Performance of a Thermodynamic Model for Predicting Inorganic Aerosols in the Southeastern U.S.
Abstract
1. Introduction
2. Materials and Methods
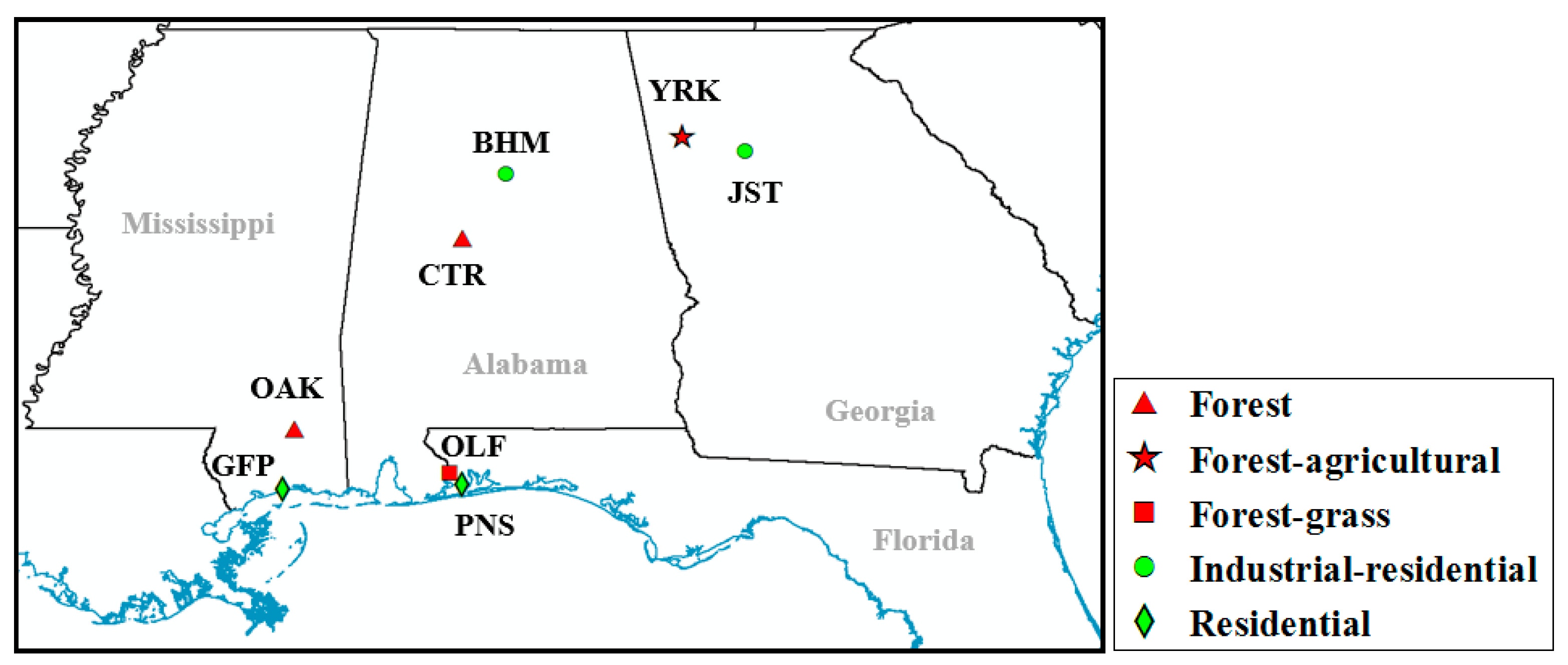
2.1. Monitoring Network Description
2.2. Data Reduction and Processing
2.3. Thermodynamic Model Assessment
2.4. Statistical Tests of the Model Performance Assessment
- 1.
- Fraction of predictions falls in a factor of two of observations (Fa2): the fraction of data with 0.5 ≤ ≤ 2.0 (Fa2 ≥ 0.8).
- 2.
- Normalized mean square error (NMSE): NMSE = (NMSE ≤ 0.5).
- 3.
- Fractional bias (FB): FB = (−0.5 ≤ FB ≤ 0.5).
- 4.
- Geometric mean bias (MG): MG = exp([lnCp] − [lnCo]) (0.75 ≤ MG ≤ 1.25).
3. Results and Discussion
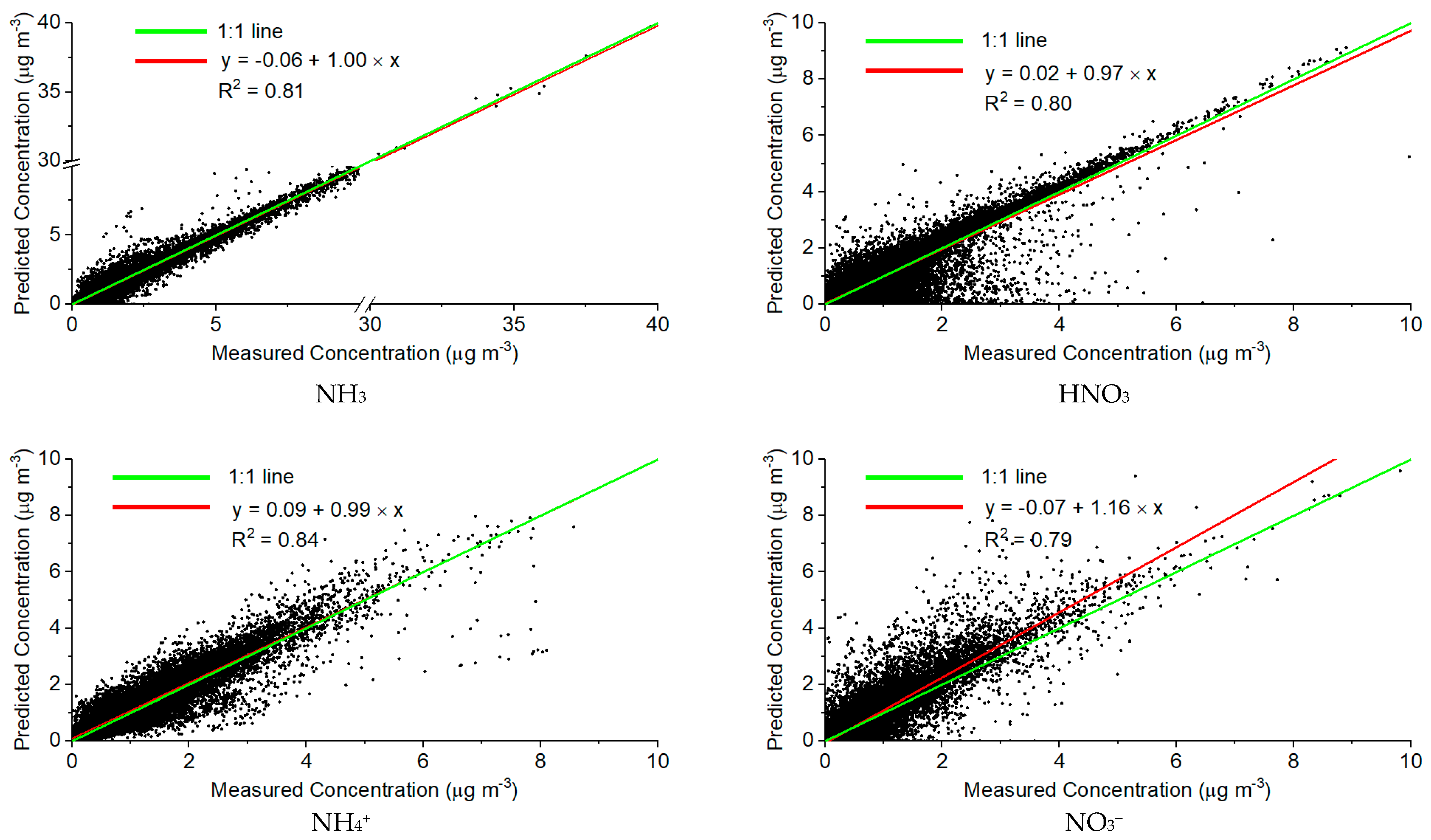
3.1. Applicability of ISORROPIA II in the Predictions of Gas- and Particle-Phase Pollutants
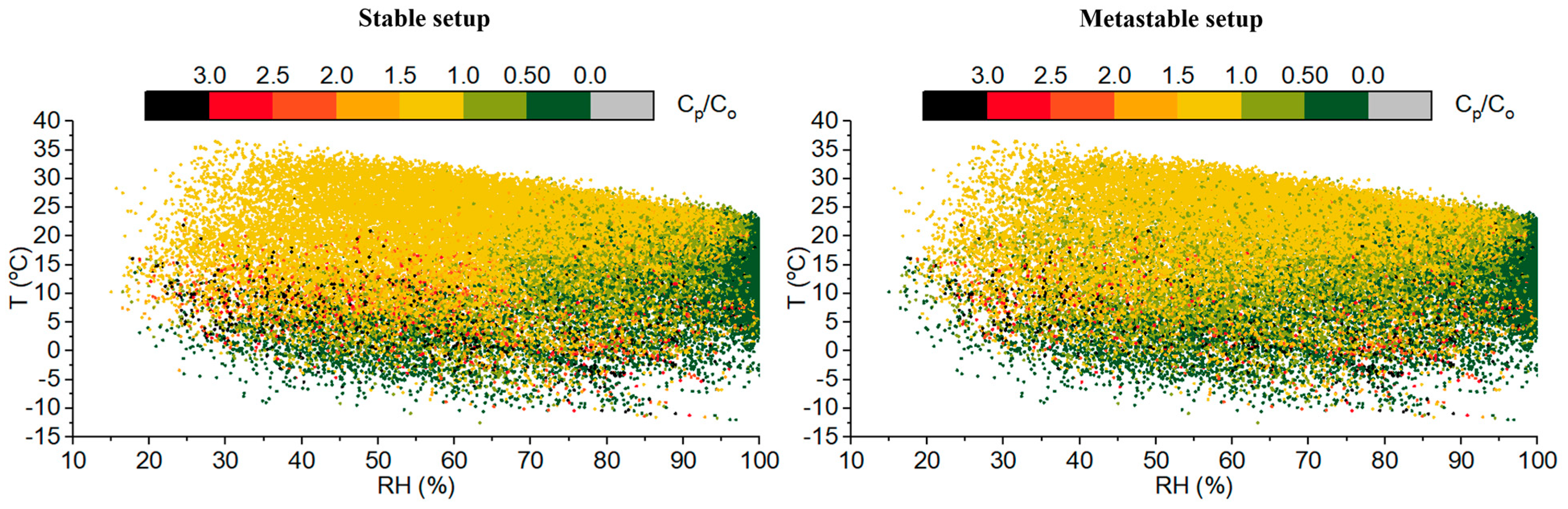
