Response of Fluorescence and Chlorophyll Physiological Characteristics of Typical Urban Trees to Ozone Stress
Abstract
1. Introduction
2. Research Method
2.1. Study Area
2.2. Ozone Concentration Control
2.3. Determination Chlorophyll Fluorescence
2.4. Determination of Chlorophyll Content
2.5. Measurement of Relative Conductivity
2.6. Data Processing and Analysis
3. Results and Analysis
3.1. Effects of Ozone Stress on the Maximum Photochemical Efficiency of Trees
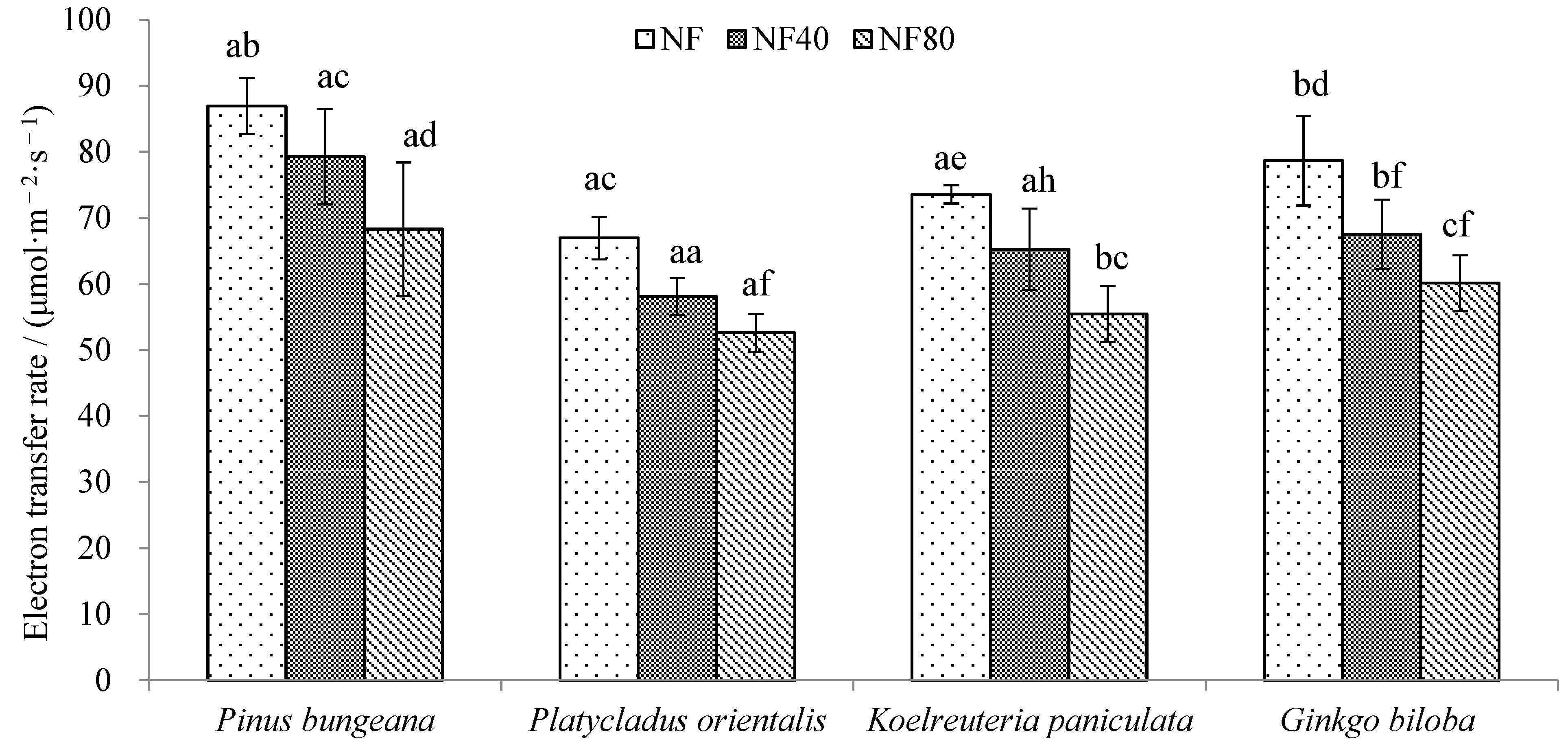
3.2. Response of tree Electron Transfer Rate (ETR) to Ozone Stress
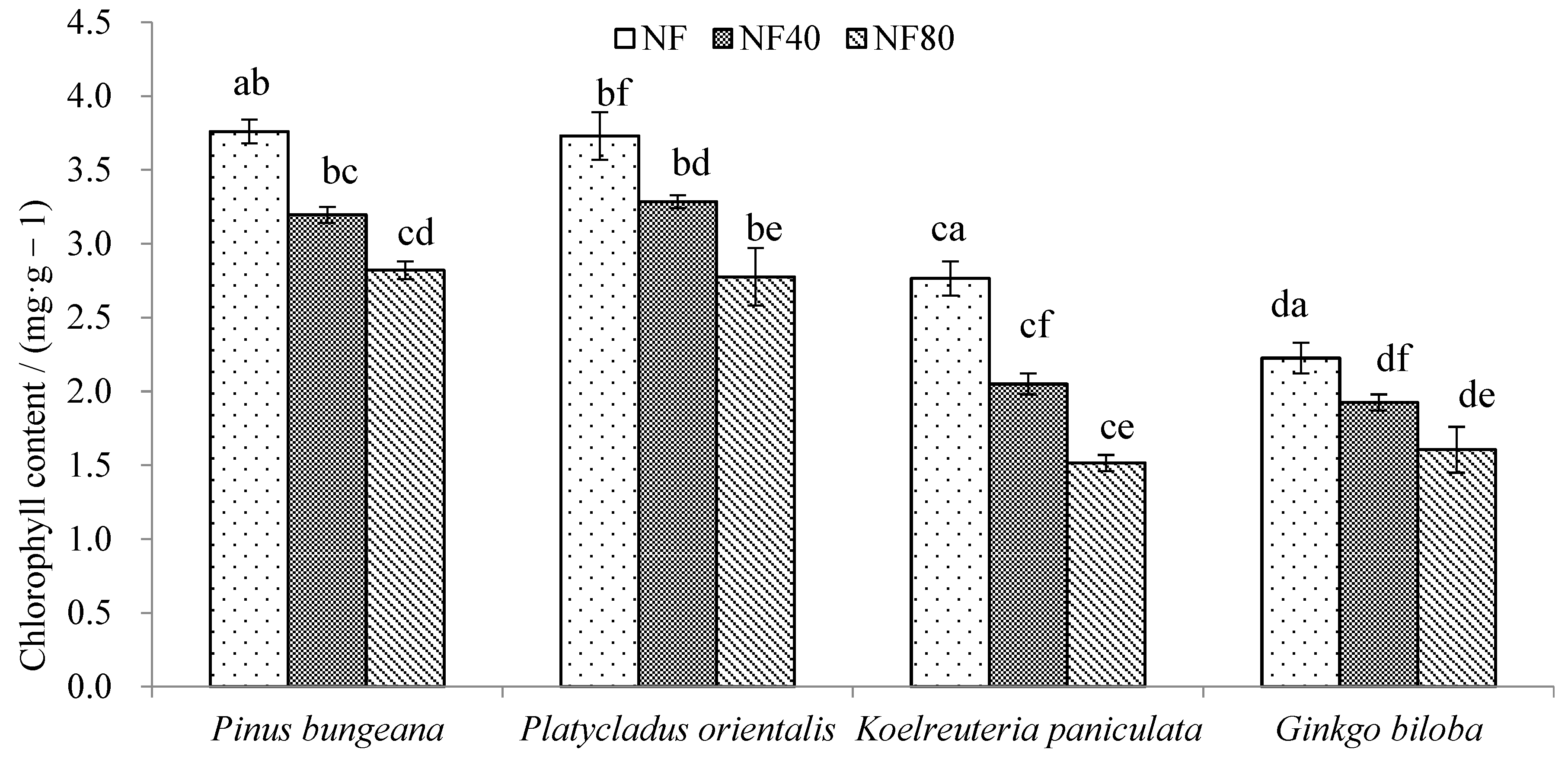
3.3. Response of Tree Chlorophyll Changes to Ozone Stress
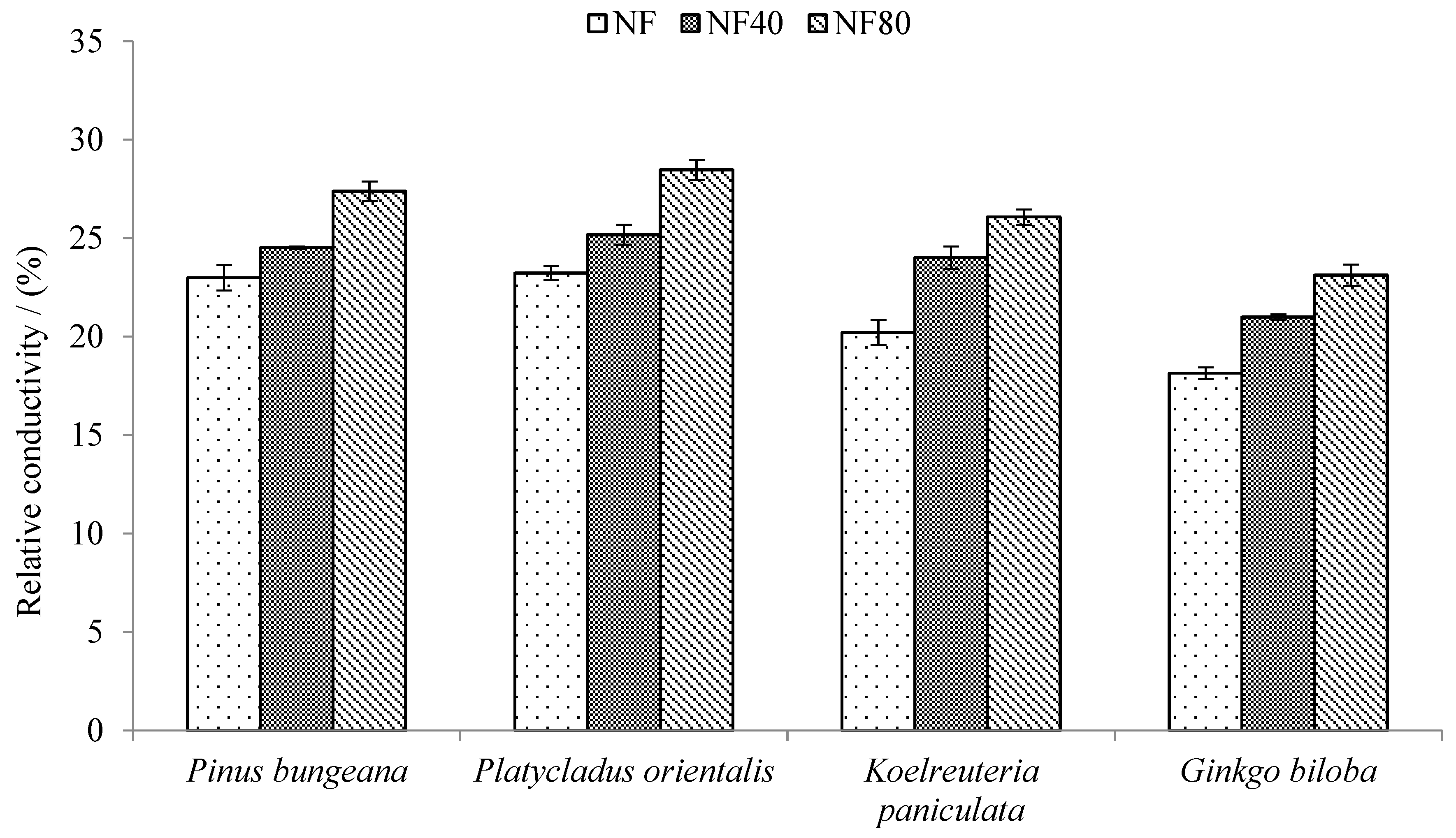
3.4. Response of Relative Conductivity of Trees to Ozone Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J.J. Effect of Ozone Stress on the Physiological and Root Characteristics of Greening Tree Species; Hebei Agriculture University: Baoding, China, 2022. [Google Scholar]
- Chen, B.; Yang, X.B.; Xu, J.J. Spatio-temporal variation and influencing factors of ozone pollution in Beijing. Atmosphere 2022, 13, 359. [Google Scholar] [CrossRef]
- Chen, B.; Lu, S.W.; Li, S.N. Ozone uptake characteristics in different dominance hierarchies of poplar plantation. J. Beijing For. Univ. 2015, 37, 29–36. [Google Scholar]
- Wang, H.; Ouyang, Z.Y.; Ren, Y.F.; Zhang, H.X.; Wang, X.K.; Hao, S.Q.; Guan, Y.F.; Gao, F.Y. Ozone uptake at the canopy level in Robinia pseudoacacia in Beijing based on sap flow measurements. Acta Ecol. Sin. 2013, 33, 7323–7331. [Google Scholar] [CrossRef][Green Version]
- Chen, B. Effects of Ozone Stress on the Growth and Physiological Characteristics of Four Tree Species; Beijing Forestry University: Beijing, China, 2018. [Google Scholar]
