Contribution of Physical and Chemical Properties to Dithiothreitol-Measured Oxidative Potentials of Atmospheric Aerosol Particles at Urban and Rural Sites in Japan
Abstract
:1. Introduction
2. Materials and Methods
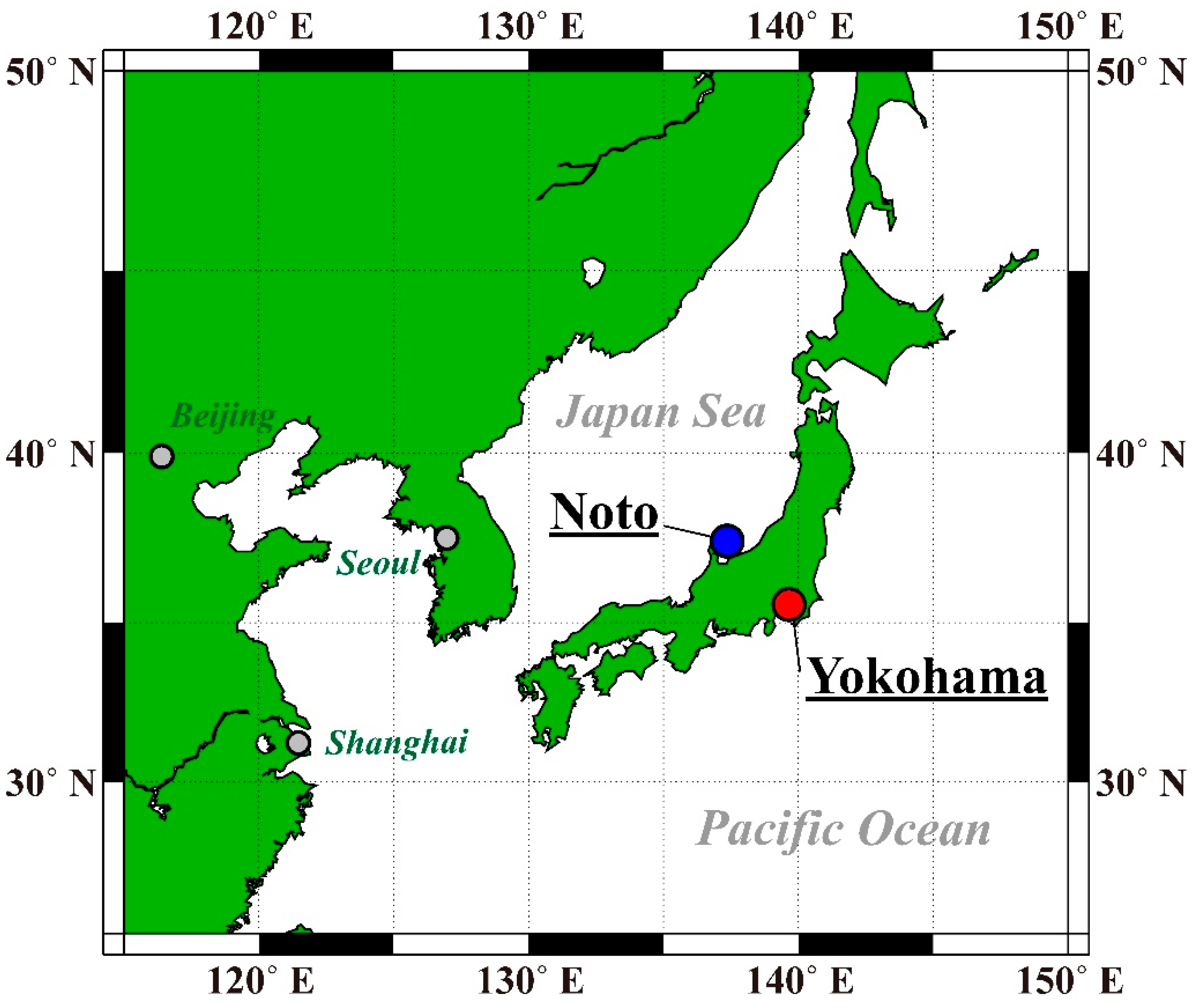
2.1. Sites and Periods of Field Observations and Sampling
2.2. Observations
2.3. Aerosol Sampling
2.4. Oxidative Potential Measurement (DTT Assay)
2.5. Chemical Component Analysis
3. Results and Discussion
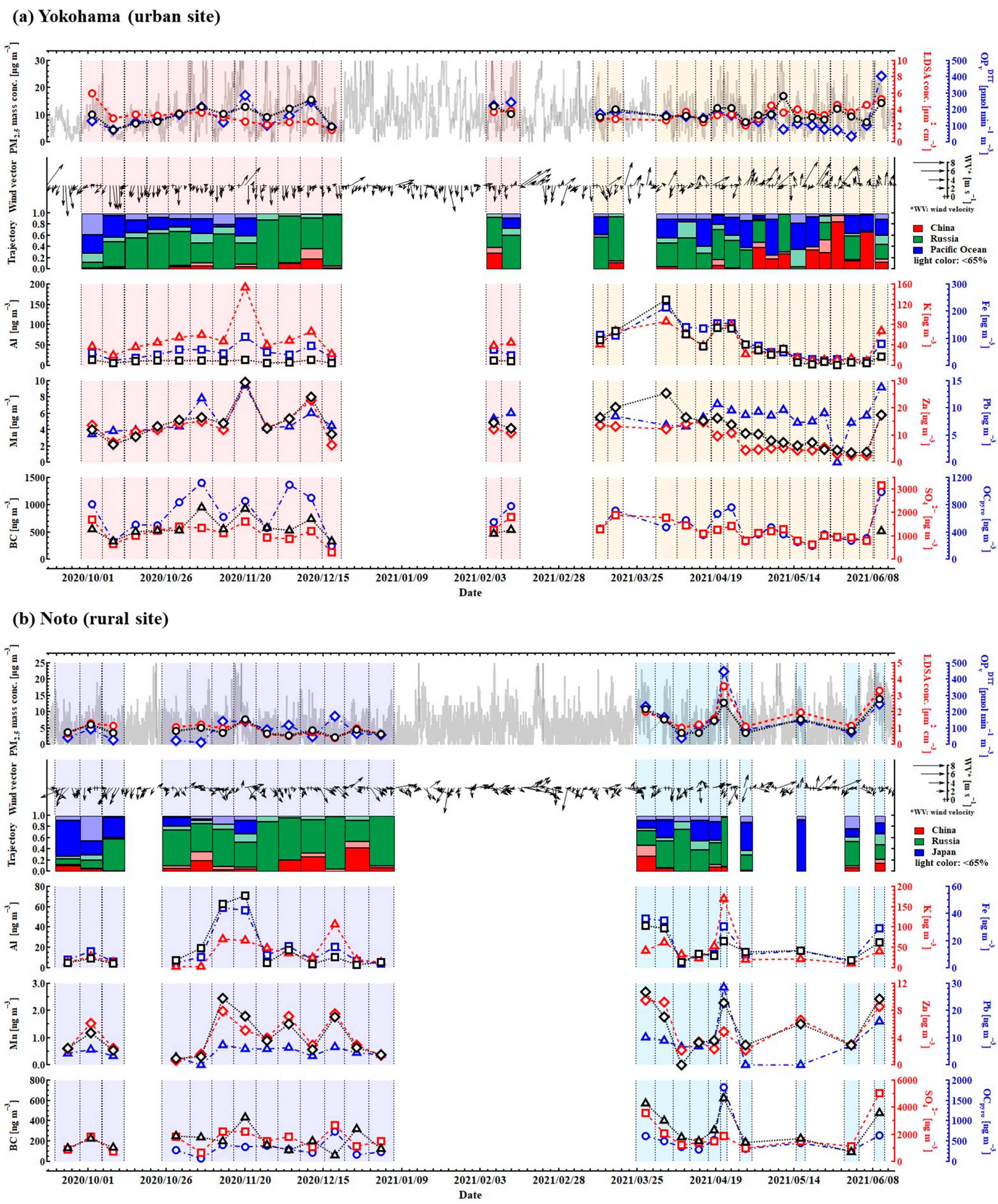
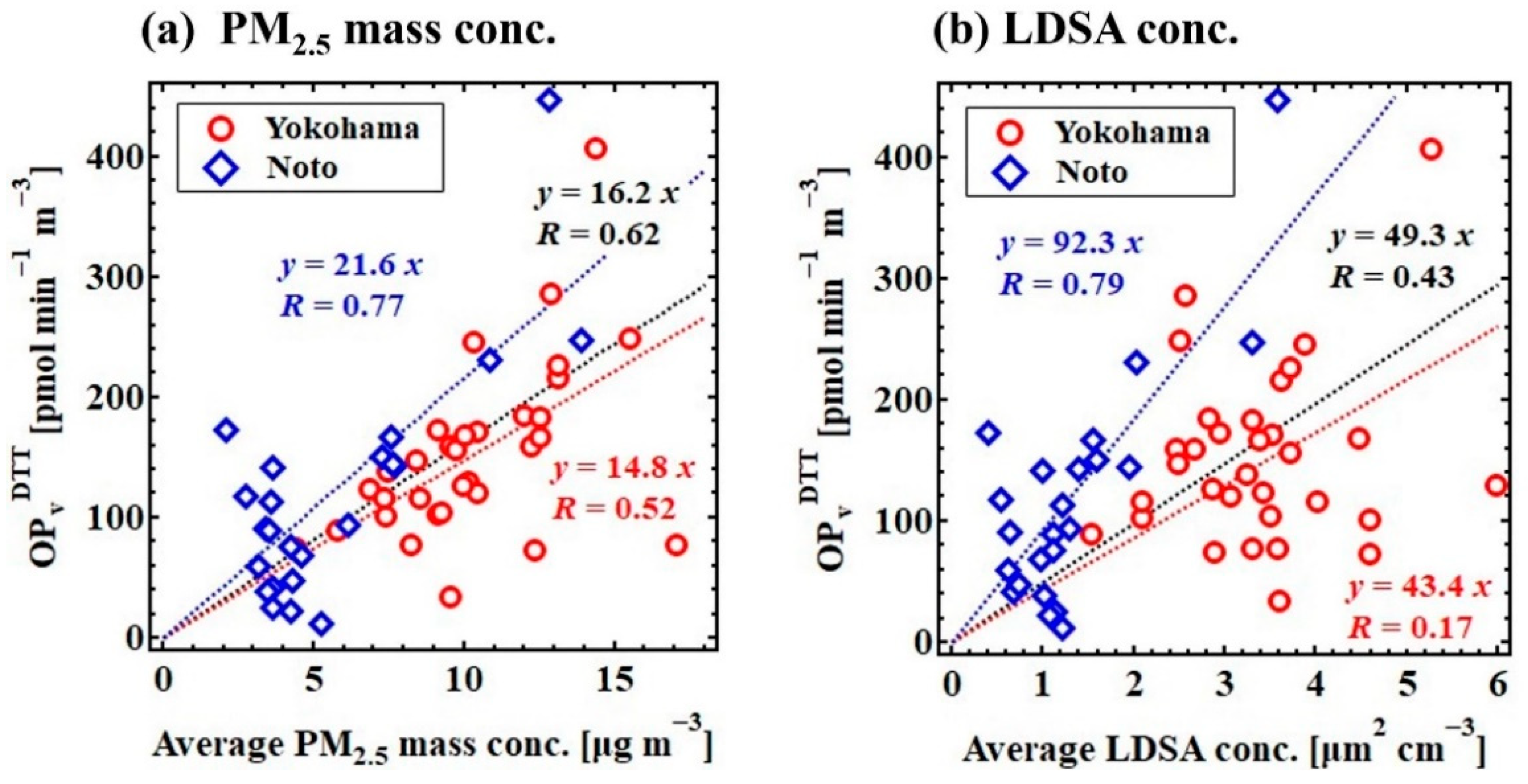
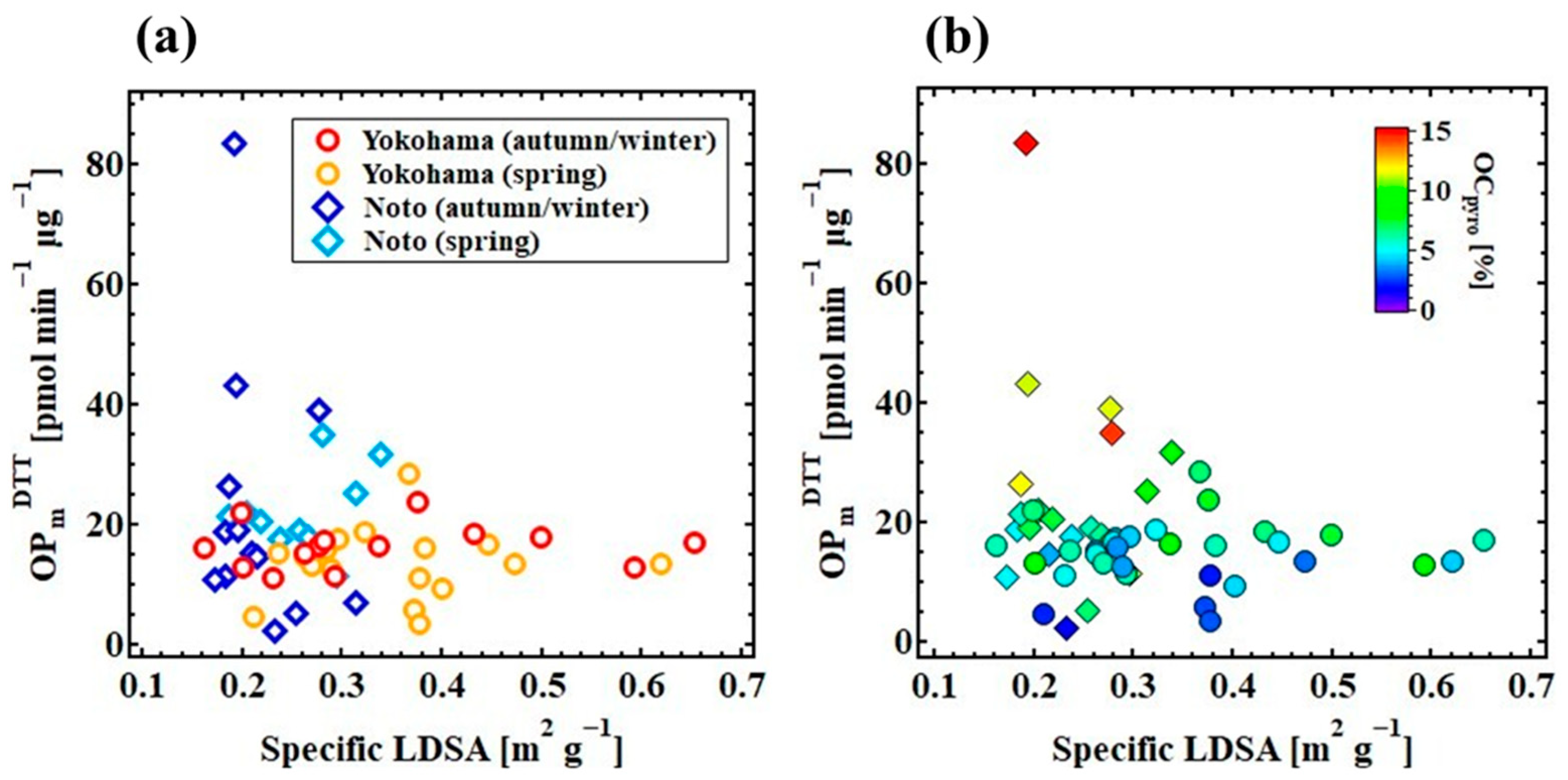
3.1. Regional Differences in Physicochemical Properties and OPDTT
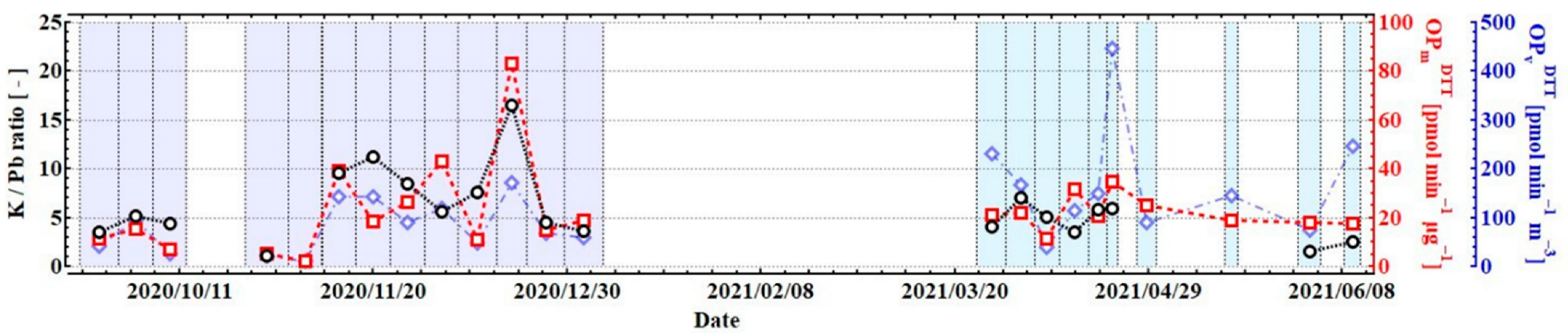
3.2. Contribution of Chemical Properties to the Variation in OPDTT
| (a) Yokohama | ||||||||
| All Samples | Autumn/Winter | Spring | ||||||
| Component | Cross-Correlation (R > 0.8) | Component | Cross-Correlation (R > 0.8) | Component | Cross-Correlation (R > 0.8) | |||
| SO42− (R = 0.79) | ↔ | S | Pb (R = 0.83) | ↔ | BC, K | S (R = 0.90) | ↔ | SO42−, NH4+, OCpyro |
| S (R = 0.79) | ↔ | SO42− | Ca (R = 0.82) | ↔ | Ti | SO42− (R = 0.88) | ↔ | NH4+, S, OCpyro |
| NH4+ (R = 0.78) | ↔ | OCpyro | Ti (R = 0.82) | ↔ | Ca | NH4+ (R = 0.87) | ↔ | SO42−, S, OCpyro |
| OCpyro (R = 0.72) | ↔ | NH4+ | Mn (R = 0.80) | ↔ | Zn, Fe, K, Cu | OCpyro (R = 0.84) | ↔ | NH4+, SO42−, S |
| Zn (R = 0.72) | ↔ | Mn, K | Fe (R = 0.78) | ↔ | Mn, Zn, K, BC | Zn (R = 0.76) | ↔ | Mn |
| Mn (R = 0.69) | ↔ | K, Zn | Zn (R = 0.74) | ↔ | Mn, K, Fe, Cu, BC | |||
| K (R = 0.66) | ↔ | Mn, Zn | ||||||
| Pb (R = 0.65) | ↔ | N/A | ||||||
| (b) Noto | ||||||||
| All samples | Autumn/Winter | Spring (without the Extremely High OPvDTT Sample) | ||||||
| Component | Cross-Correlation (R > 0.85) | Component | Cross-Correlation (R > 0.85) | Component | Cross-Correlation (R > 0.85) | |||
| OCpyro (R = 0.93) | ↔ | OC3, OC4, nss-K+, Pb, K | K (R = 0.93) | ↔ | OCpyro, EC1corr | Mn (R = 0.96) | ↔ | Zn, Fe, Al, SO42− |
| OC4 (R = 0.91) | ↔ | OC3, OCpyro | Mn (R = 0.91) | ↔ | S, Zn, Fe, Mg | OCpyro (R = 0.89) | ↔ | BC |
| Pb (R = 0.89) | ↔ | OCpyro | S (R = 0.91) | ↔ | Mn, Zn, Pb, Mg | SO42− (R = 0.88) | ↔ | NH4+, OC2, S, Mn |
| OC3 (R = 0.88) | ↔ | OCpyro, OC4, nss-K+ | Zn (R = 0.90) | ↔ | Mn, S, NH4+ | NH4+ (R = 0.88) | ↔ | SO42−, OC2, S |
| K (R = 0.84) | ↔ | nss-K+, EC1corr, OCpyro | Pb (R = 0.89) | ↔ | S | |||
| Mn (R = 0.80) | ↔ | Fe, Zn | ||||||
| nss-K+ (R = 0.79) | ↔ | K, EC1corr, OCpyro, OC3 | ||||||
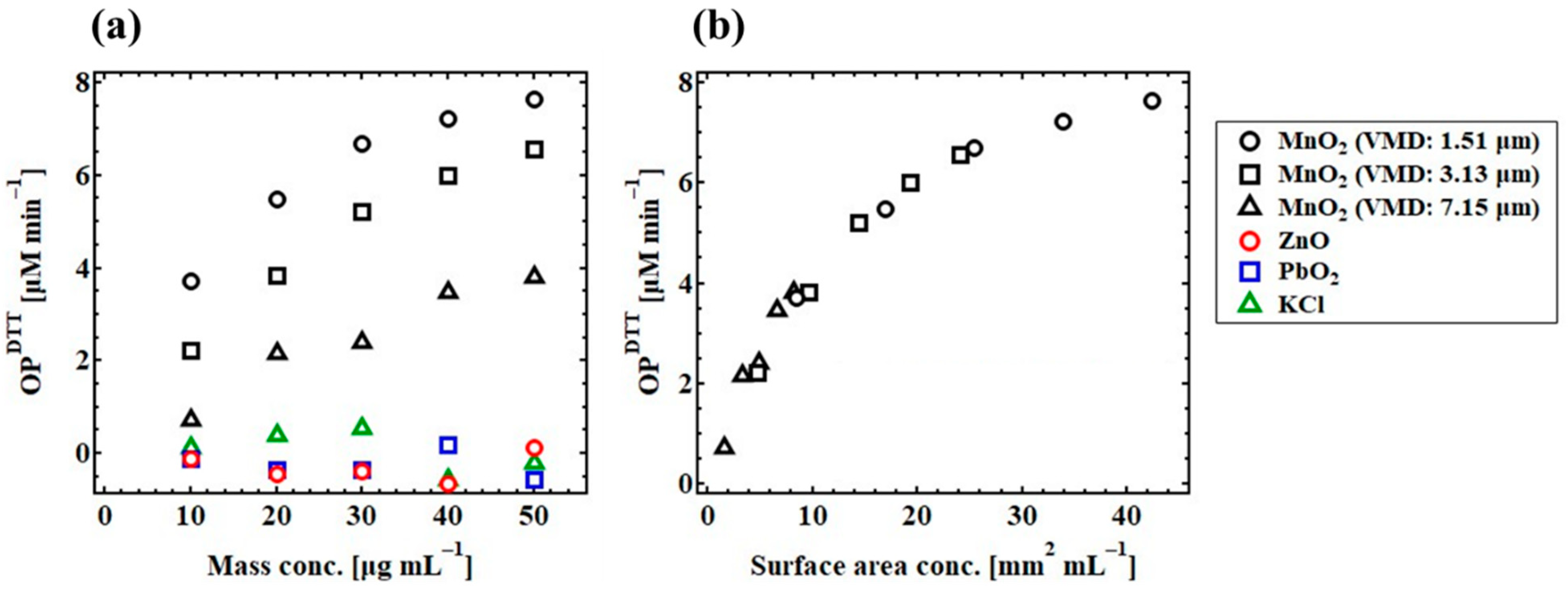
