The Effect of Drought and Sowing Date on Dry Matter Accumulation and Partitioning in the Above-Ground Organs of Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Experimental Design
2.3. Methods
2.3.1. Calculating the Degree of Drought
2.3.2. Calculating Relative Water Content of Soil
2.4. Abbreviations
3. Results
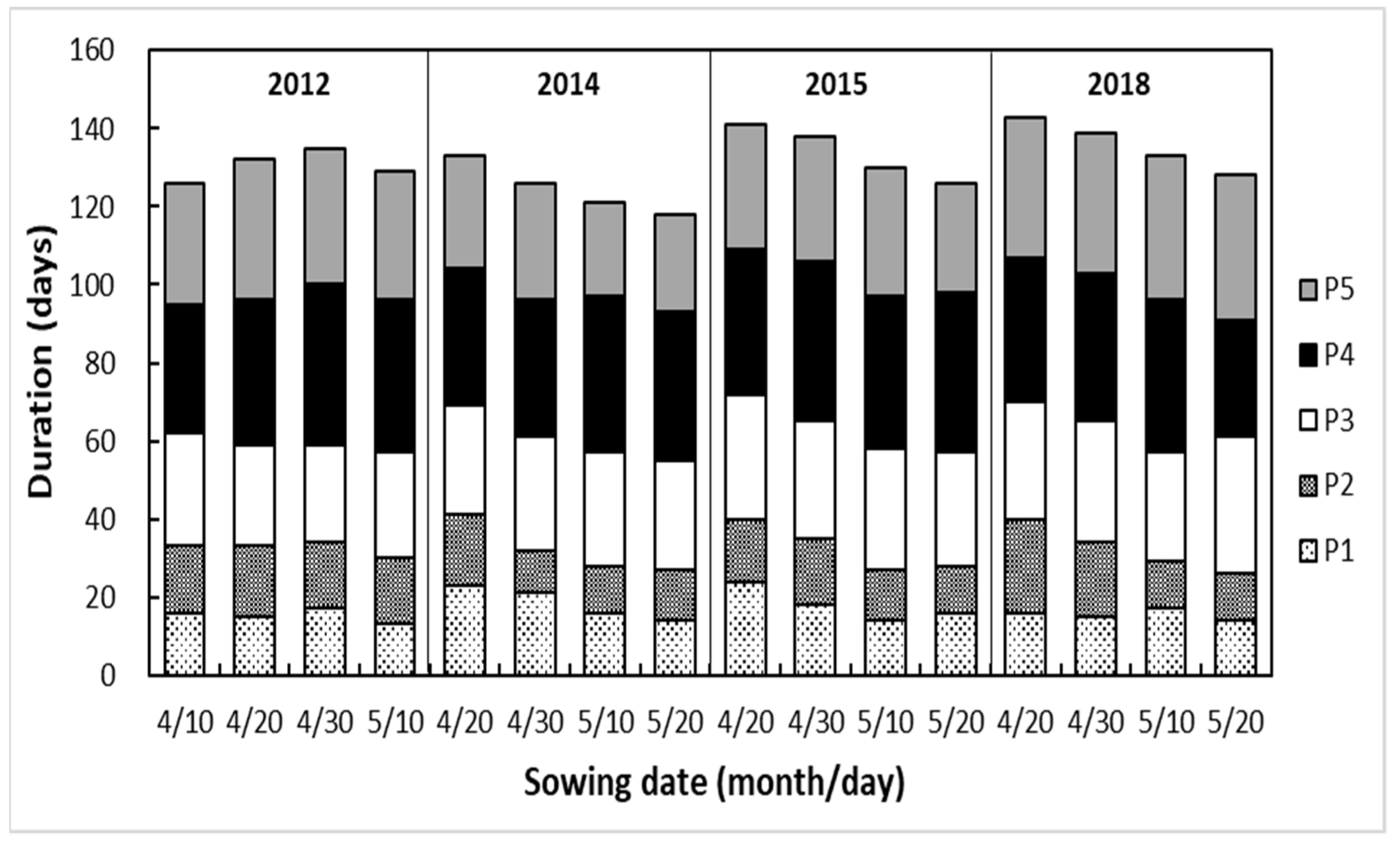