- Gao, F.; Li, P.; Feng, Z.Z. Interactive effects of ozone and drought stress on plants: A review. Chin. J. Plant Ecol. 2017, 41, 252–268. [Google Scholar]
- Xin, Y. Effects of Ozone on the Photosynthetic Physiology and Growth of Populus cathayana under Nitrogen Deposition; University of Chinese Academy of Sciences: Beijing, China, 2016. [Google Scholar]
- Xin, Y.; Gao, F.; Feng, Z.Z. Photosynthetic Characteristics and Ozone Dose-response Relationships for Different Genotypes of Poplar. Environ. Sci. 2016, 37, 2359–2367. [Google Scholar]
- Vollenweider, P.; Ottiger, M.; Güthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Niu, J.F. Effects of Elevated Ozone and Nitrogen Deposition on the Growth and Physiology of Cinnamomum Camphora Seedlings; Chinese Academy of Sciences: Beijing, China, 2012. [Google Scholar]
- Xin, Y.; Shang, B.; Chen, X.L.; Feng, Z.Z. Effects of elevated ozone and nitrogen deposition on photosynthetic characteristics and biomass of Populus cathayana. Environ. Sci. 2016, 37, 3642–3649. [Google Scholar]
- Cheng, X.Y.; Luo, C.W.; Liu, J.Q.; Zhou, Y.S.; Meng, S.W. Influence of ozone stress on spectral characteristics and chlorophyll content of woody plant leaves. Environ. Sci. Technol. 2017, 40, 1–8. [Google Scholar]
- Liu, D.H.; Zhao, S.W.; Wang, X.Q.; Fan, J.L. The effect of ozone on the leaf damage symptom and Physiological characteristics of landscape plants. Chin. Ornam. Hortic. Res. Prog. 2015, 507–512. [Google Scholar]
- Guo, C.L.; Jiang, J. Effect of ozone fumigation on membrane permeability of 13 native tree species. J. Green Sci. Technol. 2019, 118–119. [Google Scholar]
- Chen, B.; Song, Q.F.; Pan, Q.H. Study on Transpiration water consumption and photosynthetic characteristics of landscape tree species under ozone stress. Atmosphere 2022, 13, 1139. [Google Scholar] [CrossRef]
