Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Site Description
2.2. Location Allocation of Passive Diffusion Tubes
2.3. Gas Measurements: Passive Diffusion Tubes
2.4. Safety Levels of NO2 Using the Annualization Method
3. Results
3.1. Safety of NO2 Levels Using the Annualization Method
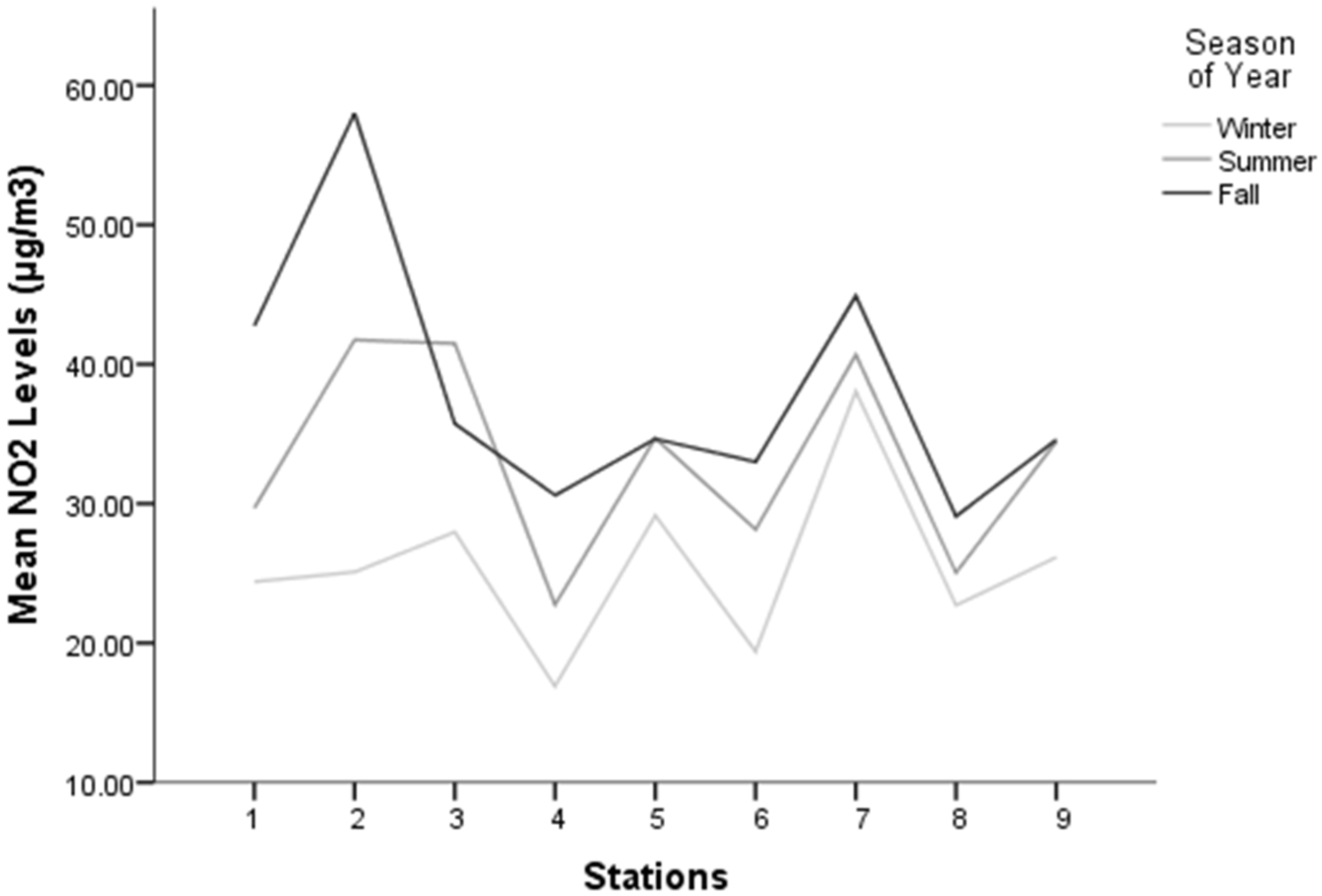
3.2. Spatial and Seasonal Variations of NO2 at Study Locations
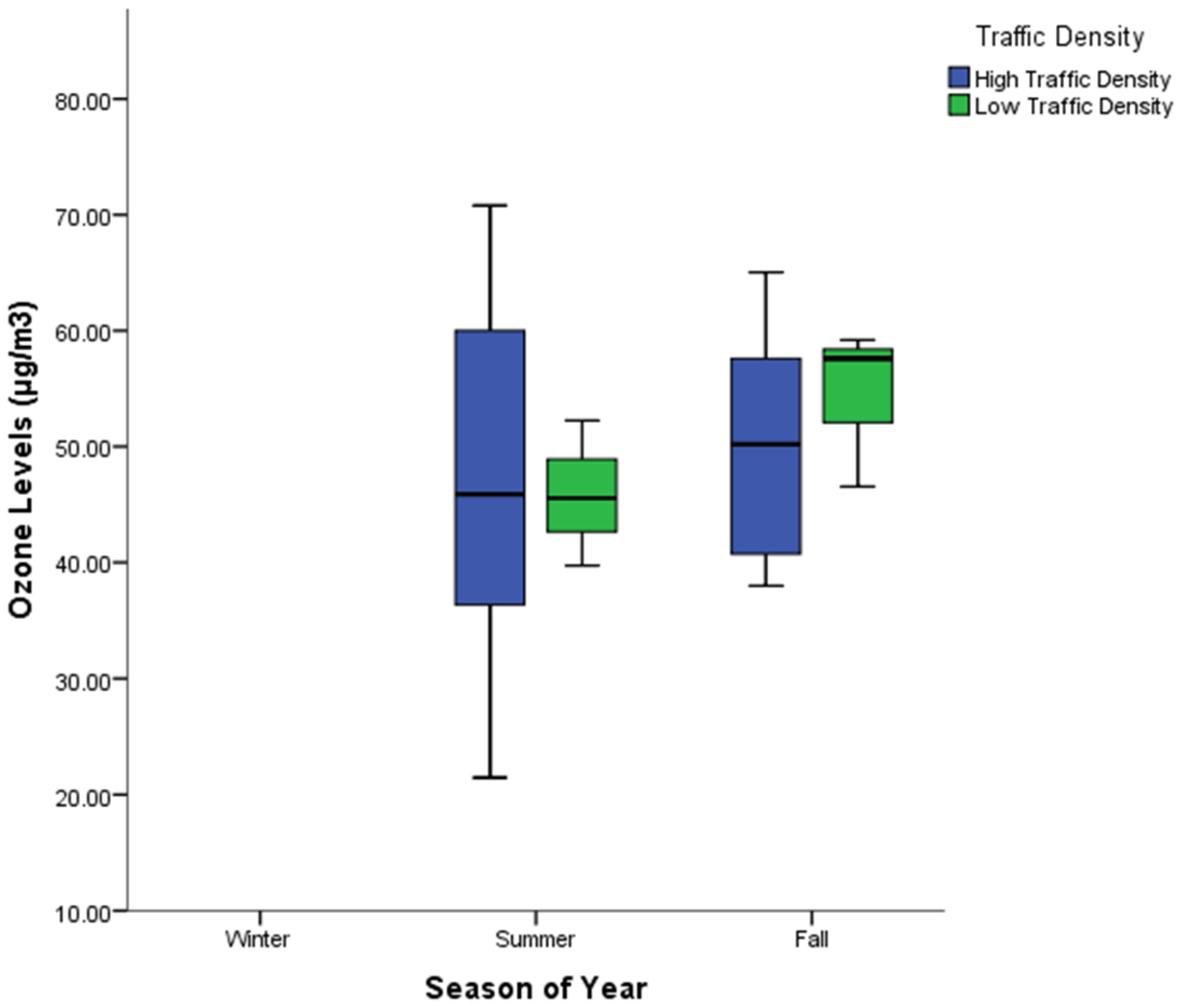
3.3. Spatial and Seasonal Variations of O3 at Study Locations
4. Discussion
4.1. Safe Levels of NO2 at Study Locations
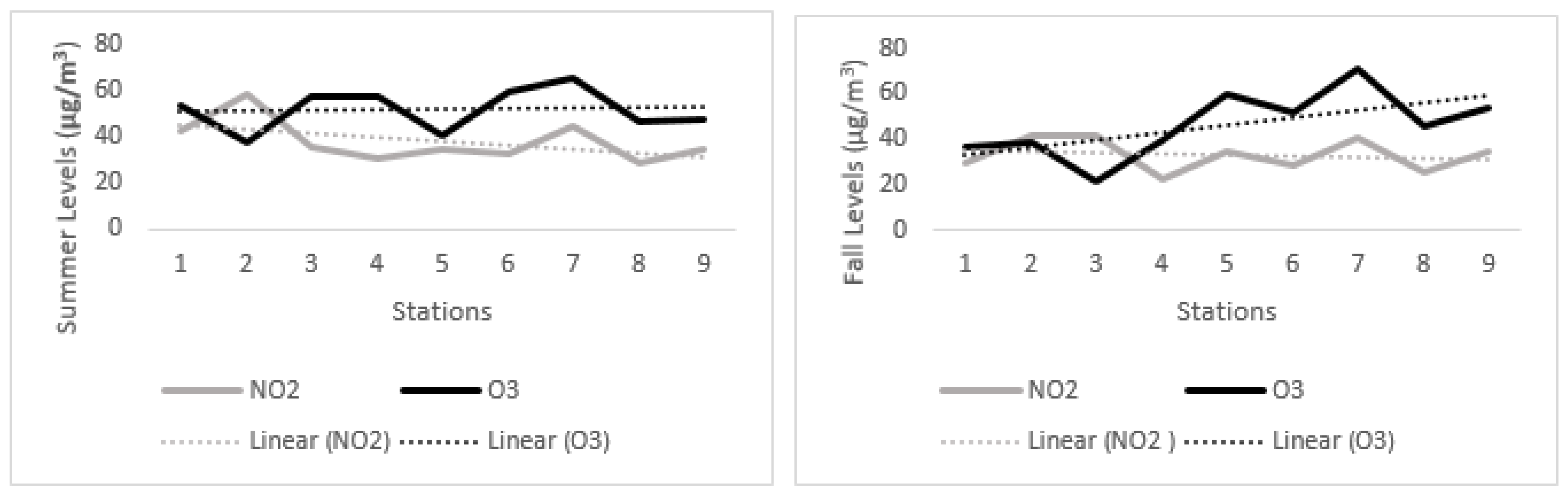
4.2. Spatial and Seasonal Variations of NO2
4.3. Spatial and Seasonal Variations of O3 at Study Locations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritz, B.; Hoffmann, B.; Peters, A. The Effects of Fine Dust, Ozone, and Nitrogen Dioxide on Health. Dtsch. Arztebl. Int. 2019, 51–52, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Li, R.; Li, W.; Wang, Z.; Ma, X.; Zhang, R.; Fang, X.; Wu, Z.; Cao, Y.; Xu, Q. Acute Effects of Nitrogen Dioxide on Cardiovascular Mortality in Beijing: An Exploration of Spatial Heterogeneity and the District-Specific Predictors. Sci. Rep. 2016, 6, 38328. