Comparative Analysis of Secondary Organic Aerosol Formation during PM2.5 Pollution and Complex Pollution of PM2.5 and O3 in Chengdu, China
Abstract
1. Introduction
2. Materials and Methods
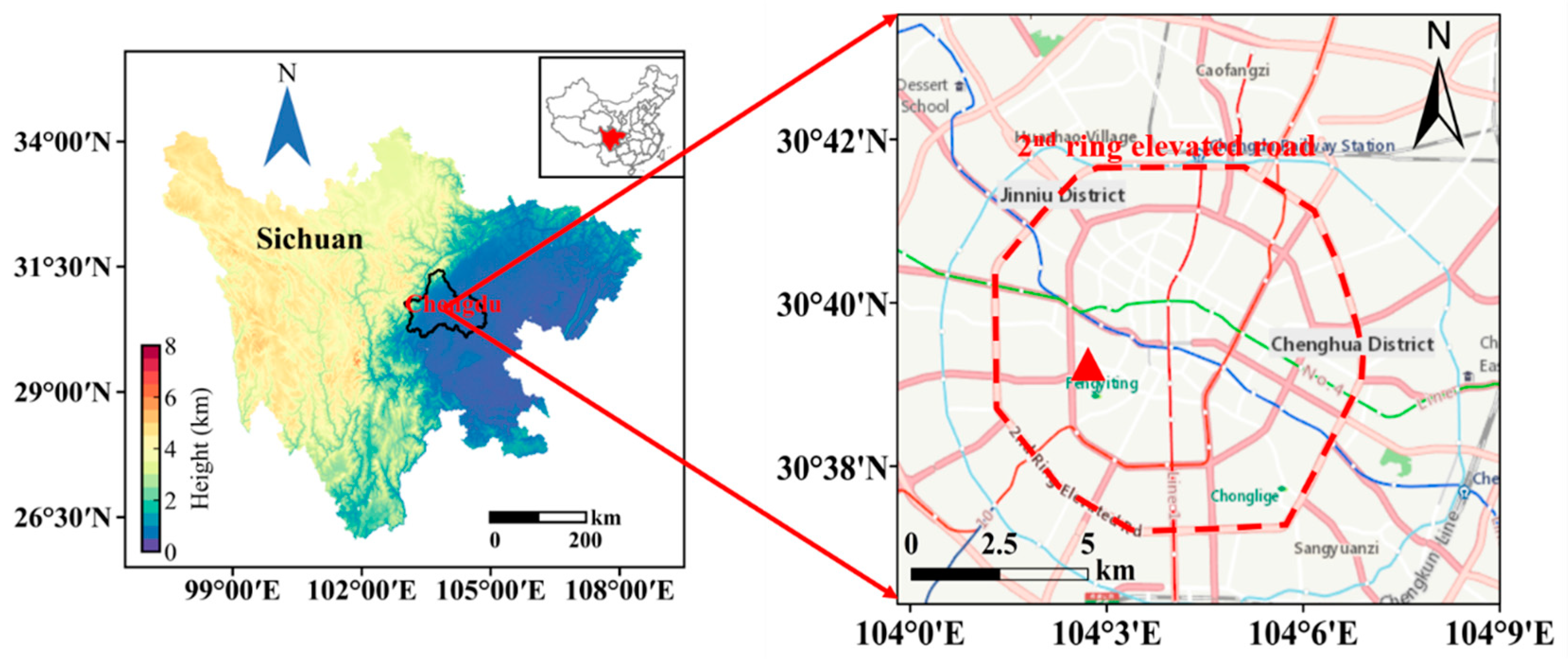
2.1. Sampling Site
2.2. Instruments
2.3. Data Analysis
2.3.1. Estimation of SOA in PM2.5
2.3.2. Estimation of SOA Generation from VOCs
3. Results and Discussion
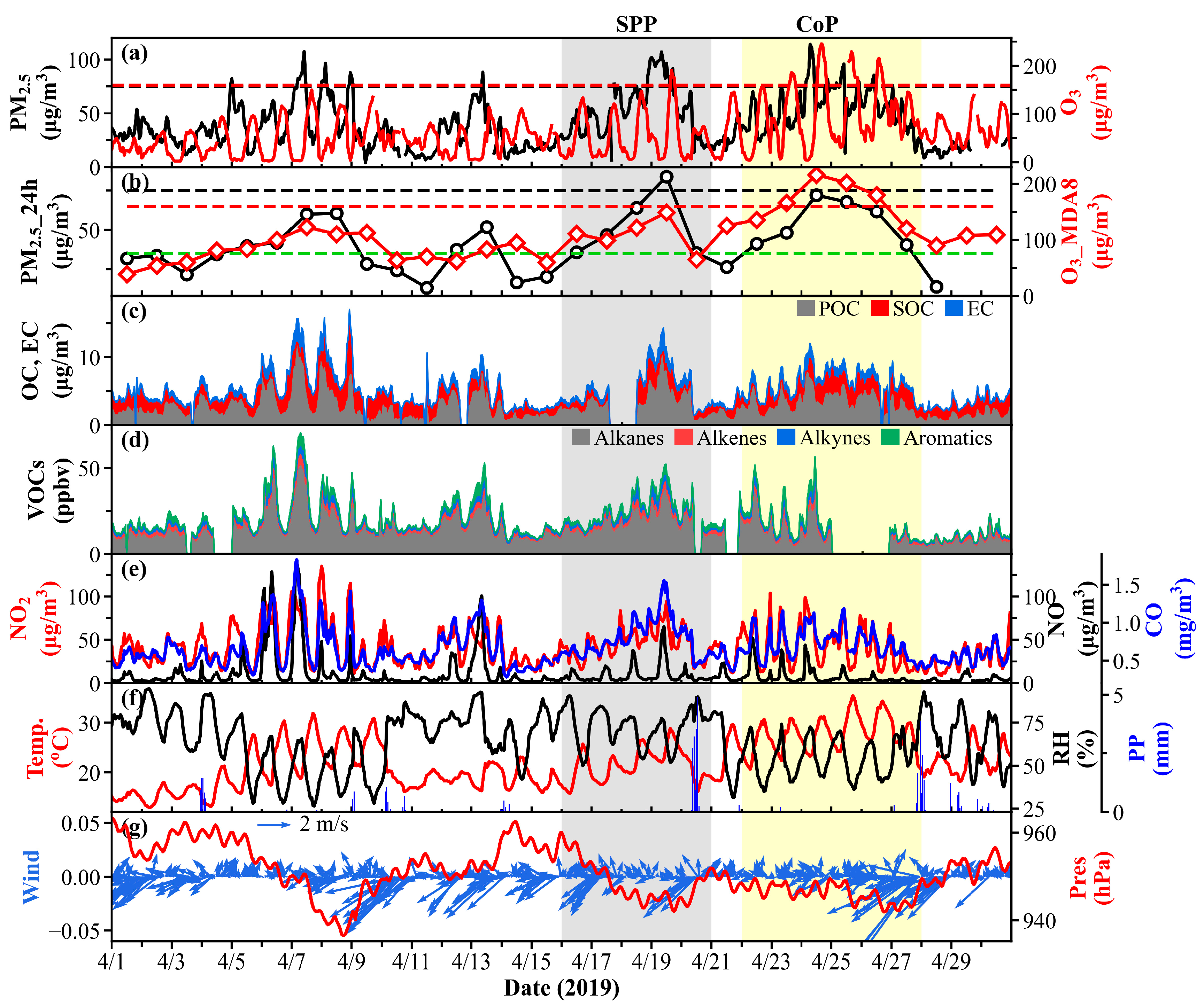
3.1. General Characteristics of Air Pollutants
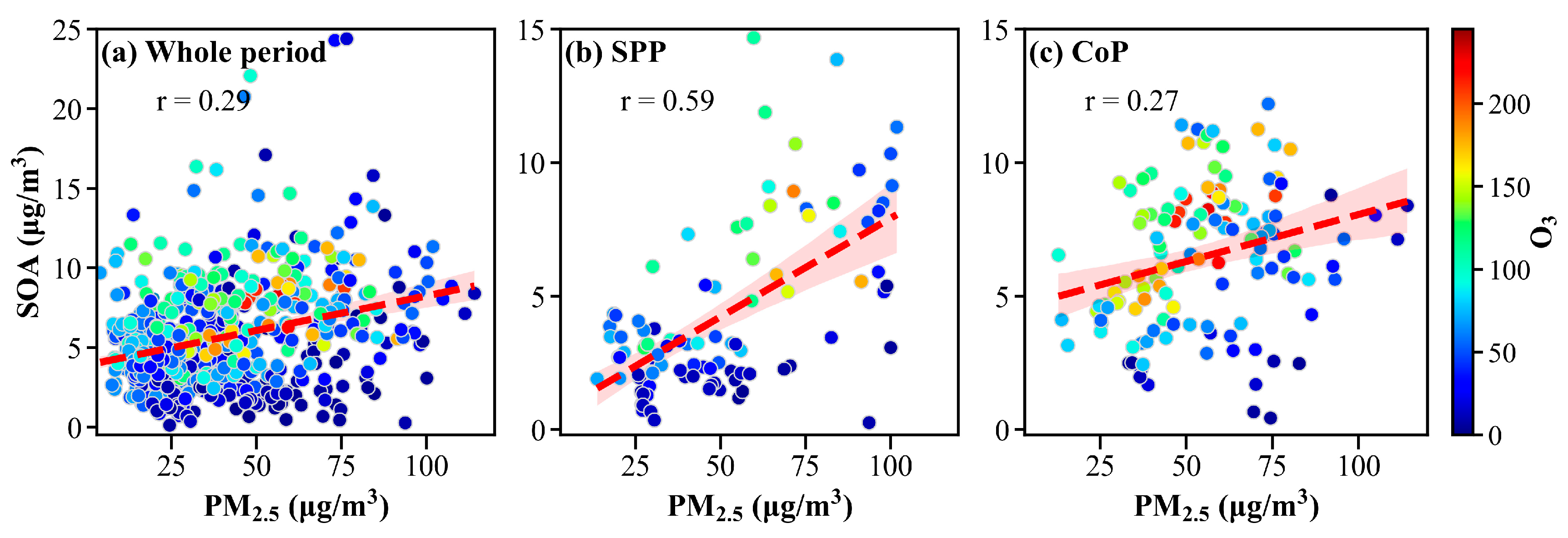
3.2. Characteristics of SOA in PM2.5
3.3. Characteristics of Measured VOCs in Different Pollution Episodes
