The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column
Abstract
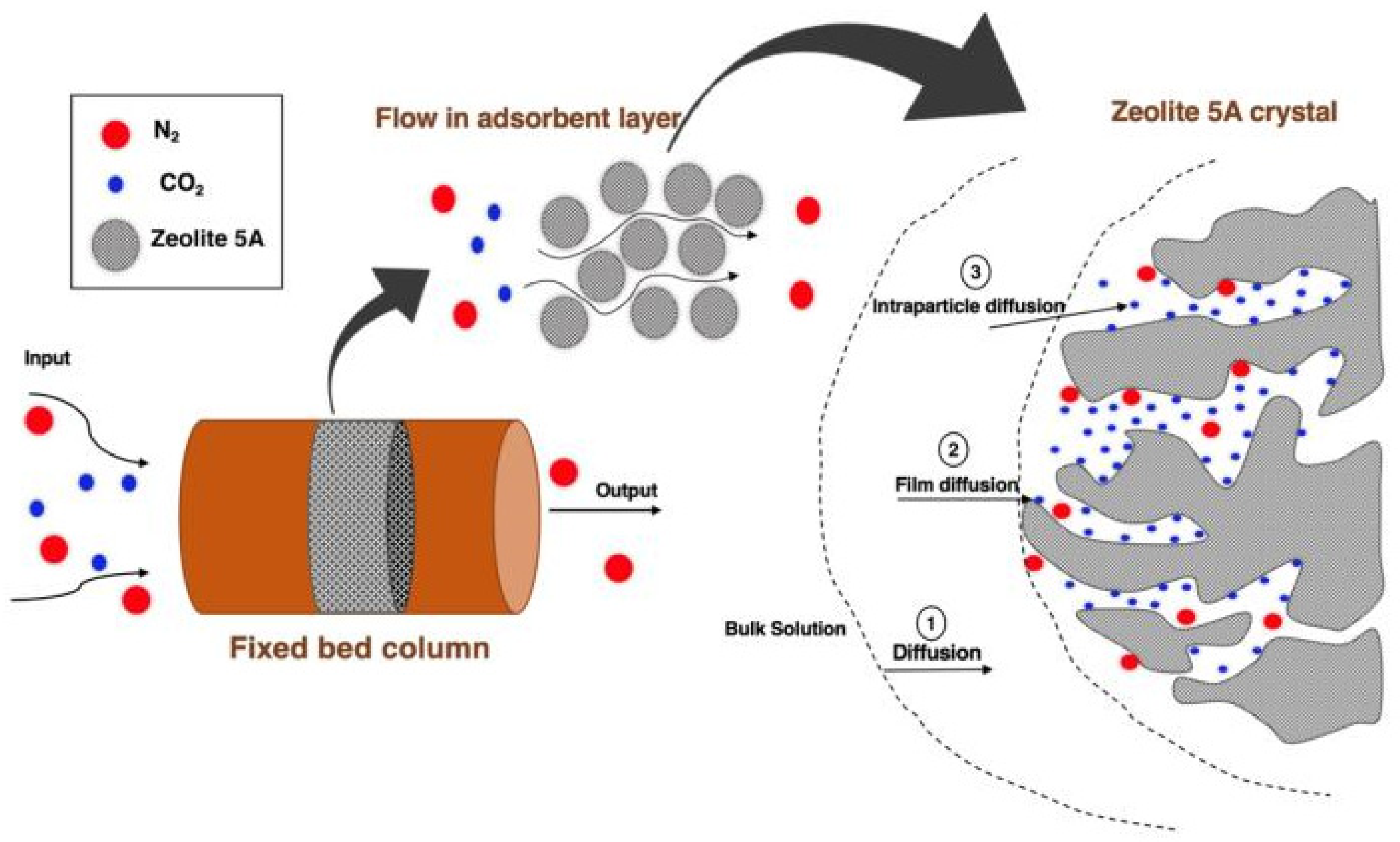
:1. Introduction
2. Materials and Methods
2.1. Materials
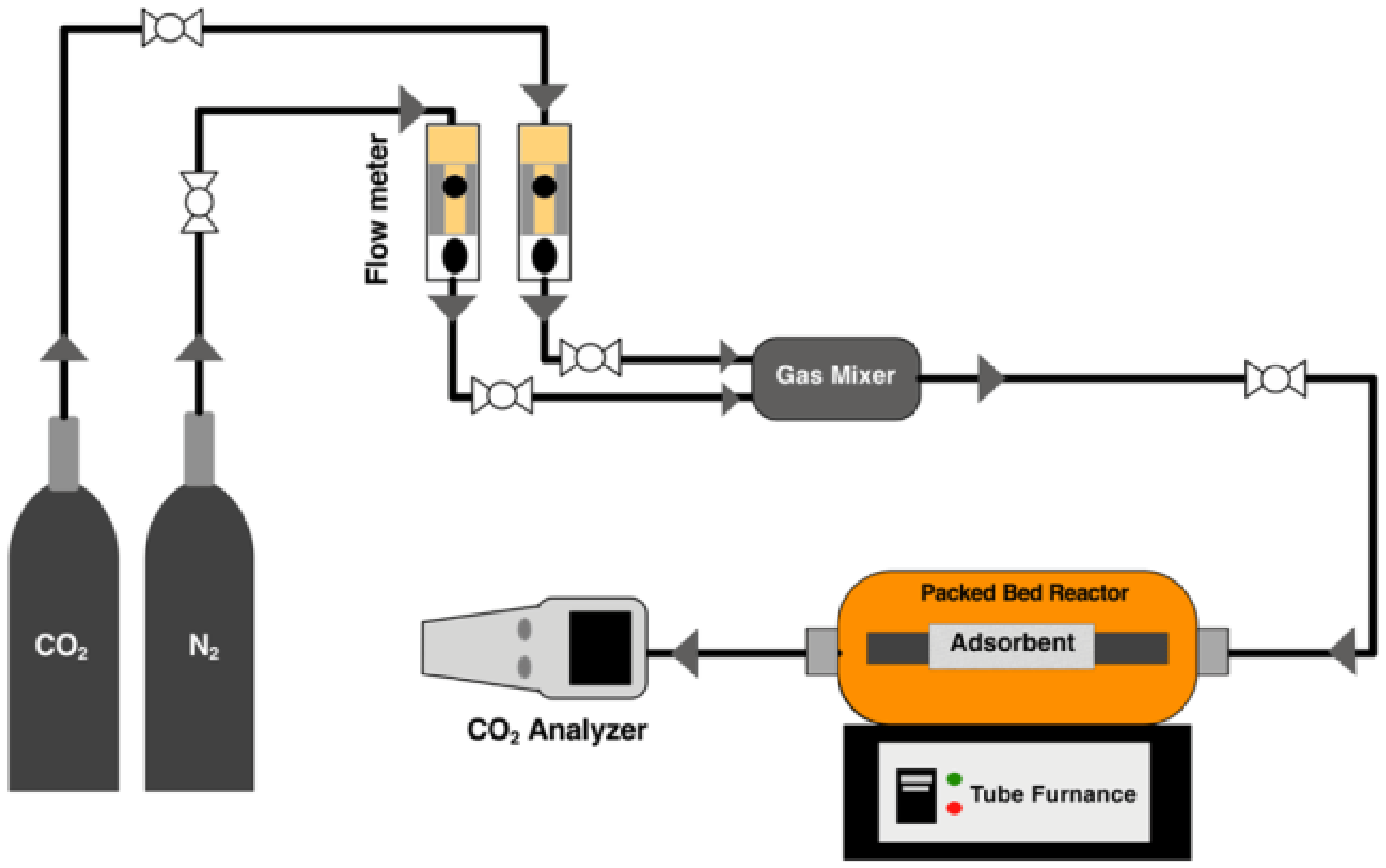
2.2. Experimental Methods
The CO2/N2 Gas Mixture Adsorption Measurements
2.3. Characterization
2.4. Theoretical Model
