Condensed Phase Kinetic Studies of Hydroxynitrates Derived from the Photooxidation of Carene, Limonene, trans-Carveol, and Perillic Alcohol
Abstract
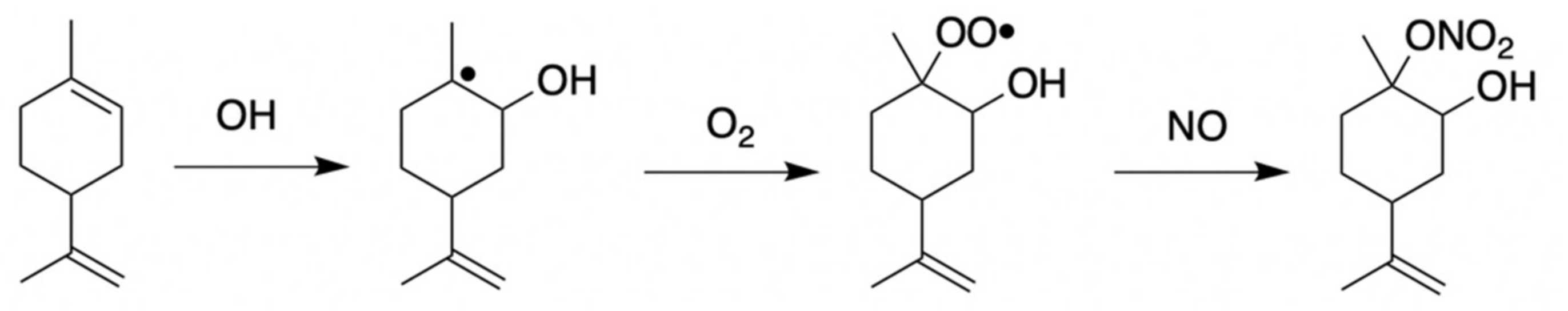
1. Introduction
2. Materials and Methods
2.1. General
2.2. NMR Kinetic Experiments
3. Results
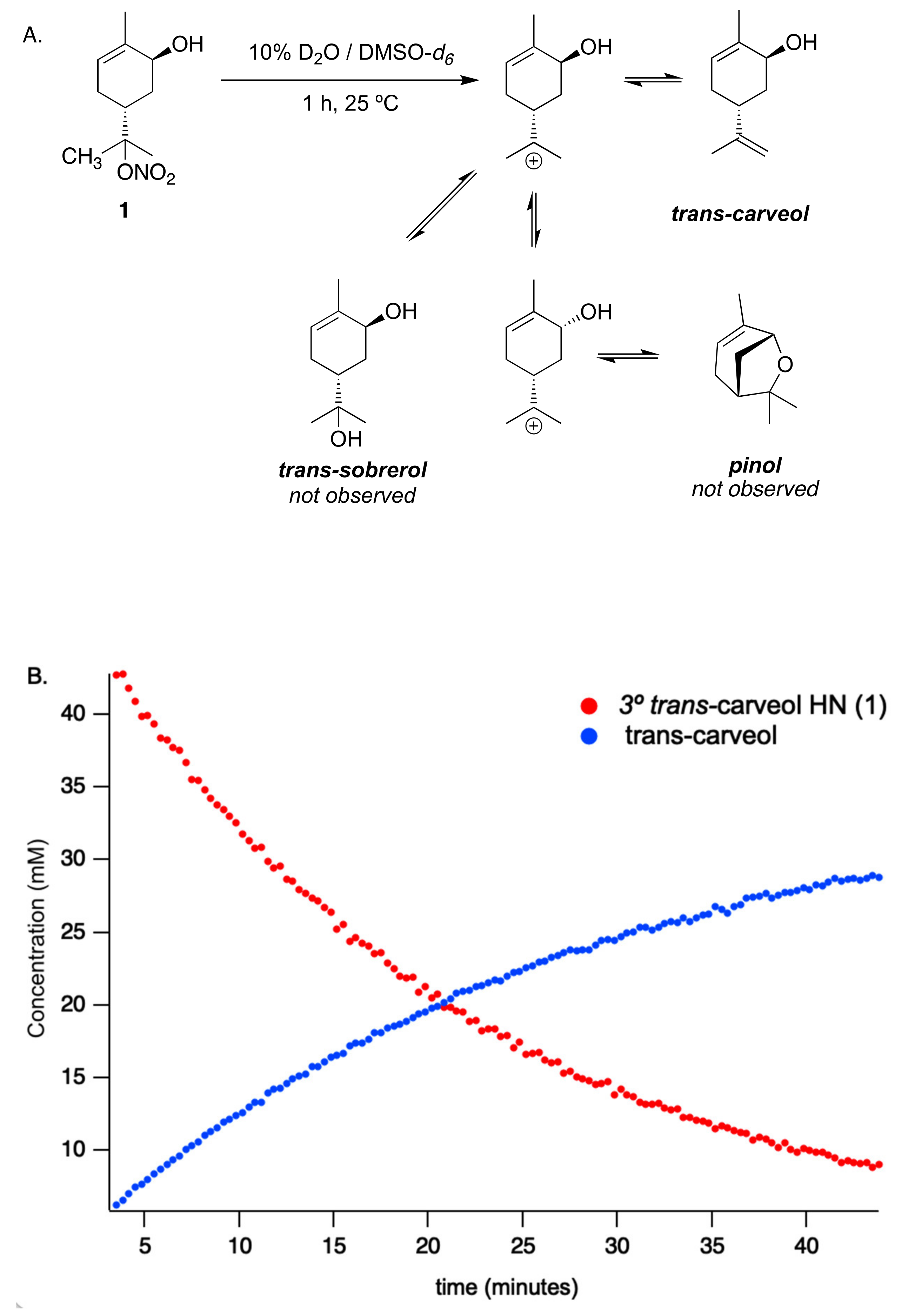
3.1. Total Conversion Kinetics of HN in a Mixed Aqueous/Organic Matrix
3.2. Carene HN
3.3. Limonene HN
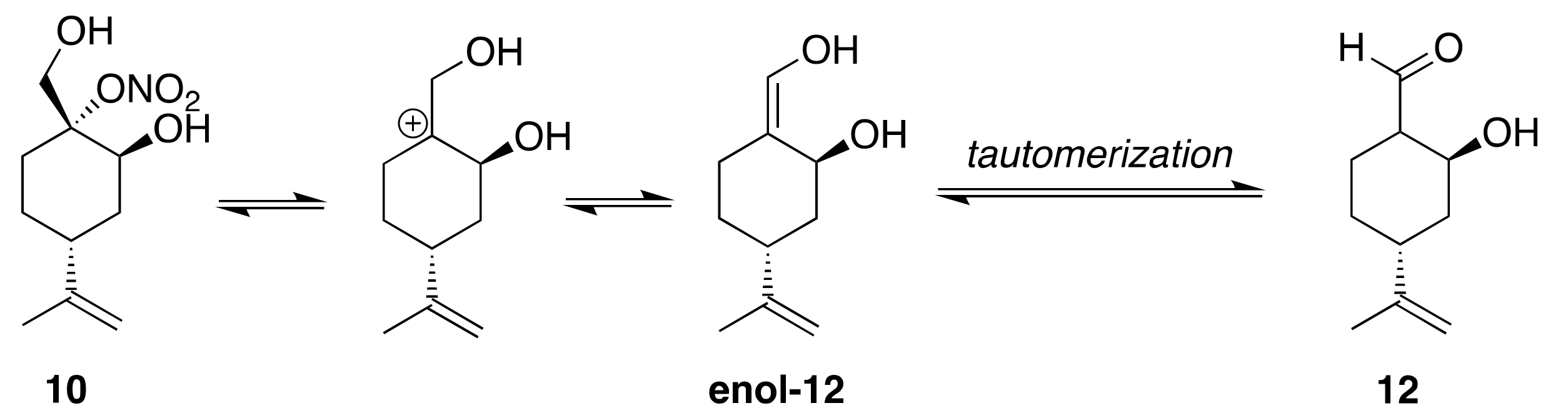
3.4. Perillic Alcohol HN
3.5. Trans-Carveol HN
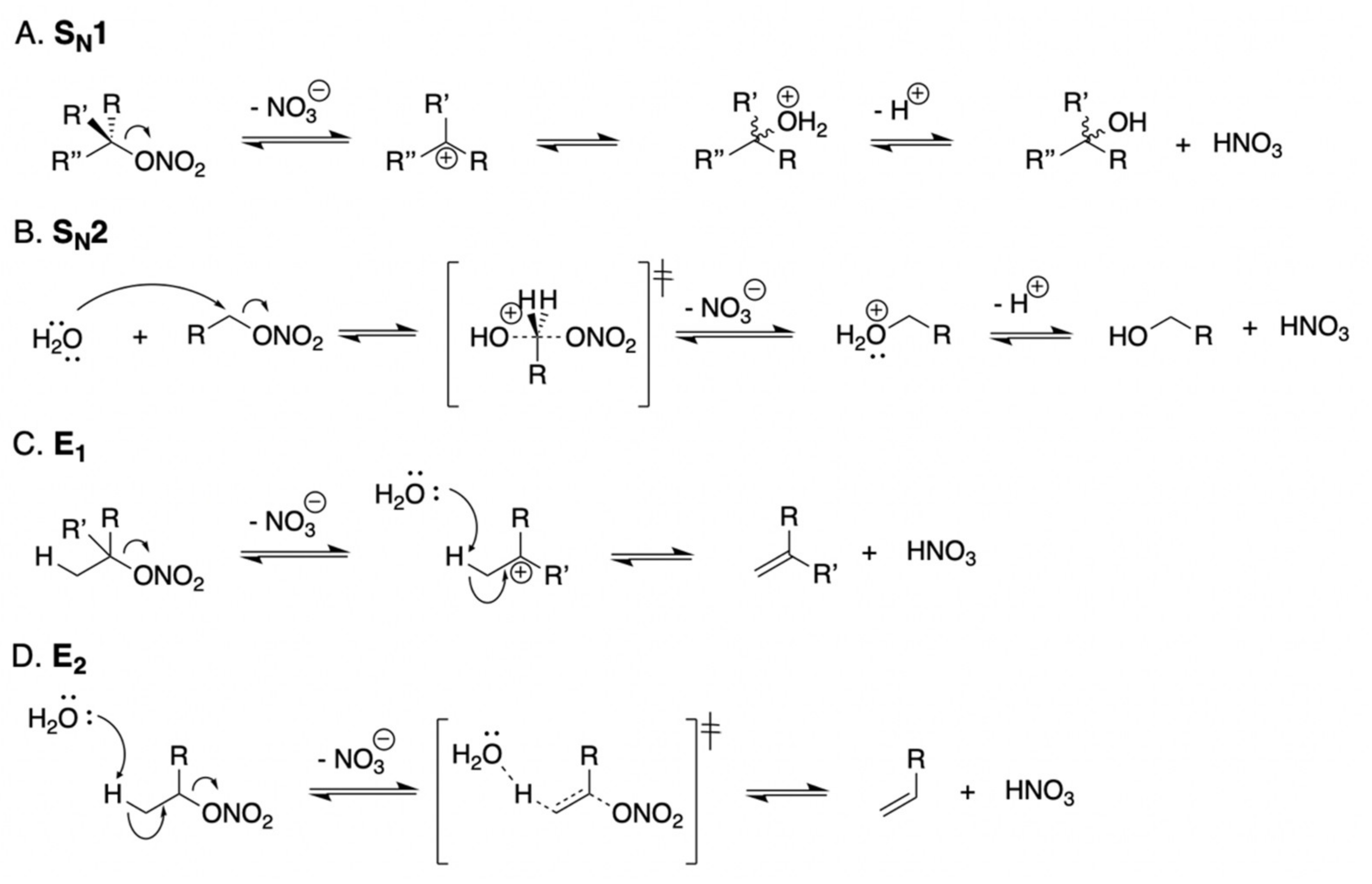
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wängberg, I.; Barnes, I.; Becker, K.H. Product and Mechanistic Study of the Reaction of NO3 Radicals with α- Pinene. Environ. Sci. Technol. 1997, 31, 2130–2135. [Google Scholar] [CrossRef]
- Hallquist, M.; Wängberg, I.; Ljungström, E.; Barnes, I.; Becker, K.H. Aerosol and Product Yields from NO3 Radical-Initiated Oxidation of Selected Monoterpenes. Environ. Sci. Technol. 1999, 33, 553–559. [Google Scholar] [CrossRef]
- Spittler, M.; Barnes, I.; Bejan, I.; Brockmann, K.J.; Benter, T.; Wirtz, K. Reactions of NO3 Radicals with Limonene and α-Pinene: Product and SOA Formation. Atmos. Environ. 2006, 40, 116–127. [Google Scholar] [CrossRef]
- Matsunaga, A.; Docherty, K.S.; Lim, Y.B.; Ziemann, P.J. Composition and Yields of Secondary Organic Aerosol Formed from OH Radical-Initiated Reactions of Linear Alkenes in the Presence of NOx: Modeling and Measurements. Atmos. Environ. 2009, 43, 1349–1357. [Google Scholar] [CrossRef]
- Kroll, J.H.; Seinfeld, J.H. Chemistry of Secondary Organic Aerosol: Formation and Evolution of Low-Volatility Organics in the Atmosphere. Atmos. Environ. 2008, 42, 3593–3624. [Google Scholar] [CrossRef]
