Abstract
Mercury (Hg) is a useful heavy metal; however, it is toxic to both humans and the environment. Tree bark is an excellent bioindicator, which has been proven to be effective in studying the level of atmospheric Hg contamination. This study aimed to determine the distribution of evaporated Hg using the total weight of Hg (THg) in tree barks in Indonesia at the artisanal and small-scale gold mining (ASGM) area of Bunut Seberang Village and Lampung University, respectively. Samples were taken using purposive sampling, based on the criteria of forestry trees at a height level of 1.3 m above ground as wide as 100 cm2. The samples were analyzed by atomic absorption spectrometry and Scanning electron microscopy to determine the levels of THg and to investigate the bark structures. Results showed that the highest THg values were found in a Magnolia champaca sample (56.5 µg), followed by Swietenia mahagoni (45.8 µg) and Swietenia mahagoni (33.5 µg). All species studied showed THg levels in the tree barks at an elevation from 30 to 320 m above sea level. The Hg amounts found in the sampled barks indicated the dispersion of Hg throughout the ASGM area, which signified hazardous atmospheric conditions in the area.
1. Introduction
Mercury (Hg) is a toxic heavy metal, which is well known for its harmful effects in the environment. The European Union Commission and World Health Organization have stated that Hg even at low concentrations could affect human health and has become a global environmental concern [1]. Reduction or elimination of Hg use [2]. Hg transport and disposal present serious risks to the environment [3]. In artisanal and small-scale gold mining (ASGM), Hg has been used to extract gold (Au) from an ore to form a stable Hg–Au amalgam. Then, the amalgam is heated to evaporate the Hg and isolate the Au. Hg amalgamation has been used for thousands of years to mine Au and Ag in this way [4].
An uncontrolled use of Hg in gold mining occurs due to low technical knowledge [5]. In Indonesia, ASGM has been the second most significant source of Hg pollution after coal burning [6]. Recognizing the problems associated with the use of Hg in mining, over 130 countries have signed the United Nation’s Minamata Convention as an agreement to significantly reduce the emission and usage of Hg [7]. During an entire ore amalgamation process, 20 g of Hg is typically used to produce 1 g of Au, where 19 and 1 g of the Hg pass or are released to the tailings and atmosphere, respectively [3]. Globally, Hg amalgamation activities in mining could lead to the annual release of up to 1000 tons of Hg to the environment. Specifically, Hg release from Indonesia has been estimated between 100 and 150 tons per year [8].
Various studies have focused on the determination of Hg concentrations in various bioindicators including tree-like foliage [9], leaf litter [10], tree bark, and roots [11]. In particular, tree barks have been used to assess the state of Hg contamination in the environment [12]. In one study, higher Hg was found in tree foliages and barks than in wood samples [13]. Foliage materials typically contain high Hg concentrations because foliage absorbs elemental Hg from the atmosphere through its stomata [14]. Although bark and wood are less exposed to atmospheric Hg than foliage, bark can capture and retain atmospheric Hg through surface sorption [13]. The enrichment of trace elements in a tree bark can be used to trace pollution sources [15]. This is because airborne particles are often trapped within the structure of a tree bark, where these particles accumulate over a period of several years [16].
This study aimed to determine the atmospheric pool of Hg in an ASGM area at Bunut Seberang Village, Lampung, Indonesia, using forestry tree barks as bioindicators.
2. Materials and Methods
2.1. Study Location
The test and control samples were collected in December 2020 in an ASGM area of Bunut Seberang Village, Pesawaran District, and in January 2021 in the University of Lampung, Lampung Province, respectively, as shown in Figure 1. The control samples were selected to compare the research main samples. The distance of main test samples obtained in the ASGM area ranged from 0 to 1.2 km. For the control samples, the location was 32 km from the ASGM location. Six forestry trees were found in the ASGM areas and three forestry trees were found in control areas, respectively. The trees were selected due to their rough bark characteristics, which could retain atmospheric pollutants such as Hg [13].
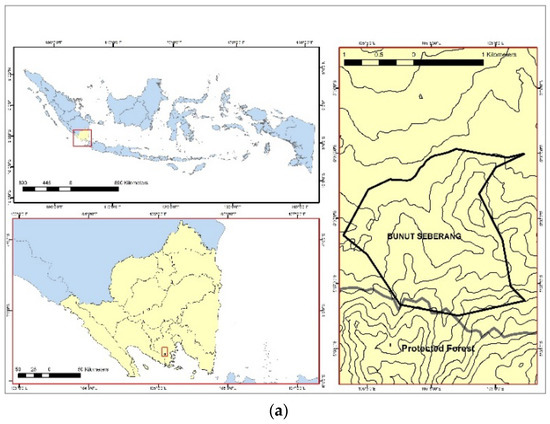
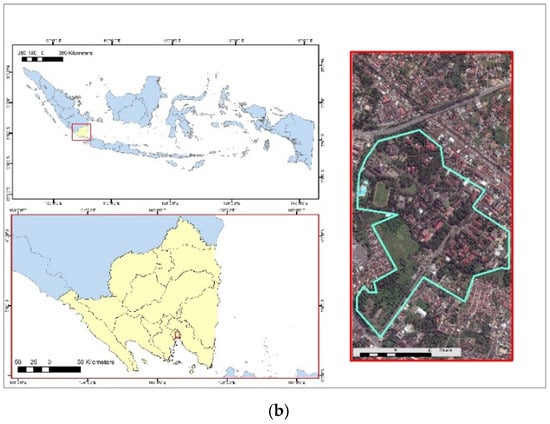
Figure 1.
