Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Kinetic Study (IMT Nord Europe)
- −
- a Pyrex laminar flow reactor (LFR) coupled to a GC with a double detection by Mass Spectrometry and Flame Ionization Detection (GC-MS/FID).
- −
- a 300 L Teflon atmospheric simulation chamber (ASC) coupled to a FTIR spectrometer.
2.2. Mechanistic Study
2.2.1. Product Identification and Quantification in the Absence of an OH Scavenger (QUAREC)
2.2.2. Product Identification and Quantification in the Presence of an OH Scavenger (RASC)
2.3. SOA Formation (IMT Nord Europe)
3. Computational Methodology (Bharathiar University)
4. Results and Discussion
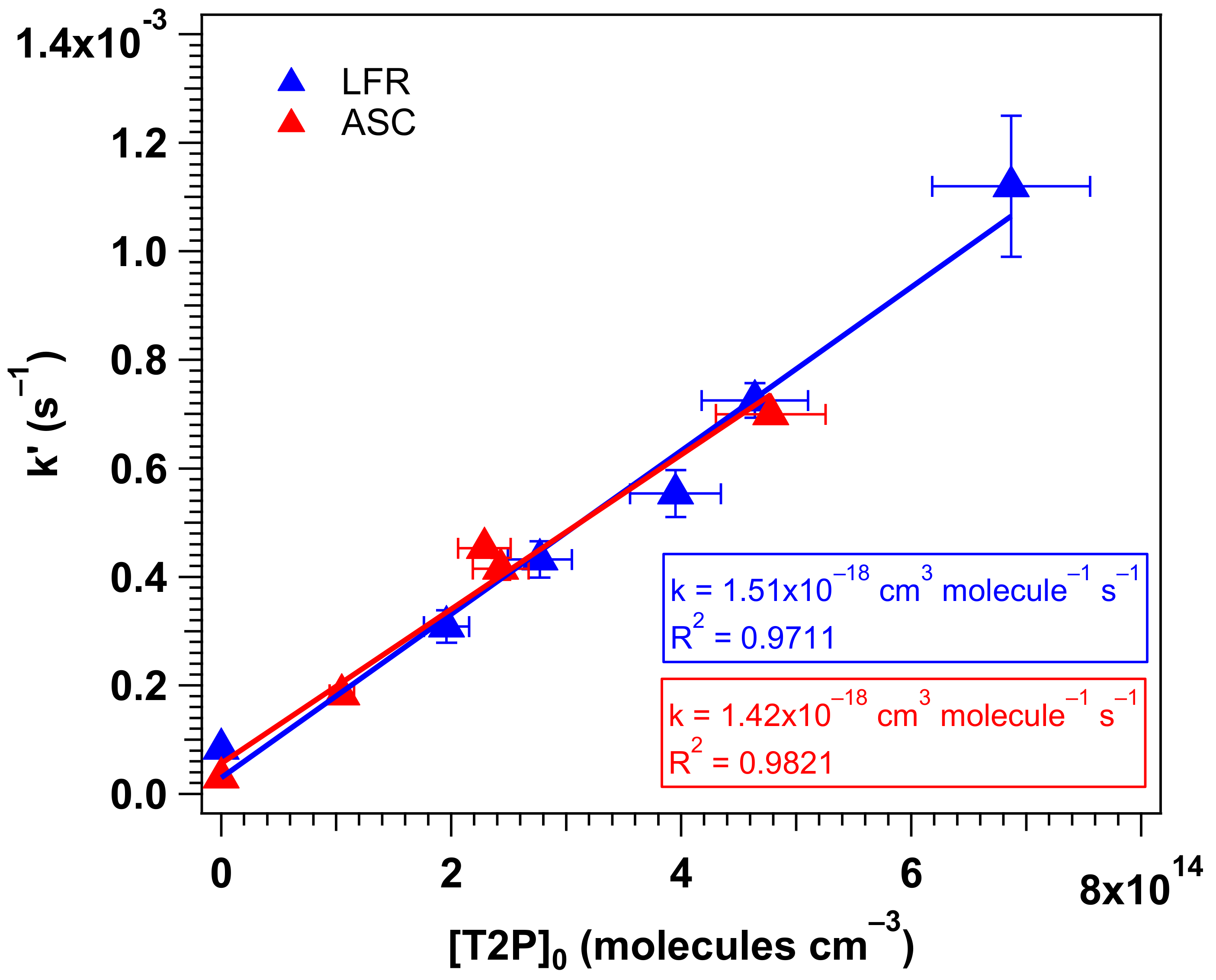
4.1. Experimental Kinetic Study
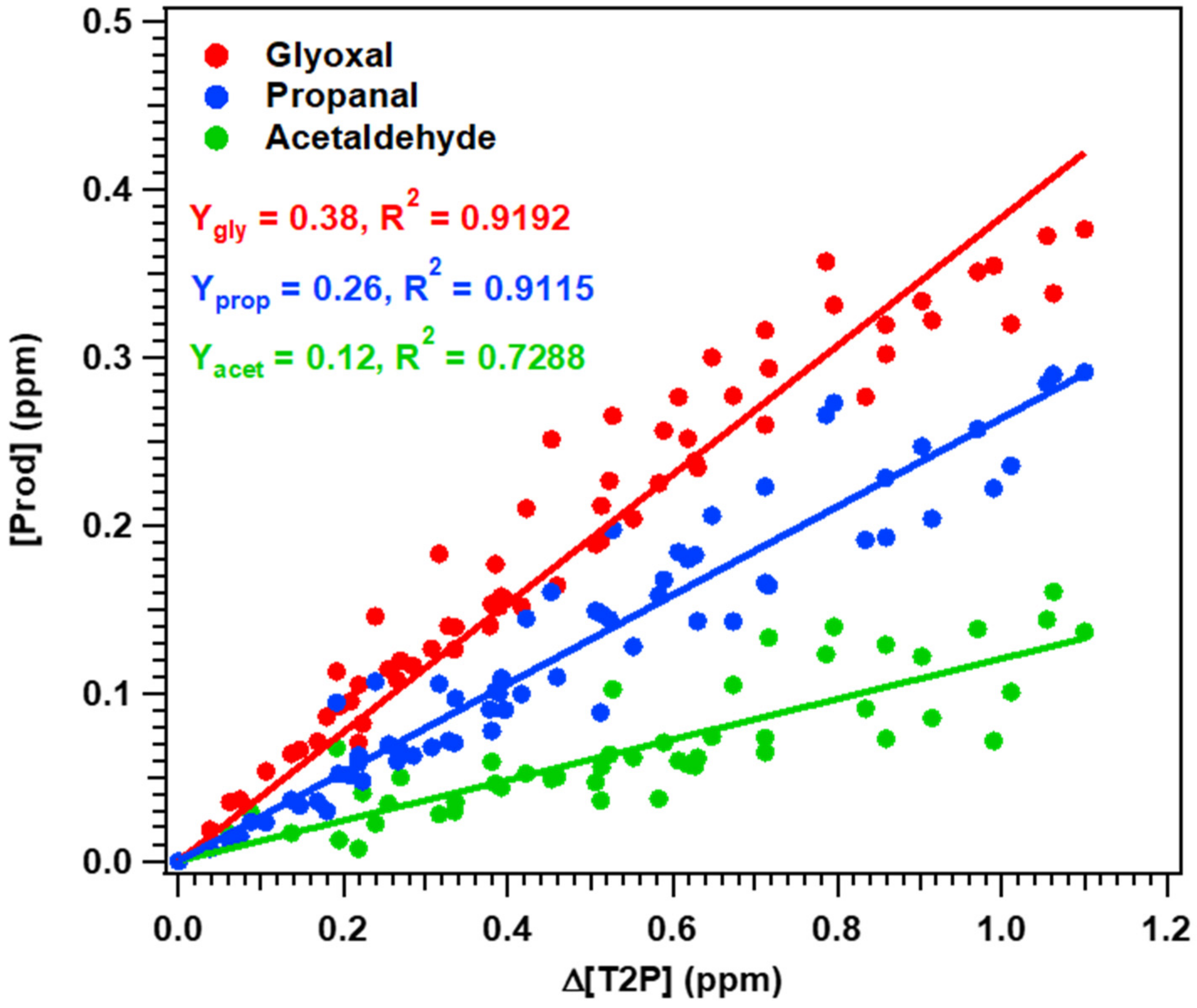
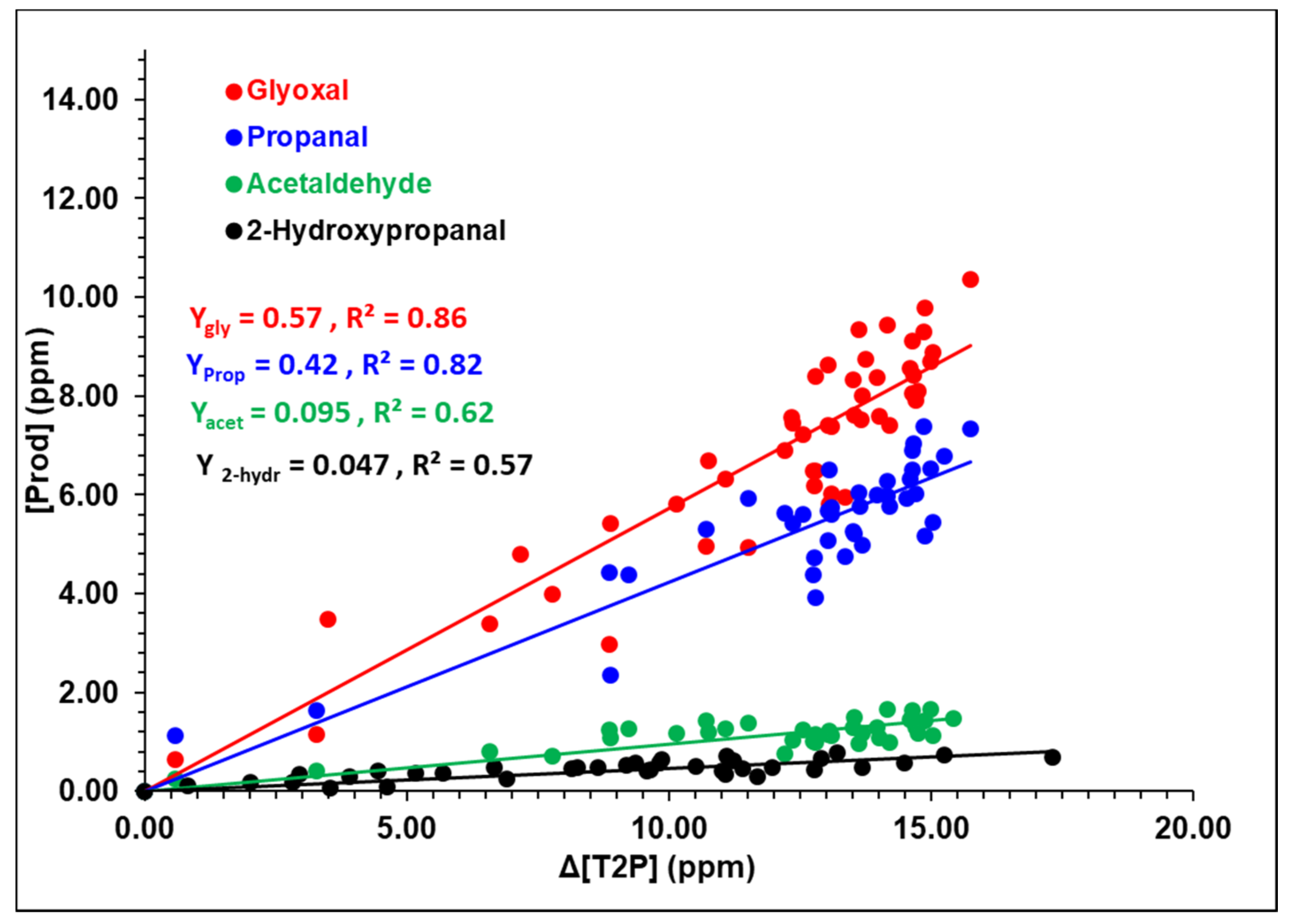
4.2. Experimental Mechanistic Study
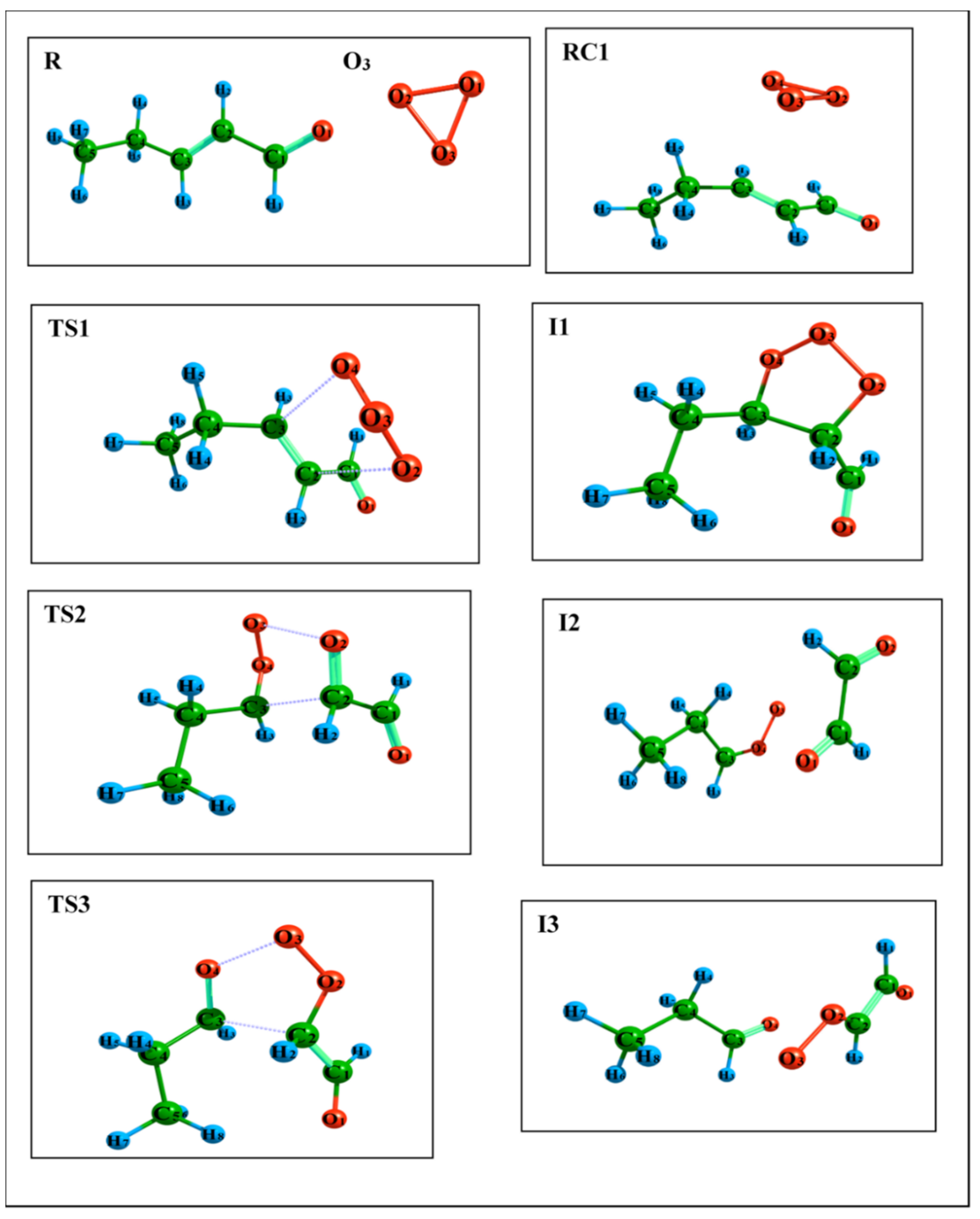
4.3. Theoretical Results
4.4. Comparison with Other Unsaturated Aldehydes
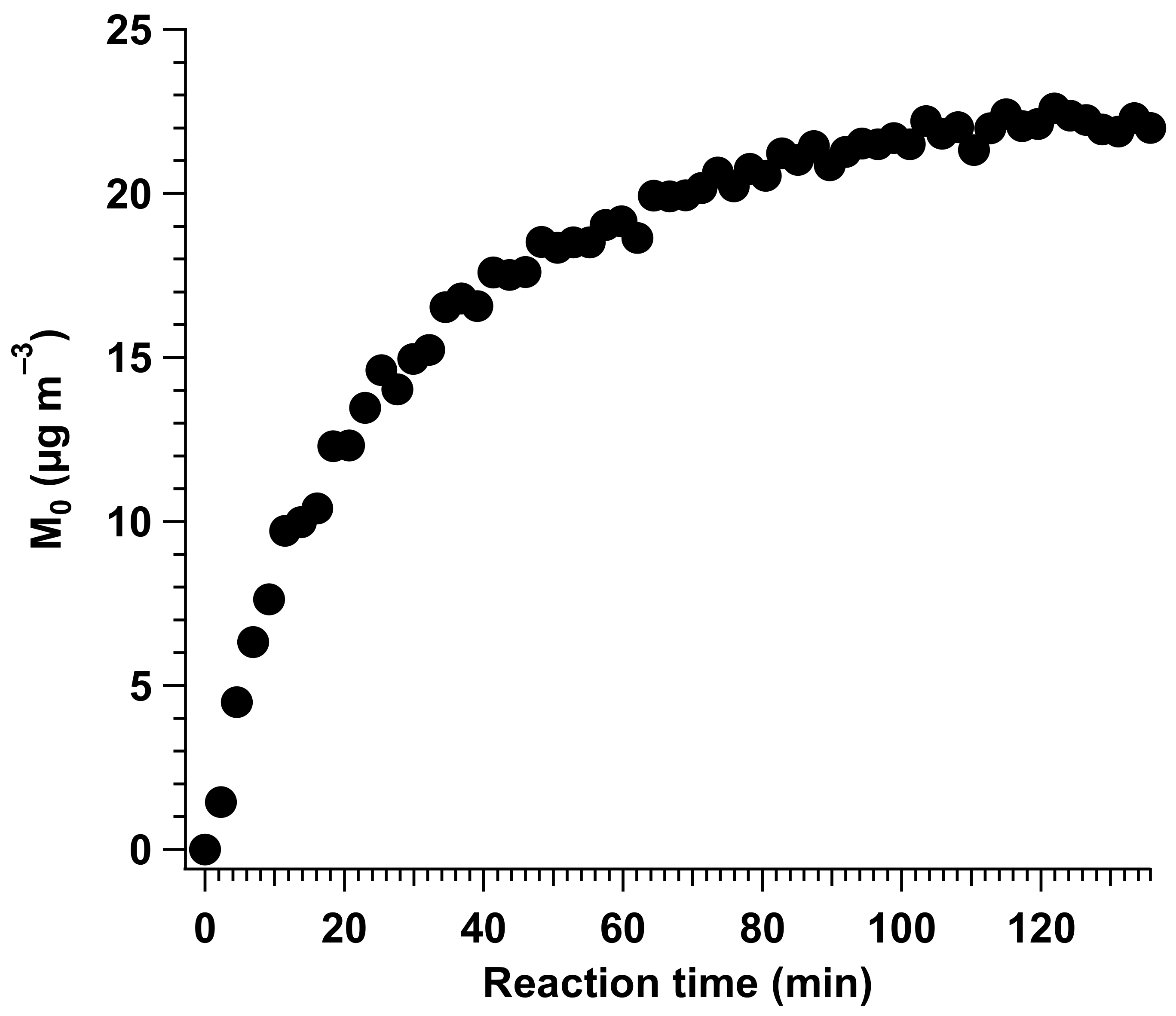
4.5. SOA Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, 8873. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.P.; Wenger, J.C.; Mellouki, A.; Wirtz, K.; Muñoz, A. The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal. Phys. Chem. Chem. Phys. 2006, 8, 5236–5246. [Google Scholar] [CrossRef] [PubMed]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compound (VOC): An Overview on Emissions, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Gardner, H.W.; Grove, M.J.; Salch, Y.P. Enzymic pathway to ethyl vinyl ketone and 2-pentenal in soybean preparations. J. Agric. Food Chem. 1996, 44, 882–886. [Google Scholar] [CrossRef]
- Grira, A.; Kalalian, C.; Illmann, J.N.; Patroescu-Klotz, I.; El Dib, G.; Coddeville, P.; Canosa, A.; Coeur, C.; Wiesen, P.; Roth, E.; et al. Gas-phase ozonolysis of trans-2-hexenal: Kinetics, products, mechanism and SOA formation. Atmos. Environ. 2021, 253, 118344. [Google Scholar] [CrossRef]