- Fiore, A.M.; Horowitz, L.W.; Purves, D.W.; Levy, H.; Evans, M.J.; Wang, Y.; Li, Q.; Yantosca, R.M. Evaluating the Contribution of Changes in Isoprene Emissions to Surface Ozone Trends over the Eastern United States. J. Geophys. Res. Atmos. 2005, 110, 1–13. [Google Scholar] [CrossRef]
- Von Kuhlmann, R.; Lawrence, M.G.; Pöschl, U.; Crutzen, P.J. Sensitivities in Global Scale Modeling of Isoprene. Atmos. Chem. Phys. 2004, 4, 1–17. [Google Scholar] [CrossRef]
- Horowitz, L.W.; Fiore, A.M.; Milly, G.P.; Cohen, R.C.; Perring, A.; Wooldridge, P.J.; Hess, P.G.; Emmons, L.K.; Lamarque, J.F. Observational Constraints on the Chemistry of Isoprene Nitrates over the Eastern United States. J. Geophys. Res. Atmos. 2007, 112, 1–13. [Google Scholar] [CrossRef]
- Ayres, B.R.; Allen, H.M.; Draper, D.C.; Brown, S.S.; Wild, R.J.; Jimenez, J.L.; Day, D.A.; Campuzano-Jost, P.; Hu, W.; de Gouw, J.; et al. Organic nitrate aerosol formation via NO3+ biogenic volatile organic compounds in the southeastern United States. Atmos. Chem. Phys. 2015, 15, 13377–13392. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Luecken, D.J.; Xu, L.; Boyd, C.M.; Ng, N.L.; Baker, K.R.; Ayres, B.R.; Bash, J.; Baumann, K.; Carter, W.P.L.; et al. Modeling the Current and Future Roles of Particulate Organic Nitrates in the Southeastern United States. Environ. Sci. Technol. 2015, 49, 14195–14203. [Google Scholar] [CrossRef]