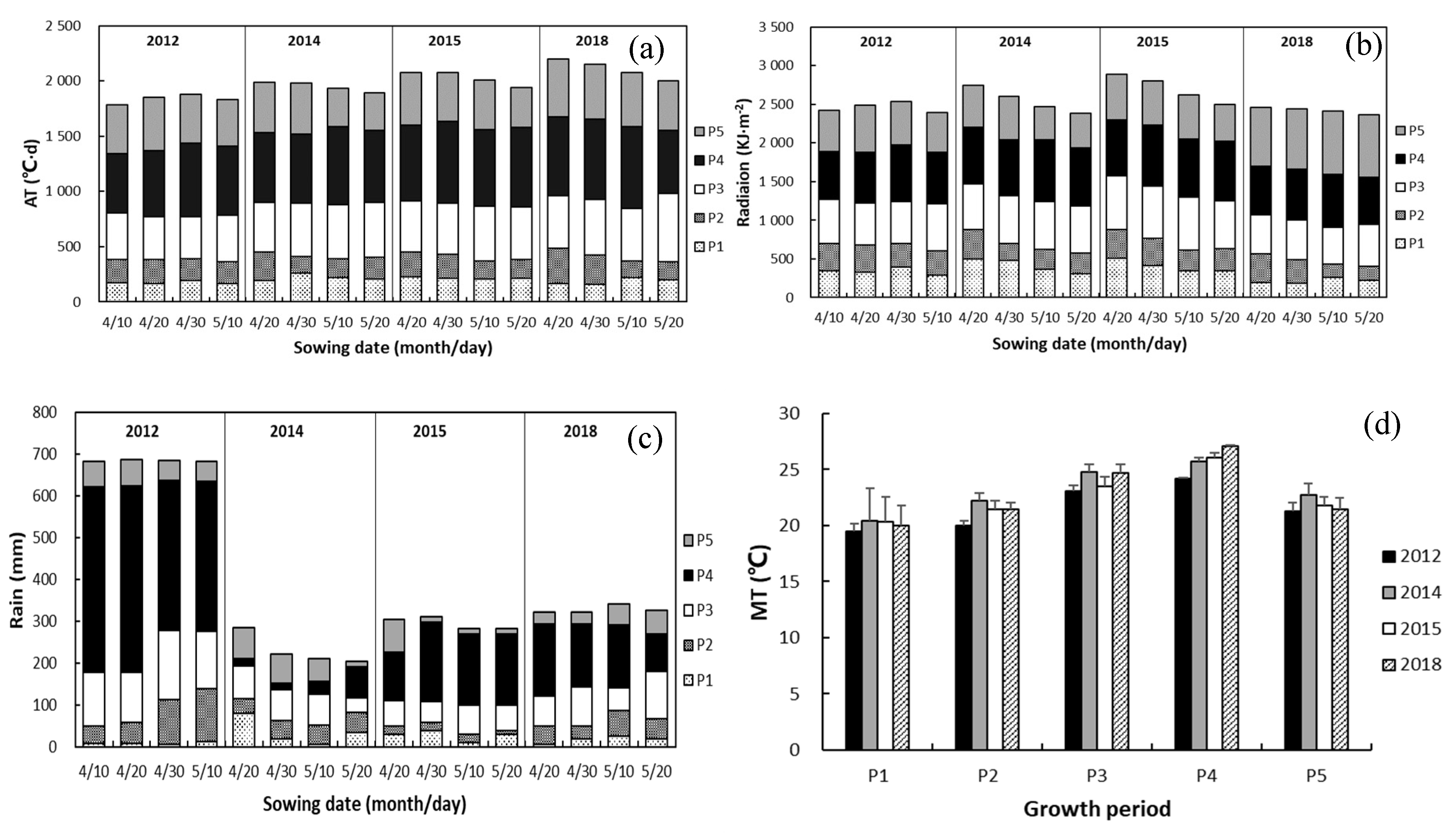
3.1. Growth Stages and Methodological Factors
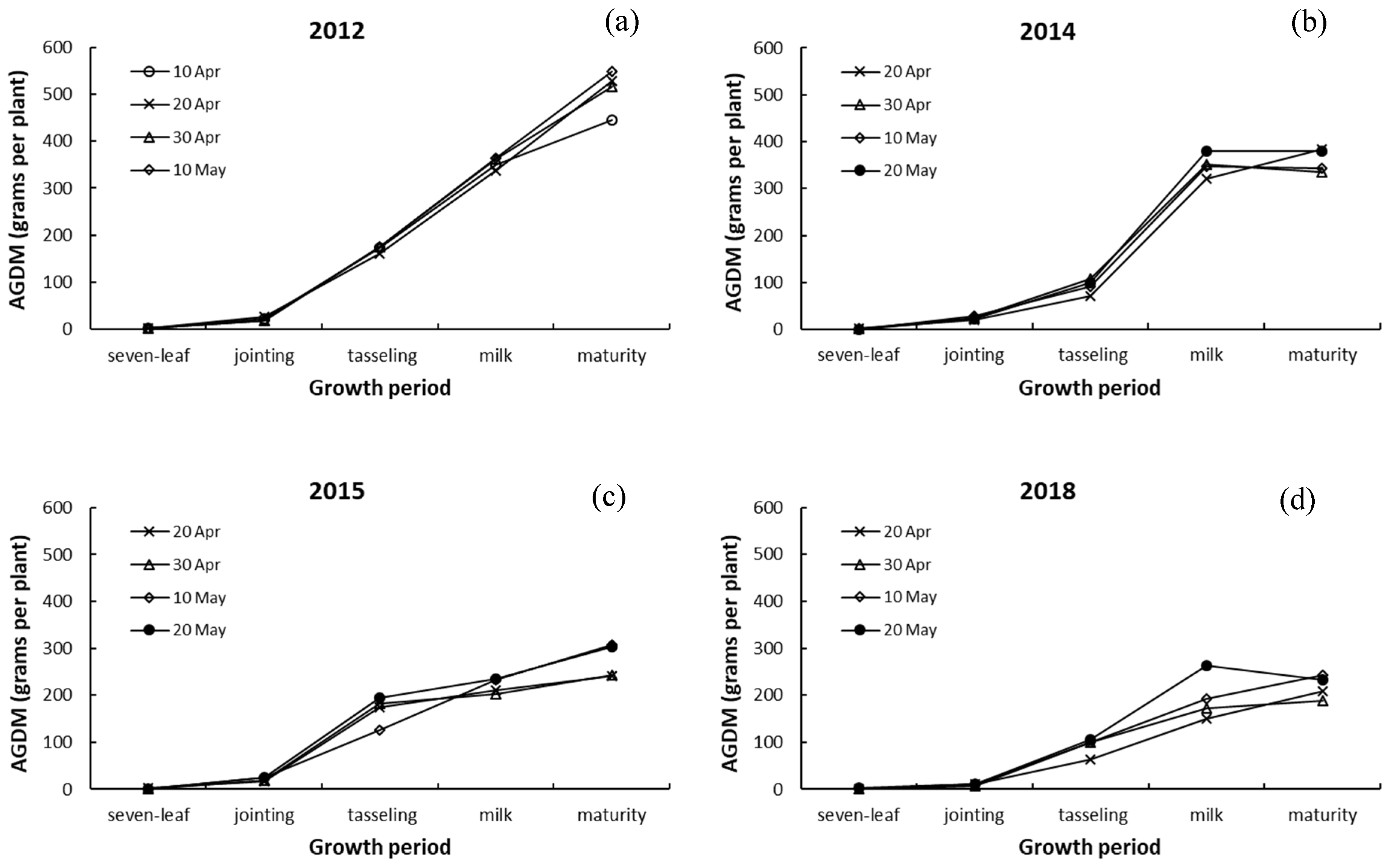
3.2. Above-Ground Dry Matter Weight and Its Response to Drought
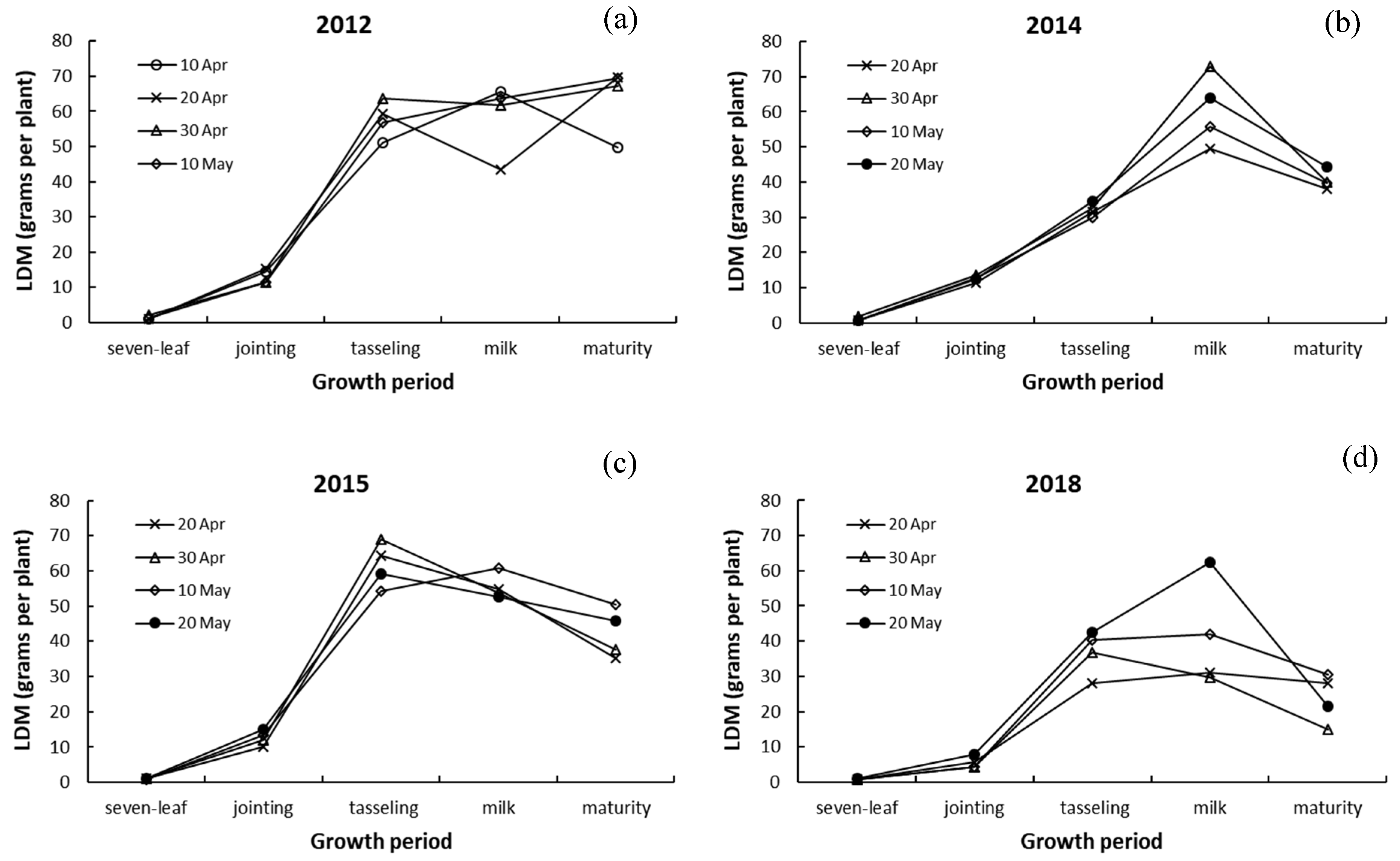
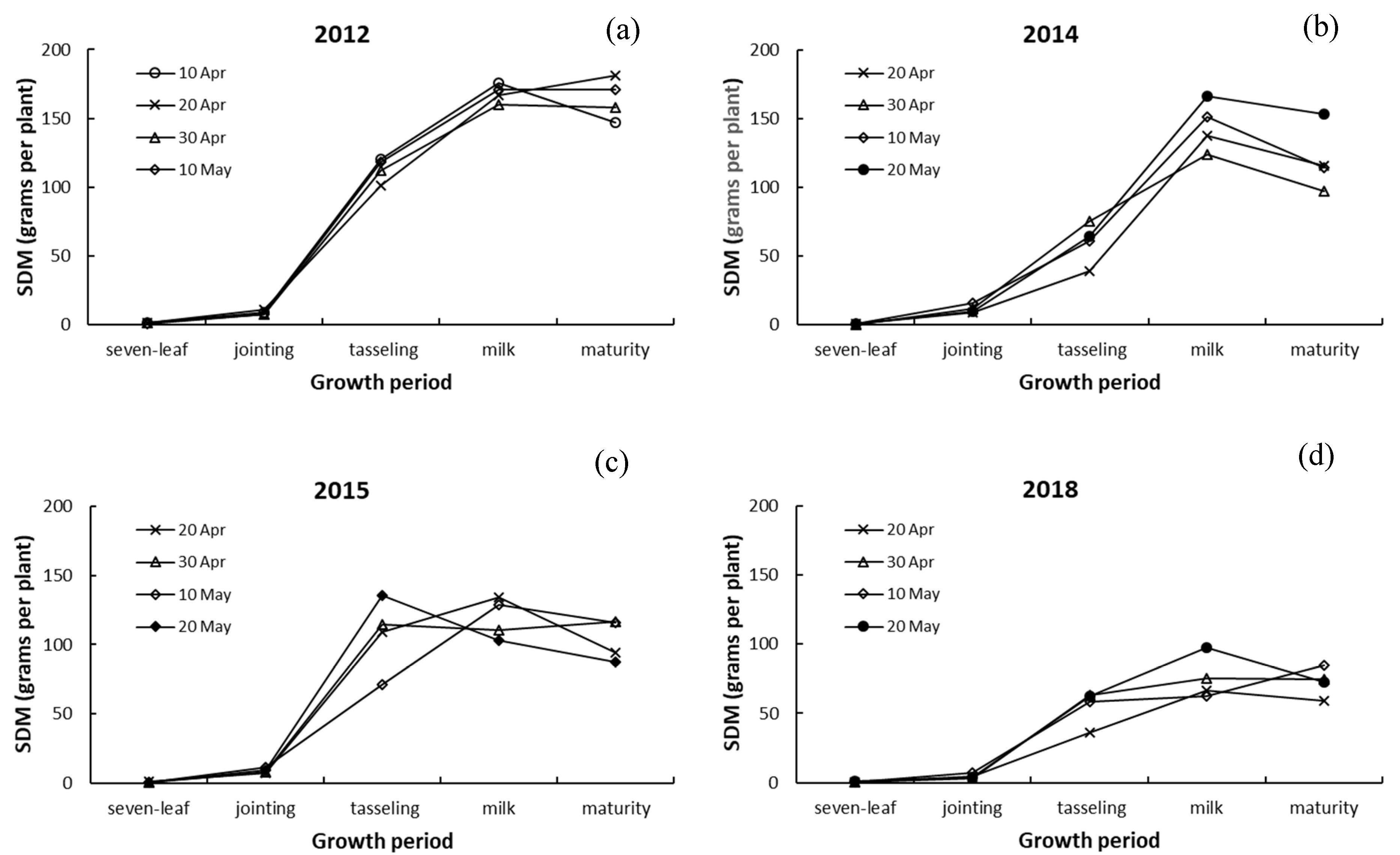
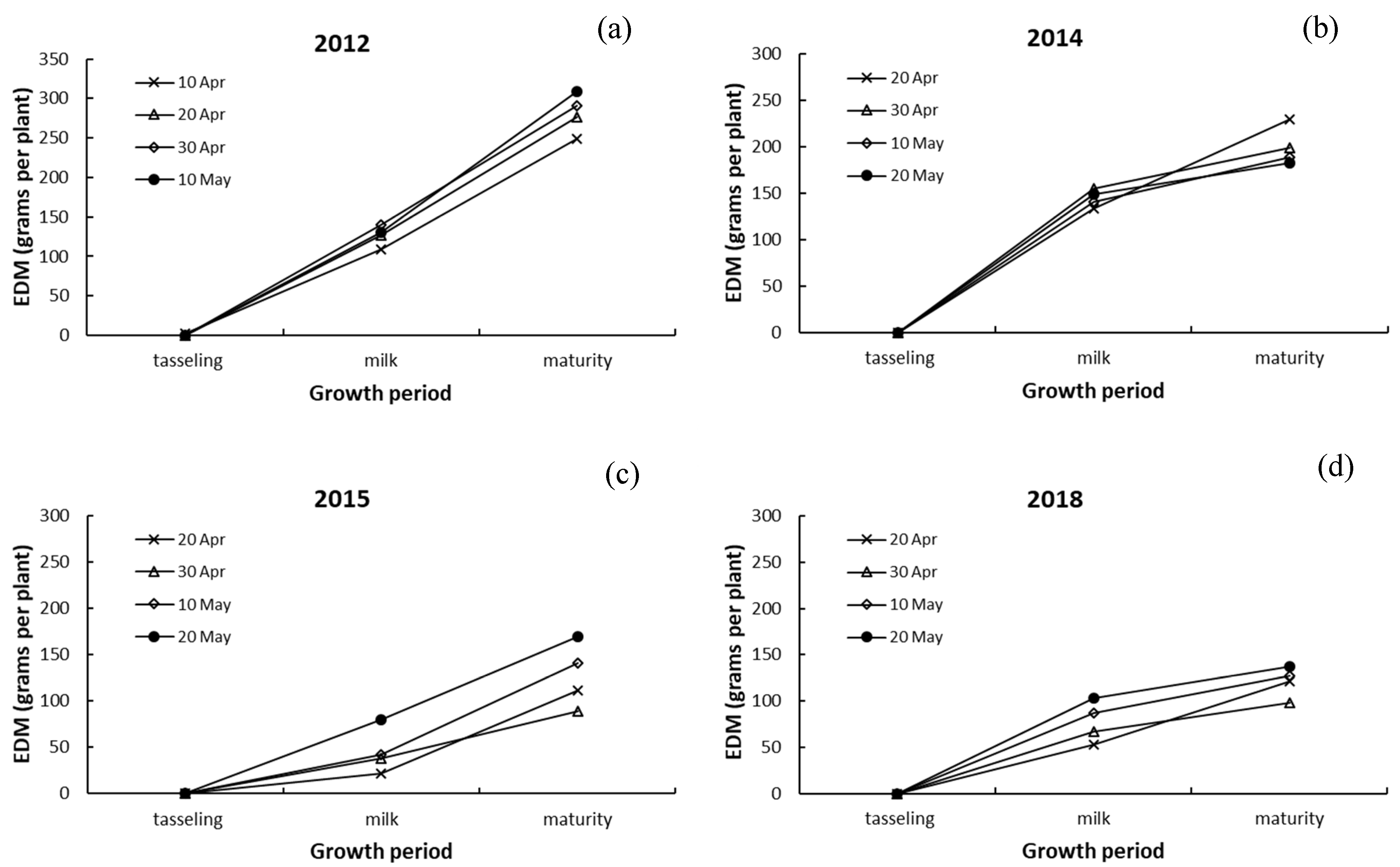
3.3. Differences in Dry Matter among Leaves, Stalk, and Ears of Maize