2.4.1. Fixed-Bed Experiment and Mathematical Models
2.4.2. Adsorption Kinetics
2.4.3. Diffusional Mass Transfer Models
3. Results
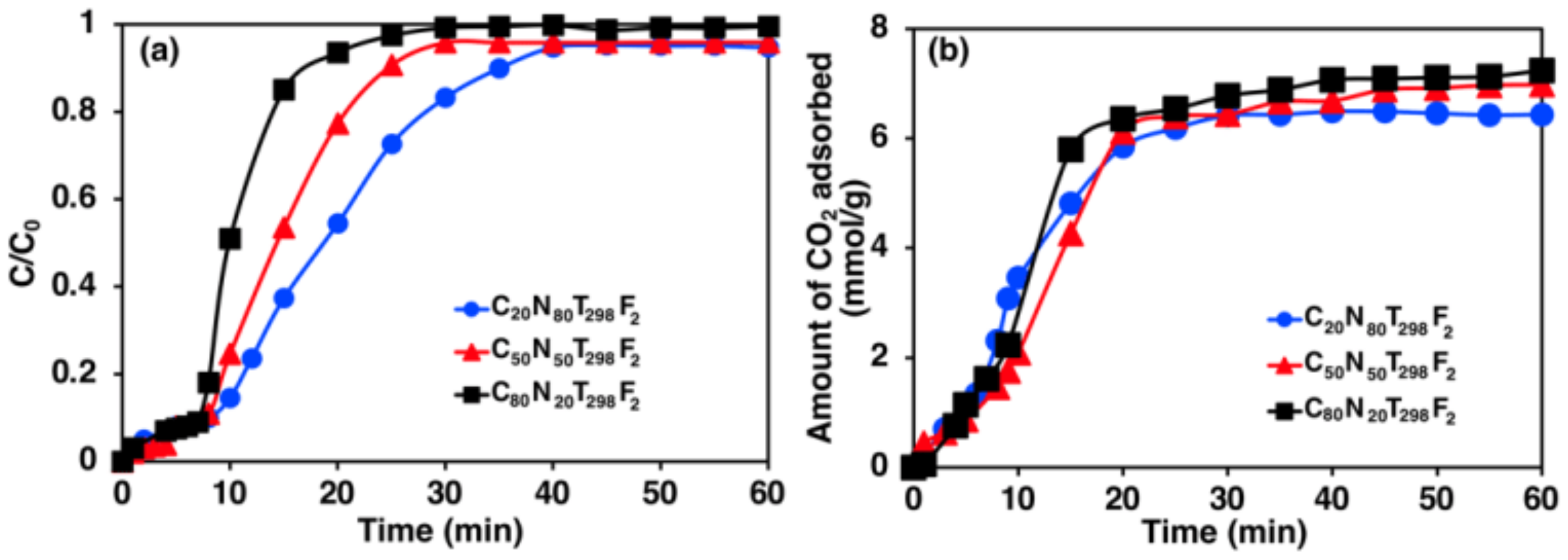
3.1. Effect of CO2 Composition in a CO2/N2 Gas Mixture for CO2 Adsorption
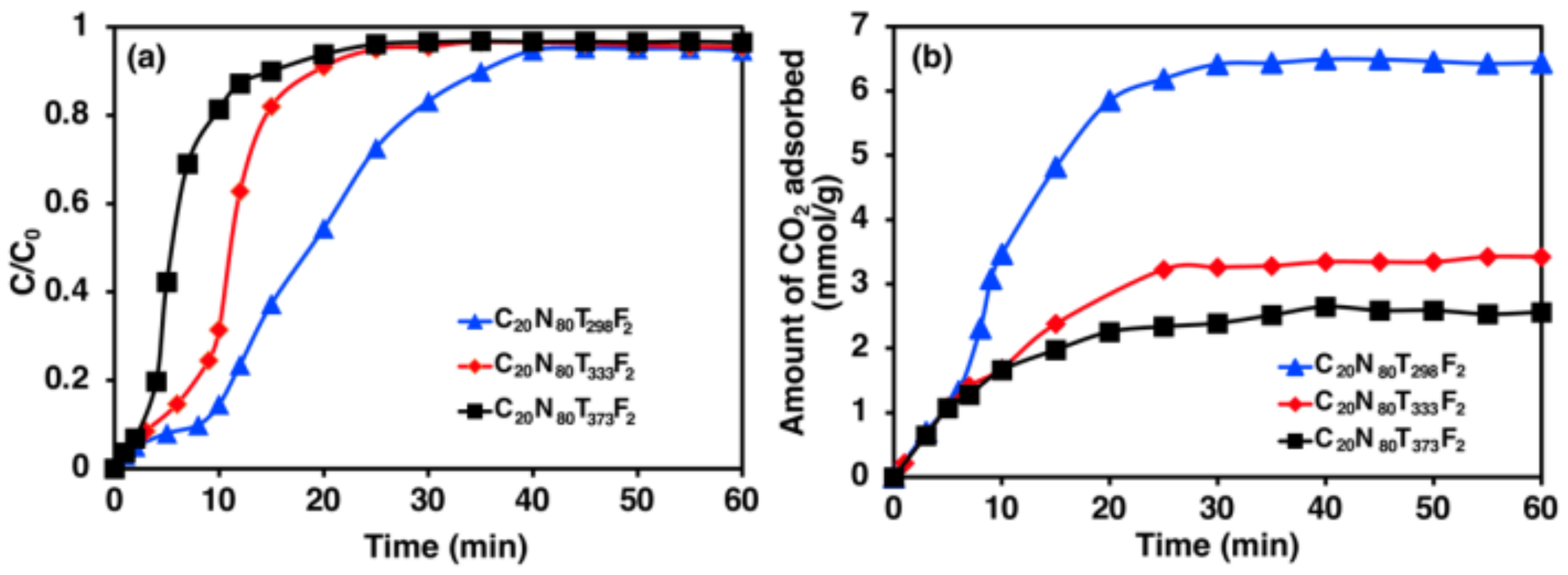
3.2. Effect of Temperature on CO2 Adsorption
