Abstract
The efficient generation of high concentrations of fine-particle, pure surfactant aerosols provides the possibility of new, rapid, and effective treatment modalities for Acute Respiratory Distress Syndrome (ARDS). SUPRAER-CATM is a patented technology by Kaer BiotherapeuticsTM, which is a new class of efficient aerosol drug generation and delivery system using Compressor Air (CA). SUPRAER-CA is capable of aerosolizing relatively viscous solutions or suspensions of proteins and surfactants and of delivering them as pure fine particle dry aerosols. In this Computational Fluid Dynamics (CFD) study, we select a number of sites within the upper 17 generations of the human respiratory tract for calculation of the deposition of dry pulmonary surfactant aerosol particles. We predict the percentage of inhaled dry pulmonary surfactant aerosol arriving from the respiratory bronchioles to the terminal alveolar sacs. The dry pulmonary surfactant aerosols, with a Mass Median Aerodynamic Diameter (MMAD) of 2.6 µm and standard deviation of 1.9 µm, are injected into the respiratory tract at a dry surfactant aerosol flow rate of 163 mg/min to be used in the CFD study at an air inhalation flow rate of 44 L/min. This CFD study in the upper 17th generation of a male adult lung has shown computationally that the penetration fraction (PF) is approximately 25% for the inhaled surfactant aerosols. In conclusion, an ARDS patient might receive approximately one gram of inspired dry surfactant aerosol during an administration period of one hour as a possible means of further inflating partly collapsed alveoli.
1. Introduction
The pulmonary route is composed of two structural parts that have different physiological characteristics (Figure 1): the (upper) conducting airways and the respiratory airways [1,2,3,4]. The conducting airways extend from the trachea to the terminal bronchioles, a series of approximately 16 generations [5]. This is followed by the respiratory bronchioles, starting at approximately 17 generations and ending at the alveolar sacs. The conducting part has pseudo-stratified columnar ciliated epithelia with mucous-secreting cells, which are responsible for the mucociliary clearance of the airstream of any exogenous particles, dust, and bacteria (Figure 1). The thickness of the epithelium is approximately 60 μm, with a thick mucus layer overlying a watery periciliary layer that contains the airway cilia [6]. The surfactant is secreted in the airways by club cells [7]. The surface area of the epithelium cells is about 2 m2 [8]. Its main functions are to transport the air to the gas exchange area and to humidify, adjust the gas temperature, and filter the incoming air stream [9]. The respiratory airways are proximal to the terminal bronchioles and end in the alveolar sacs. In the alveoli, there is a very thin (0.2–2 μm) epithelium of alveolar cell type I (main cells) and type II cells, which secrete the pulmonary surfactant [10]. This large surface area (100–140 m2) is responsible for gas exchanges and demonstrates a high permeability toward water and macromolecules, making it a suitable target for drug delivery [11,12].
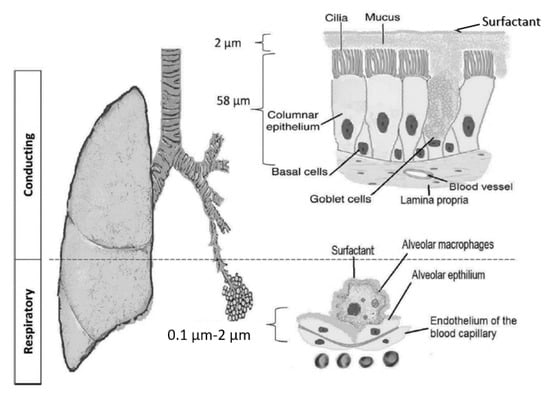
Figure 1.
The pulmonary barrier structure in the conducting and respiratory airways [5].
Acute Respiratory Distress Syndrome (ARDS) are common causes of respiratory failure in critically ill patients [13]. Such conditions are associated with insufficient surfactant in the peripheral lungs. When there is insufficient surfactant, more force is required to open and expand the airways and create a sufficient surface area for adequate gas exchange to support respiratory homeostasis. Currently, only in pediatric indications is surfactant instillation approved for administration through an endotracheal tube. In adult ARDS, despite the proven mechanistic functions and associated physiologic benefits of the surfactant, neither surfactant instillation nor aerosol administration of the surfactant has resulted in sustained, reproducible improvements in clinical outcomes or mortality [14]. The theoretical advantages of aerosol surfactant delivery include minimal manipulation of the respiratory tract, improved pulmonary gas distribution, and avoidance of the acute airway fluid load occurring immediately after surfactant instillation [15,16]. Delivery of a sufficient mass of aerosolized surfactant to the peripheral respiratory bronchi and alveoli to treat either the absence of surfactant and/or its continued depletion has been a recalcitrant problem [14]. To achieve this therapeutically effective dose in a meaningful treatment time (minutes, not hours), aerosols with high particle concentrations are required. The volume of a 10 µm diameter liquid particle is 523 µm3, which is 25 times larger than a 3.4 µm diameter particle’s volume of 20.5 µm3. Thus, the dose per 3.4 µm dry (solid-phase) particles generated then evaporated from a 40 mg/mL surfactant suspension by the SUPREAR-CA dry aerosol generator is 25 times higher than 10 µm liquid aerosols generated from a 40 mg/mL surfactant suspension by a nebulizer. This factor is high enough to substantially reduce the treatment time and/or increase the mass of the surfactant deposited [17].
2. Pulmonary Surfactant Aerosol Generation Methods
Surfactant solutions and suspensions have relatively high viscosity in the range of 10–40 cSt, which reduces or prevents adequate aerosol outputs from jet atomizers, as well as from vibrating mesh, ultrasonic nebulizers, or by the condensation of water vapor on a surfactant nucleolus using a capillary aerosol generator [18]. Dry powder inhalers, while potentially providing a higher dose of the active pharmaceutical ingredient per puff, often require the addition of excipients and the need for an excessive number of capsules [18].
Bianco et al. generated respirable phospholipid surfactant aerosols at a delivery rate of 18.5 mg/min with the customized eFlow-Neos vibrating membrane nebulizer systems [19]. Heng and Yeates [18] successfully generated 8.8 times higher dry aerosol surfactant delivery of 163 mg/min than Bianco et al. [19] with their SUPRAER-CA aerosol generation and delivery system. SUPRAER-CA is a novel dry pulmonary aerosol generation and delivery system developed by KAER Biotherapeutics. SUPRAER-CA uses compressed air at 60 psi to aerosolize an aqueous solution or suspension containing the active surfactant agent through a twin-fluid injector nozzle. The water from the generated droplets is rapidly evaporated in an IR (Infrared Radiation) evaporation chamber to form a solid-phase aerosol that is subsequently concentrated through a patented aerosol concentrator (virtual impactor) and delivered at the output section of the device as a respirable solid-phase aerosol [17]. Use of the aerosol concentrator produces an aerosol output that is comprised of a high concentration of particles in a smaller volume of gas (inhaled air).
