Uncovering the Fresh Snowfall Microbiome and Its Chemical Characteristics with Backward Trajectories in Daejeon, the Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. The 16S rRNA Gene and ITS Gene Amplification and Sequencing
2.3. Sequence Data Analysis
2.4. Water-Soluble Organic Carbon (WSOC) and Inorganic Ion Analyses
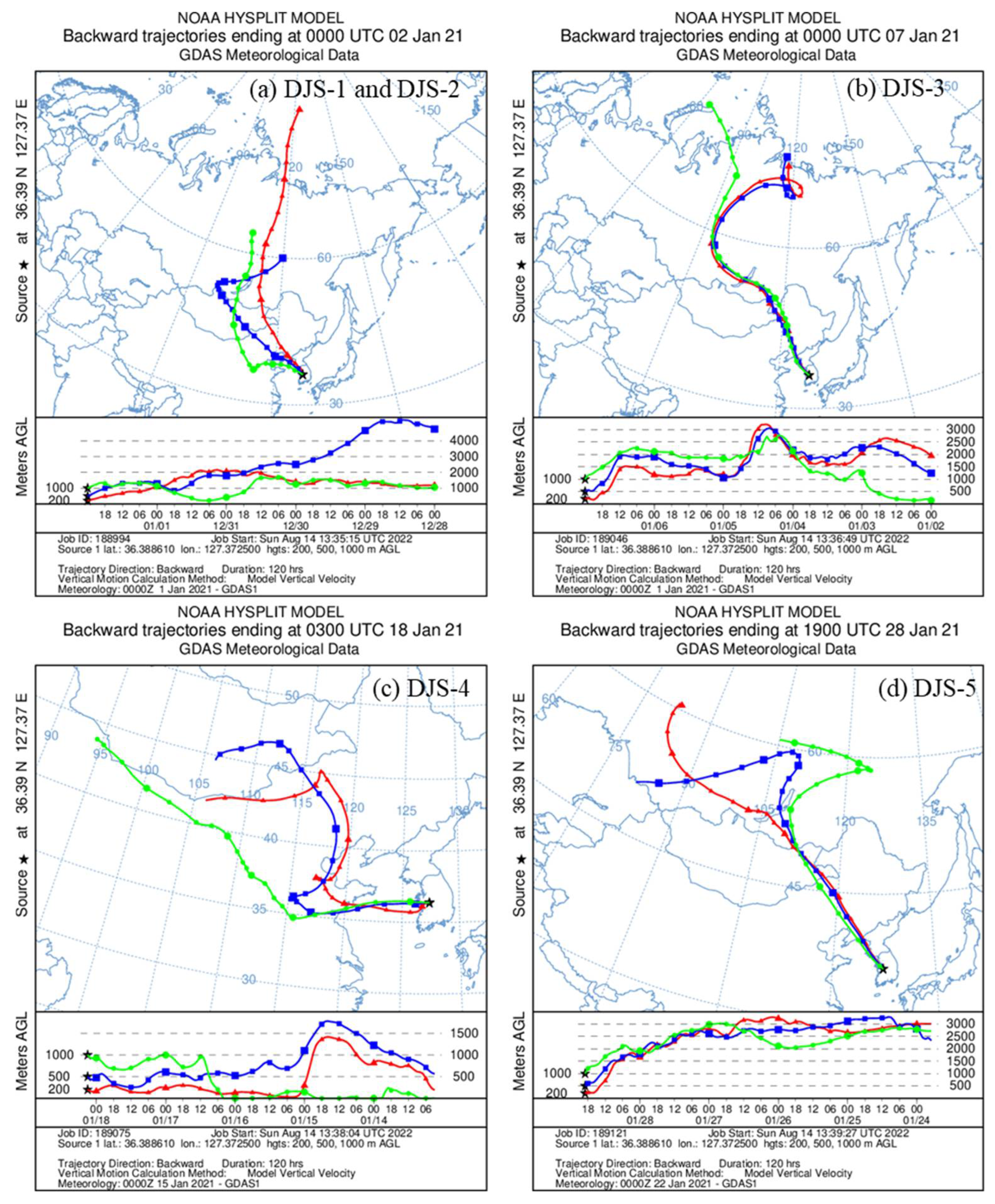
2.5. Air Mass Backward Trajectory Analysis