The research sampling locations were (a) Bunut Seberang Village, Lampung Province, Pesawaran District, Indonesia (test samples), and (b) University of Lampung, Lampung Province, Bandar Lampung, Indonesia (control samples).
2.2. Sampling Methods
Evaporated elemental Hg has been mostly found in trees, which are located around 1.3 m from the ground [12]. The dimensions of tree bark samples obtained were 10 cm × 10 cm. The samples were taken at a height of 1.3 m to ensure homogeneity [13]. The collection of tree barks was conducted by selecting features that faced gold refining locations at the ASGM site. The trees selected for sampling have a minimum diameter of 20 cm. Bark samples were collected by purposive sampling based on rough stem morphology [17]. Based on the sampling used, there were six tree species (C. pentandra (n = 1), M. champaca (n = 1), P. falcataria (n = 2), P. acerifolium (n = 2), S. mahagoni (n = 7), and T. grandis (n = 5)), all with rough bark characteristics as shown in Figure 2. The total number of samples collected was 18 (n (total) = 18). The locations of the sampled trees were selected as shown in Figure 2. The collected rough barks were in form of hard chipped, broken scaled, and grooved barks. Trees that have such rough bark characteristics include T. grandis, Swietenia sp., and Schima wallichii [18]. The collected samples were kept in coded plastic sample bags.
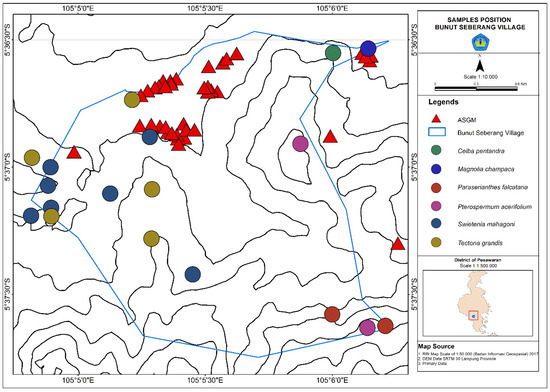
Figure 2.
The locations of the sampled trees at Bunut Seberang Village including C. pentandra (n = 1), M. champaca (n = 1), P. falcataria (n = 2), P. acerifolium (n = 2), and S. mahagoni (n = 7) and at Bunut Seberang Village such as T.grandis (n = 5) (n (total sampled trees) = 18).
The sample distances in the ASGM location were measured by conducting field observations at the location. The elevation data were obtained using the secondary data from the Digital Elevation Model and Rupa Bumi Indonesia maps of Lampung Province for 2017 with a scale of 1:10,000. The wind direction data for 2010–2020 were obtained from four stations of the Meteorology, Climatology, and Geophysical Agency, which are located in Bandar Lampung, Pesawaran, South Lampung, and North Lampung, Lampung, Indonesia.
2.3. Atomic Absorption Spectrometry (AAS) and Scanning Electron Microscopy (SEM)
The mercury concentrations were determined by Atomic Absorption Spectrometry (AAS). The barks were cut to obtain the Fragment Dimension (FD), approximately 100 cm2 (10 cm × 10 cm) from the tree. Samples were dried in an oven at 300 °C for 48 h. A dried sample was weighed and the weight was counted as the dry weight (DW) (g). Each sample was analyzed by Image J from Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda (Maryland, USA) and cut as wide as 14 to 18 cm2 to measure the Real Square (RS). Each sample was mashed using a Philips Plastic HR2115/00 mixer by Phillips Inc. (Eindhoven, Netherlands) speed of 13,000 rpm.
The AAS analyses were conducted using the AAS method described in the Indonesian National Standard 01-2896-1998. A 5 g sample was placed in a plastic tube, then dissolved in 25 mL H2SO4 18N, 20 mL HNO3 7N, and 1 mL 2% of Na2MoO4. The samples then were sent to PT Superintending Company of Indonesia (PT SUCOFINDO) for AAS analysis using an Agilent 240FS-VGA 7 from Agilent Technologies Inc. (Mulgrave, Victoria, Australia). AAS analysis was conducted using the AAS method described in the Indonesian National Standard 01-2896-1998. Hg results were reported as concentrated Hg (CHg) before conversion to total weight Hg (THg).