Aerosolizing 10.3% concentrations of pulmonary surfactant suspensions at 3 mL/min with SUPAER-CA resulted in the delivery of up to 163 mg/min (2.716 mg/s) of dry pulmonary surfactant aerosols at an inhaled airflow rate of 44 L/min. The dry pulmonary surfactant aerosols were generated by SUPRAER-CA from a 10.3% (w/v) synthetic surfactant suspension with synthetic phospholipids of palmitoyl-oleoyl-phosphatidylglycerol (POPG) (21.3 mg/mL), dipalmitoylphosphatidylcholine (DPPC) (50.4 mg/mL), and palmitoyl-oleoyl-phosphatidylcholine (POPC) (31.3 mg/mL) (weight ratio 2:5:3) in 0.9% saline. The surface tension of the surfactant was not changed by these aerosol generation processes. SEM (scanning electron microscopy) showed dimpled particles of polyvinylpyrrolidone (PVP), albumin, and gamma globulin, indicating that their aerodynamic diameter was less than their morphometric diameter [18]. In this CFD study, 10.3% pulmonary dry aerosols were used because of their high delivery rate of 163 mg/min.
2.1. Respirable Dry Pulmonary Surfactant Aerosol Sizes
Respirable dry pulmonary surfactant aerosols are often measured by cascade impactors, yielding the aerosol size distributions based on the aerodynamic diameter. Cascade impactors are the instruments of choice for measuring the particle size distribution of the aerosol present in the complex discharge from pharmaceutical inhalers. The distribution of the drug captured in the cascade impactor may be usefully represented by the log-normal mass distribution. Only two parameters must be extracted from the analysis of the cascade impactor data in order to describe the distribution. These two parameters are the mass median aerodynamic diameter (MMAD) and the geometric standard deviation [20]. The aerodynamic diameter of a particle is defined as that of a sphere whose density is 1 g/cm3 (cf. density of water), which settles in still air at the same velocity as the particle in question (or more simply as 4× the surface area divided by the perimeter of the containing structure). This diameter is obtained from aerodynamic classifiers such as cascade impactors. The MMAD is defined as the diameter at which 50% of the particles by mass (volume) are larger and 50% are smaller than the median size.
Yu et al. [21] produced respirable dry pulmonary surfactant Naringenin-loaded DPPC (dipalmitoylphosphatidylcholine) aerosols by solvent evaporation and a freeze-drying method with an aerodynamic diameter of 12.48 µm and a fine particle fraction of 23.90%. Vanbever et al. [22] also produced 4% albuterol respirable dry powders made of water-soluble excipients combined with water-insoluble materials (e.g., pulmonary surfactant) using a standard single-step spray-drying process. The tap densities of the respirable dry porous albuterol particles were between 0.08 g/cm3 and 0.31 g/cm3, with a cascade impactor measured MMAD between 6.7 µm and 7.5 µm and with standard deviations ranging from 1.4 µm to 1.5 µm. Chow et al. [23] measured the particle MMADs of the spray-dried powders for solution compositions containing mannitol, L-leucine, and nucleic acid (HSDNA or short interfering RNA). The volumetric diameter of the spray-dried powders was measured by laser diffraction, and all powder formulations had a MMAD ranging from 1.91 µm to 4.43 µm, with a geometric standard deviation ranging from 2.08 µm to 3.52 µm. Gradon and Sosnowski [24] concluded that the most promising hollow or porous nanostructured particles have a tapped density below 0.4 g/cm3 and MMAD below 5 µm by spray-drying, spray freeze-drying, and supercritical antisolvent precipitation production methods. The tapped density is obtained by mechanically tapping a graduated measuring cylinder or vessel containing the powder sample.
Heng and Yeates [18] achieved a 2.6 µm MMAD with a standard deviation of 1.9 µm for the respirable dry 10.3% concentrated surfactant particles (obtained from a 10.3% (w/v) synthetic surfactant suspension with synthetic phospholipids of POPG (21.3 mg/mL), DPPC (50.4 mg/mL), and POPC (31.3 mg/mL) (weight ratio 2:5:3) in 0.9% saline by a SUPRAER-CA aerosol generator) using a 5-stage cascade impactor. They did not measure the tap density of the dry porous surfactant particles, but they roughly estimated the diameter of the initially aqueous aerosols between 4 µm and 10 µm based on solute concentration (10%, w/v) and the MMAD of the dry solid-phase aerosols (2 µm and 4 µm). Therefore, in this CFD study, the 10.3% concentration respirable dry surfactant particles were considered to enter the trachea (G0 generation) inlet surface area homogenously distributed as monodisperse particles with 2.6 µm MMAD and an aerodynamic particle (water) density = 1 g/cm3, as well as its equivalent Mass Median Geometric Diameters (MMGD) of 4.11 µm, 3.68 µm, and 3.36 µm at the assumed geometrical particle densities (tap densities for dry porous particles) of 0.5 g/cm3 (see CFD test cases M-1 and M-2 in Table 1). The Mass Median Geometric Diameter can be calculated with the geometric particle density (which is the tap density for the dry porous particles) by using the widely accepted rule [22,25,26,27]:
where is the Mass Median Aerodynamic Diameter (MMAD) measured by the cascade impactor based on the aerodynamic particle density (i.e., water) . As expected, the CFD simulation results of the particle deposition rates on the conduction airways are approximately the same for the CFD test cases M-1 for MMAD (73.48% particles deposited on the G0–G17 generations of the conducting airways) and M-2 for the corresponding MMGDs (73.48% particles deposited on the G0–G17 generations of the conducting airways).

Table 1.
CFD test case matrix of dry pulmonary aerosol deposition in the G0–G17 airways with the SST turbulence model.
Particles with diameters greater than 5 µm have a greater chance of being deposited in the throat, while particles with a diameter smaller than 0.5 µm may fail to be deposited due to their smaller inertial impaction effect (smaller Stokes number) by following the airflow streamlines. Thus, to effectively deliver inhaled particles deeply into the lungs, the aerodynamic particle sizes of inhaled particles must be within the suitable range of 0.5–5 µm [27]. This CFD simulation study has been conducted for a particle deposition with a polydisperse particle distribution between 0.5 and 5.5 µm. Heng and Yeates [18] provided the cascade impactor particle deposition measurement for the 10.3% surfactant dry aerosol with = 2.6 µm and standard deviation = 1.9 µm for an airflow rate of 44 L/min. In commercial CFD code CFX, we used the log-normal mass distribution corresponding cascade impactor data for the polydisperse particle distribution at the tracheal inlet area (G0 generation) with particles between the minimum diameter of 0.5 µm and maximum diameter of 5.5 µm (see the CFD test case P-1 in Table 1 at the inhaled airflow rate of 44 L/min). The Ansys CFX code offers log-normal mass distribution, which requires the mass mean aerodynamic diameter (MMAD), geometric standard deviation, min diameter, and max diameter. These minimum and maximum diameters correspond to the last and first stages of the cascade impactor covering the measured aerodynamic size range. We used the dry surfactant particle sizes from Heng and Yeates [18], which were obtained by the SUPRAER-CA-produced aerosol generation and delivery system and measured by a cascade impactor in CFD test case P-1 in Table 1.