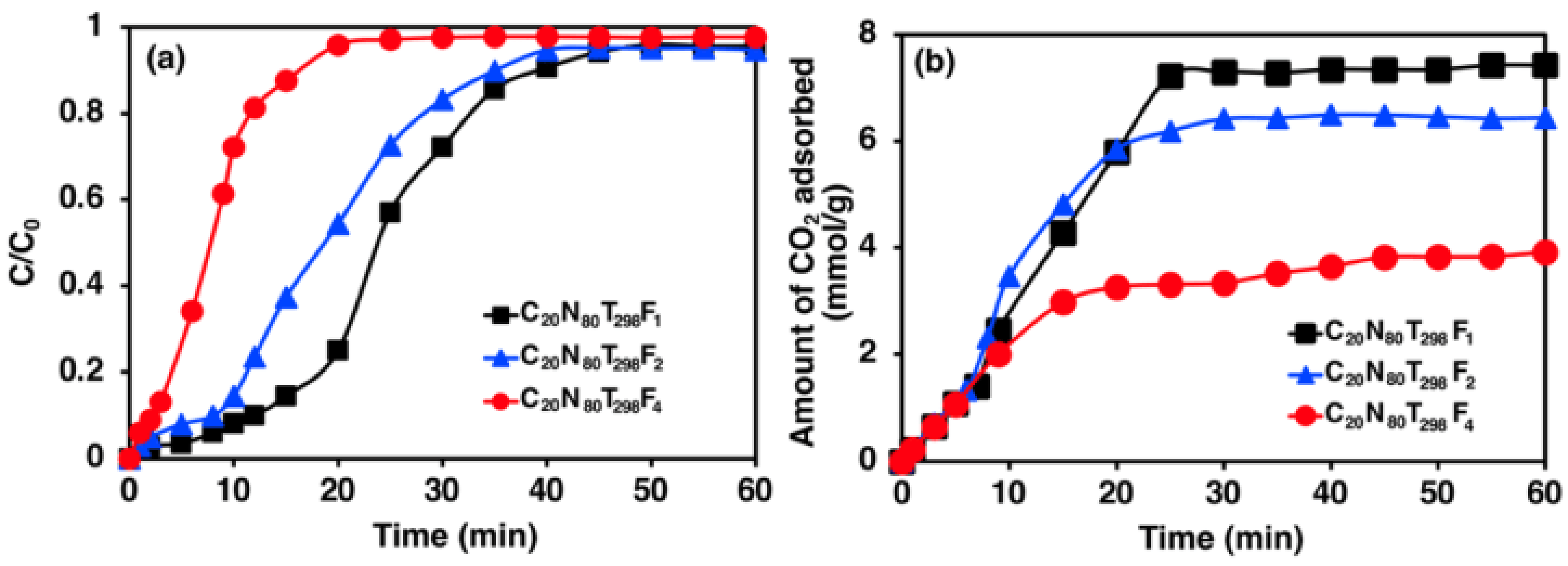
3.3. Effect of Feed Flow Rate on CO2 Adsorption
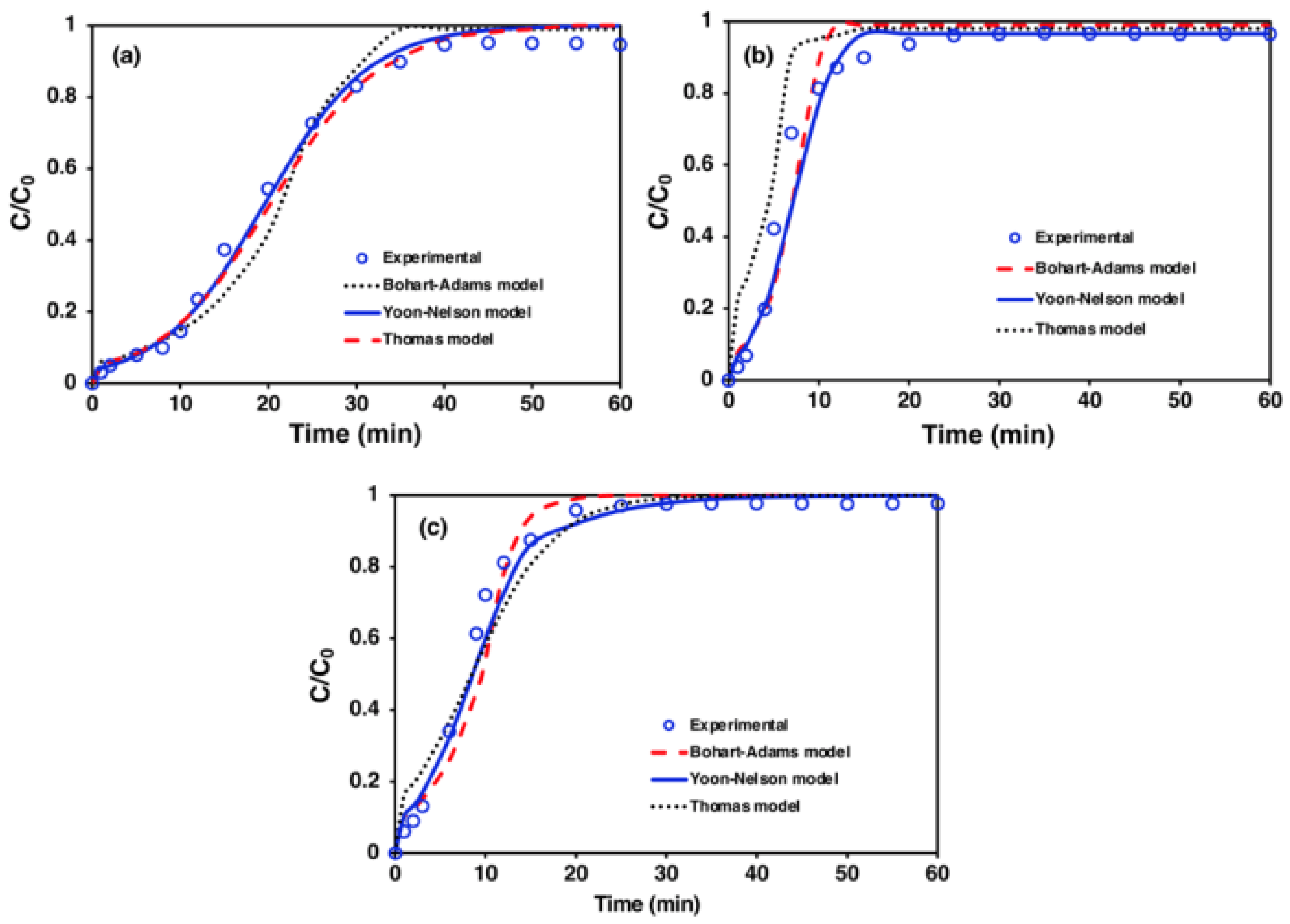
3.4. Adsorption Breakthrough Curve Models Study for a Fixed-Bed Column
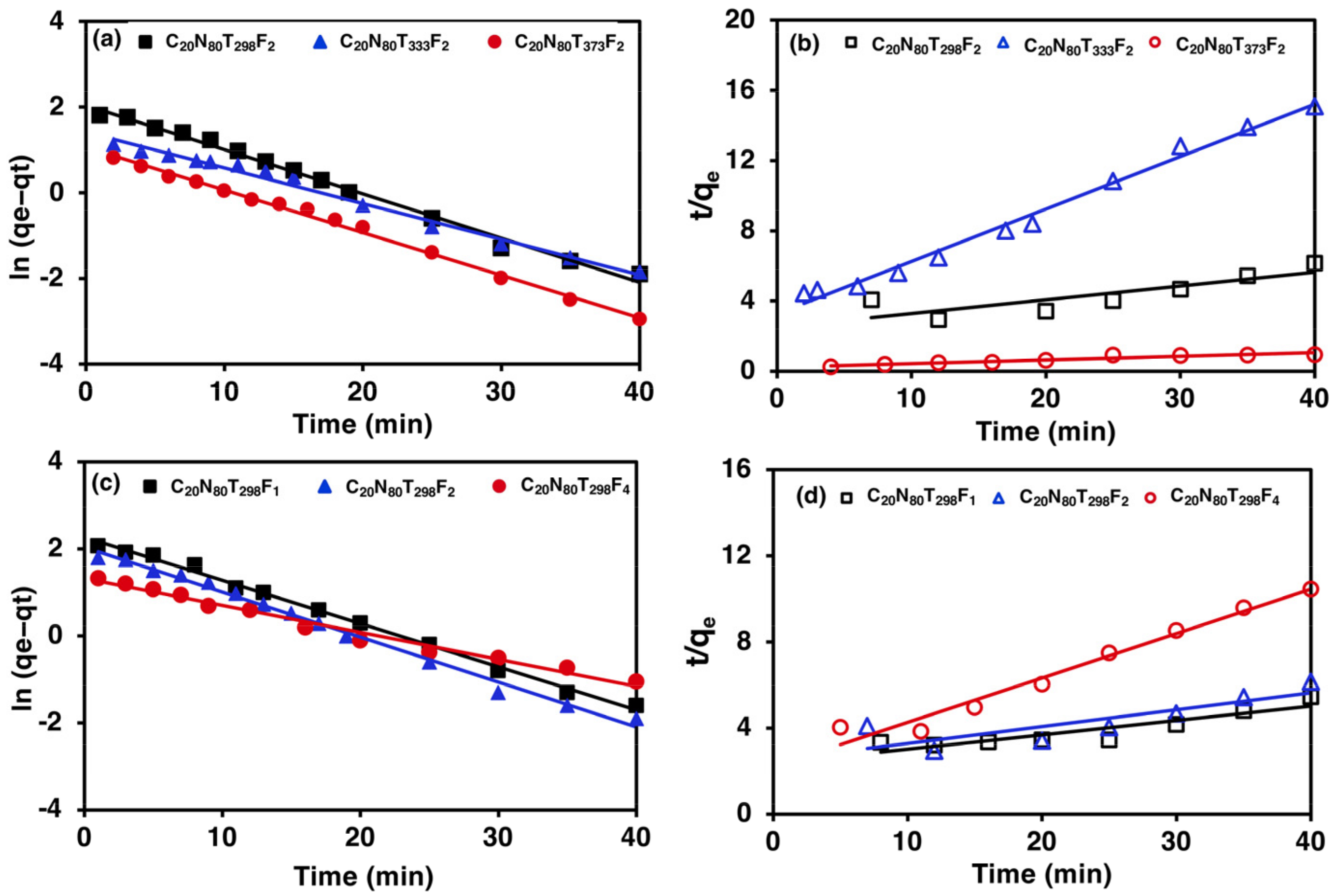
3.5. Adsorption Kinetics Study
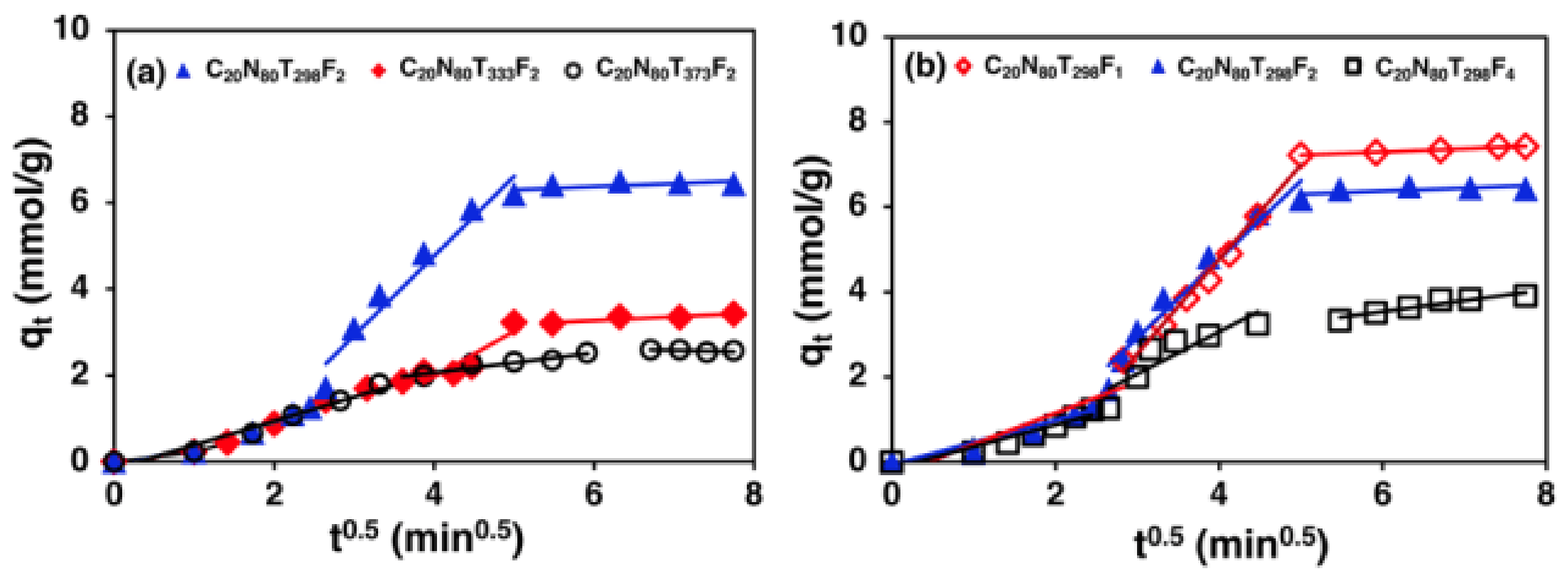