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.; Mar, K.A.; Ojha, N.; Butler, T.M. The Influence of Temperature on Ozone Production under Varying NOx Conditions—A Modelling Study. Atmos. Chem. Phys. 2016, 16, 11601–11615. [Google Scholar] [CrossRef]
- Moshammer, H.; Poteser, M.; Kundi, M.; Lemmerer, K.; Weitensfelder, L.; Wallner, P.; Hutter, H.-P. Nitrogen-Dioxide Remains a Valid Air Quality Indicator. Int. J. Environ. Res. Public Health 2020, 17, 3733. [Google Scholar] [CrossRef] [PubMed]
- Ban-Weiss, G.A.; McLaughlin, J.P.; Harley, R.A.; Kean, A.J.; Grosjean, E.; Grosjean, D. Carbonyl and Nitrogen Dioxide Emissions from Gasoline- and Diesel-Powered Motor Vehicles. Environ. Sci. Technol. 2008, 42, 3944–3950. [Google Scholar] [CrossRef]
- Voiculescu, M.; Constantin, D.-E.; Condurache-Bota, S.; Călmuc, V.; Roșu, A.; Dragomir Bălănică, C.M. Role of Meteorological Parameters in the Diurnal and Seasonal Variation of NO2 in a Romanian Urban Environment. Int. J. Environ. Res. Public Health 2020, 17, 6228. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.; Maruyama, R.; Riaz, A.; Nakai, S. Development of Land Use Regression Model for Seasonal Variation of Nitrogen Dioxide (NO2) in Lahore, Pakistan. Sustainability 2021, 13, 4933. [Google Scholar] [CrossRef]
- Romer, P.S.; Duffey, K.C.; Wooldridge, P.J.; Edgerton, E.; Baumann, K.; Feiner, P.A.; Miller, D.O.; Brune, W.H.; Koss, A.R.; De Gouw, J.A.; et al. Effects of Temperature-Dependent NOx Emissions on Continental Ozone Production. Atmos. Chem. Phys. 2018, 18, 2601–2614. [Google Scholar] [CrossRef]
- Roberts-Semple, D.; Song, F.; Gao, Y. Seasonal Characteristics of Ambient Nitrogen Oxides and Ground–Level Ozone in Metropolitan Northeastern New Jersey. Atmos. Pollut. Res. 2012, 3, 247–257. [Google Scholar] [CrossRef]
- Allen, J. Chemistry in the Sunlight. Earth Observatory. Available online: https://earthobservatory.nasa.gov/features/ChemistrySunlight (accessed on 15 November 2022).
