Abstract
In the last few decades, excessive greenhouse gas emissions into the atmosphere have led to significant climate change. Many approaches to reducing carbon dioxide (CO2) emissions into the atmosphere have been developed, with carbon capture and sequestration (CCS) techniques being identified as promising. Flue gas emissions that produce CO2 are currently being captured, sequestered, and used on a global scale. These techniques offer a viable way to encourage sustainability for the benefit of future generations. Finding ways to utilize flue gas emissions has received less attention from researchers in the past than CO2 capture and storage. Several problems also need to be resolved in the field of carbon capture and sequestration (CCS) technology, including those relating to cost, storage capacity, and reservoir durability. Also covered in this research is the current carbon capture and sequestration technology. This study proposes a sustainable approach combining CCS and methane production with CO2 as a feedstock, making CCS technology more practicable. By generating renewable energy, this approach provides several benefits, including the reduction of CO2 emissions and increased energy security. The conversion of CO2 into methane is a recommended practice because of the many benefits of methane, which make it potentially useful for reducing pollution and promoting sustainability.
1. Introduction
Energy plants, industries, as well as other sources of carbon dioxide (CO2) result in global warming and affect the planet [1]. The temperature of the Earth’s surface has risen by 0.8 °C in tandem with an increase in CO2 concentration of 280 to 400 ppm [2]. In the last century, the CO2 level has increased slightly to 408.8 ppm [3], with projections of 600 to 700 ppm, raising average surface temperatures by 4.5–5 °C [4]. Unstable financial, technological, and sociological advances, along with natural and human developments, are all important factors affecting the CO2 concentration [5]. In addition to CO2, other greenhouse gas (GHG) emissions have significantly increased in recent years, such as sulfur hexafluoride (SF6), perfluorocarbons, hydrofluorocarbons (HFC), nitrous oxide (N2O), and methane (CH4) [1,6]. According to the International Panel on Climate Change, GHG emissions should be lessened by 50–80% to prevent our planet from disintegrating [2]. Almost 190 countries met in Paris in December 2015 (Conference of Parties—COP 21) to agree on limiting global temperatures to 2 °C by the end of the 21st century. COP 21 proposed several techniques, including increasing energy efficiency, utilizing renewable fuels, and implementing geoengineering techniques such as afforestation and, more specifically, developing carbon capture and storage (CCS) technologies [3,4]. Therefore, carbon capture and storage (CCS) is generally acknowledged as a potential, if not suitable, method to minimize CO2 emissions globally [7,8].
An estimated 60% of CO2 emissions are attributed to many stationary sources, including industrial plants, fossil fuel power plants and thermoelectric cement plants, iron and steel mills, gas processing industries, refineries, and power plants. These industries serve a variety of sectors such as the industrial sector, transportation, and electricity generation [9]. According to the above analysis, stationary emission sources will continue to be the greatest drivers of greenhouse gases (GHGs) resulting from the burning of fossil fuels for several decades to come. The production of electricity is responsible for 25% of all emissions, while forestry and agriculture account for 24% [10]. Thermal power plants typically use coal as fuel, because it is cheap and readily available, yet it is considered a major emission source, with emissions approaching 2249 lbs/MWh [11]. As a result, new and improved technologies such as CCS for swift CO2 removal from the atmosphere to tackle global warming have received a lot of attention lately. Figure 1 illustrates the various energy sectors contributing the most to CO2 emissions.
The type of combustion process utilized, as well as the volume of CO2, absorbed, separated, and transported for reuse or storage, are the primary determinants of CCS [4]. CCS can reduce CO2 emissions from power plants to limit global warming to 2 °C [4]. Post-combustion is the most advanced of the three major CCS technologies, followed by pre-combustion and oxy-fuel combustion. In terms of CO2 capture, coal gasification is the best option; however, it cannot be used in gas-fired power plants. It makes use of most of the existing power plant infrastructure as a retrofit technology. There has been a demonstration of the ability to recover 300 t/day of CO2 on a small scale [4].
During oxy-fuel combustion, pure oxygen replaces air, thereby eliminating the requirement for flue gas de-NOx and the incorporation of 80–98 °C CO2 in the exhaust, resulting in a more effective and reliable CO2 removal method. Although the process requires a lot of energy, corrosion is aided by SO2 concentration due to the increased energy consumption [12]. Gasification under low O2 pressure in pre-combustion CCS produces syngas (CO + H2) from the fuel pretreatment [4]. In the presence of high CO2 concentrations in fuel gas, separation is less difficult than with other methods, resulting in the formation of H2 as a transport medium for energy. This helps to offset some of the technology’s operational costs [12].
The purpose of this paper is to provide an overview of the state of carbon capture systems, both conventional and the emerging, and present CO2 as a feedstock for producing methane (CH4). It further concentrates on improving the concept and fostering research on CO2 capture and utilization to circumvent the storage issues with the current CCS technology, and make sustainability a priority for future generations.
There are currently no studies that address all the gaps; however, this paper covers CO2 capture, sequestration, utilization, and CO2 methanation.
2. Methodology
The focus of this research on the literature was intended to identify the benefits and drawbacks of current carbon capture systems and porous carbon-based adsorbents for CO2 adsorption.
In addition, the challenges and prospects for CO2 conversion into methane were discussed. The manuscript’s section on methodology describes how the literature was found, gathered, and then arranged by identifying the knowledge gaps that exist in earlier studies. Using scientific databases and a variety of search engines, such as NCBI, Google Scholar, Scopus, Science Direct, Web of Science, and major publishers, an in-depth analysis of the articles and literature from peer-reviewed journals was carried out. The search for relevant literature was constrained to studies from the years 2000 to 2022 to draft this manuscript. The limited literature search for this manuscript’s preparation was mostly focused on studies between the years 2000 and 2022. For this paper’s main theme, several pertinent keywords were employed in the literature search. These keywords include zeolite, activated carbon, graphene, silica, and metal–organic framework, as well as CO2 capture and conversion. Research articles with the most pertinent material were selected after the articles were evaluated and the information from the abstracts was reviewed. To prevent translation challenges, articles published in languages other than English were omitted.
3. CO2 Capture Technologies
CCS technology relies on CO2 capture. Three methods for trapping and sequestering CO2 are envisaged, based on the settings of a fossil fuel-based power plant, gas steam pressure, and CO2 partial pressure. Among the carbon capture technologies are pre-combustion, oxy-fuel combustion, and post-combustion [13].
3.1. Pre-Combustion Capture Method
This method involves capturing CO2 before combustion, rather than after combustion, thus making this method more feasible. Gasifiers and catalytic reactors are involved. In a gasifier with low oxygen pressure, the following equations (Equations (1)–(3)) demonstrate how pure syngas (CO, H2) is produced. Moreso syngas is enriched by passing it through a shift reactor with a steam, as shown in Figure 1.
Natural gas could also be applied for steam reforming via this method; between 700 and 850 °C, an endothermic reaction occurs, culminating in the conversion of CH4 and H2O and the generation of syngas (CO, H2,). An exothermic reaction occurs after partial oxidation, in which CH4 burns in oxygen to create CO2 (Equations (4) and (5)) [14].
A key element of pre-combustion is the hydration of gas separated from air, which is the most promising technology today [15].
The CO2 captured in this technique produces hydrogen fuel, which is used to generate electricity in several industries while emitting little CO2 [16]. Fertilizers and hydrogen are produced through pre-combustion capture [17]. CO2 is generated from this process in concentrations ranging from 5 to 60%, making it easy to capture; however, the water gas shift reactions and gasification are costly and difficult to operate [18].
3.2. Oxy Fuel Combustion Capture Method
A system for air separation during oxy-fuel combustion capture is used to obtain pure oxygen, as flue gas and coal are fed into the oxy-fuel boiler [19,20,21]. Coal is burned at a fixed temperature and pressure. Figure 1 illustrates how the flue gases, lacking oxygen and nitrogen, are only made up of CO2 and water vapors. Additionally, this reduces the quantity of flue gas required to remove NOx and sulfur [22,23]. Condensing water vapor maintains the boiler’s temperature. Dehydrated CO2 is compressed and delivered to a storage facility or an industry where it is utilized in beverage carbonation, as a solvent, and as a fire extinguisher [24]. Flue gas is recycled in its natural state to maintain the boiler’s internal temperature [20]. When compared to other carbon capture technologies, it is the most efficient and adaptable [25]. There are, however, some obstacles to overcome. The air separation unit (ASU) uses cryogenic distillation, which consumes a great deal of energy to produce essentially pure oxygen. The integrated gasification combined cycle (IGCC) can be replaced by this method, which converts CO to CO2.
A major drawback of this method is that it demands more energy to separate oxygen from the air [4,26].
3.3. Post Combustion Capture Method
After burning carbonaceous materials (such as biomass) or fossil fuels, CO2 is removed from the flue gas using a viable adsorbent such as amine [4,27]. Currently, it is the most effective method of capturing CO2 from natural gas plants. As separating agents, it employs amine solutions including potassium carbonate (K2CO3) mono-ethanolamine (MEA), methyl-di-ethanolamine (MDEA), and di-ethanolamine (DEA) [14]. The novel sorbent piperazine collects contaminants efficiently because of its high volatility [28]. A chemical reaction deposits CO2 in the solution, which is then separated by flowing high-temperature (100–200 °C) steam over it.
An equation representing the reversible absorption–regeneration reaction is shown below [14].
However, this method has some drawbacks, such as a high parasitic load, high flue gas temperatures, and the inability to operate at low CO2 levels. Figure 1 illustrates a direct post-combustion mechanism. Gas-fired power plants use about 4% CO2, while coal-fired power plants utilize 7–14%, increasing electricity costs by 32% and 65%, respectively, in gas-fired and coal power plants [4]. As a result, cutting costs while improving system performance is still a subject of great interest [29]. Membrane technology, including ceramic or polymeric membranes, marine algae, and cryogenic distillation, can all be used for post-combustion CO2 capture. This has many benefits, including the ability to retrofit and eliminate the need for a cryogenic separator and shift reactor. In post-combustion, CO2 is usually absorbed or desorbed using a solution containing alkanol amine [30,31,32].
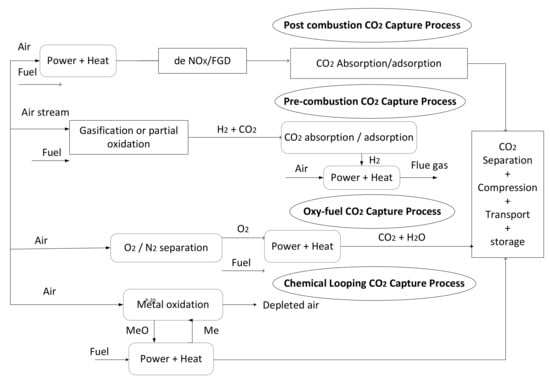
Figure 1.
CO2 capture technologies [33].
4. Various Combustion Technologies for CO2 Capture
A comparison of the three CO2 capture technologies is illustrated in Table 1. Coal gasification uses pre-combustion, while oxy-fuel combustion and post-combustion are applied to coal- and gas-fired plants. CO2 capture using post-combustion technology is presently the most advanced [28,34]. According to Gibbins and Chalmers [35], three technologies were compared in terms of the costs of both gas-fired and coal-fired power plants (Table 2). CO2 mitigation for coal-fired power plants was the cheapest using pre-combustion technology, while oxy-fuel and post-combustion technologies were comparable. For post-combustion capture, the costs per tonne of avoided CO2 were 50% lower for gas-fired plants than for the two other technologies. Moreover, the most inefficient method of CO2 capture is post-combustion CO2 capture, having an energy cost of roughly 8% for coal-fired plants and 6% for gas-fired plants [36].

Table 1.
Comparison of various CO2 capture technologies [37].

Table 2.
Comparison of the costs of various capture methods [35,37]. Costs exclude storage and shipping costs, but include CO2 compression to 110 bars.
5. CO2 Separation Techniques
CO2 is separated from flue gas during combustion using a variety of advanced separation techniques [4]. There are several techniques involved, such as absorption, adsorption, chemical looping, membrane separation, and cryogenics [18,38,39]. Figure 2 displays a flowchart outlining the methods and techniques for CO2 capture/separation technologies.
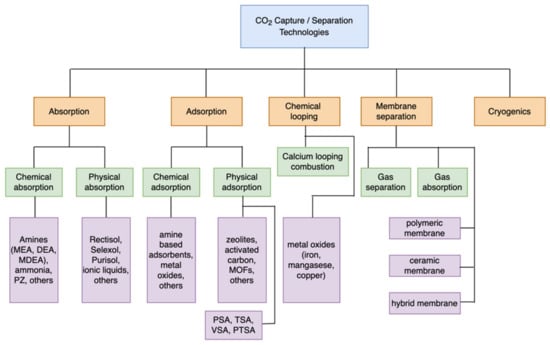
Figure 2.
Separation techniques for CO2 capture [40].
5.1. Absorption
The ability of absorption to capture huge quantities of emissions from chemical factories and power plants has gained considerable attention in recent years. Chemical absorption is a reliable technique for CO2 separation in coal-fired power plants because it is well-suited for existing plants with high operating costs and limited infrastructure [41]. The chemical absorption of CO2 is a commercially viable technology due to its many advantages, including technical efficiency, handling capacity, and sophistication [42]. The potential absorbents and processes of absorption CO2 capture are highlighted in Table 3.
CO2 is separated from flue gas by absorption using a liquid sorbent [3,43]. It is possible to regenerate the sorbent via a regenerative process or stripping by depressurizing and/or heating. This is the latest and most advanced method for separating CO2 [44]. Potassium carbonate (K2CO3), monoethanolamine (MEA), and diethanolamine (DEA) are examples of common sorbents [45]. MEA is very reactive and absorbs more quickly, and it is quite inexpensive [44]. However, their main drawback is the substantial parasitic energy load in relation to solvent regeneration, which adversely affects the total effectiveness of systems combined with aqueous amine-based absorption processes [46]. DEA and other alkanolamines have also been employed for absorption, although they have comparable defects. Methyldiethanolamine (MDEA), a mixture of MEA and DEA, has been used with moderate success. It has higher CO2 loading capacity, and degradation and corrosion resistance, as well as cheaper regeneration costs, but lower rates of absorption [47,48,49,50,51,52].
Veawab et al. [53] reported that MEA is the most efficient aqueous alkanolamine for CO2 absorption, with a performance rate greater than 90%. Additionally, Aaron and Tsouris [54] reviewed various CO2 capture technologies and determined that MEA absorption is the most viable method for CO2 capture in CCS. Applying a solvent containing 30% MEA, a 1 t CO2/h absorption pilot plant was designed and experimentally validated in conjunction with a coal-fired power plant’s post-combustion capture technology [55]. In recent times, other adsorbents, including anion-functionalized ionic liquid and piperazine, have attracted a lot of attention [56]. Even though piperazine rapidly reacts compared to MEA, its use in CO2 absorption is more costly. Due to its higher volatility, it is still in the experimental phase [28]. The risk of amine degradation, which could lead to equipment corrosion, solvent loss, and the formation of volatile degradation compounds, is a significant barrier to the widespread adoption of this technology for the CCS [57,58], while environmental degradation has gone unnoticed.
Furthermore, amine emissions can deteriorate into nitramines and nitrosamines, which are highly toxic to human health and the environment. The chilled ammonia process captures CO2 using aqueous ammonium salts (including ammonium carbonate) and can regenerate the CO2 at elevated temperatures and pressures using waste heat, thereby minimizing the downstream compression [59]. There are fewer problems with this process than those caused by amine degradation.
Water’s use as a co-solvent, which has higher thermal characteristics than other co-solvents, is one of the key precursors for the high solvent regeneration energy of MEA [20]. In the context of CO2 absorption, the predicted regeneration energy for 30 wt. % aqueous MEA showed that more than 50% of the total energy was used to heat and vaporize the water co-solvent. The remaining energy was used to reverse the chemical interaction between CO2 and MEA at the same time [60,61]. Considering this, it was thought that either totally or partially substituting other organic diluents for water as co-solvents could potentially reduce solvent regeneration energy, since they effectively create water-free/water-lean hybrid solvents with poorer thermal properties than water [62,63,64,65,66,67,68]. Instead of vaporizing and heating the co-solvent, comparable to aqueous amines, the regeneration energy will be used more effectively to reverse acid gas chemisorption.
Additionally, hybrid water-free/water-lean solvents have been thoroughly studied in recent years, primarily for their CO2 capture applications [68,69,70]. They provide a wide range of potentially alluring substitutes to conventional aqueous amines [46,71]. The main objective of water-lean solvents is to preserve the chemical selectivity of water-based solvents, while enabling step gains in efficiency due to the lower specific heats of organics than water [46]. However, two problematic regions refute the claim of their attractiveness. The stated performance of these solvents when scaled up from lab-scale to industrial-scale settings has not been adequately examined due to a lack of availability of a few essential properties. This is predicted given the labor-intensive nature of experimental work, which makes it impossible to expand experimental testing to the broad range of transport and thermophysical parameters needed for precise and representative performance evaluation on an industrial scale. The second issue is that, when carried out on a lab scale, the potentiality of a particular solvent is typically demonstrated using a limited set of parameters, most notably the low enthalpy of absorption and high absorption capacity [72,73,74,75]. These two characteristics are indeed of great concern for chemical absorption procedures, but they are still unsuitable for accurately gauging the potential of the tested solvents for their intended use. However, they ignore significant trade-offs between competing environmental, economic, and operational factors. The results of a straightforward assessment can help direct the development of novel generating solvents [76,77].
However, these difficulties can be overcome if the proper tools or novel process configurations are available. Due to recent developments in computational power and thermodynamic modeling tools, the first issue can be resolved by scaling the data from lab to industrial operating conditions. The most appealing models for this application are molecular equations of state (EoSs) centered on the Statistical Associating Fluid Theory (SAFT) [78,79], due to their strong theoretical background, demonstrated correctness for a range of complex systems, and predictive abilities.
The solution to the second problem, which is to demonstrate the viability of a chosen solvent typically acknowledged using a limited number of requirements, may appear relatively apparent: add more evaluation criteria to the already-existing standard key performance indicators (KPIs). Moreover, making such a preference is more difficult because early design phases may not have access to information on a particular criterion [80]. There must be a justification for why certain criteria should be included or excluded when narrowing the search space among the numerous properties that are available [81,82,83]. The effect of solvent characteristics on economic metrics such as total capital expenditures (CAPEX) and operating expenditures (OPEX) typically serves as the foundation for justification. Through the careful process modeling of hypothetical solvents, Mota-Martinez et al. [84] ranked various solvent characteristics according to how they affected the process’ overall economics. Leclaire and Heldebrant [43] recently recommended the use of ideas from green engineering and chemistry to address problems with the advancement of CCUS technologies. They asserted that by applying the 12 + 12 principles of engineering and green chemistry [85], they could indirectly encourage the improvement of chemical processes’ economic attractiveness and efficiency, which goes beyond their environmental motivation. Similarly to this, it may be beneficial to consider sustainability, health, and safety issues while assessing the possibility of promising solvents for the removal of acid gas [86,87]. Figure 3 displays the schematic diagram for the absorption carbon capture process.