Scanning Electron Microscopy (SEM) was used to inspect and identify the anatomical features of the tree bark samples [19]. The SEM analysis uses ZEISS/EVO MA 10, The Carl Zeiss Foundation, Munich, Germany. SEM uses a procedure involving electrons to magnify the image of a sample, which allowed the inspection of the tree bark samples in detail [20].
The accumulated Hg was measured as THg (μg), which was calculated by Equation (1) [12,21,22].
where the THg is Total Weight of Mercury, CHg is Concentrated Hg, DW is Dry Weight, FD is Fragment Dimension, RS is Real Square.
2.4. Statistical Analysis
Statistical analyses were performed using an IBM SPSS Version 26 SPSS Inc. (Chicago, IL, USA). To determine whether the THg, CHg, bark thickness, elevation, and distance values were normally distributed, a Shapiro–Wilk measurement at the 5% level was used followed by a one-way analysis of variance at the 5% level of significance [21]. A bivariate correlation analysis was used to determine the correlation between bark thickness, elevation, distance, and THg.
3. Results
THg Levels in the Tree Bark Samples Based on Distance and Elevation
The THg levels found in the Bunut Seberang Village are presented in Table 1.

Table 1.
THg Levels Found in Bunut Seberang Village.
The results in Table 1 showed that all of the sampled forestry trees contained Hg. The highest THg amount was found in M. champaca (56.5 μg), which was followed by S. mahagoni, P. acerifolium, and C. pentandra (2.21–45.8 μg). Various THg amounts were found in T. grandis from 3.50 to 17.7 μg.
The bark thickness did not show any significant correlation to the THg. The thickest bark sample was from P. falcataria 2 (bark thickness = 19.5 mm), which contained a lower THg of 4.24 μg than the thin bark sample from S. mahagoni 5 (bark thickness = 0.62 mm) with a measured THg of 45.8 μg. Another example was C. pentandra (bark thickness = 9.64 mm), which contained a lower THg of 11.7 μg than S. mahagoni 6 (bark thickness = 0.80 mm) with a measured THg of 33.5 μg.
Meanwhile, THg was affected by CHg found in the samples. Magnolia champaca gave the highest value for CHg of 1.01 µg/g. It also contained the highest amount Hg with a THg of 56.5 μg. The M. champaca and S. mahagoni 7 samples both gave the second largest CHg value of 0.78 µg/g. They also contained the second largest THg of 45.8 μg.
Based on the results, THg values found in the studied areas were not affected by elevation. Similar values for THg were generally found at any elevation level. P. falcataria 1 (THg = 5.66 μg) that was obtained at an elevation level of 310 m asl showed a similar THg with T. grandis 5 and T. grandis 1 (THg = 3.50 and 6.66 μg, respectively), which were obtained at an elevation level of 150 and 30 m asl, respectively. C. pentandra 1 which was sampled at an elevation level of 80 m asl contained a THg of 11.7 μg-DW. This was lower than the THg found in S. mahagoni 2 (32.2 μg), which was sampled at the same elevation level of 80 m asl.
The THg values found were also not affected by distance. M. champaca was the closest sample location (47 m from the ASGM location) providing the highest THg value (56.5 μg) compared to the other samples. In a further location, a high THg value (45.8 μg) was also found in S. mahagoni 5, which was sampled 723 m away from the ASGM location. These numbers showed the variation in atmospheric Hg, which was not dependent on distance.
The results from control samples are summarized in Table 2.

Table 2.
THg values found in the control location.
The control samples showed THg values of <1 μg. Plants have been classified as hyperaccumulators of Hg and toxic if the Hg concentration in a plant exceeds 10 [23] and 1 ppm [24], respectively. Therefore, the trees in the control area were not toxic.
Wind direction, elevation, and bark type have been found to determine the levels of heavy metal contamination in tree barks [25]. This was because Hg spreads in the atmosphere due to wind [26]. Wind also causes the dispersion of Hg over long distances [27].
As seen in Table 1, all the sampled trees in the ASGM area contained and were above the Hg detection limit (0.02 μg); therefore, the air near this area was most likely contaminated by Hg. Hence, M. champaca, S. mahagoni, C. pentandra, P. acerifolium, P. falcataria, and T. grandis samples in Table 1 were used to determine the atmospheric Hg contamination in the studied area. To determine the distribution rate of THg in the ASGM study area, the distance and elevation of each collected bark were defined. These are summarized in Table 2.
