Preliminary Findings on CO2 Capture over APTES-Modified TiO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of APTES-Modified TiO2
2.3. Characterization Methods
2.4. CO2 Sorption Measurement
3. Results
3.1. Characterization of Materials
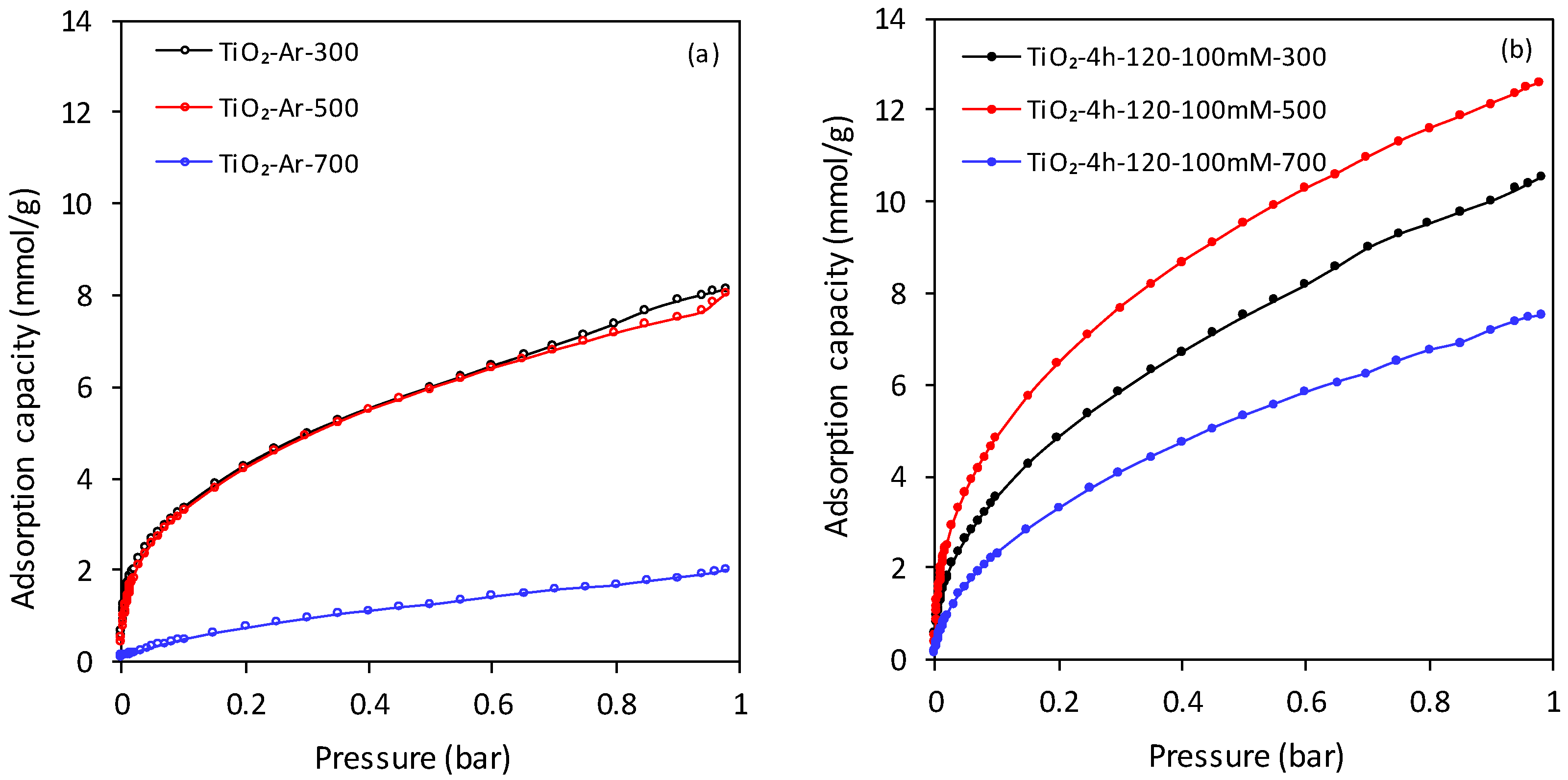
3.2. CO2 Adsorption Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kús, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R.M. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Kim, J.T.; Suh, J.K.; Lee, J.M.; Lee, C.H. Adsorption equilibria of CO2 on zeolite 13X and zeolite X/Activated carbon composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar] [CrossRef]
- Zhao, G.; Aziz, B.; Hedin, N. Carbon dioxide adsorption on mesoporous silica surfaces containing amine-like motifs. Appl. Energy 2010, 87, 2907–2913. [Google Scholar] [CrossRef]
- Liao, Y.; Cheng, Z.; Trunk, M.; Thomas, A. Targeted control over the porosities and functionalities of conjugated microporous polycarbazole networks for CO2-selective capture and H2 storage. Polym. Chem. 2017, 8, 7240–7247. [Google Scholar] [CrossRef]
- Kang, M.; Kim, J.E.; Kang, D.W.; Lee, H.Y.; Moon, D.; Hong, C.S. A diamine-grafted metal–organic framework with outstanding CO2 capture properties and a facile coating approach for imparting exceptional moisture stability. J. Mater. Chem. A 2019, 7, 8177–8183. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Qiao, X.; Shen, X.; Guo, J.; Xiang, J.; Jin, Y. Experiment and regeneration kinetic model study on CO2 adsorbent prepared from fly ash. Chem. Eng. J. 2020, 421, 127865. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Knöfel, C.; Descarpentries, J.; Benzaouia, A.; Zeleňák, V.; Mornet, S.; Llewellyn, P.L.; Hornebecq, V. Functionalised micro-/mesoporous silica for the adsorption of carbon dioxide. Microporous Mesoporous Mater. 2007, 99, 79–85. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Hu, X.; Hao, J.; Guo, Q. Mechanism and kinetics of CO2 adsorption for TEPA- impregnated hierarchical mesoporous carbon in the presence of water vapour. Powder Technol. 2020, 368, 227–236. [Google Scholar] [CrossRef]
- Kapica-Kozar, J.; Michalkiewicz, B.; Wrobel, R.J.; Mozia, S.; Piróg, E.; Kusiak-Nejman, E.; Serafin, J.; Morawski, A.W.; Narkiewicz, U. Adsorption of carbon dioxide on TEPA-modified TiO2/titanate composite nanorods. New J. Chem. 2017, 41, 7870–7885. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desolination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorp-tion: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Knowles, G.P.; Chaffee, A.L. Aminopropyl-functionalized silica CO2 adsorbents via sonochemical methods. J. Chem. 2016, 2016, 070838. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.H.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef]
- Jeon, S.; Min, J.; Kim, S.H.; Lee, K.B. Introduction of cross-linking agents to enhance the performance and chemical stability of polyethyleneimine-impregnated CO2 adsorbents: Effect of different alkyl chain lengths. Chem. Eng. J. 2020, 398, 125531. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. Development of polyethylenimine-functionalized mesoporous Si-MCM-41 for CO2 adsorption. Fuel Process Technol. 2017, 167, 622–630. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Feldmann, C. Preparation of nanoscale pigment particles. Adv. Mater. 2001, 13, 1301. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer. 2005, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lib, Y.; Deng, D.; Liu, X.; Xing, X.; Xiao, X.; Wang, Y. Acetone sensing performances based on nanoporous TiO2 sy,nthesized by a facile hydrothermal method Sensor. Actuator. B Chem. 2017, 238, 238491–238500. [Google Scholar] [CrossRef]