3.3. Contribution of Physical Properties to the Variations in OPDTT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauderman, W.J.; Vora, H.; McConnell, R.; Berhane, K.; Gilliland, F.; Thomas, D.; Lurmann, F.; Avol, E.; Kunzli, N.; Jerrett, M.; et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: A cohort study. Lancet 2007, 369, 571–577. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution: Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoek, G.; Brunekreef, B.; Goldbohm, S.; Fischer, P.; Van Den Brandt, P.A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet 2002, 360, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Rissler, J.; Nordin, E.Z.; Eriksson, A.C.; Nilsson, P.T.; Frosch, M.; Sporre, M.K.; Wierzbicka, A.; Svenningsson, B.; Löndahl, J.; Messing, M.E.; et al. Effective density and mixing state of aerosol particles in a near-traffic urban environment. Environ. Sci. Technol. 2014, 48, 6300–6308. [Google Scholar] [CrossRef]
- Silva, R.A.; West, J.J.; Zhang, Y.; Anenberg, S.C.; Lamarque, J.F.; Shindell, D.T.; Collins, W.J.; Dalsoren, S.; Faluvegi, G.; Folberth, G.; et al. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ. Res. Lett. 2013, 8, 034005. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Biol. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K. Development and laboratory testing of an automated monitor for the measurement of atmospheric particle-bound reactive oxygen species (ROS). Aerosol Sci. Technol. 2008, 42, 629–635. [Google Scholar] [CrossRef]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Nel, A. Air Pollution—Related Illness: Effects of Particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Janssen, N.; Beelen, R.; Schmitz, O.; Vaartjes, I.; Karssenberg, D.; van den Brink, C.; Bots, M.L.; Dijst, M.; Brunekreef, B.; et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ. Int. 2017, 108, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Janssen, N.A.H.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef]
- Zhang, X.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M.; Hasheminassab, S.; Pakbin, P.; Longhurst, J.; Sioutas, C.; et al. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ. Health 2016, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Part Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble\newline transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Lin, P.; Kuang, B.Y.; Yu, J.Z. Reactive oxygen species production mediated by humic-like substances in atmospheric aerosols: Enhancement effects by pyridine, imidazole, and their derivatives. Environ. Sci. Technol. 2015, 49, 6457–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Ning, Z.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 2009, 43, 6360–6368. [Google Scholar] [CrossRef]
- Daellenbach, K.R.; Uzu, G.; Jiang, J.; Cassagnes, L.E.; Leni, Z.; Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020, 587, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pietrogrande, M.C.; Bertoli, I.; Clauser, G.; Dalpiaz, C.; Dell’Anna, R.; Lazzeri, P.; Lenzi, W.; Russo, M. Chemical composition and oxidative potential of atmospheric particles heavily impacted by residential wood burning in the alpine region of northern Italy. Atmos. Environ. 2021, 253, 118360. [Google Scholar] [CrossRef]
