Long-Term Warming and Nitrogen Addition Regulate Responses of Dark Respiration and Net Photosynthesis in Boreal Bog Plants to Short-Term Increases in CO2 and Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Foliar Photosynthesis and Dark Respiration Measurements
2.4. Environmental Measurements
2.5. Statistical Analysis
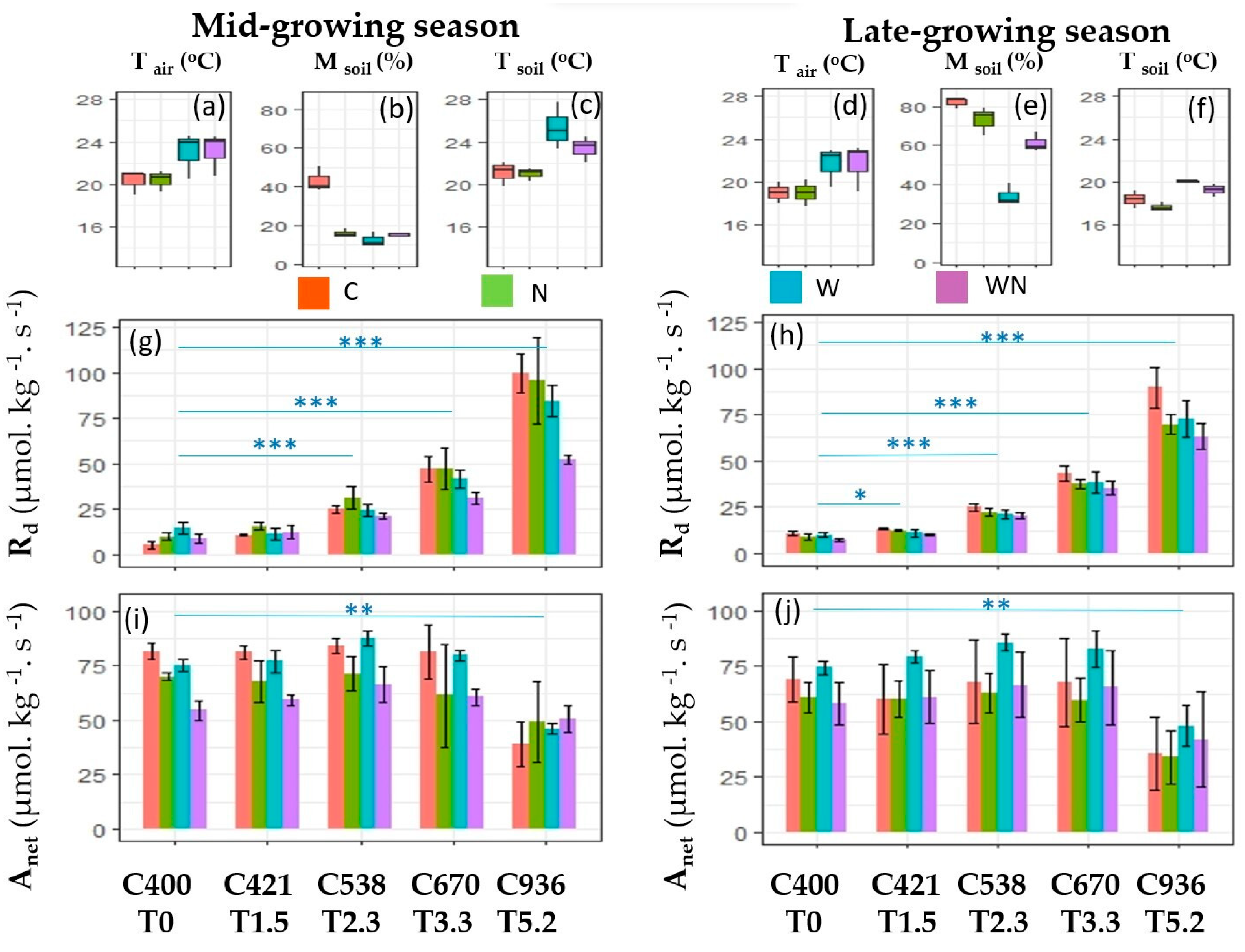
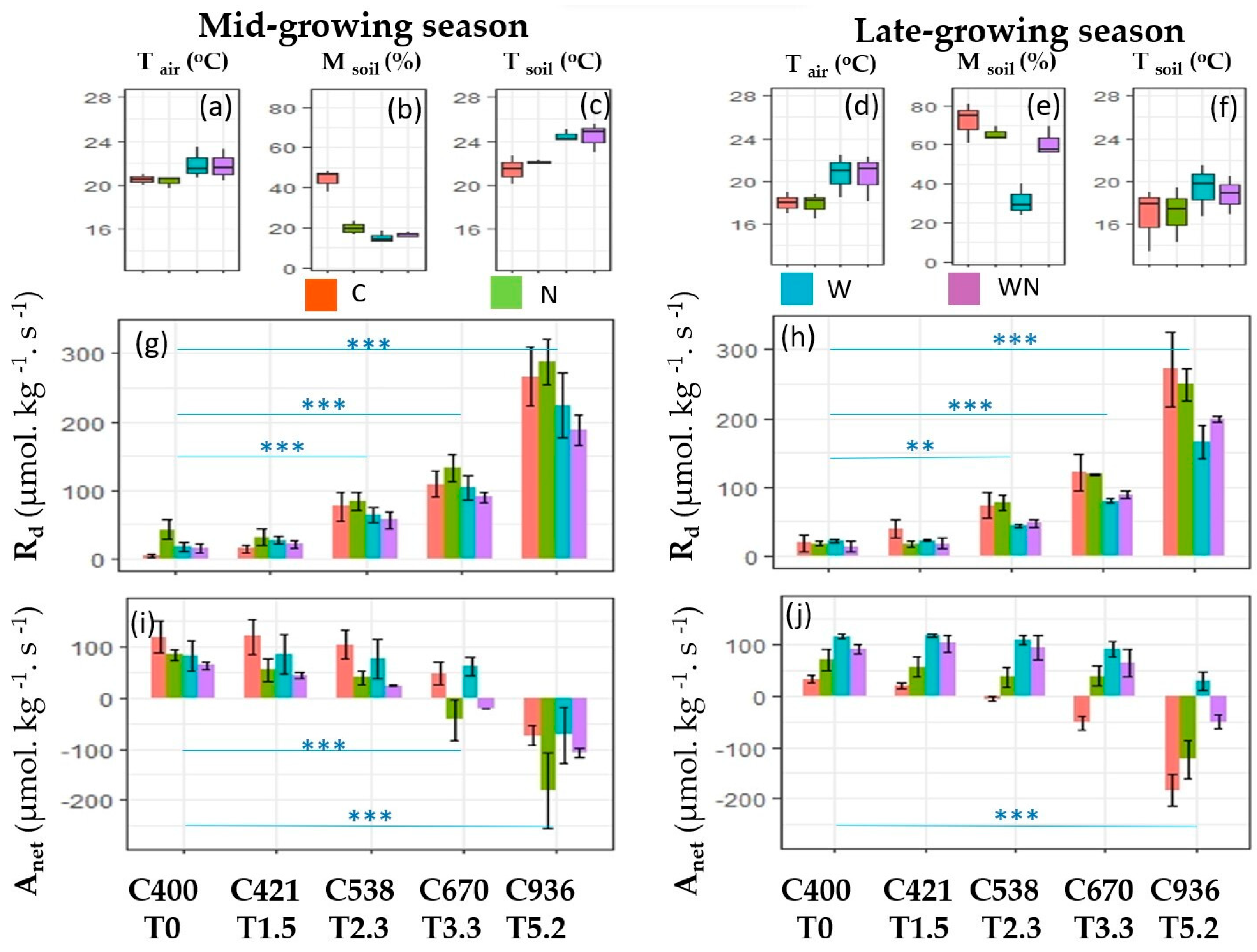
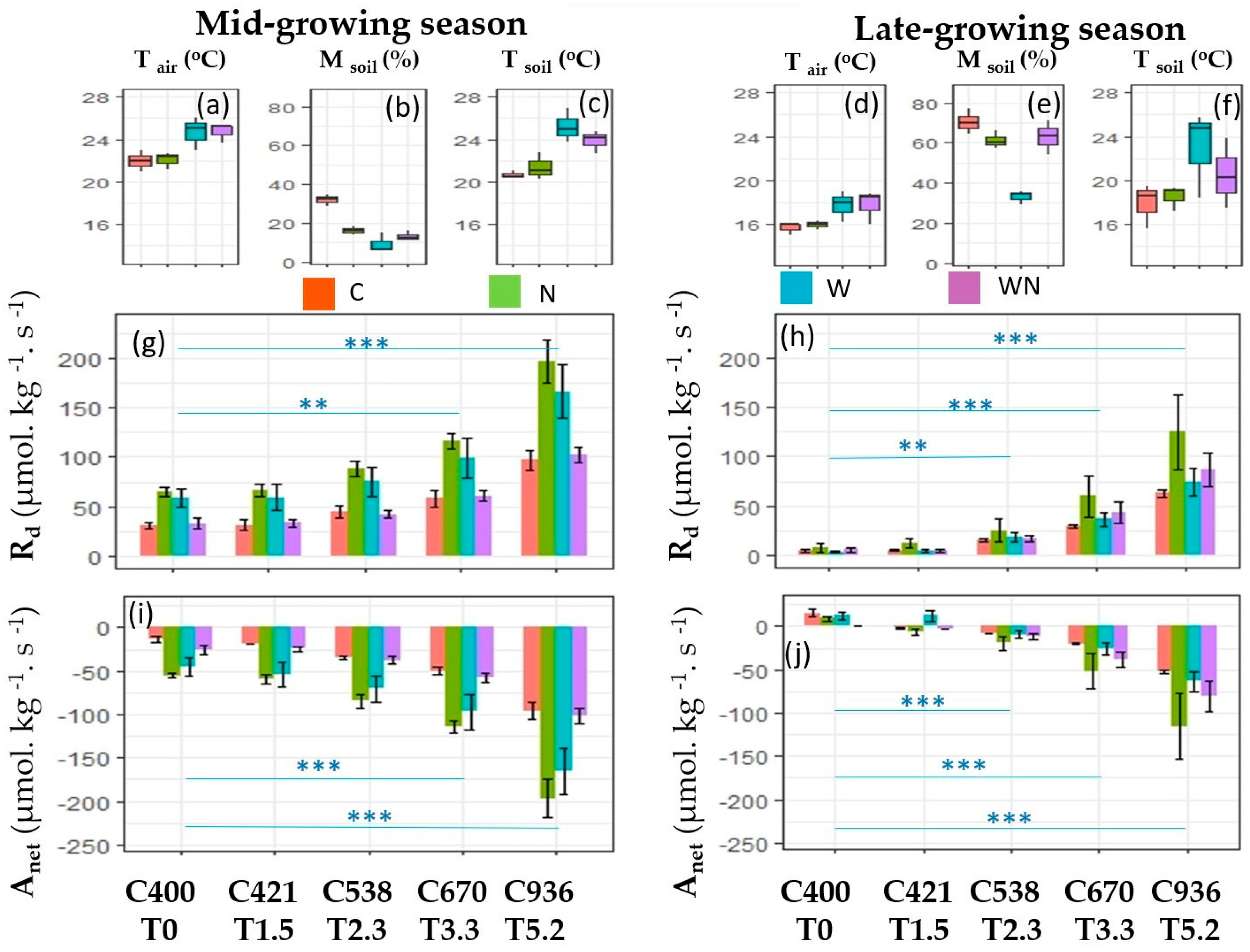
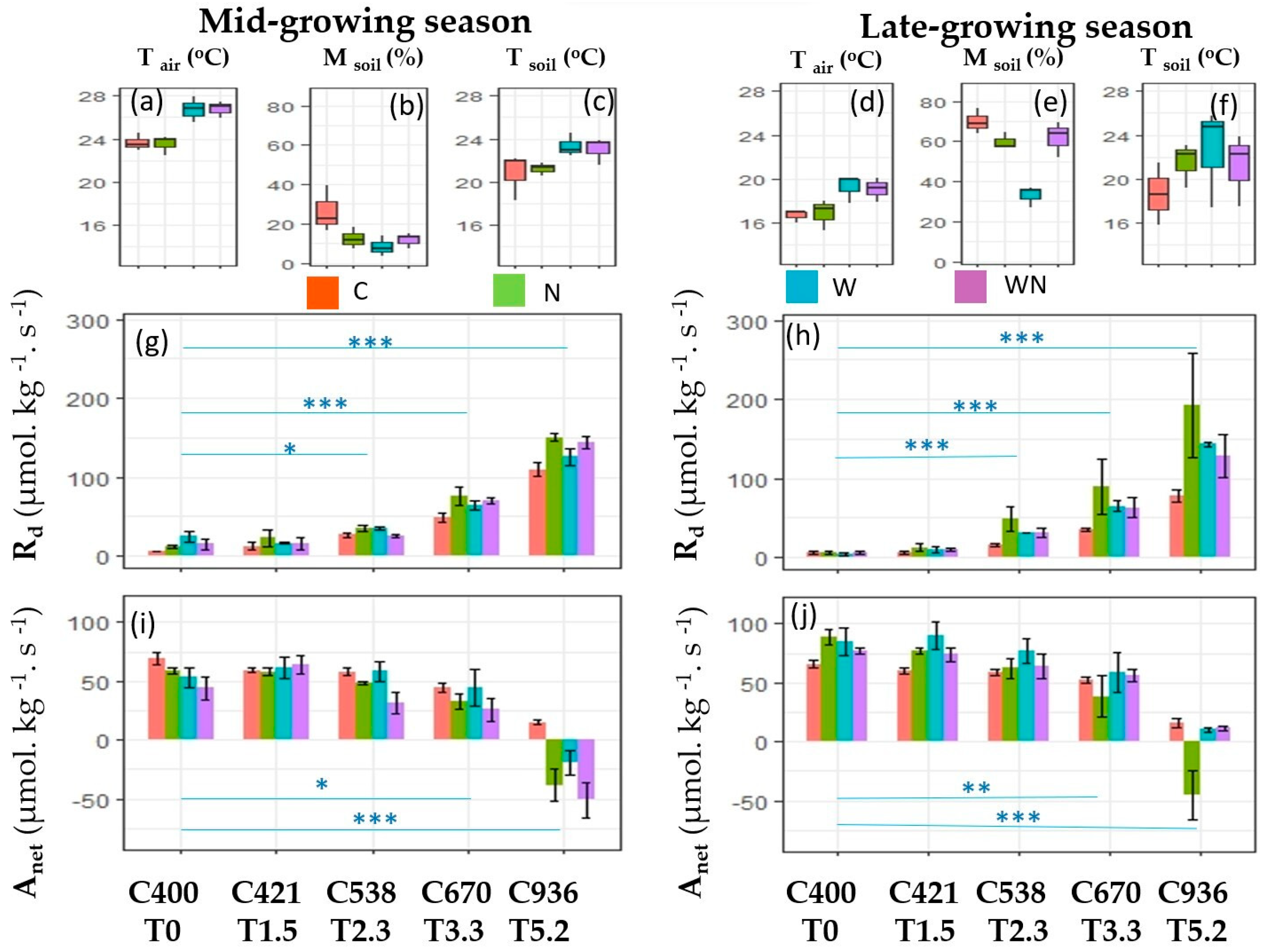
3. Results
3.1. The Response of Dark Respiration
3.2. The Response of Net Photosynthesis
3.3. Effects of W and N Addition Treatment on Environments
4. Discussion
4.1. Responses of Dark Respiration
4.2. Responses of Net Photosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob. Chang. Biol. 1999, 5, 679–691. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Wei, X.; Rich, R.L.; Montgomery, R.A. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 2016, 531, 633–636. [Google Scholar] [CrossRef]
- Kirschbaum, M. Direct and indirect climate change effects on photosynthesis and transpiration. Plant Biol. 2004, 6, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Tkemaladze, G.S.; Makhashvili, K. Climate changes and photosynthesis. Ann. Agrar. Sci. 2016, 14, 119–126. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, X.; Korpelainen, H.; Berninger, F.; Li, C. Effects of elevated CO2 and temperature on photosynthesis and leaf traits of an understory dwarf bamboo in subalpine forest zone, China. Physiol. Plant. 2013, 148, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Michelsen, A.; Ro-Poulsen, H.; van der Linden, L. Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J. Plant Physiol. 2011, 168, 1550–1561. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Bu, Z.; Hans, J.; Li, H.; Zhao, G.; Zheng, X.; Ma, J.; Zeng, J. The response of peatlands to climate warming: A review. Acta Ecol. Sin. 2011, 31, 157–162. [Google Scholar] [CrossRef]
- Hamilton III, E.W.; Heckathorn, S.A.; Joshi, P.; Wang, D.; Barua, D. Interactive effects of elevated CO2 and growth temperature on the tolerance of photosynthesis to acute heat stress in C3 and C4 species. J. Integr. Plant Biol. 2008, 50, 1375–1387. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Bubier, J.L.; Smith, R.; Juutinen, S.; Moore, T.R.; Minocha, R.; Long, S.; Minocha, S. Effects of nutrient addition on leaf chemistry, morphology, and photosynthetic capacity of three bog shrubs. Oecologia 2011, 167, 355–368. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Leeson, S.R.; Levy, P.E.; van Dijk, N.; Drewer, J.; Robinson, S.; Jones, M.R.; Kentisbeer, J.; Washbourne, I.; Sutton, M.A.; Sheppard, L.J. Nitrous oxide emissions from a peatbog after 13 years of experimental nitrogen deposition. Biogeosciences 2017, 14, 5753–5764. [Google Scholar] [CrossRef]
- Le, T.B.; Wu, J.; Gong, Y.; Vogt, J. Graminoid Removal Reduces the Increase in N2O Fluxes Due to Nitrogen Fertilization in a Boreal Peatland. Ecosystems 2021, 24, 261–271. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.A.; Wang, X.; Philpott, S.M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 2012, 169, 1–13. [Google Scholar] [CrossRef]