- Fisher, J.A.; Jacob, D.J.; Travis, K.R.; Kim, P.S.; Marais, E.A.; Miller, C.C.; Yu, K.; Zhu, L.; Yantosca, R.M.; Sulprizio, M.P.; et al. Organic nitrate chemistry and its implications for nitrogen budgets in an isoprene- and monoterpene-rich atmosphere: Constraints from aircraft (SEAC4RS) and ground-based (SOAS) observations in the Southeast US. Atmos. Chem. Phys. 2016, 16, 5969–5991. [Google Scholar] [CrossRef]
- Perring, A.E.; Pusede, S.E.; Cohen, R.C. An Observational Perspective on the Atmospheric Impacts of Alkyl and Multifunctional Nitrates on Ozone and Secondary Organic Aerosol. Chem. Rev. 2013, 113, 5848–5870. [Google Scholar] [CrossRef]
- Rollins, A.W.; Smith, J.D.; Wilson, K.R.; Cohen, R.C. Real Time in Situ Detection of Organic Nitrates in Atmospheric Aerosols. Environ. Sci. Technol. 2010, 44, 5540–5545. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Suresh, S.; Guo, H.; Weber, R.J.; Ng, N.L. Aerosol Characterization over the Southeastern United States Using High-Resolution Aerosol Mass Spectrometry: Spatial and Seasonal Variation of Aerosol Composition and Sources with a Focus on Organic Nitrates. Atmos. Chem. Phys. 2015, 15, 7307–7336. [Google Scholar] [CrossRef]
- Rollins, A.W.; Pusede, S.; Wooldridge, P.; Min, K.E.; Gentner, D.R.; Goldstein, A.H.; Liu, S.; Day, D.A.; Russell, L.M.; Rubitschun, C.L.; et al. Gas/Particle Partitioning of Total Alkyl Nitrates Observed with TD-LIF in Bakersfield. J. Geophys. Res. Atmos. 2013, 118, 6651–6662. [Google Scholar] [CrossRef]
- Zare, A.; Romer, P.S.; Nguyen, T.; Keutsch, F.N.; Skog, K.; Cohen, R.C. A Comprehensive Organic Nitrate Chemistry: Insights into the Lifetime of Atmospheric Organic Nitrates. Atmos. Chem. Phys. 2018, 18, 15419–15436. [Google Scholar] [CrossRef]