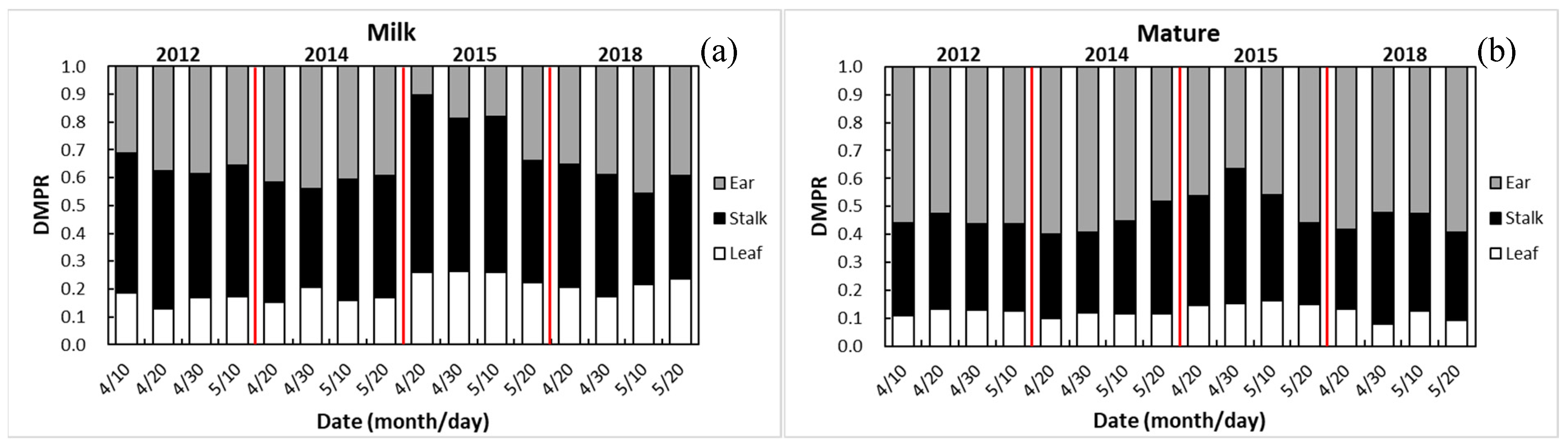
3.4. Dry Matter Partitioning among Different Organs of Maize at Different Growth Stages as Affected by Drought
3.4.1. Dry Matter Partitioning at Milk Stage
3.4.2. Dry Matter Partitioning at Maturity
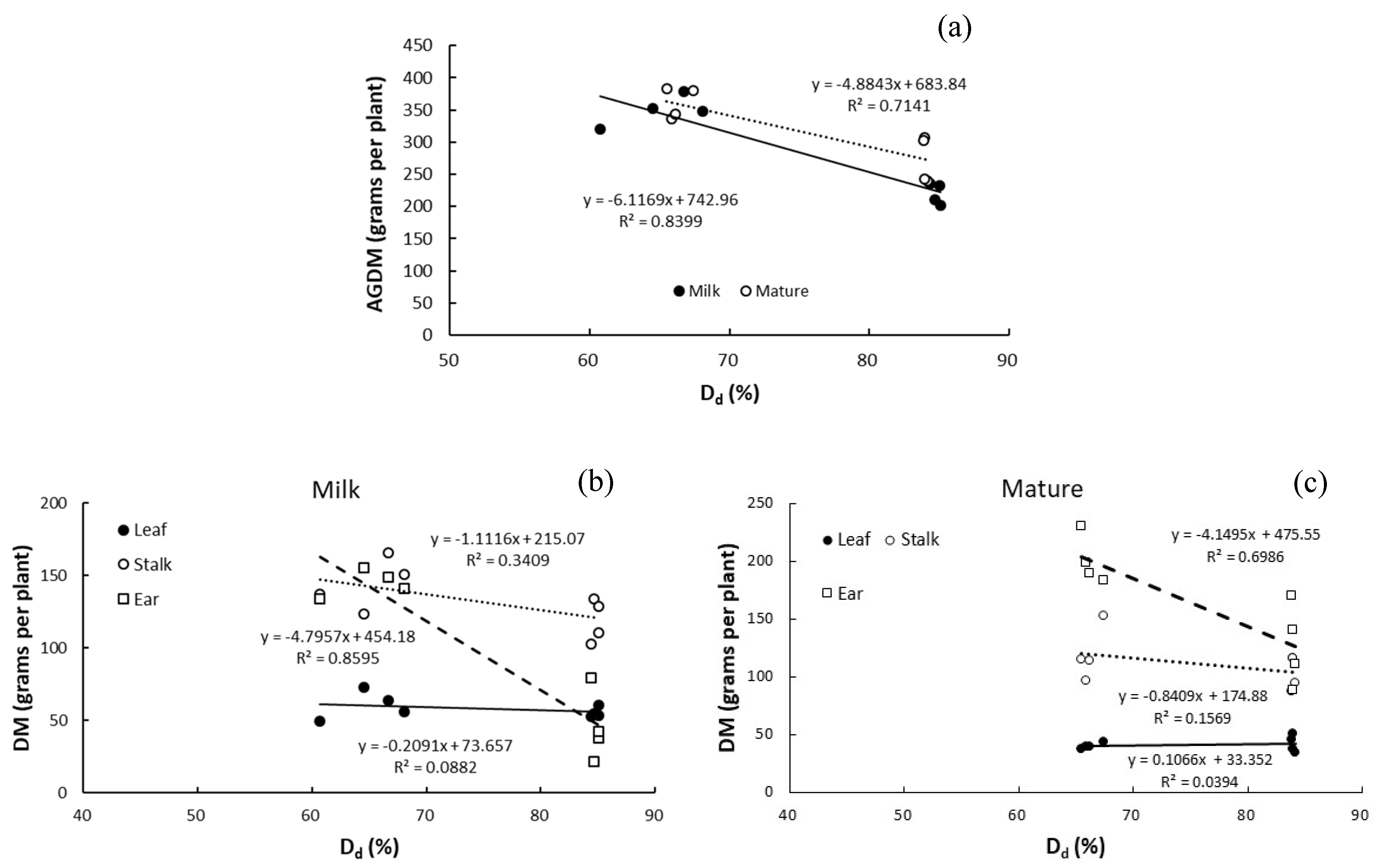
3.5. Relationship between Dry Matter and Degree of Drought
4. Discussion
4.1. Response of Length of Growth Period to Drought and Sowing Date
4.2. Response of Aggregate Above-Ground Dry Matter to Drought and Sowing Date
4.3. Responses of Dry Matter in Leaves, Stalk, and Ears to Drought and Sowing Date
4.4. Responses of DMP among Leaves, Stalk, and Ears to Drought and Sowing Date
4.5. Relationships of Magnitude of Increase in Dry Matter of Above-Ground Organs between Development Stages
5. Conclusions
- The degree of drought, corresponding closely to soil moisture and clearly reflecting the progressive accumulation of drought, is negatively correlated to AGDM, SDM, and EDM. The phenology of maize was more closely related to the sowing date than to drought.
