Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review
Abstract
1. Introduction
1.1. Physical and Chemical Properties of CO2
1.2. Trend of Atmospheric CO2 Concentration and Potential CO2 Emissions Sources
1.3. Significant Outcomes Owing to the Trend of Increasing CO2 Emissions
1.4. Approaches to Reduce Atmospheric CO2 Concentration
1.5. CO2 Emission Sources
1.6. CO2 Capture Technologies
1.6.1. Pre-Combustion Capture
1.6.2. Oxy-Fuel Capture
1.6.3. Post-Combustion Capture
1.7. Available CO2 Sequestration Methods
2. Solid Adsorbents for CO2 Capture
2.1. Adsorption Process of CO2
2.1.1. Physisorption of CO2 onto Adsorbents
2.1.2. Chemisorption of CO2 onto Adsorbents
2.2. Different Regeneration Strategies
2.3. Criteria for Selecting CO2 Adsorbents
2.4. Different Adsorbents for CO2 Capture
2.5. Importance of Carbon-Based Adsorbents for Effective CO2 Capture
3. CO2 Capture Using Porous Carbon Materials: Physisorption
3.1. Synthesis of Physisorbents
3.1.1. General Introduction
3.1.2. Porous Carbon Synthesis Using Different Precursors
3.1.3. The Effect of Synthesis Procedures on the Development of Textural Properties
3.2. CO2 Adsorption Capacities of Carbon-Based Physisorbents
| Porous Carbon Material | SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | Smic (m2/g) | Smes (m2/g) | Average Pore Size (nm) | CO2 Capture Conditions for Pure CO2 Gas Flow | CO2 Capture Capacity (mmol/g) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| KOH activated carbon nanoflakes | 2010 | 0.82 | 0.718 | 0.102 | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 7.82 4.27 | [64] |
| Mesoporous carbon synthesized using 3D silica KIT-6 as the hard template | 740 | 0.88 | - | - | - | - | 1.7 8.7 | 0 °C and 1.2 bar 25 °C and 0.01 bar | 2.29 1.62 | [114] |
| KOH activated biotar | 2595 | 1.296 | - | - | - | - | 2.5 | 0 °C and 1 bar | 5.35 | [24] |
| ZnCl2 activated Poplar cat skin-derived porous carbon | 1005.4 | 0.41 | 0.34 | - | 867.6 | 137.8 | - | 0 °C and 0.15 bar 25 °C and 0.15 bar | 1.94 1.13 | [23] |
| KOH activated date sheets | 2367 | 1.48 | 0.834 | - | 2059 | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.4 4.36 | [143] |
| NaNH2 activated lotus stalk | 1113 | 0.41 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 3.88 5.45 | [182] |
| NaNH2 activated lotus leaf | 1087 | 0.45 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 3.50 5.04 | [183] |
| KOH activated coconut shells | 1172 | 0.58 | 0.44 | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.04 4.23 | [81] |
| NaOH activated sugarcane bagasse | 1149 | 1.73 | 0.08 | - | - | - | 6.02 | 25 °C and 1 bar | 4.28 | [47] |
| NaNH2 activated water chestnut shells | 1416 | 0.53 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 4.50 6.04 | [183] |
| CO2 activated bamboo | 953 | 0.4 | 0.51 | 0.04 | - | - | - | 25 °C and 1 bar | 3.4 | [11] |
| CO2 activated solid residue | 1316 | 0.55 | 0.54 | 0.07 | - | - | - | 25 °C and 1 bar | 3.4 | [11] |
| KOH activated pinewood | 900.76 | 0.38 | 0.33 (87%) | 0.05 (13%) | - | - | 1.69 | 25 °C and 1bar | 3.92 | [17] |
| Steam activated pine sawdust | 581.74 | 0.25 | - | - | - | - | 2.24 | 25 °C and 1 bar | 2.498 | [54] |
| CO2 activated palm kernel shell | 367.8 | 0.2199 | - | - | - | - | - | 25 °C and 1 bar | 2.13 | [84] |
| KOH activated blue algae | 1018.55 | - | 0.46 | - | - | - | 2.09 | 0 °C and 1 bar 25 °C and 1 bar | 4.88 2.76 | [176] |
| Carbonized mangosteen peel | 1270 | 0.55 | 0.51 | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar 45 °C and 1 bar | 6.93 4.77 3.35 | [15] |
| NaNH2 activated hazelnut shells | 1099 | 0.45 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.06 4.23 | [151] |
| Chemically activated rice husk with prior compaction | 1190 | 0.777 | 0.422 | 0.175 | - | - | - | 25 °C and 15 kPa | 1.9 | [50] |
| KOH activated algae | 1247.2 | - | 0.69 | - | 1192.4 | 39.4 | - | 0 °C and 1 bar 25 °C and 1 bar | 5.7 3.9 | [57] |
| Potassium acetate activated sucrose | 1917 | 0.85 | - | 71% | 78.8% | - | - | 25 °C and 1 bar | 4.82 | [78] |
| Urea activated MOF-5-derived porous carbon | 1161 | 1.31 | 0.25 | 1.06 | 554 | 607 | - | 25 °C and 1 bar | 2.44 | [198] |
| Cu-BTC framework-derived porous carbon | 1364 | 0.65 | 0.59 (91%) | - | - | - | - | 25 °C and 1 bar | 4.51 | [22] |
| ZIF-8-derived porous carbon | 948 | 0.73 | 0.39 | 0.34 | 826 | 122 | - | 25 °C and 1 bar | 3.7 | [199] |
| KOH activated graphite oxide | 3240 | 2.23 | - | - | - | - | 2.75 | 25 °C and 20 bar | 21.1 | [170] |
| KOH activated graphene | 716 | 0.66 | - | - | - | - | 3.7 | 25 °C and 1 bar | 3.13 | [42] |
| CO2 activated graphene | 1315.98 | 1.07 | 0.21 | - | - | - | - | 0 °C and 1 bar | 3.36 | [150] |
| MgO nanoparticles fabricated on Graphene oxide | 12 | 0.1 | <0.01 | - | - | - | - | 25 °C and 1 bar | 0.16 | [94] |
| Urea and KOH activated graphene oxide | 1032 | 0.61 | 0.59 | - | - | - | - | 25 °C and 1 bar | 2.4 | [21] |
| KOH activated petroleum coke | 1445 | 0.52 | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.41 4.57 | [127] | |
| Urea modified and KOH activated petroleum coke | 1394 | 0.52 | - | - | - | - | - | 25 °C and 1 bar | 4.4 | [193] |
| KOH activated petroleum coke | 1433 | 0.6 | - | - | - | - | - | 25 °C and 1 bar | 3.68 | [10] |
| NaNH2 activated petroleum coke | 1666 | 0.66 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 5.93 3.84 | [180] |
| KOH activated petroleum coke | 1470 | 0.6 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar 50 °C and 1 bar | 6.7 4.17 2.45 | [146] |
| KOH activated asphalt | 4200 | 2.4 | - | - | - | - | 2.4 | 25 °C and 54 bar | 35 | [130] |
| KOH activated Iranian asphalt | 2186 | 1.3 | 0.25 | 1.05 | - | - | 2.37 | 25 °C and 1 bar 35 °C and 1 bar | 11.37 38.49 | [7] |
| KOH activated carbon fibers from anthracene oil-based pitch | 1294 | 0.6 | - | - | - | - | - | 25 °C and 1 bar | 3.5 | [166] |
| Phenolic resin electrospun carbon fibers | 650 | 0.277 | 0.249 | - | - | - | - | 25 °C and 1 bar | 2.92 | [153] |
| CO2 activated Resorcinol–formaldehyde-derived carbon | 1458 | 0.647 | - | - | - | - | - | 25 °C and 1 bar | 4.54 | [178] |
| KOH activated phenolic resin spheres | 2130 | 1.1 | 0.78 (71%) | - | - | - | - | 0 °C and 1 bar | 6.6 | [134] |
| Urea modified and KOH activated phenolic resin-derived carbon | 1404 | 0.53 | - | - | - | - | - | 25 °C and 1 bar | 4.61 | [196] |
| KOH activated commercial phenolic resin | 1040 | 0.37 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 4.12 5.66 | [177] |
| KOH activated resorcinol–formaldehyde spheres | 1235 | 0.67 | 0.52 | 1084 | - | - | - | 25 °C and 1 bar | 4.83 | [4] |
| NaNH2 activated phenolic resin | 1924 | 0.71 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 4.57 7.13 | [185] |
| Urea modified and KOH activated phenolic resin-derived carbon | 1482 | 0.56 | - | - | - | - | - | 25 °C and 1 bar | 5.01 | [173] |
| CO2 activated cellulose | 1249 | 0.53 | 0.4 | - | - | - | - | 0 °C and 0.15 bar 0 °C and 1 bar | 1.96 5.52 | [117] |
| KOH activated chitosan | 1746 | - | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.37 3.91 | [187] |
| KOH activated chitosan | 3226 | 1.35 | - | - | - | - | 3.91 | 0 °C and 1 bar | 8.3 | [88] |
| Potassium citrate activated chitosan | 2278 | 1 | 63% | - | - | - | 0.56 0.73 | 0 °C and 30 bar | 22 | [189] |
| Potassium citrate activated chitosan | 1784 | 0.78 | 74% | - | - | - | 0.56 0.66 | 0 °C and 1 bar | 6.1 | [189] |
| CO2 activated carbon aerogel by cellulose | 1364 | 1.43 | 0.37 | - | - | - | - | 25 °C and 1 bar | 3.42 | [179] |
| KOH activated lignin | 1788 | 0.91 | 0.49 | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 8.2 4.8 | [161] |
| KOH activated EHL | 2870 | 2.02 | 0.7 | 1.32 | 1000 | - | 2.8 | 30 °C and 1 bar | 1.31 | [141] |
| KOH activated starch-based packing peanut | 1354 | 0.551 | 0.539 | - | 1235 | - | - | 0 °C and 1 bar 25 °C and 1 bar 50 °C and 1 bar | 6.51 4.07 2.35 | [138] |
| KOH activated waste wool | 1352 | 0.78 | 0.54 | - | - | - | - | 25 °C and 1 bar | 2.78 | [164] |
| KOH activated starch | 1636 | 0.51 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 7.49 3.84 | [49] |
| CO2 activated starch | 3350 | 1.75 | 1.67 | - | 3281 | - | - | 25 °C and 20 bar | 1.2 | [145] |
| KOH activated chitin aerogel | 521 | 0.19 | - | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 5.02 3.44 | [52] |
| KOH activated polypyrrole | 941 | - | 0.34 | - | - | - | - | 25 °C and 0.1 bar 25 °C and 1 bar | 1.42 4.5 | [135] |
| KOH activated waste CDs and DVDs | 2710 | 1.27 | 91% | - | - | - | - | 0 °C and 1 bar 25 °C and 1 bar | 5.8 3.3 | [154] |
| PILs as the precursor and C3N4 nanosheets | 1120 | 2.28 | - | - | - | - | - | 0 °C and 1 bar | 4.37 | [159] |
| KOH activated PIL | 1742 | 1.415 | 1.078 | - | 1392 | - | - | 0 °C and 1 bar 25 °C and 1 bar | 6.2 4.5 | [157] |
| Chitosan grafted graphene oxide aerogel | 33.32 | 0.129 | - | - | - | - | - | 25 °C and 1 bar | 0.2579 | [152] |
3.3. Importance of Textural Properties in CO2 Capture by Carbon-Based Adsorbents
3.4. Selectivity of CO2 over Other Gases and Moisture
3.5. Regeneration and Cyclic Stability of Porous Carbon Materials
4. CO2 Chemisorption Using Amine-Functionalized Porous Carbon Materials
4.1. Importance of Chemisorbents
4.2. CO2 Capture by Amine-Impregnated Carbon-Based Adsorbents
4.2.1. Synthesis of Amine-Impregnated Porous Carbon Adsorbents
4.2.2. CO2 Adsorption Capacities of Amine-Impregnated Porous Carbon Materials
4.2.3. Regeneration and Cyclic Stability of Amine-Impregnated Porous Carbon Materials
4.3. CO2 Capture by Amine-Grafted Porous Carbon Adsorbents
4.3.1. Synthesis of Amine-Grafted Porous Carbon Adsorbents
4.3.2. CO2 Adsorption Capacities of Amine-Grafted Porous Carbon Materials
4.3.3. Regeneration and Cyclic Stability of Amine-Grafted Porous Carbon Materials
4.4. CO2 Selectivity of Amine-Functionalized Porous Carbons
4.5. Importance of Amine-Functionalization for Effective CO2 Capture
4.6. Importance of Moisture in the Effective Capture of CO2 by Amine-Functionalized Porous Carbon Adsorbents
5. Contactors for CO2 Adsorption Using Porous Carbon Materials
5.1. Fixed Bed Reactor
- (i)
- (ii)
- Structured fixed bed reactor: Structured fixed bed contactors are considered a better alternative to conventional fixed beds. In this arrangement, sophisticated packing materials are employed to maximize the surface area per volume of the adsorbent and heat transfer [248,261,262] while maintaining better temperature control [263,264]. Additionally, this reactor configuration is capable of lowering the pressure drop (50% reduction can be achieved compared to pellets) [264] and improves the gas throughput and productivity 3–10 times [265] while reducing the cycle time [247].
5.2. Moving Bed
- (i)
- (ii)
- Rotary bed: The concept of a rotary bed has been innovated as an alternative to traditional moving beds [248]. This reactor configuration comprises a rotating reactor that can effectively separate CO2 from industrial flue gas [248]. Even though the rotary bed enables steady-state operation, there might be sealing and leakage problems [248].
5.3. Fluidized Bed
- (i)
- Single-stage fluidized bed: Single-stage fluidized bed operated at steady-state with a low-pressure drop while providing a high heat transfer coefficient in the range of 300–600 W/m2 K [281]. On the contrary, this bed configuration possesses some disadvantageous properties, including attrition of sorbents and lower working capacity due to back mixing [275].
- (ii)
- Multistage fluidized bed: Compared to the single-stage fluidized bed reactor, the multi-stage fluidized bed reduces the internal back mixing by introducing a plug flow behavior while enhancing the CO2 capture performance as in packed beds just after 3–5 stages [285]. Moreover, the cost associated with the multi-stage fluidized bed is notably higher than that of the single-stage fluidized bed configuration. Even though high driving forces and improved CO2 capture could be achieved in multi-stage fluidized beds, the complexity of this bed configuration hinders the industrial scale deployment [286].
- (iii)
- Transient fluidized bed: The concept of transient fluidized bed reactor, which is also known as the swing adsorption reactor cluster (SARC), was initially proposed by Zaabat et al. [287]. In this bed configuration, there is no solid particle circulating, which enables the application of VSA during the regeneration step [287]. In this reactor, the back mixing is further reduced concerning the multi-stage fluidized bed [288], and a significant reduction of the energy penalty compared to other benchmarking technologies [289] improved CO2 capture efficiencies, which meant adsorber working capacities [290] could be achieved. Interestingly, this contactor configuration can be easily retrofitted into existing plants while applying both TSA and steam regeneration processes [291].