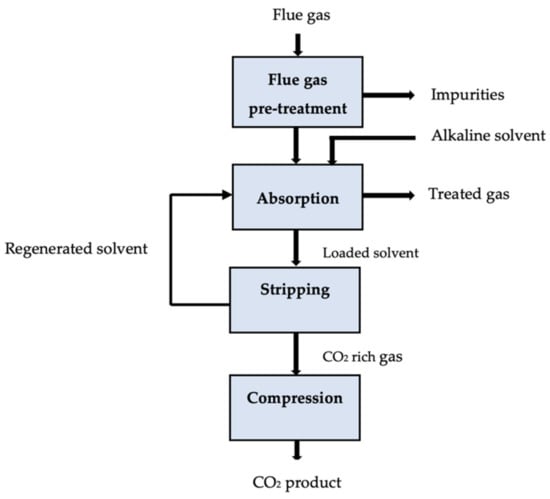
Figure 3.
Schematic of an absorption carbon capture process [110].

Table 3.
Summary of absorption-based carbon capture.
Table 3.
Summary of absorption-based carbon capture.
| Type | Absorbent | Reactive Separator | Operating Conditions P, C, T, G | CO2 Capture (%), AC (kg/kg) | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|---|
| Single solvent | MEA | Flow (SC) | C:8 −16; T:10–40; G:2–10 | 94, 0.4 | [88,89,90] | |
| K2CO3 | Fixed-bed (Con-O, bench scale) | T:60 G:40 mL/min | 99.4, NA | NA | [91,92] | |
| Ammonia | Sieve plate (CC) | C:10–14; T:25–55 °C | 95–99, 1.2 | [88,89] | ||
| Piperazine | Stirred cell (SC, BS) | P:0.032 T:42 and 0.042 | 100, 0.32 | 1st order partial reaction occurs | [93] | |
| Ionic liquids | Double stirred cell (BS) | T:25–50; P:0.1; A:0.5–1.2 | 99.11 at 60 °C, | NA | [94,95,96,97] | |
| Mixed Solvents | DEA-K2CO3 | Split flow (CC, bench scale) | T:115 L:63.66 m3/h | 99, NA | Promoter selection is very critical. It is a reversible exothermic reaction | [98] |
| PEI-SiO2 Alcohol/amine/water | Packed (bench scale) | L:33.66 m3/h | NA, NA | [99,100] | ||
| BDA-DEEA | Packed (CC, BS) | T:40 (absorption) T:90 (desorption) G: 24.78 m3/h | 46 (HCL), 48 (HCC), 11(HCE) than MEA with 5 M | Carbamate and bicarbamate formations | [101] | |
| AMP-PZ | Packed (pilot) | L/G:2.9; packing height=10 m | 90, NA | - | [102,103,104,105,106,107,108,109] |
5.2. Adsorption
The process of adsorption [43] involves molecules in liquids and gases adhering to solid surfaces by weak van der Waals interactions. Unlike liquid absorbent processes, solid adsorbents bind CO2 to their surfaces during adsorption. Selection criteria for this sorbent include a large surface area, high regeneration capability, and high selectivity. Common adsorbents include activated carbon, molecular sieves, zeolites, lithium zirconate, and hydrotalcite [27]. Table 4 highlights the potential adsorbents and adsorption parameters for CO2 capture.
It is possible to achieve CO2 adsorption by changing the pressure or temperature of a saturated sorbent. Pressure swing adsorption (PSA) is a commercially applied technology that recovers more than 85% of CO2 from power plants [111,112]. A solid adsorbent selectively adsorbs CO2 at high pressures, then the solid desorbs, releasing CO2 for low-pressure transport (usually atmospheric pressure). The temperature swing adsorption (TSA) releases the CO2 in the system by increasing its temperature through steam injection or hot air distribution [113]. A CO2 purity of over 95% and recovery of over 80% are possible when using CO2 regeneration, although regeneration is more time-consuming than PSA [114]. It was estimated that the operating costs of a particular TSA process ranged between USD 80 and 150 per tonne of CO2 captured [115]. Significant attention has been paid to developing CO2 capture sorbents from agricultural and industrial wastes to lower the overall cost of CO2 capture. An adsorption carbon capture process is shown in Figure 4.
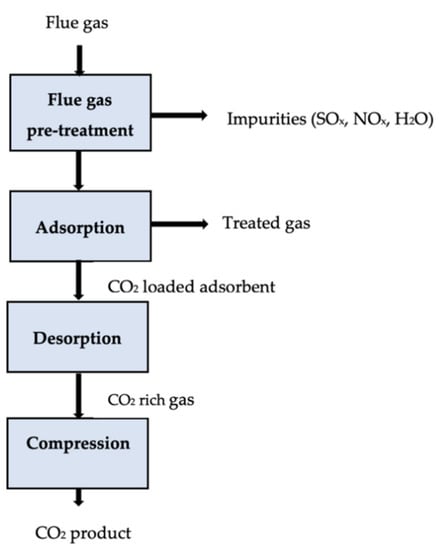
Figure 4.
Schematic of an adsorption carbon capture process [110].

Table 4.
Summary of adsorption-based carbon capture.
Table 4.
Summary of adsorption-based carbon capture.
| Adsorbent | Reactive Separator | Operating Conditions P, T, C, G | CO2 Capture (%), Ad-C (gCO2/gads) | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|
| TEPA-Mg-MOF-74 | PBR (LS) | Regeneration temp is 250–300 °C | 4–4.9 wt. %, 8.31 mmol CO2/g absorbent, NA | N2 adsorption–desorption isotherm | [109] |
| ZX-APG, | PBR (3-bed, 8-step, VPSA, LS) | T:35; P: 0.007–0.008 | 85–95, NA, 73–82% CO2 purity | Langmuir adsorption isotherm is adopted | [116] |
| Activated carbon | PBR (1 bed, 3 step, VSA, LS) | Water vapour (H2O): 4.6 mol%, Vf: 44; TDes:100T: 60, ICC:11.2, Bd:0.493, Lg:50, P:0.113, PVP = 3, Trpt:3; SA:921.7, PV:0.37, Tads:35, | 69.5, NA | Dual-site Langmuir equation has been adopted | [117] |
| NPC10 | PBR (TSA, LS) | T: 25, P: 0.1, SA: 639 | NA, 0.041 | Langmuir adsorption isotherm | [118] |
| Fly ash + PEI + PEG | PBR (LS, TSA) | St: 24 h, P: 0.11, T: 70 | 4.5 at 85 °C | [119] | |
| ZX | MBA (LS, PSA) | Bed dimensions (m): FRR: 0.5; CT: 650; AT: 950; SA: 1873.9; 2b: 0.03, Nm: 36, W: 1.5; L:1.5; Xpth: 0.012 Bd: 0.65, Cs: 1.07, Dp: 3420, ε: 0.31 ks: 0.275 | 80, NA, 97% purity | Extended Langmuir isotherm was used | [120] |
| Rayon–HCM | PBR (TSA) | 97, 0.2 | Langmuir adsorption isotherm adopted | [121,122] |
5.3. Chemical Looping Combustion
In contrast to oxy-fuel combustion, which uses pure oxygen for combustion, metal oxides are used as oxygen carriers in combustion. Metal oxides are reduced to metal during the process, while fuels are oxidized to create CO2 and water. In a subsequent stage, the metal is oxidized and recycled. The removal of water by condensation from the process byproducts is easy, but the separation of pure CO2 requires no energy. Numerous low-cost metal oxides, including Mn2O3, CuO, NiO, and Fe2O3, are suitable for this process. The potential sorbents and processes of chemical looping combustion are highlighted in Table 5.
Several researchers [123,124,125,126,127] have examined the performance efficiency of various metal oxides in this process. According to Adánez, de Diego [126], a metal oxide can be optimized by using support inert materials, but the selection of an inert material will vary depending on the characteristics of the metal oxide. Chemical looping combustion (CLC) was studied by Lyngfelt, Leckner [128] in a boiler consisting of two fluidized beds. Lyngfelt, Leckner [128] recently reviewed this technology. This process has been demonstrated to be a very promising CO2 capture technology by both Lyngfelt, Leckner [128] and Adánez, de Diego [126]. The IGCC’s CO2 separation is based on pre-combustion, but Erlach, Schmidt [113] found chemical looping combustion to have a 2.8% higher net plant efficiency than the former method. Figure 5 illustrates the basic CLC system.
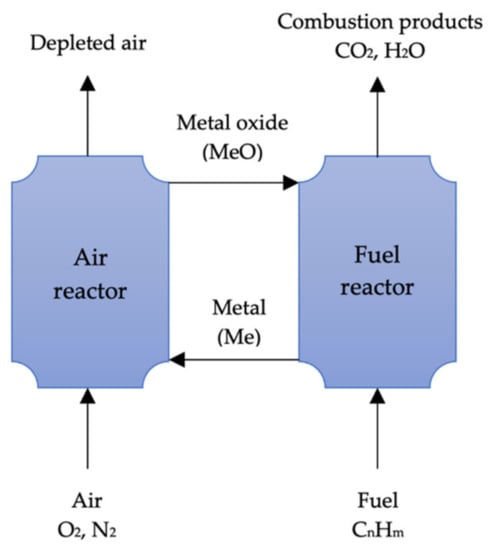
Figure 5.
Schematic of chemical-looping combustion (adapted from Yang [145]).

Table 5.
An overview of chemical looping combustion-based carbon capture.
Table 5.
An overview of chemical looping combustion-based carbon capture.
| Fuel Type | Operating Conditions P, T, C, G | Reactive Separator | CC (%), Purity (%) | Challenges | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|---|
| Coal, C2H5OH, Isooctane, C3H8 and CH4. | T: 200–1200; molar ratios of carbon/CaSO4 = 0.5 and carbon/steam = 1 | TGA | NA, 93 (with CaSO4 at 850–975 °C) | The ΔHr is dependent on the fuel but not the amount of OC utilized. The yield depends on OC. | Combustion of iso-octane (−5101.58 kJ/mol) with Na2SO4 and CaSO4 produces without SO2 formation between 200 °C and 344.3 °C. | [129,130] |
| Syngas, H2 | XOC: 80–95, HR: 90–99, T: 370–1030 | 2-stage PBR- CLC | 100, NA | PP of O2 in reactors; high solid inventories. | The packed bed of OC reduces the need for highly efficient cyclone to reduce costs; boron nitride (BN) used as the dense support material due to high thermal conductivity, low thermal expansion and high thermal stability. | [131,132,133,134] |
| Coal, kerosene, biomass | Bd: 4.750; Dp: 128 Umf: 0.0129, Φ: 0.64 | IFBR | 83–99.3% at 800–950 °C, NA | Scale-up, fuel conversion, agglomeration and attrition. | increases linearly with solid flow rate. | [135,136,137,138,139,140,141] |
| CH4, coal | Iron oxide: 950 °C, FF: 1.18, CO2 EF: 10, DT: 5.25 | CMBS or RPBR (1 MWth) | >99, >95 | Reaction heat exceeds the convective heat-transfer rate to the gas flow. | The reduction kinetics and activation energy parameters are critical to find fuel conversion efficiency, temperature distribution and carbon separation efficiency. | [142,143] |
| CH4, syngas | T: 700–975; SITC:20–30; SFRR: 8–10 for CO SFRR:4–12 for H2 Fsolids:1.7–2.5 | CC-MBR | >99% CH4 and 100% syngas conversion. >99.99% H2 purity. | The formation of FeO and FeAl2O4 indicates further utilization of oxygen in iron-based OC׳s can be achieved.–ϕ > 1.14. | At 900 °C, the reduction of Fe2O3 to Fe with CO generates 37.7 kJ/mol Fe2O3 of heat but its reduction with H2 gas needs 61.8 kJ/mol Fe2O3 of heat. | [143,144] |
5.4. Membrane Separation
Membrane separation uses a semipermeable membrane or barrier to physically separate CO2 from other flue gases [146]. Membrane separation uses less energy than traditional solvent absorption methods, making it less expensive [147]. Membrane separation has successfully been used for selective gas separation in a variety of fields for the past two decades, including natural gas sweetening, air separation, hydrogen production, and biogas upgrading. Researchers are working on developing membrane-based materials to separate CO2 released by various industries. Furthermore, this technology has produced increased efficiency in terms of both the economy and the environment [148]. Scientists have developed a variety of different membranes for CO2 separation, including inorganic membranes, polymers, carbon molecular sieve membranes (CMSMs), microporous organic polymers (MOPs), and mixed matrix membranes (MMMs) [149]. The potential sorbents and processes of membrane separation are highlighted in Table 6.
In addition, membrane separation technology can also separate gases in CCS processes such as pre- and post-combustion capture. It is generally considered that polymeric membranes are more flexible, durable, and efficient at capturing CO2 from industrial processes. An upper bound relationship analysis describes how selectivity and permeability are related to CO2 capture by polymeric membranes [150]. To improve results, glassy and rubbery materials with varying separation principles based on their size and diffusion ability can be used to synthesize polymeric membranes. The condensability and differences in kinetic properties of gas molecules are responsible for gas separation by glassy and rubbery polymers [151]. Considering how difficult it is to examine operating conditions for rapid performance, membranes applied in gas separation systems are typically modeled to determine their working capacity [152]. For optimal results in industrial settings, membrane performance must not be interfered with by flue gas impurities [149]. Researchers were able to separate CO2 from other gases with an efficiency of 82–88% [153,154]. In fact, despite membrane materials having poor permeability and selectivity [155], it is also problematic to use this extraction method in flue gas with low pressure and CO2 concentration in flue gas conditions [156]. A membrane carbon capture process is displayed in Figure 6.
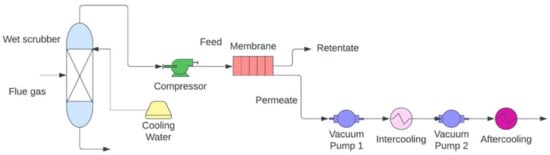
Figure 6.
Schematic of membrane carbon capture process (adapted from Wang [161]).

Table 6.
Summary of membrane-based carbon capture.
Table 6.
Summary of membrane-based carbon capture.
| Membrane | Reactive Separator | Operating Parameters | Challenges | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|
| Dense membranes | Hollow fiber and flat-sheet | S-P, T, P, La, pressure ratio of the permeate side to the feed side, pore size and porosity | Lower selectivity at higher permeability | Solution–diffusion; among the mechanisms are Knudsen diffusion and the molecular sieve effect | [157] |
| Micro-porous Membranes | Hollow fiber and flat-sheet | P, T, pore size and ε of the membrane–membrane wettability | Wetting of the membrane | Reaction kinetics depend on solvent | [157] |
| Gas flow area | There are other compounds present in the gas stream | Even at high pressures, Ko is controlled by the resistance of the liquid film | [158] | ||
| Liquid flow area | Solvent volatility and limited long-term stability | Pore diffusion depends on membrane support | [159] | ||
| Liquid in the membrane pores | Flat-sheet only | Ga, La, VVIS, P, T | Solvent “wash-out” causes the membrane’s stability to decrease | The overall mass transfer coefficient | [160] |
5.5. Cryogenic Distillation
This process separates CO2 from gas mixtures by focusing on their boiling points at temperatures ranging from 100 to 135 °C [6,43]. In the presence of high pressures (100–200 atm), solidified CO2 provides two significant benefits: a lack of solvents and liquefied CO2 for more convenient transport and injection [37]. It does, however, have some drawbacks that need to be investigated further, as do other processes. When cold and pressurized nitrogen is used as a refrigerant, ice formation compromises equipment safety, causing pressure fluctuations and pipe blockages, as well as increasing the consumption of energy [37,162]. This enhanced CO2 separation can nullify the need for refrigerant preparation and storage [162,163]. However, CO2 is separated using cryogenic distillation coupled with biogas upgrading. A comparison of different separation methods for CO2 capture is shown in Table 7.

Table 7.
Current status of different separation technologies for CO2 capture [16,37,54,111,112,113,114,161,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187].
6. CO2 Capture Using Dry Solid Sorbents
The selective separation of CO2 based on interactions between gases and solids is required for CO2 capture using a dry adsorbent [188]. In packed columns, universal dry adsorbents are typically utilized, including activated carbon and molecular sieves [189]. The surface tension and pore size of the adsorbent, as well as the process temperature and partial pressure, are critical factors in a dry adsorption process [190]. Adsorption and desorption cycles are repeated throughout the process (regeneration).
The following are the several adsorption types: (i) pressure swing adsorption (PSA) [4,5,6]; (ii) temperature swing adsorption (TSA) [191,192], which combines two processes—low-temperature adsorption followed by desorption or regeneration by raising the pressure; (iii) electric swing adsorption (ESA) [193], which entails adsorption and desorption by altering the electricity supply while a low-voltage current flows through the adsorbent; and (iv) vacuum swing adsorption (VSA) [194]. Additionally, due to their low energy needs and relative simplicity, adsorption-based technologies such as pressure/vacuum swing adsorption (PVSA) have been extensively researched [195,196,197].
To effectively treat high volumes of combustion emissions from numerous sources, it is imperative to increase a dry adsorbent’s CO2 capture selectivity and adsorptive capacity. The CO2 adsorption can be enhanced and stabilized by adding functional groups to the surface of the adsorbent material that has a high affinity for CO2 to react with it. CO2 can then be selectively adsorbed using the adsorbent’s sizeable specific surface area and pore structure [198,199,200,201,202,203].
The addition of different amine groups to solid materials used as CO2 capture sorbents is anticipated to increase polarization and CO2 capture to achieve high selectivity and capture performance. These adsorbents have several benefits, including the potential eradication of corrosion issues and reduced costs of energy for regeneration. Under anhydrous conditions, the reactions of CO2 with amine functional groups result in ammonium carbamates, as follows [204]:
The reversible nature of the adsorption process and the possibility to increase adsorption efficiency by altering the composition of adsorbent materials make this process important. Therefore, by choosing a suitable adsorbent material, the CO2 adsorption efficiency can be improved. Activated carbons [205,206], zeolites [207], hollow fibers, and alumina are currently the major commercially available adsorbents. Each material has a unique surface area, pore structure, and surface functional groups, and their application areas are very specific. The following section discusses and provides descriptions of some typical carbonaceous and non-carbonaceous dry sorbents for CO2 capture.