- Under drought conditions, DMA is especially sensitive to total precipitation during the developmental period: under a severe drought, AGDM and dry matters in different organs are impacted earlier and decrease to a greater extent, with wider differences among the sowing dates compared to those under a mild drought.
- A mild drought promoted the transfer of SDM into the ears at the milk stage, whereas it promoted DMA in the early-sown crop, but inhibited it in the late-sown crop at maturity. A severe drought inhibited DMA of the ears at both the stages, especially for the milk stage, and dramatically varied the pattern of DMP as well as increased the difference of the patterns among the sowing dates.
- There are negative correlations for the IDMs in different organs between P3 and P4, and between P4 and P5, proving the complementary effect of the DMA in different organs between the two adjacent developmental stages.
- High temperatures and drought have a synergistic and larger effect on DMA than that of drought alone, and lower DMAs in all the organs, but have only a limited impact on the DMP pattern.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Li, Y.B.; Song, H.; Zhou, L.; Xu, Z.Z.; Zhou, G.S. Vertical distributions of chlorophyll and nitrogen and their associations with photosynthesis under drought and rewatering regimes in a maize field. Agric. For. Meteorol. 2019, 272–273, 40–54. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, X.F.; Liu, Y.; Guo, R.; Chen, L.Y. Effects of drought on maize yield under climate change in China. Trans. Chin. Soc. Agric. Mach. 2020, 36, 149–158. [Google Scholar]
- Cai, F.; Zhang, Y.S.; Mi, N.; Ming, H.Q.; Zhang, S.J.; Zhang, H.; Zhao, X.L.; Zhao, X.L. Maize (Zea mays L.) physiological responses to drought and rewatering, and the associations with water stress degree. Agric. Water Manag. 2020, 241, 106379. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, R.H.; Wang, W.B.; Li, Z.W.; Xue, J.Q.; Aamp, N. Effects of drought stress on performance of photosystem Ⅱ in maize seedling stage. Chin. J. Appl. Ecol. 2015, 26, 1391–1396. [Google Scholar]
- Djaman, K.; Irmak, S.; Rathje, W.R.; Martin, D.L.; Eisenhauer, D.E. Maize Evapotranspiration, Yield Production Functions, Biomass, Grain Yield, Harvest Index, and Yield Response Factors under Full and Limited Irrigation. Trans. ASABE 2013, 56, 373–393. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Sprent, J.I. Environmental effects on dry matter partitioning between shoot and root of crop plants: Relations with growth and shoot protein concentration. Ann. Appl. Biol. 2001, 138, 57–68. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E. Concepts of modelling carbon allocation among plant organs. In Functional-Structural Plant Modelling in Crop Production; Vos, J., Marcelis, L.F.M., de Visser, P.H.B., Struik, P.C., Evers, J.B., Eds.; Springer: Cham, The Netherlands, 2007; pp. 103–111. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.W.; Wang, K.; Harris, W.; Zhong, H.P.; Hu, Z.M.; Han, B.; Zhang, W.Y.; Wang, J.B. Allocation of vegetation biomass across a climate-related gradient in the grasslands of Inner Mongolia. J. Arid Environ. 2009, 73, 521–528. [Google Scholar] [CrossRef]
- Berendse, F.; Möller, F. Effects of competition on root-shoot allocation in Plantago lanceolata L.: Adaptive plasticity or ontogenetic drift? Plant Ecol. 2009, 201, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Schenk, H.J. Root competition: Beyond resource depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- Weiner, J.; Campbell, L.G.; Pino, J.; Echarte, L. The allometry of reproduction within plant populations. J. Ecol. 2009, 97, 1220–1233. [Google Scholar] [CrossRef]
- Bonato, O.; Schulthess, I.F.; Baumgartner, J. Simulation model for maize crop growth based on acquisition and allocation processes for carbohydrate and nitrogen. Ecol. Model. 1999, 124, 11–28. [Google Scholar] [CrossRef]
- Penning de Vries, F.W.T.; Jansen, D.M.; Ten Berge, H.F.M.; Bakema, A. Simulation of Ecophysiological Processes of Growth in Several Annual Crops; Simulation Monographs; PUDOC: Wageningen, The Netherlands, 1989; p. 271. [Google Scholar]
- Yang, J.C.; Zhang, J.H. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Araya, A.; Keesstra, S.D.; Stroosnijder, L. Simulating yield response to water of Teff (Eragrostis tef) with FAO’s AquaCrop model. Field Crops Res. 2010, 116, 196–204. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Raes, D.; Fereres, E. AquaCrop: The FAO crop model to simulate yield response to Water: I. Concepts and underlying principles. Agron. J. 2009, 101, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Toumi, J.; Er-Raki, S.; Ezzahar, J.; Khabba, S. Performance assessment of AquaCrop model for estimating evapotranspiration, soil water content and grain yield of winter wheat in Tensift Al Haouz (Morocco): Application to irrigation management. Agric. Water Manag. 2016, 163, 219–235. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.S.; et al. Decision Support System for Agrotechnology Transfer (DSSAT), Version 4.7.5; DSSAT Foundation: Gainesville, FL, USA, 2019; Available online: https://DSSAT.net (accessed on 1 January 2022).
