. Surv. Asia 2018, 22, 95–104. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Zhang, H.; Zhao, Q.; Xue, F.; Li, X. Cu-BTC metal-organic framework as a novel catalyst for low temperature selective catalytic reduction (SCR) of NO by NH3: Promotional effect of activation temperature. Integr. Ferroelectr. 2016, 172, 169–179. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; He, C.; Miao, J.; Chen, J. In situ Growth Synthesis of CuO@ Cu-MOFs Core-shell Materials as Novel Low-temperature NH3-SCR Catalysts. ChemCatChem 2019, 11, 979–984. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, H.; Sun, H.; Yu, H.; Quan, X. Porous metal–organic framework MIL-100 (Fe) as an efficient catalyst for the selective catalytic reduction of NOx with NH3. RSC Adv. 2014, 4, 48912–48919. [Google Scholar] [CrossRef]
- Sun, X.; Shi, Y.; Zhang, W.; Li, C.; Zhao, Q.; Gao, J.; Li, X. A new type Ni-MOF catalyst with high stability for selective catalytic reduction of NOx with NH3. Catal. Commun. 2018, 114, 104–108. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Li, C.; Zhao, Q.; Li, X. Synthesis of bimetallic MOFs MIL-100 (Fe-Mn) as an efficient catalyst for selective catalytic reduction of NO x with NH3. Catal. Lett. 2016, 146, 1956–1964. [Google Scholar] [CrossRef]
- Jiang, H.; Niu, Y.; Wang, Q.; Chen, Y.; Zhang, M. Single-phase SO2-resistant to poisoning Co/Mn-MOF-74 catalysts for NH3-SCR. Catal. Commun. 2018, 113, 46–50. [Google Scholar] [CrossRef]
- Yao, Z.; Qu, D.; Guo, Y.; Yang, Y.; Huang, H. Fabrication and characteristics of Mn@Cu3(BTC)2 for low-temperature catalytic reduction of NOx with NH3. Adv. Mater. Sci. Eng. 2019, 2019, 2935942–2935950. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sun, H.; Quan, X.; Chen, S. Enhanced catalytic activity over MIL-100 (Fe) loaded ceria catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. J. Hazard. Mater. 2016, 301, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Sun, W.; Wang, Q.; Cao, L.; Yang, J. Sparsely loaded Pt/MIL-96 (Al) MOFs catalyst with enhanced activity for H2-SCR in a gas diffusion reactor under 80 °C. Chem. Eng. J. 2018, 335, 612–620. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, B.; Jiang, H.; Chen, Y. Metal-organic framework loaded manganese oxides as efficient catalysts for low-temperature selective catalytic reduction of NO with NH3. Front. Chem. Sci. Eng. 2017, 11, 594–602. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Yu, F.; Chu, Q.; Sun, X. Preparation of metal-organic framework Cu+/Ni-MOF catalyst with enhanced catalytic activity for selective catalytic reduction of NOx. Ferroelectrics 2020, 565, 26–34. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, J.; Hou, Y.; Guo, Y.; Liu, Y.; Li, Y.; Han, X.; Huang, Z. Co3O4@ PC derived from ZIF-67 as an efficient catalyst for the selective catalytic reduction of NOx with NH3 at low temperature. Chem. Eng. J. 2019, 361, 703–712. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Huang, W.; Pan, Z.; Zhou, H. Metal organic frameworks-assisted fabrication of CuO/Cu2O for enhanced selective catalytic reduction of NOx by NH3 at low temperatures. J. Hazard. Mater. 2019, 364, 499–508. [Google Scholar] [CrossRef]
- Gong, Z.; Niu, S.-L.; Zhang, Y.-J.; Lu, C.-M. Facile synthesis of porous α-Fe2O3 nanostructures from MIL-100 (Fe) via sacrificial templating method, as efficient catalysts for NH3-SCR reaction. Mater. Res. Bull. 2020, 123, 110693. [Google Scholar] [CrossRef]