- Liu, S.-K.; Cai, S.; Chen, Y.; Xiao, B.; Chen, P.; Xiang, X.-D. The Effect of Pollutional Haze on Pulmonary Function. J. Thorac. Dis. 2016, 8, E41–E56. [Google Scholar] [PubMed]
- Jarvis, D.J.; Adamkiewicz, G.; Heroux, M.-E.; Rapp, R.; Kelly, F.J. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Copenhagen, Denmark, 2010; pp. 201–248. [Google Scholar]
- Di, Q.; Amini, H.; Shi, L.; Kloog, I.; Silvern, R.; Kelly, J.; Sabath, M.B.; Choirat, C.; Koutrakis, P.; Lyapustin, A.; et al. Assessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ. Sci. Technol. 2020, 54, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.C.; Heald, C.L. The Mechanisms and Meteorological Drivers of the Summertime Ozone–Temperature Relationship. Atmos. Chem. Phys. 2019, 19, 13367–13381. [Google Scholar] [CrossRef]
- World Health Organization; Occupational and Environmental Health Team. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- United States Environmental Protection Agency. Ground-Level Ozone Basics. Available online: https://www.epa.gov/ground-level-ozone-pollution/ground-level-ozone-basics (accessed on 19 August 2022).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A. Hypothesis: Ill Health Associated with Low Concentrations of Nitrogen Dioxide—An Effect of Ultrafine Particles? Thorax 2003, 58, 1012–1015. [Google Scholar] [CrossRef]
- Gorai, A.K.; Tuluri, F.; Tchounwou, P.B.; Ambinakudige, S. Influence of Local Meteorology and NO2 Conditions on Ground-Level Ozone Concentrations in the Eastern Part of Texas, USA. Air Qual. Atmos. Health 2015, 8, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Rowell, A.; Terry, M.E.; Deary, M.E. Comparison of Diffusion Tube–Measured Nitrogen Dioxide Concentrations at Child and Adult Breathing Heights: Who Are We Monitoring for? Air Qual. Atmos. Health 2021, 14, 27–36. [Google Scholar] [CrossRef]
- Kirby, C.; Fox, M.; Waterhouse, J. Reliability of Nitrogen Dioxide Passive Diffusion Tubes for Ambient Measurement: In Situ Properties of the Triethanolamine Absorbent. J. Environ. Monit. 2000, 2, 307–312. [Google Scholar] [CrossRef]
- Larkin, A.; Hystad, P. Towards Personal Exposures: How Technology Is Changing Air Pollution and Health Research. Curr. Environ. Health Rep. 2017, 4, 463–471. [Google Scholar] [CrossRef]
- Apte, J.S.; Messier, K.P.; Gani, S.; Brauer, M.; Kirchstetter, T.W.; Lunden, M.M.; Marshall, J.D.; Portier, C.J.; Vermeulen, R.C.H.; Hamburg, S.P. High-Resolution Air Pollution Mapping with Google Street View Cars: Exploiting Big Data. Environ. Sci. Technol. 2017, 51, 6999–7008. [Google Scholar] [CrossRef]
- Brugge, D.; Patton, A.P.; Bob, A.; Reisner, E.; Lowe, L.; Bright, O.-J.M.; Durant, J.L.; Newman, J.; Zamore, W. Developing Community-Level Policy and Practice to Reduce Traffic-Related Air Pollution Exposure. Environ. Justice 2015, 8, 95–104. [Google Scholar] [CrossRef]
- U.S. Census Bureau. American Community Survey 1-Year Estimates. Census Reporter Profile Page for NYC-Queens Community District 12—Jamaica, Hollis & St. Albans PUMA, NY. Available online: http://censusreporter.org/profiles/79500US3604112-nyc-queens-community-district-12-jamaica-hollis-st-albans-puma-ny/ (accessed on 15 November 2022).