- Wanbayor, R.; Ruangpornvisuti, V. Adsorption of di-, tri- and polyatomic gases on the anatase TiO2 (0 0 1) and (1 0 1) surfaces an,d their adsorption abilities. J. Mol. Stuct. 2010, 952, 103–108. [Google Scholar] [CrossRef]
- Aquino, C.C.; Richner, G.; Kimling, M.C.; Chen, D.; Puxty, G.; Feron, P.H.M.; Caruso, R.A. Amine-functionalized titania-based po,rous structures for carbon dioxide postcombustion capture. J. Phys. Chem. C. 2013, 117, 9747–9757. [Google Scholar] [CrossRef]
- Muller, K.; Lu, D.; Senanayake, S.D.; Starr, D.E. Monoethanolamine adsorption on TiO2(110): Bonding, structure, and implications for use as a model solid-supported CO2 capture material. J. Phys. Chem. C. 2014, 18, 1576–1586. [Google Scholar] [CrossRef]
- Kapica-Kozar, J.; Pirog, E.; Kusiak-Nejman, E.; Wrobel, R.J.; Gesikiewicz-Puchalska, A.; Morawski, A.W.; Nakiewicz, U.; Michalkiewicz, B. Titanium dioxide modified with various amines used as sorbents of carbon dioxide. New J. Chem. 2017, 41, 1549–1557. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Xu, G.Q.; Zheng, Z.X.; Wu, Y.C.; Feng, N. Effect of silica on the microstructure and photocatalytic properties of titania. Ceram. Int. 2009, 35, 1–5. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Tucci, A.; Skapin, A.S.; Esposito, L. Effects of SiO2 addition on TiO2 crystal structure and photocatalytic activity. J. Eur. Ceram. Soc. 2010, 30, 2481–2490. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Morawski, A.W. Preparation and characterisation of TiO2 thermally modified with cyclohexane vapours. Int. J. Mater. Prod. Techn. 2016, 52, 286–297. [Google Scholar] [CrossRef]
- Wanag, A.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Influences of modification of titanium dioxide by silane coupling agents on the photocatalytic activity and stability. J. Environ. Chem. Eng. 2020, 8, 103917. [Google Scholar] [CrossRef]
- Colón, G.; Sánchez-España, J.M.; Hidalgo, M.C.; Navío, J.A. Effect of TiO2 acidic pre-treatment on the photocatalytic properties for phenol degradation. J. Photochem. Photobiol. A 2006, 179, 20–27. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Wanag, A.; Kusiak-Nejman, E.; Ekiert, E.; Rokicka-Konieczna, P.; Morawski, A.W. Effect of calcination on the photocatalytic activity and stability of TiO2 photocatalysts modified with APTES. J. Environ. Chem. Eng. 2021, 9, 104794. [Google Scholar] [CrossRef]
- Winter, M.; Hamal, D.; Yang, X.; Kwen, H.; Jones, D.; Rajagopalan, S.H.; Klabunde, K.J. Defining reactivity of solid sorbents: What is the most appropriate metric? Chem. Mater. 2009, 21, 2367–2374. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating photodegradation properties of anatase and rutile TiO2 nanoparticles for organic compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- Cheng, F.; Sajedin, S.M.; Kelly, S.M.; Lee, A.F.; Kornherr, A. UV-stable paper coated with APTES-modified P25 TiO2 nanoparticles. Carbohydr. Polym. 2016, 114, 246–252. [Google Scholar] [CrossRef]
- Maira, A.J.; Coronado, J.M.; Augugliaro, V.; Yeung, K.L.; Conesa, J.C.; Soria, J. Fourier transform infrared study of the performance of nanostructured TiO2 particles for the photocatalytic oxidation of gaseous toluene. J. Catal. 2001, 202, 413–420. [Google Scholar] [CrossRef]
- Saliba, P.A.; Mansur, A.A.P.; Mansur, H.S. Advanced nanocomposite coatings of fusion bonded epoxy reinforced with amino-functionalized nanoparticles for applications in underwater oil pipelines. J. Nanomater. 2016, 2016, 7281726. [Google Scholar] [CrossRef]
- Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-cleaning ceramic tiles produced via stable coating of TiO2 nanoparticles. Materials 2018, 11, 1003. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chena, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, M. The effect of surface modification with silane coupling agent on suppressing the photo-catalytic activity of fine TiO2 particles as inorganic UV filter. Appl. Surf. Sci. 2007, 254, 563–569. [Google Scholar] [CrossRef]
- Youssef, Z.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Moussaron, A.; Baros, F.; Toufaily, J.; Harmieh, T.; Roques-Carmes, T.; Frochot, C. Titania and silica nanoparticles coupled to Chlorin e6 for anti-cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2018, 22, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wachs, I.E. Titania-silica as catalysts: Molecularstructural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Milanesi, F.; Cappelletti, G.; Annunziata, R.; Bianchi, C.L.; Meroni, D.; Ardizzone, S. Siloxane-TiO2 hybrid nanocomposites. The structure of the hydrophobic layer. J. Phys. Chem. C 2010, 114, 8287–8293. [Google Scholar] [CrossRef]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M.-A. Functionalized TiO2 nanoparticles by single-step hydrothermal synthesis: The role of the silane coupling agents. Beilstein, J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A.; MacKenzie, K.J.D. Effect of silica additive on the anatase-to-rutile phase transition. Ceram. Soc. 2001, 84, 1591–1596. [Google Scholar] [CrossRef]
- Cheng, P.; Zheng, M.; Jin, Y.; Huang, Q.; Gu, M. Preparation and characterization of silica-doped titania photocatalyst through sol–gel method. Mater. Lett. 2003, 57, 2989–2994. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I. Effect of calcination temperatures of CaO/Nb2O5 mixed oxides catalysts on biodiesel production. Sains Malays. 2014, 43, 783–790. [Google Scholar]
- Galaburdaa, M.V.; Bogatyrova, V.M.; Skubiszewska-Zieba, J.; Oranskaa, O.I.; Sternik, D.; Gun’ko, V.M. Synthesis and structural features of resorcinol-formaldehyde resin chars containing nickel nanoparticles. Appl. Surf. Sci. 2016, 360, 722–730. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, W.P.; Hunter, D.; Singh, R.A. Vaia, Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem. Mat. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Song, Z.; Srinivasakannan, C.; Peng, J. A new highly efficient method for the synthesis of rutile TiO2. J. Alloys Compd. 2014, 585, 75–77. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Kern, F.; Dufner, L.; Dubiel, A.; Antonowicz, M.; Matus, K. Effect of calcination temperature on the phase composition, morphology, and thermal properties of ZrO2 and Al2O3 modified with APTES (3-aminopropyltriethoxysilane). Materials 2021, 14, 6651. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C. 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Rhee, K.Y.; Ryu, S.H. Influence of dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay materials. J. Colloid Interface Sci. 2006, 298, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay: The effect of dispersing media on intercalation and chemical grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, J.; Cui, G.; Shang, X.; Tang, Q.; Wang, J.; Fan, M. Highly efficient and reversible CO2 adsorption by amine-grafted platelet SBA-15 with expanded pore diameters and short mesochannels. Green Chem. 2014, 16, 4009–4016. [Google Scholar] [CrossRef]
- Fauth, D.J.; Gray, M.L.; Pennline, H.W. Investigation of porous silica supported mixed-amine sorbents for post-combustion CO2 capture. Energy Fuels 2012, 26, 2483–2496. [Google Scholar] [CrossRef]
- Zhang, L.; Aziz, N.; Ren, T.; Wang, Z. Influence of Temperature on the Gas Content of Coal and Sorption Modelling 2011 Underground Coal Operators Conference. Available online: https://ro.uow.edu.au/coal/367/ (accessed on 1 November 2022).