3. CFD Simulation of Respirable Pulmonary Surfactant Dry Aerosols
One of the problems in computer simulations of the surfactant penetrability of adult human lungs is the geometric growth of the number of bronchi. The bronchial tree begins from one trachea and ends with approximately 223 terminal airways, while the bronchial scale decreases approximately by a factor of 240 [28]. A recent review of human lung airway CFD simulations and particle depositions was completed by Islam et al. [29]. The currently used upper 17 generations of the human respiratory tract were first introduced in the airflow study by Gemci et al. [30] and, then, for the particle deposition studies by Islam et al. [31,32]. The computational mesh of the 17 generations of pulmonary human conducting airways with 720 bronchi outlets was based on abstracted topological graph data of an adult male human lung from High-Resolution Computed Tomography (HRTC) by Schmidt et al. [33]. The generated mesh model has 720 limited bronchi outlets because of the lack of high-resolution HRTC. The 17-generation airway model used unstructured tetrahedral elements and an inflation layer mesh for the bifurcating airways. The details of the computational mesh can be found in the co-author’s previous study [31]. Ansys CFX (Version 19.2) CFD software [34] was used here to predict the 3D airflow field in the upper 17 generations of human conducting airways tract geometry with 720 bronchi outlets by solving the Navier–Stokes equation numerically within the domain decomposed into computationally affordable finite-volume mesh elements of 6.744 × 106 tetrahedral cells and 12.5 million triangular interior faces. The number of cells needed to fully resolve the geometry of this 17-generation computational model with 30,000 particle tracking is currently not practical. Due to the current hardware limitations, one can only use approximately 6.744 million control volumes. A highly tetrahedral mesh has been generated at the deeper airway level, along with their bifurcation angles, to assess the complex flow behavior at the downstream (terminal bronchi) location. The mass and momentum equations were solved to calculate the fluid flow field. The continuum phase air was treated as a homogenous Newtonian fluid with a constant viscosity and air density . The governing continuity and momentum equations for the flow velocity vector are given, respectively, as
where is the fluid static pressure, is the body force due to gravity, and is the body force due to the external (particle–fluid interaction) force [35].
Figure 2 shows the CFD computational domain of the modeled G0–G17 conducting airways.
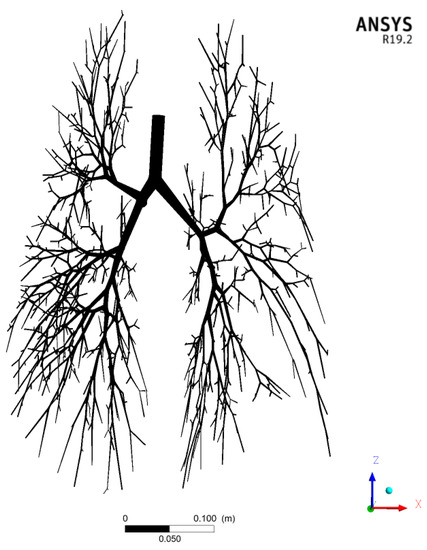
Figure 2.
Computational domain of the G0–G17 human conducting airways with 720 bronchi outlets.
We adapted the anatomical graph data, free of pathological alterations, of Schmidt et al. [33] based on the measurements derived from high-resolution computer tomography (HRCT). The mouth, pharynx, epiglottis, larynx, and upper section of the trachea are not included in the present 17-generation pulmonary model. The tracheal inlet section (G0) has a surface area of 162.86 mm2 with a 14.4 mm tracheal diameter. The quasi-steady-state inlet velocity is 4.503 m/s, corresponding to an inhalation airflow rate of 44 L/min. A uniform inlet velocity field boundary condition corresponding to the airflow rate per inlet surface area is applied at the tracheal inlet section (G0). Particles are injected into the inlet section once the airflow field reaches a steady state after running the CFD simulation for 0.01 s. A fully developed parabolic velocity field is achieved within 1 cm downstream of the inlet section. The pressure at each outlet (total of 720 outlets) must be known a priori, reflecting the effect of the remaining truncated generations G18–G26 at the alveolar level. An alternative approach to setting the flow division ratios would be to use the flow ratio values given by Horsfield et al. [36]. Ma and Lutchen [37] attempted this, but the pressures computed at the 13 outlets of the G6 large airway model were unacceptably inaccurate, since this was a pressure-driven flow. Accordingly, we recommend the use of mass distributions to determine the outflow boundary conditions. The conclusion of Ma and Lutchen [37] regarding flow versus outlet pressure boundary conditions at the end of G6 do not appear relevant to our model, which comprises almost the complete anatomical replica of the pulmonary tree down to the 17th generation level, at which level a constant output p = 0 boundary condition is clearly more appropriate than at the upstream G6 level. From Ma and Lutchen [37], it appears that the turbulence does not have a significant effect on the total input impedance.
We used an inflow boundary condition at G0 and specified the open pressure outlet boundary conditions (p = 0) at all the terminal bronchi exits. The total diameter-dependent mass flow rate at all bronchial exits approximated the inlet value within 11.1 × 10−6 g/s. No-slip boundary conditions were assumed at the tracheobronchial airway walls. Generation G0 and the first bifurcation were represented by a less dense mesh (1.063 × 106 cells), as the flow was more uniform at this upstream location. The computational mesh, with a total of 6.744 × 106 cells, was divided into 1.063 × 106 tetrahedral cells in the G0 zone and into 5.680 × 106 unstructured tetrahedral cells in the remaining zones [30].
The respirable pulmonary surfactant dry aerosols were injected at an inhalation airflow velocity corresponding to the above-noted range of 15–60 L/min with a homogenous distribution throughout the tracheal inlet surface area. The dry aerosols were injected into the pulmonary airways in the direction normal to the face of their inlet (tracheal) cross-section.
3.1. The SST Turbulence Model for the Airflow in Human Respiratory Airways
The airflow in the human respiratory airways is in a transitional laminar-to-turbulent flow and must be considered accurately and effectively. Most industrial flows include turbulent structures that cannot be resolved numerically on the currently available computers. To overcome these limitations, CFD methods are employed to solve Reynolds-averaged Navier–Stokes equations, using turbulence models to compute the averaged turbulent stresses. These models often limit the accuracy of CFD simulations. Ansys CFX CFD software offers a Shear Stress Transport (SST) k-ω turbulence model, which is one of the most effective turbulence models tested for accuracy [38], and Ansys Guide 2018. The SST model is developed by solving a turbulent/frequency-based model (k-ω) at the wall and k-ε in the bulk flow. A blending function ensures a smooth transition between the two models. In a NASA Technical Memorandum [39], the SST model was rated the most accurate model for aerodynamic applications. The SST k-ω model is used in the current CFD simulations of the particle deposition in the G0–G17 conducting airway generations for its accuracy. Kolanjiyil and Kleinstreuer [40] also used the Ansys CFX’s SST k-ω turbulence model in their computational analysis of the aerosol dynamics in a human whole-lung airway model at an inhalation airflow rate of 15 L/min. Figure 3 and Figure 4 show the gas velocity streamline and pressure contours for the CFD test case P-1 in Table 1.