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- North, M. Chapter I-What is CO2? Thermodynamics, basic reactions and physical chemistry. In Carbon Dioxide Utilization; Styring, P., Quadrelli, E.A., Armstrong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–17. [Google Scholar]
- Salehi, S.; Anbia, M.; Hosseiny, A.H.; Sepehrian, M. Enhancement of CO2 adsorption of polyethylenimine functionalized multiwalled carbon nanotubes/Cd-nanozeolite composites. J. Mol. Struct. 2018, 1173, 792–800. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies. J. Environ. Manag. 2019, 242, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Xing, W.; Lui, B.; Zhang, J.; Lin, H.; Cui, H.; Zhou, S. Resorcinol-formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013. [Google Scholar] [CrossRef]
- Qin, F.; Guo, Z.; Wang, J.; Qu, S.; Zuo, P.; Shen, W. Nitrogen-doped asphaltene-based porous carbon nanosheet for carbon dioxide capture. Appl. Surf. Sci. 2018, 491, 607–615. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Lia, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Alivand, M.S.; Maklarany, D.M.; Rashidi, A.; Samipoorgini, M.; Seif, A.; Yousefian, Z. Novel asphaltene-derived nanoporous carbon with N-S-rich-micro-mesoporous structure fro superior gas adsorption: Experimental and DFT study. Chem. Eng. J. 2018, 358, 1126–1138. [Google Scholar] [CrossRef]
- Sepahvand, S.; Jonobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.J.H.; Oksman, K.; Yu, Q. A promising process to modify cellulose nanotubes for carbon dioxide (CO2) adsorption. Carbohydr. Polym. 2019, 230, 115571. [Google Scholar] [CrossRef]
- Rahimi, K.; Riahi, S.; Abbasi, M.; Fakhroueian, Z. Modification of multi-walled carbon nanotubes by 1,3-diaminepropane to increase CO2 adsorption capacity. J. Environ. Manag. 2019, 242, 81–89. [Google Scholar] [CrossRef]
- Jang, E.; Choi, S.W.; Hong, S.; Shin, S.; Lu, K.B. Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation. Appl. Surf. Sci. 2017, 429, 62–71. [Google Scholar] [CrossRef]
- Khuong, D.A.; Nguyen, H.N.; Tsubota, T. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Energy 2021, 148, 106039. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, R.K.; Bhunia, H. CO2 capture on activated carbon from PET (polyethylene terephthalate) waste: Kinetics and modelling studies. Chem. Eng. Commun. 2019, 207, 1031–1047. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Development of chemically activated N-enriched carbon adsorbents from urea-formaldehyde resin for CO2 adsorption: Kinetics, isotherm, and thermodynamics. J. Environ. Manag. 2018, 218, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified carbon nanotubes/tetraethylpentamine for CO2 capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Cao, M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors. J. CO2 Util. 2018, 27, 204–216. [Google Scholar] [CrossRef]
- Liu, Z. National carbon emissions from the industry process: Production of glass, soda ash, ammonia, calcium carbide and alumina. Appl. Energy 2016, 66, 239–244. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashidi, T. Development of biomass derived highly porous fast adsorbents for post-combustion CO2 capture. Fuel 2020, 282, 118506. [Google Scholar] [CrossRef]
- Lal, R. Acceleration soil erosion as a source of atmospheric CO2 soil. Soil Tillage Res. 2019, 199, 35–40. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Zhang, T.; Wang, B.; Wang, Y.; Wang, L.; Wei, J. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation. Energy 2020, 211, 118561. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped porous carbon derived from KOH-activated waste wool: A promising material for selective adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef]
- An, L.; Liu, S.; Wang, L.; Wu, J.; Wu, Z.; Ma, C.; Yu, Q.; Hu, X.C. Novel nitrogen-doped porous carbons derived from graphene for effective CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 3349–3358. [Google Scholar] [CrossRef]
- Liu, Y.; Ghimie, P.; Jaroniec, M. Copper Benzene-1,3,5-Tricarboxylate (Cu-BTC) metal-organic framework (MOF) and porous carbon composites as effective carbon dioxide adsorbents. J. Colloid Interface Sci. 2018, 535, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lee, J.G.; Yun, H.; Deng, S.; Kim, Y.J.; Lee, J.E.; Kwak, S.K.; Lee, K.B. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2. Chem. Eng. J. 2020, 397, 125350. [Google Scholar] [CrossRef]
- Tu, R.; Sun, Y.; Wu, Y.; Wang, J.; Chen, S.; Jia, Z.; Jiang, E.; Xu, X. Bio-tar derived porous carbon with high gas uptake capacities. Renew. Energy 2021, 167, 82–90. [Google Scholar] [CrossRef]
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 adsorption study on pure and chemically activated chars derived from commercial biomass gasifiers. J. CO2 Util. 2019, 33, 46–54. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavez, C.T. Carbon dioxide capture adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef]
- Kukulka, W.; Cendrowski, K.; Michalkiewicz, B.; Mkijowska, E. MOF-5 derived carbon as material for CO2 adsorption. R. Soc. Chem. 2019, 9, 18527–18537. [Google Scholar] [CrossRef]
- Dilokekunakul, W.; Teerachawanwong, P.; Klomkliang, N.; Supasitmouskol, S.; Chaemucheun, S. Effects of nitrogen and oxygen functional groups and pore width of activated carbon on carbon dioxide capture: Temperature dependence. Chem. Eng. J. 2020, 389, 124413. [Google Scholar] [CrossRef]
- Arifin, N.P.T.A.; Zulkipils, N.A.N.; Yusof, N.; Ismail, A.F.; Azizi, F.; Salleh, W.N.W.; Jalefar, J.; Nordin, N.A.H.M.; Sazali, N. Preparation and characterization of APTES-functionalized graphene oxide for CO2 adsorption. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 61, 297–305. [Google Scholar]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-based material from polysaccharides: Properties, processing, characterization and sorption applications. Adv. Sorpt. Process Appl. 2018, 1–24. [Google Scholar] [CrossRef]
- Omidfar, N.; Mohamadalizadeh, A.; Mousavi, S.H. Carbon dioxide adsorption by modified carbon nanotubes. Asia-Pac. J. Chem. Eng. 2015, 10, 885–892. [Google Scholar] [CrossRef]
- Idrees, M.; Rangari, V.; Jeelani, S. Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 2018, 26, 380–387. [Google Scholar] [CrossRef]
- Lee, S.; Park, S. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Kamran, U.; Choi, J.R.; Park, S. A role of activation for efficient CO2 affinity on polyacronitrile based porous carbon materials. Front. Chem. 2020, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasalu, B.; Gayatri, D.V.; Sreedhar, I.; Ragharan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Urea-formaldehyde derived porous carbons for adsorption of CO2. R. Soc. Chem. 2016, 6, 111842–111855. [Google Scholar] [CrossRef]
- Deng, M.; Park, H.G. Spacer-assisted amine-coiled carbon nanotubes for CO2 capture. Langmuir 2019, 35, 4453–4459. [Google Scholar] [CrossRef]
- Jena, K.K.; Panda, A.P.; Verma, S.; Mani, G.K.; Swain, S.K.; Alhassan, S.M. MWCNTs-ZnO-SiO2 mesoporous nano-hybrid materials for CO2 capture. J. Alloy Compd. 2019, 800, 279–285. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Ismail, A.F. Activated-carbon nanofibers/graphene nanocomposites and their adsorption performance towards carbon dioxide. Chem. Engl. Technol. 2020, 43, 2023, 2030. [Google Scholar] [CrossRef]
- Shao, L.; Song, Y.; Huang, J.; Liu, Y. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture. Chem. Eng. J. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M. Highly efficient CO2 capture with a metal-organic framework-derived porous carbon impregnated with polyethylenimine. Appl. Organomet. Chem. 2018, 32, e4390. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Adsorption of CO2 on KOH activated N-enriched carbon derived from urea-formaldehyde resin: Kinetics, isotherm and thermodynamic studies. Appl. Surf. Chem. 2018, 439, 760–771. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidek, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modelling and simulations-a review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Tiwari, D.; Kaur, S.; Bhunia, H.; Bajpai, P.K. CO2 adsorption on oxygen enriched nanostructured carbon derived from silica templated resorcinol-formaldehyde. J. Ind. Eng. Chem. 2018, 65, 146–155. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S. Role of heteroatoms (nitrogen and sulfur)-dual doped corn-starch based porous carbons for selective CO2 adsorption and separation. J. CO2 Util. 2021, 51, 101641. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbon derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized microcrystalline cellulose-mesoprous silica composites for carbon dioxide sorption at elevated temepratures. J. Mater. Chem. A 2016, 4, 4808–4819. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaue, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, H.; Tan, W.H.; Wang, W.; Snape, C. Developing hierarchical ultra-micro mesoporous biocarbons for highly selective carbon dioxide adsorption. Chem. Eng. J. 2018, 361, 199–208. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized nanocrystalline cellulose-mesoporous silica composites for carbon dioxide sorption of ambient and elevated temperatures. J. Mater. Chem. A 2017, 5, 7462–7473. [Google Scholar] [CrossRef]
- Dassanayake, R.; Gunathilake, C.; Abidi, N. Activated carbon derived from chitin aerogels: Preparation and CO2 desorption. Cellulose. 2018, 25, 1911–1920. [Google Scholar] [CrossRef]
- Lashake, M.J.; Khiavi, S.; Sayari, A. Stabilizing of amine-functionlaozed CO2 adsorbents: A multifunctional puzzle. Chem. Soc. Rev. 2019, 48, 3320–3405. [Google Scholar] [CrossRef] [PubMed]
- Igalavithana, A.D.; Choi, S.W.; Shang, J.; Hanif, A.; Dissanayake, P.D.; Tsang, D.C.W.; Kwon, J.; Lu, K.B.; Ok, Y.S. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. [Google Scholar] [CrossRef] [PubMed]
- Estevez, L.; Barpaga, D.; Zheng, J.; Sabale, S.R.; Patel, R.L.; Zhang, J.; McGrail, B.P.; Motkuri, B.K. Hierarchically porous carbon materials for CO2 capture: The role of pore structure. Ind. Eng. Chem. Res. 2017, 57, 1262–1268. [Google Scholar] [CrossRef]
- Mehrrarz, E.; Ghreyshi, A.A.; Jahanshaki, M. Adsorptive separation of CO2 and CH4 by the broom sorghum based activated carbon functionalized by diethanolamine. Korean J. Chem. Eng. 2016, 34, 413–424. [Google Scholar] [CrossRef]
- Liu, K.; Jin, B.; Meng, L. Glucose/Graphene-based aerogels for gas adsorption and electric double layer capacitors. Polymers 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- He, J.; To, J.W.F.; Psarras, P.C.; Yan, H.; Atkinson, T.; Holmes, R.T.; Norduland, D.; Bao, Z.; Wilcox, J. Tunable polyaniline-based porous carbon with ultrahigh surface area for CO2 capture at elevated pressure. Adv. Energy Mater. 2016, 6, 1502491. [Google Scholar] [CrossRef]