- Li, Q.; Wyatt, A.; Kamens, R.M. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ. 2009, 43, 1037–1042. [Google Scholar] [CrossRef]
- Kramer, A.J.; Rattanavaraha, W.; Zhang, Z.; Gold, A.; Surratt, J.D.; Lin, Y.H. Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol. Atmos. Environ. 2016, 130, 211–218. [Google Scholar] [CrossRef]
- Biswas, S.; Verma, V.; Schauer, J.J.; Cassee, F.R.; Cho, A.K.; Sioutas, C. Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ. Sci. Technol. 2009, 43, 3905–3912. [Google Scholar] [CrossRef]
- Gerlofs-Nijland, M.E.; Totlandsdal, A.I.; Tzamkiozis, T.; Leseman, D.L.A.C.; Samaras, Z.; Låg, M.; Schwarze, P.; Ntziachristos, L.; Cassee, F.R. Cell toxicity and oxidative potential of engine exhaust particles: Impact of using particulate filter or biodiesel fuel blend. Environ. Sci. Technol. 2013, 47, 5931–5938. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Li, X.Y.; MacNee, W. Ultrafine (nanometre) particle mediated lung injury. J. Aerosol Sci. 1998, 29, 553–560. [Google Scholar] [CrossRef]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zeng, L.; Gao, D.; Verma, V.; Stefaniak, A.B.; Weber, R.J. Ambient Size Distributions and Lung Deposition of Aerosol Dithiothreitol-Measured Oxidative Potential: Contrast between Soluble and Insoluble Particles. Environ. Sci. Technol. 2017, 51, 6802–6811. [Google Scholar] [CrossRef]
- McWhinney, R.D.; Badali, K.; Liggio, J.; Li, S.M.; Abbatt, J.P.D. Filterable redox cycling activity: A comparison between diesel exhaust particles and secondary organic aerosol constituents. Environ. Sci. Technol. 2013, 47, 3362–3369. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global perspective on the oxidative potential of airborne particulate matter: A synthesis of research findings. Environ. Sci. Technol. 2014, 48, 7576–7583. [Google Scholar] [CrossRef]
- Sauvain, J.J.; Rossi, M.J.; Riediker, M. Comparison of three acellular tests for assessing the oxidation potential of nanomaterials. Aerosol Sci. Technol. 2013, 47, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Arangio, A.M.; Tong, H.; Socorro, J.; Pöschl, U.; Shiraiwa, M. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles. Atmos. Chem. Phys. 2016, 16, 13105–13119. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.J.G.; Schmitz, D.A.; Cho, A.K.; Froines, J.; Fukuto, J.M. Inherent redox properties of diesel exhaust particles: Catalysis of the generation of reactive oxygen species by biological reductants. Toxicol. Sci. 2004, 81, 225–232. [Google Scholar] [CrossRef]