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.-S.; Roulet, N. Peatlands in the Earth’s 21st century climate system. Environ. Rev. 2011, 19, 371–396. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands Role in the carbon cycle. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group: Greifswald, Germany, 2002. [Google Scholar]
- Page, S.; Baird, A. Peatlands and global change: Response and resilience. Annu. Rev. Environ. Resour. 2016, 41, 35–57. [Google Scholar] [CrossRef]
- Walker, T.N.; Ward, S.E.; Ostle, N.J.; Bardgett, R.D. Contrasting growth responses of dominant peatland plants to warming and vegetation composition. Oecologia 2015, 178, 141–151. [Google Scholar] [CrossRef]
- Bobbink, R.; Hornung, M.; Roelofs, J.G. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 1998, 86, 717–738. [Google Scholar] [CrossRef]
- Whitaker, J.; Richardson, H.R.; Ostle, N.J.; Armstrong, A.; Waldron, S. Plant functional type indirectly affects peatland carbon fluxes and their sensitivity to environmental change. Eur. J. Soil Sci. 2021, 72, 1042–1053. [Google Scholar] [CrossRef]
- McPartland, M.Y.; Montgomery, R.A.; Hanson, P.J.; Phillips, J.R.; Kolka, R.; Palik, B. Vascular plant species response to warming and elevated carbon dioxide in a boreal peatland. Environ. Res. Lett. 2020, 15, 124066. [Google Scholar] [CrossRef]
- Heijmans, M.M.; Mauquoy, D.; Van Geel, B.; Berendse, F. Long-term effects of climate change on vegetation and carbon dynamics in peat bogs. J. Veg. Sci. 2008, 19, 307–320. [Google Scholar] [CrossRef]
- Heijmans, M.M.; van der Knaap, Y.A.; Holmgren, M.; Limpens, J. Persistent versus transient tree encroachment of temperate peat bogs: Effects of climate warming and drought events. Glob. Chang. Biol. 2013, 19, 2240–2250. [Google Scholar] [CrossRef]
- Oke, T.A.; Hager, H.A. Plant community dynamics and carbon sequestration in Sphagnum-dominated peatlands in the era of global change. Glob. Ecol. Biogeogr. 2020, 29, 1610–1620. [Google Scholar] [CrossRef]
- Le, T.B.; Wu, J.; Gong, Y. Vascular plants regulate responses of boreal peatland Sphagnum to climate warming and nitrogen addition. Sci. Total Environ. 2021, 819, 152077. [Google Scholar] [CrossRef]
- Lamers, L.P.; Bobbink, R.; Roelofs, J.G. Natural nitrogen filter fails in polluted raised bogs. Glob. Chang. Biol. 2000, 6, 583–586. [Google Scholar] [CrossRef]
- Berendse, F.; Van Breemen, N.; Rydin, H.; Buttler, A.; Heijmans, M.; Hoosbeek, M.R.; Lee, J.A.; Mitchell, E.; Saarinen, T.; Vasander, H. Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob. Chang. Biol. 2001, 7, 591–598. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bledzki, L.A. Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Glob. Chang. Biol. 2007, 13, 1168–1186. [Google Scholar] [CrossRef]