- Gaona Colmán, E.; Blanco, M.B.; Barnes, I.; Wiesen, P.; Teruel, M.A. Mechanism and Product Distribution of the O 3 -Initiated Degradation of (E)-2-Heptenal, (E)-2-Octenal and (E)-2-Nonenal. J. Phys. Chem. A 2017, 121, 5147–5155. [Google Scholar] [CrossRef]
- Colmán, E.G.; Blanco, M.B.; Barnes, I.; Teruel, M.A. Ozonolysis of a series of C7–C9 unsaturated biogenic aldehydes: Reactivity study at atmospheric pressure. RSC Adv. 2015, 5, 30500–30506. [Google Scholar] [CrossRef] [Green Version]
- Gaona Colman, E.; Blanco, M.B.; Barnes, I.; Teruel, M.A. Kinetics of the gas-phase reaction between ozone and three unsaturated oxygenated compounds: Ethyl 3, 3-dimethyl acrylate, 2-methyl-2-pentenal and 6-methyl-5-hepten-2-one at atmospheric pressure. Atmos. Environ. 2015, 109, 272–278. [Google Scholar] [CrossRef]
- Albaladejo, J.; Ballesteros, B.; Jiménez, E.; Martín, P.; Martínez, E. A PLP-LIF kinetic study of the atmospheric reactivity of a series of C4-C7 saturated and unsaturated aliphatic aldehydes with OH. Atmos. Environ. 2002, 36, 3231–3239. [Google Scholar] [CrossRef]
- Sato, K.; Taketsugu, T.; Takayanagi, T.; Division, A.E.; Klotz, B.; Taketsugu, T.; Takayanagi, T.; Sato, K.; Division, A.E.; Klotz, B.; et al. Kinetic measurements for the reactions of ozone with crotonaldehyde and its methyl derivatives and calculations of transition-state theory. Phys. Chem. 2004, 6, 3969–3976. [Google Scholar] [CrossRef]
- Kalalian, C.; Roth, E.; Chakir, A. Rate Coefficients for the Gas-Phase Reaction of Ozone with C5 and C6 Unsaturated Aldehydes. Int. J. Chem. Kinet. 2017, 50, 47–56. [Google Scholar] [CrossRef]
- Duncianu, M.; Olariu, R.I.; Riffault, V.; Visez, N.; Tomas, A.; Coddeville, P. Development of a new flow reactor for kinetic studies. Application to the ozonolysis of a series of alkenes. J. Phys. Chem. A 2012, 116, 6169–6179. [Google Scholar] [CrossRef] [PubMed]