- Sauvain, J.J.; Rossi, M.J. Quantitative Aspects of the Interfacial Catalytic Oxidation of Dithiothreitol by Dissolved Oxygen in the Presence of Carbon Nanoparticles. Environ. Sci. Technol. 2016, 50, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, E.; Kobayashi, T. Chemical and biological oxidative effects of carbon black nanoparticles. Chemosphere 2006, 65, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Polidori, A.; Arhami, M.; Shafer, M.M.; Schauer, J.J.; Cho, A.; Sioutas, C. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long Beach harbor. Atmos. Chem. Phys. 2008, 8, 6439–6451. [Google Scholar] [CrossRef] [Green Version]
- Kajino, M.; Hagino, H.; Fujitani, Y.; Morikawa, T.; Fukui, T.; Onishi, K.; Okuda, T.; Igarashi, Y. Simulation of the transition metal-based cumulative oxidative potential in East Asia and its emission sources in Japan. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Iwata, A.; Fujioka, K.; Yonemichi, T.; Fukagata, K.; Kurosawa, K.; Tabata, R.; Kitagawa, M.; Takashima, T.; Okuda, T. Seasonal variation in atmospheric particle electrostatic charging states determined using a parallel electrode plate device. Atmos. Environ. 2019, 203, 62–69. [Google Scholar] [CrossRef]
- Ueda, S.; Nakayama, T.; Taketani, F.; Adachi, K.; Matsuki, A.; Iwamoto, Y.; Sadanaga, Y.; Matsumi, Y. Light absorption and morphological properties of soot-containing aerosols observed at an East Asian outflow site, Noto Peninsula, Japan. Atmos. Chem. Phys. 2016, 16, 2525–2541. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, Y.; Kinouchi, K.; Watanabe, K.; Yamazaki, N.; Matsuki, A. Simultaneous measurement of CCN activity and chemical composition of fine-mode aerosols at noto Peninsula, Japan, in autumn 2012. Aerosol Air Qual. Res. 2016, 16, 2107–2118. [Google Scholar] [CrossRef] [Green Version]
- Iwata, A.; Matsuki, A. Characterization of individual ice residual particles by the single droplet freezing method: A case study in the Asian dust outflow region. Atmos. Chem. Phys. 2018, 18, 1785–1804. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, K.; Iwata, A.; Kiriya, M.; Yoshino, A.; Takami, A.; Matsuki, A.; Nishita-Hara, C.; Hara, K.; Hayashi, M.; Kaneyasu, N.; et al. Lung deposited surface area of atmospheric aerosol particles at three observatories in Japan. Atmos. Environ. 2021, 262, 118597. [Google Scholar] [CrossRef]
- Asbach, C.; Fissan, H.; Stahlmecke, B.; Kuhlbusch, T.A.J.; Pui, D.Y.H. Conceptual limitations and extensions of lung-deposited Nanoparticle Surface Area Monitor (NSAM). J. Nanopart. Res. 2009, 11, 101–109. [Google Scholar] [CrossRef]