- Romonosky, D.E.; Nguyen, L.Q.; Shemesh, D.; Nguyen, T.B.; Epstein, S.A.; Martin, D.B.C.; Vanderwal, C.D.; Gerber, R.B.; Nizkorodov, S.A. Absorption Spectra and Aqueous Photochemistry of β-Hydroxyalkyl Nitrates of Atmospheric Interest. Mol. Phys. 2015, 113, 2179–2190. [Google Scholar] [CrossRef]
- Hu, K.S.; Darer, A.I.; Elrod, M.J. Thermodynamics and Kinetics of the Hydrolysis of Atmospherically Relevant Organonitrates and Organosulfates. Atmos. Chem. Phys. 2011, 11, 8307–8320. [Google Scholar] [CrossRef]
- Darer, A.I.; Cole-Filipiak, N.C.; O’Connor, A.E.; Elrod, M.J. Formation and Stability of Atmospherically Relevant Isoprene-Derived Organosulfates and Organonitrates. Environ. Sci. Technol. 2011, 45, 1895–1902. [Google Scholar] [CrossRef]
- Bean, J.K.; Hildebrandt Ruiz, L. Gas-Particle Partitioning and Hydrolysis of Organic Nitrates Formed from the Oxidation of α-Pinene in Environmental Chamber Experiments. Atmos. Chem. Phys. 2016, 16, 2175–2184. [Google Scholar] [CrossRef]
- Sato, K. Detection of Nitrooxypolyols in Secondary Organic Aerosol Formed from the Photooxidation of Conjugated Dienes under High-NOx Conditions. Atmos. Environ. 2008, 42, 6851–6861. [Google Scholar] [CrossRef]
- Wang, Y.; Piletic, I.R.; Takeuchi, M.; Xu, T.; France, S.; Ng, N.L. Synthesis and Hydrolysis of Atmospherically Relevant Monoterpene-Derived Organic Nitrates. Environ. Sci. Technol. 2021, 55, 14595–14606. [Google Scholar] [CrossRef] [PubMed]
- McAlister, A.B.; Vesto, J.I.; Huang, A.; Wright, K.A.; Emily, E.J.; Bailey, G.M.; Kretekos, N.P.; Baldwin, P.R.; Carrasquillo, A.J.; Lalonde, R.L. Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications. Atmosphere 2021, 12, 1617. [Google Scholar] [CrossRef]