3.3.1. Measured VOCs Composition and Dominant Species
3.3.2. Diurnal Variations of VOC Groups
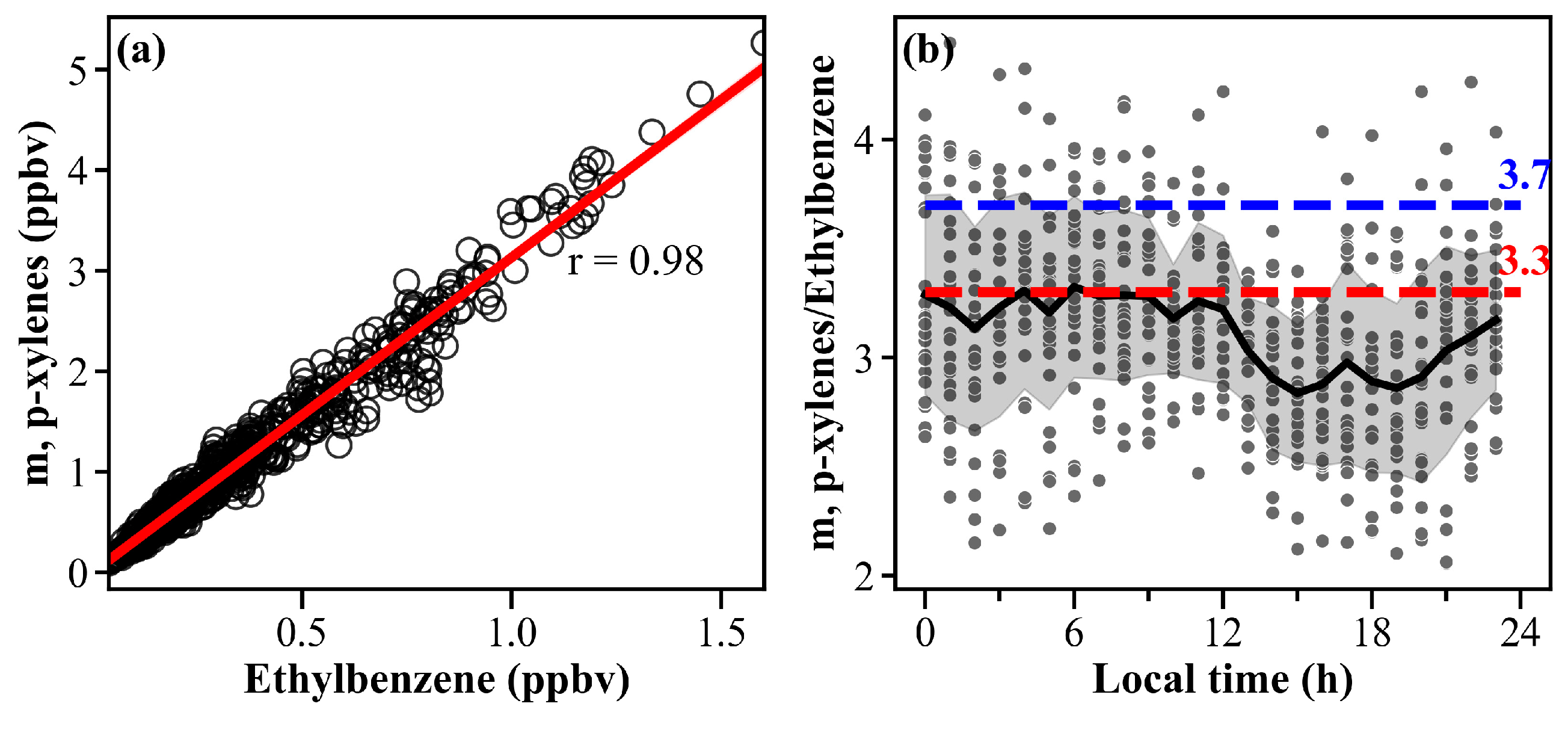
3.3.3. Source Analysis of VOCs Based on Species-Specific Ratios
3.4. Characteristics of Consumed VOCs in Different Pollution Episodes
3.4.1. Composition and Diurnal Patterns of Consumed VOCs
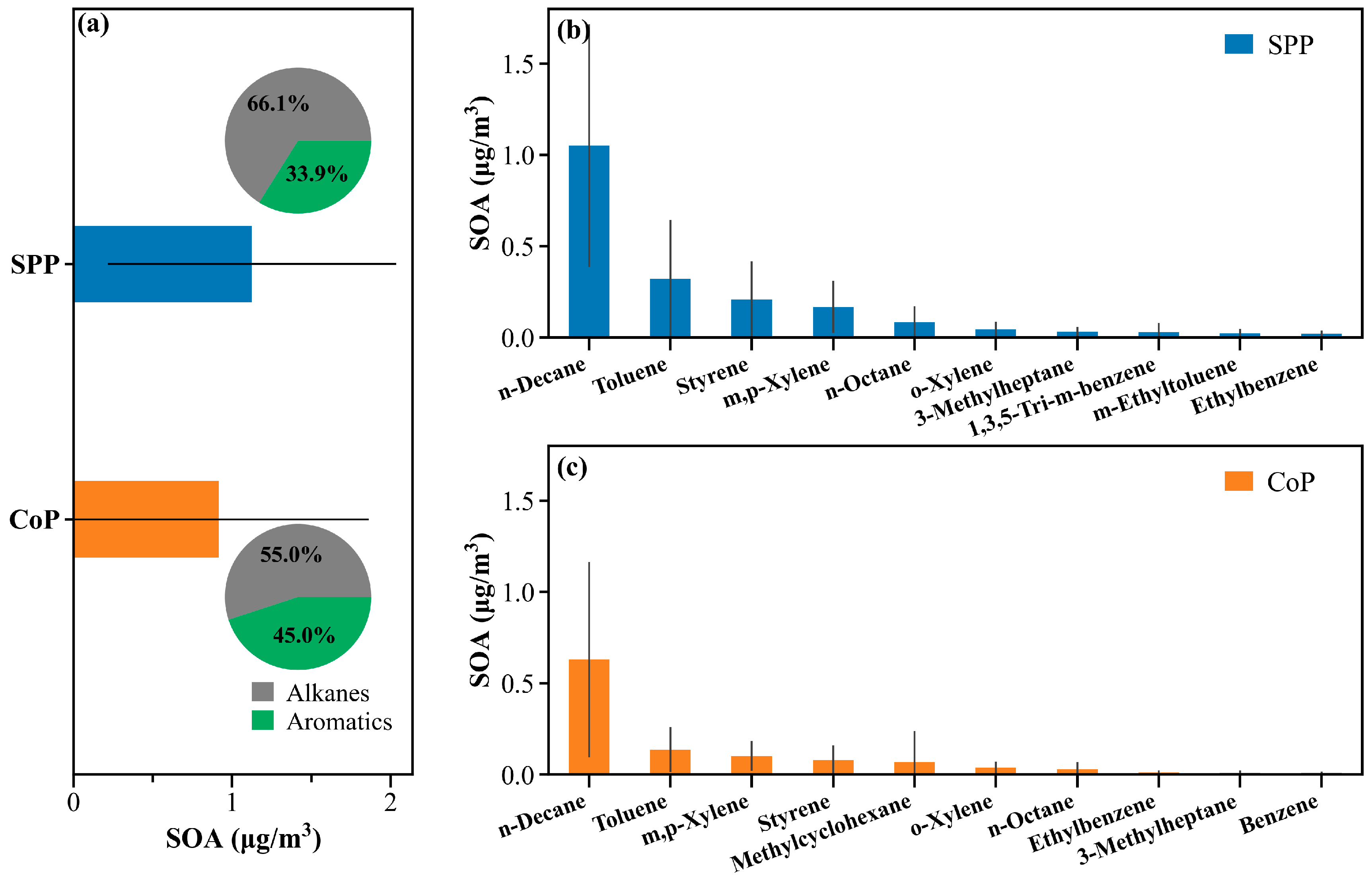
3.4.2. Estimation of SOA Based on VOC Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, C.; Tian, S.; Li, Z.W.; Li, K. Spatiotemporal characteristics of PM2.5 and ozone concentrations in Chinese urban clusters. Chemosphere 2022, 295, 133813. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, K.; Liu, Z.; Zhang, Y.; Shao, T.; Zhang, H. Coordinated control of PM2.5 and O3 is urgently needed in China after implementation of the “Air pollution prevention and control action plan”. Chemosphere 2021, 270, 129441. [Google Scholar] [CrossRef]
- State Council of the People’s Republic of China. Air pollution Prevention and Control Action Plan 2013; State Council of the People’s Republic of China: Beijing, China, 2013.
- State Council of the People’s Republic of China. Three-Year Action Plan of Win the Blue Sky Defence War 2018; State Council of the People’s Republic of China: Beijing, China, 2018.