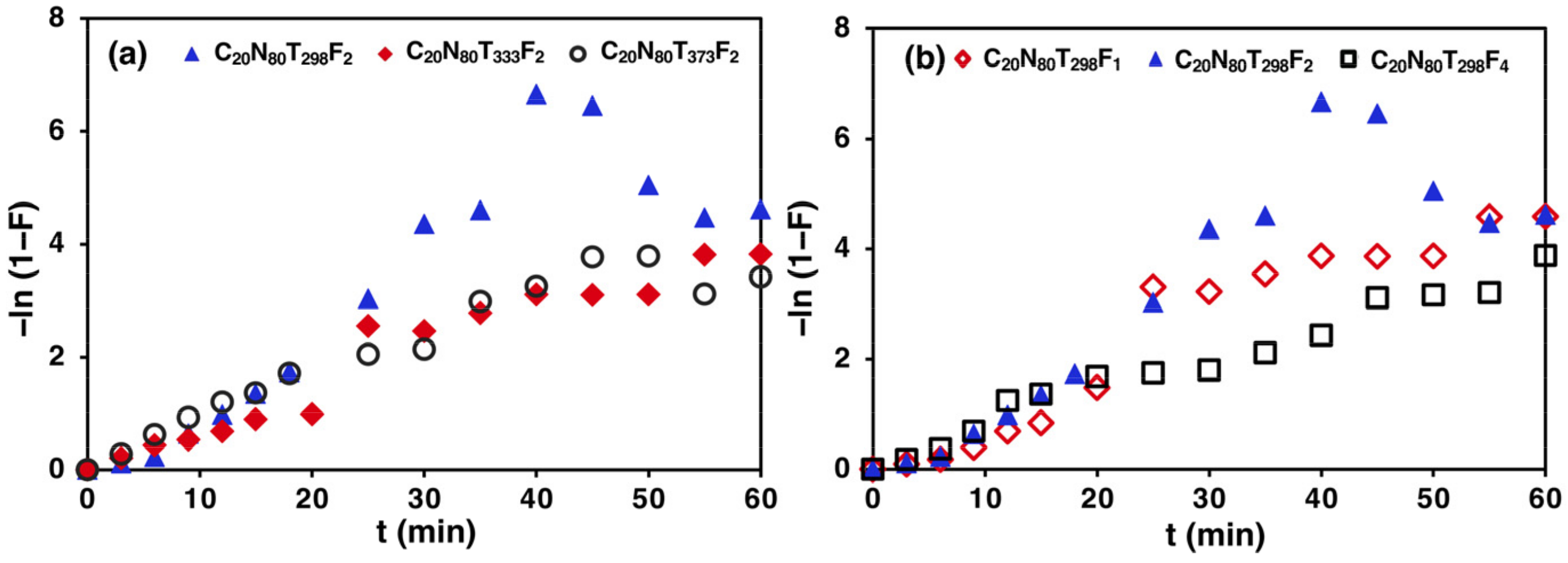
3.6. Adsorption Diffusion Study
4. Discussion
4.1. Effect of CO2 Composition in CO2/N2 Gas Mixture for CO2 Adsorption
4.2. Effect of Temperature on CO2 Adsorption
4.3. Effect of Feed Flow Rate on CO2 Adsorption
4.4. Adsorption Breakthrough Curve Models Study for Fixed-Bed Column
4.5. Adsorption Kinetics and Diffusion Models Study
5. Conclusions
- (1)
- At higher CO2 compositions, a CO2/N2 gas mixture resulted in an increase in CO2 adsorption capacities. Improving the partial pressure of CO2 and mass transfer played a significant role in the rapid adsorption of CO2 onto zeolite 5A.