- Ji, B.; Lee, J.; Kwak, S.-Y. Manganese oxides with hierarchical structures derived from coordination polymers and their enhanced catalytic activity at low temperature for selective catalytic reduction of NOx. Dalton Trans. 2019, 48, 16395–16401. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, S.; Sun, B.; Lu, Y.; Li, L.; Zou, W.; Wang, P.; Gao, F.; Tang, C.; Dong, L. Synthesis of CrOx/C catalysts for low temperature NH 3-SCR with enhanced regeneration ability in the presence of SO2. RSC Adv. 2018, 8, 3858–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Shi, J.-W.; Gao, C.; Gao, G.; Wang, B.; Niu, C. Rationally Designed Porous MnO x–FeO x Nanoneedles for Low-Temperature Selective Catalytic Reduction of NOx by NH3. ACS Appl. Mater. Interfaces 2017, 9, 16117–16127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fu, P.; Lv, D.; Chen, Y.; Fan, M.; Wu, J.; Meshram, A.; Mu, B.; Li, X.; Xia, Q. Unusual positive effect of SO2 on Mn-Ce mixed-oxide catalyst for the SCR reaction of NOx with NH3. Chem. Eng. J. 2021, 407, 127071. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational Design of High-Performance DeNOx Catalysts Based on MnxCo3–xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Sun, H.; Gao, Y.; Zhang, Y.; Liu, Y.; Ge, C.; Chen, H.; Dai, X.; Zhang, X. Cobalt doped Fe-Mn@ CNTs catalysts with highly stability for low-temperature selective catalytic reduction of NOx. Nano Res. 2021, 15, 3001–3009. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, W.; Xie, T.; Cao, L.; Yang, J. Metal–Organic Framework (MOF) template based efficient Pt/ZrO2@ C catalysts for selective catalytic reduction of H2 below 90 °C. Chem.–Asian J. 2019, 14, 416–421. [Google Scholar]
- Han, X.; Hong, Y.; Ma, Y.; Lu, W.; Li, J.; Lin, L.; Sheveleva, A.M.; Tuna, F.; McInnes, E.J.L.; Dejoie, C. Adsorption of Nitrogen Dioxide in a Redox-Active Vanadium Metal–Organic Framework Material. J. Am. Chem. Soc. 2020, 142, 15235–15239. [Google Scholar] [CrossRef]
- Xie, S.; Qin, Q.; Liu, H.; Jin, L.; Wei, X.; Liu, J.; Liu, X.; Yao, Y.; Dong, L.; Li, B. MOF-74-M (M= Mn, Co, Ni, Zn, MnCo, MnNi, and MnZn) for low-temperature NH3-SCR and in situ DRIFTS study reaction mechanism. ACS Appl. Mater. Interfaces 2020, 12, 48476–48485. [Google Scholar] [CrossRef]
- Ko, S.; Tang, X.; Gao, F.; Wang, C.; Liu, H.; Liu, Y. Selective catalytic reduction of NOx with NH3 on Mn, Co-BTC-derived catalysts: Influence of thermal treatment temperature. J. Solid State Chem. 2022, 307, 122843. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Gong, S.-Y.; Chen, B.; Xing, W.-H.; Fei, Y.-F.; Hu, Z.-T.; Pan, Z. Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review. Atmosphere 2022, 13, 793. https://doi.org/10.3390/atmos13050793
Gao Y, Gong S-Y, Chen B, Xing W-H, Fei Y-F, Hu Z-T, Pan Z. Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review. Atmosphere. 2022; 13(5):793. https://doi.org/10.3390/atmos13050793
Chicago/Turabian StyleGao, Yuan, Si-Yan Gong, Baixiao Chen, Wen-Hao Xing, Yan-Fei Fei, Zhong-Ting Hu, and Zhiyan Pan. 2022. "Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review" Atmosphere 13, no. 5: 793. https://doi.org/10.3390/atmos13050793
APA StyleGao, Y., Gong, S.-Y., Chen, B., Xing, W.-H., Fei, Y.-F., Hu, Z.-T., & Pan, Z. (2022). Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review. Atmosphere, 13(5), 793. https://doi.org/10.3390/atmos13050793