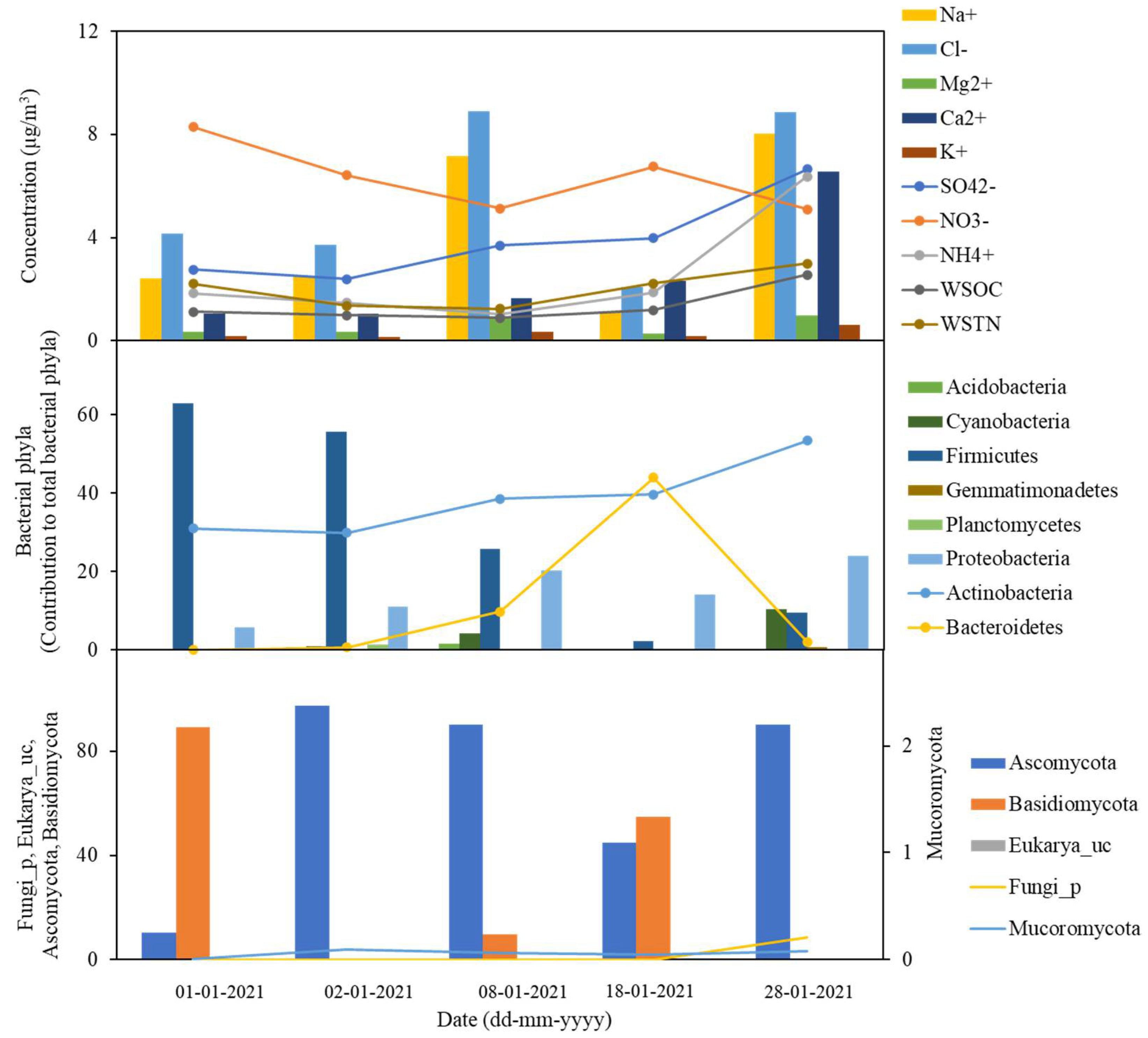
3. Results
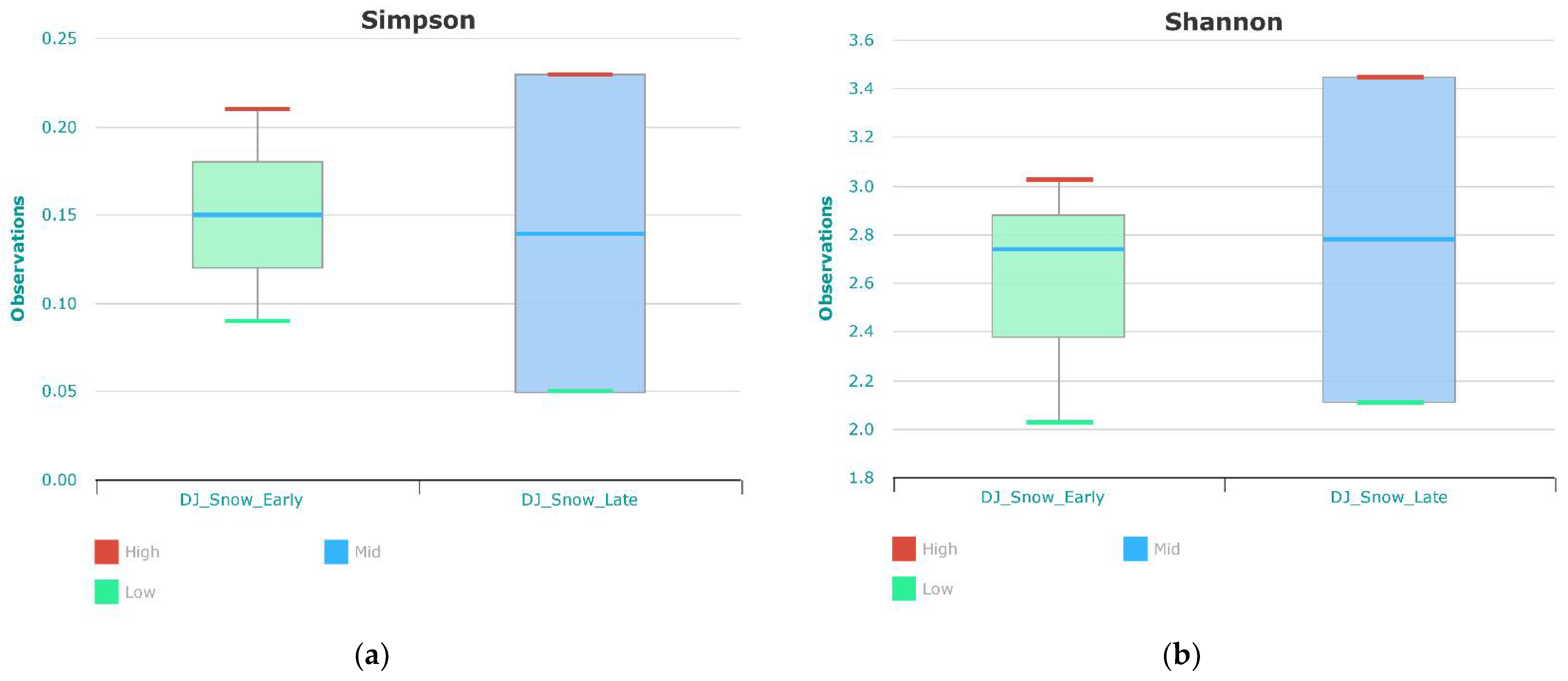
3.1. Bacterial Compositions and Diversity