- Larmola, T.; Bubier, J.L.; Kobyljanec, C.; Basiliko, N.; Juutinen, S.; Humphreys, E.; Preston, M.; Moore, T.R. Vegetation feedbacks of nutrient addition lead to a weaker carbon sink in an ombrotrophic bog. Glob. Chang. Biol. 2013, 19, 3729–3739. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F. Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: The role of amino acid nitrogen concentration. Oecologia 2003, 135, 339–345. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytologist. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Ward, E.J.; Warren, J.M.; McLennan, D.A.; Dusenge, M.E.; Way, D.A.; Wullschleger, S.D.; Hanson, P.J. Photosynthetic and respiratory responses of two bog shrub species to whole ecosystem warming and elevated CO2 at the boreal-temperate ecotone. Front. For. Glob. Chang. 2019, 2, 54. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Vogt, J.; Le, T.B. Warming reduces the increase in N2O emission under nitrogen fertilization in a boreal peatland. Sci. Total Environ. 2019, 664, 72–78. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430. [Google Scholar] [CrossRef]
- Juutinen, S.; Moore, T.R.; Bubier, J.L.; Arnkil, S.; Humphreys, E.; Marincak, B.; Roy, C.; Larmola, T. Long-term nutrient addition increased CH4 emission from a bog through direct and indirect effects. Sci. Rep. 2018, 8, 3838. [Google Scholar] [CrossRef]
- Levy, P.; van Dijk, N.; Gray, A.; Sutton, M.; Jones, M.; Leeson, S.; Dise, N.; Leith, I.; Sheppard, L. Response of a peat bog vegetation community to long-term experimental addition of nitrogen. J. Ecol. 2019, 107, 1167–1186. [Google Scholar] [CrossRef]
- Lund, M.; Christensen, T.R.; Mastepanov, M.; Lindroth, A.; Strom, L. Effects of N and P fertilization on the greenhouse gas exchange in two northern peatlands with contrasting N deposition rates. Biogeosciences 2009, 6, 2135–2141. [Google Scholar] [CrossRef]
- Nykänen, H.; Vasander, H.; Huttunen, J.T.; Martikainen, P.J. Effect of experimental nitrogen load on methane and nitrous oxide fluxes on ombrotrophic boreal peatland. Plant Soil 2002, 242, 147–155. [Google Scholar] [CrossRef]
- Zhang, X.; Flato, G.; Kirchmeier-Young, M.; Vincent, L.; Wan, H.; Wang, X.; Rong, R.; Fyfe, J.; Li, G.; Kharin, V. Changes in temperature and precipitation across Canada. Chapter 4; In Canada’s Changing Climate, Report; Bush, E., Lemmen, D.S., Eds.; Government of Canada: Ottawa, ON, Canada, 2019; pp. 112–193. [Google Scholar]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.; Lamarque, J.-F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.; Riahi, K. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–241. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Zhang, D. R-Squared and Related Measures, 2.1; Department of Statistics, Purdue University: West Lafayette, IN, USA, 2020. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ‘Ggplot2’ Based Publication Ready Plots. 2020. Available online: https://cloud.r-project.org/web/packages/ggpubr/ggpubr (accessed on 3 October 2022).
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Matson, P.; Lohse, K.A.; Hall, S.J. The Globalization of Nitrogen Deposition Consequences for Terrestrial Ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef]