- Prasetyo, I.; Maukti, N.I.F.; Cahyono, R.B.; Prasetya, A.; Ariyanto, T. Nanoporous carbon prepared from palm kernel shell for CO2/CH4 separation. Waste Biomass Volatilization 2020, 11, 5599–5606. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Nordin, A.R.; Wilfred, C.D. The improvement of activated carbon as CO2 adsorbent with supported amine functionalized ionic liquids. J. Environ. Chem. Eng. 2019, 7, 103319. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liu, L.; Zhang, Y.; Yang, J.; Zeng, Z.; Deng, S. Controllable synthesis of bifunctional porous carbon for efficient gas-mixture separation and high-performance supercapacitor. Chem. Eng. J. 2018, 348, 57–66. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.; Wang, M.; Yao, L.; Qiao, W.; Long, D.; Ling, L. Direct capture of low-concentration CO2 on mesoporous carbon-supported solid amine adsorbents at ambient temperature. Ind. Eng. Chem. Res. 2015, 54, 5319–5327. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbon utilization of the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Zhu, X.; Tsang, D.C.W.; Wang, C.; Su, Z.; Hou, D.; Liangchun, L.; Shang, J. Machine learning exploration of the critical factors for CO2 adsorption capacity on porous carbon materials at different pressures. J. Clean. Prod. 2020, 263, 122915. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chane, W.; Mattern, D.L.; Hamner, N.; Raman, V.; Dorris, A. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Singh, J.; Bhunia, H.; Basu, S. Development of sulfur-doped carbon monolith derived from phenol-formaldehyde resin for fixed bed CO2 adsorption. Environ. Innov. 2020, 20, 101104. [Google Scholar] [CrossRef]
- Cueller-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilization technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2014, 9, 82–102. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Jafarinejad, S.; Rashidi, A. Lignocellulose-based adsorbents: A spotlight review of the effective parameters on carbon dioxide capture process. Chemosphere 2020, 246, 125746. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.H.; Li, J.R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 capture and separations using MOFs: Computational and experimental studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Wang, P.; Guo, Y.; Zhao, C.; Yan, J.; Lu, P. Biomass derived wood ash with amine modification for post-combustion CO2 capture. Appl. Energy 2017, 201, 34–44. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Najmi, B. Operation of Power Cycles with Integrated CO2 Capture Using Advances High-Temperature Technologies; Department of Energy and Process Engineering, Norwegian University of Science and Technology: Trondheim, Norway, 2015; p. 77. [Google Scholar]
- Zhang, Z.; Borhani, T.N.G.; El-Naas, M.H. Carbon Capture. In Exegetic and Environmental Dimensions; Academic Press, Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate-based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Carrasco-Maldonado, F.; Sporl, R.; Fleiger, K.; Hoenia, V.; Maier, J.; Scheffknecht, G. Oxy-fuel combustion technology for cement production-state of the art research and technology development. Int. J. Greenh. Gas Control 2016, 45, 189–199. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Song, X.; Shmulsky, R.; Hasgan, E.B. Microporous carbon nanoflakes derived from biomass cork waste for CO2 capture. Sci. Total Environ. 2020, 748, 142465. [Google Scholar] [CrossRef] [PubMed]
- Henry, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Seira, T.; Henni, A.; Sumon, K.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine-based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar]
- Singh, G.; Ismail, I.S.; Bilen, C.; Shanbhas, D.; Sathish, C.I.; Ramadass, K.; Vinu, A. A facile synthesis of activated porous carbon spheres from D-glucose using a non-corrosive activating agent for efficient carbon dioxide capture. Appl. Energy 2019, 255, 113831. [Google Scholar] [CrossRef]
- Khalilis, S.; Khoshandam, B.; Jahanshahi, M. Synthesis of activated carbon/polyaniline nanocomposites for enhanced CO2 adsorption. RCS Adv. 2016, 6, 35692–35704. [Google Scholar]
- Hu, H.; Zhang, T.; Yuan, S.; Tang, S. Functionalization of multi-walled carbon nanotubes with phenylenediamine for enhanced CO2 adsorption. Adsorption 2016, 23, 73–85. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Hu, X.; Wang, L.; Zhao, Y.; Lin, Y.; Sun, Y.; DaCosta, H.; Guo, L. Efficient CO2 capture by porous carbons derived from coconut shell. Energy Fuels 2017, 31, 4287–4293. [Google Scholar] [CrossRef]
- Auta, M.; Umaru, M.; Yahya, M.D.; Adeniyi, O.D.; Aris, I.M.; Suleiman, B. Diethanolamine functionalized waste tea activated carbon for CO2 adsorption. In Proceedings of the International Conference on Chemical, Environmental and Biological Sciences, Dubai, United Arab Emirates, 18–19 March 2015; pp. 96–99. [Google Scholar]
- Bamdad, H.; Hawboldt, K.A.; MacQuarre, S.C. Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels. 2018, 32, 11742–11748. [Google Scholar] [CrossRef]
- Kim, H.R.; Yoon, T.; Kim, S.; An, J.; Bae, Y.; Lee, C.Y. Beyond pristine MOFs: Carbon dioxide capture by metal-organic frameworks (MOFs)-derived porous carbon materials. R. Soc. Chem. 2017, 7, 1266–1270. [Google Scholar] [CrossRef]
- Yuan, X.; Li, S.; Jeon, S.; Deng, S.; Zhao, L.; Lee, K.B. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis. J. Hazard. Mater. 2020, 399, 123010. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Potential of pal, kernel shell as activated carbon precursors through single stage activated technique for carbon dioxide adsorption. J. Clean. Prod. 2017, 168, 474–486. [Google Scholar] [CrossRef]
- Das, S.; Meikap, B.C. Comparison of adsorbent capacity of mono-ethanolamine and diethanolamine impregnated activated carbon in a multi-staged fluidized bed reactor for carbon-dioxide capture. Fuel 2018, 224, 47–56. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Jalilov, A.S.; Ruan, G.; Hwang, C.; Schipper, D.E.; Tour, J.J.; Li, Y.; Fei, H.; Samuel, E.L.G.; Tour, J.M. Asphalt-derived high surface area activated porous carbons for carbon dioxide capture. ACS Appl. Mater. Interfaces 2015, 7, 1376–1382. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, Y.; Hagio, T.; Hu, Y.H. Surface-microporous graphene for CO2 adsorption. Catal. Today 2020, 356, 514–518. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Processing Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Gadipelli, S.; Patel, A.A.; Guo, Z. An ultrahigh pore volume drives up the amine stability and cyclic CO2 capacity of a solid-amine@carbon sorbent. Adv. Mater. 2015, 27, 4903–4909. [Google Scholar] [CrossRef]
- Gunathilake, C.A.; Ranathunga, G.G.T.A.; Dassanayake, R.S.; Illesighne, S.D.; Mnchanda, A.S.; Kalpage, C.S.; Rajapakshe, R.M.G.; Karunaratne, D.G.G.P. Emerging investigator series: Synthesis of magnesium oxide nanoparticles fabricated on a graph oxide nanocomposite for CO2 sequestration at elevated temperatures. R. Soc. Chem. 2020, 7, 1225–1239. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, C.M.; Abidov, A.; Palanichainy, M.; Jang, H.T. Facile synthesis of amine modified silica reduced graphene oxide composite sorbent for CO2 adsorption. Mater. Lett. 2019, 247, 44–47. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Lu, A. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Suo, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenh. Gases Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Xu, C.; Stromme, M. Sustainable porous carbon materials derived from wood-based biopolymers for CO2 capture. Nanomaterials 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Proc. 2011, 4, 562–567. [Google Scholar] [CrossRef]
- Nandi, M.; Uyama, H. Exceptional CO2 adsorbents materials under different conditions. Chem. Rec. 2014, 14, 1134–1148. [Google Scholar] [CrossRef]
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for efficient CO2 capture. Chem. Eng. J. 2018, 358, 1507–1518. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, S.; Wei, H.; Wang, B.; Huang, J.; Yu, G. Activated carbons and amine-modified materials for carbon dioxide capture—A review. Front. Environ. Sci. Eng. 2013, 7, 326–340. [Google Scholar] [CrossRef]
- Tiwari, D.; Geol, C.; Bhunia, H.; Bajpai, P.K. Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Geol, C.; Bhunia, H.; Bajpai, P.K. Novel nitrogen enriched porous carbon adsorbents for CO2 capture: Breakthrough adsorption study. J. Environ. Chem. Eng. 2016, 4, 346–356. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes towards highly efficient CO2 capture. Chem. Eng. J. 2018, 360, 250–259. [Google Scholar] [CrossRef]
- Durante, L.A.; Walton, K.S.; Soll, D.S.; Jones, C.W. CO2 capture via adsorption in amine-functionalized sorbents. Curr. Opin. Chem. Eng. 2016, 12, 82–90. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.; Wang, J.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Adsorption and regeneration study of polyethylenemine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture. Appl. Energy 2016, 168, 282–290. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Abdel-Salam, N.M.; Ghaffar, A. CVD synthesis, functionalization and CO2 adsorption attribute of multiwalled carbon nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef]
- Faisal, M.; Pamungkas, A.Z.; Krisnandi, Y.K. Study of Amine functionalized mesoporous carbon as CO2 storage materials. Processes 2021, 9, 456. [Google Scholar] [CrossRef]
- Cabriga, C.K.C.; Clarete, K.V.R.; Zhang, J.A.T.; Pacia, R.M.P.; Ko, Y.S.; Castro, J.C. Evaluation of biochar derived from the slow pyrolysis of rice straw as a potential adsorbent for carbon dioxide. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; He, Y. CO2 capture using solid sorbents. In Handbook of Climate Change Mitigation and Adaptation; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Gunathilake, C.; Manchanda, A.S.; Chrimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. Nano 2016, 3, 806–817. [Google Scholar] [CrossRef]
- Yao, M.; Wang, L.; Hu, X.; Hu, G.; Luo, M.; Fan, M. Synthesis of nitrogen-doped carbon with three-dimensional mesostructures for CO2 capture. J. Mater. Sci. 2015, 50, 1221–1227. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 311, 1919–1934. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, C.; Li, Y.; Lindh, J.; Stromne, M. High performance activated carbons synthesized from nanocellulose for CO2 capture and extremely selectivity removal of volatile organic compounds. Adv. Sustain. Syst. 2017, 2, 1700147. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Shamiri, A.; Aghamohammadi, N. Adsorption equilibrium of carbon dioxide onammonia-modified activated carbon. Chem. Eng. Res. Des. 2015, 104, 42–54. [Google Scholar] [CrossRef]
- Gomez-Pozuelo, G.; Sanz-Perez, E.S.; Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 adsorption on amine-functionalized clays. Microporous Mesoporous Mater. 2019, 282, 38–47. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Zarandi, S.M.; Masoundinejad, M.; Amini, M.M. Modelling and optimization by response surface technique for adsorption of carbon dioxide by aminated basilica/alginate composite: Experiments characterization and regeneration studies. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Sahequi, H.; Galvez, M.E.; Bacatirini, V.; Cheng, Y.; Steinfeld, A.; Zimmermann, T.; Tingant, P. Fast and reversible direct CO2 capture from air onto all-polymer nanofibrillated cellulose-polyethylenimine foams. Environ. Sci. Technol. 2015, 49, 3167–3174. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon aerogels for environmental clean-up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141. [Google Scholar] [CrossRef]