- Fissan, H.; Neumann, S.; Trampe, A.; Pui, D.Y.H.; Shin, W.G. Rationale and principle of an instrument measuring lung deposited nanoparticle surface area. J. Nanopart. Res. 2007, 9, 53–59. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, T.; Zhu, Z.; Ma, Y.; Ma, X.; Wang, W. Characteristics of PM1.0, PM2.5, and PM10, and their relation to black carbon in Wuhan, central China. Atmosphere 2015, 6, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yonemochi, S.; Umezawa, N. Parallel continuous observation of submicron particle (PM1) and PM2.5, and characterization of PM1 in a suburban of Tokyo. J. Jpn. Soc. Atmos. Environ. 2010, 45, 271–278. [Google Scholar]
- Konishi, T.; Yonemochi, S.; Murata, M. Chemical composition and sources estimation of PM2.5 and submicron particles (PM1) in urban atmosphere based on chemical components. Bunseki Kagaku 2018, 67, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Rolph, G.; Stein, A.; Stunder, B. Real-time Environmental Applications and Display sYstem: READY. Environ. Model. Softw. 2017, 95, 210–228. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. Noaa’s hysplit atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Alimov, Z.B.; Kusakari, H.; Okuda, T. Development of A Low-cost Simultaneous Low Volume Air Sampler Controlled with Sonic Venturi. Asian J. Atmos. Environ. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Matthews, P.S.J.; Baeza-Romero, M.T.; Whalley, L.K.; Heard, D.E. Uptake of HO2 radicals onto Arizona test dust particles using an aerosol flow tube. Atmos. Chem. Phys. 2014, 14, 7397–7408. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Schauer, J.J.; Shafer, M.M. Improved methods for elemental analysis of atmospheric aerosols for evaluating human health impacts of aerosols in East Asia. Atmos. Environ. 2014, 97, 552–555. [Google Scholar] [CrossRef]
- Bukowiecki, N.; Dommen, J.; Prévôt, A.S.H.; Richter, R.; Weingartner, E.; Baltensperger, U. A mobile pollutant measurement laboratory—Measuring gas phase and aerosol ambient concentrations with high spatial and temporal resolution. Atmos. Environ. 2002, 36, 5569–5579. [Google Scholar] [CrossRef]
- Ketzel, M.; Wåhlin, P.; Kristensson, A.; Swietlicki, E.; Berkowicz, R.; Nielsen, O.J.; Palmgren, F. Particle size distribution and particle mass measurements at urban, near city and rural level in the Copenhagen area and Southern Sweden. Atmos. Chem. Phys. 2004, 4, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, P.C.; Gomes, J.F.; Bordado, J.C. Assessment of exposure to airborne ultrafine particles in the urban environment of Lisbon, Portugal. J. Air Waste Manag. Assoc. 2012, 62, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takegawa, N.; Miyakawa, T.; Kondo, Y.; Jimenez, J.L.; Zhang, Q.; Worsnop, D.R.; Fukuda, M. Seasonal and diurnal variations of submicron organic aerosol in Tokyo observed using the Aerodyne aerosol mass spectrometer. J. Geophys. Res. Atmos. 2006, 111, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Kikuchi, M.; Iwabuchi, M.; Hara, M.; Takahashi, A.; Kidokoro, Y. Study of Suspended Particulate Matter in Kawasaki City (1991–1998). Annu. Rep. Kawasaki Munic. Res. Inst. Environ. Prot. 2000, 27, 12–25. [Google Scholar]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- McMurry, P.H. Chapter 17 A review of atmospheric aerosol measurements. Dev. Environ. Sci. 2002, 1, 443–517. [Google Scholar] [CrossRef]