- Zhai, S.; Jacob, D.J.; Wang, X.; Shen, L.; Li, K.; Zhang, Y.; Gui, K.; Zhao, T.; Liao, H. Fine particulate matter (PM2.5) trends in China, 2013–2018: Separating contributions from anthropogenic emissions and meteorology. Atmos. Chem. Phys. 2019, 19, 11031–11041. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Zhu, J.; Shah, V.; Shen, L.; Bates, K.H.; Zhang, Q.; Zhai, S. A two-pollutant strategy for improving ozone and particulate air quality in China. Nat. Geosci. 2019, 12, 906–910. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, Y.; Yin, D.; Qin, D.; He, J.; Dong, L. Spatial patterns and temporal variations of six criteria air pollutants during 2015 to 2017 in the city clusters of Sichuan Basin, China. Sci. Total Environ. 2018, 624, 540–557. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, J.; Liao, H.; Li, J.; Liang, M.; Yang, Y.; Yue, X. Co-occurrence of ozone and PM2.5 pollution in the Yangtze River Delta over 2013–2019: Spatiotemporal distribution and meteorological conditions. Atmos. Res. 2021, 249, 105363. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Wang, H.; Xu, X.; Li, Y.; Fan, S. A cold front induced co-occurrence of O3 and PM2.5 pollution in a Pearl River Delta city: Temporal variation, vertical structure, and mechanism. Environ. Pollut. 2022, 306, 119464. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, T.; Yang, Y.; Zong, L.; Kumar, K.R.; Wang, H.; Meng, K.; Zhang, L.; Lu, S.; Xin, Y. Seasonal changes in the recent decline of combined high PM2.5 and O3 pollution and associated chemical and meteorological drivers in the Beijing–Tianjin–Hebei region, China. Sci. Total Environ. 2022, 838, 156312. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Gao, Y.; Zhou, M.; Jing, S.; Qiao, L.; Yuan, B.; Huang, D.; Huang, C.; Lou, S.; et al. Estimation of Secondary Organic Aerosol Formation During a Photochemical Smog Episode in Shanghai, China. J. Geophys. Res. Atmos. 2020, 125, e2019JD032033. [Google Scholar] [CrossRef]
- Li, Q.; Su, G.; Li, C.; Liu, P.; Zhao, X.; Zhang, C.; Sun, X.; Mu, Y.; Wu, M.; Wang, Q.; et al. An investigation into the role of VOCs in SOA and ozone production in Beijing, China. Sci. Total Environ. 2020, 720, 137536. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef]
- Lu, K.; Guo, S.; Tan, Z.; Wang, H.; Shang, D.; Liu, Y.; Li, X.; Wu, Z.; Hu, M.; Zhang, Y. Exploring atmospheric free-radical chemistry in China: The self-cleansing capacity and the formation of secondary air pollution. Natl. Sci. Rev. 2019, 6, 579–594. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC / EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Guo, Q.; Shang, D. Comparison of Secondary Organic Aerosol Estimation Methods. Acta Chim. Sinica 2014, 72, 658–666. [Google Scholar] [CrossRef]
- Ren, H.; Hu, W.; Wei, L.; Yue, S.; Zhao, J.; Li, L.; Wu, L.; Zhao, W.; Ren, L.; Kang, M.; et al. Measurement report: Vertical distribution of biogenic and anthropogenic secondary organic aerosols in the urban boundary layer over Beijing during late summer. Atmos. Chem. Phys. 2021, 21, 12949–12963. [Google Scholar] [CrossRef]