- Morales, A.C.; Jayarathne, T.; Slade, J.H.; Laskin, A.; Shepson, P.B. The Production and Hydrolysis of Organic Nitrates from OH Radical Oxidation of β-Ocimene. Atmos. Chem. Phys. 2021, 21, 129–145. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; Borca, C.H.; Hostetler, M.A.; Slade, J.H.; Lipton, M.A.; Slipchenko, L.V.; Shepson, P.B. The Acid-Catalyzed Hydrolysis of an α-Pinene-Derived Organic Nitrate: Kinetics, Products, Reaction Mechanisms, and Atmospheric Impact. Atmos. Chem. Phys. 2016, 16, 15425–15432. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; McAvey, K.M.; Shepson, P.B. The Photochemical Production of Organic Nitrates from α-Pinene and Loss via Acid-Dependent Particle Phase Hydrolysis. Atmos. Environ. 2015, 100, 193–201. [Google Scholar] [CrossRef]
- Jacobs, M.I.; Burke, W.J.; Elrod, M.J. Kinetics of the Reactions of Isoprene-Derived Hydroxynitrates: Gas Phase Epoxide Formation and Solution Phase Hydrolysis. Atmos. Chem. Phys. 2014, 14, 8933–8946. [Google Scholar] [CrossRef]
- Cortés, D.A.; Elrod, M.J. Kinetics of the Aqueous Phase Reactions of Atmospherically Relevant Monoterpene Epoxides. J. Phys. Chem. A 2017, 121, 9297–9305. [Google Scholar] [CrossRef]
- Vasquez, K.T.; Crounse, J.D.; Schulze, B.C.; Bates, K.H.; Teng, A.P.; Xu, L.; Allen, H.M.; Wennberg, P.O. Rapid Hydrolysis of Tertiary Isoprene Nitrate Efficiently Removes NOx from the Atmosphere. Proc. Natl. Acad. Sci. USA 2021, 117, 33011–33016. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ng, N.L. Chemical Composition and Hydrolysis of Organic Nitrate Aerosol Formed from Hydroxyl and Nitrate Radical Oxidation of α-Pinene and β-Pinene. Atmos. Chem. Phys. 2019, 19, 12749–12766. [Google Scholar] [CrossRef]