- Garratt, J.R. Review: The atmospheric boundary layer. Earth-Sci. Rev. 1994, 37, 89–134. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.J. Species contributions to pm2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Yoshino, A.; Takami, A.; Sato, K.; Shimizu, A.; Kaneyasu, N.; Hatakeyama, S.; Hara, K.; Hayashi, M. Influence of trans-boundary air pollution on the urban atmosphere in Fukuoka, Japan. Atmosphere 2016, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Takami, A.; Miyoshi, T.; Irei, S.; Yoshino, A.; Sato, K.; Shimizu, A.; Hayashi, M.; Hara, K.; Kaneyasu, N.; Hatakeyama, S. Analysis of organic aerosol in Fukuoka, Japan using a PMF method. Aerosol Air Qual. Res. 2016, 16, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Nishita-Hara, C.; Hirabayashi, M.; Hara, K.; Yamazaki, A.; Hayashi, M. Dithiothreitol-Measured Oxidative Potential of Size-Segregated Particulate Matter in Fukuoka, Japan: Effects of Asian Dust Events. GeoHealth 2019, 3, 160–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujitani, Y.; Furuyama, A.; Tanabe, K.; Hirano, S. Comparison of oxidative abilities of PM2.5 collected at traffic and residential sites in Japan. contribution of transition metals and primary and secondary aerosols. Aerosol Air Qual. Res. 2017, 17, 574–587. [Google Scholar] [CrossRef] [Green Version]
- Brehmer, C.; Lai, A.; Clark, S.; Shan, M.; Ni, K.; Ezzati, M.; Yang, X.; Baumgartner, J.; Schauer, J.J.; Carter, E. The Oxidative Potential of Personal and Household PM2.5 in a Rural Setting in Southwestern China. Environ. Sci. Technol. 2019, 53, 2788–2798. [Google Scholar] [CrossRef]
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Fushimi, A.; Saitoh, K.; Hayashi, K.; Ono, K.; Fujitani, Y.; Villalobos, A.M.; Shelton, B.R.; Takami, A.; Tanabe, K.; Schauer, J.J. Chemical characterization and oxidative potential of particles emitted from open burning of cereal straws and rice husk under flaming and smoldering conditions. Atmos. Environ. 2017, 163, 118–127. [Google Scholar] [CrossRef]
- Shafer, M.M.; Perkins, D.A.; Antkiewicz, D.S.; Stone, E.A.; Quraishi, T.A.; Schauer, J.J. Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore, Pakistan: An important role for transition metals. J. Environ. Monit. 2010, 12, 704–715. [Google Scholar] [CrossRef]
- Yu, J.Z.; Xu, J.; Yang, H. Charring characteristics of atmospheric organic particulate matter in thermal analysis. Environ. Sci. Technol. 2002, 36, 754–761. [Google Scholar] [CrossRef]