- Alveraz-Gutierrez, N.; Gil, M.V.; Rubiera, F.; Peviada, C. Kinetics of CO2 adsorption on cherry stone-based carbons in CO2/CH4 separations. Chem. Eng. J. 2017, 307, 249–257. [Google Scholar] [CrossRef]
- Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnihowski, J. Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 2017, 21, 436–443. [Google Scholar] [CrossRef]
- Marin, L.; Dragoi, B.; Olaru, N.; Perju, E.; Coroaba, A.; Doraftei, F.; Scavia, G.; Destri, S.; Zappia, S.; Porzro, W. Nanoporous furfuryl-imine-chitosan fibers as a new pathway towards eco-materials for CO2 adsorption. Eur. Polym. J. 2019, 120, 109214. [Google Scholar] [CrossRef]
- Linga, Z.; Kun, C.; Feng, Z.; Qunfeng, Y. Adsorption of CO2 and H2 on nitrogen-doped porous carbon from Ionic Liquid precursor. Chem. Res. Chin. Univ. 2015, 1, 130–137. [Google Scholar]
- Ma, X.; Li, L.; Wang, S.; Lu, M.; Li, H.; Ma, W.; Keener, T.C. Ammonia-treated porous carbon derived from ZIF-8 for enhanced CO2 adsorption. Appl. Surf. Sci. 2016, 369, 390–397. [Google Scholar] [CrossRef]
- Yang, M.; Guo, L.; Hu, G.; Hu, X.; Chen, J.; Shen, S.; Dai, W.; Fan, M. Adsorption of CO2 by petroleum coke nitrogen-doped porous carbons synthesized by combining ammoxidation with KOH activation. Am. Chem. Soc. 2016, 55, 757–765. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Datta, K.K.R.; Shiva, K.; Bhattacharyya, A.J.; Eswaramoortrhy, M.; Maji, T.K. Controlled synthesis of tunable nanoporous carbons for gas storage and supercapacitor application. Microporous Mesoporous Mater. 2015, 206, 127–135. [Google Scholar] [CrossRef]
- Psarras, P.; He, J.; Wilcox, J. Effect of water on the CO2 adsorption capacity of amine-functionalized carbon sorbents. Ind. Energy Chem. Res. 2017, 56, 6317–6325. [Google Scholar] [CrossRef]
- Jalilov, A.S.; Li, Y.; Tian, J.; Tour, J.M. Ultra-high surface area activated porous asphalt for CO2 capture through competitive adsorption at high pressures. Adv. Energy Mater. 2016, 7, 1600693. [Google Scholar] [CrossRef]
- Bai, B.C.; Kim, E.A.; Lee, C.W.; Lee, Y.; Im, J.S. Effects of surface chemical properties of activated carbon fibers modified by liquid oxidation for CO2 adsorption. Appl. Surf. Sci. 2015, 353, 158–164. [Google Scholar] [CrossRef]
- Laing, T.; Chen, C.; Li, X.; Zhang, J. Popcorn-derived porous carbon for energy storage and CO2 capture. Langmuir 2016, 32, 8042–8049. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Mi, L. Porous carbons derived from metal organic framework for gas storage and separation: The size effect. Inorg. Chem. Commun. 2020, 118, 107999. [Google Scholar] [CrossRef]
- Ludwinowicz, J.; Jaroniec, M. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 2015, 82, 297–303. [Google Scholar] [CrossRef]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosuwa, T.; Chen, S.; Bae, W.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-doped carbon as CO2 adsorbents with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2015, 138, 1001–1009. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Synthesis of nitrogen enriched porous carbons for urea formaldehyde resin and their carbon dioxide adsorption capacity. J. CO2 Util. 2017, 21, 302–313. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, S.; Quan, C.; Gao, N.; Johnston, C.; Wu, C. One-pot synthesis of digestate-derived biochar for carbon dioxide capture. Fuel 2020, 279, 118525. [Google Scholar] [CrossRef]
- Hong, S.; Yoon, H.J.; Choi, Y.; Cho, Y.; Mun, S.; Pol, U.S.; Lee, K.B. Solving two environmental problems simultaneously: Scalable production of carbon microsheets from structured packing peanuts with tailored microporosity for efficient CO2 capture. Chem. Eng. J. 2020, 379, 122219. [Google Scholar] [CrossRef]
- Wu, X.; Li, D.; Cheng, W.; Zhou, J.; Zhang, H. Progress in methods for preparation of monolith active carbons. Nanosci. Nanotechnol. Lett. 2017, 9, 839–848. [Google Scholar] [CrossRef]
- Sun, Y.; Sui, Z.; Li, X.; Xiao, P.; Wei, Z.; Han, B. Nitrogen-doped porous carbons derived from polypyrrole-based aerogels for gas uptake and supercapacitors. Appl. Nanomater. 2018, 1, 609–616. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Hashiso, Z.; Feizbakhshan, M.; Shariaty, P.; Wiknaddaf, S.; Zhou, X. Template-free and fast one-step synthesis from enzymatic hydrolysis lignin to hierarchical porous carbon for CO2 capture. Microporous Mesoporous Mater. 2019, 280, 57–65. [Google Scholar] [CrossRef]
- Tajer, M.; Anbia, M.; Salehi, S. Fabrication of polyacrylonitrile hybrid nanofiber scaffold containing activated carbon by electrospinning process as nanofilter media for SO2, CO2 and CH4 adsorption. Environ. Prog. Sustain. Energy 2020, 40, e13498. [Google Scholar]
- Li, L.; Wang, X.; Zhong, J.; Qian, X.; Song, S.; Zhang, Y.; Li, D. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Yun, S.; Lee, H.; Lee, W.; Park, H.S. Multiscale textured, ultralight graphene monoliths for enhanced CO2 and SO2 adsorption capacity. Fuel 2016, 174, 36–42. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Zhao, X.; Wu, M. Superior CO2, CH4 and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Jang, E.; Choi, S.W.; Lee, K.B. Effect of carbonization temperature on the physical properties and CO2 adsorption behavior of petroleum-coke derived porous carbon. Fuel 2019, 248, 85–92. [Google Scholar] [CrossRef]
- Tobi, A.R.; Dennis, J.O.; Zid, H.M.; Adekoya, A.A.; Yar, A.; Usman, F. Comparative analysis of physicochemical properties of physically activated carbon from palm bio-waste. J. Mater. Res. Technol. 2019, 8, 3688–3695. [Google Scholar] [CrossRef]
- Kuch, B.; Kapsi, M.; Veziri, C.; Athanasekou, C.; Pilatos, G.; Reddy, S.K.; Raj, A.; Karanikolas, G.N. Asphaltene-derived activated carbon and carbon nanotube membranes for CO2 separation. Energy Fuels 2018, 32, 11718–11730. [Google Scholar]
- Jalilov, A.S.; Li, Y.; Kittrell, C.; Tour, J.M. Increased CO2 selectivity of asphalt-derived porous carbon through introduction of water into pore spaces. Nat. Energy 2017, 2, 932–938. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Three-dimensional graphene-based porous adsorbents for post-combustion CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 7906–7916. [Google Scholar] [CrossRef]
- Liu, Y.; Sjjadi, S.; Chen, W.; Chatterjee, R. Ultrasound assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 2019, 247, 10–18. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel—Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.; Liu, J.; Ma, W. Electrospun phenolic resin-based carbon-based ultrafine fibers with abundant ultra-small micropores for CO2 adsorption. Chem. Eng. J. 2015, 276, 44–50. [Google Scholar] [CrossRef]
- Choma, J.; Marsszewski, M.; Osuchowiski, L.; Jagiello, J.; Dziura, A.; Jaroniec, M. Adsorption properties of activated carbons prepared from waste CDs and DVDs. Sustain. Chem. Eng. 2015, 3, 733–742. [Google Scholar] [CrossRef]
- Park, H.; Lee, C.H.; Cho, D.; Lee, C.; Park, J. Synthesis of porous carbon derived from poly (vinylidenefluoride) and its adsorption characteristics for CO2 and CH4. Microporous Mesoporous Mater. 2020, 299, 110121. [Google Scholar] [CrossRef]
- Ge, C.; Song, J.; Qin, Z.; Wang, J.; Fan, W. Polyurethane foam-based ultra-microporous carbons for CO2 capture. Appl. Mater. Interfaces 2016, 8, 18849–18859. [Google Scholar] [CrossRef]
- Gong, J.; Lin, H.; Grygiel, K.; Yuan, J. Main-chain poly (ionic liquid)-derived nitrogen-doped micro-mesoporous carbons for CO2 capture and selective aerobic oxidation of alcohols. Appl. Mater. Today 2017, 7, 159–168. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, C.; Xing, F.; Shi, W.; Meng, J.; Wan, H.; Guan, G. Constructing hierarchical porous N-doped carbon derived from poly (ionic liquids) with the multifunctional Fe-based template for CO2 adsorption. American Chemical Society. Omega 2021, 6, 7186–7198. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lin, H.; Antonietti, M.; Yuan, J. Nitrogen-doped porous carbon nanosheets derived from poly (ionic liquid): Hierarchical pore structures for efficient CO2 capture and dye removal. R. Soc. Chem. 2013, 4, 7313–7321. [Google Scholar] [CrossRef]
- Saha, D.; Bramer, S.E.V.; Orkoulas, G.; Ho, H.; Chen, J.; Henley, D.K. CO2 capture in lignin-derived and nitrogen doped hierarchical porous carbons. Carbon 2017, 121, 257–266. [Google Scholar] [CrossRef]
- Deneir, M.; Tessema, T.; Farghlay, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-derived heteroatom-doped porous carbons for supercapacitor and CO2 capture applications. Int. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Moroto-Vater, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Gao, A.; Guo, N.; Yan, M.; Li, M.; Wang, F.; Yang, R. Hierarchical porous carbon activated by CaCO3 from pigskin collagen for CO2 and H2 adsorption. Microporous Mesoporous Mater. 2017, 260, 172–179. [Google Scholar] [CrossRef]
- Li, Y.; Wnag, X.; Wnag, B.; Cao, J.; Yang, J.; Wei, J. Waste wool derived nitrogen-doped hierarchical porous carbon for selective CO2 capture. R. Soc. Chem. 2018, 8, 19818–19826. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, C.; Kook, J.; Kim, J.H.; Hwang, K.; Lee, J. Amine functionalized wheat bran husk as bio-based organic adsorbent for low-density polyethylene composite of carbon dioxide capture. Macromol. Res. 2020, 28, 1289–1296. [Google Scholar] [CrossRef]
- Diez, N.; Alveraz, P.; Granda, M.; Blanco, C.; Santamaria, R.; Menendez, R. CO2 adsorption capacity and kinetics in nitrogen-enriched activated carbon fibers prepared by different methods. Chem. Eng. J. 2015, 281, 704–712. [Google Scholar] [CrossRef]
- Liu, J.; Jin, B.; Meng, L.; Lee, K. Synthesis of polypyrrole-based nitrogen-containing porous carbon nanotubes for CO2 adsorption. Carbon Lett. 2018, 28, 111–115. [Google Scholar]
- Arami-Niya, A.; Rufford, T.E.; Zhu, Z. Activated carbon monoliths with hierarchical pore structure from tar pitch and coal powder for the adsorption of CO2, CH4 and N2. Carbon 2016, 103, 115–124. [Google Scholar] [CrossRef]
- Gao, S.; Ge, L.; Rufford, T.E.; Zhu, Z. The preparation of activated carbon discs from tar pitch and coal powder for adsorption of CO2, CH4 & N2. Microporous Mesoporous Mater. 2016, 238, 19–26. [Google Scholar]
- Ganesan, A.; Shaijumon, M.M. Activated graphene derived porous carbon with exceptional gas adsorption properties. Microporous Mesoporous Mater. 2015, 220, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, T.C.; Yuan, S.; Liang, B. N-doped porous carbon derived from rGO-incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. J. Power Sources 2020, 472, 228610. [Google Scholar] [CrossRef]
- Shao, L.; Wang, S.; Liu, M.; Huang, J.; Liu, Y. Triazine-based hyper-cross-linked polymers derived porous carbons for CO2 capture. Chem. Eng. J. 2018, 339, 509–518. [Google Scholar] [CrossRef]