- Hedwall, P.O.; Brunet, J.; Rydin, H. Peatland plant communities under global change: Negative feedback loops counteract shifts in species composition. Ecology 2017, 98, 150–161. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Le, T.B. Counteractions between biotic and abiotic factors on methane dynamics in a boreal peatland: Vegetation composition change vs warming and nitrogen deposition. Geoderma 2021, 395, 115074. [Google Scholar] [CrossRef]
- Gonzàlez-Meler, M.A.; Ribas-CarbÓ, M.; Siedow, J.N.; Drake, B.G. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996, 112, 1349–1355. [Google Scholar] [CrossRef]
- Markelz, R.C.; Lai, L.X.; Vosseler, L.N.; Leakey, A.D. Transcriptional reprogramming and stimulation of leaf respiration by elevated CO2 concentration is diminished, but not eliminated, under limiting nitrogen supply. Plant Cell Environ. 2014, 37, 886–898. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Sun, B.; Zhang, S.; Zhang, Y.; Liao, Y.; Zhou, Y.; Xia, X.; Shi, K.; Yu, J. Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci. Rep. 2013, 3, 3433. [Google Scholar] [CrossRef]
- Tjoelker, M.; Oleksyn, J.; Lee, T.; Reich, P. Direct inhibition of leaf dark respiration by elevated CO2 is minor in 12 grassland species. New Phytol. 2001, 150, 419–424. [Google Scholar] [CrossRef]
- Amthor, J.S. Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol. 2000, 20, 139–144. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Madhavji, S.; Way, D.A. Contrasting acclimation responses to elevated CO2 and warming between an evergreen and a deciduous boreal conifer. Glob. Chang. Biol. 2020, 26, 3639–3657. [Google Scholar] [CrossRef]
- Curtis, P. A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 1996, 19, 127–137. [Google Scholar] [CrossRef]
- Ayub, G.; Zaragoza-Castells, J.; Griffin, K.L.; Atkin, O.K. Leaf respiration in darkness and in the light under pre-industrial, current and elevated atmospheric CO2 concentrations. Plant Sci. 2014, 226, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Aspinwall, M.J.; Jacob, V.K.; Blackman, C.J.; Smith, R.A.; Tjoelker, M.G.; Tissue, D.T. The temperature response of leaf dark respiration in 15 provenances of Eucalyptus grandis grown in ambient and elevated CO2. Funct. Plant Biol. 2017, 44, 1075–1086. [Google Scholar] [CrossRef]
- Slot, M.; Kitajima, K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 2015, 177, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Heskel, M.A.; O’Sullivan, O.S.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Egerton, J.J.; Creek, D.; Bloomfield, K.J.; Xiang, J. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proc. Natl. Acad. Sci. USA 2016, 113, 3832–3837. [Google Scholar] [CrossRef]
- Zha, T.; Kellomäki, S.; Wang, K.Y. Seasonal variation in respiration of 1-year-old shoots of Scots pine exposed to elevated carbon dioxide and temperature for 4 years. Ann. Bot. 2003, 92, 89–96. [Google Scholar] [CrossRef][Green Version]