- Chen, B.; Xu, J.J.; Liu, D.H.; Yang, X.B. Response of Ginkgo biloba growth and physiological traits to ozone stress. Glob. Ecol. Conserv. 2022, 34, e02020. [Google Scholar] [CrossRef]
- Zhang, W.W. Effect of Elevated Ozone Level on Native Tree Species in Subtropical China; Research Center of Ecological Environment, Chinese Academy: Beijing, China, 2011. [Google Scholar]
- Wang, X.N.; Agathokleous, E.; Qu, L.Y.; Watanabe, M.; Koike, T. Effects of CO2 and O3 on the interaction between root of woody plants and ectomycorrhizae. J. Agric. Meteorol. 2016, 72, 95–105. [Google Scholar] [CrossRef]
- Xu, W.Y.; Qi, S.Y.; He, X.Y.; Chen, W.; Zhao, G.L.; Zhou, Y. Effects of elevated CO2 and O3 concentrations on quantitative characteristics of mature leaf stomata in Ginkgo biloba. Chin. J. Ecol. 2008, 27, 1059–1063. [Google Scholar]
- Li, P.; Feng, Z.Z.; Shang, B.; Yuan, X.Y.; Dai, L.L.; Xu, Y.S. Stomatal characteristics and ozone dose-response relationships for six greening tree species. Acta Ecol. Sin. 2018, 38, 2710–2721. [Google Scholar]
- Li, L. The Effects of Elevated Ozone on Growth and Physiology of Acer truncatum under Drought Stress; University of Chinese Academy of Sciences: Beijing, China, 2016. [Google Scholar]
- Feng, Z.Z.; Li, P.; Yuan, X.Y.; Gao, F.; Jiang, L.J.; Dai, L.L. Progress in ecological and environmental effects of ground-level O3 in China. Acta Ecol. Sin. 2018, 38, 1530–1541. [Google Scholar]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Koike, T. O3-induced stomatal sluggishness develops progressively in siebold’s beech (Fagus crenata). Environ. Pollut. 2012, 166, 152–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hao, J.; Luo, M. Seasonal and spatial variability of surface ozone over China: Contributions from background and domestic pollution. Atmos. Chem. Phys. Discuss. 2010, 10, 27853–27891. [Google Scholar] [CrossRef]