- Liu, S.; Rao, L.; Yang, P.; Wang, X.; Wang, L.; Ma, R.; Yue, L.; Hu, X. Superior CO2 uptake on nitrogen doped carbonaceous adsorbents from commercial phenolic resin. J. Environ. Sci. 2020, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lu, T.; Shao, J.; Hung, J.; Hu, X.; Wang, L. Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption. Sep. Purif. Technol. 2022, 281, 119899. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, C.; Chen, F.; Xu, G.; Pang, R.; Qiao, X.; Shao, J.; Hu, X. Water caltrop shell-derived nitrogen-doped porous carbons with high CO2 adsorption capacity. Biomass Bioenergy 2021, 145, 105969. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liu, G.; Yan, Q. In-situ pyrolysis of Taihu blue algae biomass as appealing porous carbon adsorbent for CO2 capture: Role of the intrinsic N. Sci. Total Environ. 2021, 771, 145424. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Wang, L.; Ma, R.; Zou, J.; Huang, L.; Hu, X. Facile single-step synthesis of porous carbons as efficient CO2 adsorbents. Energy Fuels 2019, 33, 11544–11551. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, M.S.; Yoo, W.C. Highly enhanced gas sorption capacities of N-doped porous carbon spheres by hot NH3 and CO2 treatments. J. Phys. Chem. 2015, 119, 28512–28522. [Google Scholar] [CrossRef]
- Rao, L.; Liu, S.; Chen, J.; Wang, L.; An, L.; Yang, P.; Hu, X. Single step synthesis of nitrogen-doped porous carbons for CO2 capture by low-temperature sodium amide activation of petroleum coke. Energy Fuels 2018, 32, 12787–12794. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitors and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Hu, X.; Wang, L.; Wang, X.; Radosz, M.; Fan, M. CO2 adsorption on hazelnut-shell derived nitrogen-doped porous carbons synthesized by single-step sodium amine activation. Ind. Eng. Chem. Res. 2019, 59, 7046–7053. [Google Scholar] [CrossRef]
- Rao, L.; Yue, L.; Wang, L.; Wu, Z.; Ma, C.; An, L.; Hu, X. Low-temperature and single-step synthesis of N-doped porous carbons with a high CO2 adsorption performance by sodium amide activation. Energy Fuels 2018, 32, 10830–10837. [Google Scholar] [CrossRef]
- Liu, S.; Yang, P.; Wang, L.; Li, Y.; Wu, Z.; Ma, R.; Wu, J.; Hu, X. Nitrogen doped porous carbons from lotus leaf for CO2 capture and supercapacitor electrodes. Energy Fuels 2019, 33, 6568–6576. [Google Scholar] [CrossRef]
- Rao, L.; Liu, S.; Wang, L.; Ma, C.; Wu, J.; An, L.; Hu, X. N-doped porous carbons from low-temperature and single-step sodium amide activation of carbonized water chestnut-shell with excellent CO2 capture performance. Chem. Eng. J. 2018, 359, 428–435. [Google Scholar] [CrossRef]
- Wang, L.; Rao, L.; Xia, B.; Wang, L.; Yue, L.; Liang, Y.; DaCosta, H.; Hu, X. Highly efficient CO2 adsorption bu nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation. Carbon 2018, 130, 31–40. [Google Scholar] [CrossRef]
- Yu, D.; Hu, J.; Zhou, L.; Li, J.; Tang, J.; Peng, C.; Liu, H. Nitrogen-doped coal tar pitch based microporous carbons with superior CO2 capture performance. Energy Fuels 2018, 32, 3726–3732. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. Carbon 2020, 159, 625–637. [Google Scholar] [CrossRef]
- Fujiki, J.; Yogo, K. Increased CO2 adsorption performance of chitosan derived activated carbon with nitrogen-doping. Chem. Comm. 2016, 52, 186–189. [Google Scholar] [CrossRef]
- Singh, G.; Tiburcius, S.; Ruban, S.M.; Shanbhas, D.; Sathish, C.I.; Kamadass, K.; Vinu, A. Pure and strontium carbonate nanoparticles functionalized microporous carbons with high specific surface areas derived from chitosan for CO2 adsorption. Emergent Mater. 2019, 2, 337–349. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Duan, H.; Li, Y.; Xu, J.; Huang, Y.; Liu, B.; Zhang, T. Polyvinyl chloride-derived carbon spheres for CO2 adsorption. ChemSusChem 2020, 13, 1–8. [Google Scholar]
- Politakos, N.; Barbarn, I.; Cantador, L.S.; Cecilia, J.A.; Mehrarar, E.; Tamovska, R. Grpahene-based monolirhoc nanostructures for CO2 capture. Ind. Eng. Chem. Res. 2020, 58, 3349–3358. [Google Scholar]
- Li, P.; Zeng, H.C. Hierarchical nanocomposites by integrating reduced graphene oxide and amorphous carbon with ultrafine MgO nanocrystallites for enhanced CO2 capture. Environ. Sci. Technol. 2017, 51, 12998–13007. [Google Scholar] [CrossRef]
- Bai, R.; Yang, M.; Hu, G.; Xu, L.; Hu, X.; Li, Z.; Wang, S.; Dai, W.; Fan, M. A new nanoporous nitrogen-doped highly-efficient carbonaceous CO2 sorbent synthesized with inexpensive urea and petroleum coke. Carbon 2015, 81, 465–473. [Google Scholar] [CrossRef]
- Wang, W.; Motuzas, J.; Zhao, X.S.; Costa, J.C.D. 2D/3D assembles of amine-functionalized graphene silica (templated) aerogel for enhanced CO2 sorption. Appl. Mater. Interfaces 2019, 11, 30391–30400. [Google Scholar] [CrossRef]
- Plaza, M.G.; Duran, I.; Querejeta, N.; Rubiera, F.; Pevida, C. Experimental and Simulation Study of Adsorption in Post combustion Conditions using a Microporous Biochar.2.H2O, CO2 and N2 Adsorption. Ind. Eng. Chem. Res. 2016, 55, 6854–6865. [Google Scholar] [CrossRef]
- Yue, L.; Rao, L.; Wang, L.; Sun, Y.; Wu, Z.; DaCosta, H.; Hu, X. Enhanced CO2 adsorption on nitrogen-doped porous carbons derived from commercial phenolic resin. Energy Fuels 2018, 32, 2081–2088. [Google Scholar] [CrossRef]
- Gong, J.; Antonietti, M.; Yuan, J. Poly (ionic liquid)-derived carbon with site-specific N-doping and Biphasic Heterojunction for enhanced CO2 capture and sensing. Angewandate Chem. 2017, 129, 7665–7671. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Ruofei, C.; Chunhao, W.; Li, H.; Shaobin, W. Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Appl. Surf. Sci. 2017, 435, 494–502. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Li, H.; Li, H. Highly nitrogen-doped porous carbon derived from zeolitic imidazolate framework-8 for CO2 capture. Chem. Asian J. 2018, 13, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Borchardts, L.; Zhu, Q.; Casco, M.E.; Berger, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Li, Z.; Chen, T.; Wu, X.; Luo, L.; Zhang, Z.; Li, Z.; Fan, M.; Su, Z.; Zhao, W. Nitrogen-containing high surface area carbon cryogel from co-condensed phenol-urea-formaldehyde resin for CO2 capture. J. Porous Mater. 2018, 26, 847–854. [Google Scholar] [CrossRef]
- Zohdi, S.; Anbia, M.; Salehi, S. Improved CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity in novel hollow silica particles by modification with multi-walled carbon nanotubes containing amine groups. Polyhedron 2019, 166, 175–185. [Google Scholar] [CrossRef]
- You, Y.Y.; Liu, X.J. Modelling of CO2 Adsorption and Recovery from wet Flue Gas by Using Activated Carbon. Chem. Eng. J. 2019, 369, 672–685. [Google Scholar] [CrossRef]
- Plaza, M.G.; Gonzalez, A.S.; Rubeira, F.; Pevida, C. Evaluation of Microporous biochars produced by single-step oxidation for post combustion CO2 capture under humid conditions. Energy Procedia 2014, 63, 693–702. [Google Scholar] [CrossRef]
- Lou, Y.; Qi, S.; Xue, D.; Gu, C.; Zhou, R.; Liu, X.; Sun, L. Solvent-free synthesis of N-containing polymers with high cross-linking degree to generate N-doped porous carbons for high-efficiency CO2 capture. Chem. Eng. J. 2020, 399, 125845. [Google Scholar] [CrossRef]
- Das, D.; Meikap, B.C. Role of amine-impregnated activated carbon in carbon dioxide capture. Indian Chem. Eng. 2020, 63, 425–447. [Google Scholar] [CrossRef]
- Chai, S.; Liu, Z.; Huang, K.; Tan, S.; Dai, S. Amine-functionalization of microsized and nanosized mesoporous carbons for carbon dioxide capture. Ind. Eng. Chem. Res. 2016, 55, 7355–7361. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the begening of 2013—A Review. Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Ohs, B.; Krodel, M.; Kiessimg, M. Adsorption of carbon dioxide on solid amine-functionalized sorbents: A dual kinetic model. Sep. Purif. Technol. 2018, 204, 13–20. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Zhang, J.; Zhong, F.; Wu, P.; Huang, K.; Fan, J.; Liu, F. Chitosan-derived mesoporous carbon with ultrahigh pore volume for amine-impregnated and highly efficient CO2 capture. Chem. Eng. J. 2018, 359, 1159–1165. [Google Scholar] [CrossRef]
- Das, D.; Samal, D.P.; Meikap, B.C. Removal of CO2 in a multistage fluidized bed reactor by diethanol amine impregnated activated carbon. J. Environ. Sci. Health Part A 2016, 51, 769–775. [Google Scholar] [CrossRef]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.; Gunathilake, C.; Jaroniec, M. CO2 adsorption on amine-functionalized periodic mesoporous benzenesilicas. Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef]
- Didas, S.A.; Kulkrani, A.R.; Sholl, D.S.; Jones, C.W. Role of amine structure on carbon dioxide adsorption from ultra dilute gas streams such as ambient air. ChemSusChem. 2012, 5, 2058–2064. [Google Scholar] [CrossRef]
- Gholidoust, A.; Atkinson, J.D.; Hashisho, Z. Enhancing CO2 adsorption via amine impregnated activated carbon from oil sands coke. Energy Fuels 2017, 31, 1756–1763. [Google Scholar] [CrossRef]
- Chen, L.; Gong, M.; Cheng, Y.; Liu, Y.; Yin, S.; Luo, D. Effect of pore structure of supports on CO2 adsorption of tetraethylenepentamine/carbon aerogels prepared by incipient wetness impregnation method. Pol. J. Environ. Stud. 2019, 28, 4127–4137. [Google Scholar] [CrossRef]
- Kongnoo, A.; Intharapat, P.; Worathanakul, P.; Phalakormkule, C. Diethanolamine impregnated palm shell activated carbon for CO2 adsorption at elevated temperatures. J. Environ. Chem. Eng. 2015, 4, 73–81. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.; Park, S. Preparation and characterization of multi-walled carbon nanotubes impregnated with polyethylenimine for carbon dioxide capture. Int. J. Hydrogen Energy 2015, 40, 3415–3421. [Google Scholar] [CrossRef]
- Gibson, J.A.A.; Gromov, A.V.; Brandani, S.; Camphell, E.E.B. The effect of pore structure on the CO2 adsorption efficiency of polyamine impregnated porous carbons. Microporous Mesoporous Mater. 2015, 208, 129–139. [Google Scholar] [CrossRef]
- Numaguchi, R.; Fujiki, J.; Yamada, H.; Chowdhury, F.A.; Kida, K.; Goto, K.; Okumura, T.; Yoshizawa, K.; Yogo, K. Development of post-combustion CO2 capture system using amine-impregnated solid sorbent. Energy Procedia 2017, 114, 2304–2312. [Google Scholar] [CrossRef]
- Pen, H.; Zhong, F.; Zhang, J.; Zhang, J.; Wu, P.; Huang, K.; Fan, J.; Jiang, L. Graphitic carbon nitride functionalized with polyethylenimine for highly effective capture of carbon dioxide. Ind. Eng. Chem. Res. 2018, 57, 11031–11038. [Google Scholar]
- Yaumi, A.L.; Bakar, M.Z.; Hameed, B.H. Melamine-nitrogenated mesoporous activated carbon derived from rice husk for carbon dioxide adsorption in fixed-based. Energy 2018, 155, 46–55. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.M.; Hong, W.G.; Huh, Y.S.; Park, S.Y.; Lee, S.C.; Lee, J.; Lee, J.B.; Lee, H.U.; Kim, H.J. Carbon dioxide capture on primary amine groups entrapped in activated carbon at low temperatures. J. Ind. Eng. Chem. 2014, 23, 16–20. [Google Scholar] [CrossRef]
- Lee, M.; Park, S. Silica-coated multi-walled carbon nanotubes impregnated with polyethylenimine for carbon dioxide capture under the flue gas conditions. J. Solid-State Chem. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Alhassan, M.; Auta, M.; Sabo, J.K.; Umaru, M.; Kovo, A.S. CO2 capture using amine-impregnated activated carbon from Jatropha curas shell. Br. J. Appl. Sci. Technol. 2016, 14, 1. [Google Scholar] [CrossRef]