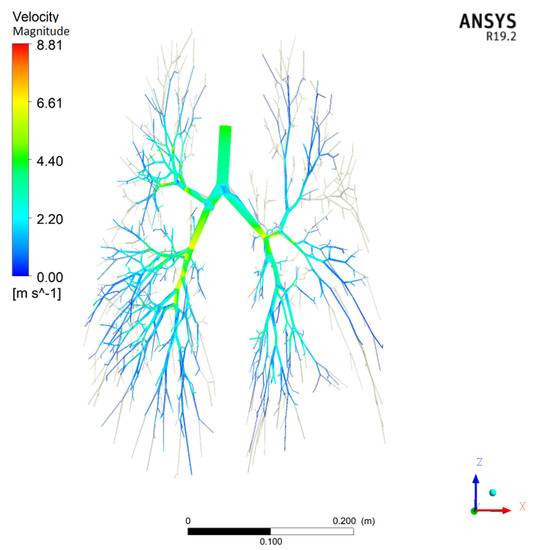
Figure 3.
Magnitude of the air velocity for CFD test case P-1 in Table 1 with = 44 L/min and = 4.503 m/s at the tracheal inlet.
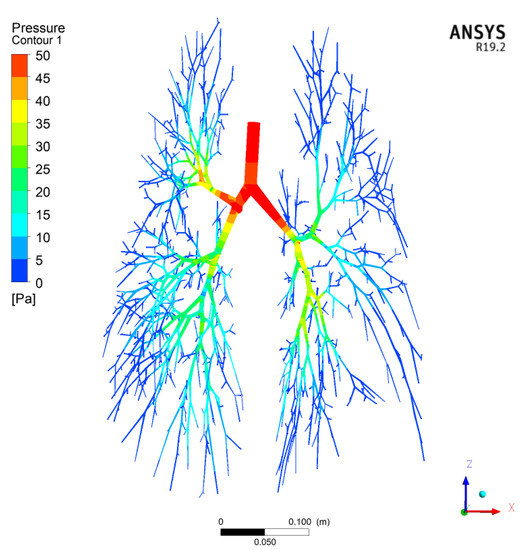
Figure 4.
Pressure contours for CFD Test Case P-1 in Table 1.
3.2. Lagrangian Particle Tracking in the Ansys CFX Code
For inhaled particles involving the tracking of discrete particles, it is not practical to track all physically existing particles in the conducting respiratory airways. Instead, representative particles (computational particle parcels) are used to track these discrete particles. Each representative particle parcel characterizes a certain number of actual particles (). In Ansys CFX, the enabled forces in the particle equation of motion (Newton’s second law) are viscous drag and buoyancy force:
where is the Schiller Naumann drag coefficient and is the buoyancy force. The Cunningham slip correction factor is not considered in Equation (4), because the particles less than 1 µm in diameter represent only 4.3% of the polydisperse particle size population. Additionally, the turbulent tracking for the particle-laden flow was switched on in all CFD runs. The particle position is obtained by . Particles hitting the interior airway surfaces are assumed to be deposited on the airways regardless of their impact velocity or impact angle. Particles escaping through one of the 720 bronchi outlets are assumed to escape into the further G18–G23 respiratory airways. Particles were distributed homogenously at the tracheal inlet surface (G0 generation) and were injected into the gas inlet velocity corresponding to the inhaled airflow rate.
The hygroscopic growth of particles is not considered in CFD simulations, because the particles consist of dry surfactant molecules, and the particles less than 1 µm in diameter represent only 4.3% of the polydisperse particle size population. Haddrell et al. [41] concluded in their growth dynamics study that the inhaled particles smaller than 1 µm in diameter can be assumed to respond instantaneously to the moisture content of the surrounding environment, except when they contain surfactant molecules or start as crystalline. In these instances, the absorption of moisture from the vapor phase and the ensuing growth in the particle size may be delayed by many seconds or longer. Inhaled particles larger than 1 µm in diameter absorb water much more slowly, over time scales typically longer than 5–10 s [41].
4. Results and Discussions for Respirable Dry Surfactant Aerosols Deposition
Respirable dry aerosols (particles) with high momentum (mass times velocity) struck the walls of the air ducts due to inertial impaction, mostly when the airflow rapidly turned. Particles that are able to penetrate these regions but have a relatively high mass can still fall gravitationally at an appreciable rate. Therefore, the chance of their penetration beyond the small bronchi, where the aerosol residence time is extended due to a decrease of the average flow velocity, is minute. Moreover, the diameter of the airways in this region is reduced so that the gravitational settling becomes effective. Submicron particles, which have a low inertia and a negligible sedimentation rate, penetrate the lung periphery to a greater extent. They can reach the airway walls of that region by Brownian diffusion [42].
The CFD test case matrix is shown in Table 1, which consists of monodisperse and polydisperse particle distributions: (1) the CFD test cases for monodisperse particles are completed at 44 L/min (CFD test cases M-1 and M-2), and (2) the CFD test cases for polydisperse particle distribution between 0.5 µm and 5.5 µm are completed at a 44 L/min inhalation airflow rate (see CFD test case P-1) with a MMAD 2.6 µm and standard deviation 1.9 µm (see Section 2.1 for these values taken from Heng and Yeates [18]. Heng and Yeates [18] were able to generate a high aerosol delivery rate of 163 mg/min (2.716 mg/s) dry pulmonary surfactant aerosols at an air inhalation flow rate of 44 L/min with their novel SUPRAER-CA aerosol generation and delivery system. The goal of this CFD study is to estimate the percentage of inhaled dry surfactant aerosol at 2.716 mg/s that is deposited on the G0–G17 conducting airways and the percentage that escapes into the further downstream pulmonary airways (G18–G23). The mass flow rate of 2.716 mg/s for dry surfactant particles is inhaled at a steady-state air inhalation velocity between 1.535 m/s and 6.140 m/s (corresponding to air inhalation flow rates between 15 L/min and 60 L/min). The particles are injected into the tracheal inlet during the period = 0.1 s ( = 0.01 s and = 0.11 s). The Courant number () used in all CFD simulations was less than 0.614, which evaluates the time step requirements () of a transient simulation for a given mesh size () and flow velocity (). After the particle injection is stopped, the CFD computation is continued until all particles are either deposited on the conducting airway inner wall surfaces or they exit one of the 720 bronchi outlets. Figure 5 shows the progress of the computational particle parcel at the different time points inside the conducting airways (without showing the deposited particles) for CFD test case P-1.
Figure 5.