- Boyd, C.M.; Sanchez, J.; Xu, L.; Eugene, A.J.; Nah, T.; Tuet, W.Y.; Guzman, M.I.; Ng, N.L. Secondary Organic Aerosol Formation from the β-Pinene+NO3 System: Effect of Humidity and Peroxy Radical Fate. Atmos. Chem. Phys. 2015, 15, 7497–7522. [Google Scholar] [CrossRef]
- McKnight, E.A.; Kretekos, N.P.; Owusu, D.; LaLonde, R.L. Technical Note: Preparation and Purification of Atmospherically Relevant Alpha-Hydroxynitrate Esters of Monoterpenes. Atmos. Chem. Phys. 2019, 20, 4241–4254. [Google Scholar] [CrossRef]
- Lu, T.J.; Lin, C.K. Asymmetric Synthesis of α-Methyl-α-Amino Acids via Diastereoselective Alkylation of (1s)-(+)-3-Carene Derived Tricyclic Iminolactone. J. Org. Chem. 2011, 76, 1621–1633. [Google Scholar] [CrossRef]
- Bleier, D.B.; Elrod, M.J. Kinetics and Thermodynamics of Atmospherically Relevant Aqueous Phase Reactions of α-Pinene Oxide. J. Phys. Chem. A 2013, 117, 4223–4232. [Google Scholar] [CrossRef]

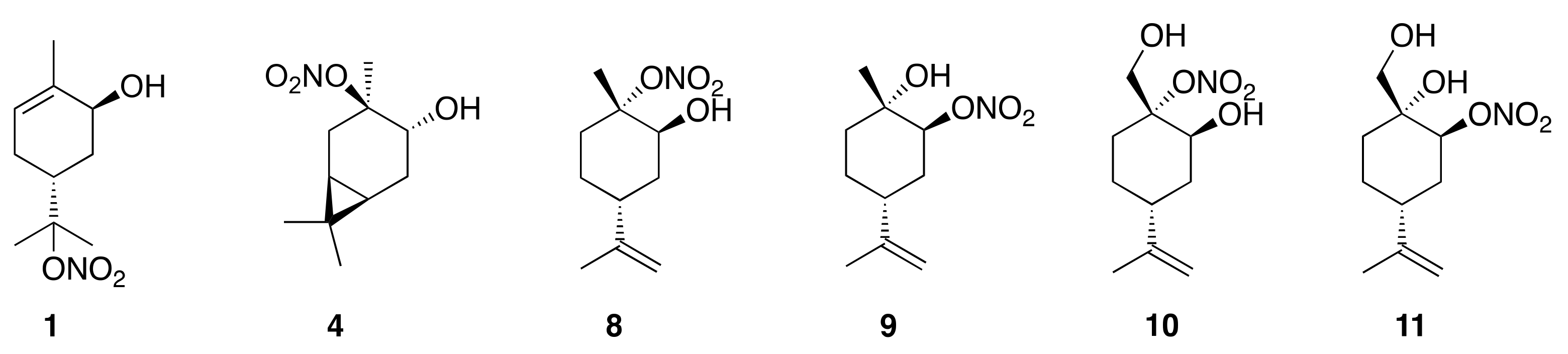

| HN Structure | Temp (°C) | No Acid (h) | 5 Molar Equivalent D2SO4 (h) |
|---|---|---|---|
| 3-Car (4) | 30 | 0.28 (0.02) | 0.25 (0.02) |
| 3-Lim (8) | 30 | 1.45 (0.06) | 1.53 (0.1) |
| 2-Lim (9) | 80 | 3.34 (0.3) | 2.91 (0.04) |
| 3-Per (10) | 50 | 0.63 (0.03) | 0.55 (0.03) |
| 2-Per (11) | 80 | 12.6 (0.6) | 9.42 (0.6) |
| HN Structure | 5 Molar Equivalent D2SO4 (h)/30 °C |
|---|---|
| 3-Car (4) | 0.25 (0.02) |
| 3-Lim (8) | 1.53 (0.1) |
| 2-Lim (9) | 1340 (200) 1 |
| 3-Per (10) | 5.94 (0.3) 1 |
| 2-Per (11) | 18,600 (4000) 1 |
| trans-Carv (1) | 0.37 (0.003) 2 |
| HN Structure | Temp (°C) | 25% D2O/DMSO-d6 | 25% D2O/EtOD-d6 |
|---|---|---|---|
| 3-Car (4) | 30 | 0.25 (0.02) | 0.63 (0.01) |