- van de Weg, M.J.; Shaver, G.R.; Salmon, V.G. Contrasting effects of long term versus short-term nitrogen addition on photosynthesis and respiration in the Arctic. Plant Ecol. 2013, 214, 1273–1286. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.; Tjoelker, M.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Heskel, M.A.; Anderson, O.R.; Atkin, O.K.; Turnbull, M.H.; Griffin, K.L. Leaf-and cell-level carbon cycling responses to a nitrogen and phosphorus gradient in two Arctic tundra species. Am. J. Bot. 2012, 99, 1702–1714. [Google Scholar] [CrossRef]
- Shapiro, J.; Griffin, K.; Lewis, J.; Tissue, D. Response of Xanthium strumarium leaf respiration in the light to elevated CO2 concentration, nitrogen availability and temperature. New Phytol. 2004, 162, 377–386. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Heskel, M.A.; Greaves, H.E.; Turnbull, M.H.; O’Sullivan, O.S.; Shaver, G.R.; Griffin, K.L.; Atkin, O.K. Thermal acclimation of shoot respiration in an Arctic woody plant species subjected to 22 years of warming and altered nutrient supply. Glob. Chang. Biol. 2014, 20, 2618–2630. [Google Scholar] [CrossRef]
- Smith, N.G.; Dukes, J.S. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob. Chang. Biol. 2013, 19, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.; Abuarghub, S.; Read, D. The biology of mycorrhiza in the Ericaceae: X. The utilization of proteins and the production of proteolytic enzymes by the mycorrhizal endophyte and by mycorrhizal plants. New Phytol. 1985, 101, 469–486. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Ellsworth, D.S.; Thomas, R.; Crous, K.Y.; Palmroth, S.; Ward, E.; Maier, C.; DeLucia, E.; Oren, R. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: A synthesis from Duke FACE. Glob. Chang. Biol. 2012, 18, 223–242. [Google Scholar] [CrossRef]
- Gunderson, C.A.; Sholtis, J.; Wullschleger, S.D.; Tissue, D.T.; Hanson, P.J.; Norby, R.J. Environmental and stomatal control of photosynthetic enhancement in the canopy of a sweetgum (Liquidambar styraciflua L.) plantation during 3 years of CO2 enrichment. Plant Cell Environ. 2002, 25, 379–393. [Google Scholar] [CrossRef]
- Medlyn, B.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.; Kirschbaum, M.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.; Xia, J.; Han, Y.; Wu, M.; Wan, S. Climatic warming changes plant photosynthesis and its temperature dependence in a temperate steppe of northern China. Environ. Exp. Bot. 2008, 63, 91–101. [Google Scholar] [CrossRef]
- Teskey, R. Combined effects of elevated CO2 and air temperature on carbon assimilation of Pinus taeda trees. Plant Cell Environ. 1997, 20, 373–380. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lucash, M.; Olszyk, D.; Tingey, D.T. Seasonal patterns of photosynthesis in Douglas fir seedlings during the third and fourth year of exposure to elevated CO2 and temperature. Plant Cell Environ. 2001, 24, 539–548. [Google Scholar] [CrossRef]