Movement progress of the computational particle parcels (without showing the deposited particles) at times t = 0.01 s (a), t = 0.09 s (b), t = 0.13 s (c), and t = 0.20 s (d) for the CFD test case P-1. Polydisperse particles (0.5 µm ≤ ≤ 5.5 µm) are injected, noted by the different (to scale) sphere sizes.
Table 2 compares the current CFD results with the literature available from in vivo experimental studies and CFD studies for the MMAD around 2.5–3.5 µm particles in the total thoracic region particle deposition (throat, tracheobronchial, and alveolar airways), excluding oral throat deposition. The CFD particle deposition study of a symmetrical 16-generation model by Zhang et al. [43] estimated the 3 µm particle depositions as 10% for a 30 L/min flow rate. Kim and Hu [44] obtained a thoracic deposition of 68% for male subjects and 76% for female subjects for a 3 µm particle diameter and flow rate 30 L/min compared to the 10% result of Zhang et al. [43]. The CFD deposition study of the dual-path whole lung model by Kolanjiyil and Kleinstreuer [40] predicted a particle deposition of 57% for the 3 µm particle diameter and a flow rate of 15 L/min with a tidal volume of 1 L.

Table 2.
Comparison of the total thoracic particle deposition with the available in vivo experiments and CFD models for 2.5–3.5 µm particles (chronological order).
Zhang et al. [43] performed a CFD particle deposition study of the monodisperse aerodynamic particles from 1 µm to 11 µm in a (Weibel Type A) 16-generation model at the inhalation airflow rates of 15 and 30 L/min, respectively. For example, they received a 10% deposition fraction for 3 µm particles at 30 L/min, which was three-fold underestimated compared to an experiment by Lippmann [51] (see Figure 2 in Zhang et al. [43]). The current CFD study with polydisperse particles of a MMAD = 2.6 µm and standard deviation of 1.9 µm at 44 L/min in CFD test case P-1 produced a 74.65 % deposition fraction in a realistic human lung airway of the G0–G17 generations (see Table 1).
Katz et al. [50] studied the influence of lung volume on the particle deposition during imaging within realistic G0–G7 generation airway models. They used two different airways models from 24 subjects: (1) a Mean Lung Volume (MLV) airway model, where the images taken by High-Resolution Computed Tomography (HRTC) with an average of 30 airway exits from 24 subjects, and (2) a Total Lung Capacity (TLC) airway model, where the images were taken by Positron Emission Tomography (PET) with an average 46 airway exits from 24 subjects. For example, in their deposition study, an average 15% (MLV model) and 9% (TLC model) of the injected 3 µm monodisperse aerodynamic particles ( = 1 g/cm3) at a 30 L/min airflow rate were deposited in the G0–G7 conducting airway generations (see Table 5 in Katz et al. [50]).
Islam et al. [31] studied coal dust particle ( = 1.55 g/cm3) deposition in the G0–G17 generation human airways for monodisperse geometrical particle sizes of 1 µm, 5 µm, and 10 µm at three different airflow rates of 15 L/min, 30 L/min, and 60 L/min. They concluded, for example, that the deposition fraction of the 1 µm monodisperse geometrical particle diameter was 43.24% at an airflow rate of 30 L/min.
Conway et al. [48] performed radioactive aerosol deposition imaging measurements in the respiratory tract of 11 healthy human male subjects for model validation. The aerosol distribution in the lungs was measured by combined single-photon emission computed tomography and X-ray computer tomography (SPECT/CT). They used large aerosols with an average MMAD value of 5.7 µm (standard deviation 1.6) and small aerosols with an average MMAD value of 3.1 µm (standard deviation 1.5). Table 2 lists the results of their main study with three male subjects for inhaled small aerosols of an average diameter MMAD of 3.1 µm. Their total thoracic aerosol deposition fractions at an 18 L/min inhalation flow rate were, on average, 80% for a shallow breathing pattern with a 600 mL tidal volume and an average 91% for a deep breathing pattern with a 1000 mL tidal volume (see Table 6 in Conway et al. [48]).
4.1. Effect of Computational Particle Parcel Number on the Particle Deposition
The goal of this CFD dry particle deposition study of the inhaled dry surfactant medication is to quantify the total medication amount that penetrates into the deep lung region, i.e., into the respiratory bronchioles and alveoli. A simulation of the couple of hundred million particles/droplets using current computers, and even supercomputers, requires high computation times and memory and storage capacities. Therefore, the stochastic particle (parcel) techniques used in CFD codes are necessary, requiring that certain numbers of droplets or particles be represented by a single computational parcel and assumes that all particles in a parcel will act together as one. The CFD simulation approach to the quantitative description of particle deposition in the realistic human reparatory airways model is based on Lagrangian particle tracking during their flights/passages through the respiratory airways. The tracking of each particle parcel in the airflow field is done by the Lagrangian particle tracking approach in the Ansys CFX code, i.e., solving the mass balance of a single particle. Graham and Moyeed [52] proposed a strategy to determine in an efficient way how large the particle parcel sample size should be to produce results with the given confidence limits. The main task to answer this important concern is to perform repeated calculations with various samples of a given size. For example, CFD test case P-1 in Table 1 is repeated twice and yields the same deposition fraction of 74.65%. Lagrangian particles track models to dictate that the statistical reliability has been achieved when it can be demonstrated that the results are independent of increasing the number of particles in a parcel. An example includes Pascal and Oesterle [53], who compared results using 104, 2 × 104, and 4 × 104 particle parcels in a simple shear flow and concluded that 2 × 104 particle parcels were sufficient.
For the sake of limiting the computational time and memory, the CFD test cases were run with the 3 × 104 particle parcels injected in 0.1 sec, which corresponds to 3 × 105 parcels per second (see Table 1). Kolanjiyil and Kleinstreuer [40] used 5 × 104 particles injected per second for their 3 µm particle deposition study simulation in a symmetric dual-path human whole-lung airway model. Our study was conducted with 720 paths of airway generations (G0–G17). The particle-independent study of Kolanjiyil and Kleinstreuer [40] ensured that increasing the number of particles by a factor of two (105 particles per second) resulted in less than a 2% difference in the total and regional deposition results. We carried out the monodisperse CFD test case M-1 and polydisperse particle CFD test cases with 3 × 105 parcels per second (3 × 104 parcels in 0.1 s) with appropriate accuracy.
4.2. Effect of Particle Size Distribution on the Particle Deposition Fraction
The monodisperse particle size represented by the MMAD 2.6 µm (CFD test case M-1 at 44 L/min) is compared with polydisperse particle distribution represented by a MMAD of 2.6 µm and a standard deviation of 1.9 µm with a Normal Mass (Volume) Distribution, as set out in the Ansys CFX code (CFD test case P-1 at 44 L/min) from the 0.5 µm to 5.5 µm particle size range. The particle deposition fraction in our G0–G17 generations model increased by 1.59% from 73.48% to 74.65% at a 44 L/min airflow rate by using monodisperse vs. polydisperse particle sizes. This was to be expected, because 50% of the particles are now greater than the median value of 2.6 µm, and they have deposited more frequently because of their larger inertia compared to the smaller-sized particles.