- Luo, S.; Chen, S.; Chen, S.; Zhuang, L.; Ma, N.; Xu, T.; Li, Q.; Hou, X. Preparation and characterization of amine-functionalized sugarcane bagasse for CO2 capture. J. Environ. Manag. 2016, 168, 142–148. [Google Scholar] [CrossRef]
- Ali, U.F.M.; Azmi, N.H.; Isa, K.M.; Aroua, M.K.; Shien, T.R.; Khamidun, M.H. Optimization study on preparation of amine functionalized sea mango (Cerbia Odollam) activated carbon for carbon dioxide (CO2) adsorption. Combust. Sci. Technol. 2018, 190, 1259–1282. [Google Scholar] [CrossRef]
- Sreedhar, I.; Aniruddha, R.; Malik, S. Carbon capture using amine modified porous carbons derived from starch (starbons). SN Appl. Sci. 2019, 1, 463. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Song, M.; Xin, C.; Zeng, W. Tetraethylenepentamine-modified activated semi coke for CO2 capture from the flue gas. Energy Fuels 2017, 31, 3055–3061. [Google Scholar] [CrossRef]
- Shin, G.; Rhee, K.; Park, S. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrog. Energy 2016, 41, 14351–14359. [Google Scholar] [CrossRef]
- Ardhyarini, N.; Krisnandi, Y.K. Carbon dioxide capture by activated methyl diethanol amine impregnated mesoporous carbon. Int. Symp. Curr. Prog. Math. Sci. 2017, 1862, 0300090. [Google Scholar]
- Pruna, A.; Carcel, A.C.; Benedito, A.; Gimenez, E. Effect of synthesis conditions on CO2 capture of ethylenediamine-modified graphene aerogels. Appl. Surf. Sci. 2019, 487, 228–235. [Google Scholar] [CrossRef]
- Chi, Y.Z.Y.; Liu, C.Z.Y.; Zhao, Y.; Jiang, L.; Song, Y. CO2 adsorption behaviour of graphite oxide modified with Tetraethylenepentamine. J. Chem. Eng. 2018, 63, 202–207. [Google Scholar]
- Iqbal, N.; Wang, X.; Yu, J.; Ding, B. Robust and flexible carbon nanofibers doped with amine functionalized carbon nanotubes for efficient CO2 capture. Adv. Sustain. Syst. 2017, 1, 1600028. [Google Scholar] [CrossRef]
- Lourenco, M.A.O.; Fontana, M.; Jagdale, P.; Pirris, C.F.; Bocchini, S. Improved CO2 adsorption properties through amine functionalization of multi-walled carbon nanotubes. Chem. Eng. J. 2021, 414, 128763. [Google Scholar] [CrossRef]
- Park, J.M.; Woo, H.C.; Jhung, S.H. Effective CO2 adsorption of low pressure over nitrogen-enriched porous carbons derived from melamine-loaded polyaniline. Chem. Eng. J. 2021, 412, 128641. [Google Scholar] [CrossRef]
- Peng, A.; Qi, S.; Liu, X.; Xue, D.; Peng, S.; Yu, G.; Liu, X.; Sun, L. Fabrication of N-doped porous carbons for enhanced CO2 capture: Rational design of an ammoniated polymer precursor. Chem. Eng. J. 2019, 369, 170–179. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zheng, M.; Zhao, H.; Zhao, Y.; Sun, Z. Hierarchical structured porous nitrogen-doped carbon for highly selective CO2 capture. Sustain. Chem. Eng. 2015, 4, 298–304. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, Y.; Bao, A. Preparation of nitrogen-doped hierarchical porous carbon materials by a template-free method and its application to CO2 capture. J. Environ. Chem. Eng. 2020, 8, 103732. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and efficient nitrogen modifies porous carbon derived from sugarcane bagasse for CO2 capture: Experimental and DFT investigations of nitrogen atoms on carbon frameworks. Chem. Eng. J. 2019, 382, 122964. [Google Scholar]
- Keller, L.; Chs, B.; Lenhart, J.; Abduly, L.; Blanke, F.; Wessling, M. High capacity polyethylenimine impregnated microtubes made of carbon nanotubes for CO2 capture. Carbon 2017, 126, 338–345. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, B.; Shi, J.; Tao, M.; He, Y.; Shi, Y. Enhanced Tolerance to Flue Gas Contaminants on Carbon Dioxide Capture Using Amine-Functionalized Multiwalled Carbon Nanotubes. Energy Fuels 2014, 28, 6494–6501. [Google Scholar] [CrossRef]
- Ning, H.; Yang, Z.; Wang, D.; Meng, Z.; Li, Y.; Ju, X.; Wang, C. Graphene-based semi-coke porous carbon with N-rich hierarchical sandwich-like structure for efficient separation of CO2/N2. Microporous Mesoporous Mater. 2021, 311, 110700. [Google Scholar] [CrossRef]
- Du, N.; Ma, R.; Liu, Z.; Yang, G.; Chen, J. Study on the adsorption properties of graphene oxide/Laponite RD/chitosan composites. Materials 2021, 14, 3224. [Google Scholar] [CrossRef]
- Andreoli, E.; Cullum, L.; Barron, A.R. Carbon dioxide adsorption by polyethylenimine-functionalized nanocarbons: A kinetic study. Ind. Eng. Chem. Res. 2014, 54, 878–889. [Google Scholar] [CrossRef]
- Ye, Q.; Jiang, J.; WANG, c.; Liu, Y.; Pan, H.; Shi, Y. Adsorption of Low-Concentration Carbon Dioxide on Amine-Modified Carbon Nanotubes at Ambient Temperature. Energy Fuels 2012, 26, 2497–2504. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers Manag. 2018, 49, 346–356. [Google Scholar] [CrossRef]
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattison, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Barandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control 2015, 40, 126. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on reactor Configurations for Adsorption-Based CO2 capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Chalmers, H.; Leach, M.; Lucquiaud, M.; Gibbins, J. Valuing flexible operation of power plants with CO2 capture. Energy Procedia 2009, 1, 4289–4296. [Google Scholar] [CrossRef]
- Normann, F.; Gardarsdottir, S.O.; Skagestad, R.; Mathisen, A.; Johnsson, F. Partial capture of Carbon Dioxide from Industrial Sources-A discussion on Cost optimization and the CO2 Capture Rate. Energy Procedia 2017, 114, 113–121. [Google Scholar] [CrossRef]
- Auta, M.; Darbis, N.D.A.; Din, A.T.M.; Hamed, B.H. Fixed-bed column adsorption of carbon dioxide by sodium hydroxide modifies activated alumina. Chem. Eng. J. 2013, 233, 80–87. [Google Scholar] [CrossRef]
- Webley, P.A.; Zhang, J. Microwave assisted vacuum regeneration for CO2 capture from wet flue gas. Adsorption 2014, 20, 201–210. [Google Scholar] [CrossRef]
- Luberti, M.; Oreggion, G.D.; Ahn, H. Design of rapid vacuum pressure swing adsorption (RVPSA) process for post-combustion CO2 capture from a biomass-filled CHP plant. J. Environ. Chem. Eng. 2017, 5, 3973–3982. [Google Scholar] [CrossRef]
- Grande, C.A.; Ribeiro, R.P.L.; Olivera, E.G.L.; Rodrigues, A.E. Electric swing adsorption as emerging CO2 capture technology. Energy Procedia 2009, 1, 1219–1225. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Evaluating pressure swing adsorption as a CO2 separation technique in coal-fired power plants. Int. J. Greenh. Gas Control 2015, 39, 1–16. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Pre-combustion captuire of carbon dioxide in a fixed bed reactor using the clathrate hydrate process. Energy 2013, 50, 364–373. [Google Scholar] [CrossRef]
- Dantas, T.L.P.; Luna, I.M.T.; Sivar, J.I.J.; Tores, A.E.B.; de-Azevedo, D.C.S.; Rodrigues, A.E.; Moreira, R.F.P.M. Modeling of the fixed-bed adsorption of carbon dioxide and a carbon-dioxide-nitrogen mixture on zeolite 13X. Braz. J. Chem. Eng. 2011, 28, 533–544. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Kong, X.; Li, P.; Yu, J.; Rodrigues, A.E. Onsite CO2 capture from flue gas by an adsorption process in a coal-fired power plant. Ind. Eng. Chem. Res. 2012, 51, 7355–7363. [Google Scholar] [CrossRef]
- Joss, L.; Gazzani, M.; Mazzotti, M. Rational design of temperature swing adsorption cycles for post-combustion CO2 capture. Chem. Eng. Sci. 2017, 158, 381–394. [Google Scholar] [CrossRef]
- Razaei, F.; Webley, P. Structured adsorbents in gas separation process. Sep. Purif. Technol. 2010, 70, 243–256. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Thaeron, C. Performance of a parallel passage adsorbent contactor. Gas. Sep. Purif. 1996, 10, 63–73. [Google Scholar] [CrossRef]
- Razaei, F.; Grahn, M. Thermal management of structured adsorption in CO2 capture process. Ind. Eng. Chem. Res. 2012, 51, 4025–4034. [Google Scholar] [CrossRef]
- Lively, R.P.; Chance, R.R.; Kelley, B.T.; Deckman, H.W.; Drese, J.H.; Jones, C.W.; Koros, W.J. Hollow fiber adsorbents for CO2 removal from flue gas. Ind. Eng. Chem. Res. 2009, 48, 7314–7324. [Google Scholar] [CrossRef]
- Plaza, M.G.; Rubiera, F.; Penda, C. Evaluating the feasibility of a TSA process based on steam stripping in combination with structured carbon adsorbent to capture CO2 from a coal power plant. Energy Fuels 2017, 31, 9760–9775. [Google Scholar] [CrossRef]
- Shen, C.; Liu, Z.; Li, P.; Yu, J. Two-stage VPSA process for CO2 capture from flue gas using Activated carbon beads. Ind. Eng. Chem. Res. 2012, 51, 5011–5021. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide caoture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-doped porous carbons with exceptionally high CO2 selectivity for CO2 Capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, Y.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Kim, K.; Son, Y.; Lee, K.S. Moving bed adsorption process with internal heat integration for carbon dioxide capture. Int. J. Greenh. Gas Control 2013, 17, 13–24. [Google Scholar] [CrossRef]
- Mondino, G.; Grande, C.A.; Blom, R.; Nord, L.O. Moving bed temperature swing adsorption for CO2 capture from a natural gas combined cycle power plant. Int. J. Greenh. Gas Control 2019, 85, 58–70. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Performance of polyethylenimine-silica adsorbents for post-combustion CO2 capture in a bubbling fluidized bed. Chem. Eng. J. 2014, 251, 293–303. [Google Scholar] [CrossRef]
- Hornbostel, M.C.; Bao, J.; Krishnan, G.; Nagar, A.; Jyaweera, I.; Kobayashi, T.; Sanjurjo, A.; Sweeney, J.; Carruttler, D.; Petruska, M.; et al. Characterizatiom of an advanced carbon sorbent for CO2 capture. Carbon 2013, 56, 77–85. [Google Scholar] [CrossRef]
- Okummura, T.; Ogino, T.; Nishihe, S.; Nonaka, Y.; Shoji, T.; Higashi, T. CO2 Capture test for a moving bed system utilizaing low-temperature steam. Energy Procedia 2014, 63, 2249–2254. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaoubout, A.; Cloete, S.; Seo, H.; Park, Y.K.; Demoulin, L.; Amini, S. Demonstration of the novel swing adsorption reactor cluster concept in multistage fluidized bed with heat transfer surface for post-combustion CO2 capture. Ind. Eng. Chem. Res. 2020, 59, 22281–22291. [Google Scholar] [CrossRef]
- MCDonogh, J.R.; Law, R.; Reany, D.A.; Zivkovic, V. Intensified carbon capture using adsorption. Heat Prog. 2018, 8, 17–30. [Google Scholar]
- Monazam, E.R.; Spenk, J.; Shadi, L.J. Fluid bed adsorption of carbon dioxide on immobilized polyethyleneimine (PEI): Kinetic analysis and breakthrough behaviour. Chem. Eng. J. 2013, 223, 795–805. [Google Scholar] [CrossRef]
- Yashoobi-Khankhanej, S.; Alizadeh, R.; Zarghami, R. Adsorption modeling of CO2 in fluidized bed reactor. Chem. Eng. Res. Des. 2018, 129, 111–121. [Google Scholar]
- Raganati, F.; Chirone, R.; Ammmendola, P. Calcium-looping for thermomechanical energy storage in concentrating solar power applications: Evaluation of the effect of acoustic perturbation on the fluidized bed carbonation. Chem. Eng. J. 2020, 392, 123658. [Google Scholar] [CrossRef]
- Hofer, G.; Schony, G.; Fuchi, J.; Proli, T. Investigating wall-to-bed heat transfer in view of a continuous temperature swing adsorption process. Fuel Process Technol. 2018, 169, 157–159. [Google Scholar] [CrossRef]
- Yang, W.C.; Hoffman, J. Exploration Design Study on Reactor Configurations for Carbon Dioxide Capture from Conventional Power Plants Employing Regenerable Solid Sorbents. Ind. Eng. Chem. Res. 2009, 48, 341–356. [Google Scholar] [CrossRef]