4. Discussion
4.1. THg in the Tree Bark Samples
Barks contain Hg (Table 1) and main organic matters of carbon and oxygen. Hg found in a bark sample was either from within or between the atmosphere in ASGM area and stored in the tree bark, especially in the external layer of the barks [28]. The amount of Hg stored in tree bark is often defined by the factors of bark roughness and sample location [29]. The differences in Hg concentrations between tree species in the studied location could be explained by the variations in wind flow during 2010–2020 as shown in Figure 3 [30] and different rates of absorption in the six tree species considered [13].
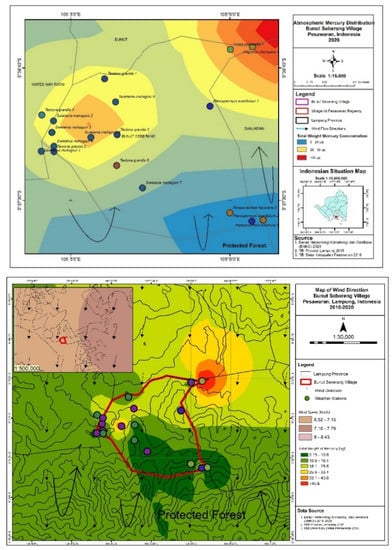
Figure 3.
Atmospheric Hg distribution in the Bunut Seberang Village between 2010 and 2020.
THg contamination in tree barks did not exhibit significant variation in this study, with the exception of M. champaca 1, S. mahagoni 2, S. mahagoni 5, and S. mahagoni 6 with THg values of >20 µg. The surface magnification of the samples is shown in Figure 4. The samples of M. champaca and S. mahagoni showed textures of clustered and fibrous-grouped, respectively. These samples have a rough-textured bark. The THg found in P. acerifolium was low, because its bark was less rough especially when compared with M. champaca [31] (see Figure 4). The SEM results showed that the Hg levels found in M. champaca ranged from 19.6 to 25.1%. The level was 19.6% in S. mahagoni. After considering the SEM data, the low roughness of a tree bark seemed to have minimized the possibility of accumulation of atmospheric Hg. In fact, rougher tree barks have been known to be better accumulators of heavy metals [32].
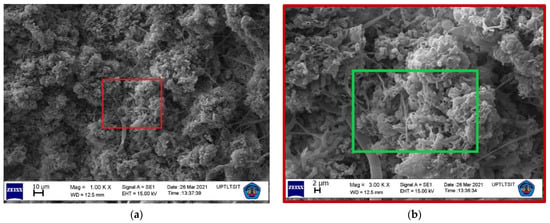
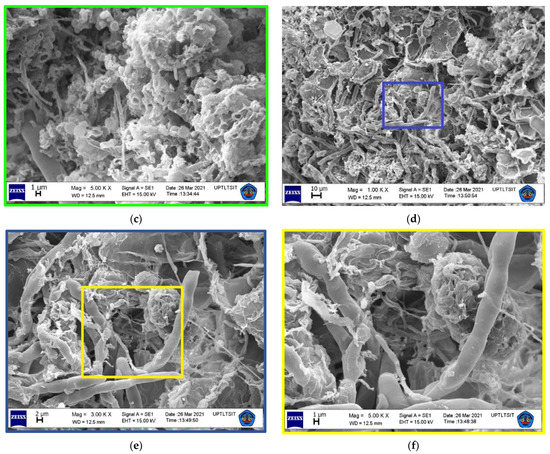
Figure 4.
SEM micrographs of M. champaca at magnifications of (a) 1000, (b) 3000, and (c) 5000×. SEM micrographs of S. mahagoni at magnifications of (d) 1000, (e) 3000, and (f) 5000×. The colored box (  :
:  ,
,  , or
, or  ) in a panel depicts the analyzed area.
) in a panel depicts the analyzed area.
 :
:  ,
,  , or
, or  ) in a panel depicts the analyzed area.
) in a panel depicts the analyzed area.
We found that the tree bark thickness did not define the THg level found in bark (see Figure 5). The bark thickness only affected the THg value by 0.6%. The bark thickness varied from 0.17 to 19.5 mm as shown in Figure 5. The differences in the bark thickness can vary in shape and thickness, depending on the species and ecological factors [33]. In a study with bark thickness values from 0 to 0.6 mm of samples, the heavy metal adsorption capacity was found to be higher in the outer bark than in the inner bark [34]. This was because the uptake of heavy metals by the tree inner bark (via phloem) was lower than the outer bark (via rhytidome).