- Verma, V.; Wang, Y.; El-Afifi, R.; Fang, T.; Rowland, J.; Russell, A.G.; Weber, R.J. Fractionating ambient humic-like substances (HULIS) for their reactive oxygen species activity—Assessing the importance of quinones and atmospheric aging. Atmos. Environ. 2015, 120, 351–359. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.; Zhao, J.; Wang, Y.; Shi, Q.; Peng, J.; Wang, Y.; Wang, C.; Zhang, F.; Wang, Y.; et al. Unexpected Oligomerization of Small α-Dicarbonyls for Secondary Organic Aerosol and Brown Carbon Formation. Environ. Sci. Technol. 2021, 55, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Sartelet, K.; Couvidat, F.; Wang, Z.; Flageul, C.; Kim, Y. SSH-aerosol v1.1: A modular box model to simulate the evolution of primary and secondary aerosols. Atmosphere 2020, 11, 525. [Google Scholar] [CrossRef]
- Xiao, H.W.; Xiao, H.Y.; Shen, C.Y.; Zhang, Z.Y.; Long, A.M. Chemical composition and sources of marine aerosol over the western north Pacific ocean in winter. Atmosphere 2018, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Keene, W.C.; Pszenny, A.A.P.; Galloway, J.N.; Hawley, M.E. Sea-salt corrections and interpretation of constituent ratios in marine precipitation. J. Geophys. Res. 1986, 91, 6647. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J. Atmospheric heavy metals and Arsenic in China: Situation, sources and control policies. Atmos. Environ. 2013, 74, 93–101. [Google Scholar] [CrossRef]
- Calvo, A.I.; Alves, C.; Castro, A.; Pont, V.; Vicente, A.M.; Fraile, R. Research on aerosol sources and chemical composition: Past, current and emerging issues. Atmos. Res. 2013, 120–121, 1–28. [Google Scholar] [CrossRef]
- Yu, J.; Yan, C.; Liu, Y.; Li, X.; Zhou, T.; Zheng, M. Potassium: A tracer for biomass burning in Beijing? Aerosol Air Qual. Res. 2018, 18, 2447–2459. [Google Scholar] [CrossRef] [Green Version]
- Sakata, M.; Kurata, M.; Tanaka, N. Estimating contribution from municipal solid waste incineration to trace metal concentrations in Japanese urban atmosphere using lead as a marker element. Geochem. J. 2000, 34, 23–32. [Google Scholar] [CrossRef]
- Sakata, M.; Marumoto, K. Formation of atmospheric particulate mercury in the Tokyo metropolitan area. Atmos. Environ. 2002, 36, 239–246. [Google Scholar] [CrossRef]
- Choël, M.; Deboudt, K.; Flament, P. Development of time-resolved description of aerosol properties at the particle scale during an episode of industrial pollution plume. Water Air Soil Pollut. 2010, 209, 93–107. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.Z. Uncertainties in charring correction in the analysis of elemental and organic carbon in atmospheric particles by thermal/optical methods. Environ. Sci. Technol. 2002, 36, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Han, B.; Huo, J.; Lu, B.; Ding, X.; Chen, L.; Kong, S.F.; Bai, Z.P.; Wang, B. Characterization, meteorological influences and source identification of carbonaceous aerosols during the autumn-winter period in Tianjin, China. Aerosol Air Qual. Res. 2012, 12, 283–294. [Google Scholar] [CrossRef]