4.3. Effect of Inhalation Airflow Rate on the Particle Deposition Fraction
Kolanjiyil and Kleinstreuer [40] estimated a total 57% deposition fraction in the tracheobronchial and bronchoalveolar regions (see Figure 8 in Kolanjiyil and Kleinstreuer [40]) in their dual-path human whole-lung airway model for a 3 µm aerodynamic particle diameter at 15 L/min for an inhalation tidal volume of 1 L. The in vivo experimental studies from Heyder et al. [54] for nasal breathing are shown in Table 2 as a summary that the total deposition fractions of 3 µm aerodynamic particles in the laryngeal, bronchial, and alveolar regions are 19% at 45 L/min with a 1.5 L tidal volume and a 4 s breathing cycle period. Darquenne et al. [49] also studied in vivo experimental particle deposition for nose inhalation in human lungs and obtained a deposition fraction of 79% at a 45 L/min inhalation airflow rate for 2.9 µm diameter monodisperse dry polystyrene particles at a unit density (see Table 2). We estimate in our current CFD study the on 17 generations shown in Table 1 a deposition fraction value of 74.65% at 44 L/min for the polydisperse particle distribution (0.5 µm ≤ ≤ 5.5 µm with = 2.6 µm; = 1.9 µm). It is highly expected that the deposition fraction can reach the 90–95% range if the CFD study includes the complete generations (G0–G23). Kim and Hu [44] studied in vivo experimental particle deposition for the aerodynamic particle diameters of 1, 3, and 5 µm in healthy adults (eight male and seven female) in a wide range of breathing patterns, tidal volumes, and respiratory flow rates. They measured the particle size by an aerodynamic particle sizer (APS 3310, TSI, St. Paul, MN, USA). For example, for 3 µm particles, they obtained a total DF of 68% for the males (a mean of eight males) and 76% for the females (a mean of seven females) at the inhaled airflow rate of 15 L/min and tidal volume of 1.5 L at a breathing frequency of five breaths per minute (see Table 2). The current CFD study in our 17 generations of a male adult human lung permits us to estimate the deposition fraction at 74.65% for a flow rate of 44 L/min with a polydisperse particle distribution (0.5 µm ≤ ≤ 5.5 µm with = 2.6 µm; = 1.9 µm), as set out in CFD test case P-1 in Table 1.
The overall penetration fraction is around 25% in the current CFD test case for the polydisperse particle distribution at the inhalation airflow rate of 44 L/min. The PF value in the current CFD study showed that these escaped particles will penetrate the bronchioles (G18–G23). The bronchioles end in tiny air sacs called alveoli, where oxygen is transferred from the inhaled air to the blood. For humans, the typical respiratory rate for a healthy adult at rest is 12 breaths per minute. The respiratory center sets the quiet respiratory rhythm at around two (2) seconds for inhalation and three (3) seconds for exhalation. Therefore, the total inhalation time is 24 s per minute. An ARDS patient during a one-minute respiratory duration might inspire and deposit 16.296 mg (=0.25 × 24 s/min × 2.716 mg/s) of active surfactant ingredients transferred to the alveolar surfaces when the patient is administered the surfactant aerosols generated by the novel SUPRAER-CA aerosol generation and delivery system. In conclusion, an ARDS patient might get around 1 g of respirable dry surfactant aerosol during 1 h of SUPRAER-CA aerosol administration time. It remains to be estimated the resulting transmural pressure on the alveolar sacs during that administration time, as excessive levels could lead to bursting and inadequate levels, resulting in alveolar collapse.
5. Conclusions
The ultimate goal is to deliver the inhaled active surfactant ingredients in respiratory drug administration to the alveolar sacs in the respiratory airways (generations G18–G23) of the deep lung region. A novel aerosol generation and delivery system SUPREAR-CA is able to produce respirable dry pulmonary surfactant active ingredients with a Mass Median Aerodynamic Diameter (MMAD) of 2.6 µm and standard deviation of 1.9 µm in the amount of 163 mg/min at an inhalation airflow rate of 44 L/min [6]. This CFD study in the upper 17th generation of a male adult lung showed computationally that the penetration fraction (PF) is approximately 25% of the inhaled surfactant aerosols amount, under the assumption of a total inhalation time of 24 s per minute (2 s of inhalation at 12 breaths per minute and 3 s of exhalation at 12 breaths per minute) Therefore, theoretically, during 12 inhalations (inspirations) per minute, an ARDS patient can be administered 16.296 mg of active surfactant ingredients during each one-minute inhalation cycle to where the pulmonary bronchioles end in tiny air sacs called alveoli, the locations at which oxygen is transferred from the inhaled air to the blood. In conclusion, an ARDS patient might receive approximately 1 g (977 mg) of inspired dry surfactant aerosol during an administration period of one hour as a possible means of further inflating partly collapsed alveoli. The pool size of the extracellular surfactant ranges from about 10 to 15 mg/kg body weight in adults. Therefore, a 70 kg person would have an estimated alveolar surfactant pool of approximately 0.7–1.0 g. An adequate single dose for the treatment of ARDS is thought to be in the range of 50–300 mg phospholipids/kg body weight. Alternatively, for example, a cumulative total dose of 4 g has been used [55,56]. Therefore 1 g of dry surfactant aerosol administration per hour can markedly improve the ARDS treatment time.