- Yang, C.; Hong, Z.; Chen, J.; Xu, L.; Zhuang, M.; Huang, Z. Characteristics of secondary organic aerosols tracers in PM2.5 in three central cities of the Yangtze river delta, China. Chemosphere 2022, 293, 133637. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Chen, N.; Niu, Z.; Zhang, Y.; Jiang, S.; Yan, Y.; Qi, S. Source apportionment of volatile organic compounds: Implications to reactivity, ozone formation, and secondary organic aerosol potential. Atmos. Res. 2021, 249, 105344. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, D.; Yu, Y.; Kang, S.; Qin, D.; Dong, L. PM2.5 and O3 pollution during 2015–2019 over 367 Chinese cities: Spatiotemporal variations, meteorological and topographical impacts. Environ. Pollut. 2020, 264, 114694. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.K.; Luo, B.; Wang, L.L.; Tang, G.Q.; Liu, Z.R.; Song, H.Y.; Zhang, W.; Yuan, L.; Wang, Y.S. Water-soluble ions in PM2.5 during spring haze and dust periods in Chengdu, China: Variations, nitrate formation and potential source areas. Environ. Pollut. 2018, 243, 1740–1749. [Google Scholar] [CrossRef]
- Li, L.; Tan, Q.W.; Zhang, Y.H.; Feng, M.; Qu, Y.; An, J.L.; Liu, X.G. Characteristics and source apportionment of PM2.5 during persistent extreme haze events in Chengdu, southwest China. Environ. Pollut. 2017, 230, 718–729. [Google Scholar] [CrossRef]
- Tian, M.; Liu, Y.; Yang, F.; Zhang, L.; Peng, C.; Chen, Y.; Shi, G.; Wang, H.; Luo, B.; Jiang, C.; et al. Increasing importance of nitrate formation for heavy aerosol pollution in two megacities in Sichuan Basin, southwest China. Environ. Pollut. 2019, 250, 898–905. [Google Scholar] [CrossRef]
- Song, T.; Feng, M.; Song, D.; Zhou, L.; Qiu, Y.; Tan, Q.; Yang, F. Enhanced nitrate contribution during winter haze events in a megacity of Sichuan Basin, China: Formation mechanism and source apportionment. J. Clean. Prod. 2022, 370, 133272. [Google Scholar] [CrossRef]
- Yang, X.; Wu, K.; Wang, H.; Liu, Y.; Gu, S.; Lu, Y.; Zhang, X.; Hu, Y.; Ou, Y.; Wang, S.; et al. Summertime ozone pollution in Sichuan Basin, China: Meteorological conditions, sources and process analysis. Atmos. Environ. 2020, 226, 117392. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zu, K.; Lian, C.; Chen, S.; Dong, H.; Feng, M.; Liu, H.; Liu, J.; Lu, K.; et al. Elucidating the effect of HONO on O3 pollution by a case study in southwest China. Sci. Total Environ. 2021, 756, 144127. [Google Scholar] [CrossRef]
- Tan, Z.; Lu, K.D.; Jiang, M.Q.; Su, R.; Dong, H.B.; Zeng, L.M.; Xie, S.D.; Tan, Q.W.; Zhang, Y.H. Exploring ozone pollution in Chengdu, southwestern China: A case study from radical chemistry to O3-VOC-NOx sensitivity. Sci. Total Environ. 2018, 636, 775–786. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, Y.Q.; Wu, R.R.; Xie, S.D. Characteristics of volatile organic compounds, NO2, and effects on ozone formation at a site with high ozone level in Chengdu. J. Environ. Sci. 2019, 75, 334–345. [Google Scholar] [CrossRef]
- Kong, L.; Feng, M.; Liu, Y.; Zhang, Y.; Zhang, C.; Li, C.; Qu, Y.; An, J.; Liu, X.; Tan, Q.; et al. Elucidating the pollution characteristics of nitrate, sulfate and ammonium in PM2.5 in Chengdu, southwest China, based on 3-year measurements. Atmos. Chem. Phys. 2020, 20, 11181–11199. [Google Scholar] [CrossRef]