- Turpin, E.; Tomas, A.; Fittschen, C.; Devolder, P.; Galloo, J.C. Acetone-h6 or -d6 + OH reaction products: Evidence for heterogeneous formation of acetic acid in a simulation chamber. Environ. Sci. Technol. 2006, 40, 5956–5961. [Google Scholar] [CrossRef] [PubMed]
- Grosiean, E.; Grosjean, D. Rate Constants for the Gas-Phase Reaction of Ozone with 1, 1-Disubstituted Alkenes. Int. J. Chem. Kinet. 1996, 28, 911–918. [Google Scholar] [CrossRef]
- Illmann, J.N.; Patroescu-Klotz, I.; Wiesen, P. Gas-phase reactivity of acyclic α,β-unsaturated carbonyls towards ozone. Phys. Chem. Chem. Phys. 2021, 23, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Illmann, N.; Gastón Gibilisco, R.; Bejan, I.G.; Patroescu-Klotz, I.; Wiesen, P. Atmospheric oxidation of α,β-unsaturated ketones: Kinetics and mechanism of the OH radical reaction. Atmos. Chem. Phys. Discuss. 2021, 1–34. [Google Scholar]
- Volkamer, R.; Spietz, P.; Burrows, J.; Platt, U. High-resolution absorption cross-section of glyoxal in the UV-vis and IR spectral ranges. J. Photochem. Photobiol. A Chem. 2005, 172, 35–46. [Google Scholar] [CrossRef]
- Ahmad, W.; Coeur, C.; Tomas, A.; Fagniez, T.; Brubach, J.-B.; Cuisset, A. Infrared spectroscopy of secondary organic aerosol precursors and investigation of the hygroscopicity of SOA formed from the OH reaction with guaiacol and syringol. Appl. Opt. 2017, 56, E116–E122. [Google Scholar] [CrossRef]
- Laversin, H.; Masri, A.E.; Rashidi, M.A.; Roth, E.; Chakir, A. Kinetic of the gas-phase reactions of OH radicals and Cl atoms with diethyl ethylphosphonate and triethyl phosphate. Atmos. Environ. 2016, 126, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Kalalian, C.; Roth, E.; El Dib, G.; Singh, H.J.; Rao, P.K.; Chakir, A. Product investigation of the gas phase ozonolysis of 1-penten-3-ol, cis-2-penten-1-ol and trans-3-hexen-1-ol. Atmos. Environ. 2020, 238, 117732. [Google Scholar] [CrossRef]