- Seo, Y.; Jo, S.H.; Ryu, H.J.; DalBae, H.; Ryu, C.K.Y. Effect of water pre-treatment on CO2 capture using a potassium-based solid sorbent in a bubbling fluidized bed reactor. Korean J. Chem. Eng. 2007, 24, 457–460. [Google Scholar] [CrossRef]
- Yi, C.K.; Jo, S.H.; Ryu, H.J.; Yoo, Y.W.; Lee, J.B.; Ryu, C.K.; Rubin, E.S.; Keith, D.W.; Gilboy, C.F.; Wilson, M.; et al. CO2 reaction characteristics of dry sorbents in fluidized reactors. Greenh. Gas Control Technol. 2005, 7, 1765–1769. [Google Scholar]
- Das, D.; Meikap, B.C. Removal of CO2 in a multistage fluidized bed reactor by monoethanolamine impregnated activated carbon. Miner. Processing Extr. Metall. 2019. [Google Scholar] [CrossRef]
- Varma, Y.B.G. Pressure drop of the fluid and the flow patterns of the phases in multistage fluidization. Power Technol. 1975, 12, 167–174. [Google Scholar] [CrossRef]
- Schony, G.; Zehetner, E.; Fuchi, J.; Proll, T.; Sprachmann, G.; Hofbauer, H. Design of a bench scale unit for continuous CO2 capture via temperature swing adsorption-Fluid Dynamic feasibility study. Chem. Eng. Res. Des. 2016, 106, 155–167. [Google Scholar] [CrossRef]
- Zaabout, A.; Romano, M.C.; Cloete, S.; Guiffrida, A.; Morud, J.; Chiesa, P.; Amini, S. Thermodynamic assessment of the swing adsorption reactor cluster (SARC) concept for post-combustion CO2 capture. Int. J. Greenh. Gas Control 2017, 60, 74–92. [Google Scholar] [CrossRef]
- Cloete, S.; Guiffrida, A.; Romano, M.C.; Zaabout, A. The swing adsorption reactor cluster for post-combustion CO2 capture from cement plants. J. Clean. Prod. 2019, 223, 692–703. [Google Scholar] [CrossRef]
- Cloete, S.; Guiffrida, A.; Romano, M.C.; Zaabout, A. The effect of sorbent regeneration enthalpy on the performance of the novel swing adsorption reactor cluster (SARC) for post-combustion CO2 capture. Chem. Eng. J. 2018, 377, 119810. [Google Scholar] [CrossRef]
- Zaabout, A.; Romano, M.C.; Cloete, S.; Guiffrida, A.; Morud, J.; Chiesa, P.; Amini, S.A. Novel swing adsorption reactor cluster (SARC) for cost effective post combustion CO2 capture: A Thermodynamic Assessment. Energy Procedia 2017, 114, 2488. [Google Scholar] [CrossRef][Green Version]
- Cloete, S.; Guiffrida, A.; Romano, M.C.; Zaabout, A. Economic assessment of the swing adsorption reactor cluster for CO2 capture from cement production. J. Clean. Prod. 2020, 275, 123024. [Google Scholar] [CrossRef]
- Das, D.; Behran, K.B.; Meikap, B.C. Removal of CO2 in a multistage fluidized bed reactor by amine impregnated activated carbon: Optimization using response surface methodology. Int. J. Coal. Sci. Technol. 2019, 6, 445–458. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. CO2 adsorption on fine activated carbon in a sound assisted fluidized bed: Effect of sound intensity and frequency, CO2 partial pressure and fluidization velocity. Appl. Energy 2014, 113, 1269–1282. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R. Effect of operating conditions on the CO2 recovery from a fine activated carbon by means of TSA in a fluidized bed assisted by acoustic fields. Fuel Processing Technol. 2015, 134, 494–501. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. Effect of acoustic field on CO2 desorption in a fluidized bed on fine activated carbon. Particuology 2015, 23, 8–15. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. On improving the CO2 recovery efficiency of a conventional TSA process in a sound assisted fluidized bed by separating heating and purging. Sep. Purif. Technol. 2016, 167, 24–31. [Google Scholar] [CrossRef]
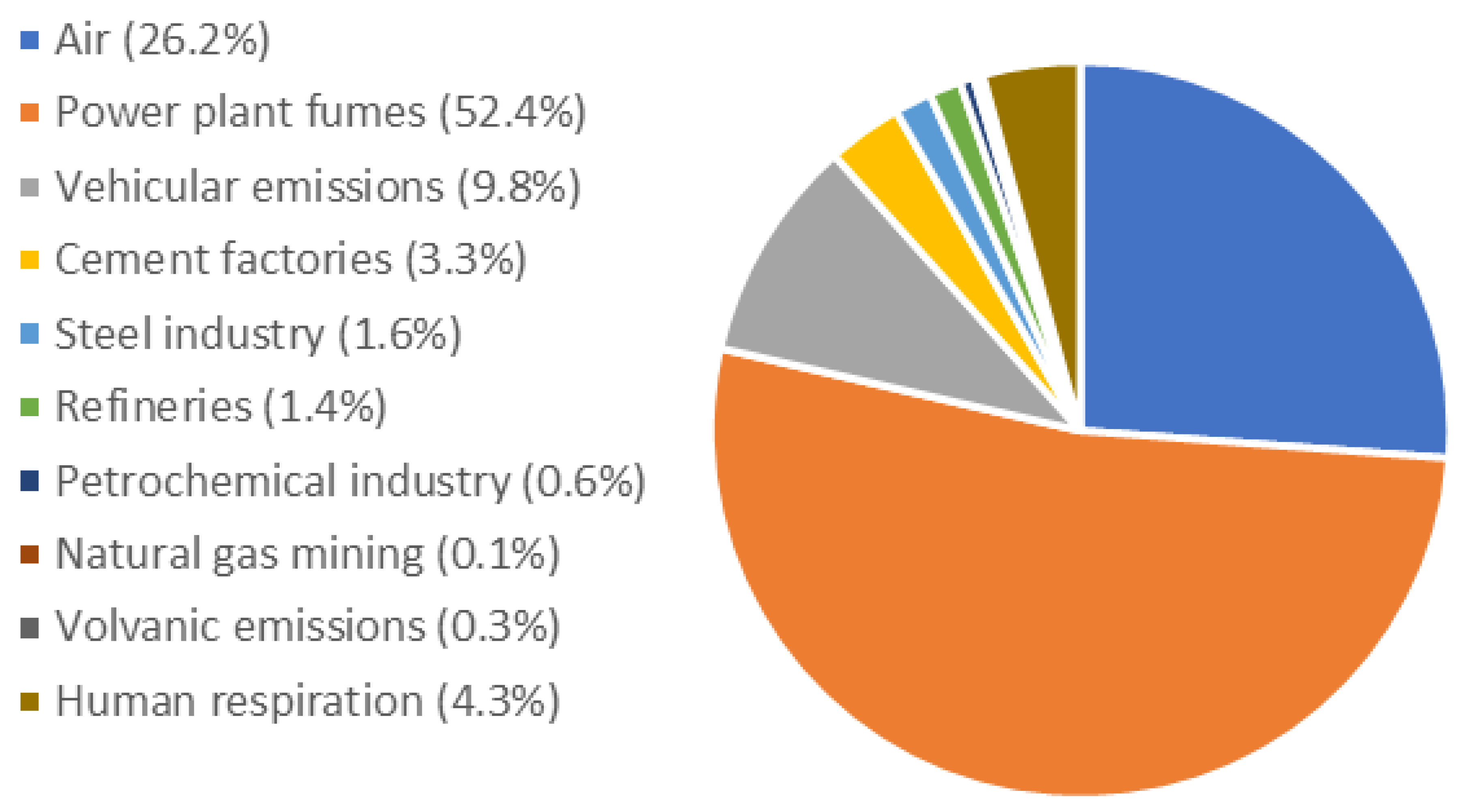
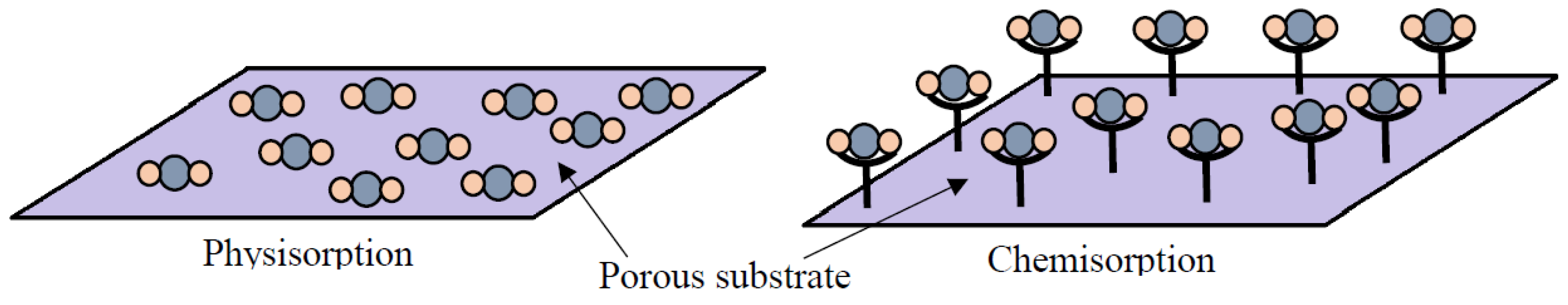

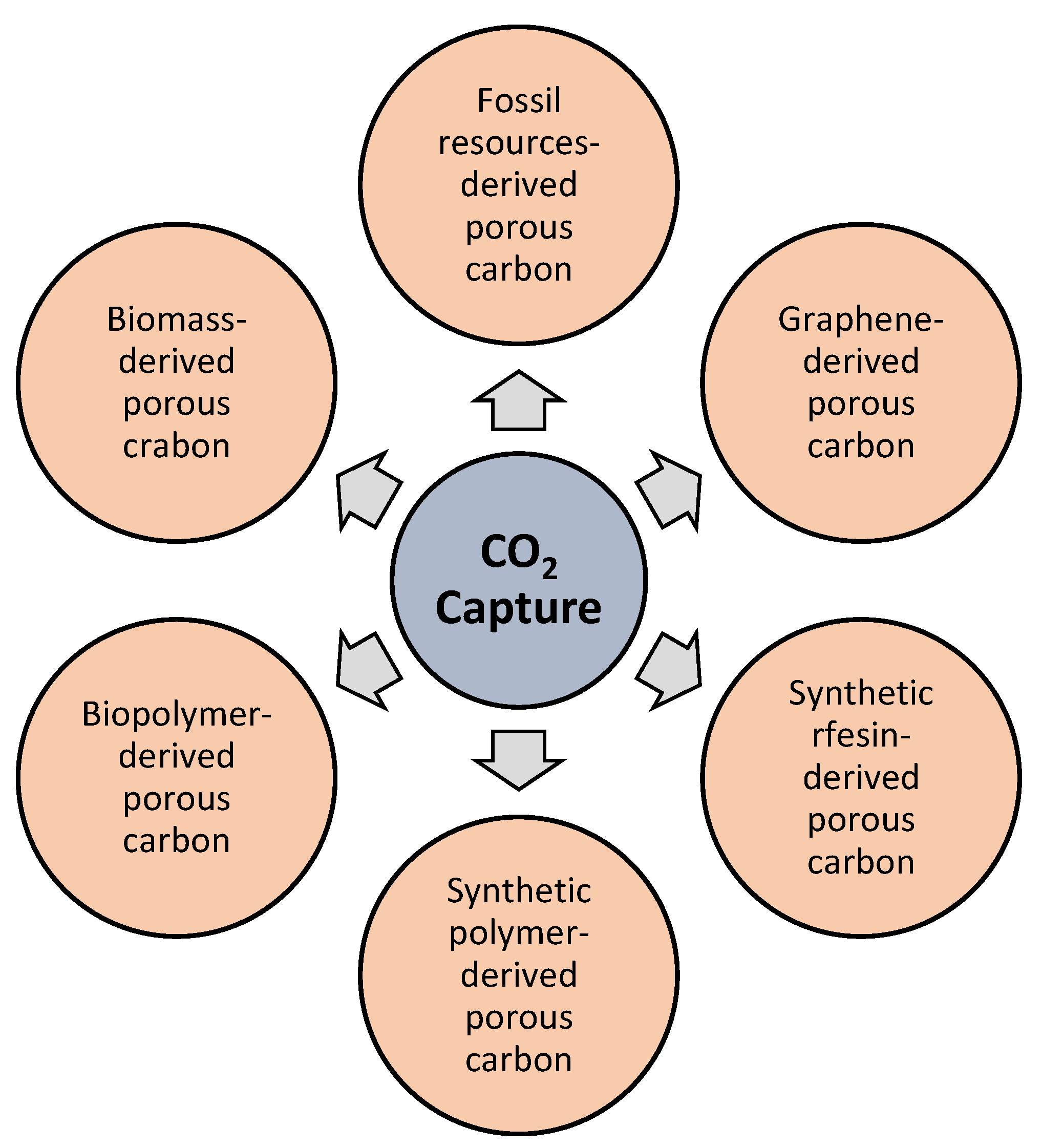

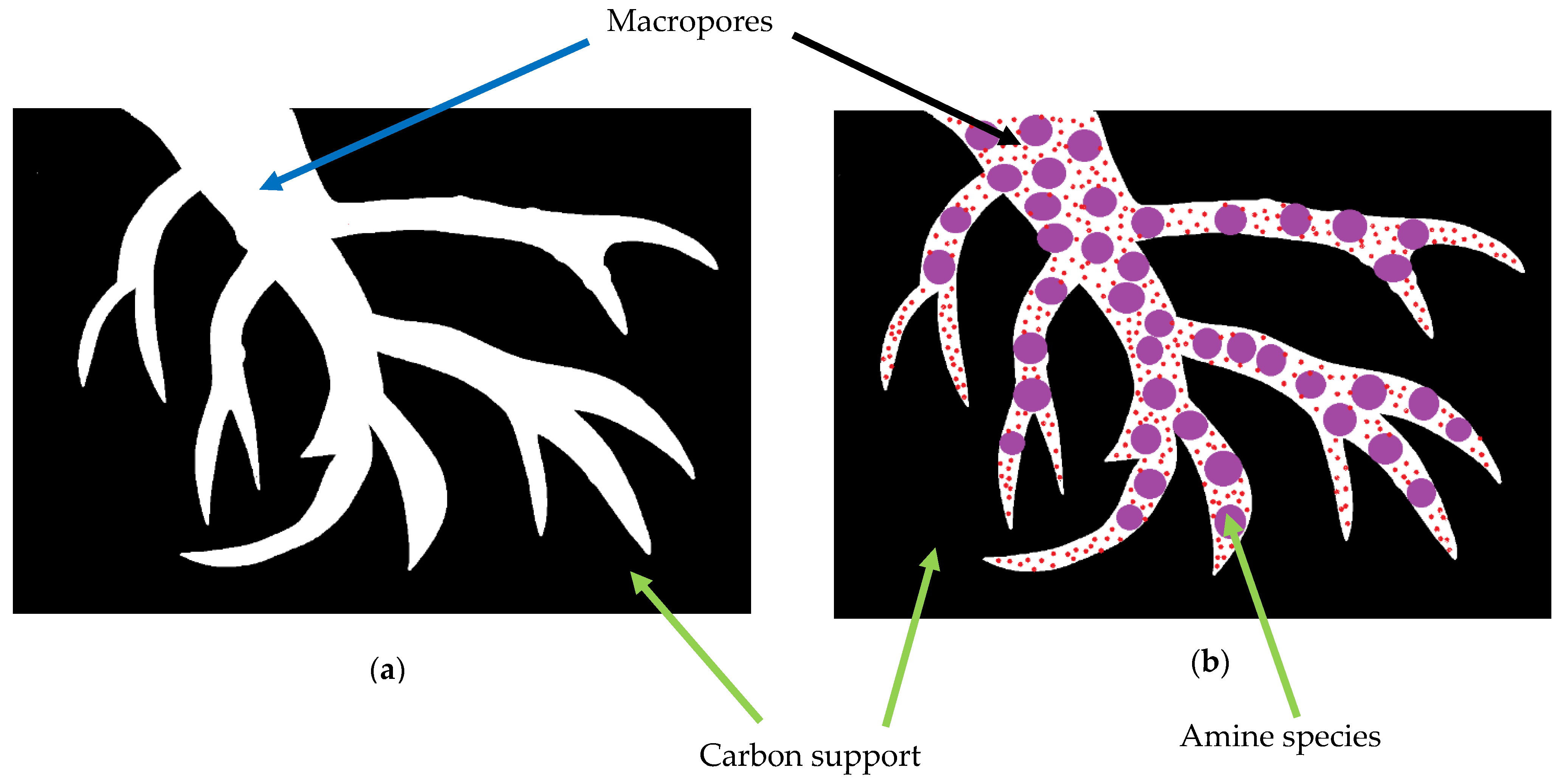
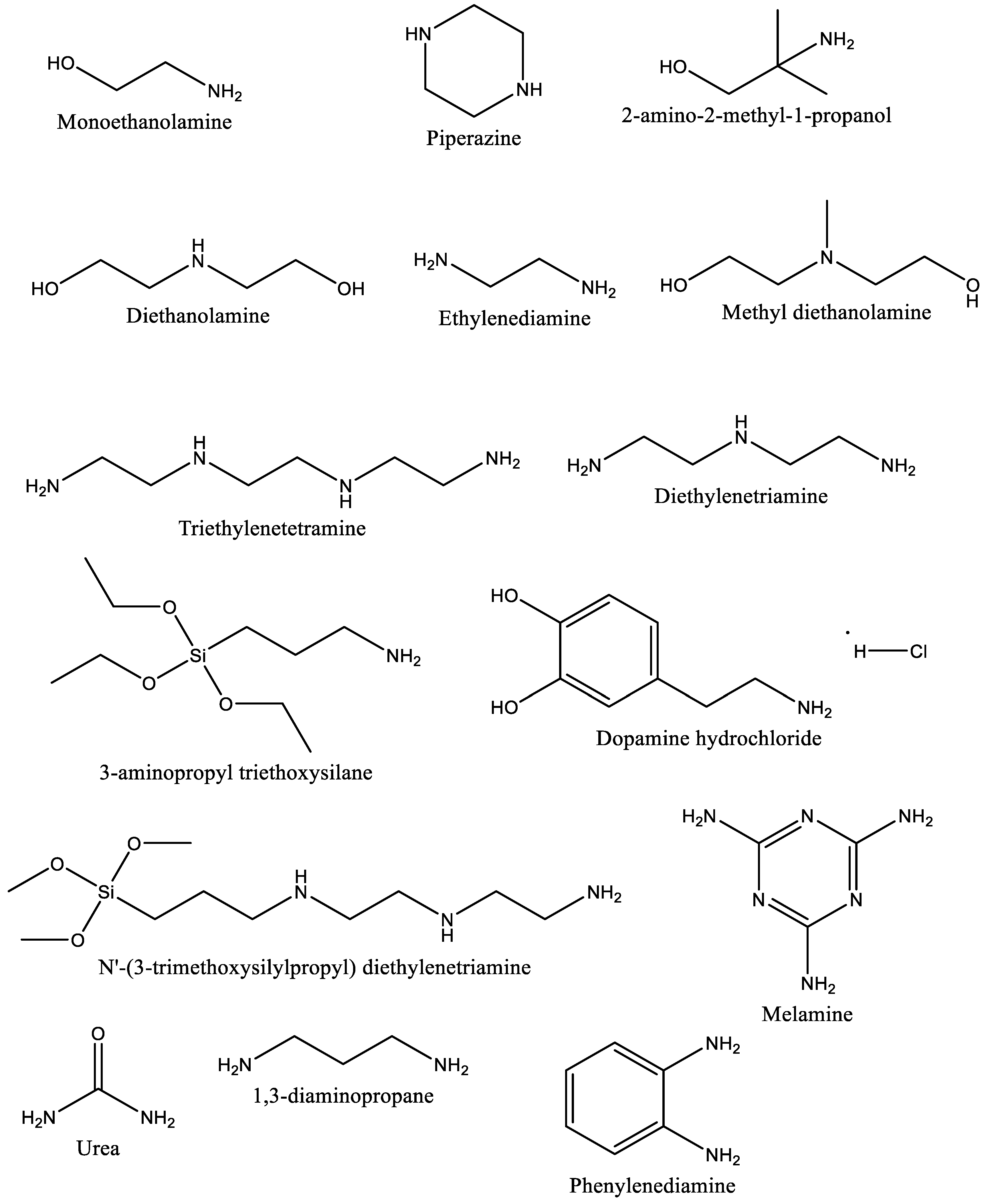

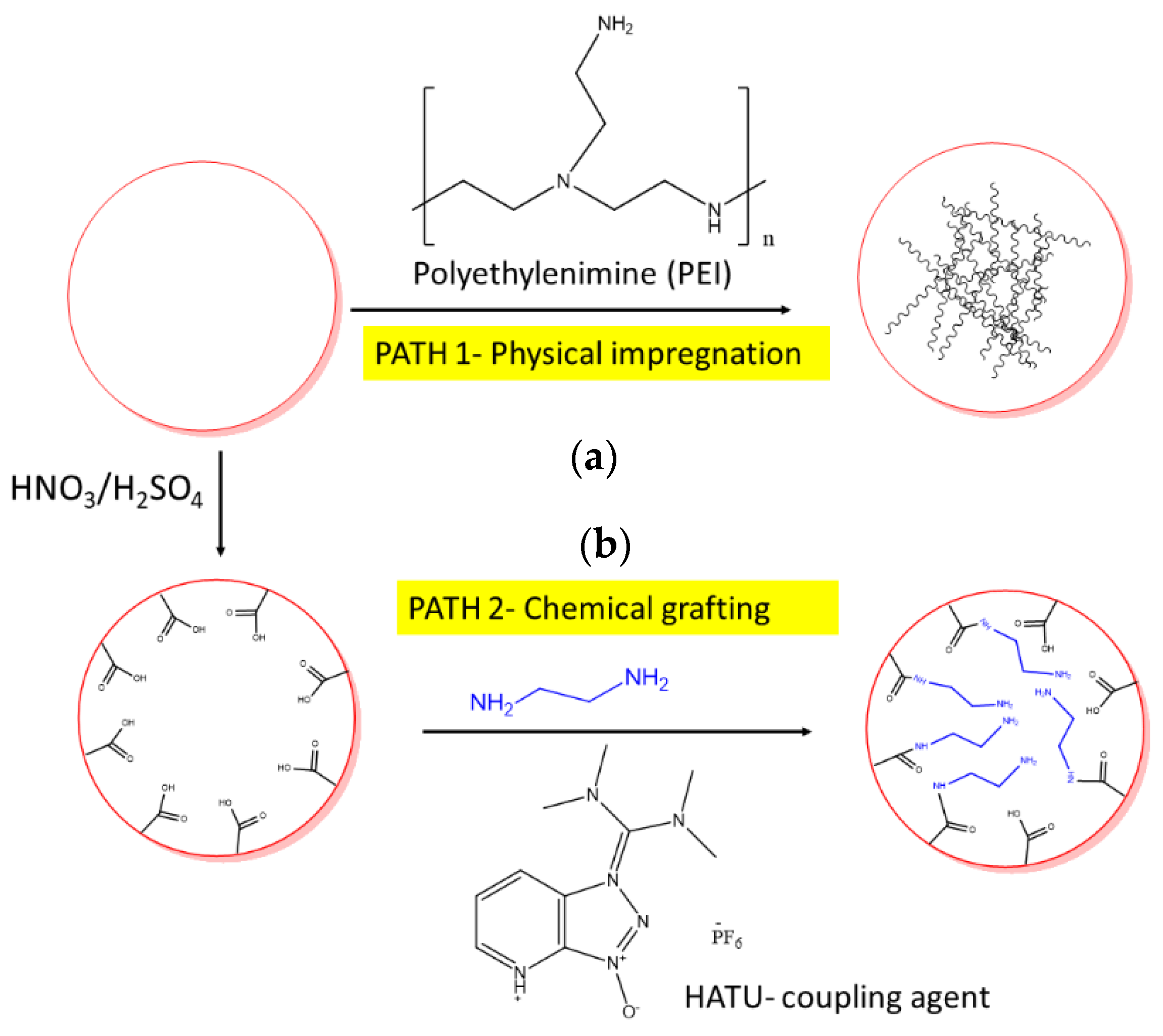
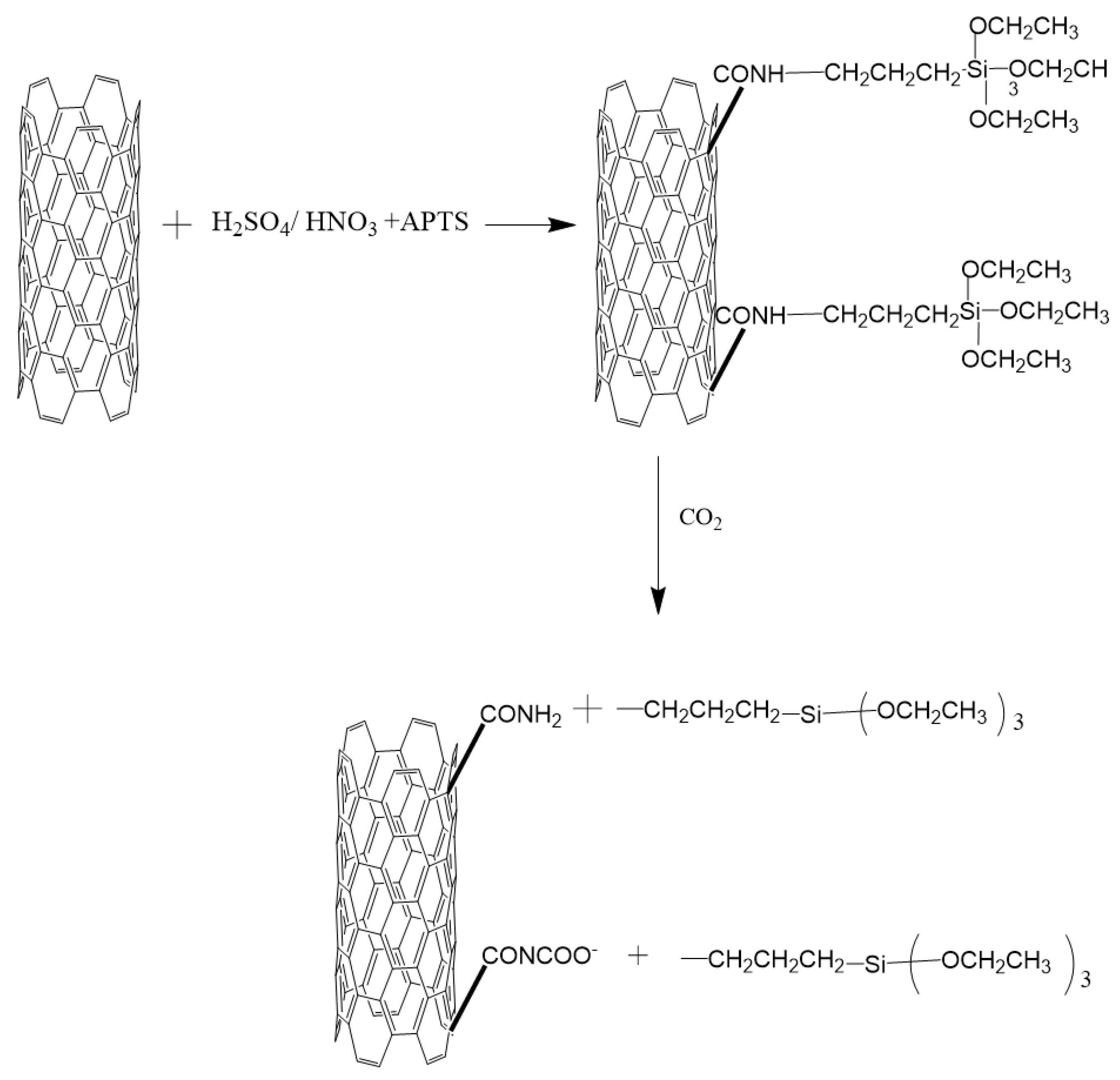
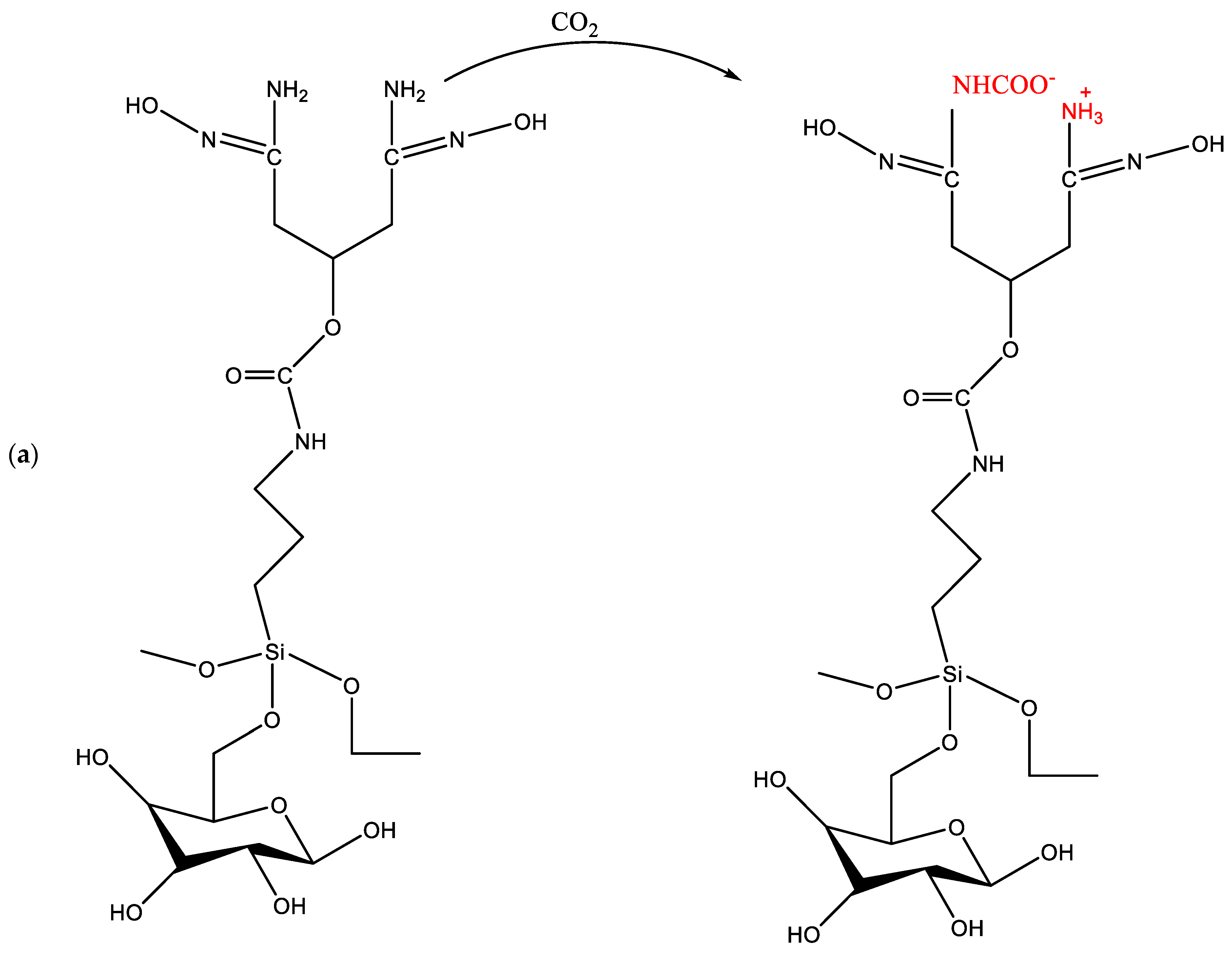
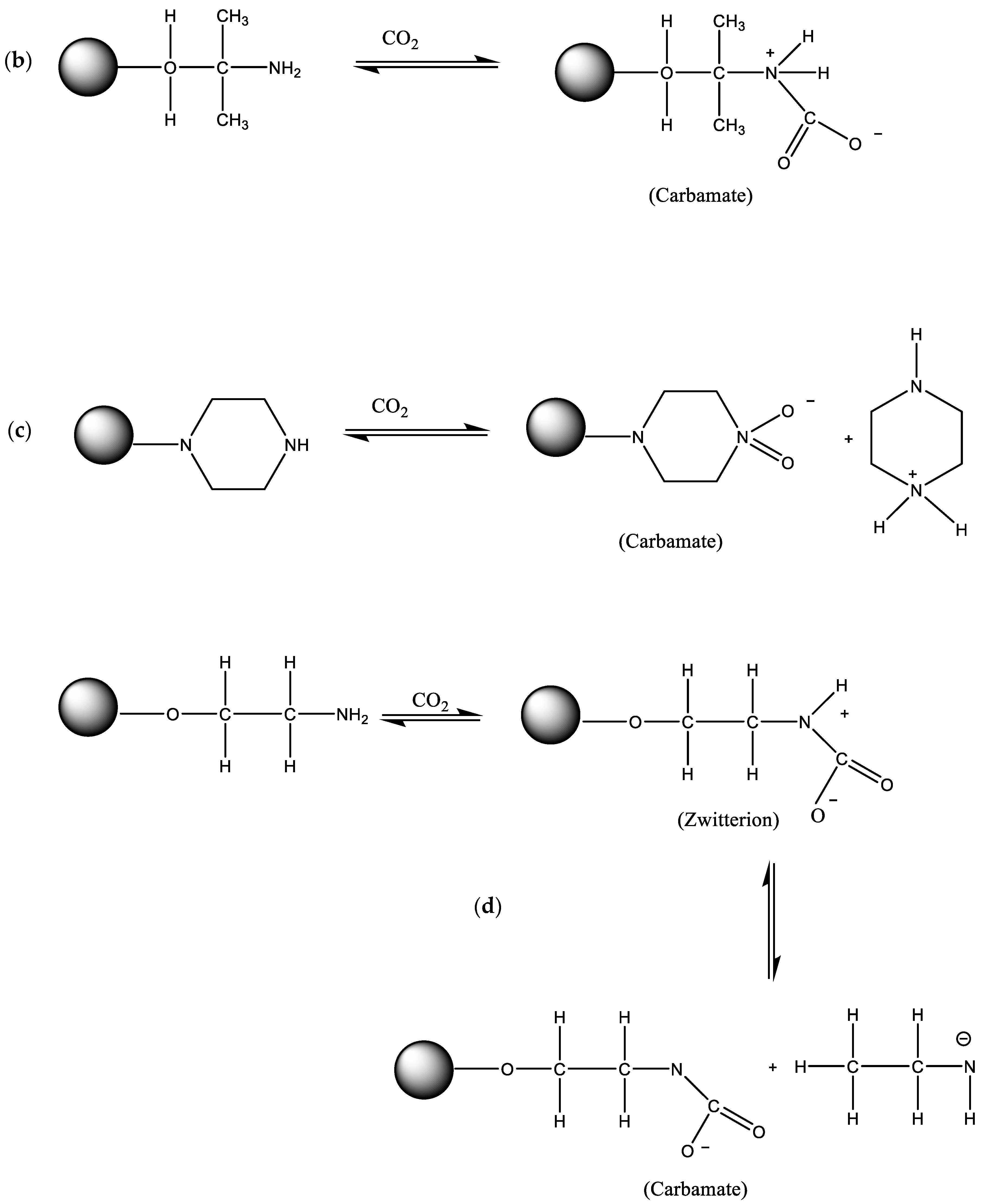
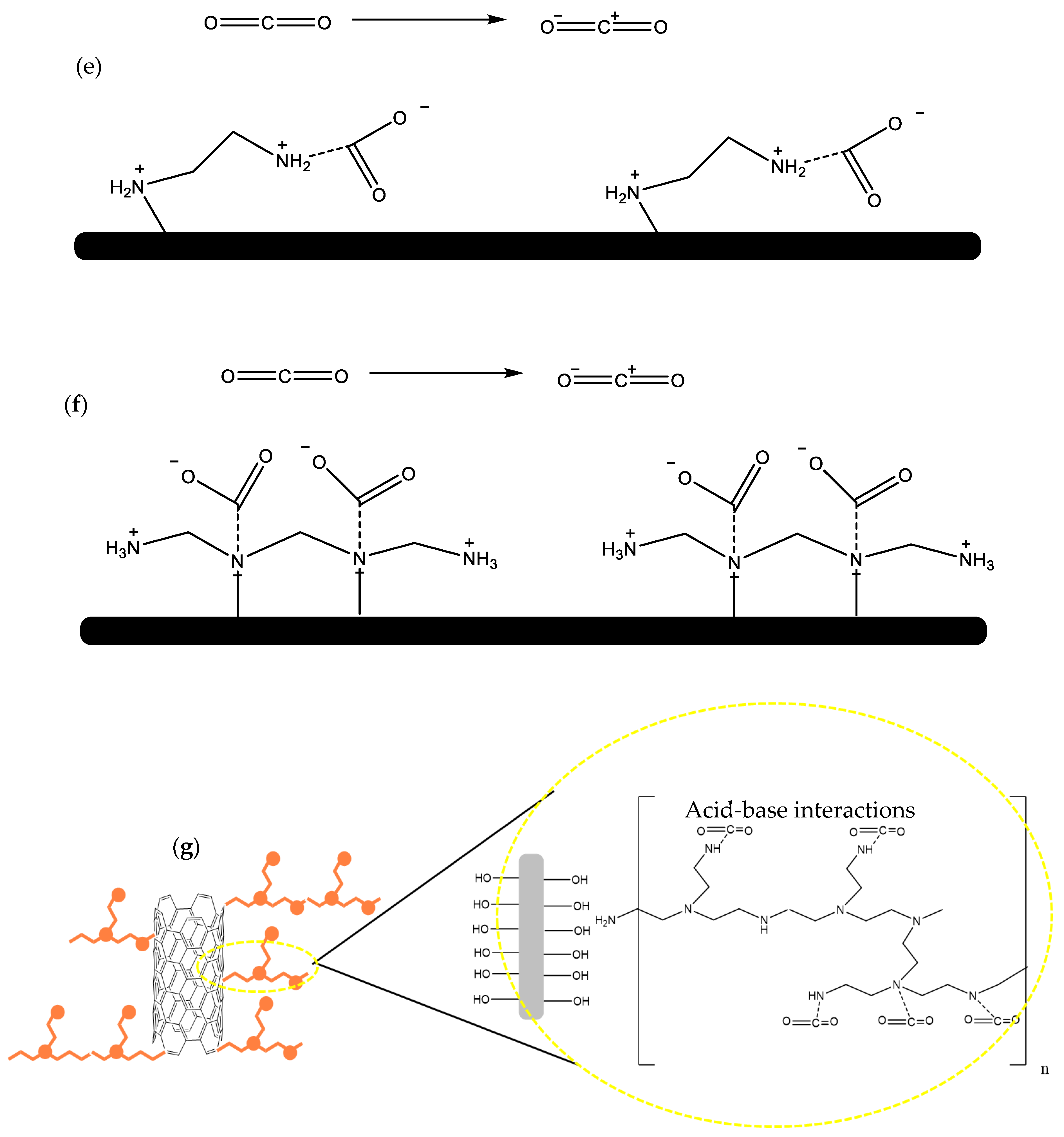
| Component | Cement Rotary Kiln | Dry Atmospheric Air | Biogas Generated from Waste Water Treatment Plant Sludge | Natural Gas Fired Flue Gas | Coal-Fired Flue Gas |
|---|---|---|---|---|---|
| N2 | 59 vol % | 70 vol % | 0–1 vol % | 73–80 vol % | 70–80 vol % |
| CO2 | 19 vol % | 410 ppm | 19–33 vol % | 3–8 vol % | 11–15 vol % |
| H2O | 13 vol % | - | - | 7–14.6 vol % | 5–12 vol % |
| O2 | 7 vol % | 21 vol % | <0.5 vol % | 4.5–15 vol % | 3–6 vol % |
| SO2 | 5–1200 ppm | - | - | <10 ppm | 200–4000 ppm |
| SO3 | - | - | - | - | 0–20 ppm |
| NOX | 100–1500 ppm | - | - | 50–70 ppm | 200–800 ppm |
| CO | - | - | - | - | 50–100 ppm |
| H2 | - | 0.5 vol % | - | 5–300 ppm | 5–20 g/m3 |
| Particulate matter | - | - | - | - | - |
| H2S | - | - | 100–4000 ppm | - | - |
| Ar | - | 0.9 vol % | - | - | - |
| Xe | - | 0.1 vol % | - | - | - |
| Ne | - | 18 ppm | - | - | - |
| He | - | 5.2 ppm | - | - | - |
| CH4 | - | 1.6 vol % | 60–75 vol % | - | - |
| Kr | - | 1.1 vol % | - | - | - |
| N2O | - | 0.3 vol % | - | - | - |
| CO2 Capture Technology | Advantages | Disadvantages |
|---|---|---|
| Pre-combustion capture |
|
|
| Oxy-fuel combustion |
| |
| Post-combustion capture |
| Separation Technology | Advantages | Disadvantages |
|---|---|---|
| Adsorption using solid sorbents (proposed) | ||
| Amine-based absorption (Liquid amine) (current) |
| Process | Advantages | Disadvantages |
|---|---|---|
| Physisorption | ||
| Chemisorption |
|
|
| Regeneration Strategy | Advantages | Disadvantages |
|---|---|---|
| Temperature swing adsorption (TSA) | ||
| Pressure swing adsorption (PSA) | ||
| Electric swing adsorption (ESA) | ||
| Vacuum swing adsorption (VSA) |
|
|
| Parameter | Requirement |
|---|---|
| CO2 adsorption capacity | 3–4 mmol/g |
| Regenerability | >1000 cycles |
| CO2 gas selectivity over other gases | >100 |
| Adsorption/desorption kinetics | >1 mmol/g.min |
| Adsorbent cost | $5–15/kg sorbent |
| Synthesis Route | Carbon Precursor | |||||
|---|---|---|---|---|---|---|
| Biomass-Derived Porous Carbon | Biopolymer-Derived Porous Carbon | Fossil–Resources-Derived Porous Carbon | Graphene-Derived Porous Carbon | Synthetic Polymer-Derived Porous Carbon | Synthetic Resin-Derived Porous Carbon | |
| Carbonization followed by KOH activation | Biomass cork dust [76], Bio-tar [24], Date seeds [141], Coconut shells [81], Rice husk [50], Mangosteel peel [15] | Starch [32,46,49,138], Chitin [52], Waste wool [165], Chitosan [88], Lignin [161] | Anthracene oil-based pitch [166], Petroleum coke [10,81,146], Asphalt [89,167], Iranian asphaltene [7], Petroleum tar pitch [168], Coal particles [169] | Graphene oxide [170], Graphene [91] | PAN [34,58], Polypyrrole [135,171], Triazine-based hyper cross-linked polymer [172], PET [3,12,101], Waste CDs and DVDs [154], PUF [156] | Commercial phenolic resin [173], Urea–formaldehyde [17,42] |
| Carbonization followed by ZnCl2 activation | Biomass cork dust [76], Poplar catkin [101] | - | - | - | Triazine-based hyper cross-linked polymer [172] | - |
| Thio-urea modification of the carbonized product followed by KOH activation | Hazelnut shells [174] | - | - | - | - | - |
| Carbonization followed by NaOH activation | Waste sugarcane bagasse [47] | Chitosan [88] | - | - | PAN [34], PET [101] | - |
| Post nitridation of the carbonized product using melamine followed by KOH activation | Water caltrop shells [175] | - | - | - | - | - |
| Single step KOH activation | Pine wood [17], Taihu blue algae [176], Peanut shell [17], Chars derived from biomass gasifiers [25], Walnut shell [17], N-Salina algae [62] | EHL [141] | - | - | Main-chain PIL [157] | Commercial phenolic resin [177] |
| ZnCl2 activation | Chars derived from biomass gasifiers [25] | - | - | - | Polypyrrole [130] | - |
| Carbonization followed by steam activation | Paper mill sludge [54], Palm kernel shell [59], Pine sawdust [54] | - | - | Reduced graphene oxide [144] | - | - |
| Carbonization followed by CO2 activation | Sucrose [55], Rice husk [90], Bamboo solid residue [11], Waste sugarcane bagasse [47] | Starch [145] | - | Graphene/Glucose composite [61] | Polyvinylidene fluoride [155], Polypyrrole [140], Waste CDs and DVDs [162] | Phenolic resin [178] |
| Carbonization flowed by NH3 activation | - | - | - | - | - | Phenolic resin [179] |
| Carbonization followed by air activation | Waste sugarcane bagasse [47] | - | - | - | - | - |
| Carbonization followed by H3PO3 activation | Waste sugarcane bagasse [47] | - | - | - | - | - |
| Carbonization followed by potassium acetate activation | Waste sugarcane bagasse [47] | - | - | - | - | - |
| Single step CO2 activation | Palm kernel shell [86] | Cellulose [117,180] | Petroleum coke [179] | Reduced graphene oxide [150] | - | - |
| Single step low temperature NaNH2 activation | Hazelnut shell [181], Lotus stalk [182], Lotus leaf [183] | - | - | - | - | - |
| Carbonization followed by NaNH2 activation | Water chestnut shells [184] | - | - | - | - | Phenolic resin [185] |
| Carbonization followed by FeCl3 activation | - | - | Coal tar pitch [186] | - | Polypyrrole [172] | - |
| Direct carbonization | - | - | - | - | - | Resorcinol–formaldehyde [4] |
| Electrospinning followed by carbonization | - | - | - | - | - | Phenolic resin [153] |
| Nanocasting | - | - | - | - | - | Urea–formaldehyde [36,42], Resorcinol–formaldehyde [45], Phenol–formaldehyde [66], Hexamethoxymethylmelamine (HMMM) [105] |
| In-situ activation using potassium organic salt during precursor synthesis followed by carbonization | - | - | - | - | - | Resorcinol–formaldehyde [135] |
| Carbonization followed by K2C2O4 activation | - | Corn starch [46] | - | - | - | - |
| Carbonization followed by K2CO3 activation | - | Corn starch [46] | - | - | - | - |
| Carbonization followed by KOH and Urea activation | - | Chitosan [187] | - | - | - | - |
| One step carbonization/activation with N2 | - | Cellulose [116] | - | - | - | - |
| Carbonization followed by alkali metal carbonate activation | - | Chitosan [188] | - | - | - | - |
| Carbonization followed by potassium citrate activation | - | Chitosan [189] | - | - | - | - |
| Carbonization followed by CaCO3 activation | - | Pigskin collagen [163] | - | - | - | - |
| Carbonization followed by CH4 activation | - | Starch [145] | - | - | - | |
| Carbonization followed by H2 activation | - | Starch [145] | - | - | - | - |
| Microwave treatment | - | - | - | - | Polyacrylonitrile [142] | - |
| Spheroidization, oxidation, cross-linking and KOH activation | - | - | - | - | PVC [190] | - |
| Cross-linking, pre-oxidation and carbonization | - | - | - | - | PAN [143] | - |
| Spheroidization followed by alkaline activation | - | - | - | - | PVC [61] | - |
| C3N4 nanosheets as sacrificial template | - | - | - | - | PIL [159] | - |
| Carbonization followed by Fe-Based template removal | - | - | - | - | PIL [158] | - |
| Reduction-induced self-assembly process of graphene oxide nano platelets in aqueous dispersion at 45–90 °C | - | - | - | Graphene [191] | - | - |
| Sol–gel method | - | - | - | Magnesium oxide nanoparticle fabricated on graphene oxide [94] | - | - |
| Polyol-mediated self-assembly and subsequent thermal annealing treatment | - | - | - | Reduced graphene oxide and nanocrystalline composite [192] | - | - |