- (2)
- The CO2 adsorption process onto zeolite 5A is an exothermic reaction based on the physical adsorption influenced by Van der Waals forces, because the adsorption capacities are low at high temperature, which results in greater gas kinetics.
- (3)
- The CO2 adsorption capacity decreased when the feed flow rates increased because the contact time between adsorbate and adsorbent in a fixed-bed column decreased, thereby improving mass transfer, which was greatly influenced by the shallower adsorption zone.
- (4)
- The Yoon–Nelson model is an excellent model to describe the behavior of CO2 adsorption in a fixed-bed column. The high temperature and high feed flow rate increase the value of the Yoon–Nelson kinetic rate constant, which results in low adsorption capacities.
- (5)
- The CO2 adsorption from a CO2/N2 gas mixture using zeolite 5A over a fixed-bed column can be described using a PFO model because it is a physical adsorption process controlled by diffusion. In addition, the CO2 adsorption process in a fixed-bed column involves more than one diffusion step, including film diffusion, intraparticle diffusion and adsorption on adsorbent surface. The intraparticle diffusion was observed to be the rate-limiting step controlling the CO2 adsorption process on zeolite 5A in a fixed-bed column.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papaolias, G.; Mavroidis, I. Atmospheric emissions from oil and gas extraction and production in Greece. Atmosphere 2017, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Shi, Y. Estimating CO2 emission from large scale coal-fired power plants using OCO-2 observations and emission inventories. Atmosphere 2021, 12, 811. [Google Scholar] [CrossRef]
- Patiño, L.I.; Padilla, E.; Alcántara, V.; Raymond, J.L. The relationship of energy and CO2 emissions with GDP per capita in Colombia. Atmosphere 2020, 11, 778. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. A non-contract microwave sensor for monitoring the interaction of zeolite 13X with CO2 and CH4 in gaseous streams. Sens. Actuators B Chem. 2017, 71, 243–247. [Google Scholar]
- Abid, H.R.; Rada, Z.H.; Shang, J.; Wang, S. Synthesis, characterization, and CO2 adsorption of three metal-organic frameworks (MOFs): MIL-53, MIL-96, and amino-MIL-53. Polyhedron 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Williams, T.C.; Shaddix, C.R.; Schefer, R.W. Effect of Syngas Composition and CO2-Diluted Oxygen on Performance of a Premixed Swirl-Stabilized Combustor. Combust. Sci. Technol. 2007, 180, 64–88. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Syngas in perspective. Catal. Today 2002, 71, 243–247. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Layered Pressure Swing Adsorption for Methane Recovery from CH4/CO2/N2 Streams. Adsorption 2005, 11, 549–554. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kilpinen, P. Control of Pollutants in Flue Gases and Fuel Gases; Helsinki University of Technology: Espoo, Finland, 2001. [Google Scholar]
- Artioli, Y.; Jørgensen, S.E.; Fath, B.D. Encyclopedia of Ecology, 1st ed.; Academic Press: Oxford, UK, 2008; pp. 60–65. [Google Scholar]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Losch, J. Adsorption of CO2 on Zeolites at Moderate Temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Lange, R.S.A.; Hekkink, J.H.A.; Keizer, K.; Burggraaf, A.J.; Ma, Y.H. Sorption studies of microporous sol-gel modified ceramic membranes. J. Porous Mater. 1995, 2, 141–149. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Nam, G.M.; Jeong, B.M.; Kang, S.H.; Lee, B.K.; Choi, D.K. Equilibrium Isotherms of CH4, C2H6, C2H4, N2, and H2 on Zeolite 5A Using a Static Volumetric Method. J. Chem. Eng. Data 2005, 50, 72–76. [Google Scholar] [CrossRef]
- Mendes, P.A.P.; Ribeiro, A.M.; Gleichmann, K.; Ferreira, A.F.P.; Rodrigues, A.E. Separation of CO2/N2 on binderless 5A zeolite. J. CO2 Util. 2017, 20, 224–233. [Google Scholar] [CrossRef]
- Liu, Z.; Grande, C.A.; Li, P.; Yu, Y.; Rodrigues, A.E. Adsorption and Desorption of Carbon Dioxide and Nitrogen on Zeolite 5A. Sep. Sci. Technol. 2011, 46, 434–451. [Google Scholar] [CrossRef]
- Sudani, D.H.A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Kusuma, H.S.; Darmokoesoemo, H.; Osibote, O.A.; Balogun, V.A.; Widyaningrum, B.A. Malachite Green Removed by Activated Potassium Hydroxide Clove Leaf Agrowaste Biosorbent: Characterization, Kinetics, Isotherm, and Thermodynamics Studies. Adsorpt. Sci. Technol. 2021, 2021, 1145312. [Google Scholar]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Vilvanathan, S.; Shanthakumar, S. Column adsorption studies on nickel and cobalt removal from aqueous solution using native and biochar form of Tectona grandis. Environ. Prog. Sustain. Energy 2017, 36, 1030–1038. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1667. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-called Adsorption of Soluble Substances. K. Sven. Vetensk. Handl. 1989, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G.A. Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–400. [Google Scholar] [CrossRef] [Green Version]