- Reisen, F.; Aschmann, S.M.; Atkinson, R.; Arey, J. Hydroxyaldehyde products from hydroxyl radical reactions of Z-3-hexen-1-ol and 2-methyl-3-buten-2-ol quantified by SPME and API-MS. Environ. Sci. Technol. 2003, 37, 4664–4671. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, Å.M.; Hallquist, M.; Ljungström, E. Influence of OH scavenger on the water effect on secondary organic aerosol formation from ozonolysis of limonene, Δ3-carene, and α-pinene. Environ. Sci. Technol. 2008, 42, 5938–5944. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Ding, X.; Hakala, J.; Hofbauer, V.; Robinson, E.S.; Donahue, N.M. Vapor wall loss of semi-volatile organic compounds in a Teflon chamber. Aerosol Sci. Technol. 2016, 50, 822–834. [Google Scholar] [CrossRef] [Green Version]
- Odum, J.R.; Hoffmann, T.; Bowman, F.; Collins, D.; Flagan, R.C.; Seinfeld, J.H.; Odum Jay, R.; Hoffmann, T.; Bowman, F.; Collins, D.; et al. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 1996, 30, 2580–2585. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Gonzalez, C.; Bernhard Schlegel, H. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Aazaad, B.; Lakshmipathi, S. OH initiated oxidation mechanism of monoterpene (linalool)—A first comprehensive theoretical study. Atmos. Environ. 2018, 189, 235–243. [Google Scholar] [CrossRef]
- Aazaad, B.; Lakshmipathi, S. Reaction of NO3 radical with benzyl alcohol—A DFT study. Comput. Theor. Chem. 2017, 1102, 51–59. [Google Scholar] [CrossRef]