- Schneider, P.; Castell, N.; Vogt, M.; Dauge, F.R.; Lahoz, W.A.; Bartonova, A. Mapping Urban Air Quality in near Real-Time Using Observations from Low-Cost Sensors and Model Information. Environ. Int. 2017, 106, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kendler, S.; Nebenzal, A.; Gold, D.; Reed, P.M.; Fishbain, B. The Effects of Air Pollution Sources/Sensor Array Configurations on the Likelihood of Obtaining Accurate Source Term Estimations. Atmos. Environ. 2021, 246, 117754. [Google Scholar] [CrossRef]
- Kanaroglou, P.S.; Jerrett, M.; Morrison, J.; Beckerman, B.; Arain, M.A.; Gilbert, N.L.; Brook, J.R. Establishing an Air Pollution Monitoring Network for Intra-Urban Population Exposure Assessment: A Location-Allocation Approach. Atmos. Environ. 2005, 39, 2399–2409. [Google Scholar] [CrossRef]
- Yoshida, I.; Tasaki, Y.; Tomizawa, Y. Optimal Placement of Sampling Locations for Identification of a Two-Dimensional Space. Georisk Assess. Manag. Risk Eng. Syst. Geohazards 2022, 16, 98–113. [Google Scholar] [CrossRef]
- Mano, Z.; Kendler, S.; Fishbain, B. Information Theory Solution Approach to the Air Pollution Sensor Location-Allocation Problem. Sensors 2022, 22, 3808. [Google Scholar] [CrossRef]
- New York City Department of Transportation. Queens Village/Jamaica Avenue Transportation Study: Final Report (Report Number: P.I.N. PTDT14D00.G05); New York City Department of Transportation: New York, NY, USA, 2015. [Google Scholar]
- New York City Department of Transportation. Downtown Jamaica Transportation Study Final Report. (Report Number: PIN PTDT18D00.G12). Available online: https://www.nyc.gov.downtown-jamaica-study-final-report.pdf (accessed on 28 November 2022).
- Practical Guidance: NO2 Diffusion Tubes for LAQM. Gov.uk. Available online: https://laqm.defra.gov.uk/air-quality/air-quality-assessment/practical-guidance/ (accessed on 1 December 2022).
- Niepsch, D.; Clarke, L.J.; Tzoulas, K.; Cavan, G. Spatiotemporal Variability of Nitrogen Dioxide (NO2) Pollution in Manchester (UK) City Centre (2017–2018) Using a Fine Spatial Scale Single-NOx Diffusion Tube Network. Environ. Geochem. Health 2022, 44, 3907–3927. [Google Scholar] [CrossRef]
- Air Quality Guide for Nitrogen Dioxide Air Quality Index Protect Your Health near Roadways. Airnow.gov. Available online: https://www.airnow.gov/sites/default/files/2018-06/no2.pdf (accessed on 10 August 2022).
- Vardoulakis, S.; Lumbreras, J.; Solazzo, E. Comparative Evaluation of Nitrogen Oxides and Ozone Passive Diffusion Tubes for Exposure Studies. Atmos. Environ. 2009, 43, 2509–2517. [Google Scholar] [CrossRef]
- Varshney, C.K.; Singh, A.P. Passive Samplers for NOx Monitoring: A Critical Review. Environmentalist 2003, 23, 127–136. [Google Scholar] [CrossRef]
- Tang, Y.S.; Cape, J.N.; Sutton, M.A. Development and Types of Passive Samplers for Monitoring Atmospheric NO2 and NH3 Concentrations. Sci. World J. 2001, 1, 513–529. [Google Scholar] [CrossRef]
- Nash, D.G.; Leith, D. Use of Passive Diffusion Tubes to Monitor Air Pollutants. J. Air Waste Manag. Assoc. 2010, 60, 204–209. [Google Scholar] [CrossRef][Green Version]
- United States Environmental Protection Agency. NAAQS Table 2014. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 10 August 2022).