- Hidemori, T.; Nakayama, T.; Matsumi, Y.; Kinugawa, T.; Yabushita, A.; Ohashi, M.; Miyoshi, T.; Irei, S.; Takami, A.; Kaneyasu, N.; et al. Characteristics of atmospheric aerosols containing heavy metals measured on Fukue Island, Japan. Atmos. Environ. 2014, 97, 447–455. [Google Scholar] [CrossRef]
- Tian, H.Z.; Wang, Y.; Xue, Z.G.; Cheng, K.; Qu, Y.P.; Chai, F.H.; Hao, J.M. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in China, 1980–2007. Atmos. Chem. Phys. 2010, 10, 11905–11919. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Li, M.; Zou, C.; Fan, X.; Song, J.; Jia, W.; Yu, C.; Yu, Z.; Peng, P. Chemical composition, optical properties, and oxidative potential of water- and methanol-soluble organic compounds emitted from the combustion of biomass materials and coal. Atmos. Chem. Phys. Discuss. 2021, 21, 13187–13205. [Google Scholar] [CrossRef]
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392. [Google Scholar] [CrossRef]
- Yu, H.; Wei, J.; Cheng, Y.; Subedi, K.; Verma, V. Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environ. Sci. Technol. 2018, 52, 2261–2270. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, K.; Iwata, A.; Murray Horwitz, S.G.; Ogane, K.; Sugioka, T.; Matsuki, A.; Okuda, T. Contribution of Physical and Chemical Properties to Dithiothreitol-Measured Oxidative Potentials of Atmospheric Aerosol Particles at Urban and Rural Sites in Japan. Atmosphere 2022, 13, 319. https://doi.org/10.3390/atmos13020319
Kurihara K, Iwata A, Murray Horwitz SG, Ogane K, Sugioka T, Matsuki A, Okuda T. Contribution of Physical and Chemical Properties to Dithiothreitol-Measured Oxidative Potentials of Atmospheric Aerosol Particles at Urban and Rural Sites in Japan. Atmosphere. 2022; 13(2):319. https://doi.org/10.3390/atmos13020319
Chicago/Turabian StyleKurihara, Kazuki, Ayumi Iwata, Samuel Gray Murray Horwitz, Kako Ogane, Tomoki Sugioka, Atsushi Matsuki, and Tomoaki Okuda. 2022. "Contribution of Physical and Chemical Properties to Dithiothreitol-Measured Oxidative Potentials of Atmospheric Aerosol Particles at Urban and Rural Sites in Japan" Atmosphere 13, no. 2: 319. https://doi.org/10.3390/atmos13020319
APA StyleKurihara, K., Iwata, A., Murray Horwitz, S. G., Ogane, K., Sugioka, T., Matsuki, A., & Okuda, T. (2022). Contribution of Physical and Chemical Properties to Dithiothreitol-Measured Oxidative Potentials of Atmospheric Aerosol Particles at Urban and Rural Sites in Japan. Atmosphere, 13(2), 319. https://doi.org/10.3390/atmos13020319