- Kong, L.; Tan, Q.W.; Feng, M.; Qu, Y.; An, J.L.; Liu, X.G.; Cheng, N.L.; Deng, Y.J.; Zhai, R.X.; Wang, Z. Investigating the characteristics and source analyses of PM2.5 seasonal variations in Chengdu, Southwest China. Chemosphere 2020, 243, 125267. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, N.; Zhou, L.; Yang, F.; Qiu, Y.; Chen, J.; Han, L.; Li, J. Component characteristics and source apportionment of volatile organic compounds during summer and winter in downtown Chengdu, southwest China. Atmos. Environ. 2021, 258, 118485. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, Y.; Qin, D.H.; Yin, D.Y.; Dong, L.X.; He, J.J. Analyses of regional pollution and transportation of PM2.5 and ozone in the city clusters of Sichuan Basin, China. Atmos. Pollut. Res. 2019, 10, 374–385. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.G.; Tan, Q.W.; Feng, M.; Qu, Y.; An, J.L.; Zhang, Y.H. Characteristics and formation mechanism of persistent extreme haze pollution events in Chengdu, southwestern China. Environ. Pollut. 2019, 251, 1–12. [Google Scholar] [CrossRef]
- Song, M.; Tan, Q.; Feng, M.; Qu, Y.; Liu, X.; An, J.; Zhang, Y. Source Apportionment and Secondary Transformation of Atmospheric Nonmethane Hydrocarbons in Chengdu, Southwest China. J. Geophys. Res. Atmos. 2018, 123, 9741–9763. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos. Environ. 1995, 29, 3527–3544. [Google Scholar] [CrossRef]
- Hu, W.; Hu, M.; Hu, W.-W.; Niu, H.; Zheng, J.; Wu, Y.; Chen, W.; Chen, C.; Li, L.; Shao, M.; et al. Characterization of submicron aerosols influenced by biomass burning at a site in the Sichuan Basin, southwestern China. Atmos. Chem. Phys. 2016, 16, 13213–13230. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ng, N.L.; Kroll, J.H.; Chan, A.W.H.; Chhabra, P.S.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 2007, 7, 3909–3922. [Google Scholar] [CrossRef]
- Lim, Y.B.; Ziemann, P.J. Effects of Molecular Structure on Aerosol Yields from OH Radical-Initiated Reactions of Linear, Branched, and Cyclic Alkanes in the Presence of NOx. Environ. Sci. Technol. 2009, 43, 2328–2334. [Google Scholar] [CrossRef]
- Zhang, X.; Cappa, C.D.; Jathar, S.H.; McVay, R.C.; Ensberg, J.J.; Kleeman, M.J.; Seinfeld, J.H. Influence of vapor wall loss in laboratory chambers on yields of secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2014, 111, 5802–5807. [Google Scholar] [CrossRef]
- Shao, M.; Wang, B.; Lu, S.; Yuan, B.; Wang, M. Effects of Beijing Olympics Control Measures on Reducing Reactive Hydrocarbon Species. Environ. Sci. Technol. 2011, 45, 514–519. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Wang, S.; Wang, W.; Yuan, B.; Qi, J.; Wang, B.; Wang, H.; Wang, C.; Song, W.; et al. Measurement report: Important contributions of oxygenated compounds to emissions and chemistry of volatile organic compounds in urban air. Atmos. Chem. Phys. 2020, 20, 14769–14785. [Google Scholar] [CrossRef]