Non-carbonaceous adsorbents
Non-carbonaceous adsorbents include zeolites, metalorganic frameworks, silica, etc. The CO2 uptake capacity of these materials is high; however, they are expensive and highly sensitive to moisture. As a result, zeolites can capture CO2 molecules of 0.33 nm due to their suitable channel diameters (0.3–1.0 nm) [208].
6.1. Adsorbents Based on Zeolite
Adsorbents with a natural structure, such as zeolites, are made up of an interlocking structure of AlO4 and SiO4 tetrahedrons that share atoms of oxygen [209]. Small pore size and high porosity are responsible for zeolite’s excellent CO2 adsorption at temperatures as low as 30 °C.; however, their CO2 capture capacity rapidly declines above this temperature and is almost non-existent above 200 °C [209]. Zeolite’s high hydrophilicity significantly reduces its CO2 adsorption capacity when the gas contains water. In this case, high temperatures are required for the regeneration [210], resulting in significant energy losses as a result of the CO2 regeneration [200].
The CO2 selectivity of zeolites remains low despite their ability to separate gases [211,212,213]. The moisture content of the gas is a constraint on zeolites’ capacity to adsorb CO2 from flue gas. Water competes for adsorption with CO2, reducing the amount of CO2 adsorbed by Y-type zeolites (CaY and NiY) [214]. By contacting an ion and a dipole at low desorption temperatures, physisorption occurs, resulting in a linear orientation of the CO2 molecule [209,215].
Additionally, zeolites exhibit excellent chemical reactivity, recyclability, high stability, excellent recyclability, and structural diversity, which make them a promising candidate for the CO2 adsorption [216]. This structure consists of a network of streams into which gas molecules are adsorbable, ranging from 0.5 to 1.2 nm in size [200,209,217]. Flue gas mixtures (CO2, H2O, CO) are not suitable for zeolites because they are not CO2 selective, along with their limited capacity to adsorb at high temperatures [209,218,219].
Zeolite contains exchangeable cations including K+, Mg2+, and Na+ that act as a balance to the negative charge that results from a SiO4 tetrahedron replacing an AlO4 tetrahedron. Specifically, the charge density, cation distribution, and size of the zeolites influence their adsorption and separation properties. Zeolite particles have a large pore size that allows CO2 to diffuse into them. The adsorption of CO2 molecules by zeolites is increased by the presence of cations in their structure via molecules and the adsorbent’s interaction electrostatically [220]. CO2 adsorption has been studied mostly for the zeolites 13X and 5A. Using a type 13X zeolite, Moura, Bezerra [221] investigated the effect of cation exchange on CO2 adsorption, concluding that zeolites rich in alkali-cations, such as Li+ and Na+, could effectively adsorb CO2. The order of decreasing adsorption capacity was Li+ > Na+ > NH4+ > Ba2+ > Fe3+. According to Walton [222], it is believed that the ionic charge of zeolites, the ionic radius, the shielding effects, as well as the nuclear charge are the main causes of the observed decline in CO2 adsorption capacity. According to Calleja, Jimenez [223], zeolites 13X and 5A have CO2 adsorption capacities of 3–25 wt. % and 2–12 wt. %, respectively, at a CO2 partial pressure of 15% and a CO2 pressure of 100%.
6.2. Adsorbents Based on Metal-Organic Frameworks
The molecular organic framework (MOF) is a crystalline material containing metal ions or clusters of metal ions combined with organic molecules (ligands) [9,43]. As the MOF has been adapted to a variety of chemical processes and applications, over 20,000 MOFs have now been created, each with distinct geometries, pore sizes, and functions [224,225,226,227,228].
Having large mesopores, high surface areas (approximately 10,000 m2/g), and an adaptability to different geometries, MOFs are ideal chemisorbents for CO2 capture [228,229]. Although MOFs have some advantages when it comes to CO2 capture at high temperatures, they also have some drawbacks. For example, moisture is absorbed while CO2 is being captured, the manufacturing process is difficult, and the MOFs are not very durable [200].
In a study by Szczęśniak and Choma [230], copper-based MOFs (Cu-BTC), with benzene-1,3,5-tricarboxylate, had an uptake capacity for CO2 of 9.59 mmol/g adsorbent and a surface area of 1760 m2/g at 1 atm at 273 K. The CO2 capacity of most MOFs decreases noticeably as the temperature increases during the CO2 capture phase. At 298 K, 313 K, and 328 K, the temperatures for CO2 capture, Aarti et al. [231] reported 4 mmol/g adsorbent for Cu-BTC-PEI-2.5, 2.61 mmol/g adsorbent for Cu-BTC-PEI-2.5, and 1.66 mmol/g adsorbent for Cu-BTC-PEI-2.5, respectively, due to MOFs’ structural stability’s degradation with temperature.
There has been a breakthrough in developing microporous coordination polymers (MCPs) to decrease the production cost of MOFs. As the name implies, MCPs are composed of repeating channels of metal ions linked by ligands whose function is to link ions. MCPs have porous structures because of their inherited organic functionality [232]. The magnesium-based MOF Mg-MOF-74 was found to have better CO2 adsorption with 8.61 mmol/g adsorbents at 1 bar partial pressure and 298 K [233]. Magnesium dioxybenzenedicarboxylate (Mg/DOBDC) is an MCP composed of Mg2+ ions connected to 2,5-dioxide-1,4-benzene-dicarboxylate [232]. Researchers have claimed that the high performance can be attributed to the improved ionic behavior of the magnesium oxide Mg-O bond when CO2 partial pressures are as low as 0.1 bar. As opposed to MgO, exothermic carbonation does not form magnesium carbonate (MgCO3) from Mg/DOBDC. MCP networks are inflexible, and prevent insertion into the MgO bonds likely due to their inflexibility. Due to the highly ionic nature of the Mg-O bonds in Mg/DOBDC, the material can capture CO2 reversibly. Low CO2 partial pressures can be improved by these techniques [232].
The ability of many early MOF adsorbents to adsorb large amounts of CO2 was demonstrated. To be used as CO2 adsorbents, MOFs must be stable in an aqueous medium. Mahdipoor, Halladj [234] reported an MOF CO2 adsorbent containing iron-based on poly terephthalate (BDC). Observations revealed that the amino-functionalized MOF MIL 101(FE) is water- and ethanol-stable, and capable of capturing 13 mmol/g CO2 per adsorbent. Additionally to the physical CO2 adsorption by the amino MIL 101(FE) MOF’s structure, the chemisorption of CO2 by the MIL 101(FE) MOF structure affects the total CO2 adsorption by the MOF [234].
6.3. Mesoporous Silica Materials
Mesoporous silica has also been proposed as a viable candidate material for capturing CO2 because of its large surface area and high capacity to be synthesized with a variety of pore hole sizes [235]. The modification of mesoporous silica for CO2 capture has been proven to be an effective method for developing adsorbents, despite its low adsorption capacity [235]. The possibility of chemically treating their surface OH groups to promote their CO2 selectivity and adsorption capacity may be further investigated to process flue gases at low pressures using CO2 [235].
Sánchez-Zambrano, Lima Duarte [236] investigated the chemical modifications of mesoporous silica treated with 3-aminopropyl triethoxysilane (APTES), and then impregnated it with polyethyleneimine (PEI) to find out how the modifications affected kinetic mechanisms, site energy distributions, and CO2 adsorption under post-combustion conditions [10,43]. As determined by microcalorimetry, the functionalization process resulted in new adsorption sites. When amine groups were added to the support, physisorption accounted for the vast majority of adsorption, and CO2 capture capacity and selectivity increased as the temperature increased (50 and 75 °C). Due to sites that could not be restored due to strong chemical bonds formed by adsorption products, using a turbomolecular vacuum pump was the only way to regenerate after adsorption at 25 °C with CO2. In the first stage of CO2 capture, these sites became available for regeneration, but only at higher temperatures [236]. Although mesoporous silicas are highly effective adsorbents for CO2 capture due to their large pores with a tunable size, high surface area, and mechanical and thermal stability, they do not possess sufficient CO2 adsorption abilities to be of any use, especially at a 1-atmosphere pressure [235].
6.4. Alkali Metal-Based Materials
Alkali metal carbonates, including those made of Na, K, and Al, have been reported to be efficient dry adsorbents for CO2 capture from flue gas operating below 473 K in relatively moderate conditions [237,238]. Various inorganic supports, including alumina, silica, ceramics, zirconia, and carbon materials, are added with alkali metal carbonates during this procedure; Equation (9) explains how moisture and CO2 react, facilitating CO2 adsorption, and how decarbonization (Equation (10)) regenerates the absorbent (10) [38]:
141 kJ/mol and ΔH = –135 kJ/mol for M = K and Na, respectively
As shown in Equation (8), alkali–metal bicarbonates are typically formed when CO2 and H2O react with carbonate sorbents between 333 and 383 K, which then regenerate alkali–metal carbonates at 373–473 K, releasing CO2. Theoretically, Na2CO3 and K2CO3 have CO2 adsorption capacities of 41.5 and 31.8 wt. %, respectively.
The use of lithium-based materials such as lithium-based silicate (Li4SiO4) and lithium-based zirconate (Li2ZrO3) for direct CO2 capture from flue gas at temperatures of 700–900 K is another promising method for capturing CO2 [239]. Li4SiO4, specifically, is a promising CO2 captor because of its low volume change during CO2-adsorption–desorption and its high CO2 sorption capacity of 36.7% [240].
In a study conducted by Kato et al., Li4SiO4 and Li2ZrO3 were examined at low CO2 concentrations, i.e., 50 ppv. They found that Li4SiO4 was 30 times more capable of absorbing CO2 than Li2ZrO3. Additionally, zirconia materials are more expensive than silica materials [241]. Recently, Seggiani et al. [242] reported that Li4SiO4, with the addition of 30% Na2CO3 or K2CO3, demonstrated a CO2 sorption capacity of 23 wt. % at an ideal sorption temperature of 853 K and low CO2 partial pressure of 0.04 bar, equating to a Li4SiO4 conversion of almost 80%. Li8SiO6 was proposed by Durán-Muoz et al., as a substitute dry adsorbent for CO2 capture. It demonstrated a high sorption capacity of roughly 51.9 wt. % over a wide temperature range, with an efficiency of 71.1% [243].
It is technically and economically desirable to use alkali–metal-based materials to capture CO2 post-combustion at low temperatures and in low concentrations, since they do not require additional cooling processes; although, the long-term stability and sustained performance of such adsorbents under real flue gas conditions must be addressed.
6.5. Alkaline Metal-Based Ceramics
In addition to alkaline ceramics, binary metal oxides are also referred to as alkaline oxides because they contain more than one alkaline element. Metal-based alkaline ceramics include Li5AlO4, Li2CuO2, Li2ZrO3, and Li4SiO4. Notably, these alkaline metal ceramics can be regenerated at elevated temperatures. Consequently, they are ideal for adsorption and desorption cycles, such as those associated with post-combustion capture. The robustness of alkaline metal ceramics allows them to be made without refractory supports, resulting in a simpler synthesis method [244]. Alkaline ceramics are synthesized in a variety of ways, depending on the desired type. When synthesizing zirconates, co-precipitation, sol-gel, or soft chemistry methods are preferred for achieving the desired morphology of an adsorbent. A sorbent needs to have a morphology with a large surface area and a small particle size to capture CO2 to its maximum capacity [244]. The sol-gel method can be used to create a mesoporous structure for silicates that is advantageous [244].
These materials, including zirconate Na2ZrO3, are important for process intensification [245], owing to their catalytic activity [244]. Following CO2 chemisorption, the carbonated derivate, Na2CO3, is formed. Equation (11) depicts the reaction pathway for Na2ZrO3 [244,245].
Sutton, Kelleher [246] described alkaline metal-based ceramics as catalysts of the water gas shift reaction (WGSR).
Compared to other ceramics made of alkaline metal, lithium cuprate (Li2CuO2) has demonstrated the ability to adsorb CO2 over a wide temperature range (120 to 690 °C), and it retains its efficacy as a CO2 adsorbent at low CO2 partial pressures [247,248]. At CO2 concentrations less than 15%, the alkaline metal zirconate (Li2ZrO3) exhibits excellent CO2 selectivity and CO2 acceptor performance in flue gas streams [244]. In summary, Li2CuO2 and Li2ZrO3 are two CO2 adsorbents that have been subjected to extensive research and have been demonstrated to be promising dependable CO2 adsorbents [244].
Much of the research on alkaline ceramics in recent years has focused on CO2 capture at high temperatures. The CO2 adsorption capacities of two alkaline yttrium oxides (NaYO2 and LiYO2) at high temperatures were recently evaluated. Since lithium ions are not as close together as sodium ions are in an octahedron, LiYO2 has a greater capacity for CO2 capture than NaYO2. However, both ceramics were capable of adsorption/desorption for at least ten cycles [249]. Table 8 lists the basic CO2 adsorption capacities of the main adsorbent types that are commonly addressed in the literature.

Table 8.
The CO2 adsorption capacity of the most common adsorbents [250,251,252].
Carbonaceous Adsorbents
Even though carbon is the only component of carbonaceous materials, they offer several advantages, such as high thermal/chemical stability, heat and electrical conductivity, bio-affinities, elasticities, and strengths [253,254,255]. Being lightweight, having a very high specific surface area, and having a large pore volume make them especially suitable for applications involving the storage of gases or adsorption [256,257]. Additionally, they offer benefits for CO2 capture: (i) they are not moisture-sensitive; (ii) they are reasonably priced; (iii) their desorption/adsorption temperatures are below 373 K.; (iv) they may be employed at atmospheric pressure; and (v) their energy usage is minimal. Each of these factors has had an impact on recent research in this field.
6.6. Activated Carbons
The textural characteristics and surface groups of carbon-based adsorbents are highly correlated with CO2 adsorption capability [258,259]. Due to their materials’ wide range of pore sizes, from micropores to macropores, activated carbons are not suitable for selective gas adsorption. Adsorption temperatures of less than 25 kJ/mol are typically seen in pristine carbon-based adsorbents, which have weak CO2 affinities [260]. At 298 K and 0.1 pressure, the standard CO2 adsorption capacity of activated carbon is ~5 wt. % [261,262].
By modifying the activation and preparation conditions, the pore structures of activated carbons can be easily regulated [263,264]. Additionally, the activated carbon’s surface functional groups can be easily modified utilizing a variety of treatment methods [265,266]. Incorporating different basic groups into activated carbon has been extensively researched for improving the CO2 affinity by enhancing the CO2 adsorption capacity [267,268].
NiO-loaded activated carbons (NiO-ACs) were synthesized by Jang et al., utilizing a post-oxidation technique that involved nickel electroless plating at 573 K in an air stream. The NiO-AC samples’ ability to adsorb CO2 increased as oxidation duration increased. The maximal CO2 adsorption capacity was 49.9 cm3/g, above the 41.2 cm3/g capacity of unaltered activated carbon at 298 K and 1 bar. They reported that the acid-base characteristics of the NiO caused it to serve as an electron donor on the carbon surface, increasing the CO2 adsorption, which acts as an electron acceptor [269].
6.7. Graphene
A graphene derivative known as graphene oxide (GO) can be created using different functional groups on the edges and basal planes [270]. Researchers have extensively studied modifying the surfaces of GO with functional groups for applications including gas storage, separation, conversion of energy, and synthesizing newly developed GO-like derivatives with lightweight frameworks [271,272,273].
Thermally exfoliated graphene nanoplates were reported by Meng et al. as being innovative, highly effective sorbents for CO2 capture. At 298 K and 30 bar, the produced graphene nanoplates demonstrated remarkable capture efficiencies of 248 wt. %. The graphene nanoplates’ wider inter-layer spacing and substantial inner void volume were attributed to the higher CO2 capture capacity [273].
6.8. Ordered Porous Carbons
Since they are widely used as electrode materials, catalyst supports, and other types of materials, ordered porous carbon materials have drawn a lot of study interest. [274,275,276]. There are numerous ways to make ordered porous carbons, including (i) direct synthesis utilizing organic self-assembly, which uses a mixture of carbon precursors and blocks co-polymers as soft templates, and (ii) nano-casting, employing silica materials as structure-controlling hard templates [277,278].
Yoo et al., examined the impact of the phenolic resins’ carbonization temperature on the total pore volumes and the specific surface areas of ordered nanoporous carbons (ONCs). Due to its greater specific surface area and smaller micropore size distribution, ONC carbonized at 1173 K had the highest CO2 adsorption capacities at 298 K (15.8 wt. % at 1 pressure and 68.5 wt. % at 30 bar) [279].
6.9. Activated Carbon Fibers (ACFs)
ACFs are attractive adsorbent materials because of their numerous micrometer porosities, nanostructures, and other characteristics, including narrow pore size distributions and large specific surface areas [280,281]. Compared to granular and powdered adsorbents, ACFs are more flexible due to their fibrous structure [282].
For large-scale CO2 capture testing, Thiruvenkatachari et al. created huge honeycomb-shaped carbon fiber composite (HMCFC) adsorbents (the adsorbent mass in one column was 4.486 kg). For a simulated flue gas with 13% CO2, 5.5% O2, and the remaining N2 at 293 K, the average CO2 adsorption capacity was 11.9 wt. %. Additionally, they demonstrated that the thermal decomposition process and combined vacuum improve the CO2 collection efficiency of the HMCFC adsorbents [283].
7. Research Progress in Converting CO2 into Valuable Fuels
One of the most stable compounds with carbon in its greatest valence state is CO2. Due to its low electron affinity, electrophilic reactions are challenging. Thus, a nucleophilic assault on the carbon atom is necessary for the conversion of CO2. As is well known, the dissociation energy required to rupture the C=O bond within a CO2 molecule is greater than 750 kJ mol−1 [284]. From a thermodynamic perspective, this is an uphill reaction. To provide the necessary energy for such a reaction to be completed, high temperatures, high pressures, or extremely effective catalysts are frequently required.
To date, a range of methods have been employed to reduce CO2, including the thermal catalysis [285,286,287,288,289], photocatalysis [290,291,292,293], electrocatalysis [294,295,296,297], and photoelectrochemical (PEC) reactions [298,299,300,301], in which heat, light, or electricity are utilized to supply the reaction’s necessary energy. Eight electrons are required for each CO2 molecule to convert into a hydrocarbon molecule completely. Due to the large number of compounds that are created during the reduction process, the purification procedure is complicated, and the yield of the desired products is decreased. Table 9 presents a clear comparison of the performance of different CO2 conversion methods employed in recent years. Extremely concentrated and efficient enzymatic processes were added to the aforementioned reduction technologies to boost the efficiency and accuracy of the CO2 conversion [302,303]. These reactions were inspired by natural photosynthesis. The schematic of the most modern CO2 chemical conversion techniques is shown in Figure 7.