- Choy, K.K.H.; Porter, J.F.; Mackey, G. Film-Pore Diffusion Models-Analytical and Numerical Solutions. Chem. Eng. Sci. 2014, 59, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Kavand, M.; Asasian, N.; Soleimani, M.; Kaghazchi, T.; Bardestani, R. Film-Pore-[Concentration-Dependent] Surface Diffusion model for heavy metal ions adsorption: Single and multi-component systems. Process Saf. Environ. Prot. 2017, 107, 486–497. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, K.; Rudzinski, W. Modeling of Sorption Kinetics: The Pseudo-Second Order Equation and the Sorbate Intraparticle Diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Crittenden, J.; Hristovski, K.; Hand, D.; Westerhoff, P. User-Oriented Batch Reactor Solutions to the Homogeneous Surface Diffusion Model for Different Activated Carbon Dosages. Water Res. 2009, 43, 1859–1866. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem. Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Yang, B.; Lui, Y.; Li, M. Separation of CO2-N2 using zeolite NaKA with high selectivity. Chin. Chem. Lett. 2016, 27, 933–937. [Google Scholar] [CrossRef]
- Al-Junabi, N.; Vakiti, R.; Kalumpasut, P.; Gorgojo, P.; Siperstein, F.R.; Fan, X. Velocity Variation Effect in Fixed Bed Columns: A Case Study of CO2 Capture Using Porous Solid Adsorbents. AIChE J. 2018, 64, 2189–2197. [Google Scholar] [CrossRef] [Green Version]
- Golden, T.C.; Sircar, S. Gas Adsorption on Silicalite. J. Colloid Interface Sci. 1994, 162, 82–188. [Google Scholar] [CrossRef]
- Heymans, N.; Alban, B.; Moreau, S.; De Weireld, G. Experimental and theoretical study of the adsorption of pure molecules and binary systems containing methane, carbon monoxide, carbon dioxide and nitrogen: Application to the syngas generation. Chem. Eng. Sci. 2011, 66, 3850–3858. [Google Scholar] [CrossRef]
- Mulgundmath, V.P.; Tezel, F.H.; Saatcioglu, T.; Golden, T.C. Adsorption and separation of CO2/N2 and CO2/CH4 by 13X zeolite. Can. J. Chem. Eng. 2012, 90, 730–738. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279C. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina. Chem. Eng. Sci. 2001, 56, 3781–3796. [Google Scholar] [CrossRef]
- Kongnoo, A.; Intharapat, P.; Worathanakul, P.; Phalakornkul, C. Diethanolamine impregnated palm shell activated carbon for CO2 adsorption at elevated temperatures. J. Environ. Chem. Eng. 2016, 4, 73–78. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: Oxford, UK, 2014; pp. 25–56. [Google Scholar]
- Yi, Y.J.; Wang, Z.; Zhang, K.; Yu, G.; Duan, X. Sediment pollution and its effect on fish through food chain in the Yangtze River. Int. J. Sediment Res. 2008, 23, 338–347. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.; Shamiri, A.; Aghamohammadi, N. Modeling of Carbon Dioxide Adsorption onto Ammonia-Modeified Activated Carbon: Kinetic Analysis and Breakthrough Behavior. Energy Fuels 2015, 29, 6565–6577. [Google Scholar] [CrossRef]
- Monazam, E.R.; Spenik, J.; Shadle, L.J. Fluid bed adsorption of carbon dioxide on immobilized polyethylenimine (PEI): Kinetic analysis and breakthrough behavior. Chem. Eng. J. 2013, 223, 795–805. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Theoretical and experimental SO2 adsorption onto pistachio-nut-shell activated carbon for a fixed-bed column. Chem. Eng. J. 2009, 155, 175–183. [Google Scholar] [CrossRef]
- Mulgundmath, V.P.; Jones, R.A.; Tezel, F.H.; Thibault, J. Fixed bed adsorption for the removal of carbon dioxide from nitrogen: Breakthrough behavior and modeling for heat and mass transfer. Sep. Purif. Technol. 2012, 85, 17–27. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, Y.; Bao, A. Preparation of nitrogen-doped hierarchical porous carbon materials by a template-free method and application to CO2 capture. J. Environ. Chem. Eng. 2020, 8, 103732. [Google Scholar] [CrossRef]
- Tobarameekul, P.; Sangsuradet, S.; Worathanakul, P. Comparative Study of Zn Loading on Advanced Functional Zeolite NaY from Bagasse Ash and Rice Husk Ash for Sustainable CO2 Adsorption with ANOVA Factorial Design. Atmosphere 2022, 13, 314. [Google Scholar] [CrossRef]
- Yoro, K.O.; Amosa, M.K.; Sekoai, P.T.; Mulopo, J.; Daramola, M.O. Diffusion mechanism and effect of mass transfer limitation during the adsorption of CO2 in a packed-bed column. Int. J. Sustain. Eng. 2020, 13, 54–67. [Google Scholar] [CrossRef]
- Gabruś, E.; Wojtacha-Rychter, K.; Aleksandrzak, T.; Smoliński, A.; Król, M. The feasibility of CO2 emission reduction by adsorptive storage on Polish hard coals in the Upper Silesia Coal Basin: An experimental and modeling study of equilibrium, kinetics and thermodynamics. Sci. Total Environ. 2021, 796, 149064. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Carrillo, J.; Garrido, F.; Barrios, L.; García-González, M.T. Sorption of As, Cd, and Tl as influenced by industrial by-products applied to an acidic soil: Equilibrium and kinetic experiments. Chemosphere 2006, 65, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Das, S.K. Biosorption of Cr (VI) ions from aqueous solutions: Kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf. B 2011, 84, 221–232. [Google Scholar] [CrossRef]