- Zou, Y.; Charlesworth, E.; Wang, N.; Flores, R.M.; Liu, Q.Q.; Li, F.; Deng, T.; Deng, X.J. Characterization and ozone formation potential (OFP) of non-methane hydrocarbons under the condition of chemical loss in Guangzhou, China. Atmos. Environ. 2021, 262, 118630. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, W.W.; Shao, M.; Wang, M.; Chen, W.T.; Lu, S.H.; Zeng, L.M.; Hu, M. VOC emissions, evolutions and contributions to SOA formation at a receptor site in eastern China. Atmos. Chem. Phys. 2013, 13, 8815–8832. [Google Scholar] [CrossRef]
- Wang, H.; Tian, M.; Chen, Y.; Shi, G.; Liu, Y.; Yang, F.; Zhang, L.; Deng, L.; Yu, J.; Peng, C.; et al. Seasonal characteristics, formation mechanisms and source origins of PM2.5 in two megacities in Sichuan Basin, China. Atmos. Chem. Phys. 2018, 18, 865–881. [Google Scholar] [CrossRef]
- Sbai, S.E.; Li, C.; Boreave, A.; Charbonnel, N.; Perrier, S.; Vernoux, P.; Bentayeb, F.; George, C.; Gil, S. Atmospheric photochemistry and secondary aerosol formation of urban air in Lyon, France. J. Environ. Sci. 2021, 99, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, S.; Li, G.; Lu, Z.; Song, H.; Gao, J.; Sun, Z.; Xu, K. VOCs characteristics and their ozone and SOA formation potentials in autumn and winter at Weinan, China. Environ. Res. 2022, 203, 111821. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Bao, Y.; Xiang, F.; Wang, Z.; Ren, L.; Pang, X.; Wang, J.; Han, X.; Ning, P. Pollution Characteristics and Health Risk Assessment of VOCs in Jinghong. Atmosphere 2022, 13, 613. [Google Scholar] [CrossRef]
- Niu, Y.; Yan, Y.; Chai, J.; Zhang, X.; Xu, Y.; Duan, X.; Wu, J.; Peng, L. Effects of regional transport from different potential pollution areas on volatile organic compounds (VOCs) in Northern Beijing during non-heating and heating periods. Sci. Total Environ. 2022, 836, 155465. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Wu, Z.; Zhang, W.; Liu, K.; Cheng, X.; Zhang, Y.; Li, B.; Chen, Y. Characteristics of atmospheric volatile organic compounds in urban area of Beijing: Variations, photochemical reactivity and source apportionment. J. Environ. Sci. 2020, 95, 190–200. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.; Kumar, K.; Singh, B.B.; Jain, V.K. Distribution of VOCs in urban and rural atmospheres of subtropical India: Temporal variation, source attribution, ratios, OFP and risk assessment. Sci. Total Environ. 2018, 613–614, 492–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Yuan, M.; Zhang, R.; Zhang, M.; Yu, S.; Li, Y. Characteristics and source apportionment of ambient VOC in spring in Zhengzhou. Environ. Sci. 2019, 40, 4372–4381. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, L.; Li, R.; Li, Y.; Li, L.; Bai, H. Concentration, ozone formation potential and source analysis of volatile organic compounds (VOCs) in a thermal power station centralized area: A study in Shuozhou, China. Environ. Pollut. 2017, 223, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kong, S.; Xing, X.; Mao, Y.; Hu, T.; Ding, Y.; Li, G.; Liu, D.; Li, S.; Qi, S. Monitoring of volatile organic compounds (VOCs) from an oil and gas station in northwest China for 1 year. Atmos. Chem. Phys. 2018, 18, 4567–4595. [Google Scholar] [CrossRef]
- Zhan, J.; Feng, Z.; Liu, P.; He, X.; He, Z.; Chen, T.; Wang, Y.; He, H.; Mu, Y.; Liu, Y. Ozone and SOA formation potential based on photochemical loss of VOCs during the Beijing summer. Environ. Pollut. 2021, 285, 117444. [Google Scholar] [CrossRef] [PubMed]