- Hauchhum, L.; Mahanta, P.R. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 53, 49–356. [Google Scholar] [CrossRef]






| Sample Name | Anatase in Crystallite Phase [%] | Anatase Crystallite Size [nm] | Rutile in Crystallite Phase [%] | Rutile Crystallite Size [nm] |
|---|---|---|---|---|
| TiO2-Ar-300 °C | 96 | 14 | 4 | 51 |
| TiO2-Ar-500 °C | 95 | 18 | 5 | 41 |
| TiO2-Ar-700 °C | 88 | 22 | 12 | >100 |
| TiO2-4 h-120 °C-100 mM-300 °C | 96 | 15 | 4 | 46 |
| TiO2-4 h-120 °C-100 mM-500 °C | 96 | 15 | 4 | 45 |
| TiO2-4 h-120 °C-100 mM-700 °C | 96 | 18 | 4 | 73 |
| Sample Name | SBET [m2/g] | Vtotala [cm3/g] | Vmicrob [cm3/g] | Vmesoc [cm3/g] | CO2 Adsorption at 0 °C [mmol/g] | CO2 Adsorption at 25 °C [mmol/g] |
|---|---|---|---|---|---|---|
| TiO2-Ar-300 °C | 112 | 0.31 | 0.04 | 0.27 | 0.36 | 0.25 |
| TiO2-Ar-500 °C | 75 | 0.22 | 0.03 | 0.19 | 0.36 | 0.16 |
| TiO2-Ar-700 °C | 23 | 0.10 | 0.01 | 0.09 | 0.09 | 0.07 |
| TiO2-4 h-120 °C-100 mM-300 °C | 178 | 0.26 | 0.06 | 0.20 | 0.47 | 0.29 |
| TiO2-4 h-120 °C-100 mM-500 °C | 153 | 0.28 | 0.06 | 0.22 | 0.56 | 0.29 |
| TiO2-4 h-120 °C-100 mM-700 °C | 108 | 0.27 | 0.04 | 0.23 | 0.33 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanag, A.; Kapica-Kozar, J.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Preliminary Findings on CO2 Capture over APTES-Modified TiO2. Atmosphere 2022, 13, 1878. https://doi.org/10.3390/atmos13111878
Wanag A, Kapica-Kozar J, Sienkiewicz A, Rokicka-Konieczna P, Kusiak-Nejman E, Morawski AW. Preliminary Findings on CO2 Capture over APTES-Modified TiO2. Atmosphere. 2022; 13(11):1878. https://doi.org/10.3390/atmos13111878
Chicago/Turabian StyleWanag, Agnieszka, Joanna Kapica-Kozar, Agnieszka Sienkiewicz, Paulina Rokicka-Konieczna, Ewelina Kusiak-Nejman, and Antoni W. Morawski. 2022. "Preliminary Findings on CO2 Capture over APTES-Modified TiO2" Atmosphere 13, no. 11: 1878. https://doi.org/10.3390/atmos13111878
APA StyleWanag, A., Kapica-Kozar, J., Sienkiewicz, A., Rokicka-Konieczna, P., Kusiak-Nejman, E., & Morawski, A. W. (2022). Preliminary Findings on CO2 Capture over APTES-Modified TiO2. Atmosphere, 13(11), 1878. https://doi.org/10.3390/atmos13111878