- Chouch, P.K.; Mishra, I.M.; Chand, S. Decolorization and removal of chemical oxygen demand (COD) with energy recovery, Treatment of biodigester effluent of a molasses based alcohol distillery using inorganic coagulants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 296, 238–247. [Google Scholar]
- Kaosuah, F.; Kaouah, B.; Berrama, T.; Trai, M.; Bendjama, B. Preparation and characterization of activated carbon from wild olive cores (oleaster) by H3PO4 for the removal of basic Red 46. J. Clean. Prod. 2013, 54, 296–306. [Google Scholar] [CrossRef]
- Moyo, M.; Chikazaza, L.; Chomunorwa, B.; Guyo, U. Adsorption batch studies on the removal of Pb(II) using Maize Tassel based activated carbon. J. Chem. 2013, 2013, 508934. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Adsorbent | Zeolite 5A |
| Reactor size | 0.07752 cm.id |
| stainless steel tube | |
| Weight of adsorbent | 1 g |
| Length of bed | 5 cm |
| Pressure | 100 kPa |
| C20N80T298F2 | CO2 20 %vol, N2 80 %vol, 298 K and 2 L/h |
| C50N50T298F2 | CO2 50 %vol, N2 50 %vol, 298 K and 2 L/h |
| C80N20T298F2 | CO2 80 %vol, N2 20 %vol, 298 K and 2 L/h |
| C20N80T333F2 | CO2 20 %vol, N2 80 %vol, 333 K and 2 L/h |
| C20N80T373F2 | CO2 20 %vol, N2 80 %vol, 373 K and 2 L/h |
| C20N80T298F1 | CO2 20 %vol, N2 80 %vol, 298 K and 1 L/h |
| C20N80T298F4 | CO2 20 %vol, N2 80 %vol, 298 K and 4 L/h |
| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |||
|---|---|---|---|---|---|
| BET Surface Area a | External Surface Area b | Micropore Surface Area b | Micropore Volume b | Total Pore Volume c | |
| Pure zeolite 5A | 517.44 | 18.21 | 499.23 | 0.260 | 0.277 |
| C20N80T298F1 | 496.51 | 21.80 | 474.71 | 0.254 | 0.274 |
| C20N80T298F2 | 498.15 | 20.30 | 477.85 | 0.241 | 0.260 |
| C20N80T298F4 | 502.64 | 19.67 | 482.97 | 0.256 | 0.277 |
| Model | Conditions | Parameters | ||
|---|---|---|---|---|
| kBA (L mg−1 min−1) | N0 (mg L−1) | R2 | ||
| Bohart–Adams | C20N80T298F2 | 2.959 × 10−6 | 1.419 × 1010 | 0.936 |
| C20N80T373F2 | 6.024 × 10−6 | 0.669 × 1010 | 0.833 | |
| C20N80T298F4 | 3.730 × 10−6 | 1.854 × 1010 | 0.890 | |
| Yoon–Nelson | Conditions | kYN (min−1) | (min) | R2 |
| C20N80T298F2 | 0.169 | 19.569 | 0.990 | |
| C20N80T373F2 | 0.429 | 7.164 | 0.990 | |
| C20N80T298F4 | 0.286 | 8.605 | 0.992 | |
| Thomas | Conditions | kTH (L mg−1 min−1) | qTH (mg g−1) | R2 |
| C20N80T298F2 | 4.414 × 10−6 | 2.387 × 107 | 0.987 | |
| C20N80T373F2 | 4.611 × 10−6 | 1.249 × 107 | 0.811 | |
| C20N80T298F4 | 4.396 × 10−6 | 2.829 × 107 | 0.905 | |
| Conditions | Experiment | Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | ||||
|---|---|---|---|---|---|---|---|
| qexp (mmol g−1) | qcal (mmol g−1) | k1 (min−1) | R2 | qcal (mmol g−1) | k2 (g mmol−1min−1) | R2 | |
| C20N80T298F2 | 6.44 | 7.69 | 0.1034 | 0.993 | 12.77 | 2.45 × 10−3 | 0.701 |
| C20N80T333F2 | 3.42 | 4.09 | 0.0834 | 0.986 | 47.85 | 1.92 × 10−3 | 0.910 |
| C20N80T373F2 | 2.56 | 2.89 | 0.0996 | 0.996 | 3.35 | 27.20 × 10−3 | 0.989 |
| C20N80T298F1 | 7.42 | 9.02 | 0.0994 | 0.996 | 14.97 | 1.90 × 10−3 | 0.831 |
| C20N80T298F4 | 3.83 | 4.77 | 0.0620 | 0.988 | 4.84 | 19.40 × 10−3 | 0.972 |
| Conditions | ki,1 (mmol g−1min0.5) | ki,2 (mmol g−1min0.5) | ki,3 (mmol g−1min0.5) |
|---|---|---|---|
| C20N80T298F2 | 0.5212 | 1.8460 | 0.0717 |
| C20N80T333F2 | 0.5711 | 1.0573 | 0.0894 |
| C20N80T373F2 | 0.5589 | 0.2299 | 0.0395 |
| C20N80T298F1 | 0.7363 | 2.2231 | 0.0767 |
| C20N80T298F4 | 0.5148 | 0.9935 | 0.2587 |
| Adsorption Conditions | Time Taken for Film Diffusion (min) | Time Taken for Intraparticle Diffusion (min) | Time Taken for Adsorption Equilibrium (min) | Ratio of Time For Film Diffusion to Intraparticle Diffusion |
|---|---|---|---|---|
| C20N80T298F2 | 6 | 18 | 20 | 0.33 |
| C20N80T333F2 | 11 | 12 | 20 | 0.92 |
| C20N80T373F2 | 12 | 22 | 15 | 0.50 |
| C20N80T298F1 | 6 | 17 | 25 | 0.35 |
| C20N80T298F4 | 7 | 13 | 30 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonchuay, A.; Worathanakul, P. The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column. Atmosphere 2022, 13, 513. https://doi.org/10.3390/atmos13040513
Boonchuay A, Worathanakul P. The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column. Atmosphere. 2022; 13(4):513. https://doi.org/10.3390/atmos13040513
Chicago/Turabian StyleBoonchuay, Arunaporn, and Patcharin Worathanakul. 2022. "The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column" Atmosphere 13, no. 4: 513. https://doi.org/10.3390/atmos13040513
APA StyleBoonchuay, A., & Worathanakul, P. (2022). The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column. Atmosphere, 13(4), 513. https://doi.org/10.3390/atmos13040513