Table 9.
Performance evaluation of various CO2 reduction systems.
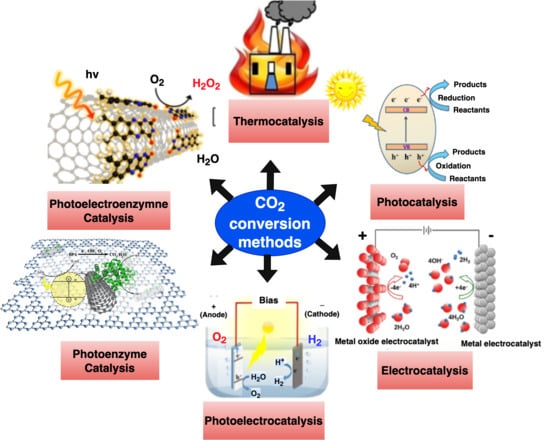
Figure 7.
A schematic of the CO2 chemical conversion methods [304].
As a result of the use of different CO2 conversion strategies, such as thermal catalysis, photocatalysis, electrocatalysis, photoenzymatic catalysis, photoelectroenzymatic catalysis, and photoelectrochemical (PEC), a range of yields and products were obtained. Table 10 the numerous value-added products made using different CO2 utilization strategies and the catalysts used throughout the conversion process.

Table 10.
List of catalysts utilized in CO2 conversion processes to manufacture valuable products.
8. Technologies for CO2 Capture at Various Technological Readiness Levels (TRL)
The global concern over the effects of industrial operations, such as chemical processes, on the environment has increased interest in green technologies. This issue has fueled significant growth in research and new technologies across many fields since the turn of the 21st century. Due to their significant contributions to high greenhouse gas emissions, the power generation, agricultural, and chemical industries stand out numerically in developing green technologies. It is crucial to track how new technologies are being developed and how old ones are being improved. This allows for future projections for a given area of Research, Development and Innovation (RDI).
A tool called technological maturity analysis, created by NASA in 1990 and modified by The Electric Power Research Institute (EPRI), can rank existing technologies by highlighting the stage they are at. This system is called the Technology Readiness Level (TRL) system [323]. TRL has a straightforward nomenclature (TRL1, TRL2, TRL3, etc.) and enables analysis of the stages of technological development. As illustrated in Table 11, these stages advance progressively, and each one is necessary for the next.

Table 11.
Stages of TRL development as defined by EPRI. Source: adapted from Freeman and Bhown (2011) [323].
TRL analysis assesses current technologies, aids in decision-making, and provides for forecasts of time, cost, and environmental implications. Politicians and businesspeople use it as a tool today across the globe, notably in RDI.
Carbon capture, storage and use (CCSU) technologies are typically expensive technologies, hence it is crucial to conduct a technological maturity analysis to identify potential solutions that can direct RDI investments by analyzing the TRL levels the technology can achieve [324].
The International Energy Agency (2020) [325] conducted a survey of opinions on CO2 capture systems based on TRL levels. Table 12 presents information from this review’s four categories of technologies—adsorption, absorption, membrane separation, and chemical capture.

Table 12.
TRL assessments for adsorption, absorption, membrane separation, and chemical capture technologies (2020) conducted by the International Energy Agency (2020) [325].
TRL studies have been carried out using information from technology suppliers. Data from Kearns et al. (2021) [326] were used as a comparative source.
The main drawback of the TRL scale, according to Freeman and Bhown (2011) [323], is the absence of requirements at each stage of development to move on to the next stage. Specifically, the amount of effort needed to move from TRL1 to TRL2 and beyond is unknown.
9. The Use of CO2 as a Feedstock for Fuel and Chemical Production
Finding alternatives to fossil fuels is viewed as being of great priority globally due to the increasing reliance on them and the depletion of resources. In general, it has become crucial to find a sustainable solution for transforming CO2, a toxic greenhouse gas that contributes to global warming, into a renewable carbon supply. CO2 can be directly converted into several useful chemicals through endergonic or exergonic processes [327]. CO2 can be used to meet the demands of different industries, including those for beverages, food, and chemicals [328]. Because of the financial and environmental benefits, technologies that allow CO2 to be converted into value-added products are continuously being researched.
The valence state of CO2 can be altered, in contrast to physical processes [329]. This procedure can be utilized to create chemical feedstock (carbonates, plastics, polymers [330]) as well as energy forms (syngas, methanol, ethane, methane). There are three types of chemical conversions that can be distinguished: thermochemical, electrochemical (photoelectrochemical [331]), and biological processes that involve enzymes [332].
There is a thermodynamic hurdle in CO2 conversion due to its high stability [333]. Hydrogen is a key element in many processes that convert CO2 into value-added chemicals. It should be produced from renewable energy sources to have an environmentally benign effect. Syngas (intermediate products) is produced as a byproduct of the reforming process that converts waste materials into useful fuels and chemicals. It often contains significant amounts of hydrogen and carbon monoxide, as well as small amounts of water and carbon dioxide [334]. Through the pyrolysis or gasification of biomass or natural gas conversion, respectively, reforming can occur in a solid state and with or without a gaseous state into syngas. Significant amounts of CO2 released from various industrial facilities, such as fossil fuel-fired power stations, can be used as feedstock in diverse CO2 recycling processes. The primary obstacle limiting the development of large-scale applications for biofuel is the availability of source feedstocks (namely, CO2 and H2). Methane (CH4), methanol (CH3OH), and dimethyl ether (CH3OCH3) are just a few of the many biofuels that can be made from CO2. This path makes it possible to create a wide range of fuels for both stationary and mobile applications.
9.1. Production of Chemicals
A wide variety of fine compounds can be made from CO2, in addition to synthetic fuels. The most significant uses are urea alkylene carbonates (a few kt year−1), polycarbonates (4 Mt year−1), acrylic acid and acrylates (10 Mt year−1), polyurethane (≈18 Mt year−1), inorganic carbonates (≈60 Mt year−1), and urea (≈160 Mt year−1) [335]. The greatest market for the use of carbon dioxide is urea, a key fertilizer [335,336]. It is also frequently utilized as a feedstock in producing fine chemicals, polymers, medicines, and inorganic compounds such as urea resins and melamine [337,338].
Other chemicals that can be generated from CO2 capture are also beneficial in various sectors, including lubricants, polymers, agrochemicals, pharmaceuticals, coatings, and catalytic processes. These chemical classes include organic carbonates such as diphenyl carbonate (DPC), dimethyl carbonate (DMC), diallyl carbonate (DAC) and diethyl carbonate (DEC), as well as cyclic carbonates such as styrene carbonate (SC), cyclohexene carbonate (CC), propylene carbonate (PC) and ethylene carbonate (EC), and even polycarbonates such as bisphenol polycarbonate (BPA-PC) and poly(propylene carbonate) [339,340].
This process faces difficulties since it requires a large amount of catalyst inventory and operates at high temperatures and pressures. Additionally, this procedure faces additional challenges in separating the catalyst from the products [339,340]. Commercially accessible Al-based catalysts are frequently utilized, but they are not eco-friendly in manufacturing polycarbonates from the reaction of CO2 with epoxides. In this context, an alternate method with a lot of potential for producing polycarbonates from CO2 and olefins is the oxidative carboxylation approach [341]. Another chemical made from the interaction of CO2 and cyclic amines, such as azetidines and aziridines, or the N-analogues of epoxides, is polyurethane [341,342].
Formic acid is another significant chemical that can be created using CO2. The hydrogenation of CO2 into formic acid has lately attracted some study interest because of the benign reaction conditions, the absence of byproduct formation, its ability to store hydrogen in liquid form, and the ease of formic acid’s decomposition into hydrogen and CO2 [337,343].
The biological use of CO2 provides an additional route for producing biodiesel and numerous commodity chemicals produced from biomass (used in food, silage, biogas, and fertilizer) [344]. This method has the benefits of a faster development rate, a shorter growth cycle, no competition with other plants for land, and the creation of various valuable by-products. To remove contaminants such as heavy metals, NOx, and SOx that are harmful to microalgae growth, the captured CO2 should be filtered before being fed into a photobioreactor [345].
Using CO2 as a technological fluid without converting it into chemicals has found applications outside of EOR in a variety of industries, such as the air conditioning (as a coolant), food-preservation, dry-washing, solvent, and beverage industries [335,343,346]. Generally, EOR consumes 50 Mt year−1 CO2, compared to the 8 Mt year−1 CO2 used by the beverage and food industries [347].
Overall, the proposed laboratory-scale solutions are still far from being commercialized for industrial use, even though there is a huge market for converting captured CO2 into chemicals and fuels. This is partly due to the exorbitant manufacturing costs of the materials under investigation thus far, which are also not chemically stable, and in part to the generally low CO2 conversion rates and total yields of the primary products. As a result, they do not satisfy the criteria for widespread deployment. Furthermore, knowledge of the mechanisms underlying the chemical reactions involved in the transformations of CO2 is still in its infancy. Evaluations of the requirements and factors of the procedure have also been neglected in this discipline.
9.2. Production of Fuels
The most effective method of utilizing CO2 is its conversion to fuels. Alkanes, methane, methanol, syngas, and other compounds can be produced from the captured CO2. In addition to transportation, power plants, and fuel cells, the fuel produced can be used in many different industries [335]. The number of methods for using CO2 to produce fuels is enormous. Since CO2 is a molecule with a steady thermodynamic state, its use requires a lot of heat and catalysts to achieve high fuel yields [348]. The hydrogenation and dry reforming of methane (DRM) are the two main processes for producing fuel from captured CO2 [349].
The possibility of creating fuel, storing H2, recycling CO2, and resolving the difficulty of storing electric energy makes CO2 hydrogenation a very attractive method of using CO2 [343]. DRM is also regarded as one of the most significant routes for the Fischer–Tropsch (FT) process’ production of methanol and numerous other liquid fuels [341,350,351]. The source of hydrogen from fossil fuels seems to be a concern in the hydrogenation of CO2 into methane [352], methanol [353], carbon monoxide [352], and formic acid [354], as this can result in a rise in the atmospheric emissions of CO2.
However, renewable energy sources, such as solar, wind, and biomass, can be used as a substitute for fossil fuels to further reduce CO2 emissions during hydrogenation [355]. Recently, the “e-gas” created by the German Audi Motor Company using CO2 hydrogenation produced 1000 Mt year−1 of methane [356].
The volumetric gas density of methane is low, which makes it an unsuitable fuel for use in vehicles. Additionally, its global warming potential (GWP) is 30 [348]. Methane is readily available, thus producing more will not be profitable for CO2 capture (methane is plentiful in landfill gas, coal gas, shale gas, and natural gas). It appears that a preferable mechanism is CO2 hydrogenation to methanol [357]. However, it is extremely difficult to activate C–H bonds with the currently used (mostly Cu-based) catalysts for the manufacture of methanol, and the catalysts that have been tried so far are not particularly profitable [358,359,360].
Methanol has numerous uses in organic solvents, combustion engines, plastics, and paints [348]; nevertheless, only 0.1% of CO2 emissions are reduced by its synthesis [361]. One of the most significant methods for using CO2 is the reverse water–gas shift (RWGS) reaction, which converts CO2 into CO because CO is a starting material for the FT reaction, which produces methanol and hydrocarbon fuels [359]. Despite this, the RWGS reaction’s endothermic nature and the low conversion at moderate temperatures provide the two biggest challenges to the implementation of large-scale methanol production from CO2 using the FT process. Another major obstacle is the development of active catalysts that can speed up the reaction rate and enhance the yield.
DRM has recently gained a lot of study interest in terms of utilizing CO2 to produce syngas [340,362,363]. According to Ofélia de Queiroz et al. [364], DRM often produces syngas with a greater purity than partial oxidation and steam reforming. Additionally, the DRM process only produces 2% of unreacted methane, which is less than steam reforming, allowing it to be used at remote natural gas locations to produce liquid fuels, which are more transport-friendly than gaseous fuels [341]. The DRM reaction has been thoroughly examined for Rh, Ir, Ru, Ni–Co, and Ni supported on lanthanum oxide, alumina, and silica [341]. Despite major advancements in the design of catalysts with high activity and ideal stability for DRM, finding a good catalyst for this reaction still poses a significant difficulty, especially at high operation temperatures when deactivation by coke formation is inevitable (>700 °C) [365,366,367,368,369].
Another appealing method that can lessen the formation of coke and maintain the stability of catalysts at high temperatures is the oxidative dehydrogenation of light alkanes to alkenes (ODA), which uses CO2 as a soft oxidant instead of the usual dehydrogenation oxidant, O2 [370,371,372,373,374]. Additionally, by eliminating hydrogen via the RWGS process, CO2 enhances the light alkanes’ oxidative dehydrogenation equilibrium conversion [375]. However, it is important to monitor the temperature since too much heat might lead to the olefins overoxidizing, which produces carbon oxides and reduces selectivity [376]. The redox cycle and active oxygen species are both formed by CO2. The active site type, the reduction capability of the metal, and the supporting material all affect how CO2 functions in the ODA and how this reaction works [377]. The catalysts studied thus far have little stability, while having a high initial activity.
The aforementioned discussion makes it clear that creating novel catalysts with high catalytic activity under a variety of reaction conditions, coke resistance, and long-term structural and chemical stability is the main obstacle to using captured CO2 as a feedstock for the creation of synthetic fuels.
9.2.1. Production of Methane (CH4) Based on CO2 (Methanation), Challenges and Prospects
Chemical feedstocks such as CO2 can be used to transform renewable energy. One promising approach to combating CO2-induced climate change is CO2 methanation for value-added products, where water electrolysis is a potential energy storage technique for generating H2 with renewable energy and would contribute to the creation of a carbon-based cycle that is sustainable. Due to their inherent intermittency, renewable energy sources are currently limited by the need for scalable storage methods [378]. Therefore, the most practical and easiest method of storing significant volumes of intermittent energy generated from renewable sources for extended periods is the generation of synthetic natural gas or liquid fuels. Power to gas (PtG) is one concept that has gained a lot of attention over the years (Figure 8) [379]. In this method, CO2 interacts with H2, which is created by water electrolysis using renewable solar or wind energy to generate CH4 as a substitute for natural gas. In Copenhagen, a commercial-scale PtG plant with 1.0 M2 of capacity was successfully operating in 2016, exploiting the change of the energy system towards a sustainable system [380]. Five projects, using CO2 methanation at a commercial or pilot plant size with capacities varying from 25 kW to 6300 kW, were implemented in Germany between 2009 and 2013 [381]. In fact, natural gas, or methane, is Germany’s main source of heat and a significant contributor to natural gas supplies. Due to their robust dynamic properties, natural gas power plants now generate a larger portion of Germany’s electricity than the country’s present coal-fired power plants do [382]. Because it has a higher H:C ratio than its conventional counterpart, its use in automobiles instead of gasoline minimizes CO2 emissions. The following reactions in Table 13 take place in the methanation reactor [383]:
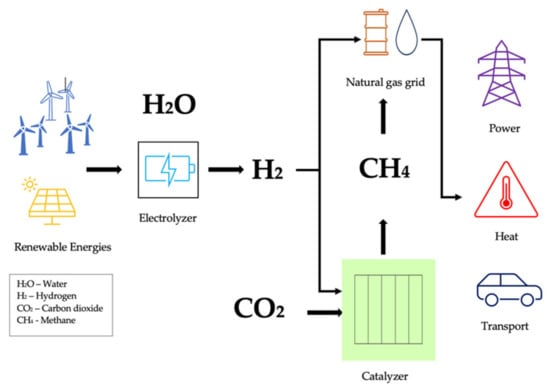
Figure 8.
Schematic illustration of “power to gas” (PtG) technology (adapted from Younas [380]).

Table 13.
CO2 methanation: main reactions and side reactions [384].
It is difficult to apply CO2 because of its inertness, which prevents it from being converted into chemicals with added value. The use of specific catalysts can, however, help to resolve this problem [385]. According to Park, Kwak [386], utilizing a double-layered TiO2/Cu-TiO2 catalyst instead of a typical TiO2 catalyst resulted in a two-fold improvement in the yield of CH4 production from CO2 (film catalyst). To clean syngas in ammonia factories, carbon oxides were also hydrogenated to methane. Additionally, this might result in carbon-neutral fuel (methane) [327]. The conversion of CO2 to methane can also occur biologically, for example, using methanogens. Methane-producing organisms are produced via the anoxic enrichment of waste-activated sludge (methanogens). The effectiveness of methane generation was improved by almost 70-fold when the organism’s activated cultures were used [387].
Due to the increasing demand for storing renewable energy and mitigating global warming, CO2 methanation has gained renewed attention due to the French chemist Paul Sabatier’s discovery in 1902 [388]. Storing energy from renewable resources such as wind and solar, the efficient conversion of biogas to biomethane, and the conversion of CO2 into chemical feedstocks and fuels are all made possible by the Sabatier reaction [389,390].
Figure 9 illustrates the exothermic nature of CO2 methanation, which has a high equilibrium conversion between 25 and 400 °C [391,392]. By using the right catalysts, CO2 methanation can achieve 99% CH4 selectivity, avoid product separation, and circumvent the challenges of dispersed product distribution. Because of this thermodynamic characteristic, CO2 methanation is more important in terms of energy effectiveness and economic viability.
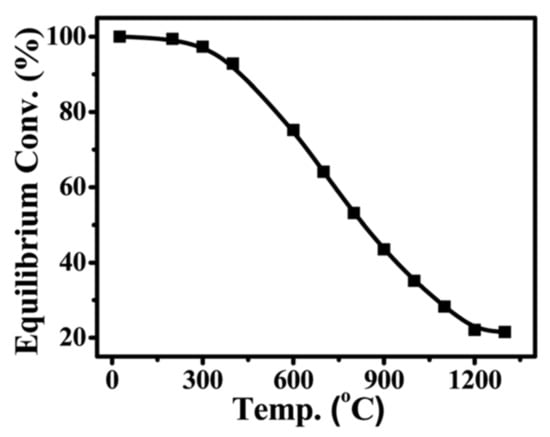
Figure 9.
A plot of equilibrium CO2 conversion in methanation at various temperatures (based on literature data) [393,394].
9.2.2. Challenges
In CO2 methanation, deactivating metal catalysts is a major challenge. There are two different ways that methanation catalysts can be deactivated: (a) chemically and (b) physically.