- Priya, A.M.; Senthilkumar, L. Degradation of methyl salicylate through Cl initiated atmospheric oxidation-a theoretical study. RSC Adv. 2014, 4, 23464–23475. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Criterion of minimum state density in the transition state theory of bimolecular reactions. J. Chem. Phys. 1979, 70, 1593–1598. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized Transition State Theory. Bond Energy-Bond Order Method for Canonical Variational Calculations with Application to Hydrogen Atom Transfer Reactions. J. Am. Chem. Soc. 1979, 101, 4534–4548. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G.; Grev, R.S.; Magnuson, A.W. Improved treatment of threshold contributions in variational transition-state theory. J. Phys. Chem. 1980, 84, 1730–1748. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lynch, G.C.; Truong, T.N.; Lu, D.H.; Truhlar, D.G.; Garrett, B.C. Molecular Modeling of the Kinetic Isotope Effect for the [1,5] Sigmatropic Rearrangement of cis-1,3-Pentadiene. J. Am. Chem. Soc. 1993, 115, 2408–2415. [Google Scholar] [CrossRef]
- Lu, D.H.; Truong, T.N.; Melissas, V.S.; Lynch, G.C.; Liu, Y.P.; Garrett, B.C.; Steckler, R.; Isaacson, A.D.; Rai, S.N.; Hancock, G.C.; et al. POLYRATE 4: A new version of a computer program for the calculation of chemical reaction rates for polyatomics. Comput. Phys. Commun. 1992, 71, 235–262. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, S.; Corchado, J.C.; Chuang, Y.Y.; Coitino, E.L.; Ellingson, B.A.; Truhlar, D.G. Gaussrate, Version 2009-A. Univ. Minnesota Minneapolis, MN 2010. Available online: https://comp.chem.umn.edu/gaussrate/gaussrate_Manual_v2009A.2010.03.17.pdf (accessed on 4 February 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision B. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Zheng, J.; Zhang, S.; Lynch, B.J.; Corchado, J.C.; Chuang, Y.; Fast, P.L.; Hu, W.; Liu, Y.; Lynch, G.C.; Nguyen, K.A.; et al. Manual POLYRATE—Version 2010-A. 2010. Available online: https://comp.chem.umn.edu/polyrate/polyrate_Manual_v2010A.2010.6.16.pdf (accessed on 4 February 2022).