- Yazdi, M.D.; Wang, Y.; Di, Q.; Requia, W.J.; Wei, Y.; Shi, L.; Sabath, M.B.; Dominici, F.; Coull, B.; Evans, J.S.; et al. Long-Term Effect of Exposure to Lower Concentrations of Air Pollution on Mortality among US Medicare Participants and Vulnerable Subgroups: A Doubly-Robust Approach. Lancet Planet. Health 2021, 5, e689–e697. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Aga, E.; Touloumi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-Term Effects of Nitrogen Dioxide on Mortality: An Analysis within the APHEA Project. Eur. Respir. J. 2006, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Chiusolo, M.; Cadum, E.; Stafoggia, M.; Galassi, C.; Berti, G.; Faustini, A.; Bisanti, L.; Vigotti, M.A.; Dessì, M.P.; Cernigliaro, A.; et al. Short-Term Effects of Nitrogen Dioxide on Mortality and Susceptibility Factors in 10 Italian Cities: The EpiAir Study. Environ. Health Perspect. 2011, 119, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Afif, C.; Dutot, A.L.; Jambert, C.; Abboud, M.; Adjizian-Gérard, J.; Farah, W.; Perros, P.E.; Rizk, T. Statistical Approach for the Characterization of NO2 Concentrations in Beirut. Air Qual. Atmos. Health 2009, 2, 57–67. [Google Scholar] [CrossRef]
- Perkauskas, D.; Mikelinskiene, A. Evaluation of SO2 and NO2 Concentration Levels in Vilnius (Lithuania) Using Passive Diffusion Samplers. Environ. Pollut. 1998, 102, 249–252. [Google Scholar] [CrossRef]
- Mavroidis, I.; Ilia, M. Trends of NOx, NO2 and O3 Concentrations at Three Different Types of Air Quality Monitoring Stations in Athens, Greece. Atmos. Environ. 2012, 63, 135–147. [Google Scholar] [CrossRef]
- New York City. NY Weather History. Wunderground.com. Available online: https://www.wunderground.com/history/monthly/us/ny/new-york-city (accessed on 2 August 2022).
- Hargreaves, P.R.; Leidi, A.; Grubb, H.J.; Howe, M.T.; Mugglestone, M.A. Local and Seasonal Variations in Atmospheric Nitrogen Dioxide Levels at Rothamsted, UK, and Relationships with Meteorological Conditions. Atmos. Environ. 2000, 34, 843–853. [Google Scholar] [CrossRef]
- Kubota, T.; Miura, M.; Tominaga, Y.; Mochida, A. Wind Tunnel Tests on the Relationship between Building Density and Pedestrian-Level Wind Velocity: Development of Guidelines for Realizing Acceptable Wind Environment in Residential Neighborhoods. Build. Environ. 2008, 43, 1699–1708. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe: Europe, UK. Proximity to Roads, NO2, Other Air Pollutants and Their Mixtures; WHO Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Grange, S.K.; Lewis, A.C.; Moller, S.J.; Carslaw, D.C. Lower Vehicular Primary Emissions of NO2 in Europe than Assumed in Policy Projections. Nat. Geosci. 2017, 10, 914–918. [Google Scholar] [CrossRef]
- How Does Transportation Impact Health? RWJF. Available online: https://www.rwjf.org/en/library/research/2012/10/how-does-transportation-impact-health-.html (accessed on 2 August 2022).