- Hijmans, R.J.; Guiking-lens, I.M.; Van Diepen, C.A. WOFOST, User Guide for the WOFOST 6.0 Crop Growth Simulation Model; Technical Document; DLO Win and Staring Centre: Wageningen, The Netherlands, 1994. [Google Scholar]
- Cavero, J.; Farre, I.; Debaeke, P.; Faci, J.M. Simulation of Maize Yield under Water Stress with the EPICphase and CROPWAT Models. Agron. J. 2000, 92, 679–690. [Google Scholar] [CrossRef]
- Mi, N.; Zhang, Y.S.; Ji, R.P.; Cai, F.; Yu, W.Y.; Zhang, S.J. Analysis on optimum sowing date of maize in Jinzhou using crop growth model and optimum season method. Chin. J. Agrometeorol. 2016, 37, 67–75. [Google Scholar]
- Anothai, J.; Soler, C.M.T.; Green, A. Evaluation of two evapotranspiration approaches simulated with the CSM–CERES–Maize model under different irrigation strategies and the impact on maize growth, development and soil moisture content for semi-arid conditions. Agric. For. Meteorol. 2013, 176, 64–76. [Google Scholar] [CrossRef]
- Balemi, T.; Kebede, M.; Golla, B.; Tufa, T.; Chala, G.; Abera, T. Phenological and grain yield response of hybrid maize varieties, released for differing agro-ecologies, to growing temperatures and planting dates in Ethiopia. Afr. J. Agric. Res. 2020, 16, 1730–1739. [Google Scholar]
- Zhang, Z.X.; Yu, K.L.; Jin, X.L.; Nan, Z.B.; Wang, J.F.; Niu, X.L.; Whish, J.P.M.; Bell, L.W.; Siddique, K.H.M. Above- and belowground dry matter partitioning of four warm-season annual crops sown on different dates in a semiarid region. Eur. J. Agron. 2019, 109, 125918. [Google Scholar] [CrossRef]
- Bonelli, L.E.; Monzon, J.P.; Cerrudo, A.; Rizzalli, R.H. Maize grain yield components and source-sink relationship as affected by the delay in sowing date. Field Crops Res. 2016, 198, 215–225. [Google Scholar] [CrossRef]
- Mi, N.; Cai, F.; Zhang, Y.S.; Ji, R.P.; Yu, W.Y.; Zhang, S.J.; Fang, Y. Effects of continuous drought during different growth stages on maize and its quantitative relationship with yield loss. Chin. J. Appl. Ecol. 2017, 28, 1563–1570. [Google Scholar]
- Mi, N.; Cai, F.; Zhang, Y.S.; Ji, R.P.; Zhang, S.J.; Wang, Y. Differential responses of maize yield to drought at vegetative and reproductive stages. Plant Soil Environ. 2018, 64, 260–267. [Google Scholar]
- Ma, X.Y.; Zhou, G.S. Maize biomass simulation based on dynamic photosynthate allocation. Chin. J. Appl. Ecol. 2016, 27, 2292–2300. [Google Scholar]
- FAO. Faostat. Verified. 3 March 2019. Available online: http://www.fao.org/faostat/en/#data (accessed on 1 January 2022).
- Cheng, Z.Q.; Meng, J.H.; Wang, Y.M. Improving Spring Maize Yield Estimation at Field Scale by Assimilating Time-Series HJ-1 CCD Data into the WOFOST Model Using a New Method with Fast Algorithms. Remote Sens. 2016, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, L.; Wang, H.; Huang, Q.; Leng, W.; Fang, W.; Zhang, Y. Spatio-temporal characteristics of drought structure across China using an integrated drought index. Agric. Water Manag. 2019, 218, 182–192. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, H.Y.; Fu, Q.; Li, T.X.; Gan, T.Y.; Liu, S.N. Assessing spatiotemporal characteristics of drought and its effects on climate-induced yield of maize in Northeast China. J. Hydrol. 2020, 588, 125097. [Google Scholar] [CrossRef]
- Mi, N.; Zhang, Y.S.; Cai, F.; Ji, R.P.; Zhang, S.J.; Chen, N.N. Higher yields of rain-fed maize affected by drought and sowing date in a semi-humid region of North-eastern China. Int. J. Agric. Biol. 2019, 22, 83–90. [Google Scholar]
- Cai, F.; Mi, N.; Ji, R.P.; Ming, H.Q.; Feng, R.; Zhang, S.J.; Zhang, H.; Zhao, X.L.; Zhang, Y.S. Determination of crop parameters for WOFOST model and its performance evaluation based on field experiment of spring maize in Jinzhou, Liaoning. Chin. J. Ecol. 2019, 38, 1238–1248. [Google Scholar]
- Zargar, A.; Sadiq, R.; Naser, B.; Khan, F.I. A review of drought indices. Environ. Rev. 2011, 19, 333–349. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration. In Guideline for Computing Crop Water Requirements; FAO Irrigation and Drainage Papa 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Ma, X.Y. The Occurrence and Development of Drought on Summer Maize and Its Quantitative Research; Chinese Academy of Meteorological Sciences: Beijing, China, 2017. [Google Scholar]
- Zhang, S.J.; Zhou, G.S.; Li, R.P. Daily crop coefficient of spring maize using eddy covariance observation and its actual evapotranspiration simulation. J. Appl. Meteorol. Sci. 2015, 26, 695–704. [Google Scholar]
- Cao, Y.Q.; Feng, X.X.; Li, L.H.; Lu, J. Temporal and spatial variation of spring corn in Liaoning Province under climate change. Acta Ecol. Sin. 2021, 41, 1092–1105. [Google Scholar]
- Song, H.; Li, Y.B.; Zhou, L.; Xu, Z.Z.; Zhou, G.S. Maize leaf functional responses to drought episode and rewatering. Agric. For. Meteorol. 2018, 249, 57–70. [Google Scholar] [CrossRef]