- Ghannoum, O.; Phillips, N.G.; Sears, M.A.; Logan, B.A.; Lewis, J.D.; Conroy, J.P.; Tissue, D.T. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant Cell Environ. 2010, 33, 1671–1681. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; Siebers, M.; Gray, S.B.; Drag, D.W.; Rosenthal, D.M.; Kimball, B.A.; Ort, D.R.; Bernacchi, C.J. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 2013, 162, 410–423. [Google Scholar] [CrossRef]
- Wang, K.; Kellomäki, S.; Laitinen, K. Effects of needle age, long-term temperature and CO2 treatments on the photosynthesis of Scots pine. Tree Physiol. 1995, 15, 211–218. [Google Scholar] [CrossRef]
- Lewis, J.D.; Phillips, N.G.; Logan, B.A.; Smith, R.A.; Aranjuelo, I.; Clarke, S.; Offord, C.A.; Frith, A.; Barbour, M.; Huxman, T. Rising temperature may negate the stimulatory effect of rising CO2 on growth and physiology of Wollemi pine (Wollemia nobilis). Funct. Plant Biol. 2015, 42, 836–850. [Google Scholar] [CrossRef]
- Breeuwer, A.; Heijmans, M.M.; Gleichman, M.; Robroek, B.J.; Berendse, F. Response of Sphagnum species mixtures to increased temperature and nitrogen availability. Plant Ecol. 2009, 204, 97–111. [Google Scholar] [CrossRef]
- Kosykh, N.; Koronatova, N.; Granath, G. Effect of temperature and precipitation on linear increment of Sphagnum fuscum and S. magellanicum in Western Siberia. Russ. J. Ecol. 2017, 48, 203–211. [Google Scholar] [CrossRef]
- Sheppard, L.J.; Leith, I.D.; Leeson, S.R.; van Dijk, N.; Field, C.; Levy, P. Fate of N in a peatland, Whim bog: Immobilisation in the vegetation and peat, leakage into pore water and losses as depend on the form of N. Biogeosciences 2013, 10, 149–160. [Google Scholar] [CrossRef]
- Turetsky, M.; Wieder, R.; Vitt, D.; Evans, R.; Scott, K. The disappearance of relict permafrost in boreal north America: Effects on peatland carbon storage and fluxes. Glob. Chang. Biol. 2007, 13, 1922–1934. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Bond-Lamberty, B.; Euskirchen, E.; Talbot, J.; Frolking, S.; McGuire, A.D.; Tuittila, E.-S. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 2012, 196, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Lombardozzi, D.L.; Bonan, G.B.; Smith, N.G.; Dukes, J.S.; Fisher, R.A. Temperature acclimation of photosynthesis and respiration: A key uncertainty in the carbon cycle-climate feedback. Geophys. Res. Lett. 2015, 42, 8624–8631. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, M.; Shen, R.; Yang, Q.; Huang, B.; Wan, S. Acclimation of foliar respiration and photosynthesis in response to experimental warming in a temperate steppe in northern China. PLoS ONE 2013, 8, e56482. [Google Scholar] [CrossRef]
- Smith, N.G.; Malyshev, S.L.; Shevliakova, E.; Kattge, J.; Dukes, J.S. Foliar temperature acclimation reduces simulated carbon sensitivity to climate. Nat. Clim. Chang. 2016, 6, 407–411. [Google Scholar] [CrossRef]
- Cornic, G.; Fresneau, C. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann. Bot. 2002, 89, 887–894. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source–sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on dry matter partitioning and photosynthesis before and after cutting of nodulated alfalfa. Plant Sci. 2006, 170, 1059–1067. [Google Scholar] [CrossRef]