| 3-Lim (8) | 30 | 1.38 (0.05) | 4.72 (0.4) |
| 2-Lim (9) | 65 | 15.87 (0.6) | 39.57 (2) |
| 3-Per (10) | 50 | 0.54 (0.03) | 1.38 (0.04) |
| 2-Per (11) | 65 | 55.56 (0.6) | 143.93 (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesto, J.I.; McAlister, A.B.; Wright, K.A.; Huang, A.; Baldwin, P.R.; McLaughlin Sta. Maria, E.J.; LaLonde, R.L.; Carrasquillo, A.J. Condensed Phase Kinetic Studies of Hydroxynitrates Derived from the Photooxidation of Carene, Limonene, trans-Carveol, and Perillic Alcohol. Atmosphere 2022, 13, 592. https://doi.org/10.3390/atmos13040592
Vesto JI, McAlister AB, Wright KA, Huang A, Baldwin PR, McLaughlin Sta. Maria EJ, LaLonde RL, Carrasquillo AJ. Condensed Phase Kinetic Studies of Hydroxynitrates Derived from the Photooxidation of Carene, Limonene, trans-Carveol, and Perillic Alcohol. Atmosphere. 2022; 13(4):592. https://doi.org/10.3390/atmos13040592
Chicago/Turabian StyleVesto, James I., Addison B. McAlister, Kathryn A. Wright, Aaron Huang, Petra R. Baldwin, Emily J. McLaughlin Sta. Maria, Rebecca Lyn LaLonde, and Anthony J. Carrasquillo. 2022. "Condensed Phase Kinetic Studies of Hydroxynitrates Derived from the Photooxidation of Carene, Limonene, trans-Carveol, and Perillic Alcohol" Atmosphere 13, no. 4: 592. https://doi.org/10.3390/atmos13040592
APA StyleVesto, J. I., McAlister, A. B., Wright, K. A., Huang, A., Baldwin, P. R., McLaughlin Sta. Maria, E. J., LaLonde, R. L., & Carrasquillo, A. J. (2022). Condensed Phase Kinetic Studies of Hydroxynitrates Derived from the Photooxidation of Carene, Limonene, trans-Carveol, and Perillic Alcohol. Atmosphere, 13(4), 592. https://doi.org/10.3390/atmos13040592