Author Contributions
Conceptualization, T.G., V.P., R.C. and T.E.C.; methodology, T.G., V.P., R.C., T.E.C. and M.S.I.; software, T.G., V.P., R.C., T.E.C. and M.S.I.; validation, T.G.; formal analysis, T.G., V.P., R.C., T.E.C. and M.S.I.; investigation, T.G.; data curation, T.G., V.P., R.C., T.E.C. and M.S.I.; writing—original draft preparation T.G.; writing—review and editing T.G., V.P., R.C., T.E.C., M.S.I. and S.C.S.; visualization, T.G. and M.S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Symbols | Definitions | SI Unit |
| and | Aerodynamic and geometrical particle diameters | µm |
| Mass Median Aerodynamic Diameter defined as the diameter at which 50% of the particles by mass are larger and 50% are smaller | µm | |
| Mass Median Geometrical Diameter based on the porous particle tap density (see Equation (1)) | µm | |
| Inhalation gas flow (airflow) rate | L/min | |
| Number of physical particles in each computational particle parcel | - | |
| Total number of injected computational particle parcels in = 0.1 s | - | |
| Inhaled particle mass flow rate | mg/s | |
| Particle injection time duration (0.1 s) | s | |
| Gas velocity at tracheal inlet | m/s | |
| Standard deviation of MMAD measurement by Cascade impactor | µm | |
| Aerodynamic particle (water) density | g/m3 | |
| geometrical particle density (tap density for dry porous particle) | g/m3 |
References
- Gu, Q.; Qi, S.; Yue, Y.; Shen, J.; Zhang, B.; Sun, W.; Qian, W.; Islam, M.S.; Saha, S.C.; Wu, J. Structural and functional alterations of the tracheobronchial tree after left upper pulmonary lobectomy for lung cancer. J. Biomed. Eng. Online 2019, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Gu, Y.; Ristovski, Z. Numerical investigation of aerosol particle transport and deposition in realistic lung airway. In Proceedings of the International Conference on Computational Methods, Auckland, New Zealand, 14–17 July 2015. [Google Scholar]
- Ghosh, A.; Islam, M.S.; Saha, S.C. Targeted drug delivery of magnetic nano-particle in the specific lung region. Computation 2020, 8, 10. [Google Scholar] [CrossRef]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Ong, H.; Young, P.; Gu, Y. Euler–Lagrange approach to investigate respiratory anatomical shape effects on aerosol particle transport and deposition. Toxicol. Res. Appl. 2019, 3. [Google Scholar] [CrossRef]
- Osman, N.; Kaneko, K.; Carini, V.; Saleem, I. Carriers for the Targeted Delivery of Aerosolized Macromolecules for Pulmonary Pathologies. Expert Opin. Drug Deliv. 2018, 15, 821–834. [Google Scholar] [CrossRef]
- Siekmeier, R.; Scheuch, G. Systemic Treatment by Inhalation of Macromolecules Principles, Problems, and Examples. J. Physiol. Pharmacol. 2008, 59, 53–79. [Google Scholar]
- Harkema, J.R.; Nikula, K.J.; Haschek, W.M. Respiratory System. In Fundamentals of Toxicologic Pathology, 3rd ed.; Wallig, M.A., Haschek, W.M., Rousseaux, C.G., Bolon, B., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 12; pp. 351–393. ISBN 978-0-12-809841-7. [Google Scholar]
- Gallo, R.L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef]
- Chalon, J.; Ali, M.; Ramanathan, S.; Turndorf, H. The humidification of anaesthetic gases: Its importance and control. J. Can. Anaesth. Soc. J. 1979, 26, 361–366. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, R.F.; Dobbs, L.G. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. In Epithelial Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–159. [Google Scholar]
- Mason, G.R.; Peters, A.M.; Bagdades, E.; Myers, M.J.; Snook, M.; Hughes, J.M. Evaluation of Pulmonary Alveolar Epithelial Integrity by the Detection of Restriction to Diffusion of Hydrophilic Solutes of Different Molecular Sizes. Clin. Sci. 2001, 100, 231–236. [Google Scholar] [CrossRef]
- Islam, M.S.; Larpruenrudee, P.; Paul, A.R.; Paul, G.; Gemci, T.; Gu, Y.; Saha, S.C. SARS CoV-2 aerosol: How far it can travel to the lower airways? Phys. Fluids 2021, 33, 061903. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Premiers 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Yeates, D.B. Surfactant Aerosol Therapy for nRDS and ARDS. In Inhalation Aerosols, Physical and Biological Basis for Therapy, 3rd ed.; Hickey, A.J., Mansour, H.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; Chapter 21; pp. 327–342. ISBN 9781138064799. [Google Scholar]
- Mazela, J.; Merritt, T.A.; Finer, N.N. Aerosolized Surfactants. Curr. Opin. Pediatr. 2007, 19, 155–162. [Google Scholar] [CrossRef]
- Dijk, P.H.; Heikamp, A.; Oetomo, S.M. Surfactant Nebulisation: Lung Function, Surfactant Distribution and Pulmonary Blood Flow Distribution in Lung Lavaged Rabbits. Intensive Care Med. 1997, 23, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Yeates, D.B.; Heng, X. Generation of Respirable Particles from Surfactant Suspensions and Viscous Solutions at High Dose Rates. In Proceedings of the Drug Delivery to The Lungs (DDL27) Conference, Edinburgh, UK, 6–8 December 2017; pp. 205–208. [Google Scholar]
- Heng, X.; Yeates, D.B. Generation of High Concentrations of Respirable Solid-Phase Aerosols from Viscous Fluids. Aerosol Sci. Technol. 2018, 52, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Ricci, F.; Catozzi, C.; Murgia, X.; Schlun, M.; Buckolski, A.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Pasini, E.; et al. From Bench to Bedside: In Vitro and In Vivo Evaluation of a Neonate-Focused Nebulized Surfactant Delivery Strategy. Respir. Res. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.G. Cascade Impactor Data and the Lognornal Distribution: Nonlinear Regression for a Better Fit. J. Aerosol Med. 2002, 15, 369–378. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Chen, H.; Zhu, L. Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33. [Google Scholar] [CrossRef]
- Vanbever, R.; Mintzes, J.D.; Wang, J.; Nice, J.; Chen, D.; Batycky, R.; Langer, R.; Edwards, D.A. Formulation and Physical Characterization of Large Porous Particles for Inhalation. Pharm. Res. 1999, 16, 1735–1742. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Qiu, Y.; Lo, F.F.K.; Lin, H.H.S.; Chan, H.-K.; Kwok, P.C.L.; Lam, J.K.W. Inhaled Powder Formulation of Naked siRNA Using Spray Drying Technology with L-leucine as Dispersion Enhancer. Int. J. Pharm. 2017, 530, 40–52. [Google Scholar] [CrossRef]
- Gradon, L.; Sosnowski, T.R. Formation of Particles for Dry Powder Inhalers. Adv. Powder Technol. 2014, 25, 43–55. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Yan Chan, J.G.; Lip Kwok, P.C.; Prud’homme, R.K.; Chan, H.-K. Constant Size, Variable Density Aerosol Particles by Ultrasonic Spray Freeze Drying. Int. J. Pharm. 2012, 427, 185–191. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Lopez-Arraiza, A.; Rey-Santano, C.; Mielgo, V.; Basterretxea, F.J.; Sancho, J.; Homez-Solaetxe, M.A. Experimental and Numerical Modeling of Aerosol Delivery for Preterm Infants. Int. J. Environ. Res. Public Health 2018, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Sosnowski, T.R.; Tobita, S.; Tachibana, I.; Tse, J.Y.; Uchiyama, H.; Tozuka, Y. A Particle Technology Approach toward Designing Dry-Powder Inhaler Formulations for Personalized Medicine in Respiratory Diseases. Adv. Powder Technol. 2020, 31, 219–226. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Fomin, V.M.; Gafurova, P.S. Three-Dimensional Model of the Human Bronchial Tree—Modeling of the Air Flow in Normal and Pathological Cases. J. Appl. Mech. Tech. Phys. 2020, 61, 1–13. [Google Scholar] [CrossRef]
- Islam, M.S.; Paul, G.; Ong, H.X.; Young, P.M.; Gu, Y.T.; Saha, S.C. A Review of respiratory Anatomical Development, Air Flow Characterization and Particle Deposition. Int. J. Environ. Res. Public Health 2020, 17, 380. [Google Scholar] [CrossRef]
- Gemci, T.; Ponyavin, V.; Chen, Y.; Chen, H.; Collins, R. Computational Model of Airflow in Upper 17 Generations of Human Respiratory Tract. J. Biomech. 2008, 41, 2047–2054. [Google Scholar] [CrossRef]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Gemci, T.; Gu, Y.T. Pulmonary Aerosol Transport and Deposition Analysis in Upper 17 Generations of the Human Respiratory Tract. J. Aerosol Sci. 2017, 108, 29–43. [Google Scholar] [CrossRef]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Gemci, T.; Yang, I.A.; Gu, Y.T. Ultrafine Particle Transport and Deposition in a Large Scale 17-Generation Lung Model. J. Biomech. 2017, 64, 16–25. [Google Scholar] [CrossRef][Green Version]
- Schmidt, A.; Zidowitz, S.; Kriete, A.; Denhard, T.; Krass, S.; Peitgen, H.-O. A Digital Reference Model of the Human Bronchial Tree. Comput. Med. Imaging Graph. 2004, 28, 203–211. [Google Scholar] [CrossRef]
- Ansys Technical Brief CFX-SST: Innovative Turbulence Modeling: SST Model in Ansys® CFX®. December 2006. Available online: http://fluid.itcmp.pwr.wroc.pl/~pblasiak/CFD/UsefulInformation/sst.pdf (accessed on 28 April 2022).