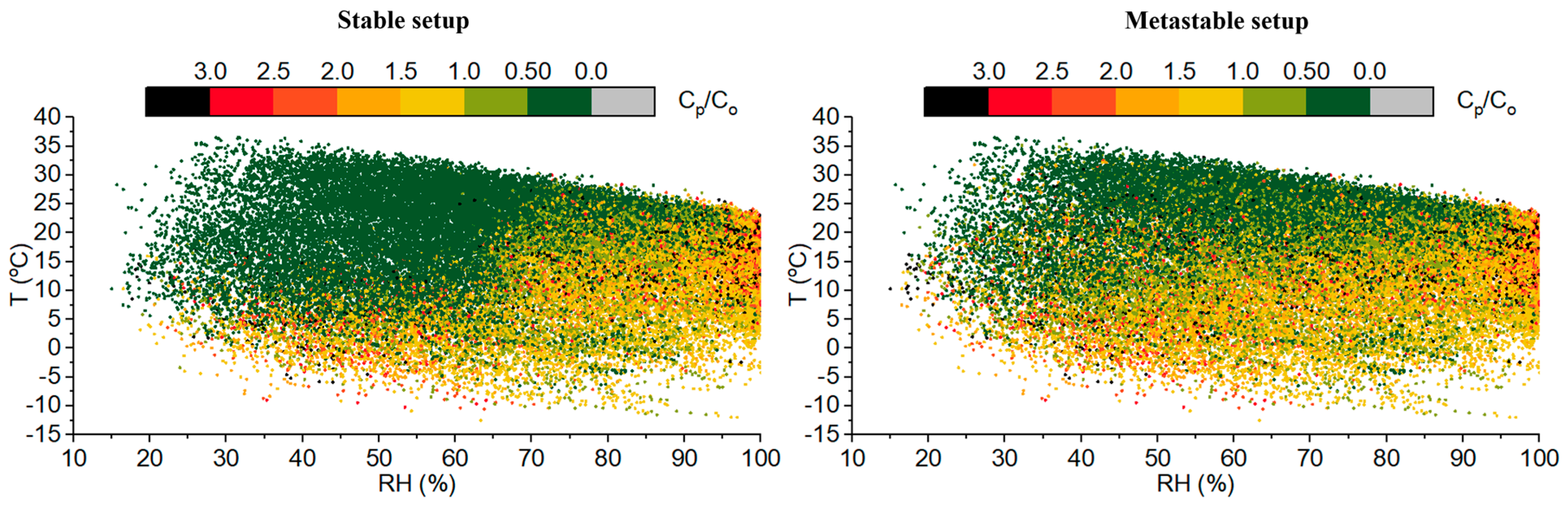
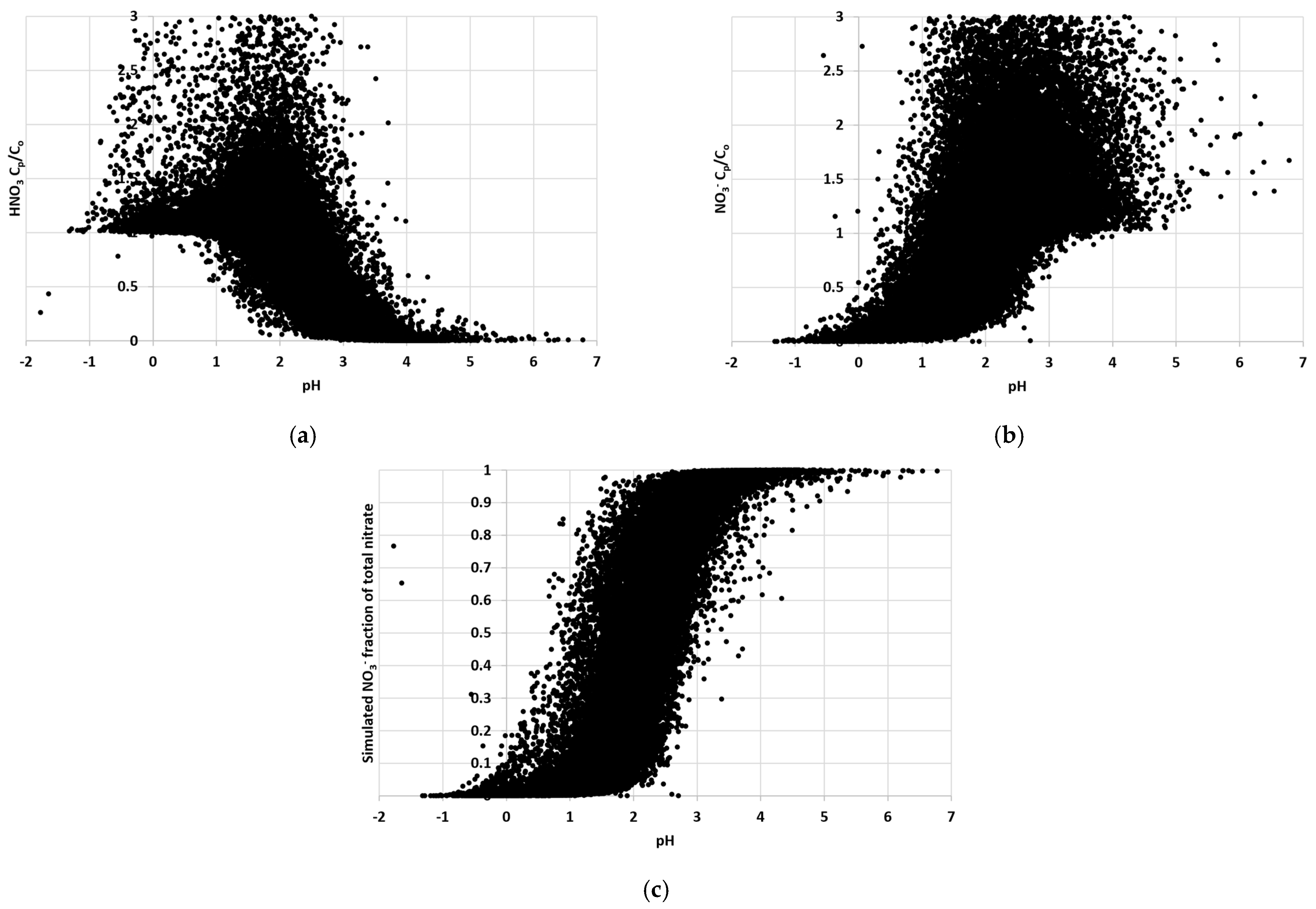
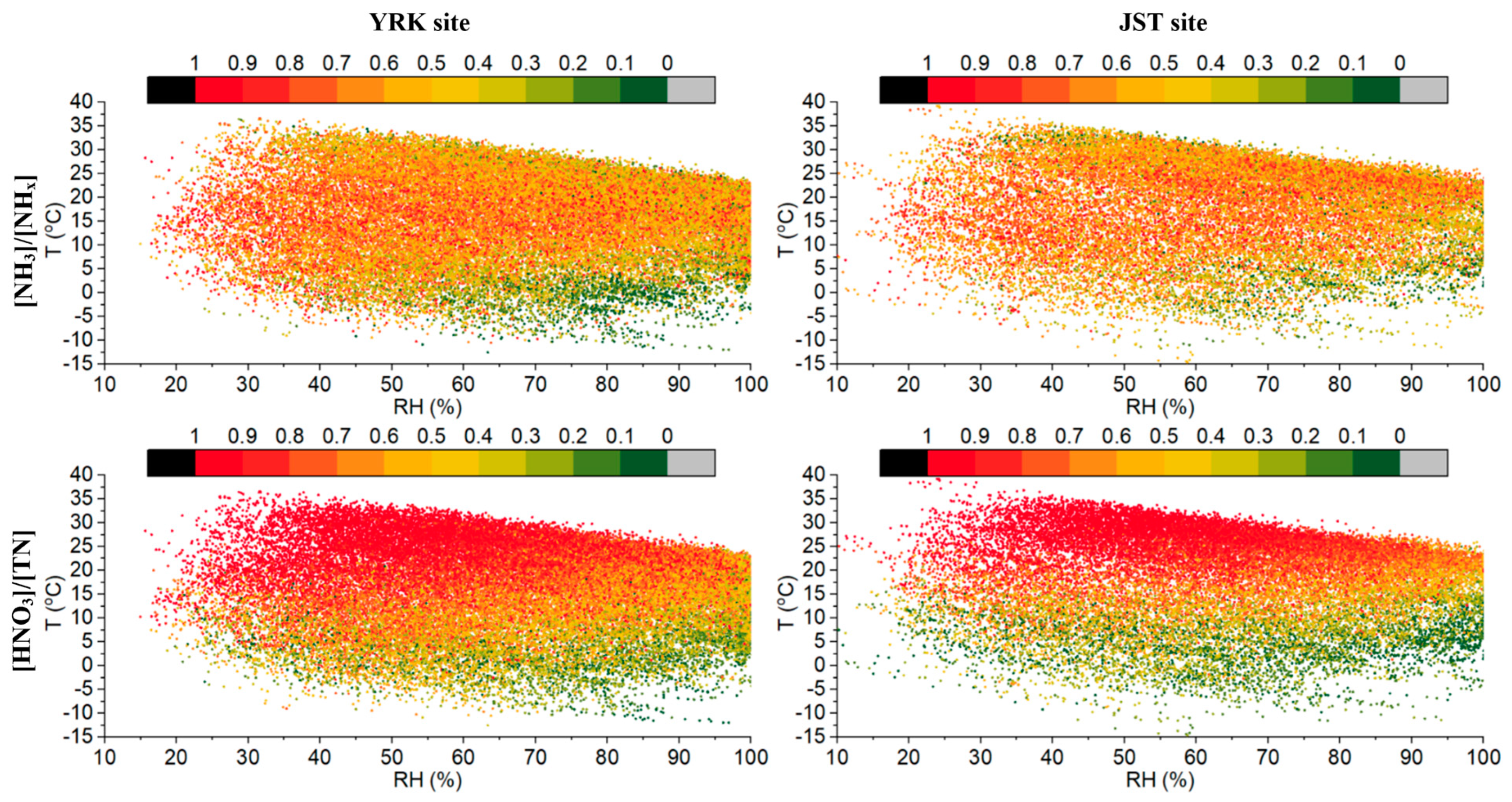
3.1.1. Ambient T-RH, Aerosol pH, and ISORROPIA II Stable and Metastable Setups
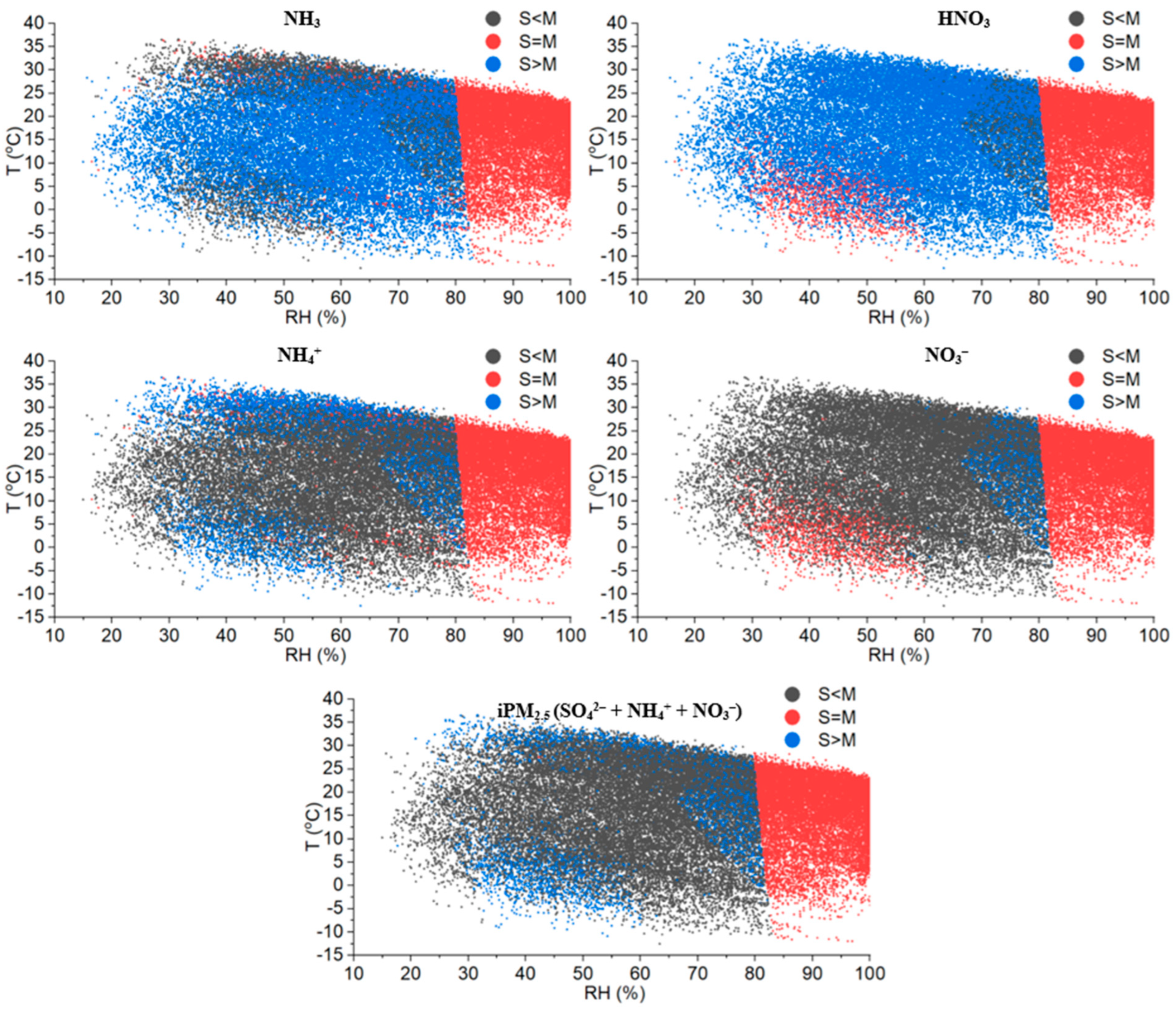
3.1.2. Statistical Tests of Model Performance under Different T-RH Conditions
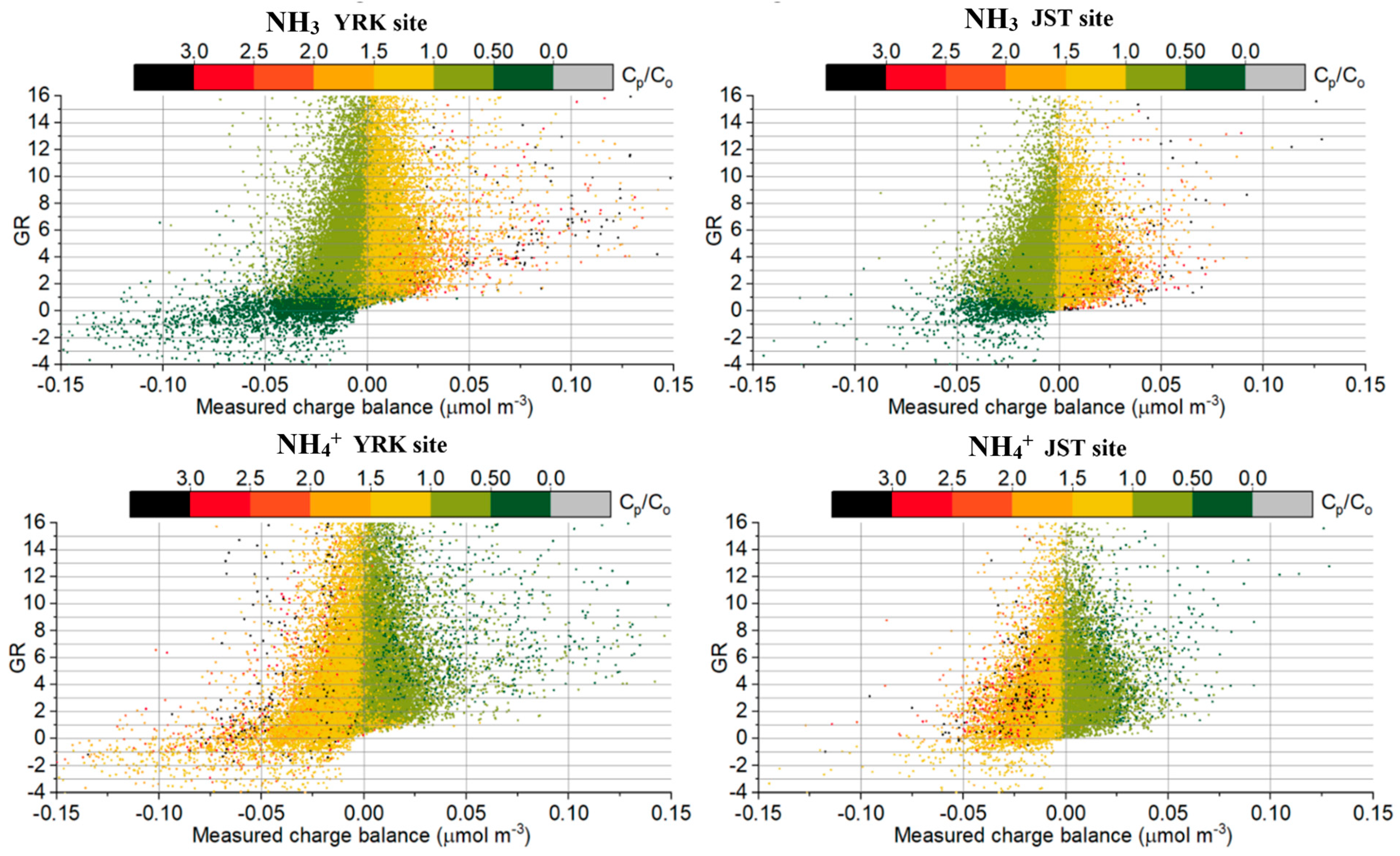
- The measurement uncertainties in total H2SO4 may explain part of the disagreement between predictions and measurements. Zhang et al., (2002) used 5 min measurements of iPM2.5 chemical compositions and their precursor gases to test the validity of the thermodynamic equilibrium assumption for the partitioning of NH3-NH4+ [33]. Good agreement was found between field measurements and ISORROPIA II predictions in NO3− and NH4+ when ~15% downward correction in SO42− concentration was applied. Yu et al., (2005) used different time