- Why Are Ozone Concentrations Higher in Rural Areas than in Cities? Irceline.Be. Available online: https://www.Irceline.Be/En/Documentation/Faq/Why-Are-Ozone-Concentrations-Higher-in-Rural-Areas-than-in-Cities (accessed on 13 June 2022).
- Austin, E.; Zanobetti, A.; Coull, B.; Schwartz, J.; Gold, D.R.; Koutrakis, P. Ozone Trends and Their Relationship to Characteristic Weather Patterns. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 532–542. [Google Scholar] [CrossRef]
- Simon, H.; Reff, A.; Wells, B.; Xing, J.; Frank, N. Ozone Trends across the United States over a Period of Decreasing NOx and VOC Emissions. Environ. Sci. Technol. 2015, 49, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ohara, T.; Uno, I.; Tanimoto, H.; Kurokawa, J.; Akimoto, H. Analysis of the Seasonal Variation of Ozone in the Boundary Layer in East Asia Using the Community Multi-Scale Air Quality Model: What Controls Surface Ozone Levels over Japan? Atmos. Environ 1994, 40, 1856–1868. [Google Scholar] [CrossRef]
- Cape, J.N. The Use of Passive Diffusion Tubes for Measuring Concentrations of Nitrogen Dioxide in Air. Crit. Rev. Anal. Chem. 2009, 39, 289–310. [Google Scholar] [CrossRef]
- Ren, C.; Melly, S.; Schwartz, J. Modifiers of Short-Term Effects of Ozone on Mortality in Eastern Massachusetts—A Casecrossover Analysis at Individual Level. Environ. Health 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, F.; Stafoggia, M.; Picciotto, S.; Bellander, T.; D’Ippoliti, D.; Lanki, T.; von Klot, S.; Nyberg, F.; Paatero, P.; Peters, A.; et al. A Case-Crossover Analysis of out-of-Hospital Coronary Deaths and Air Pollution in Rome, Italy. Am. J. Respir. Crit. Care Med. 2005, 172, 1549–1555. [Google Scholar] [CrossRef]
- Varadarajan, C. Development of a Source-Meteorology-Receptor (SMR) Approach Using Fine Particulate Intermittent Monitored Concentration Data for Urban Areas in Ohio; University of Toledo: Toledo, OH, USA, 2007. [Google Scholar]
- Kan, H.; Chen, B.; Zhao, N.; London, S.J.; Song, G.; Chen, G. Part 1. A Timeseries Study of Ambient Air Pollution and Daily Mortality in Shanghai, China. Res. Rep. Health Eff. Inst. 2010, 1, 17–78. [Google Scholar]
- Giles, L.V.; Barn, P.; Künzli, N.; Romieu, I.; Mittleman, M.A.; van Eeden, S.; Allen, R.; Carlsten, C.; Stieb, D.; Noonan, C.; et al. From Good Intentions to Proven Interventions: Effectiveness of Actions to Reduce the Health Impacts of Air Pollution. Environ. Health Perspect. 2011, 119, 29–36. [Google Scholar] [CrossRef]
- Pannullo, F.; Lee, D.; Waclawski, E.; Leyland, A.H. Improving Spatial Nitrogen Dioxide Prediction Using Diffusion Tubes: A Case Study in West Central Scotland. Atmos. Environ. 2015, 118, 227–235. [Google Scholar] [CrossRef]
- Cox, R.M. The Use of Passive Sampling to Monitor Forest Exposure to O3, NO2 and SO2: A Review and Some Case Studies. Environ. Pollut. 2003, 126, 301–311. [Google Scholar] [CrossRef]