In contrast to Co/Al2O3, which deactivated quickly in the same amount of time, the Co/ZrO2 catalysts showed a greater CO2 methanation activity and practically consistent performance even after 300 h on stream. Through thermogravimetric analysis and hydrothermal (H2O) treatment verification experiments, the deactivation of the Co/Al2O3 catalyst was further researched. An excessive amount of CoAl2O4 was produced because of the addition of extra H2O to the reaction system, which hastened the deactivation of the Co/Al2O3 catalyst. As a result, the product H2O encourages the production of the inactive phase CoAl2O4, which causes Co/Al2O3 catalysts to deactivate quickly. One of the causes of deactivation is the deposition of carbon, but the primary cause is the creation of the CoAl2O4 spinel structure in the inactive phase.
Active metal sintering and carbon deposition are the main causes of physical deactivation. Ni/YSZ catalysts were synthesized by Kesavana et al. [395] using several techniques. Thin coatings of carbon are generated on Ni0 particles with a spherical shape, and graphitic laminations are formed on Ni0 particles that are exposed to surfaces on the Ni/YSZ catalyst created by the impregnation procedure. When Ni0/Ni2+ oxidation with a high CO2:H2 ratio was used, the Ni/YSZ(EDTA) catalyst displayed outstanding stability, and operand XAS demonstrated that it was not deactivated. Increasing the H2/CO2 ratio or adding steam can prevent carbon from depositing on the catalyst because hydrogen reacts with the carbon deposits to keep them from deactivating the catalyst.
Increased metal dispersion via strong metal–support contact, the addition of catalyst promoters, the development of novel synthesis techniques, and other typical techniques are used to reduce metal sintering [396]. When the active Co particles are separated by graphite-like carbon, metal sintering is averted effectively. In a study by Li et al. [397], NiO–MgO@ SiO2 catalysts were successfully prepared and maintained their activity after 100 h on stream. Therefore, particles with the right size are beneficial to CH4 formation.
9.2.3. Prospects
Increasing atmospheric CO2 concentrations are believed to be one of the primary drivers of climate change. Although CO2 capture technologies are quite advanced, using captured CO2 remains a significant difficulty that will require extensive future research. In the short to medium term, the creation of new life cycle assessments (LCA), techno-economic tools, and benchmark assessments will make it possible to evaluate CO2 conversion pathways consistently and transparently. Current barriers to furthering CO2 conversion include energy and water usage, the need for costly catalysts, and problems with gas infrastructure. Cost-effective CO2 conversion techniques are especially needed, and their pilot-scale testing is essential for their commercial application. A thorough introduction to the various CO2 conversion processes aids in comprehension of the mechanisms and the selection of suitable methods for using captured CO2. It is preferable to use the prospective integrated approaches to close the current gaps for widespread industrial applications. Additionally, it is advantageous to demonstrate research and development (R&D) initiatives for CO2 conversion and analyze CO2 utilization markets to determine the likelihood of the full-scale implementation of these technologies.
Economic viability, whether it is attained through technical progress or legislative changes, is crucial to promoting the development of the industrial feasibility of CO2 conversion technology. Because of this, future key research objectives should be concentrated on CO2 conversion and CCS legislation, policy, and assessments, as well as the integration of CO2 use with other strategies to minimize costs and energy consumption, especially at a larger scale. Public education and publicity on the capture, sequestration and methanation of CO2 (CSCM) should be highlighted, and global collaborations need to be further strengthened in the meantime, to increase the general public’s awareness of the environmental repercussions. Additionally, it should be highlighted that CCS is necessary to achieve our climate goals, and that CO2 is not a substitute for it. To keep the rise in the global mean temperature below 1.5 °C, governments should strengthen their commitment to CSCM and play a critical role in supporting its deployment (e.g., through laws, financial options, and tax incentives). The encouragement of private sector funding for larger-scale demonstration programs and the commercialization of CO2 technologies is another successful approach. The market for CO2-based products may soon be impacted by regulations and policies as CO2 becomes a resource that diverse sectors of the global economy want.
The following are general directions for future CO2 methanation research:
- To increase the H/C ratio of catalyst surface and facilitate C–C coupling for high-value-added products;
- To promote CO2 adsorption and activation by enhancing the oxygen vacancies and support basicity;
- To investigate potential novel catalytic materials and enhance the stability of the catalyst;
- To develop more effective catalysts for CO2 hydrogenation at low temperatures and with little energy consumption;
- To analyze the process intensification and optimization of CO2 conversion technologies, which are crucial to understanding how various operating parameters interact, improve process effectiveness, and reduce costs.
10. Conclusions
The above sections provide a comprehensive overview of different CO2 separation and capture technologies. The ideal separation techniques to lower CO2 emissions are also highlighted, along with their benefits and drawbacks, in this review paper. Additionally, dry solid sorbents, with a focus on emerging adsorbents, were addressed. A discussion of the challenges involved in converting CO2 into value-added products was also conducted, and future perspectives were laid out.
This study leads to the conclusion that the CO2 capture and separation (CCS) problem cannot be solved by a single method. Multiple technologies covered in this paper would need to be incorporated to solve this complex problem. To solve the technological problems in the CCS, it is necessary to scale-up innovative technologies from a laboratory to an industrial scale. This requires further research on new physical and chemical sorbents and procedures with improved cost-effectiveness and efficiency.
Author Contributions
Conceptualization, S.O.A.; investigation, S.OA.; methodology, S.O.A.; writing—original draft preparation, S.OA.; writing—review and editing, S.O.A. and Y.M.I.; supervision, Y.M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors used data and information from various research papers and reports as indicated in the references, and gratefully acknowledge the assistance of the facilitators, namely, Joseph Gbadeyah, Elutunji Buraimoh, and Emmanuel Tetteh, at the Scientific research retreat organized by the Faculty of Engineering and the Built Environment, Durban University of Technology, South Africa.
Conflicts of Interest
The authors declare no known competing financial interest or personal relationships that could influence the information given in this work.
References
- Al-Mamoori, A.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Improving adsorptive performance of CaO for high-temperature CO2 capture through Fe and Ga doping. Energy Fuels 2019, 33, 1404–1413. [Google Scholar] [CrossRef]
- Rojas-Cuevas, I.-D.; Caballero-Morales, S.-O.; Sánchez-Partida, D.; Martínez-Flores, J.-L. A Proposal to the Reduction of Carbon Dioxide Emission in Inventory Replenishment: Mitigating the Climate Change. In Humanitarian Logistics from the Disaster Risk Reduction Perspective; Springer: Cham, Switzerland, 2022; pp. 189–203. [Google Scholar]
- Edrisi, S.A.; Tripathi, V.; Dubey, P.K.; Abhilash, P. Carbon sequestration and harnessing biomaterials from terrestrial plantations for mitigating climate change impacts. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 299–313. [Google Scholar]
- Dai, Y.; Lai, F.; Ni, J.; Liang, Y.; Shi, H.; Shi, G. Evaluation of the impact of CO2 geological storage on tight oil reservoir properties. J. Pet. Sci. Eng. 2022, 212, 110307. [Google Scholar] [CrossRef]
- Triyanti, A.; Marfai, M.A.; Mei, E.T.W.; Rafliana, I. Review of Socio-Economic Development Pathway Scenarios for Climate Change Adaptation in Indonesia: Disaster Risk Reduction Perspective. In Climate Change Research, Policy and Actions in Indonesia; Springer: Cham, Switzerland, 2021; pp. 13–31. [Google Scholar]
- Gimžauskaitė, D.; Aikas, M.; Tamošiūnas, A. Recent progress in thermal plasma gasification of liquid and solid wastes. Recent Adv. Renew. Energy Technol. 2022, 2, 155–196. [Google Scholar] [CrossRef]
- Ouyang, T.; Xie, S.; Pan, M.; Qin, P. Peak-shaving scheme for coal-fired power plant integrating flexible carbon capture and wastewater treatment. Energy Convers. Manag. 2022, 256, 115377. [Google Scholar] [CrossRef]
- Holz, F.; Scherwath, T.; del Granado, P.C.; Skar, C.; Olmos, L.; Ploussard, Q.; Ramos, A.; Herbst, A. A 2050 perspective on the role for carbon capture and storage in the European power system and industry sector. Energy Econ. 2021, 104, 105631. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Lee, J.-M. Recent progress on transition metal diselenides from formation and modification to applications. Nanoscale 2022, 14, 1075–1095. [Google Scholar] [CrossRef]
- Li, K.; Zhang, D.; Niu, X.; Guo, H.; Yu, Y.; Tang, Z.; Lin, Z.; Fu, M. Insights into CO2 adsorption on KOH-activated biochars derived from the mixed sewage sludge and pine sawdust. Sci. Total Environ. 2022, 826, 154133. [Google Scholar] [CrossRef]
- Zhang, D.; Zou, R.; Wang, X.; Liu, J.; Mo, F. Carbon Capture, Utilization & Storage: A General Overview. In Climate Mitigation and Adaptation in China; Springer: Singapore, 2022; pp. 61–107. [Google Scholar]
- Alobaid, F.; Ströhle, J. Thermochemical Conversion Processes for Solid Fuels and Renewable Energies; MDPI: Basel, Switzerland, 2021; Volume 11, p. 1907. [Google Scholar]
- Alrebei, O.F.; Amhamed, A.I.; El-Naas, M.H.; Hayajnh, M.; Orabi, Y.A.; Fawaz, W.; Al-tawaha, A.S.; Medina, A.V. State of the Art in Separation Processes for Alternative Working Fluids in Clean and Efficient Power Generation. Separations 2022, 9, 14. [Google Scholar] [CrossRef]
- Nawkarkar, P.; Ganesan, A.; Kumar, S. Carbon dioxide capture for biofuel production. In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 605–619. [Google Scholar]
- Xu, C.-G.; Li, X.-S. Research progress of hydrate-based CO2 separation and capture from gas mixtures. Rsc. Adv. 2014, 4, 18301–18316. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. A new porous material to enhance the kinetics of clathrate process: Application to precombustion carbon dioxide capture. Environ. Sci. Technol. 2013, 47, 13191–13198. [Google Scholar] [CrossRef]
- Pires, J.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Sheng, C. An overview on oxyfuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Buhre, B.J.; Elliott, L.K.; Sheng, C.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T. Sulphur impacts during pulverised coal combustion in oxy-fuel technology for carbon capture and storage. Prog. Energy Combust. Sci. 2011, 37, 69–88. [Google Scholar] [CrossRef]
- Chen, S.S.; Curcic, M. Ocean surface waves in Hurricane Ike (2008) and Superstorm Sandy (2012): Coupled model predictions and observations. Ocean Model. 2016, 103, 161–176. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre-and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 capture and separations using MOFs: Computational and experimental studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.; Bamidele, O.; Basha, M.; Qasem, N.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. CO2 absorption in aqueous piperazine solutions: Experimental study and modeling. J. Chem. Eng. Data 2011, 56, 1547–1554. [Google Scholar] [CrossRef]
- Kim, H.S.; Gwak, I.C.; Lee, S.H. Numerical analysis of heat transfer area effect on cooling performance in regenerator of free-piston Stirling cooler. Case Stud. Therm. Eng. 2022, 32, 101875. [Google Scholar] [CrossRef]
- Kazemi, A.; Mehrabani-Zeinabad, A. Post combustion carbon capture: Does optimization of the processing system based on energy and utility requirements warrant the lowest possible costs? Energy 2016, 112, 353–363. [Google Scholar] [CrossRef]
- Li, K.; Leigh, W.; Feron, P.; Yu, H.; Tade, M. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: Techno-economic assessment of the MEA process and its improvements. Appl. Energy 2016, 165, 648–659. [Google Scholar] [CrossRef]
- Li, K.; Cousins, A.; Yu, H.; Feron, P.; Tade, M.; Luo, W.; Chen, J. Systematic study of aqueous monoethanolamine-based CO2 capture process: Model development and process improvement. Energy Sci. Eng. 2016, 4, 23–39. [Google Scholar] [CrossRef]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef]
- Bailey, K.; Kurz, K.; Sushkova, T. Carbon Offset Solutions for International Travel Emissions; University of Richmond: Richmond, VA, USA, 2014. [Google Scholar]
- Gibbins, J.; Chalmers, H. Preparing for global rollout: A ‘developed country first’demonstration programme for rapid CCS deployment. Energy Policy 2008, 36, 501–507. [Google Scholar] [CrossRef]
- Ketzer, J.M.; Iglesias, R.S.; Einloft, S. Reducing greenhouse gas emissions with CO2 capture and geological storage. In Handbook of Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2015; Volume 2, pp. 1–40. [Google Scholar]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, J.; Jung, J.; Lee, C.S.; Han, C. Modeling and simulation of CO2 capture process for coal-based power plant using amine solvent in South Korea. Energy Procedia 2013, 37, 1855–1862. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Wang, K.; Wang, J. Studies of CO2 absorption/regeneration performances of novel aqueous monothanlamine (MEA)-based solutions. J. Clean. Prod. 2016, 112, 4012–4021. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Khurana, N.; Goswami, N.; Sarmah, R. Carbon Capture: Innovation for a Green Environment. In Advances in Carbon Capture and Utilization; Springer: Berlin/Heidelberg, Germany, 2021; pp. 11–31. [Google Scholar]
- Kashyap, N.; Deka, B.; Choudhari, B.; Singh, B.P.; Pachani, S. Present status of capture of carbon dioxide and its storage technologies: A review. Pharma Innov. J. 2019, 8, 327–332. [Google Scholar]
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-lean solvents for post-combustion CO2 capture: Fundamentals, uncertainties, opportunities, and outlook. Chem. Rev. 2017, 117, 9594–9624. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Austgen, D.M.; Rochelle, G.T.; Chen, C.C. Model of vapor-liquid equilibria for aqueous acid gas-alkanolamine systems. 2. Representation of H2S and CO2 solubility in aqueous MDEA and CO2 solubility in aqueous mixtures of MDEA with MEA or DEA. Ind. Eng. Chem. Res. 1991, 30, 543–555. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Continuous cycles of CO2 absorption and amine regeneration with aqueous alkanolamines: A comparison of the efficiency between pure and blended DEA, MDEA and AMP solutions by 13C NMR spectroscopy. Energy Environ. Sci. 2010, 3, 772–779. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Physical and chemical solubility of carbon dioxide in aqueous methyldiethanolamine. Fluid Phase Equilibria 2000, 168, 241–258. [Google Scholar] [CrossRef]
- Xu, G.-W.; Zhang, C.-F.; Qin, S.-J.; Gao, W.-H.; Liu, H.-B. Gas− liquid equilibrium in a CO2 − MDEA− H2O system and the effect of piperazine on it. Ind. Eng. Chem. Res. 1998, 37, 1473–1477. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A. Integration of CO2 capture unit using single-and blended-amines into supercritical coal-fired power plants: Implications for emission and energy management. Int. J. Greenh. Gas Control 2007, 1, 143–150. [Google Scholar] [CrossRef]
- Veawab, A.; Aroonwilas, A.; Tontiwachwuthikul, P. CO2 absorption performance of aqueous alkanolamines in packed columns. Fuel Chem. Div. Prepr. 2002, 47, 49–50. [Google Scholar]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Andersen, J.; Jensen, J.N.; Biede, O. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energy Procedia 2011, 4, 1558–1565. [Google Scholar] [CrossRef]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Rochelle, G.T. Thermal degradation of amines for CO2 capture. Curr. Opin. Chem. Eng. 2012, 1, 183–190. [Google Scholar] [CrossRef]
- Fredriksen, S.; Jens, K.-J. Oxidative degradation of aqueous amine solutions of MEA, AMP, MDEA, Pz: A review. Energy Procedia 2013, 37, 1770–1777. [Google Scholar] [CrossRef]
- Kozak, F.; Petig, A.; Morris, E.; Rhudy, R.; Thimsen, D. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1419–1426. [Google Scholar] [CrossRef]
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases Sci. Technol. 2018, 8, 998–1031. [Google Scholar] [CrossRef]
- Tan, J.; Shao, H.; Xu, J.; Du, L.; Luo, G. Mixture absorption system of monoethanolamine-triethylene glycol for CO2 capture. Ind. Eng. Chem. Res. 2011, 50, 3966–3976. [Google Scholar] [CrossRef]
- Rivas, O.; Prausnitz, J. Sweetening of sour natural gases by mixed-solvent absorption: Solubilities of ethane, carbon dioxide, and hydrogen sulfide in mixtures of physical and chemical solvents. AIChE J. 1979, 25, 975–984. [Google Scholar] [CrossRef]
- Barzagli, F.; Di Vaira, M.; Mani, F.; Peruzzini, M. Improved Solvent Formulations for Efficient CO2 Absorption and Low-Temperature Desorption. ChemSusChem 2012, 5, 1724–1731. [Google Scholar] [CrossRef]
- Liu, A.H.; Li, J.J.; Ren, B.H.; Lu, X.B. Development of High-Capacity and Water-Lean CO2 Absorbents by a Concise Molecular Design Strategy through Viscosity Control. ChemSusChem 2019, 12, 5164–5171. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Jiang, W.; Gao, G.; Wu, F.; Luo, C.; Zhang, L. Low energy-consuming CO2 capture by phase change absorbents of amine/alcohol/H2O. Sep. Purif. Technol. 2021, 275, 119181. [Google Scholar] [CrossRef]