resolution (5 min, 2 h, and 12 h) measurements of NH3, NH4+, HNO3, NO3−, and SO42− to assess the ability of ISORROPIA II in the prediction of HNO3-NO3− partitioning. The sensitivity test indicated that the measurement uncertainties in the SO42− and total NH3 concentrations may explain the errors in the prediction of NO3− [34].
- The positive measured charge balance may be explained by the fact that part of the NH4+ cations are associated with organic anions, which the ISORROPIA II model does not consider in the modeling system. In addition, NH4+ cations may also be associated with Cl−, which is not incorporated in the model input; the negative measured charge balance may be explained by the exclusion of the NVCs in the modeling system. Metzger et al., (2006) investigated the partitioning of NH3–NH4+ and HNO3–NO3− using three thermodynamic models [55], the model performance was assessed with/without the inclusion of NVCs and organic acid (R-COOH) in the input data. The comparison between model prediction and observations indicated that it is necessary to include NVCs and R-COOH in the model input of a thermodynamic model to accurately predict the gas-particle partitioning of NH3-NH4+ and HNO3-NO3−.
3.1.3. Nonvolatile Cations (NVCs)
3.1.4. Organic Aerosol (OA)
3.2. Possible Reasons for the Disagreement between Model Predictions and Measurements
- The hourly and daily data were used in this research to assess the performance of the ISORROPIA II model. The duration of the daily input data may not be adequate to detect the impact of atmospheric transport and ambient T and RH on the thermodynamic equilibrium partitioning of NH3-NH4+. Thus, the daily data of NH3, HNO3, T, and RH may not be able to represent the thermodynamic equilibrium state of the gas-particle partitioning process.
- The gas-particle system was not in a chemical equilibrium state. The mixture of PM1 and PM1–2.5 in secondary iPM2.5 may hinder the applicability of the ISORROPIA II model. The simple thermodynamic equilibrium assumption for iPM2.5 may not adequately characterize the partitioning of NH3-NH4+ in ambient air.
- The inorganic PM2.5 particles were assumed to be internally mixed; therefore, the particles were treated as an ensemble bulk. However, Koo et al., (2003) observed that dynamic model may perform better in the prediction of iPM2.5 chemical compositions. The dynamic change of particle size distribution may require vigorous treatment of different physical processes such as condensation, evaporation, and coagulation. The size-resolved measurements with high time resolution are not available in this research, thus the dynamic approach cannot be tested.