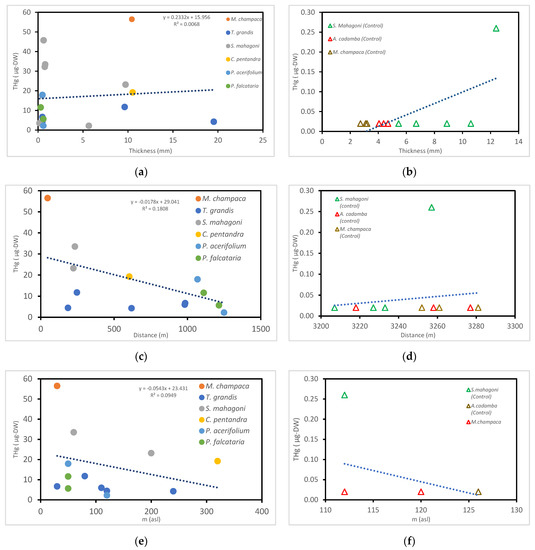
Figure 5.
(a) Bark thickness (mm) versus THg found, (b) bark thickness versus THg found (control), (c) distance from ASGM (m) versus THg found, (d) distance from ASGM (m) versus THg found (control), (e) elevation versus THg found, and (f) elevation versus THg found (control).
4.2. Elevation, Distance, and Bark Thickness
A bark accumulates atmospheric Hg as it is continually exposed to air pollution for many years; therefore, it could provide precise information about the changes in the atmospheric condition of a certain ecosystem [35]. A study of the chemical constituents in barks is an appropriate method for tracing pollution sources [12,13,15,21,22]. The distances shown in Figure 4 showed a negative trend toward THg levels in tree barks. All tree barks contained Hg in both areas near and far from the ASGM location studied. The bark has been found to have the highest capacity for heavy metal absorption, followed by cones, needles, and wood [33]. Tolerant tree species have been used as an indicator for the accumulation of air pollutants [36]. Hg concentrations have been determined by the analyses of tree tissues including bark [15].
Hg spreads in the atmosphere [26] and disperses over long distances due to wind [27]. Tree barks have been known to retain and capture atmospheric Hg through surface sorption [13] even over long distances. This was observed in our work. A high THg value of 45.8 μg was found for the S. mahagoni 5, which was sampled at a long distance of 723 m from the ASGM location (see Figure 5).
Heavy metal adsorption on biomass such as bark is a physiochemical process [35]. In the case of a bark, heavy metals typically accumulate through the wet and dry deposition processes [37]. Briefly, a tree bark absorbs gaseous Hg at its surface. The absorbed Hg is then bound to thiol-containing molecules such as tannins [38]. The wet and dry depositions of Hg have been defined as the air-to-surface flux in the presence and absence of precipitation, respectively [39]. For these reasons, tree bark can be used as a heavy metal bioindicator where their outer portion consists of dead materials, which do not interfere with their growth cycles [35].
All the tested samples contained atmospheric Hg. THg values were observed in the samples obtained from 0 to 320 m asl. A previous study found that atmospheric Hg increased with altitude [40]. Conversely, our measurements indicated that atmospheric Hg did not increase with altitude as shown in Figure 4 and Figure 5. The THg values were rather grouped and dispersed throughout all the altitudes studied. Additionally, shown in Figure 5, the group with an elevation from 0 to 100 m asl provided a maximum value of 56.5 μg, with median and minimum values of 17.8 and 5.63 μg, respectively. For the group with an elevation from 100 to 320 m asl, the maximum value was 23.2 μg, with median and minimum values of 4.32–2.19 μg, respectively.
P. falcataria 2 from an elevation of 240 m asl showed a lower THg than S. mahagoni 6 from an elevation of 60 m asl. In addition, the sources of Hg in the ASGM site were dispersed due to the wind factor. The dispersion of windblown atmospheric Hg in the 2010–2020 study period occurred from north to south. Therefore, wind largely controlled the movement of atmospheric Hg in our research sites [41] as shown in Figure 6.
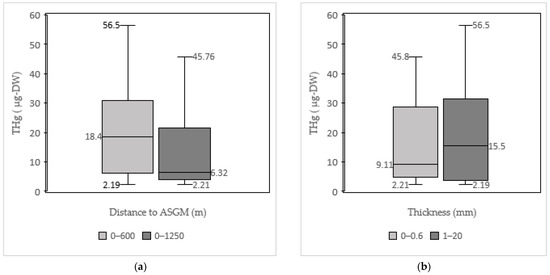
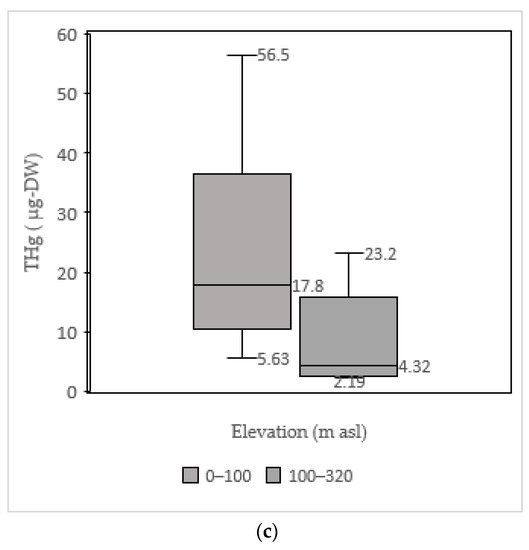
Figure 6.