- Ansys CFX-Solver Modeling Guide (Release 19.2); ANSYS, Inc.: Canonsburg, PA, USA, 2018.
- Horsfield, K.; Dart, G.; Olson, D.E.; Filley, G.F.; Cumming, G. Models of the Human Bronchial Tree. J. Appl. Physiol. 1971, 31, 207–217. [Google Scholar] [CrossRef]
- Ma, B.; Lutchen, K.R. An Anatomically Based Hybrid Computational Model of the Human Lung and its Application to Flow Frequency Oscillatory Mechanics. Ann. Biomed. Eng. 2006, 34, 1691–1704. [Google Scholar] [CrossRef]
- Menter, F.R. Zonal Two Equation k-ω Turbulence Models for Aerodynamic Flows. In Proceedings of the 23rd Fluid Dynamics, Plasmadynamics, and Lasers Conference, Orlando, FL, USA, 6–9 July 1993. [Google Scholar]
- Bardina, J.E.; Huang, P.G.; Coakley, T.J. Turbulence Modeling, Validation, Testing and Development; NASA Technical Memorandum 110446; NASA Ames Research Center: Moffett Field, CA, USA, 1997.
- Kolanjiyil, A.V.; Kleinstreuer, C. Computational Analysis of Aerosol-Dynamics in a Human Whole-Lung Airway Model. J. Aerosol Sci. 2017, 114, 301–316. [Google Scholar] [CrossRef]
- Haddrell, A.E.; Lewis, D.; Church, T.; Vehring, R.; Murnane, D.; Reid, J.P. Pulmonary Aerosol Delivery and The Importance of Growth Dynamics. Ther. Deliv. 2017, 8, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R. Powder Particles and Technologies for Medicine Delivery to the Respiratory System: Challenges and Opportunities. KONA Powder Part. J. 2018, 35, 122–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Kleinstreuer, C.; Kim, C.S. Comparison of Analytical and CFD Models with Regard to Micron Particle Deposition in a Human 16-Generation Tracheobronchial Airway Model. Aerosol Sci. 2009, 40, 16–28. [Google Scholar] [CrossRef]
- Kim, C.S.; Hu, S.-C. Total Respiratory Tract Deposition of Fine Micrometer-Sized Particles in Healthy Adults: Empirical Equations for Sex and Breathing Pattern. J. Appl. Physiol. 2006, 101, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Foord, N.; Black, A.; Walsh, M. Regional Deposition of 2.5–7.5 µm Diameter Inhaled Particles in Healthy Male Non-Smokers. J. Aerosol Sci. 1978, 9, 343–357. [Google Scholar] [CrossRef]
- Emmett, P.C.; Aitken, R.J. Measurement of the Total and Regional Deposition of Inhaled Particles in the Human Respiratory Tract. J. Aerosol Sci. 1982, 13, 549–560. [Google Scholar] [CrossRef]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of Particles in the Human Respiratory Tract in the Size Range 0.005–15 µm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Conway, J.; Fleming, J.; Majoral, C.; Katz, I.; Perchet, D.; Peebles, C.; Tossici-Bolt, L.; Collier, L.; Caillibotte, G.; Pichelin, M.; et al. Controlled, Parametric, Individualized, 2-D and 3-D Imaging Measurements of Aerosol Deposition in the Respiratory Tract of Healthy Human Subjects for Model Validation. J. Aerosol Sci. 2012, 52, 1–17. [Google Scholar] [CrossRef]
- Darquenne, C.; Lamm, W.J.; Fine, J.M.; Corley, R.A.; Glenny, R.W. Total and Regional Deposition of Inhaled Aerosols in Supine Healthy Subjects and Subjects with Mild-to-Moderate COPD. J. Aerosol Sci. 2016, 99, 27–39. [Google Scholar] [CrossRef]
- Katz, I.; Pichelin, M.; Montesantos, S.; Murdock, A.; Fromont, S.; Veneges, J.; Caillibotte, G. The Influence of Lung Volume during Imaging on CFD with Realistic Airway Models. Aerosol Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Lippmann, M. Regional Deposition of Particles in the Human Respiratory Tract. In Handbook of Physiology—Reaction to Environmental Agent; Lee, D.H.K., Ed.; American Physiology Society: Bethesda, MD, USA, 1977; pp. 213–232. [Google Scholar]
- Graham, D.I.; Moyeed, R.A. How many particles for my Lagrangian simulations? Powder Technol. 2002, 125, 179–186. [Google Scholar] [CrossRef]
- Pascal, P.; Oesterle, B. On the Dispersion of Discrete Particles Moving in a Turbulent Shear Flow. Int. J. Multiph. Flow 2000, 26, 293–325. [Google Scholar] [CrossRef]
- Heyder, J.; Armbruster, L.; Gebhart, J.; Grein, E.; Stahlhofen, W. Total Deposition of Aerosol Particles in the Human Respiratory Tract for Nose and Mouth Breathing. J. Aerosol Sci. 1975, 6, 311–328. [Google Scholar] [CrossRef]
- Hamm, H.; Fabel, H.; Bartsch, W. The Surfactant System of the Adult Lung: Physiology and Clinical Perspectives. Clin. Investig. 1992, 70, 637–657. [Google Scholar] [CrossRef]
- Richman, P.S.; Spragg, R.G.; Robertson, B.; Merritt, T.A.; Cursted, T. The Adult Respiratory Distress Syndrome: First Trials with Surfactant Replacement. Eur. Respir. J. 1989, 2 (Suppl. S3), 109–111. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).