- Field measurement uncertainty. These uncertainties can be divided into two aspects: the measurement uncertainties caused by instruments and techniques, and the uncertainties caused by the atmospheric transport of air mass. The values below the instrument’s detection limit were included in the ISORROPIA II model assessment; this may cause some disagreement between model predictions and measurements. The small values are especially sensitive to the prediction uncertainty. In addition, the ideal assessment of ISORROPIA II prediction skill should be based on the high time resolution measurements under controlled conditions. Furthermore, the thermodynamic equilibrium models should simulate the equilibrium partitioning of NH3-NH4+ and HNO3-NO3− happening in the same air parcel. While in the field, air parcels laden with different concentrations of gas-phase and particle-phase pollutants may travel from and to any direction. Thus, the average measurements in one hour or day may not represent the thermodynamic equilibrium state of the same air parcel.
- The history of RH experienced by air mass from different wind directions is not a priori; thus, the decision regarding the selection of stable and metastable setups is quite difficult, and this may also add some uncertainties to the model simulation.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USEPA. Health and Environmental Effects of Particulate Matter. Available online: https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (accessed on 31 October 2022).
- Pui, D.Y.H.; Chen, S.; Zuo, Z. PM2.5 in China: Measurements, sources, visibility and health effects, and mitigation. Particuology 2014, 13, 1–26. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Y.; Lian, Y. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.G.; Harrison, R.M.; Erisman, J.-W. Field measurements of the dissociation of ammonium nitrate and ammonium chloride aerosols. Atmos. Environ. 1989, 23, 1591–1599. [Google Scholar] [CrossRef]
- Hildemann, L.M.; Russell, A.G.; Cass, G.R. Ammonia and nitric acid concentration in equilibrium with atmospheric aerosols: Experiment vs. theory. Atmos. Environ. 1984, 18, 1737–1750. [Google Scholar] [CrossRef][Green Version]
- Stelson, A.W.; Seinfeld, J.H. Relative humidity and temperature dependence of the ammonium nitrate dissociation constant. Atmos. Environ. 1982, 16, 983–992. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Zuend, A.; Bertram, A.K.; Seinfeld, J.H. Gas-particle partitioning of atmospheric aerosols: Interplay of physical state, non-ideal mixing and morphology. Phys. Chem. Chem. Phys. 2013, 15, 11441–11453. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: New York, NY, USA, 2016; ISBN 978-1-118-94740-1. [Google Scholar]
- Cheng, B.; Wang-Li, L.; Meskhidze, N.; Classen, J.; Bloomfield, P. Partitioning of NH3-NH4+ in the Southeastern U.S. Atmosphere 2021, 12, 1681. [Google Scholar] [CrossRef]
- Saxena, P.; Hudischewskyj, A.B.; Seigneur, C. A comparative study of equilibrium approaches to the chemical characterization of secondary aerosols. Atmos. Environ. 1986, 20, 1471–1483. [Google Scholar] [CrossRef]
- Tanner, R.L.; Marlow, W.H.; Newman, L. Chemical composition correlations of size-fractionated sulfate in New York City. Am. Chem. Soc. 1979, 13, 75–78. [Google Scholar] [CrossRef]
- Tolocka, M.P.; Solomon, P.A.; Mitchell, W.; Norris, G.A.; Gemmill, D.B.; Wiener, R.W.; Vanderpool, R.W.; Homolya, J.B.; Rice, J. East versus west in the US: Chemical characteristics of PM2.5 during the winter of 1999. Aerosol Sci. Technol. 2001, 34, 88–96. [Google Scholar] [CrossRef]
- Walker, J.T.; Whitall, D.R.; Robarge, W.; Paerl, H.W. Ambient ammonia and ammonium aerosol across a region of variable ammonia emission density. Atmos. Environ. 2004, 38, 1235–1246. [Google Scholar] [CrossRef]
- Cheng, B.; Wang-Li, L. Spatial and temporal variations of PM2.5 in North Carolina. Aerosol Air Qual. Res. 2019, 19, 698–710. [Google Scholar] [CrossRef]
- Cheng, B.; Wang-Li, L.; Meskhidze, N.; Classen, J.; Bloomfield, P. Spatial and temporal variations of PM2.5 mass closure and inorganic PM2.5 in the Southeastern U.S. Environ. Sci. Pollut. Res. 2019, 26, 33181–33191. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B. and Wang-Li, L. Responses of secondary inorganic PM2.5 to precursor gases in an ammonia abundant area in North Carolina. Aerosol Air Qual. Res. 2019, 19, 1126–1138. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L., Jr.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.S.; Pandis, S.N. Prediction of multicomponent inorganic atmospheric aerosol behaviour. Atmos. Environ. 1999, 33, 745–757. [Google Scholar] [CrossRef]
- Zhang, Y.; Seigneur, C.; Seinfeld, J.H.; Jacobson, M.; Clegg, S.L.; Binkowski, F.S. A comparative review of inorganic aerosol thermodynamic equilibrium modules: Similarities, differences, and their likely causes. Atmos. Environ. 2000, 34, 117–137. [Google Scholar] [CrossRef]
- Cheng, B. Dynamics of Rural and Urban Atmospheric Chemical Conditions and Inorganic Aerosols; North Carolina State University: Raleigh, NC, USA, 2018. [Google Scholar]
- Ravishankara, A.R. Heterogeneous and multiphase chemistry in the troposphere. Science 1997, 276, 1058–1065. [Google Scholar] [CrossRef]
- Wexler, A.S.; Seinfeld, J.H. Analysis of aerosol ammonium nitrate: Departures from equilibrium during SCAQS. Atmos. Environ. 1992, 26, 579–591. [Google Scholar] [CrossRef]
- Moya, M.; Pandis, S.N.; Jacobson, M.Z. Is the size distribution of urban aerosols determined by thermodynamic equilibrium? An application to Southern California. Atmos. Environ. 2002, 36, 2349–2365. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH4+-Na+-SO42−-NO3−-Cl−-H2O aerosols. Atmos. Chem. Phys. Discuss. 2007, 7, 1893–1939. [Google Scholar] [CrossRef]
- Ansari, A.S.; Pandis, S.N. An analysis of four models predicting the partitioning of semi-volatile inorganic aerosol components. Aeros. Sci. Technol. 1999, 31, 129–153. [Google Scholar] [CrossRef]
- Nenes, A.; Pilinis, C.; Pandis, S.N. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aqua. Geochem. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Nenes, A.; Pilinis, C.; Pandis, S.N. Continued development and testing of a new thermodynamic aerosol module for urban and regional air quality models. Atmos. Environ. 1999, 33, 1553–1560. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A.; Pandis, S.; Pilinis, C. ISORROPIA v2.1 Reference Manual. 2009. Available online: https://www.epfl.ch/labs/lapi/wp-content/uploads/2018/12/ISORROPIA21Manual.pdf (accessed on 21 November 2022).