- Shen, L.; Liu, F.; Shen, Y.; Sun, C.; Zhang, Y.; Wang, Q.; Li, S.; Li, W. Novel biphasic solvent of AEP/1-propanol/H2O for CO2 capture with efficient regeneration performance and low energy consumption. Sep. Purif. Technol. 2021, 270, 118700. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Makhool, H.; Karlsson, M.; Svensson, H. Chemical absorption of carbon dioxide in non-aqueous systems using the amine 2-amino-2-methyl-1-propanol in dimethyl sulfoxide and N-methyl-2-pyrrolidone. Sep. Purif. Technol. 2021, 256, 117789. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Khalifa, O.; Bahamon, D.; Abu-Zahra, M.R.; Vega, L.F. Sustainability criteria as a game changer in the search for hybrid solvents for CO2 and H2S removal. Sep. Purif. Technol. 2021, 277, 119516. [Google Scholar] [CrossRef]
- Mathias, P.M.; Afshar, K.; Zheng, F.; Bearden, M.D.; Freeman, C.J.; Andrea, T.; Koech, P.K.; Kutnyakov, I.; Zwoster, A.; Smith, A.R. Improving the regeneration of CO2-binding organic liquids with a polarity change. Energy Environ. Sci. 2013, 6, 2233–2242. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Screening study of different amine-based solutions as sorbents for direct CO2 capture from air. ACS Sustain. Chem. Eng. 2020, 8, 14013–14021. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents–A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Zhang, Y.; Chen, W.; Wang, Q.; Li, S.; Zhang, S.; Li, W. Sustainable ionic liquid organic solution with efficient recyclability and low regeneration energy consumption for CO2 capture. Sep. Purif. Technol. 2021, 275, 119123. [Google Scholar] [CrossRef]
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP–DETA blend for post–combustion CO2 capture. Sep. Purif. Technol. 2018, 194, 89–95. [Google Scholar] [CrossRef]
- Muchan, P.; Narku-Tetteh, J.; Saiwan, C.; Idem, R.; Supap, T. Effect of number of amine groups in aqueous polyamine solution on carbon dioxide (CO2) capture activities. Sep. Purif. Technol. 2017, 184, 128–134. [Google Scholar] [CrossRef]
- Singto, S.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Tantayanon, S.; Al-Marri, M.J.; Benamor, A. Synthesis of new amines for enhanced carbon dioxide (CO2) capture performance: The effect of chemical structure on equilibrium solubility, cyclic capacity, kinetics of absorption and regeneration, and heats of absorption and regeneration. Sep. Purif. Technol. 2016, 167, 97–107. [Google Scholar] [CrossRef]
- Zhou, T.; McBride, K.; Linke, S.; Song, Z.; Sundmacher, K. Computer-aided solvent selection and design for efficient chemical processes. Curr. Opin. Chem. Eng. 2020, 27, 35–44. [Google Scholar] [CrossRef]
- Malhotra, D.; Koech, P.K.; Heldebrant, D.J.; Cantu, D.C.; Zheng, F.; Glezakou, V.A.; Rousseau, R. Reinventing design principles for developing low-viscosity carbon dioxide-binding organic liquids for flue gas clean up. ChemSusChem 2017, 10, 636–642. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilibria 1989, 52, 31–38. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. New reference equation of state for associating liquids. Ind. Eng. Chem. Res. 1990, 29, 1709–1721. [Google Scholar] [CrossRef]
- Papadokonstantakis, S.; Badr, S.; Hungerbühler, K.; Papadopoulos, A.I.; Damartzis, T.; Seferlis, P.; Forte, E.; Chremos, A.; Galindo, A.; Jackson, G. Toward sustainable solvent-based postcombustion CO2 capture: From molecules to conceptual flowsheet design. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015; Volume 36, pp. 279–310. [Google Scholar]
- Limleamthong, P.; Gonzalez-Miquel, M.; Papadokonstantakis, S.; Papadopoulos, A.I.; Seferlis, P.; Guillén-Gosálbez, G. Multi-criteria screening of chemicals considering thermodynamic and life cycle assessment metrics via data envelopment analysis: Application to CO2 capture. Green Chem. 2016, 18, 6468–6481. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Badr, S.; Chremos, A.; Forte, E.; Zarogiannis, T.; Seferlis, P.; Papadokonstantakis, S.; Adjiman, C.S.; Galindo, A.; Jackson, G. Efficient screening and selection of post-combustion CO2 capture solvents. Chem. Eng. 2014, 39, 211–216. [Google Scholar]
- Papadopoulos, A.I.; Badr, S.; Chremos, A.; Forte, E.; Zarogiannis, T.; Seferlis, P.; Papadokonstantakis, S.; Galindo, A.; Jackson, G.; Adjiman, C.S. Computer-aided molecular design and selection of CO2 capture solvents based on thermodynamics, reactivity and sustainability. Mol. Syst. Des. Eng. 2016, 1, 313–334. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Hallett, J.P.; Mac Dowell, N. Solvent selection and design for CO2 capture–how we might have been missing the point. Sustain. Energy Fuels 2017, 1, 2078–2090. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J.; Pena-Pereira, F. A solvent selection guide based on chemometrics and multicriteria decision analysis. Green Chem. 2015, 17, 4773–4785. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Yeh, A.C.; Bai, H. Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions. Sci. Total Environ. 1999, 228, 121–133. [Google Scholar] [CrossRef]
- Diao, Y.-F.; Zheng, X.-Y.; He, B.-S.; Chen, C.-H.; Xu, X.-C. Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing. Energy Convers. Manag. 2004, 45, 2283–2296. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.; Sreedhar, I.; Raghavan, K. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Russo, M.; Olivieri, G.; Marzocchella, A.; Salatino, P.; Caramuscio, P.; Cavaleiro, C. Post-combustion carbon capture mediated by carbonic anhydrase. Sep. Purif. Technol. 2013, 107, 331–339. [Google Scholar] [CrossRef]
- Kumar, N.; Rao, D.P. Design of a packed column for absorption of carbon dioxide in hot K2CO3 solution promoted by arsenious acid. Gas Sep. Purif. 1989, 3, 152–155. [Google Scholar] [CrossRef]
- Derks, P.; Kleingeld, T.; van Aken, C.; Hogendoorn, J.; Versteeg, G. Kinetics of absorption of carbon dioxide in aqueous piperazine solutions. Chem. Eng. Sci. 2006, 61, 6837–6854. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, Z.; Jing, G. Kinetics of carbon dioxide absorption into aqueous [Hmim][Gly] solution. Int. J. Greenh. Gas Control 2013, 16, 197–205. [Google Scholar] [CrossRef]
- Wappel, D.; Gronald, G.; Kalb, R.; Draxler, J. Ionic liquids for post-combustion CO2 absorption. Int. J. Greenh. Gas Control 2010, 4, 486–494. [Google Scholar] [CrossRef]
- Rogers, R.D. Reflections on ionic liquids. Nature 2007, 447, 917–918. [Google Scholar] [CrossRef]
- Davis, J.H., Jr. Task-Specific Ionic Liquids for Separations of Petrochemical Relevance: Reactive Capture of CO2 Using Amine Incorporating Ions; ACS Publications: Washnigton, DC, USA, 2005. [Google Scholar]
- Rahimpour, M.; Kashkooli, A. Enhanced carbon dioxide removal by promoted hot potassium carbonate in a split-flow absorber. Chem. Eng. Process. Process Intensif. 2004, 43, 857–865. [Google Scholar] [CrossRef]
- Lin, P.-H.; Wong, D.S.H. Carbon dioxide capture and regeneration with amine/alcohol/water blends. Int. J. Greenh. Gas Control 2014, 26, 69–75. [Google Scholar] [CrossRef]
- Hoffman, J.S.; Hammache, S.; Gray, M.L.; Fauth, D.J.; Pennline, H.W. Parametric study for an immobilized amine sorbent in a regenerative carbon dioxide capture process. Fuel Process. Technol. 2014, 126, 173–187. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Chen, C. CO2 absorption by biphasic solvents: Mixtures of 1, 4-Butanediamine and 2-(Diethylamino)-ethanol. Int. J. Greenh. Gas Control 2013, 16, 107–115. [Google Scholar] [CrossRef]
- Freeman, S.A.; Dugas, R.; van Wagener, D.H.; Nguyen, T.; Rochelle, G.T. Carbon dioxide capture with concentrated, aqueous piperazine. Int. J. Greenh. Gas Control 2010, 4, 119–124. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Tan, C.-S. Reduction of CO2 concentration in a zinc/air battery by absorption in a rotating packed bed. J. Power Sources 2006, 162, 1431–1436. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, C.; Qin, S.; Wang, Y. Kinetics study on absorption of carbon dioxide into solutions of activated methyldiethanolamine. Ind. Eng. Chem. Res. 1992, 31, 921–927. [Google Scholar] [CrossRef]
- Notz, R.; Asprion, N.; Clausen, I.; Hasse, H. Selection and pilot plant tests of new absorbents for post-combustion carbon dioxide capture. Chem. Eng. Res. Des. 2007, 85, 510–515. [Google Scholar] [CrossRef]
- Chen, X.; Closmann, F.; Rochelle, G.T. Accurate screening of amines by the wetted wall column. Energy Procedia 2011, 4, 101–108. [Google Scholar] [CrossRef]
- Rochelle, G.; Chen, E.; Freeman, S.; van Wagener, D.; Xu, Q.; Voice, A. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. J. 2011, 171, 725–733. [Google Scholar] [CrossRef]
- Adeosun, A.; Abbas, Z.; Abu-Zahra, M.R. Screening and characterization of advanced amine based solvent systems for CO2 post-combustion capture. Energy Procedia 2013, 37, 300–305. [Google Scholar] [CrossRef][Green Version]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. Simulation and parametric study of post combustion CO2 capture process using (AMP+ PZ) blended solvent. Int. J. Greenh. Gas Control 2014, 21, 130–139. [Google Scholar] [CrossRef]
- Spigarelli, B.P. A Novel Approach to Carbon Dioxide Capture and Storage. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2013. [Google Scholar]
- Takamura, Y.; Aoki, J.; Uchida, S.; Narita, S. Application of high-pressure swing adsorption process for improvement of CO2 recovery system from flue gas. Can. J. Chem. Eng. 2001, 79, 812–816. [Google Scholar] [CrossRef]
- Wyczalek, F. Energy Independence-A Nation Running on Empty. In Proceedings of the 3rd International Energy Conversion Engineering Conference, San Francisco, CA, USA, 15–18 August 2022; p. 5545. [Google Scholar]
- Erlach, B.; Schmidt, M.; Tsatsaronis, G. Comparison of carbon capture IGCC with pre-combustion decarbonisation and with chemical-looping combustion. Energy 2011, 36, 3804–3815. [Google Scholar] [CrossRef]
- Clausse, M.; Merel, J.; Meunier, F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption. Int. J. Greenh. Gas Control 2011, 5, 1206–1213. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of equilibrium-based TSA processes for direct capture of CO2 from air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Shen, W.; Kong, X.; Li, P.; Yu, J.; Rodrigues, A.E. Experimental evaluation of adsorption technology for CO2 capture from flue gas in an existing coal-fired power plant. Chem. Eng. Sci. 2013, 101, 615–619. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, P.; Zhang, J.; Li, G.; Xiao, G.; Webley, P.A.; Zhai, Y. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem. Eng. J. 2013, 230, 64–72. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, Y.; Tsang, S.C.E. Nitrogen-enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chem. Eng. J. 2012, 185, 374–379. [Google Scholar] [CrossRef]
- Arenillas, A.; Smith, K.; Drage, T.; Snape, C. CO2 capture using some fly ash-derived carbon materials. Fuel 2005, 84, 2204–2210. [Google Scholar] [CrossRef]
- Kim, K.; Son, Y.; Lee, W.B.; Lee, K.S. Moving bed adsorption process with internal heat integration for carbon dioxide capture. Int. J. Greenh. Gas Control 2013, 17, 13–24. [Google Scholar] [CrossRef]
- Kumar, R. Adsorption column blowdown: Adiabatic equilibrium model for bulk binary gas mixtures. Ind. Eng. Chem. Res. 1989, 28, 1677–1683. [Google Scholar] [CrossRef]
- An, H.; Feng, B.; Su, S. Effect of monolithic structure on CO2 adsorption performance of activated carbon fiber–phenolic resin composite: A simulation study. Fuel 2013, 103, 80–86. [Google Scholar] [CrossRef]
- Ishida, M.; Yamamoto, M.; Ohba, T. Experimental results of chemical-looping combustion with NiO/NiAl2O4 particle circulation at 1200 C. Energy Convers. Manag. 2002, 43, 1469–1478. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Reactivity of iron oxide with methane in a laboratory fluidized bed: Application of chemical-looping combustion. In Proceedings of the 7th International Conference on Circulating Fluidized Bed Technology, Niagara Falls, ON, Canada, 5–8 May 2002. [Google Scholar]
- Guo, Q.; Zhang, J.; Tian, H. Recent advances in CaSO4 oxygen carrier for chemical-looping combustion (CLC) process. Chem. Eng. Commun. 2012, 199, 1463–1491. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Integrated hydrogen and power production with CO2 capture using chemical-looping reforming redox reactivity of particles of CuO, Mn2O3, NiO, and Fe2O3 using SiO2 as a support. Ind. Eng. Chem. Res. 2005, 44, 3485–3496. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Kale, G. Feasibility study of sulfates as oxygen carriers for chemical looping processes. QScience Connect 2012, 2012, 1. [Google Scholar] [CrossRef]
- Zheng, M.; Shen, L.; Xiao, J. Reduction of CaSO4 oxygen carrier with coal in chemical-looping combustion: Effects of temperature and gasification intermediate. Int. J. Greenh. Gas Control 2010, 4, 716–728. [Google Scholar] [CrossRef]
- Spallina, V.; Gallucci, F.; Romano, M.C.; Chiesa, P.; Lozza, G.; van Sint Annaland, M. Investigation of heat management for CLC of syngas in packed bed reactors. Chem. Eng. J. 2013, 225, 174–191. [Google Scholar] [CrossRef]
- Hamers, H.; Gallucci, F.; Cobden, P.; Kimball, E.; van Sint Annaland, M. A novel reactor configuration for packed bed chemical-looping combustion of syngas. Int. J. Greenh. Gas Control 2013, 16, 1–12. [Google Scholar] [CrossRef]
- Harper, R.N.; Boyce, C.M.; Scott, S.A. Oxygen carrier dispersion in inert packed beds to improve performance in chemical looping combustion. Chem. Eng. J. 2013, 234, 464–474. [Google Scholar] [CrossRef]
- Cotton, A.; Patchigolla, K.; Oakey, J. Hydrodynamic characteristics of a pilot-scale cold model of a CO2 capture fluidised bed reactor. Powder Technol. 2013, 235, 1060–1069. [Google Scholar] [CrossRef]
- Yazdanpanah, M.M.; Forret, A.; Gauthier, T.; Delebarre, A. An experimental investigation of loop-seal operation in an interconnected circulating fluidized bed system. Powder Technol. 2013, 237, 266–275. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Chemical-looping combustion and chemical-looping with oxygen uncoupling of kerosene with Mn-and Cu-based oxygen carriers in a circulating fluidized-bed 300 W laboratory reactor. Fuel Process. Technol. 2012, 104, 378–389. [Google Scholar] [CrossRef]
- Ku, Y.; Wu, H.-C.; Chiu, P.-C.; Tseng, Y.-H.; Kuo, Y.-L. Methane combustion by moving bed fuel reactor with Fe2O3/Al2O3 oxygen carriers. Appl. Energy 2014, 113, 1909–1915. [Google Scholar] [CrossRef]
- Tong, A.; Sridhar, D.; Sun, Z.; Kim, H.R.; Zeng, L.; Wang, F.; Wang, D.; Kathe, M.V.; Luo, S.; Sun, Y. Continuous high purity hydrogen generation from a syngas chemical looping 25 kWth sub-pilot unit with 100% carbon capture. Fuel 2013, 103, 495–505. [Google Scholar] [CrossRef]
- Wang, J.; Anthony, E.J. Clean combustion of solid fuels. Appl. Energy 2008, 85, 73–79. [Google Scholar] [CrossRef]
- Jin, H.; Ishida, M. A new type of coal gas fueled chemical-looping combustion. Fuel 2004, 83, 2411–2417. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels 2006, 20, 1836–1844. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Process. Technol. 2014, 124, 104–114. [Google Scholar] [CrossRef]
- Gayán, P.; Abad, A.; de Diego, L.; García-Labiano, F.; Adánez, J. Assessment of technological solutions for improving chemical looping combustion of solid fuels with CO2 capture. Chem. Eng. J. 2013, 233, 56–69. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Performance of CLOU process in the combustion of different types of coal with CO2 capture. Int. J. Greenh. Gas Control 2013, 12, 430–440. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef]
- He, X.; Fu, C.; Hägg, M.-B. Membrane system design and process feasibility analysis for CO2 capture from flue gas with a fixed-site-carrier membrane. Chem. Eng. J. 2015, 268, 1–9. [Google Scholar] [CrossRef]
- Turi, D.M.; Ho, M.; Ferrari, M.-C.; Chiesa, P.; Wiley, D.; Romano, M.C. CO2 capture from natural gas combined cycles by CO2 selective membranes. Int. J. Greenh. Gas Control 2017, 61, 168–183. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Lee, P.S.; Lim, M.S.; Park, A.; Park, H.; Nam, S.-E.; Park, Y.-I. A zeolite membrane module composed of SAPO-34 hollow fibers for use in fluorinated gas enrichment. J. Membr. Sci. 2017, 542, 123–132. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Favre, E. Membrane processes and postcombustion carbon dioxide capture: Challenges and prospects. Chem. Eng. J. 2011, 171, 782–793. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Wang, L.; Boutilier, M.S.; Kidambi, P.R.; Jang, D.; Hadjiconstantinou, N.G.; Karnik, R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 2017, 12, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Luis, P.; van Gerven, T.; van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Paul, S.; Ghoshal, A.K.; Mandal, B. Theoretical studies on separation of CO2 by single and blended aqueous alkanolamine solvents in flat sheet membrane contactor (FSMC). Chem. Eng. J. 2008, 144, 352–360. [Google Scholar] [CrossRef]
- Andersen, A.; Divekar, S.; Dasgupta, S.; Cavka, J.H.; Nanoti, A.; Spjelkavik, A.; Goswami, A.N.; Garg, M.; Blom, R. On the development of Vacuum Swing adsorption (VSA) technology for post-combustion CO2 capture. Energy Procedia 2013, 37, 33–39. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Cryogenic technology for biogas upgrading combined with carbon capture-a review of systems and property impacts. Energy Procedia 2017, 142, 3741–3746. [Google Scholar] [CrossRef]
- Damen, K.; van Troost, M.; Faaij, A.; Turkenburg, W. A comparison of electricity and hydrogen production systems with CO2 capture and storage. Part A: Review and selection of promising conversion and capture technologies. Prog. Energy Combust. Sci. 2006, 32, 215–246. [Google Scholar] [CrossRef]
- Rubin, E.S.; Rao, A.B.; Chen, C. Comparative Assessments of Fossil Fuel Power Plants with CO. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004. [Google Scholar]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]