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G. MOLPRO, Version 2010.1, a Package of Ab Initio Programs. 2010. Available online: http//www.molpro.net (accessed on 4 February 2022).
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Johnson, D.; Marston, G. The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem. Soc. Rev. 2008, 37, 699–716. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosjean, D. Carbonyl products of the gas phase reaction of ozone with symmetrical alkenes. Environ. Sci. Technol. 1996, 30, 2036–2044. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, F.; Klippenstein, S.J.; Lester, M.I. Direct observation of unimolecular decay of CH3CH2CHOO Criegee intermediates to OH radical products. J. Chem. Phys. 2016, 145. [Google Scholar] [CrossRef]
- Grosjean, E.; Williams, E.L., II; Grosjean, D. Atmospheric chemistry of acrolein. Sci. Total Environ. 1994, 153, 195–202. [Google Scholar] [CrossRef]
- Grosjean, D.; Williams II, E.L.; Grosjean, E. Atmospheric Chemistry of Isoprene and of Its Carbonyl Products. Environ. Sci. Technol 1993, 27, 830–840. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosjean, D. The gas phase reaction of unsaturated oxygenates with ozone: Carbonyl products and comparison with the alkene-ozone reaction. J. Atmos. Chem. 1997, 27, 271–289. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosiean, D.; Selnfeldt, J.H. Gas-Phase Reaction of Ozone with Trans-2-Hexenal, Trans-2-Hexenyl Acetate, Ethylvinyl Ketone, and 6-Methyl-5-Hepten-2-One. Int. J. Chem. Kinet. 1996, 28, 373–382. [Google Scholar] [CrossRef]
- Tuazon, E.C.; Aschmann, S.M.; Arey, J.; Atkinson, R. Products of the gas-phase reactions of O3 with a series of methyl- substituted ethenes. Environ. Sci. Technol. 1997, 31, 3004–3009. [Google Scholar] [CrossRef]