- Koo, B.; Gaydos, T.M.; Pandis, S.N. Evaluation of the equilibrium, dynamic, and hybrid aerosol modeling approaches. Aerosol Sci. Technol. 2003, 37, 53–64. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhang, Y.; Jacobson, M.Z.; Chan, C.K. Coupling and evaluating gas/particle mass transfer treatments for aerosol simulation and forecast. J. Geophys. Res. 2008, 113, 1–20. [Google Scholar] [CrossRef]
- Meng, Z.; Seinfeld, J.H. Time scale to achieve atmospheric gas-aerosol equilibrium for volatile species. Atmos. Environ. 1996, 30, 2889–2900. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A.; Sullivan, A.; Weber, R.; Van Reken, T.; Fischer, M.; Matias, E.; Moya, M.; Farmer, D.; Cohen, R.C. Thermodynamic characterization of Mexico City aerosol during MILAGRO 2006. Atmos. Chem. Phys. 2009, 9, 2141–2156. [Google Scholar] [CrossRef]
- Zhang, J.; Chameides, W.L.; Weber, R.; Cass, G.; Orsini, D.; Edgerton, E.S.; Jongejan, P.; Slanina, J. An evaluation of the thermodynamic equilibrium assumption for fine particulate composition: Nitrate and ammonium during the 1999 Atlanta Supersite Experiment. J. Geophys. Res. 2002, 107, 8414. [Google Scholar] [CrossRef]
- Yu, S.; Dennis, R.; Roselle, S.; Nenes, A.; Walker, J.; Eder, B.; Schere, K.; Swall, J.; Robarge, W. An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict aerosol NO3−. J. Geophys. Res. 2005, 110, D07S13. [Google Scholar] [CrossRef]
- Takahama, S.; Wittig, A.E.; Vayenas, V.; Davidson, C.I.; Pandis, S.N. Modeling the diurnal variation of nitrate during the Pittsburgh Air Quality Study. J. Geophys. Res. 2004, 109, D16S06. [Google Scholar] [CrossRef]
- Moya, M.; Ansari, A.S.; Pandis, S.N. Partitioning of nitrate and ammonium between the gas and particulate phases during the 1997 IMADA-AVER study in Mexico City. Atmos. Environ. 2001, 35, 1791–1804. [Google Scholar] [CrossRef]
- Goetz, S.; Aneja, V.P.; Zhang, Y. Measurement, analysis, and modeling of fine particulate matter in Eastern North Carolina. J. Air Waste Manag. Assoc. 2008, 58, 1208–1214. [Google Scholar] [CrossRef]
- Walker, J.T.; Robarge, W.P.; Shendrikar, A.; Kimball, H. Inorganic PM2.5 at a U.S. agricultural site. Environ. Pollut. 2006, 139, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Silvern, R.F.; Jacob, D.J.; Kim, P.S.; Marais, E.A.; Turner, J.R. Inconsistency of ammonium-sulfate aerosol ratios with thermodynamic models in the eastern U.S.: A possible role of organic aerosol. Atmos. Chem. Phys. 2017, 17, 5107–5118. [Google Scholar] [CrossRef]
- Gill, P.S.; Graedel, T.E.; Weschler, C.J. Organic films on atmospheric aerosol particles, fog droplets, cloud droplets, raindrops, and snowflakes. Rev. Geophys. 1983, 21, 903–920. [Google Scholar] [CrossRef]
- Li, Q.-F.; Wang-Li, L.; Shah, S.B.; Jayanty, R.K.M.; Bloomfield, P. Ammonia concentrations and modeling of inorganic particulate matter in the vicinity of an egg production facility in Southeastern USA. Environ. Sci. Pollut. Res. 2014, 21, 4675–4685. [Google Scholar] [CrossRef]
- Li, Q.-F.; Wang-Li, L.; Liu, Z.; Jayanty, R.K.M.; Shah, S.B.; Bloomfield, P. Major ionic composition of fine particulate matter in an animal feeding operation facility and its vicinity. J. Air Waste Manag. Assoc. 2014, 64, 1279–1287. [Google Scholar] [CrossRef]
- Liggio, J.; Li, S.M.; Vlasenko, A.; Stroud, C.; Makar, P. Depression of ammonia uptake to sulfuric acid aerosols by competing uptake of ambient organic gases. Environ. Sci. Technol. 2011, 45, 2790–2796. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Zuend, A.; Fry, J.L.; Isaacman-VanWertz, G.; Capps, S.L.; Appel, K.W.; Foroutan, H.; Xu, L.; Ng, N.L.; Goldstein, A.H. Coupling of organic and inorganic aerosol systems and the effect on gas–particle partitioning in the southeastern US. Atmos. Chem. Phys. 2018, 18, 357–370. [Google Scholar] [CrossRef]
- Guo, H.; Nenes, A.; Weber, R.J. The underappreciated role of nonvolatile cations on aerosol ammonium-sulfate molar ratios. Atmos. Chem. Phys. 2018, 18, 17307–17323. [Google Scholar] [CrossRef]
- Zheng, G.; Su, H.; Wang, S.; Andreae, M.O.; Pöschl, U.; Cheng, Y. Multiphase buffer theory explains contrasts in atmospheric aerosol acidity. Science 2020, 369, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Electric Power Research Institute (EPRI). 2008. Available online: https://yosemite.epa.gov/sab%5CSABPRODUCT.NSF/B99E5E7B1FC13632852575B50069AAC2/$File/EPRI-SEARCH+for+EPA+SAB+INC+May+14-15+2009+Meeting.pdf (accessed on 21 November 2022).