| Source of Variation | df | Rd (µmol·kg −1·s −1) | |||
|---|---|---|---|---|---|
| Mid-Growing Season | Late-Growing Season | ||||
| F | p | F | p | ||
| Evergreen shrub: A. glaucophylla (n = 60) | |||||
| CTI | 4 | 91.3 | <0.0001 | 297.3 | <0.0001 |
| N | 1 | 0.2 | 0.6508 | 9.4 | 0.0039 |
| W | 1 | 2.1 | 0.1520 | 10.7 | 0.0023 |
| CTI × N | 4 | 1.2 | 0.3380 | 0.7 | 0.6025 |
| CTI × W | 4 | 2.7 | 0.0450 | 0.2 | 0.9565 |
| N × W | 1 | 7.9 | 0.0076 | 0.3 | 0.5716 |
| CTI × N × W | 4 | 0.7 | 0.6088 | 0.1 | 0.9773 |
| Full model adjusted R2 | 0.864 | 0.953 | |||
| Deciduous shrub: G. bigeloviana (n = 60) | |||||
| CTI | 4 | 77.7 | <0.0001 | 133.5 | <0.0001 |
| N | 1 | 2.2 | 0.1453 | 0.1 | 0.8215 |
| W | 1 | 2.2 | 0.1506 | 22.1 | <0.0001 |
| CTI × N | 4 | 1.4 | 0.2566 | 0.3 | 0.8771 |
| CTI × W | 4 | 0.8 | 0.5088 | 4.5 | 0.0045 |
| N × W | 1 | 10.6 | 0.0024 | 1.5 | 0.2352 |
| CTI × N × W | 4 | 1.9 | 0.1275 | 0.7 | 0.5943 |
| Full model adjusted R2 | 0.847 | 0.907 | |||
| Sphagnum moss: S. fuscum (n = 60) | |||||
| CTI | 4 | 44.9 | <0.0001 | 73.5 | <0.0001 |
| N | 1 | 2.8 | 0.1044 | 7.4 | 0.0099 |
| W | 1 | 1.2 | 0.2721 | 2.0 | 0.1699 |
| CTI × N | 4 | 0.1 | 0.9948 | 0.2 | 0.9194 |
| CTI × W | 4 | 0.1 | 0.9929 | 0.4 | 0.8217 |
| N × W | 1 | 95.8 | <0.0001 | 2.5 | 0.1258 |
| CTI × N × W | 4 | 0.1 | 0.9827 | 0.3 | 0.878 |
| Full model adjusted R2 | 0.816 | 0.831 | |||
| Graminoid: T. cespitosum (n = 60) | |||||
| CTI | 4 | 229.0 | <0.0001 | 96.4 | <0.0001 |
| N | 1 | 11.2 | 0.0018 | 10.0 | 0.0031 |
| W | 1 | 1.7 | 0.2052 | 0.1 | 0.7916 |
| CTI × N | 4 | 4.1 | 0.0071 | 0.2 | 0.9551 |
| CTI × W | 4 | 0.5 | 0.7030 | 0.4 | 0.8265 |
| N × W | 1 | 9.6 | 0.0037 | 7.1 | 0.0115 |
| CTI × N × W | 4 | 0.1 | 0.9723 | 1.7 | 0.1766 |
| Full model adjusted R2 | 0.941 | 0.870 | |||
| Source of Variation | df | Anet (µmol·kg−1·s−1) | |||
|---|---|---|---|---|---|
| Mid-Growing Season | Late-Growing Season | ||||
| F | p | F | p | ||
| Evergreen shrub: A. glaucophylla (n = 60) | |||||
| CTI | 4 | 8.4 | 0.0001 | 4.6 | 0.0042 |
| N | 1 | 12.2 | 0.0013 | 3.6 | 0.0649 |
| W | 1 | 1.2 | 0.2783 | 2.6 | 0.1153 |
| CTI × N | 4 | 1.9 | 0.1327 | 0.1 | 0.9824 |
| CTI × W | 4 | 0.4 | 0.7928 | 0.1 | 0.9739 |
| N × W | 1 | 0.6 | 0.4276 | 1.0 | 0.3174 |
| CTI × N × W | 4 | 0.0 | 0.9978 | 0.0 | 0.9952 |
| Full model adjusted R2 | 0.429 | 0.185 | |||
| Deciduous shrub: G. bigeloviana (n = 60) | |||||
| CTI | 4 | 31.7 | <0.0001 | 53.4 | <0.0001 |
| N | 1 | 34.4 | <0.0001 | 1.6 | 0.2097 |
| W | 1 | 1.7 | 0.1955 | 114.7 | <0.0001 |
| CTI × N | 4 | 0.8 | 0.5616 | 0.7 | 0.5983 |
| CTI × W | 4 | 1.2 | 0.3093 | 3.5 | 0.0166 |
| N × W | 1 | 0.8 | 0.3658 | 27.8 | <0.0001 |
| CTI × N × W | 4 | 0.1 | 0.9853 | 1.2 | 0.3333 |
| Full model adjusted R2 | 0.751 | 0.859 | |||
| Sphagnum moss: S. fuscum (n = 60) | |||||
| CTI | 4 | 68.0 | <0.0001 | 56.0 | <0.0001 |
| N | 1 | 6.8 | 0.0129 | 16.1 | 0.0003 |
| W | 1 | 0.4 | 0.5261 | 0.7 | 0.4037 |
| CTI × N | 4 | 0.1 | 0.9816 | 1.1 | 0.3879 |
| CTI × W | 4 | 0.2 | 0.9322 | 0.5 | 0.7114 |