- Wegener, R.; Brauers, T.; Koppmann, R.; Bares, S.R.; Rohrer, F.; Tillman, R.; Wahner, A.; Hansel, A.; Wisthaler, A. Simulation chamber investigation of the reactions of ozone with short-chained alkenes. J. Geophys. Res. Atmos. 2007, 112, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, Z.; Huang, D.D.; Wang, X.; Chan, C.K. Significant Production of Secondary Organic Aerosol from Emissions of Heated Cooking Oils. Environ. Sci. Technol. Lett. 2018, 5, 32–37. [Google Scholar] [CrossRef]


| Reactor | [T2P]0 (ppm) | [O3]0 (ppb) | k (10−18 cm3 molecule−1 s−1) | |
|---|---|---|---|---|
| This Work | Literature | |||
| LFR | 7.9−28 | ~550 | 1.42 ± 0.21 a | 1.59 ± 0.22 [11] |
| ASC | 4.3−19 | 270−440 | 1.51 ± 0.22 a | 1.24 ± 0.06 [12] |
| Average | 1.46 ± 0.31 b | 1.4 c [16] | ||
| OH Scavenger | Identified Products | Yield (%) |
|---|---|---|
| Yes | Glyoxal | 57 ± 10 |
| Propanal | 42 ± 12 | |
| 2-hydroxypropanal | 5 ± 2 | |
| Acetaldehyde | 9 ± 3 | |
| No | Glyoxal | 38 ± 8 |
| Propanal | 26 ± 5 | |
| Acetaldehyde | 12 ± 3 | |
| Propanoic acid | - |
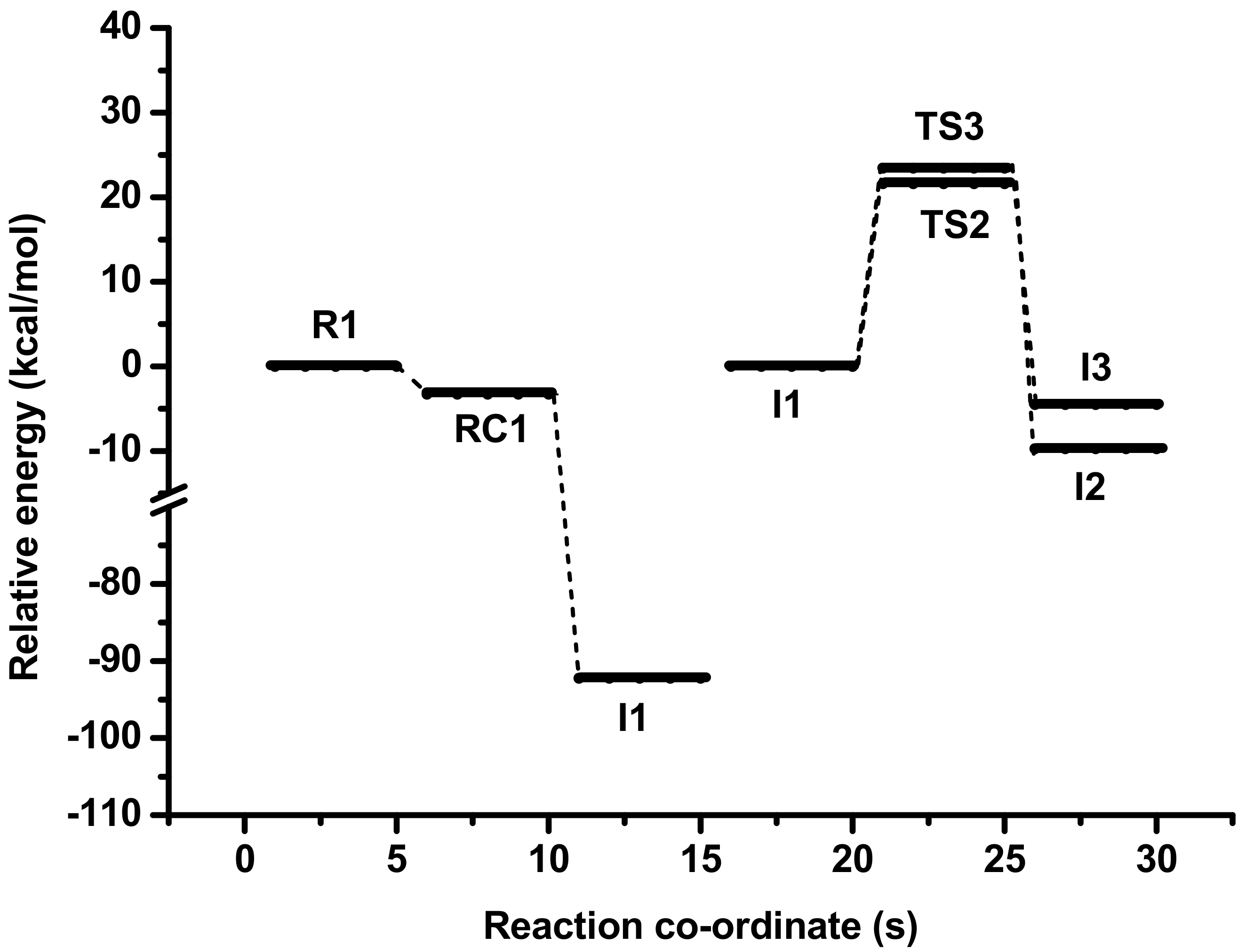
| Reaction Channel | M06−2X | BH and HLYP | MP2 | CCSD(T) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔE | ΔH | ΔG | ΔE | ΔH | ΔG | ΔE | ΔH | ΔG | ΔE | ||
| Path 1 | R1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RC1 | −3.30 | −2.63 | −6.16 | −1.75 | −1.27 | −5.18 | −3.38 | −2.99 | −3.72 | −2.27 | |
| TS1 | −22.47 | −20.70 | −20.44 | −19.69 | −17.74 | −15.46 | −38.27 | −35.76 | −29.72 | −31.10 | |
| I1 | −92.25 | −87.85 | −86.34 | −90.21 | −85.44 | −81.94 | −84.21 | −79.30 | −72.73 | −86.51 | |
| Path 2 | I1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TS2 | 21.60 | 19.81 | 19.98 | 25.63 | 23.41 | 23.32 | 3.85 | 2.47 | 2.49 | 13.71 | |
| I2 | −9.75 | −12.33 | −15.14 | −13.08 | −16.60 | −20.50 | −8.42 | −11.34 | −14.68 | −11.07 | |
| Path 3 | TS3 | 23.42 | 22.16 | 22.31 | 26.67 | 24.17 | 24.31 | 6.82 | 5.71 | 6.18 | 15.74 |
| I3 | −4.52 | −7.36 | −10.67 | −8.09 | −11.67 | −15.87 | −6.76 | −10.20 | −15.41 | −8.42 | |
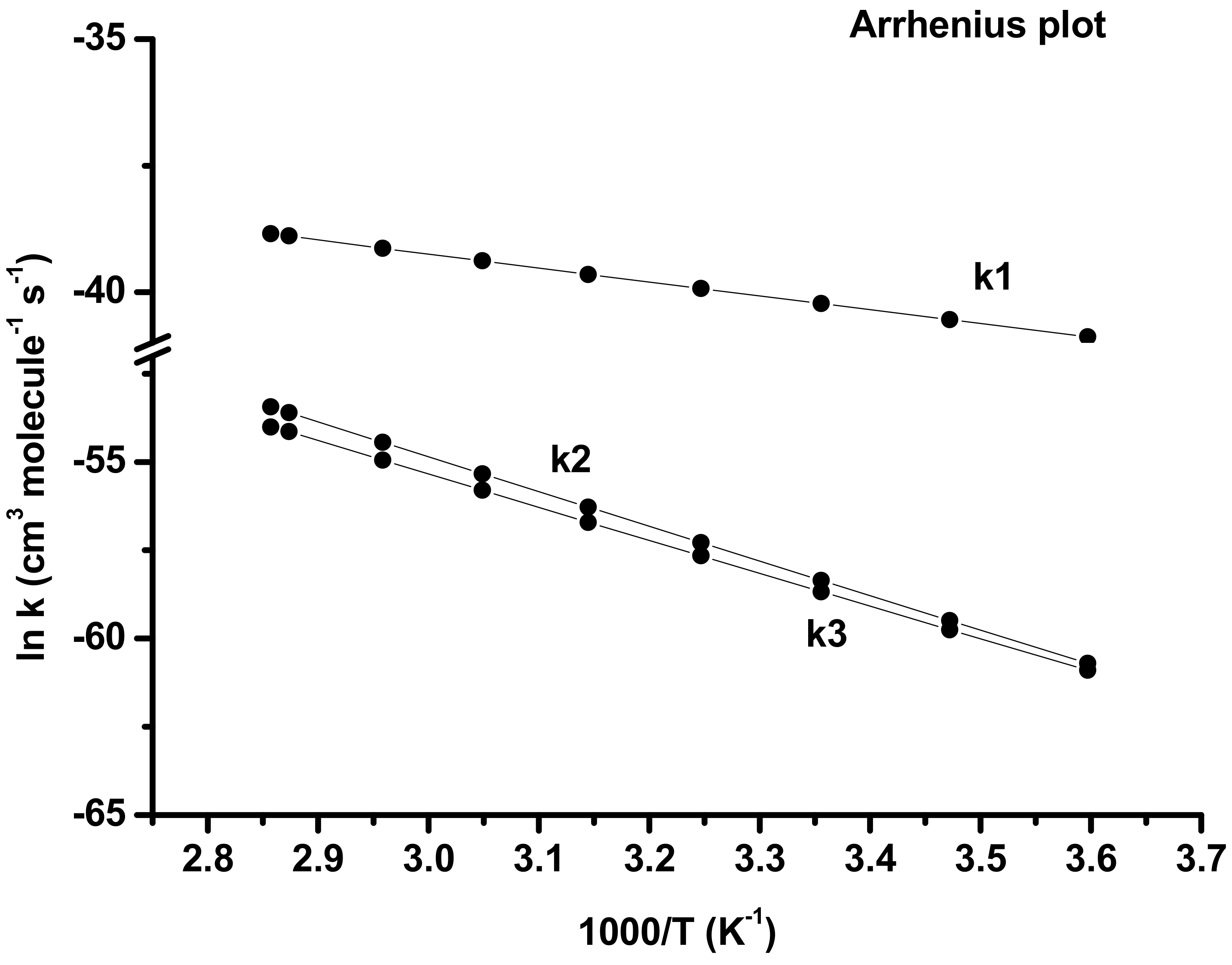
| Temp (K) | Initial (k) Path 1 | Secondary Rate (k) | Branching Ratio % | ||||
|---|---|---|---|---|---|---|---|
| Path 2 | Path 3 | Total | Path 2 | Path 3 | Total | ||