(a) Distance, (b) thickness, and (c) elevation.  = 2q box,
= 2q box,  = 3q box.
= 3q box.
 = 2q box,
= 2q box,  = 3q box.
= 3q box.
4.3. Wind Direction
The major transport of evaporated Hg comes from various sources in the atmosphere [42] and occurs in the gaseous phase [43]. The movement of heavy metals depends on numerous factors including temperature, surface waters, air masses, and wind [41]; therefore, they are transported over long distances even at global scales [44]. Wind in the study area was recorded from 2010 to 2020. Wind was found to blow mostly from north to south as shown in Figure 6. Wind movement that carried Hg was also affected by a mountainous area (e.g., P. falcataria 1 and P. falcataria were taken at the elevation level of 310 and 240 m asl, respectively), which diverted the wind to a lower elevation. A previous study that has coastal and hilly areas stated that topography significantly influences the Hg [12]. Nevertheless, in this study that has mountainous areas did not significantly affect the Hg.
The mountainous area in this study was located in the south of the ASGM area. The presence of elevation and trees have been known to cause airflow fluctuations and lower wind speeds [45]. These affect the total Hg contamination. It has been found that wind influences the distribution of heavy metals in some areas brought by pollutant sources [46]. M. champaca provided the highest Hg contamination (56.5 µg) at a distance of 47 m from the ASGM location. S. mahagoni 6, which was the sample closest to the ASGM area (distance of 233 m), also exhibited an elevated THg (33.5 µg). S. mahagoni 2 (32.2 µg) and S. mahagoni 5 (45.8 µg) exhibited high Hg contamination, although their distances (894 and 723 m, respectively) from the studied area were greater than the other samples.
5. Conclusions
In this study, about 18 samples, with 6 different species, were found during the field sampling. The M. champaca was indicated as the highest THg (56.5 µg) among other samples then followed by C. pentandra 11.7 µg, P. falcataria 1 ranged from 2.19 µg to 4.24 µg, P. acerifolium ranged from 19.2 µg to 23.2 µg, T. grandis ranged from 3.50 µg to 17.7 µg, and S. mahagoni ranged from 2.21 µg to 45.8 µg. The highest elevation on this study is at 320 m asl (P. acerifolium 2) and the lowest elevation is at 30 m asl (M. champaca and T. grandis 1). The furthest sample location is S. mahagoni 7 at 1250 m, and the closest sample is M. champaca at 47 m from ASGM site. This study showed that the wind direction and the topography (closed mountain area) is the important factor to attach evaporated mercury in the tree bark. Meanwhile, the distance, elevation, and bark thickness showed no significant effect on the attachment of evaporated mercury.
Author Contributions
A.T. was the lead author who is still doing a postgraduate program at Lampung University. M.R., H.P. and E.L.W. were the supervisors. Furthermore, S.B.Y. and C.A. were advisers. T.R. was the assistant that helped the lead author finish this research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Research Institute for Humanity and Nature (RIHN: a constituent member of NIHU), Project No.14200102.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Azhary Taufiq, one of the authors of this study, would like to thank the University of Lampung for providing the master’s degree scholarships and RIHN Project No.14200102 for funding this research, and the Pesawaran Regency’s Forestry Department.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO (World Health Organisation). Exposure to Mercury: A Major Public Health Concern. 2009. Available online: http://www.who.int/phe/news/Mercury-flyer.pdf (accessed on 4 January 2021).