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Di Sabatino, S.; Bell, M.; Norford, L.; Britter, R. The Rise of Low-Cost Sensing for Managing Air Pollution in Cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef]
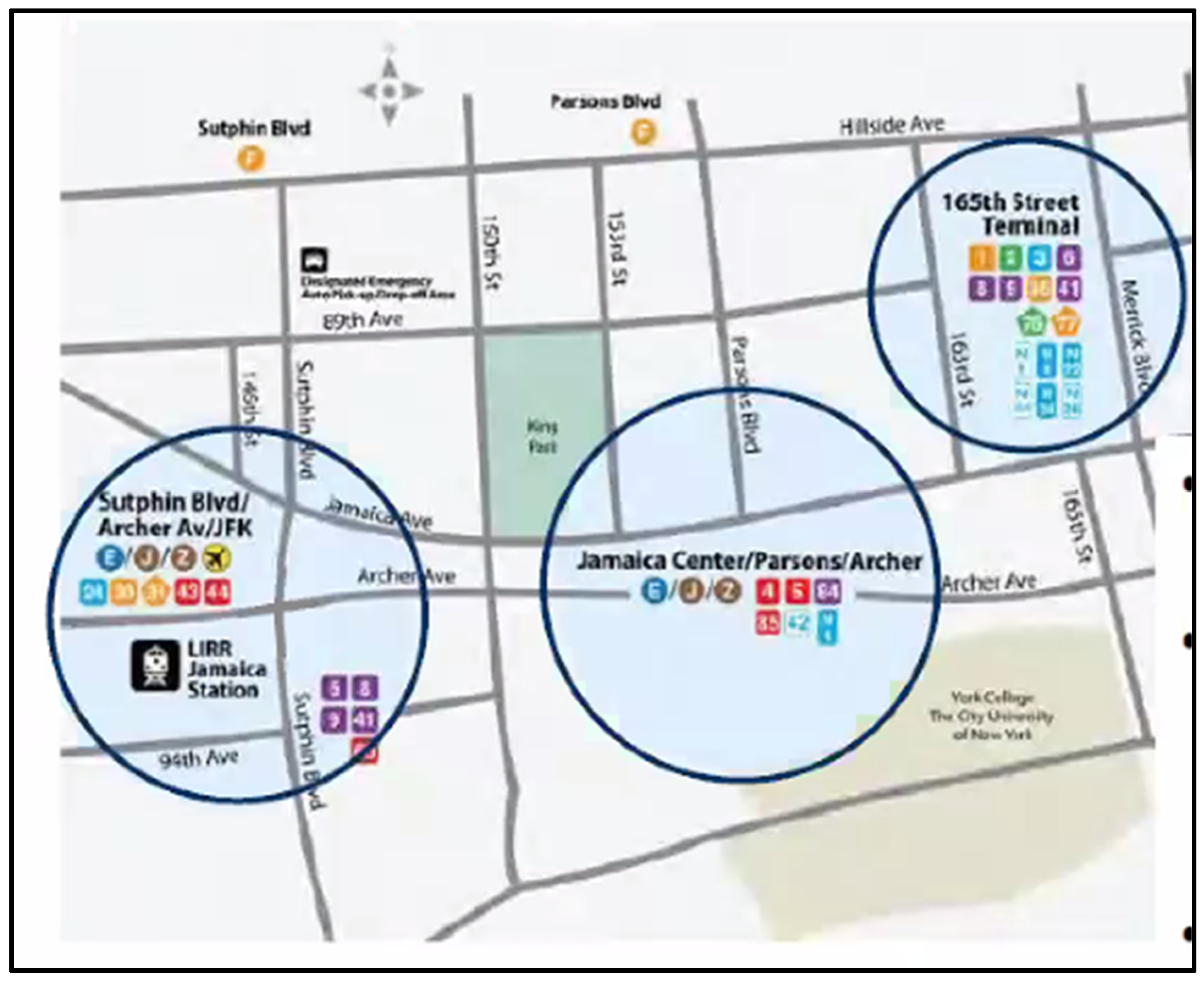


| Site | Site Name/Description | Traffic Density | Latitude | Longitude |
|---|---|---|---|---|
| 1 | ABC News Stand/Coffee Shop | High | 40.702854 | −73.799294 |
| 2 | Golden City Jewelers | High | 40.703752 | −73.799481 |
| 3 | LAZ Parking | High | 40.703132 | −73.798622 |
| 4 | Sahan Hair Braiding | Low | 40.70326 | −73.791721 |
| 5 | Archer Live Poultry | High | 40.703892 | −73.793863 |
| 6 | Dove and Matrix Salon | Low | 40.705061 | −73.794406 |
| 7 | Rattan’s Inc. | High | 40.704848 | −73.795427 |
| 8 | Long Island Beauty Supply | Low | 40.704433 | −73.799348 |
| 9 | Wendy’s Restaurant | High | 40.703442 | −73.800393 |
| Start Date | End Date | B1 | D1 | BD1 |
|---|---|---|---|---|
| 17 January 2019 | 4 February 2019 | 73.47 | 25.53 | 73.47 |
| 4 February 2019 | 9 July 2019 | 62.34 | - | - |
| 9 July 2019 | 2 August 2019 | 53.08 | 33.19 | 53.08 |
| 2 August 2019 | 2 October 2019 | 55.69 | - | - |
| 2 October 2019 | 18 October 2019 | 38.25 | 38.14 | 38.25 |
| 18 October 2019 | 31 December 2019 | 63.22 | - | - |
| Winter Mean (95%CI) | Summer Mean (95%CI) | Fall Mean (95%CI) | Total (95%CI) | p-Value | |
|---|---|---|---|---|---|
| NO2 (μg/m3) | 25.53 (20.84–30.22) | 33.19 (27.65–38.72) | 38.14 (31.18–45.11) | 32.29 (28.74–35.84) | 0.006 |
| High-Traffic Mean (95%CI) | Low-Traffic Mean (95%CI) | Mean Difference (95%CI) | p-value | ||
| NO2 (μg/m3) | 35.79 (32.81–38.77) | 25.29 (11.73- 28.85) | 10.497 (4.12–16.87) | 0.002 |
| Fall Mean (95%CI) | Summer Mean (95%CI) | Total (95%CI) | p-Value | ||
|---|---|---|---|---|---|
| O3 (μg/m3) | 51.68 (44.70–58.67) | 46.43 (35.25–57.61) | 49.06 (43.05–55.09) | 0.372 | |
| High-Traffic Mean (95%CI) | Low-Traffic Mean (95%CI) | Mean Difference (95%CI) | p-value | ||
| O3 (μg/m3) | 48.51 (40.39–56.63) | 50.14 (43.98–56.30) | −1.63 (−14.79–11.53) | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaman, M.; Roberts-Semple, D.; Aime, C.; Shin, J.; Akinremi, A. Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York. Atmosphere 2022, 13, 2042. https://doi.org/10.3390/atmos13122042
Guaman M, Roberts-Semple D, Aime C, Shin J, Akinremi A. Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York. Atmosphere. 2022; 13(12):2042. https://doi.org/10.3390/atmos13122042
Chicago/Turabian StyleGuaman, Mayra, Dawn Roberts-Semple, Christopher Aime, Jin Shin, and Ayodele Akinremi. 2022. "Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York" Atmosphere 13, no. 12: 2042. https://doi.org/10.3390/atmos13122042
APA StyleGuaman, M., Roberts-Semple, D., Aime, C., Shin, J., & Akinremi, A. (2022). Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York. Atmosphere, 13(12), 2042. https://doi.org/10.3390/atmos13122042