| 278 | 1.76 × 10−18 | 4.33 × 10−27 | 3.57 × 10−27 | 7.90 × 10−27 | 54.83 | 45.17 | 100 |
| 288 | 2.47 × 10−18 | 1.46 × 10−26 | 1.12 × 10−26 | 2.58 × 10−26 | 56.53 | 43.47 | 100 |
| 298 | 3.39 × 10−18 | 4.55 × 10−26 | 3.30 × 10−26 | 7.85 × 10−26 | 57.92 | 42.08 | 100 |
| 308 | 4.57 × 10−18 | 1.32 × 10−25 | 9.20 × 10−26 | 2.24 × 10−25 | 58.99 | 41.01 | 100 |
| 318 | 6.04 × 10−18 | 3.62 × 10−25 | 2.34 × 10−25 | 5.96 × 10−25 | 60.72 | 39.28 | 100 |
| 328 | 7.89 × 10−18 | 9.30 × 10−25 | 5.83 × 10−25 | 1.51 × 10−24 | 61.47 | 38.53 | 100 |
| 338 | 1.01 × 10−17 | 2.27 × 10−24 | 1.37 × 10−24 | 3.65 × 10−24 | 62.35 | 37.65 | 100 |
| 348 | 1.29 × 10−17 | 5.28 × 10−24 | 3.09 × 10−24 | 8.37 × 10−24 | 63.09 | 36.91 | 100 |
| 350 | 1.35 × 10−17 | 6.23 × 10−24 | 3.52 × 10−24 | 9.75 × 10−24 | 63.88 | 36.12 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalalian, C.; Grira, A.; Illmann, J.N.; Patroescu-Klotz, I.; El Dib, G.; Coddeville, P.; Canosa, A.; Wiesen, P.; Aazaad, B.; Senthilkumar, L.; et al. Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis. Atmosphere 2022, 13, 291. https://doi.org/10.3390/atmos13020291
Kalalian C, Grira A, Illmann JN, Patroescu-Klotz I, El Dib G, Coddeville P, Canosa A, Wiesen P, Aazaad B, Senthilkumar L, et al. Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis. Atmosphere. 2022; 13(2):291. https://doi.org/10.3390/atmos13020291
Chicago/Turabian StyleKalalian, Carmen, Asma Grira, Jan Niklas Illmann, Iulia Patroescu-Klotz, Gisèle El Dib, Patrice Coddeville, André Canosa, Peter Wiesen, Basheer Aazaad, Lakshmipathi Senthilkumar, and et al. 2022. "Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis" Atmosphere 13, no. 2: 291. https://doi.org/10.3390/atmos13020291
APA StyleKalalian, C., Grira, A., Illmann, J. N., Patroescu-Klotz, I., El Dib, G., Coddeville, P., Canosa, A., Wiesen, P., Aazaad, B., Senthilkumar, L., Roth, E., Tomas, A., & Chakir, A. (2022). Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis. Atmosphere, 13(2), 291. https://doi.org/10.3390/atmos13020291