- Kanniche, M.; Le Moullec, Y.; Authier, O.; Hagi, H.; Bontemps, D.; Neveux, T.; Louis-Louisy, M. Up-to-date CO2 capture in thermal power plants. Energy Procedia 2017, 114, 95–103. [Google Scholar] [CrossRef]
- Dillon, D.; White, V.; Allam, R.; Wall, R.; Gibbins, J. Oxy Combustion Processes for CO2 Capture from Power Plant. In Technology & Engineering; Engineering Investigation Report; Mitsui Babcock Energy Limited: Crawley, UK, 2005; Volume 9. [Google Scholar]
- Zheng, L. Oxy-Fuel Combustion for Power Generation and Carbon Dioxide (CO2) Capture; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Bhown, A.S.; Freeman, B.C. Analysis and status of post-combustion carbon dioxide capture technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, C. Energy Conversion: CO2 Removal from Coal-Fired Power Plant; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Ritter, J.A. Radically new adsorption cycles for carbon dioxide sequestration. In Proceedings of the University Coal Research Contractors Review Meeting, U.S. DOE National Energy Technology Laboratory, Pittsburgh, PA, USA, 10 June 2004; Volume 2, p. 2. [Google Scholar]
- Olabi, A.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Linderholm, C. Chemical-looping combustion of solid fuels–technology overview and recent operational results in 100 kW unit. Energy Procedia 2014, 63, 98–112. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; Luis, F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Rackley, S.A. Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Gielen, D. The energy policy consequences of future CO2 capture and sequestration technologies. In Proceedings of the 2nd Annual Conference on Carbon Sequestration, Alexandria, VA, USA, 5–8 May 2003; pp. 5–8. [Google Scholar]
- Audus, H. Leading options for the capture of CO2 at power stations. In Proceedings of the 5th International Conference on Greenhouse Gas Control Technologies, Cairns, Australia, 13–16 August 2000; p. 16. [Google Scholar]
- Göttlicher, G.; Pruschek, R. Comparison of CO2 removal systems for fossil-fuelled power plant processes. Energy Convers. Manag. 1997, 38, S173–S178. [Google Scholar] [CrossRef]
- Tuinier, M.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Gao, Z.; Fu, J.; Ao, W.; Dai, J. CO2 capture with chemical looping combustion of gaseous fuels: An overview. Energy Fuels 2017, 31, 3475–3524. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane processes for direct carbon dioxide capture from air: Possibilities and limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. The effects of membrane-based CO2 capture system on pulverized coal power plant performance and cost. Energy Procedia 2013, 37, 1117–1124. [Google Scholar] [CrossRef][Green Version]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B. A 1000 MWth boiler for chemical-looping combustion of solid fuels–Discussion of design and costs. Appl. Energy 2015, 157, 475–487. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Change 2020, 25, 1–24. [Google Scholar] [CrossRef]
- Ma’mum, S.; Svendsen, H.F.; Hoff, K.A.; Juliussen, O. Selection of new absorbents for carbon dioxide capture. In Greenhouse Gas Control Technologies 7; Elsevier: Amsterdam, The Netherlands, 2005; pp. 45–53. [Google Scholar]
- Balsamo, M.; Rodríguez-Reinoso, F.; Montagnaro, F.; Lancia, A.; Erto, A. Highlighting the role of activated carbon particle size on CO2 capture from model flue gas. Ind. Eng. Chem. Res. 2013, 52, 12183–12191. [Google Scholar] [CrossRef]
- Balsamo, M.; Budinova, T.; Erto, A.; Lancia, A.; Petrova, B.; Petrov, N.; Tsyntsarski, B. CO2 adsorption onto synthetic activated carbon: Kinetic, thermodynamic and regeneration studies. Sep. Purif. Technol. 2013, 116, 214–221. [Google Scholar] [CrossRef]
- Sculley, J.P.; Verdegaal, W.M.; Lu, W.; Wriedt, M.; Zhou, H.C. High-Throughput Analytical Model to Evaluate Materials for Temperature Swing Adsorption Processes. Adv. Mater. 2013, 25, 3957–3961. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Guillou, F.; Gomez, A.; Clausse, M. A theoretical analysis of the energy consumption of post-combustion CO2 capture processes by temperature swing adsorption using solid sorbents. Int. J. Greenh. Gas Control 2013, 14, 74–83. [Google Scholar] [CrossRef]
- Datta, S.; Henry, M.P.; Lin, Y.J.; Fracaro, A.T.; Millard, C.S.; Snyder, S.W.; Stiles, R.L.; Shah, J.; Yuan, J.; Wesoloski, L. Electrochemical CO2 capture using resin-wafer electrodeionization. Ind. Eng. Chem. Res. 2013, 52, 15177–15186. [Google Scholar] [CrossRef]
- Huang, Q.; Eić, M. Commercial adsorbents as benchmark materials for separation of carbon dioxide and nitrogen by vacuum swing adsorption process. Sep. Purif. Technol. 2013, 103, 203–215. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; May, R.B.; Prakash, G.S.; Olah, G.A.; Narayanan, S. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011, 133, 20164–20167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, D.G.; Moon, D.K.; Byeon, S.-H.; Ahn, H.W.; Lee, C.H. Effect of bed void volume on pressure vacuum swing adsorption for air separation. Korean J. Chem. Eng. 2014, 31, 132–141. [Google Scholar] [CrossRef]
- Chou, C.-T.; Chen, C.-Y. Carbon dioxide recovery by vacuum swing adsorption. Sep. Purif. Technol. 2004, 39, 51–65. [Google Scholar] [CrossRef]
- Prenzel, T.; Wilhelm, M.; Rezwan, K. Tailoring amine functionalized hybrid ceramics to control CO2 adsorption. Chem. Eng. J. 2014, 235, 198–206. [Google Scholar] [CrossRef]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Auta, M.; Darbis, N.A.; Din, A.M.; Hameed, B. Fixed-bed column adsorption of carbon dioxide by sodium hydroxide modified activated alumina. Chem. Eng. J. 2013, 233, 80–87. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Chang, F.-Y.; Chao, K.-J.; Cheng, H.-H.; Tan, C.-S. Adsorption of CO2 onto amine-grafted mesoporous silicas. Sep. Purif. Technol. 2009, 70, 87–95. [Google Scholar] [CrossRef]
- Plaza, M.; García, S.; Rubiera, F.; Pis, J.; Pevida, C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem. Eng. J. 2010, 163, 41–47. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Lim, Y.H.; Jo, Y.M. Surface oxidation of activated carbon pellets by hydrogen peroxide for preparation of CO2 adsorbent. J. Ind. Eng. Chem. 2014, 20, 2130–2137. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Li, G.; Huang, B.; Pan, J.; Liu, Q.; Han, J.; Xiao, L.; Lu, J.; Zhuang, L. Energy Environ. Sci 2012, 5, 5163–5185. [Google Scholar]
- Yu, F.-C.; Phalak, N.; Sun, Z.; Fan, L.-S. Activation strategies for calcium-based sorbents for CO2 capture: A perspective. Ind. Eng. Chem. Res. 2012, 51, 2133–2142. [Google Scholar] [CrossRef]
- Buckingham, J.; Reina, T.R.; Duyar, M.S. Recent advances in carbon dioxide capture for process intensification. Carbon Capture Sci. Technol. 2022, 298, 100031. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P. Adsorption of CO2, N2, and O2 on natural zeolites. Energy Fuels 2003, 17, 571–576. [Google Scholar] [CrossRef]
- Xu, X.; Andresen, J.M.; Song, C.; Miller, B.G.; Scaroni, A.W. Preparation of novel CO2 “molecular basket” of polymer modified MCM-41. ACS Div. Fuel Chem. Prepr. 2002, 47, 67–68. [Google Scholar]
- Gallei, E.; Stumpf, G. Infrared spectroscopic studies of the adsorption of carbon dioxide and the coadsorption of carbon dioxide and water on CaY-and NiY-zeolites. J. Colloid Interface Sci. 1976, 55, 415–420. [Google Scholar] [CrossRef]
- Zhao, D.; Cleare, K.; Oliver, C.; Ingram, C.; Cook, D.; Szostak, R.; Kevan, L. Characteristics of the synthetic heulandite-clinoptilolite family of zeolites. Microporous Mesoporous Mater. 1998, 21, 371–379. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Albahar, M.Z. Selective Toluene Disproportionation Over zsm-5 Zeolite; The University of Manchester: Manchester, UK, 2018. [Google Scholar]
- Omodolor, I.S.; Otor, H.O.; Andonegui, J.A.; Allen, B.J.; Alba-Rubio, A.C. Dual-function materials for CO2 capture and conversion: A review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, Y.M.; Jung, S.Y.; Lee, J.B.; Ryu, C.K.; Kim, J.C. Excellent thermal stability of potassium-based sorbent using ZrO2 for post combustion CO2 capture. Fuel 2014, 115, 97–100. [Google Scholar] [CrossRef]
- Harlick, P.J.; Sayari, A. Applications of pore-expanded mesoporous silica. 5. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind. Eng. Chem. Res. 2007, 46, 446–458. [Google Scholar] [CrossRef]
- Moura, P.; Bezerra, D.; Vilarrasa-Garcia, E.; Bastos-Neto, M.; Azevedo, D. Adsorption equilibria of CO2 and CH4 in cation-exchanged zeolites 13X. Adsorption 2016, 22, 71–80. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Calleja, G.; Jimenez, A.; Pau, J.; Dominguez, L.; Perez, P. Multicomponent adsorption equilibrium of ethylene, propane, propylene and CO2 on 13X zeolite. Gas Sep. Purif. 1994, 8, 247–256. [Google Scholar] [CrossRef]
- I Xamena, F.X.L.; Abad, A.; Corma, A.; Garcia, H. MOFs as catalysts: Activity, reusability and shape-selectivity of a Pd-containing MOF. J. Catal. 2007, 250, 294–298. [Google Scholar]
- Ravon, U.; Domine, M.E.; Gaudillere, C.; Desmartin-Chomel, A.; Farrusseng, D. MOFs as acid catalysts with shape selectivity properties. New J. Chem. 2008, 32, 937–940. [Google Scholar] [CrossRef]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.G.; Yaghi, O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. USA 2009, 106, 20637–20640. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Gandara-Loe, J.; Pastor-Perez, L.; Bobadilla, L.; Odriozola, J.; Reina, T. Understanding the opportunities of metal–organic frameworks (MOFs) for CO2 capture and gas-phase CO2 conversion processes: A comprehensive overview. React. Chem. Eng. 2021, 6, 787–814. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J. Graphene-containing microporous composites for selective CO2 adsorption. Microporous Mesoporous Mater. 2020, 292, 109761. [Google Scholar] [CrossRef]
- Aarti, A.; Bhadauria, S.; Nanoti, A.; Dasgupta, S.; Divekar, S.; Gupta, P.; Chauhan, R. [Cu3 (BTC) 2]-polyethyleneimine: An efficient MOF composite for effective CO2. Rsc Adv. 2016, 6, 93003–93009. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef]
- Mahdipoor, H.R.; Halladj, R.; Babakhani, E.G.; Amjad-Iranagh, S.; Ahari, J.S. Synthesis, characterization, and CO2 adsorption properties of metal organic framework Fe-BDC. RSC Adv. 2021, 11, 5192–5203. [Google Scholar] [CrossRef]
- Yu, C.; Huang, C.; Tan, C. A review of CO2 capture by absorption and adsorption. Aerosol. Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Sánchez-Zambrano, K.S.; Lima Duarte, L.; Soares Maia, D.A.; Vilarrasa-García, E.; Bastos-Neto, M.; Rodríguez-Castellón, E.; Silva de Azevedo, D.C. CO2 capture with mesoporous silicas modified with amines by double functionalization: Assessment of adsorption/desorption cycles. Materials 2018, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W.; Zhao, C. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Duan, Y.; Luebke, D.R.; Pennline, H.W.; Li, B.; Janik, M.J.; Halley, J.W. Ab initio thermodynamic study of the CO2 capture properties of potassium carbonate sesquihydrate, K2CO3 1.5 H2O. J. Phys. Chem. C 2012, 116, 14461–14470. [Google Scholar] [CrossRef]
- Essaki, K.; Kato, M.; Uemoto, H. Influence of temperature and CO2 concentration on the CO2 absorption properties of lithium silicate pellets. J. Mater. Sci. 2005, 40, 5017–5019. [Google Scholar] [CrossRef]
- Venegas, M.J.; Fregoso-Israel, E.; Escamilla, R.; Pfeiffer, H. Kinetic and reaction mechanism of CO2 sorption on Li4SiO4: Study of the particle size effect. Ind. Eng. Chem. Res. 2007, 46, 2407–2412. [Google Scholar] [CrossRef]
- Pacciani, R.; Torres, J.; Solsona, P.; Coe, C.; Quinn, R.; Hufton, J.; Golden, T.; Vega, L. Influence of the Concentration of CO2 and SO2 on the Absorption of CO2 by a Lithium Orthosilicate-Based Absorbent. Environ. Sci. Technol. 2011, 45, 7083–7088. [Google Scholar] [CrossRef]
- Seggiani, M.; Puccini, M.; Vitolo, S. Alkali promoted lithium orthosilicate for CO2 capture at high temperature and low concentration. Int. J. Greenh. Gas Control 2013, 17, 25–31. [Google Scholar] [CrossRef]
- Durán-Muñoz, F.; Romero-Ibarra, I.C.; Pfeiffer, H. Analysis of the CO2 chemisorption reaction mechanism in lithium oxosilicate (Li 8 SiO 6): A new option for high-temperature CO2 capture. J. Mater. Chem. A 2013, 1, 3919–3925. [Google Scholar] [CrossRef]
- Memon, M.Z.; Zhao, X.; Sikarwar, V.S.; Vuppaladadiyam, A.K.; Milne, S.J.; Brown, A.P.; Li, J.; Zhao, M. Alkali metal CO2 sorbents and the resulting metal carbonates: Potential for process intensification of sorption-enhanced steam reforming. Environ. Sci. Technol. 2017, 51, 12–27. [Google Scholar] [CrossRef]
- Alcántar-Vázquez, B.; Vera, E.; Buitron-Cabrera, F.; Lara-García, H.A.; Pfeiffer, H. Evidence of CO oxidation–chemisorption process on sodium zirconate (Na2ZrO3). Chem. Lett. 2015, 44, 480–482. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Palacios-Romero, L.M.; Pfeiffer, H. Lithium cuprate (Li2CuO2): A new possible ceramic material for CO2 chemisorption. Chem. Lett. 2008, 37, 862–863. [Google Scholar] [CrossRef]
- Oh-ishi, K.; Matsukura, Y.; Okumura, T.; Matsunaga, Y.; Kobayashi, R. Fundamental research on gas–solid reaction between CO2 and Li2CuO2 linking application for solid CO2 absorbent. J. Solid State Chem. 2014, 211, 162–169. [Google Scholar] [CrossRef]
- Bernabé-Pablo, E.; Duan, Y.; Pfeiffer, H. Developing new alkaline ceramics as possible CO2 chemisorbents at high temperatures: The lithium and sodium yttriates (LiYO2 and NaYO2) cases. Chem. Eng. J. 2020, 396, 125277. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Hao, G.-P.; Li, W.-C.; Lu, A.-H. Novel porous solids for carbon dioxide capture. J. Mater. Chem. 2011, 21, 6447–6451. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, K.-D. Adsorption Behaviors of CO2 NH3on Chemically Surface-Treated Activated Carbons. J. Colloid Interface Sci. 1999, 212, 186–189. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, S.J. A Kinetic Study on the Thermal Degradation of Multi-Walled Carbon Nanotubes-Reinforced Poly (propylene) Composites. Macromol. Mater. Eng. 2004, 289, 368–374. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.G. Sakellariou. RSC Adv. 2014, 4, 2911. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Seema, H.; Kemp, K.C.; Le, N.H.; Park, S.-W.; Chandra, V.; Lee, J.W.; Kim, K.S. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Bai, B.C.; Cho, S.; Yu, H.-R.; Yi, K.B.; Kim, K.-D.; Lee, Y.-S. Effects of aminated carbon molecular sieves on breakthrough curve behavior in CO2/CH4 separation. J. Ind. Eng. Chem. 2013, 19, 776–783. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Zhang, F.; Li, Q.; Zhu, G. One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol–urea–formaldehyde resin for CO2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Guo, B.; Chang, L.; Xie, K. Adsorption of carbon dioxide on activated carbon. J. Nat. Gas Chem. 2006, 15, 223–229. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Miao, J.; Liu, M.; Ren, B.; Zhang, L.; Liu, Z. Facile synthesis of microporous carbon xerogels for highly selective CO2 adsorption. J. Clean. Prod. 2020, 253, 120023. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Yoo, H.-M.; Lee, S.-Y.; Kim, B.-J.; Park, S.-J. Influence of phosphoric acid treatment on hydrogen adsorption behaviors of activated carbons. Carbon Lett. 2011, 12, 112–115. [Google Scholar] [CrossRef][Green Version]
- Kim, K.-S.; Park, S.-J. Synthesis of nitrogen doped microporous carbons prepared by activation-free method and their high electrochemical performance. Electrochim. Acta 2011, 56, 10130–10136. [Google Scholar] [CrossRef]
- Seo, M.-K.; Park, S.-J. Influence of air-oxidation on electric double layer capacitances of multi-walled carbon nanotube electrodes. Curr. Appl. Phys. 2010, 10, 241–244. [Google Scholar] [CrossRef]
- Pramanik, P.; Ray, P.; Maity, A.; Das, S.; Ramakrishnan, S.; Dixit, P. Nanotechnology for improved carbon management in soil. In Carbon Management in Tropical and Sub-Tropical Terrestrial Systems; Springer: Singapore, 2020; pp. 403–415. [Google Scholar]
- Meng, L.-Y.; Park, S.-J. MgO-templated porous carbons-based CO2 adsorbents produced by KOH activation. Mater. Chem. Phys. 2012, 137, 91–96. [Google Scholar] [CrossRef]
- Jang, D.-I.; Park, S.-J. Influence of nickel oxide on carbon dioxide adsorption behaviors of activated carbons. Fuel 2012, 102, 439–444. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.-J.; Kim, H.-Y. Facile preparation and characterization of poly (vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Moradi, S. Microwave assisted preparation of sodium dodecyl sulphate (SDS) modified ordered nanoporous carbon and its adsorption for MB dye. J. Ind. Eng. Chem. 2014, 20, 208–215. [Google Scholar] [CrossRef]
- Dubey, S.P.; Dwivedi, A.D.; Lee, C.; Kwon, Y.-N.; Sillanpaa, M.; Ma, L.Q. Raspberry derived mesoporous carbon-tubules and fixed-bed adsorption of pharmaceutical drugs. J. Ind. Eng. Chem. 2014, 20, 1126–1132. [Google Scholar] [CrossRef]
- How, C.; Khan, M.A.; Hosseini, S.; Chuah, T.; Choong, T.S. Fabrication of mesoporous carbons coated monolith via evaporative induced self-assembly approach: Effect of solvent and acid concentration on pore architecture. J. Ind. Eng. Chem. 2014, 20, 4286–4292. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W.; Zhou, F.; Yang, G.; Du, Y. An overview and outlook on gas adsorption: For the enrichment of low concentration coalbed methane. Sep. Sci. Technol. 2020, 55, 1102–1114. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Viswanathan, K.; Boopathy, R.; Maharaja, P.; Sekaran, G. Three dimensional electro catalytic oxidation of aniline by boron doped mesoporous activated carbon. J. Ind. Eng. Chem. 2015, 21, 942–950. [Google Scholar] [CrossRef]
- Yoo, H.-M.; Lee, S.-Y.; Park, S.-J. Ordered nanoporous carbon for increasing CO2 capture. J. Solid State Chem. 2013, 197, 361–365. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.-J.; Lee, Y.-S. Superior prospect of chemically activated electrospun carbon fibers for hydrogen storage. Mater. Res. Bull. 2009, 44, 1871–1878. [Google Scholar] [CrossRef]