- Flowers, M.D.; Fiscus, E.L.; Burkey, K.O.; Booker, F.C.; Dubois, J.B. Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris) genotypes differing in sensitivity to ozone. Environ. Exp. Bot. 2007, 61, 190–198. [Google Scholar] [CrossRef]
- Cao, J.L.; Zhu, J.G.; Zeng, Q.; Li, C.H. Research advance in the effect of elevated O3 on characteristics of photosynthesis. J. Biol. 2012, 26, 66–70. [Google Scholar]
- Liang, J.; Zeng, Q.; Zhu, J.G.; Xie, Z.B.; Liu, G.; Tang, H.Y. Review of indexes for evaluating plant response to elevated near-surface ozone concentration. Chin. J. Eco-Agric. 2010, 18, 440–445. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Q.; Feng, Z.Z.; Zhu, J.G.; Tang, H.Y.; Chen, X.; Xie, Z.B.; Liu, G.; Kobayashi, K. Photosynthetic Damage induced by elevated O3 in two varieties of winter wheat with free air controlled enrichment approach. Environ. Sci. 2009, 30, 527–534. [Google Scholar]
- Kivimäenpää, M.; Sutinen, K.; Calatayud, V.; Sanz, M.J. Visible and microscopic needle alterations of mature Aleppo pine (Pinus halepensis) trees growing on an ozone gradient in eastern Spain. Tree Physiol. 2010, 30, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Niu, J.F.; Wang, X.K.; Tian, Y.; Yao, F.F.; Feng, Z.Z. Effects of ozone exposure on growth and photosynthesis of the seedlings of Liriodendron Chinese, a native tree species of subtropical China. Photosynthetica 2011, 49, 29–36. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Mae, T. RuBiscolytics: Fate of RuBisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 2008, 59, 1615–1624. [Google Scholar] [CrossRef]