| Electrospinning process followed by physical activation | - | - | - | Activated carbon fibers/graphene nanocomposite [39] | - | - |
| Porous Carbon Material | Gas Mixture | Selectivity Value | Pressure (Bar) | Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Biomass-derived porous carbon | |||||
| Cork dust-derived porous carbon | CO2/N2 (15/85 v/v%) gas mixture | 7 | 1 | 25 | [76] |
| KOH activated starch-based sorbent | 16 | 1 | 25 | [32] | |
| Algae-derived porous carbon | 69.7 | Ambient | Ambient | [62] | |
| ZnCl2 activated poplar catkin | 22 | 1 | 25 | [101] | |
| Date sheets-derived porous carbon | 41.53 | 1 | 25 | [143] | |
| Coconut shell-based activated carbon | CO2/N2 (10/90 v/v%) gas mixture | 22 | 1 | 25 | [81] |
| Rise husk-derived activated carbon | 63 | Ambient | Ambient | [50] | |
| Rise husk-derived activated carbon | 7.6 | 1 | 25 | [92] | |
| Taihu blue algae-derived porous carbon | 39.3 | 1 | 25 | [176] | |
| Mangosteen peel-based activated carbon | 12 | 1 | 25 | [165] | |
| Hazelnut shell-based porous carbon | 17 | 1 | 25 | [181] | |
| Lotus leaf-derived activated carbon | 21 | 1 | 25 | [183] | |
| Lotus stalk-derived activated carbon | 22 | 1 | 23 | [182] | |
| Water chestnut shells-derived activated carbon | 23 | 1 | 25 | [184] | |
| Pine sawdust-based sorbent | 26.7 | Ambient | Ambient | [55] | |
| Palm kernel shell-derived activated carbon | 7 | Ambient | Ambient | [86] | |
| N-saline algae-derived porous carbon | CO2/CH4 (50/50 v/v%) gas mixture | 5.5 | Ambient | Ambient | [61] |
| Palm kernel shell-derived activated carbon | 1.7–2.5 | 0–1.1 | 25 | [59] | |
| Biopolymer-derived porous carbon | |||||
| Cellulose-derived porous carbon | CO2/N2 (15/85 v/v%) gas mixture | 41.8 | 1 | 25 | [117] |
| Starch-based peanut packaging-derived activated carbon | 15–38 | 1 | 25 | [138] | |
| Cornstarch-based activated carbon | 59–135 | 0–1 | 0 | [46] | |
| Waste wool-activated carbon | 16 | 1 | 25 | [164] | |
| Chitosan-derived porous carbon | CO2/N2 (10/90 v/v%) gas mixture | 12–25 | 1 | 25 | [187] |
| Chitosan-derived porous carbon | 17–69 | 1 | 25 | [88] | |
| Lignin-derived porous carbon | 21.8 | 1 | 25 | [161] | |
| Starch-derived porous carbon | 98 | 1 | 25 | [49] | |
| Fossil resources-derived porous carbon | |||||
| Petroleum coke-derived porous carbon | CO2/N2 (10/90 v/v%) gas mixture | 17 | 1 | 25 | [180] |
| Petroleum coke-derived porous carbon | 25 | 1 | 25 | [193] | |
| Petroleum coke-derived porous carbon | 22 | 1 | 25 | [127] | |
| Coal tar pitch-based sorbent | 23.8 | 1 | 25 | [186] | |
| Tar pitch and coal powder-derived porous carbon | 5.94 | 1 | 25 | [168] | |
| Petroleum coke-derived porous carbon | CO2/N2 (15/85 v/v%) gas mixture | 13.7 | 1 | 25 | [10] |
| Iranian asphaltene-derived porous carbon | 22.74 | 1 | 25 | [7] | |
| Graphene-derived porous carbon | |||||
| Graphene oxide-based porous carbon | CO2/N2 (10/90 v/v%) gas mixture | 12 | 1 | 25 | [32] |
| Graphene-based sorbent | 53 | 1.03 | 25 | [191] | |
| Graphene oxide-derived porous carbon | - | 162 | Simulated flue gas conditions | [150] | |
| - | 253 | Natural gas fired power plant | |||
| Synthetic resin-derived porous carbon | |||||
| Commercial phenolic resin-derived porous carbon | CO2/N2 (10/90 v/v%) gas mixture | 48 | 1 | 25 | [196] |
| Phenolic resin-derived activated carbon | 17 | 1 | 25 | [177] | |
| Phenolic resin-derived activated carbon | 14 | 1 | 25 | [185] | |
| Phenolic resin-derived porous carbon | 19 | 1 | 25 | [173] | |
| Resorcinol–formaldehyde-derived porous carbon | CO2/N2 (15/85 v/v%) gas mixture | 45 | 1 | 25 | [4] |
| Phenol–formaldehyde-based porous sorbent | 16.4 | 1 | 25 | [201] | |
| Synthetic polymer-derived porous carbon | |||||
| Polypyrrole-derived porous carbon | CO2/N2 (10/90 v/v%) gas mixture | 194 | 1 | 50 | [135] |
| PIL-derived porous carbon | 14 | 1 | 25 | [157] | |
| PIL-derived porous carbon | 44 | 1 | 0 | [197] | |
| PVC-based sorbent | 6.9 | 1 | 25 | [61] | |
| Triazine-based hyper cross-linked polymer-derived porous carbon | 8.9–42.6 | 1 | 25 | [172] | |
| K2CO3 activated polyacrylonitrile-based sorbent material | 33.6 | 1 | 0 | [34] | |
| PIL-derived porous carbon | 43.69 | 1 | 25 | [158] | |
| Pyrrole-derived porous carbon | 35 | 1.01 | 0 | [171] | |
| NaOH activated PET-derived porous carbon | CO2/N2 (15/85 v/v%) gas mixture | 13.3–31.1 | 0–1 | 50 | [23] |
| KOH activated PET-derived porous carbon | CO2/CO gas mixture | 9.09–18.94 | 0–1 | 50 | [23] |
| Polyaniline-derived porous carbon | CO2/CH4 (10/90 v/v%) gas mixture | 14.3 | 1 | 25 | [58] |
| Gas Molecule | Kinetic Diameter (Å) | Dipole Moment (×10−19 esu−1 cm−1) | Quadrupole Moment (×10−26 esu.cm2) | Polarizability (×1025 cm3) |
|---|---|---|---|---|
| CO2 | 3.3 | 0 | 4.3 | 29.1 |
| N2 | 3.64 | 0 | 1.52 | 17.6 |
| CO | 3.76 | 1.1 | - | 19.5 |
| CH4 | 3.8 | 0 | 0 | 25.9 |
| Support | Amine Attaching Method | Amine Type | CO2 Capture Conditions for Pure CO2 Gas Flow | CO2 Capture Capacity (mmol/g) | Reference | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (bar) | |||||
| Sea mango activated carbon | Impregnation | Monoethanolamine | 25 | 1 | 0.52 | [226] |
| Impregnation | Piperazine | 25 | 1 | 0.66 | ||
| Impregnation | 2-amino-2-methyl-1-propanol | 25 | 1 | 0.25 | ||
| Green coconut shell-based activated carbon | Impregnation | Monoathanolamine | 25 | 1 | 0.84 | [206] |
| Impregnation | Diethanolamine | 25 | 1 | 0.46 | ||
| Mesoporous carbon | Impregnation | Ethylenediamine | 27 | 1 | 19.68 | [110] |
| Impregnation | Triethylenetetramine | 27 | 1 | 11.24 | ||
| Carbon nanotubes | Grafting | Polyaniline | 17 | 1 | 6.3 | [233] |
| Chitosan-derived mesoporous carbon | Impregnation | Pentaethylhexamine | 100 | 1 | 3.27 | [210] |
| Mesoporous carbon | Impregnation | Methyl diethanolamine | 27 | 0.07 | 2.63 | [230] |
| Sugarcane bagasse | Grafting | Ethylenediamine | 25 | 1 | 2.2 | [225] |
| Grafting | Diethylenetriamine | 25 | 1 | 2.08 | ||
| Grafting | Tetraethylenepentamine | 25 | 1 | 2.79 | ||
| Grafting | Triethylenetetramine | 25 | 1 | 2.68 | ||
| Activated carbon | Impregnation | NH2-Cl | 0 | 1 | 3.069 | [222] |
| 25 | 1 | 1.95 | ||||
| Impregnation | 3-aminopropyl triethoxysilane | 0 | 1 | 2.433 | ||
| 25 | 1 | 1.762 | ||||
| Impregnation | Dopamine hydrochloride | 0 | 1 | 0.429 | ||
| 25 | 1 | 0.389 | ||||
| Mesoporous carbon microparticles | Grafting | Ethylenediamine | 30 | 1 | 0.75 | [207] |
| 75 | 1 | 0.37 | ||||
| Impregnation | Polyethylenimine | 30 | 1 | 0.82 | ||
| 75 | 1 | 0.40 | ||||
| Multiwalled carbon nanotubes/Cd-nanozeolite composite | Impregnation | Polyethylenimine | 25 | 1 | 5.7 | [2] |
| Graphite carbon nitride | Impregnation | Polyethylenimine | 100 | 1 | 3.77 | [229] |
| Waste tea activated mesoporous carbon | Grafting | Diethanolamine | 30 | 1 | 33.57 | [82] |
| KOH activated broom sorghum stalk-derived activated carbon | Grafting | Diethanolamine | 25 | 1 | 2.13 | [57] |
| Activated carbon | Impregnation | Monoethanolamine | 40 | 1.01325 | 1.79 | [216] |
| 50 | 1.01325 | 1.99 | ||||
| 60 | 1.01325 | 2.19 | ||||
| 70 | 1.01325 | 2.36 | ||||
| Impregnation | Diethanolamine | 40 | 1.01325 | 2.11 | ||
| 50 | 1.01325 | 2.36 | ||||
| 60 | 1.01325 | 2.57 | ||||
| 70 | 1.01325 | 2.81 | ||||
| Multiwalled carbon nanotube | Grafting | N’-(3-trimethoxysilylpropyl) diethylenetriamine | 120 | 1 | 0.48 | [234] |
| Activated carbon derived from ordos coal | Impregnation | Tetraethylenepentamine | 60 | 1.01325 | 3.24 | [228] |
| Biochar derived from rice straw | Impregnation | Tetraethylenepentamine | 25 | 1 | 5.7 | [111] |
| Phosphoric acid activated risk husk | Impregnation | Melamine | 30 | 1 | 6.877 | [221] |
| 45 | 1 | 6.518 | ||||
| 60 | 1 | 6.113 | ||||
| Microporous activated carbon | Impregnation | Triethylenetetramine | 75 | 1 | 1.05 | [218] |
| Impregnation | Polyethylenimine | 75 | 1 | 1.85 | ||
| Mesoporous activated carbon | Impregnation | Polyethylenimine | 75 | 1 | 1.4 | |
| Polyaniline | Grafting | Melamine | 25 | 0.15 | 1.3 | [235] |
| 25 | 1.01325 | 4.6 | ||||
| Multiwalled carbon nanotubes | Impregnation | Polyethylenimine | 25 | 1 | 2.14 | [217] |
| MOF-derived carbon monolith | Impregnation | Tetraethylenepentamine | 25 | 0.15 | 5.6 | [93] |
| Pinecone-based activated carbon | Grafting | Polyaniline | 25 | 1 | 3.16 | [79] |
| Multiwalled carbon nanotubes | Grafting | 3-aminopropyl triethoxysilane | 25 | 1 | 5.76 | [109] |
| Carbon nanotubes | Grafting | Polyethylenimine | 50 | 1 | 2.9 | [37] |
| Multiwalled carbon nanotubes | Impregnation | Polyethylenimine | 25 | 1 | 1.41 | [223] |
| Sugarcane bagasse | Impregnation | Urea | 25 | 1 | 4.8 | [44] |
| Multiwalled carbon nanotubes | Grafting | Phenylenediamine | 25 | 2 | 0.21 | [80] |
| Carbon nanotubes | Grafting | 1,3-diaminopropane | 30 | 17.3 | 2.11 | [80] |
| Multiwalled carbon nanotubes | Grafting | 3-aminopropyl triethoxysilane | 0 | 1 | 1.32 | [38] |
| Graphene oxide | Impregnation | Tetraethylenepentamine | 70 | 50 | 4.26 | [232] |
| Graphene oxide | Impregnation | Polyethylenimine | 25 | 1 | 1.91 | [229] |
| Graphene oxide aerogel | Impregnation | Ethylenediamine | 25 | 1 | 1.1 | [231] |
| Graphene oxide | Grafting | Tetraethylenepentamine | 70 | 0.1 | 1.2 | [151] |
| Biochar | Grafting | Aminopropyl triethoxysilane | 25 | 1.01325 | 3.7 | [90] |
| Porous carbon | Grafting | Ethylenediamine | 25 | 0.15 | 1.1 | [236] |
| Hierarchical microporous carbon | Grafting | Melamine | 0 | 1 | 3.82 | [237] |
| 25 | 1 | 2.69 | ||||
| Porous carbon | Grafting | Melamine | 30 | 1 | 1.12 | [238] |
| Grafting | Ethylenediamine | 30 | 1 | 2.84 | ||
| Grafting | Hexamethylenetetramine | 30 | 1 | 1.40 | ||
| Porous carbon | Grafting | Phenylenediamine | 25 | 5 | 4.65 | [171] |
| Nanocrystalline cellulose | Grafting | Amidoxime | 120 | 1.01325 | 5.54 | [51] |
| 25 | 1.2159 | 1.11 | ||||
| Microcrystalline cellulose | Grafting | Amidoxime | 120 | 1.01325 | 3.85 | [48] |
| 25 | 1.2159 | 1.27 | ||||
| Wheat bran husk-derived carbon | Grafting | Polyethylenimine | 75 | 1 | 0.43 | [165] |
| Sugar cane bagasse | Impregnation | Melamine | 25 | 1 | 3.34 | [239] |
| Dry Conditions (Carbamate Formation) | Humid Conditions (Bicarbonate Formation) |
|---|---|
| Primary amines | |
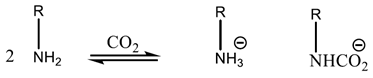 | 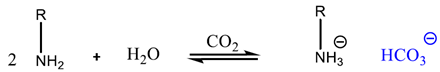 |
| Secondary amines | |
 | 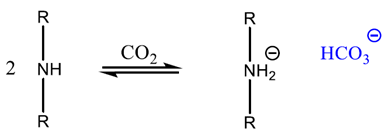 |
| Tertiary amines | |
| No carbamate formation | 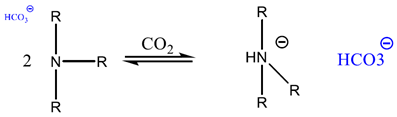 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. https://doi.org/10.3390/atmos13030397
Gunawardene OHP, Gunathilake CA, Vikrant K, Amaraweera SM. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere. 2022; 13(3):397. https://doi.org/10.3390/atmos13030397
Chicago/Turabian StyleGunawardene, Oneesha H. P., Chamila A. Gunathilake, Kumar Vikrant, and Sumedha M. Amaraweera. 2022. "Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review" Atmosphere 13, no. 3: 397. https://doi.org/10.3390/atmos13030397
APA StyleGunawardene, O. H. P., Gunathilake, C. A., Vikrant, K., & Amaraweera, S. M. (2022). Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere, 13(3), 397. https://doi.org/10.3390/atmos13030397