- Holmes, P.; James, K.A.F.; Levy, L.S. Is low level environmental mercury exposure of concern to human health? Sci. Total Environ. 2004, 408, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Telmer, K.H.; Stapper, D. Evaluating and Monitoring Small Scale Gold Mining and Mercury Use: Building a Knowledge-Base with Satellite Imagery and Fieldwork. UNDP/GEF/UNIDO Project EG/GLO/01/G34; Final Report to the United Nations Industrial Development Organization; UNIDO: Vienna, Austria, 2007. [Google Scholar]
- De Lacerda, L.D.; Salomons, W. Mercury from Gold and Silver Mining a Chemical Time Bomb? Springer: Berlin, Germany, 1998; pp. 1–151. [Google Scholar]
- Green, C.S.; Lewis, P.J.; Wozniak, J.L.; Drevnick, P.E.; Thies, M.L. A comparison of factors affecting the small-scale distribution of mercury from artisanal small-scale gold mining in a Zimbabwean stream system. Sci. Total Environ. 2019, 647, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Limbong, D.; Kumpampung, J.; Rimper, J.; Arai, T.; Miyazaki, N. Emissions and environmental implications of mercury from artisanal gold mining in North Sulawei, Indonesia. Sci. Total Environ. 2020, 302, 227–236. [Google Scholar] [CrossRef]
- Xu, J.; Garcia, A.B.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.M.; Maxson, P.A.; Hylander, L.D. Origin and comsumption of mercury in small-scale gold mining. J. Clean. Prod. 2006, 14, 436–447. [Google Scholar] [CrossRef]
- Siwik, E.L.; Campbell, L.M.; Mierle, G. Fine-scale mercury trends in temperate decidous tree leaves from Ontario, Canada. Sci. Total Environ. 2009, 407, 6275–6279. [Google Scholar] [CrossRef]
- Blackwell, B.D.; Driscoll, C.T.; Maxwell, J.A.; Holsen, T.M. Changing climate alters inputs and pathways of mercury deposition to forested ecosystems. Biogeosciences 2014, 119, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Gueguen, F.; Stille, P.; Geagea, M.L.; Boutin, R. Atmospheric pollution in an urban environment by tree bark biomonitoring-Part I: Trace element analysis. Chemosphere 2012, 86, 1013–1019. [Google Scholar] [CrossRef]
- Prasetia, H.; Sakakibara, M.; Omori, K.; Laird, J.S.; Sera, K.; Idham, A.K. Mangifera indica as Bioindicator of Mercury Atmospheric Contamination in an ASGM Area in North Gorontalo Regency, Indonesia. Geosciences 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yanai, R.D.; Driscoll, C.T.; Montesdeoca, M.; Smith, K.T. Concentrations and content of mercury in bark, wood, and leaves in hardwoods and conifers in four forested sites in the Northeastern USA. PLoS ONE 2018, 13, e0196293. [Google Scholar] [CrossRef]
- Fleck, J.A.; Grigal, D.F.; Nater, E.A. Mercury uptake by trees: And observational experiment. Water Air Soil Pollut. 1999, 115, 513–523. [Google Scholar] [CrossRef]
- Lahd Geagea, M.L.; Stiller, P.; Millet, M.; Perrone, T. Ree characteristics and Pb, Sr and Nd isotopic compositions of steel plant emissions. Sci. Total Environ. 2007, 373, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Catinon, M.; Ayrault, S.; Clocchiatti, R.; Boudouma, O.; Asta, J.; Tissut, M.; Ravanel, P. The anthropogenic atmospheric elements fraction: A new interpretation of elemental deposits on tree barks. Atmos. Environ. 2009, 43, 1124–1130. [Google Scholar] [CrossRef]
- Ismail, A.Y.; Hendrayana, Y.; Saputra, R.H. Inventarisasi dan identifikasi sebaran hutan rakyat di Kabupaten Majalengka. Wanaraksa 2016, 10, 31–40. [Google Scholar]
- Indriyanto. Dendrologi: Suatu Teori & Praktik Menyidik Pohon; Plantaxia: Bandar Lampung, Indonesia, 2012; pp. 1–124. [Google Scholar]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Poll. 2011, 12, 3560–3570. [Google Scholar] [CrossRef]
- Ji, Z. Use of compositional and combinatorial nanomaterial libraries for biological studies. Sci. Bull. 2016, 10, 755–771. [Google Scholar] [CrossRef]
- Prasetia, H.; Sakakibara, M.; Sera, K.; Laird, S.T. Evaluation of the total mercury weight exposure distribution using tree bark analysis in an artisanal and small-scale gold mining area, North Gorontalo Regency, Gorontalo Province, Indonesia. Int. J. Environ. Res. Public Health 2022, 19, 33. [Google Scholar] [CrossRef]
- Rendra, T.; Riniarti, M.; Yuwono, S.B.; Prasetia, H.; Widiastuti, E.L.; Bakri, S.; Taufiq, A. Mapping atmospheric mercury in Lampung Province, Indonesia using bark of multipurpose tree species. Atmosphere 2022, 13, 2. [Google Scholar] [CrossRef]
- Kosegeran, A.O.; Rondonuwu, S.; Simbala, H.; Rumondor, M. Kandungan merkuri pada tumbuhan paku (Diplazium accedens Blume) di daerah tambang emas Tatelu-Talawaan, Kabupaten Minahasa Utara. J. Ilm. Sains 2015, 15, 59–65. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Soils and Plants, 3rd ed.; CRC Press: New York, NY, USA, 2001; pp. 1–403. [Google Scholar]