- Zhang, W.W.; Feng, Z.Z.; Wang, X.K.; Niu, J.F. Impacts of elevated ozone on growth and photosynthesis of Metasequoia glyptostroboides Hu et Cheng. Plant Sci. 2014, 226, 182–188. [Google Scholar] [CrossRef]
- Wustman, B.A.; Oksanen, E.; Karnosky, D.F.; Noormets, A.; Isebrands, J.G.; Pregitzer, K.S.; Hendrey, G.R.; Sober, J.; Podila, G.K. Effects of elevated CO2 and O3 on aspen clones of varying O3 sensitivity. Dev. Environ. Sci. 2001, 3, 391–409. [Google Scholar]
- Liu, D.H.; Zhao, S.W.; Kang, W.J.; Wang, S.Y.; Wu, B.J. Comparation of injury on leaf adaxial and abaxial sides in Hosta plantaginea induced by ozone. Plant Physiol. J. 2015, 51, 2039–2046. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Liu, Y.; Chen, B.; Tao, Y.; Cui, C.; Wen, Y.; Deng, W.; Chen, Q.; Yuan, X. Response of Fluorescence and Chlorophyll Physiological Characteristics of Typical Urban Trees to Ozone Stress. Atmosphere 2022, 13, 1885. https://doi.org/10.3390/atmos13111885
Gao Y, Liu Y, Chen B, Tao Y, Cui C, Wen Y, Deng W, Chen Q, Yuan X. Response of Fluorescence and Chlorophyll Physiological Characteristics of Typical Urban Trees to Ozone Stress. Atmosphere. 2022; 13(11):1885. https://doi.org/10.3390/atmos13111885
Chicago/Turabian StyleGao, Yaoyao, Yuanqiu Liu, Bo Chen, Yuzhu Tao, Cheng Cui, Ye Wen, Wenping Deng, Qi Chen, and Xi Yuan. 2022. "Response of Fluorescence and Chlorophyll Physiological Characteristics of Typical Urban Trees to Ozone Stress" Atmosphere 13, no. 11: 1885. https://doi.org/10.3390/atmos13111885
APA StyleGao, Y., Liu, Y., Chen, B., Tao, Y., Cui, C., Wen, Y., Deng, W., Chen, Q., & Yuan, X. (2022). Response of Fluorescence and Chlorophyll Physiological Characteristics of Typical Urban Trees to Ozone Stress. Atmosphere, 13(11), 1885. https://doi.org/10.3390/atmos13111885