| N × W | 1 | 116.2 | <0.0001 | 1.1 | 0.3114 |
| CTI × N × W | 4 | 1.2 | 0.3294 | 1.1 | 0.3873 |
| Full model adjusted R2 | 0.867 | 0.799 | |||
| Graminoid: T. cespitosum (n = 60) | |||||
| CTI | 4 | 99.1 | <0.0001 | 50.3 | <0.0001 |
| N | 1 | 30.0 | <0.0001 | 2.8 | 0.1026 |
| W | 1 | 8.9 | 0.0049 | 9.7 | 0.0035 |
| CTI × N | 4 | 5.3 | 0.0017 | 2.3 | 0.0755 |
| CTI × W | 4 | 2.3 | 0.0736 | 0.7 | 0.5730 |
| N × W | 1 | 0.0 | 0.962 | 0.0 | 0.8582 |
| CTI × N × W | 4 | 1.1 | 0.388 | 4.5 | 0.0045 |
| Full model adjusted R2 | 0.889 | 0.792 | |||
| Treatment | Tair (°C) | Msoil (%) | Tsoil (°C) | |||
|---|---|---|---|---|---|---|
| Mid-Season | Late-Season | Mid-Season | Late-Season | Mid-Season | Late-Season | |
| C | 21.63 ± 0.46 | 17.33 ± 0.43 | 36.33 ± 2.90 | 73.60 ± 2.26 | 21.01 ± 0.36 | 17.92 ± 0.61 |
| N | 21.60 ± 0.45 | 17.40 ± 0.44 | 16.01 ± 1.20 | 64.93 ± 1.99 | 21.43 ± 0.23 | 18.68 ± 0.68 |
| W | 24.08 ± 0.68 | 19.83 ± 0.61 | 11.18 ± 1.34 | 32.85 ± 1.47 | 24.58 ± 0.43 | 21.23 ± 0.93 |
| WN | 24.09 ± 0.66 | 19.77 ± 0.64 | 14.11 ± 0.81 | 61.68 ± 1.90 | 23.69 ± 0.33 | 19.95 ± 0.68 |
| Treatment | NH4+ (mg/L) | NO3− (mg/L) | ||
|---|---|---|---|---|
| Mid-Season | Late-Season | Mid-Season | Late-Season | |
| 10 cm depth | ||||
| C | 0.366 ± 0.043 | 0.384 ± 0.080 | 0.025 ± 0.008 | 0.190 ± 0.130 |
| N | 0.982 ± 0.156 | 1.054 ± 0.355 | 0.036 ± 0.006 | 0.511 ± 0.470 |
| W | 0.454 ± 0.125 | 0.220 ± 0.063 | 0.031 ± 0.014 | 0.056 ± 0.032 |
| WN | 0.910 ± 0.178 | 1.001 ± 0.297 | 0.090 ± 0.041 | 0.504 ± 0.222 |
| 40 cm depth | ||||
| C | 0.637 ± 0.134 | 0.642 ± 0.161 | 0.038 ± 0.011 | 0.028 ± 0.010 |
| N | 1.250 ± 0.156 | 1.087 ± 0.133 | 0.020 ± 0.007 | 0.018 ± 0.003 |
| W | 0.579 ± 0.064 | 0.680 ± 0.203 | 0.056 ± 0.019 | 0.021 ± 0.006 |
| WN | 1.127 ± 0.154 | 0.980 ± 0.142 | 0.035 ± 0.013 | 0.020 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.B.; Wu, J.; Gong, Y.; Dinh, M.-V. Long-Term Warming and Nitrogen Addition Regulate Responses of Dark Respiration and Net Photosynthesis in Boreal Bog Plants to Short-Term Increases in CO2 and Temperature. Atmosphere 2022, 13, 1644. https://doi.org/10.3390/atmos13101644
Le TB, Wu J, Gong Y, Dinh M-V. Long-Term Warming and Nitrogen Addition Regulate Responses of Dark Respiration and Net Photosynthesis in Boreal Bog Plants to Short-Term Increases in CO2 and Temperature. Atmosphere. 2022; 13(10):1644. https://doi.org/10.3390/atmos13101644
Chicago/Turabian StyleLe, Thuong Ba, Jianghua Wu, Yu Gong, and Mai-Van Dinh. 2022. "Long-Term Warming and Nitrogen Addition Regulate Responses of Dark Respiration and Net Photosynthesis in Boreal Bog Plants to Short-Term Increases in CO2 and Temperature" Atmosphere 13, no. 10: 1644. https://doi.org/10.3390/atmos13101644
APA StyleLe, T. B., Wu, J., Gong, Y., & Dinh, M.-V. (2022). Long-Term Warming and Nitrogen Addition Regulate Responses of Dark Respiration and Net Photosynthesis in Boreal Bog Plants to Short-Term Increases in CO2 and Temperature. Atmosphere, 13(10), 1644. https://doi.org/10.3390/atmos13101644