- Lizaso, J.I.; Ruiz-Ramos, M.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.A.; Lorite, I.J.; Sánchez, D.; García, E.; Rodrígueza, A. Impact of high temperatures in maize: Phenology and yield components. Field Crops Res. 2018, 216, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Nouri, M.; Homaee, M.; Bannayanc, M.; Hoogenboom, G. Towards shifting planting date as an adaptation practice for rainfed wheat response to climate change. Agric. Water Manag. 2017, 186, 108–119. [Google Scholar] [CrossRef]
- Baum, M.E.; Archontoulis, S.V.; Licht, M.A. Planting Date, Hybrid Maturity, and Weather Effects on Maize Yield and Crop Stage. Agron. J. 2019, 111, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.X.; Zhou, G.S. Phenological change and its ecophysiological mechanism of spring maize responding to drought at jointing stage and rewatering. Acta Ecol. Sin. 2020, 40, 274–283. [Google Scholar]
- Fisher, M.; Abate, T.; Lunduka, R.W.; Asnake, W.; Alemayehu, Y.; Madulu, R.B. Drought tolerant maize for farmer adaptation to drought in sub-Saharan Africa: Determinants of adoption in eastern and southern Africa. Clim. Chang. 2015, 133, 283–299. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, S.; Chauhanb, Y.; Rachaputia, R.; Fekybeluc, S.; Prasannad, B. Characterising production environments for maize in eastern and southern Africa using the APSIM Model. Agric. For. Meteorol. 2017, 247, 445–453. [Google Scholar] [CrossRef]
- Zhou, W.; Guan, K.; Peng, B.; Shi, J.; Jiang, C.; Wardlow, B.; Pan, M.; Kimball, J.S.; Franz, T.E.; Gentine, P.; et al. Connections between the hydrological cycle and crop yield in the rainfed U.S. Corn Belt. J. Hydrol. 2020, 590, 125398. [Google Scholar] [CrossRef]
- Ji, R.P.; Che, Y.S.; Zhu, Y.N.; Liang, T.; Feng, R.; Yu, W.Y.; Zhang, Y.S. Impacts of drought stress on the growth and development and grain yield of spring maize in Northeast China. Chin. J. Appl. Ecol. 2012, 23, 3021–3026. [Google Scholar]
- Lobell, D.B.; Hammer, G.L.; Chenu, K.; Zheng, B.; McLean, G.; Chapman, S.C. The shifting influence of drought and heat stress for crops in northeast Australia. Glob. Chang. Biol. 2015, 21, 4115–4127. [Google Scholar] [CrossRef]
- Maddonni, G.A. Analysis of the climatic constraints to maize production in the current agricultural region of Argentina—A probabilistic approach. Arch. Meteorol. Geophys. Bioclimatol. Ser. B 2012, 107, 325–345. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Yu, W.W.; Tan, X.S.; Ye, B.X.; Bi, J.J. Effect of water stress on dry-matter partition of summer maize. Acta Agric. Boreali-Sin. 2009, 24, 149–154. [Google Scholar]
- Tan, F.Y.; Li, H.; Wang, J.L.; Wang, Z.W. Response of dry matter partitioning coefficient of summer maize to drought stress in North China. Chin. J. Appl. Ecol. 2019, 30, 217–223. [Google Scholar]
- Dang, H.K.; Li, W.; Cao, C.Y.; Zheng, C.L.; Ma, J.Y.; Li, K.J. Effects of late milk irrigation on water use efficiency and dry matter distribution of maize. Trans. Chin. Soc. Agric. Mach. 2014, 45, 131–138. [Google Scholar]
- Li, Y.B.; Song, H.; Zhou, L.; Xu, Z.Z.; Zhou, G.S. Tracking chlorophyll fluorescence as an indicator of drought and rewatering across the entire leaf lifespan in a maize field. Agric. Water Manag. 2018, 211, 190–201. [Google Scholar] [CrossRef]


| Parameter | Abbreviation |
|---|---|
| Above-ground dry matter | AGDM |
| The degree of drought | Dd |
| Dry matter | DM |
| Dry matter accumulation | DMA |
| Dry matter partitioning | DMP |
| Dry matter partitioning rate | DMPR |
| Effective accumulated temperature | AT |
| Leaf, Stalk, Ear DM | LDM, SDM, EDM |
| Increase in DM | IDM |
| Increase in AGDM | AGIDM |
| Mean temperature | MT |
| Soil relative water content | SRWC |
| Stages of growth: from emergence to 7-leaf stage, 7-leaf stage to jointing, jointing to tasselling, tasselling to milk stage, and milk stage to maturity | P1, P2, P3, P4, P5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Zhang, Y.; Mi, N.; Ming, H.; Zhang, S.; Zhang, H.; Zhao, X.; Zhang, B. The Effect of Drought and Sowing Date on Dry Matter Accumulation and Partitioning in the Above-Ground Organs of Maize. Atmosphere 2022, 13, 677. https://doi.org/10.3390/atmos13050677
Cai F, Zhang Y, Mi N, Ming H, Zhang S, Zhang H, Zhao X, Zhang B. The Effect of Drought and Sowing Date on Dry Matter Accumulation and Partitioning in the Above-Ground Organs of Maize. Atmosphere. 2022; 13(5):677. https://doi.org/10.3390/atmos13050677
Chicago/Turabian StyleCai, Fu, Yushu Zhang, Na Mi, Huiqing Ming, Shujie Zhang, Hui Zhang, Xianli Zhao, and Bingbing Zhang. 2022. "The Effect of Drought and Sowing Date on Dry Matter Accumulation and Partitioning in the Above-Ground Organs of Maize" Atmosphere 13, no. 5: 677. https://doi.org/10.3390/atmos13050677
APA StyleCai, F., Zhang, Y., Mi, N., Ming, H., Zhang, S., Zhang, H., Zhao, X., & Zhang, B. (2022). The Effect of Drought and Sowing Date on Dry Matter Accumulation and Partitioning in the Above-Ground Organs of Maize. Atmosphere, 13(5), 677. https://doi.org/10.3390/atmos13050677