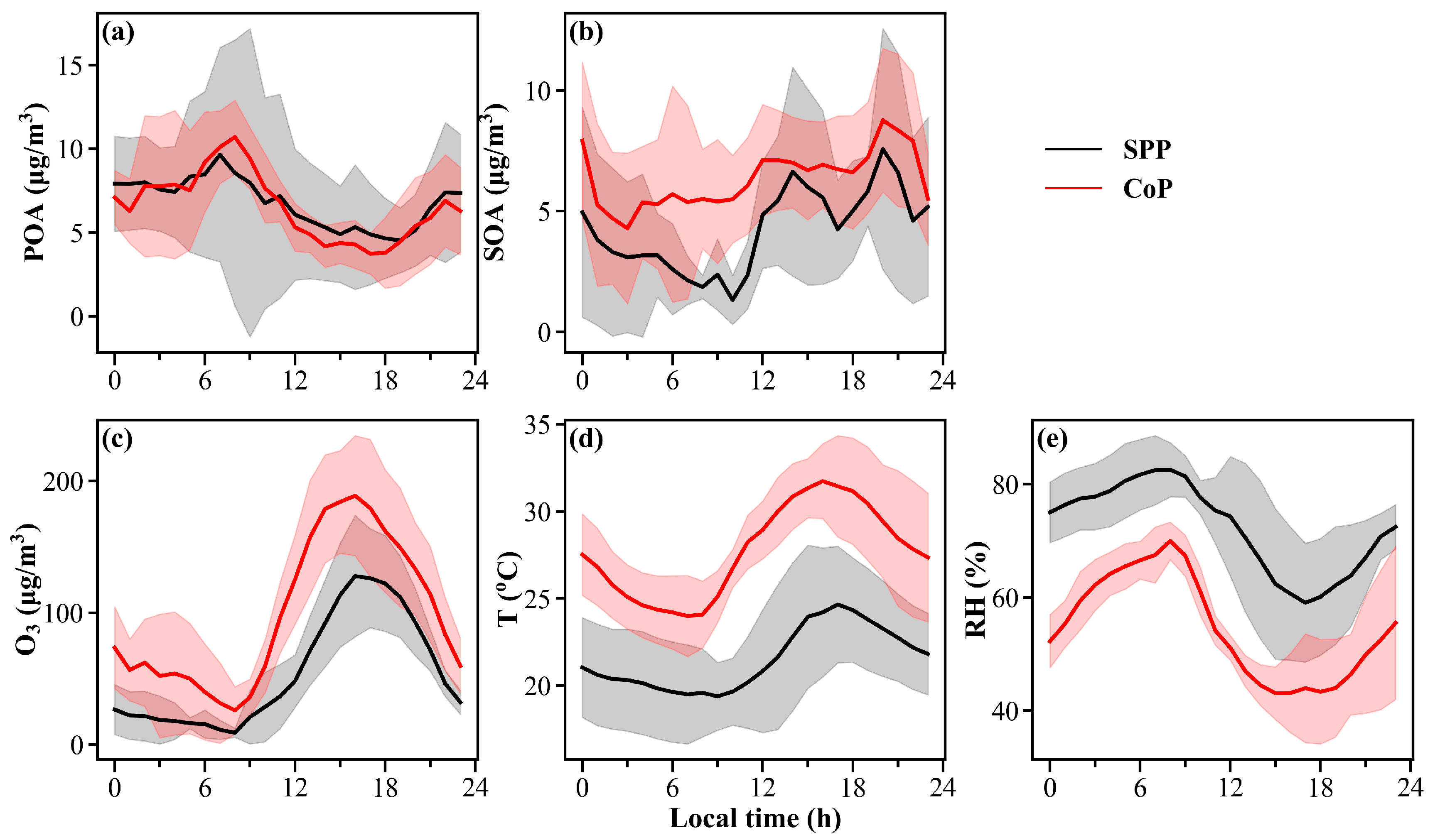
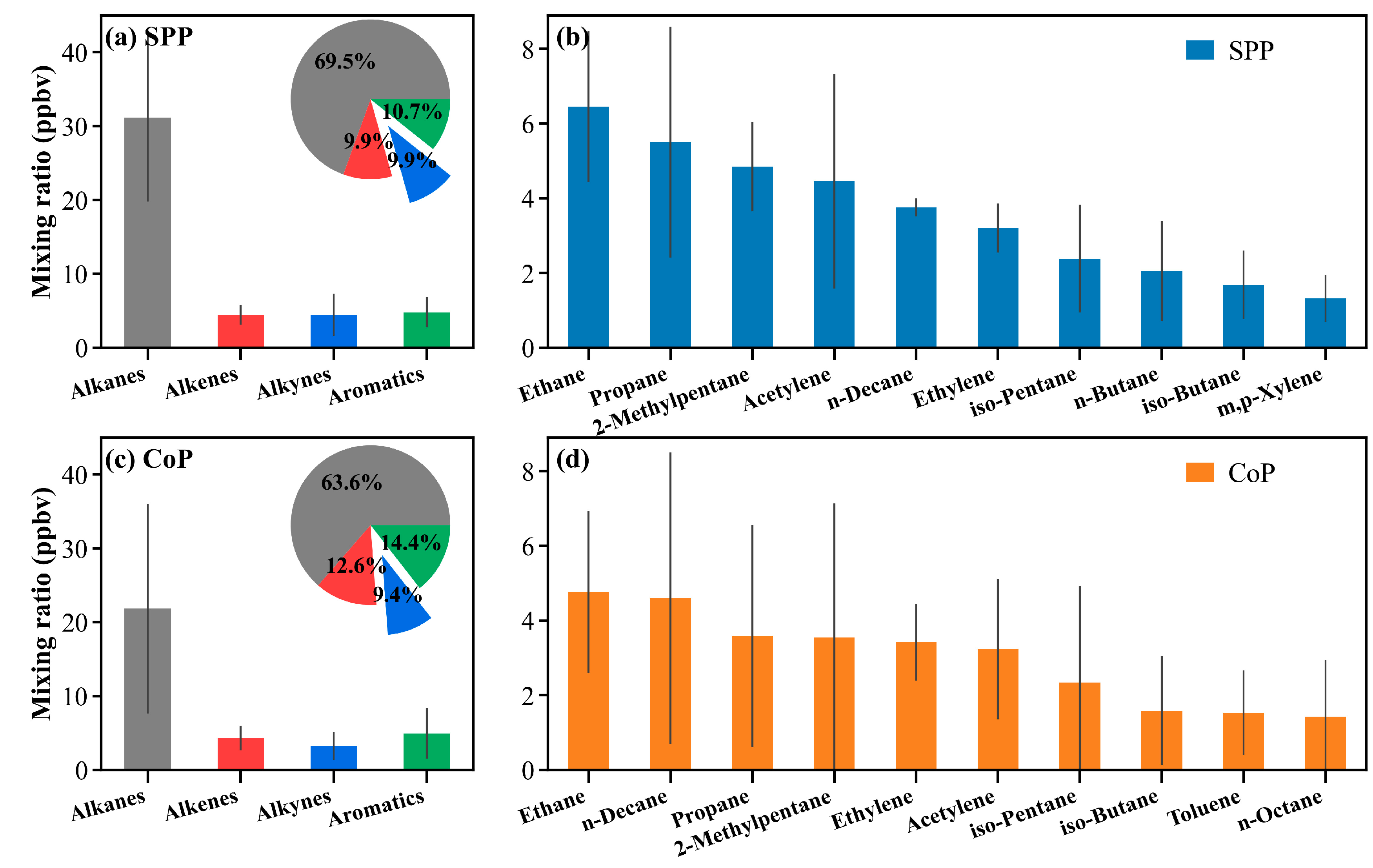

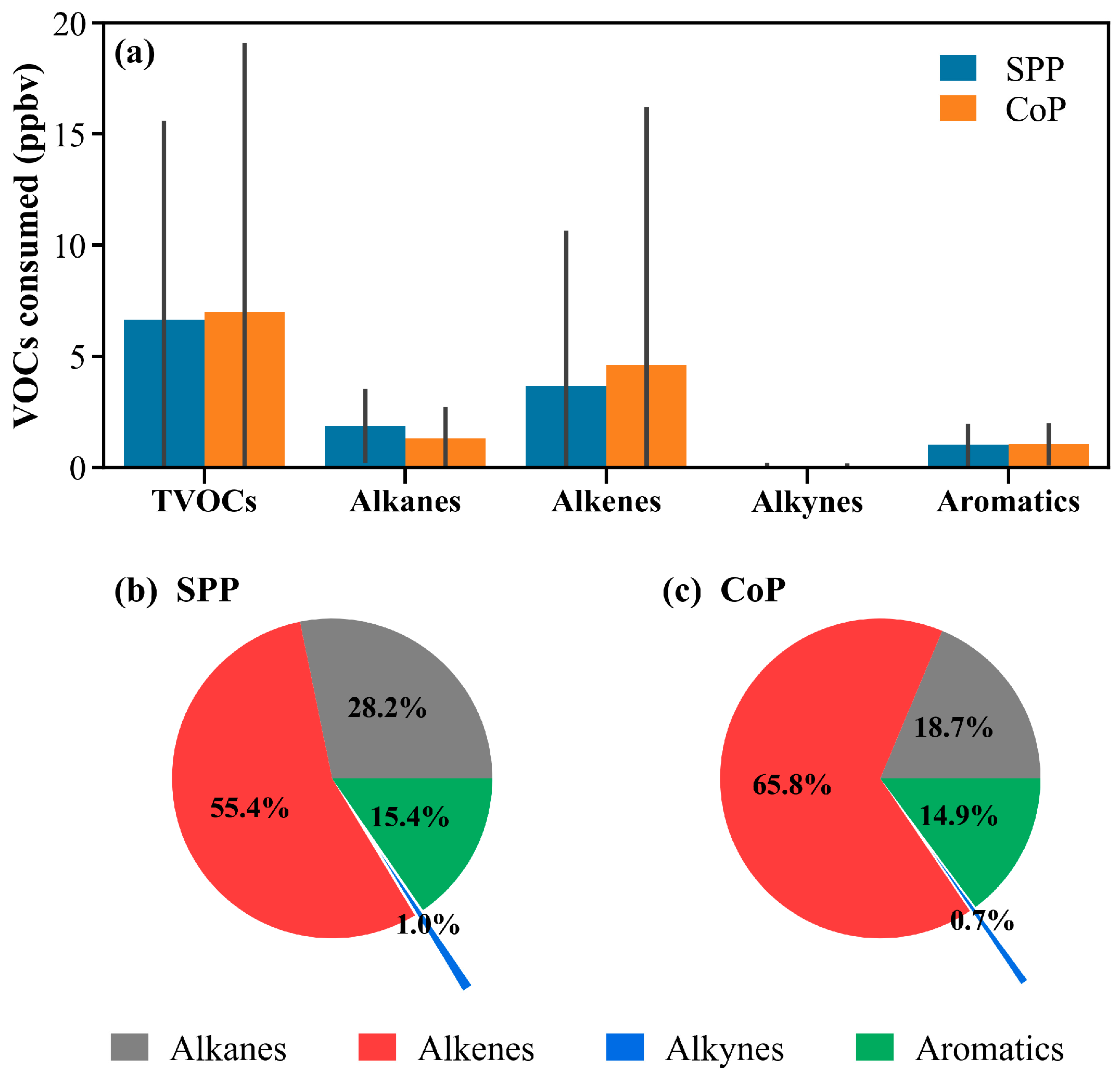
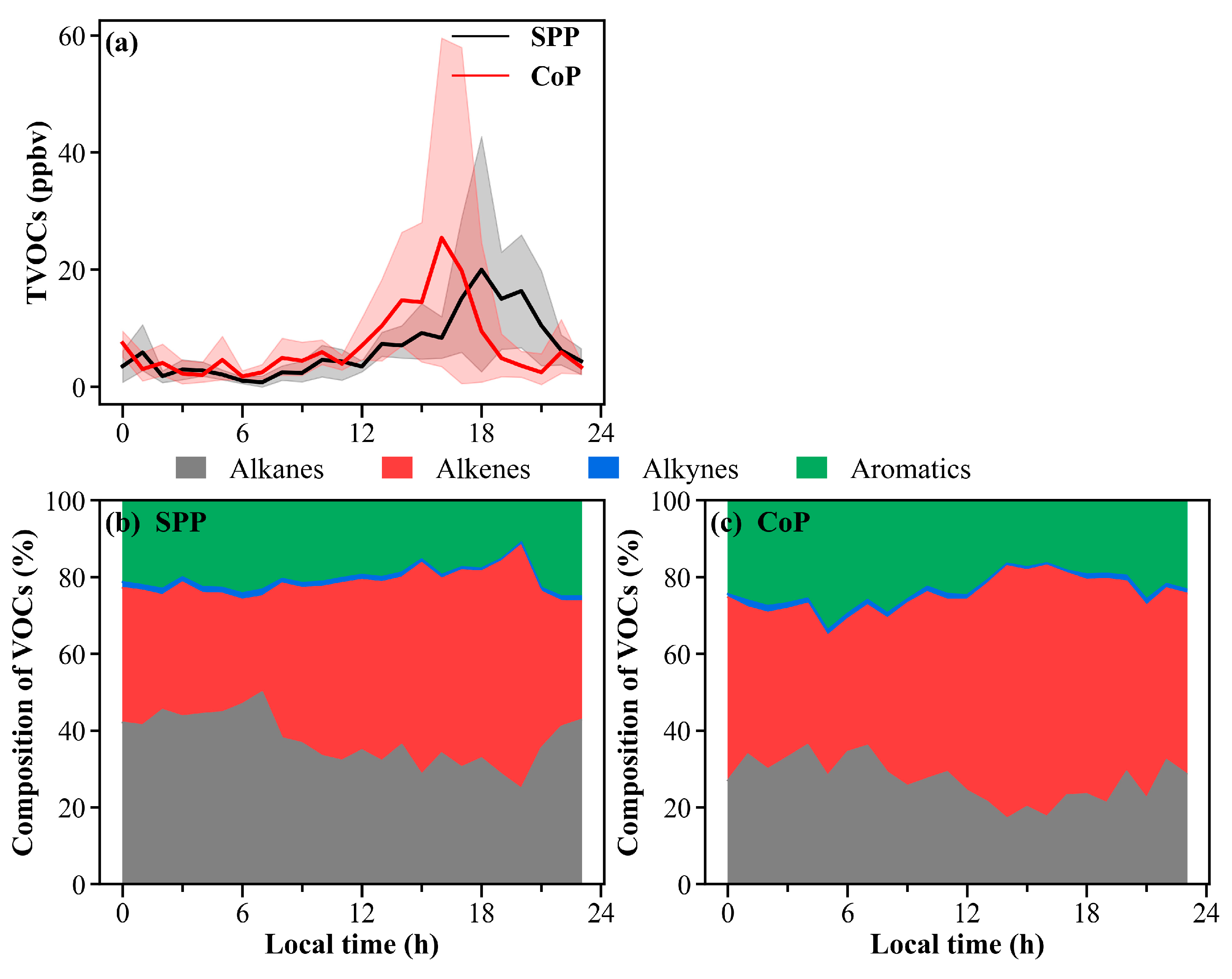
| Whole Period (1–30 April 2019) 1 | SPP (16–20 April 2019) 1 | CoP (22–27 April 2019) 1 | |
|---|---|---|---|
| Air pollutants | |||
| PM2.5 (μg/m3) | 40.6 ± 22.9 | 53.2 ± 24.7 | 55.3 ± 19.6 |
| O3 (μg/m3) | 59.9 ± 47.8 | 54.3 ± 46.7 | 97.7 ± 63.9 |
| MDA8 O3 (μg/m3) | 106.5 ± 42.6 | 109.2 ± 27.6 | 170.0 ± 33.9 |
| NO2 (μg/m3) | 41.2 ± 22.3 | 46.1 ± 17.1 | 43.0 ± 23.6 |
| NO (μg/m3) | 10.0 ± 18.1 | 9.4 ± 11.0 | 7.5 ± 9.9 |
| CO (mg/m3) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| Carbonaceous components in PM2.5 | |||
| POA (μg/m3) | 5.3 ± 3.7 | 6.8 ± 4.1 | 6.6 ± 3.1 |
| SOA (μg/m3) | 5.6 ± 3.2 | 4.3 ± 3.2 | 6.5 ± 2.6 |
| EC (μg/m3) | 1.1 ± 0.8 | 1.5 ± 0.9 | 1.4 ± 0.7 |
| Measured VOCs | |||
| TVOCs (ppbv) 2 | 36.2 ± 19.8 | 44.8 ± 16.1 | 34.4 ± 20.0 |
| Alkanes (ppbv) | 24.5 ± 14.1 | 31.1 ± 11.4 | 21.8 ± 14.2 |
| Alkenes (ppbv) | 4.1 ± 1.7 | 4.4 ± 1.3 | 4.3 ± 1.7 |
| Alkynes (ppbv) | 3.1 ± 2.2 | 4.5 ± 2.9 | 3.2 ± 1.9 |
| Aromatics (ppbv) | 4.4 ± 3.1 | 4.8 ± 2.1 | 5.0 ± 3.4 |
| Meteorological parameters | |||
| T (°C) | 22.1 ± 4.9 | 21.5 ± 3.2 | 27.7 ± 3.4 |
| Tmax (°C) 3 | 26.3 ± 5.0 | 25.7 ± 1.9 | 32.2 ± 1.9 |
| RH (%) | 63.9 ± 16.1 | 72.4 ± 10.7 | 54.6 ± 10.8 |
| WS (m/s) | 1.1 ± 0.9 | 1.0 ± 0.7 | 1.2 ± 1.2 |
| Benzene/Toluene | Isopentane/n-Pentane | |
|---|---|---|
| This study | ||
| SPP (2019.4.16–4.20) | 0.86 ± 1.57 1 | 2.65 ± 0.70 2 |
| CoP (2019.4.22–4.27) | 0.75 ± 0.60 1 | 3.42 ± 1.12 2 |
| Previous studies | ||
| Solvent usage | 0.00–0.20 3 | |
| Biomass burning | 2.50 3 | |
| Vehicle emission | 0.56–0.60 3 | 2.93 4 |
| Coal combustion | 1.05–2.20 3 | 0.56–0.80 4 |
| Natural gas | 0.82-0.89 5 | |
| Liquid gasoline | 1.50–3.00 5 | |
| Fuel volatilization | 1.84–4.46 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Feng, M.; Song, D.; Liu, S.; Tan, Q.; Wang, Y.; Luo, Y.; Chen, X.; Yang, F. Comparative Analysis of Secondary Organic Aerosol Formation during PM2.5 Pollution and Complex Pollution of PM2.5 and O3 in Chengdu, China. Atmosphere 2022, 13, 1834. https://doi.org/10.3390/atmos13111834
Song T, Feng M, Song D, Liu S, Tan Q, Wang Y, Luo Y, Chen X, Yang F. Comparative Analysis of Secondary Organic Aerosol Formation during PM2.5 Pollution and Complex Pollution of PM2.5 and O3 in Chengdu, China. Atmosphere. 2022; 13(11):1834. https://doi.org/10.3390/atmos13111834
Chicago/Turabian StyleSong, Tianli, Miao Feng, Danlin Song, Song Liu, Qinwen Tan, Yuancheng Wang, Yina Luo, Xi Chen, and Fumo Yang. 2022. "Comparative Analysis of Secondary Organic Aerosol Formation during PM2.5 Pollution and Complex Pollution of PM2.5 and O3 in Chengdu, China" Atmosphere 13, no. 11: 1834. https://doi.org/10.3390/atmos13111834
APA StyleSong, T., Feng, M., Song, D., Liu, S., Tan, Q., Wang, Y., Luo, Y., Chen, X., & Yang, F. (2022). Comparative Analysis of Secondary Organic Aerosol Formation during PM2.5 Pollution and Complex Pollution of PM2.5 and O3 in Chengdu, China. Atmosphere, 13(11), 1834. https://doi.org/10.3390/atmos13111834