- Hansen, D.A.; Edgerton, E.S.; Hartsell, B.E.; Jansen, J.J.; Kandasamy, N.; Hidy, G.M.; Blanchard, C.L. The Southeastern Aerosol Research and Characterization Study: Part 1–Overview. J. Air Waste Manag. Assoc. 2003, 53, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Hidy, G.M.; Blanchard, C.L.; Baumann, K.; Edgerton, E.; Tanenbaum, S.; Shaw, S.; Knipping, E.; Tombach, I.; Jansen, J.; Walters, J. Chemical climatology of the Southeastern United States, 1999–2013. Atmos. Chem. Phys. 2014, 14, 11893–11914. [Google Scholar] [CrossRef]
- Cohen, M.A.; Ryan, P.B. Observations less than the analytical limit of Detection: A new approach. JAPCA 1989, 39, 328–329. [Google Scholar] [CrossRef]
- USEPA. Guidance for Data Quality Assessment. 2000. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/g9-final.pdf (accessed on 21 November 2022).
- Chang, J.C.; Hanna, S.R. Air quality model performance evaluation. Meteo. Atmos. Phys. 2004, 87, 167–196. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Varadarajan, C.; Vijayan, A.; Masuraha, A. Evaluation of the AERMOD dispersion model as a function of atmospheric stability for an urban area. Environ. Prog. 2006, 25, 141–151. [Google Scholar] [CrossRef]
- Ansari, A.S.; Pandis, S.N. The effect of metastable equilibrium states on the partitioning of nitrate between the gas and aerosol phases. Atmos. Environ. 2000, 34, 157–168. [Google Scholar] [CrossRef]
- Metzger, S.; Mihalopoulos, N.; Levieveld, J. Importance of mineral cations and organics in gas-aerosol partitioning of reactive nitrogen compounds: Case study based on MINOS results. Atmos. Chem. Phys. 2006, 6, 2549–2567. [Google Scholar] [CrossRef]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef]
- Cheng, B.; Wang-Li, L.; Classen, J.; Meskhidze, N.; Bloomfield, P. Spatial and temporal variations of atmospheric chemical condition in the Southeastern U.S. Atmos. Res. 2021, 248, 105190. [Google Scholar] [CrossRef]
- Hand, J.L.; Schichtel, B.A.; Pitchford, M.; Malm, W.C.; Frank, N.H. Seasonal composition of remote and urban fine particulate matter in the United States. J. Geophys. Res. 2012, 117, D05209. [Google Scholar] [CrossRef]

| Observable | Technique | Max Resolution | Detection Limit (ppb or µg m−3) |
|---|---|---|---|
| Gases | |||
| NO | CL a | 1 min | 0.05 |
| NO2 | Photolysis/CL | 1 min | 0.1 |
| HNO3 | Denuder/Mo reduction/CL | 1 min | 0.1 |
| NOy | Mo reduction/CL | 1 min | 0.1 |
| SO2 | UV–fluorescence | 1 min | 0.2 |
| NH3 | Denuder/Pt oxidation/CL | 5 min | 0.2 |
| iPM2.5 chemical compositions | |||
| SO42− | Fe reduction/UV–fluorescence | 5 min | 0.4 |
| NO3− | Filter/Mo reduction/CL | 5 min | 0.2 |
| NH4+ | Filter/Pt oxidation/CL | 5 min | 0.1 |
| Meteorological conditions | |||
| T/RH/SR b/BP c | Various | 1 min | NA |
| WS d/WD e/Precipitation | Various | 1 min | NA |
| Discrete iPM2.5 chemical compositions | |||
| NO3− | Teflon filer + IC f | 24 h | 0.01 |
| Volatile NO3− | Nylon filer + IC | 24 h | 0.02 |
| NH4+ | Teflon filer + AC g | 24 h | 0.03 |
| Volatile NH4+ | Citric acid annular denuder + AC | 24 h | 0.04 |
| SO42− | Teflon filter + IC | 24 h | 0.05 |
| K+-Ca2+-Mg2+-Na+ | Teflon filter + ICP-MS h | 24 h | NA i |
| Temperature | Relative Humidity | |||||||
|---|---|---|---|---|---|---|---|---|
| 20–30% | 30–40% | 40–50% | 50–60% | 60–70% | 70–80% | 80–90% | 90–100% | |
| 30–35 °C | −2.00 M | −2.00 M | −2.00 M | −1.98 M | −1.81 M | NA | NA | NA |
| 25–30 °C | −2.00 M | −2.00 M | −2.00 M | −1.99 M | −1.66 M | −0.71 M | −0.48 S = M | NA |
| 20–25 °C | −2.00 M | −1.95 M | −1.84 M | −1.97 M | −1.10 M | −0.27 M | −0.09 M | 0.25 S = M |
| 15–20 °C | −0.78 M | −1.20 M | −1.33 M | −1.53 M | −0.29 M | 0.06 S | 0.12 S | 0.36 S = M |
| 10–15 °C | −0.65 M | −0.61 M | −0.49 S | −0.47 M | −0.02 S | 0.30 S | 0.29 S | 0.41 S = M |
| 5–10 °C | −0.29 M | 0.01 S | 0.09 S | 0.03 S | 0.08 S | 0.21 S | 0.17 S | 0.17 S = M |
| 0–5 °C | 0.18 S | 0.30 S | 0.29 S | 0.25 S | 0.12 S | 0.09 S | 0.07 S | 0.17 S = M |
| −5–0 °C | NA | 0.40 S | 0.44 S | 0.30 S | 0.16 S | 0.08 S | 0.06 S | 0.13 S = M |
| −10–−5 °C | NA | NA | NA | 0.37 S | 0.20 S | 0.05 S | 0.02 S | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Wang-Li, L.; Classen, J.; Bloomfield, P. Performance of a Thermodynamic Model for Predicting Inorganic Aerosols in the Southeastern U.S. Atmosphere 2022, 13, 1977. https://doi.org/10.3390/atmos13121977
Cheng B, Wang-Li L, Classen J, Bloomfield P. Performance of a Thermodynamic Model for Predicting Inorganic Aerosols in the Southeastern U.S. Atmosphere. 2022; 13(12):1977. https://doi.org/10.3390/atmos13121977
Chicago/Turabian StyleCheng, Bin, Lingjuan Wang-Li, John Classen, and Peter Bloomfield. 2022. "Performance of a Thermodynamic Model for Predicting Inorganic Aerosols in the Southeastern U.S." Atmosphere 13, no. 12: 1977. https://doi.org/10.3390/atmos13121977
APA StyleCheng, B., Wang-Li, L., Classen, J., & Bloomfield, P. (2022). Performance of a Thermodynamic Model for Predicting Inorganic Aerosols in the Southeastern U.S. Atmosphere, 13(12), 1977. https://doi.org/10.3390/atmos13121977