- Kim, B.-J.; Park, S.-J. A simple method for the preparation of activated carbon fibers coated with graphite nanofibers. J. Colloid Interface Sci. 2007, 315, 791–794. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lim, S.; Song, Y.; Ota, Y.; Qiao, W.; Tanaka, A.; Mochida, I. KOH activation of carbon nanofibers. Carbon 2004, 42, 1723–1729. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Su, S.; Yu, X.X.; Bae, J.-S. Application of carbon fibre composites to CO2 capture from flue gas. Int. J. Greenh. Gas Control 2013, 13, 191–200. [Google Scholar] [CrossRef]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Studt, F.; Abild-Pedersen, F.; Schlögl, R.; Behrens, M. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: Is there a common intermediate or not? J. Catal. 2015, 328, 43–48. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-j. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-temperature CO2 electrolysis in solid oxide electrolysis cells: Developments, challenges, and prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef]
- Currie, R.; Mottaghi-Tabar, S.; Zhuang, Y.; Simakov, D.S. Design of an Air-Cooled Sabatier Reactor for Thermocatalytic Hydrogenation of CO2: Experimental Proof-of-Concept and Model-Based Feasibility Analysis. Ind. Eng. Chem. Res. 2019, 58, 12964–12980. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the well-crystallized ordered mesoporous TiO2 with the confined space effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2–MnO x–Pt hybrid multiheterojunction film photocatalyst with enhanced photocatalytic CO2-reduction activity. ACS Appl. Mater. Interfaces 2018, 11, 5581–5589. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Huang, J.; Buonsanti, R. Colloidal nanocrystals as heterogeneous catalysts for electrochemical CO2 conversion. Chem. Mater. 2018, 31, 13–25. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and structure modulating of bimetallic CuSn nanowires boosts electrocatalytic conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Kim, C.; Cho, K.M.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Z-scheme photocatalytic CO2 conversion on three-dimensional BiVO4/carbon-coated Cu2O nanowire arrays under visible light. ACS Catal. 2018, 8, 4170–4177. [Google Scholar] [CrossRef]
- Leung, J.J.; Warnan, J.; Ly, K.H.; Heidary, N.; Nam, D.H.; Kuehnel, M.F.; Reisner, E. Solar-driven reduction of aqueous CO2 with a cobalt bis (terpyridine)-based photocathode. Nat. Catal. 2019, 2, 354–365. [Google Scholar] [CrossRef]
- Chen, J.; Yin, J.; Zheng, X.; Ait Ahsaine, H.; Zhou, Y.; Dong, C.; Mohammed, O.F.; Takanabe, K.; Bakr, O.M. Compositionally screened eutectic catalytic coatings on halide perovskite photocathodes for photoassisted selective CO2 reduction. ACS Energy Lett. 2019, 4, 1279–1286. [Google Scholar] [CrossRef]
- Kalamaras, E.; Belekoukia, M.; Tan, J.Z.; Xuan, J.; Maroto-Valer, M.M.; Andresen, J.M. A microfluidic photoelectrochemical cell for solar-driven CO2 conversion into liquid fuels with CuO-based photocathodes. Faraday Discuss. 2019, 215, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Sahoo, P.C.; Martha, S.; Parida, K. A review of harvesting clean fuels from enzymatic CO2 reduction. RSC Adv. 2016, 6, 44170–44194. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo-and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Xu, L.; Xiu, Y.; Liu, F.; Liang, Y.; Wang, S. Research progress in conversion of CO2 to valuable fuels. Molecules 2020, 25, 3653. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K.; de Tacconi, N.R.; Ghadimkhani, G.; Chanmanee, W.; Janáky, C. Tailoring copper oxide semiconductor nanorod arrays for photoelectrochemical reduction of carbon dioxide to methanol. ChemPhysChem 2013, 14, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Yadav, R.K.; Kumar, A.; Park, N.J.; Kim, J.Y.; Baeg, J.O. Fullerene polymer film as a highly efficient photocatalyst for selective solar fuel production from CO2. J. Appl. Polym. Sci. 2020, 137, 48536. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO₂ methanation on Ni–Ru/γ-Al₂O₃via modulating impregnation sequence and controlling surface active species. RSC Adv. 2014, 4, 16472–16479. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Huang, Q.; He, C.-T.; Chen, Y.; Liu, J.; Shen, F.-C.; Lan, Y.-Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Nam, D.H.; Kuk, S.K.; Choe, H.; Lee, S.; Ko, J.W.; Son, E.J.; Choi, E.-G.; Kim, Y.H.; Park, C.B. Enzymatic photosynthesis of formate from carbon dioxide coupled with highly efficient photoelectrochemical regeneration of nicotinamide cofactors. Green Chem. 2016, 18, 5989–5993. [Google Scholar] [CrossRef]
- Srikanth, S.; Maesen, M.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour. Technol. 2014, 165, 350–354. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, C. Solar-driven photoelectrochemical reduction of carbon dioxide to methanol at CuInS2 thin film photocathode. Sol. Energy Mater. Sol. Cells 2013, 108, 170–174. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Fu, J.; Yamaguchi, A.; Li, H.; Pan, H.; Hu, J.; Miyauchi, M.; Liu, M. Recent advances in the utilization of copper sulfide compounds for electrochemical CO2reduction. Nano Mater. Sci. 2020, 2, 235–247. [Google Scholar] [CrossRef]
- Zha, B.; Li, C.; Li, J. Efficient electrochemical reduction of CO2 into formate and acetate in polyoxometalate catholyte with indium catalyst. J. Catal. 2020, 382, 69–76. [Google Scholar] [CrossRef]
- Xi, G.; Ouyang, S.; Li, P.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. Ultrathin W18O49 nanowires with diameters below 1 nm: Synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angew. Chem. Int. Ed. 2012, 51, 2395–2399. [Google Scholar] [CrossRef]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective solar-driven reduction of CO2to methanol using a catalyzed p-GaP based photoelectrochemical cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-yield synthesis of ultralong and ultrathin Zn2GeO4 nanoribbons toward improved photocatalytic reduction of CO2 into renewable hydrocarbon fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, Z.; Zhao, Z.; Liu, Q.; Kou, J.; Chen, X.; Gao, J.; Yan, S.; Zou, Z. High-yield synthesis of ultrathin and uniform Bi2WO6 square nanoplates benefitting from photocatalytic reduction of CO2 into renewable hydrocarbon fuel under visible light. ACS Appl. Mater. Interfaces 2011, 3, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, H.; Li, W.; Zhuang, Z. Photocatalytic reduction of CO2 to methane over HNb3O8 nanobelts. Appl. Catal. A Gen. 2012, 413, 103–108. [Google Scholar]
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 particle size on the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Jensen, J.; Mikkelsen, M.; Krebs, F.C. Flexible substrates as basis for photocatalytic reduction of carbon dioxide. Sol. Energy Mater. Sol. Cells 2011, 95, 2949–2958. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Li, X.; Hao, Q.; Chen, H.; Ma, X. Ni-based catalysts prepared for CO2 reforming and decomposition of methane. Energy Convers. Manag. 2020, 205, 112419. [Google Scholar] [CrossRef]
- Freeman, B.C.; Bhown, A.S. Assessment of the technology readiness of post-combustion CO2 capture technologies. Energy Procedia 2011, 4, 1791–1796. [Google Scholar] [CrossRef]
- Roh, K.; Bardow, A.; Bongartz, D.; Burre, J.; Chung, W.; Deutz, S.; Han, D.; Heßelmann, M.; Kohlhaas, Y.; König, A. Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels. Green Chem. 2020, 22, 3842–3859. [Google Scholar] [CrossRef]
- IEA. Energy Technology Perspectives 2020: Special Report on Carbon Capture, Utilisation and Storage; IEA: Paris, France, 2020. [Google Scholar]
- Kearns, D.; Liu, H.; Consoli, C. Technology Readiness and Costs of CCS; Global CCS Institute: Melbourne, Australia, 2021. [Google Scholar]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Agarwal, A.S.; Rode, E.; Sridhar, N.; Hill, D. Conversion of CO to Value Added Chemicals: Opportunities and Challenges. In Handbook of Climate Change Mitigation and Adaptation; Springer: Cham, Switzerland, 2017; pp. 2487–2526. [Google Scholar]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical conversion of carbon dioxide (CO2) into fuels and value-added products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- McGrath, O.J. Biological Conversion of Carbon Dioxide to Value-Added Chemicals; West Virginia University: Morgantown, WV, USA, 2021. [Google Scholar]
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst: Effects of reactants partial pressure. J. Nat. Gas Sci. Eng. 2015, 27, 1016–1023. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; de Coninick, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- Bell, A.; Marks, T. Carbon Management: Implications for R&D in the chemical sciences and technology. In A Workshop Report to the Chemical Sciences Roundtable; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Aresta, M. Carbon Dioxide as Chemical Feedstock; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Li, Y.; Markley, B.; Mohan, A.R.; Rodriguez-Santiago, V.; Thompson, D.; Niekerk, D. Utilization of Carbon Dioxide from Coal-fired Power Plant for the Production of Value-Added Products. In Design Engineering of Energy and Geo-Environmental Systems Course (EGEE 580); Internal Document for a Course; College of Earth and Mineral Science: University Park, PA, USA, 2006. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Downturn, A.M. Global Demand for Polycarbonate Growing Again, Says IHS Chemical Report; IHS Online Pressroom: Engelwood, CO, USA, 2012. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Angunn, E.; Nada, A.; Gaëlle, B.-C. Evaluation of carbon dioxide utilisation concepts: A quick and complete methodology. Energy Procedia 2014, 63, 8010–8016. [Google Scholar] [CrossRef]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 utilization in the perspective of industrial ecology, an overview. J. CO2 Util. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Aresta, M. Carbon Dioxide Recovery and Utilization; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Ahmed, M. Greenhouse gas emissions and climate variability: An overview. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Cham, Switzerland, 2017; pp. 1–26. [Google Scholar]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Yang, V.N.; Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 2015, 137, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef]
- Laurenczy, G. Hydrogen storage and delivery: The carbon dioxide–formic acid couple. CHIMIA Int. J. Chem. 2011, 65, 663–666. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Foley, A. Sustainable Automotive Technologies 2013 Proceedings of the 5th International Conference ICSAT 2013, J. Wellnitz, A. Subic, R. Trufin. Springer International Publishing, Switzerland (2014). £126 (hardback); £100.50 (eBook), ISBN: 978-3-319-01883-6 (hardback); ISBN: 978-3-319-01884-3 (eBook). J. Transp. Geogr. 2015, 45, 83–84. [Google Scholar] [CrossRef]
- Kuld, S.; Conradsen, C.; Moses, P.G.; Chorkendorff, I.; Sehested, J. Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst. Angew. Chem. 2014, 126, 6051–6055. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014, 309, 66–70. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H 2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Gao, P.; Zhong, L.; Li, X.; Zhang, Z.; Wang, H.; Wei, W.; Sun, Y. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Today 2017, 281, 327–336. [Google Scholar] [CrossRef]
- Kattel, S.; Yu, W.; Yang, X.; Yan, B.; Huang, Y.; Wan, W.; Liu, P.; Chen, J.G. CO2 Hydrogenation over Oxide-Supported PtCo Catalysts: The Role of the Oxide Support in Determining the Product Selectivity. Angew. Chem. Int. Ed. 2016, 55, 7968–7973. [Google Scholar] [CrossRef]
- Brown, R.C. Introduction to thermochemical processing of biomass into fuels, chemicals, and power. In Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 1–12. [Google Scholar]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- De Queiroz, F.A.O.; De Medeiros, J.L.; Alves, R.M.B. CO2 Utilization: A process systems engineering vision. In CO2 Separation and Valorisation; IntechOpen: Rijeka, Croatia, 2014; pp. 35–88. [Google Scholar]
- De Queiroz, F.A.O.; De Medeiros, J.L.; Alves, R.M.B. CO2 Utilization: A Process Systems Engineering Vision. In CO2 Sequestration and Valorization; Morgado, C.D.R.V., Esteves, V.P.P., Eds.; IntechOpen: Rijeka, Croatia, 2014; pp. 44–90. [Google Scholar] [CrossRef]
- Jiang, Z.; Liao, X.; Zhao, Y. Comparative study of the dry reforming of methane on fluidised aerogel and xerogel Ni/Al2O3 catalysts. Appl. Petrochem. Res. 2013, 3, 91–99. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Kaiser, P.; Unde, R.B.; Kern, C.; Jess, A. Production of liquid hydrocarbons with CO2 as carbon source based on reverse water-gas shift and Fischer-Tropsch synthesis. Chem. Ing. Technol. 2013, 85, 489–499. [Google Scholar] [CrossRef]
- Ross, J.R. Natural gas reforming and CO2 mitigation. Catal. Today 2005, 100, 151–158. [Google Scholar] [CrossRef]
- Urlan, F.; Marcu, I.-C.; Sandulescu, I. Oxidative dehydrogenation of n-butane over titanium pyrophosphate catalysts in the presence of carbon dioxide. Catal. Commun. 2008, 9, 2403–2406. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, H.; Huang, Q. Intrinsic kinetics of oxidative dehydrogenation of propane in the presence of CO2 over Cr/MSU-1 catalyst. J. Nat. Gas Chem. 2011, 20, 311–317. [Google Scholar] [CrossRef]
- Estes, D.P.; Copéret, C. The Role of Proton Transfer in Heterogeneous Transformations of Hydrocarbons. CHIMIA Int. J. Chem. 2015, 69, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, F.; Zhang, Y.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Oxidative dehydrogenation of ethane with CO2 over Cr supported on submicron ZSM-5 zeolite. Chin. J. Catal. 2015, 36, 1242–1248. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Müller, K.; Baumgärtner, A.; Mokrushina, L.; Arlt, W. Increasing the equilibrium yield of oxidative dehydrogenation with CO2 by secondary reactions. Chem. Eng. Technol. 2014, 37, 1261–1264. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation a review. Energy Fuels 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Ansari, M.B.; Park, S.-E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci. 2012, 5, 9419–9437. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Guan, S.; Huang, J.; Wu, C. Direct and highly selective conversion of captured CO2 into methane through integrated carbon capture and utilization over dual functional materials. J. CO2 Util. 2020, 38, 262–272. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Younas, M.; Loong Kong, L.; Bashir, M.J.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation–From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Billig, E.; Decker, M.; Benzinger, W.; Ketelsen, F.; Pfeifer, P.; Peters, R.; Stolten, D.; Thrän, D. Non-fossil CO2 recycling—The technical potential for the present and future utilization for fuels in Germany. J. CO2 Util. 2019, 30, 130–141. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, Z.; Su, W.; Wang, Q.; Wang, X.; Zhang, H. Analysis of research status of CO2 conversion technology based on bibliometrics. Catalysts 2020, 10, 370. [Google Scholar] [CrossRef]
- Wannakao, S.; Artrith, N.; Limtrakul, J.; Kolpak, A.M. Engineering transition-metal-coated tungsten carbides for efficient and selective electrochemical reduction of CO2 to methane. ChemSusChem 2015, 8, 2745–2751. [Google Scholar] [CrossRef]
- Park, M.; Kwak, B.S.; Jo, S.W.; Kang, M. Effective CH4 production from CO2 photoreduction using TiO2/x mol% Cu–TiO2 double-layered films. Energy Convers. Manag. 2015, 103, 431–438. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Maeda, T.; Hu, A.; Yu, C.-P.; Wood, T.K. CO2 sequestration by methanogens in activated sludge for methane production. Appl. Energy 2015, 142, 426–434. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Tomsett, A.; Hagiwara, T.; Miyamoto, A.; Inui, T. Highly active catalysts for CO2 methanation to provide the second reactor of two stage process for high BTU SNG synthesis. Appl. Catal. 1986, 26, 391–394. [Google Scholar] [CrossRef]
- Li, Z.; Shi, R.; Ma, Y.; Zhao, J.; Zhang, T. Photodriven CO2 Hydrogenation into Diverse Products: Recent Progress and Perspective. J. Phys. Chem. Lett. 2022, 13, 5291–5303. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Liu, B.; Song, W.; Ma, Q.; Zhao, T.-S.; Wang, X.; Bae, J.W.; Zhang, X.; Zhang, J. Highly stable and selective layered Co-Al-O catalysts for low-temperature CO2 methanation. Appl. Catal. B Environ. 2022, 310, 121303. [Google Scholar] [CrossRef]
- Herrmann, F.; Grünewald, M.; Meijer, T.; Gardemann, U.; Feierabend, L.; Riese, J. Operating window and flexibility of a lab-scale methanation plant. Chem. Eng. Sci. 2022, 254, 117632. [Google Scholar] [CrossRef]
- Du, J.; Gao, J.; Gu, F.; Zhuang, J.; Lu, B.; Jia, L.; Xu, G.; Liu, Q.; Su, F. A strategy to regenerate coked and sintered Ni/Al2O3 catalyst for methanation reaction. Int. J. Hydrog Energy 2018, 43, 20661–20670. [Google Scholar] [CrossRef]
- Burger, T.; Donaubauer, P.; Hinrichsen, O. On the kinetics of the co-methanation of CO and CO2 on a co-precipitated Ni-Al catalyst. Appl. Catal. B Environ. 2021, 282, 119408. [Google Scholar] [CrossRef]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Iucci, G.; Battocchio, C.; Mobilio, S.; Casciardi, S.; Sisto, R. Nickel supported on YSZ: The effect of Ni particle size on the catalytic activity for CO2 methanation. J. CO2 Util. 2018, 23, 200–211. [Google Scholar] [CrossRef]
- Schubert, M.; Pokhrel, S.; Thomé, A.; Zielasek, V.; Gesing, T.M.; Roessner, F.; Mädler, L.; Bäumer, M. Highly active Co–Al 2 O 3-based catalysts for CO2 methanation with very low platinum promotion prepared by double flame spray pyrolysis. Catal. Sci. Technol. 2016, 6, 7449–7460. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Ma, J. Highly active and stable nano NiO–MgO catalyst encapsulated by silica with a core–shell structure for CO2 methanation. RSC Adv. 2014, 4, 17420–17428. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).