- Odukoya, O.O.; Arowolo, T.T.; Bamgbose, O. Pb, Zn and Cu levels in tree barks as indicator of atmospheric pollution. Environ. Int. 2000, 26, 11–16. [Google Scholar] [CrossRef]
- Javarabad, D.M.; Azadfar, D.; Arzanesh, H.M. The ability to filter heavy metals of lead, copper and zinc in some species of tree and shrub. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 53–60. [Google Scholar]
- Griggs, T.; Liu, L.; Talbot, R.W.; Torres, A.; Lan, X. Comparison of atmospheric mercury speciation at a coastal and an urban site in Southeastern Texas, USA. Atmosphere 2020, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Świsłowski, P.; Kříž, J.; Rajfur, M. The Use of Bark in Biomonitoring Heavy Metal Pollution of Forest Areas on the Example of Selected Areas in Poland. Ecol. Chem. Eng. S 2020, 27, 195–210. [Google Scholar] [CrossRef]
- Tye, A.M.; Hodgkinson, E.S.; Rawlins, B.G. Microscopic and chemical studies of metal particulates in attic dust: Evidence for historical atmospheric smelter emission, Humberside, UK. J. Environ. Monit. 2006, 8, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.J.; Kim, P.R.; Lee, G.S.; Lee, J.I.; Noh, S.; Yu, S.M.; Park, K.S.; Seok, K.S.; Kim, H.; Kim, Y.H. Mercury concentrations in environmental media at a hazardous solid waste landfill site and mercury emissions from the site. Environ. Earth Sci. 2017, 76, 361. [Google Scholar] [CrossRef]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Anthocephalus cadamba Miq. Ekologi, Silvikultur dan Produktivitas; CIFOR: Bogor, Indonesia, 2011; pp. 1–22. [Google Scholar]
- Barnes, D.; Hammadah, M.A.; Ottaway, J.M. The lead, copper and zinc contens of tree rings and barks, a measurement of local pollution. Sci. Total Environ. 1976, 5, 63–67. [Google Scholar] [CrossRef]
- Pásztory, Z.; Mohácsiné, I.; Gorbacheva, G.; Börcsök, Z. The Utilization of Tree Bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Aoyama, M.; Kishino, M.; Jo, T.S. Biosorption of Cr (VI) on Japanese cedar bark. Sep. Sci. Technol. 2004, 39, 1149–1162. [Google Scholar] [CrossRef]
- Patel, K.S.; Yadav, A.; Sahu, Y.K.; Lata, L.; Milosh, H.; Corns, W.T.; Martin-Ramos, P. Tree bark as a bioindicator for arsenic and heavy metal air pollution in Rajnandgaon District, Chhattisgarh, India. Hazard Toxic Radioact. Waste 2020, 24, 1–5. [Google Scholar] [CrossRef]
- Nursal, F.B. Akumulasi timbal (Pb) pada Talus Lichenes di Kota Pekanbaru. Biogenesis 2005, 1, 47–50. [Google Scholar]
- Tovar-Sanchez, E.; Surez-Rodrigues, R.; Ramires-Trujilo, A.; Valencia-Cavas, L.; Hernandez-Plata, I.; Mussali-Galante, P. The Use of Biosensors for Biomonitoring Environmental Metal Pollution. In Biosensors for Environmental Monitoring; Rinken, T., Kivirand, K., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Chiarantini, L.; Rimondi, V.; Bardelli, F.; Benvenuti, M.; Cosio, C.; Costagliola, P. Mercury speciation in Pinus nigra barks from monte Amiata (Italy): An X-ray absorption spectroscopy study. Environ. Pollut. 2017, 227, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lyman, S.; Mao, H.; Lin, C.; Gay, D.A.; Wang, S.; Gustin, M.S.; Feng, X.; Wania, F. A synthesis of research needs for improving the understanding of atmospheric mercury cycling. Atmos. Chem. Phys. 2017, 17, 9133–9144. [Google Scholar] [CrossRef] [Green Version]
- Bernhoft, A.R. Mercury Toxicity and Treatment: A Review of The Literature. Environ. Public Health 2011, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Ebinghaus, R.; Kock, H.; Dommergue, A. A review of worldwide atmospheric mercury measurements. Atmos. Chem. Phys. 2010, 10, 8245–8265. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, Z.M. Transport and Fate of Mercury (Hg) in the Environment: Need for Continuous Monitoring. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Schuster, P.F.; Krabbenhoft, D.P.; Naftz, D.L.; Cecil, L.D.; Olson, M.L.; Dewild, J.F. Atmospheric mercury deposition during the last 270 years: A glacial ice core record of natural and anthropogenic sources. Environ. Sci. Tecnol. 2002, 36, 2303–2310. [Google Scholar] [CrossRef]
- Zhang, M.; Bae, W.; Kim, J. The effects of the layouts of vegetation and wind flow in an apartment housing complex to mitigate outdoor microclimate air temperature. Sustainability 2019, 11, 3081. [Google Scholar] [CrossRef] [Green Version]
- Boylan, H.M.; Cain, R.D.; Kingston, H.M. A new method to assess mercury emissions: A study of three coal-fired electric-generating power station configurations. Air Waste Manag. Assoc. 2003, 53, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).