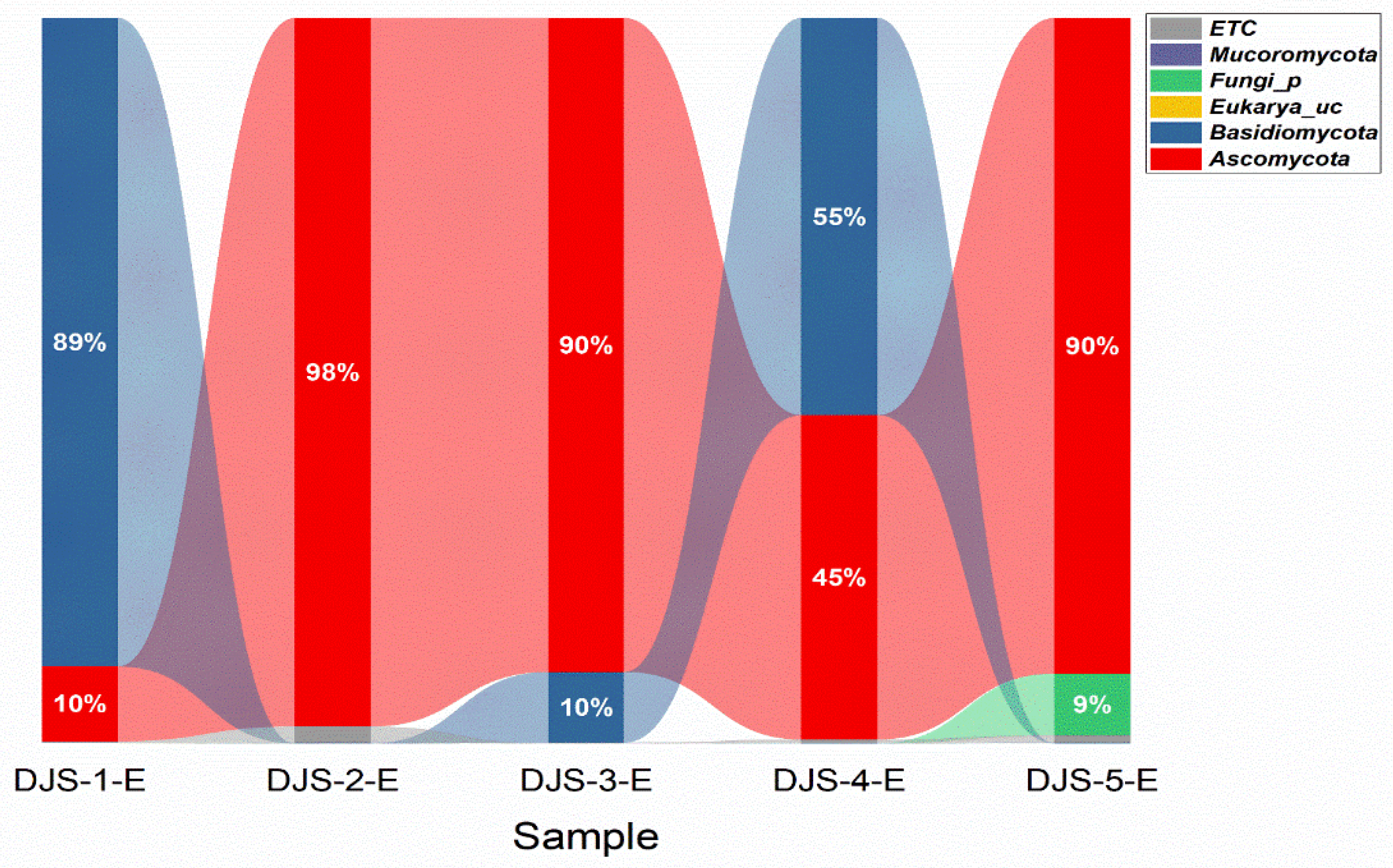
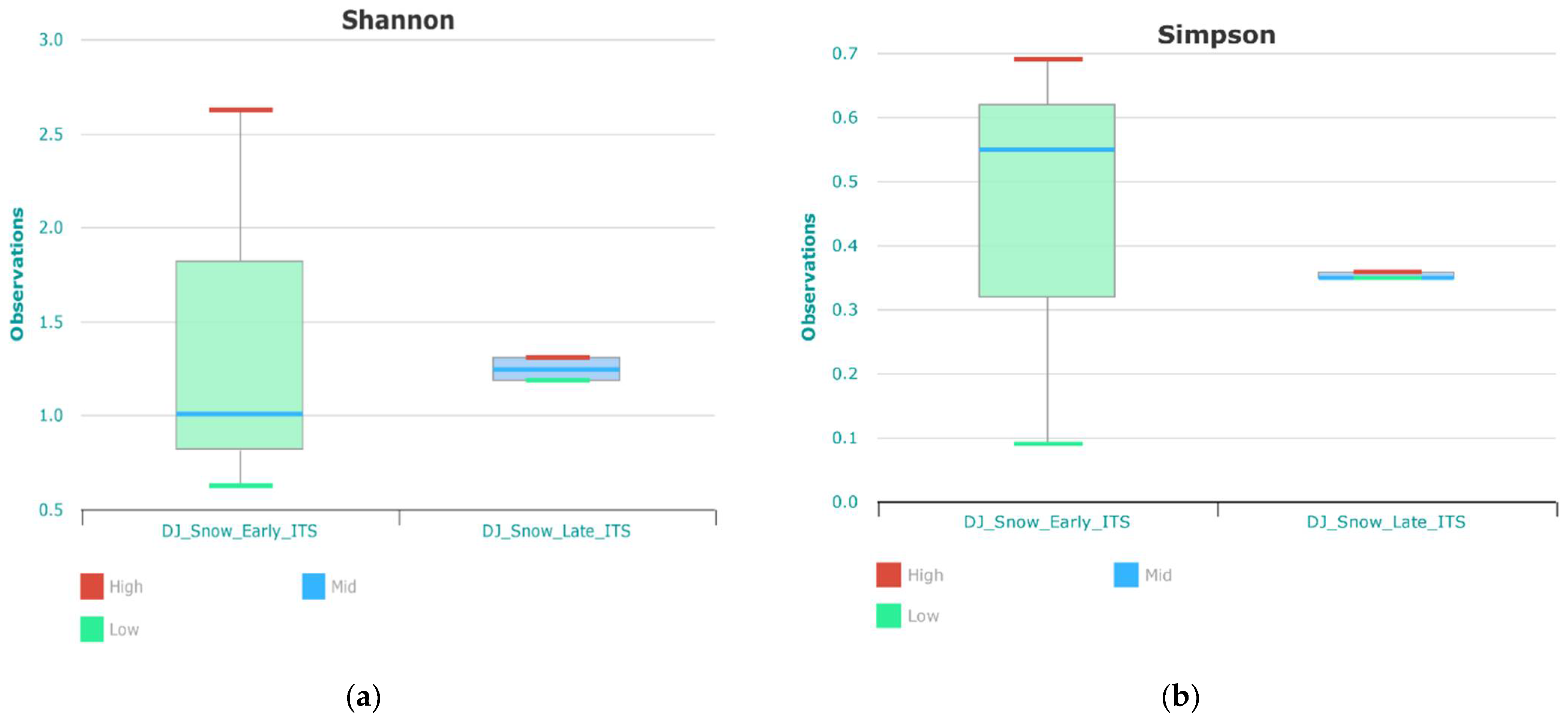
3.2. Fungal Compositions and Diversity
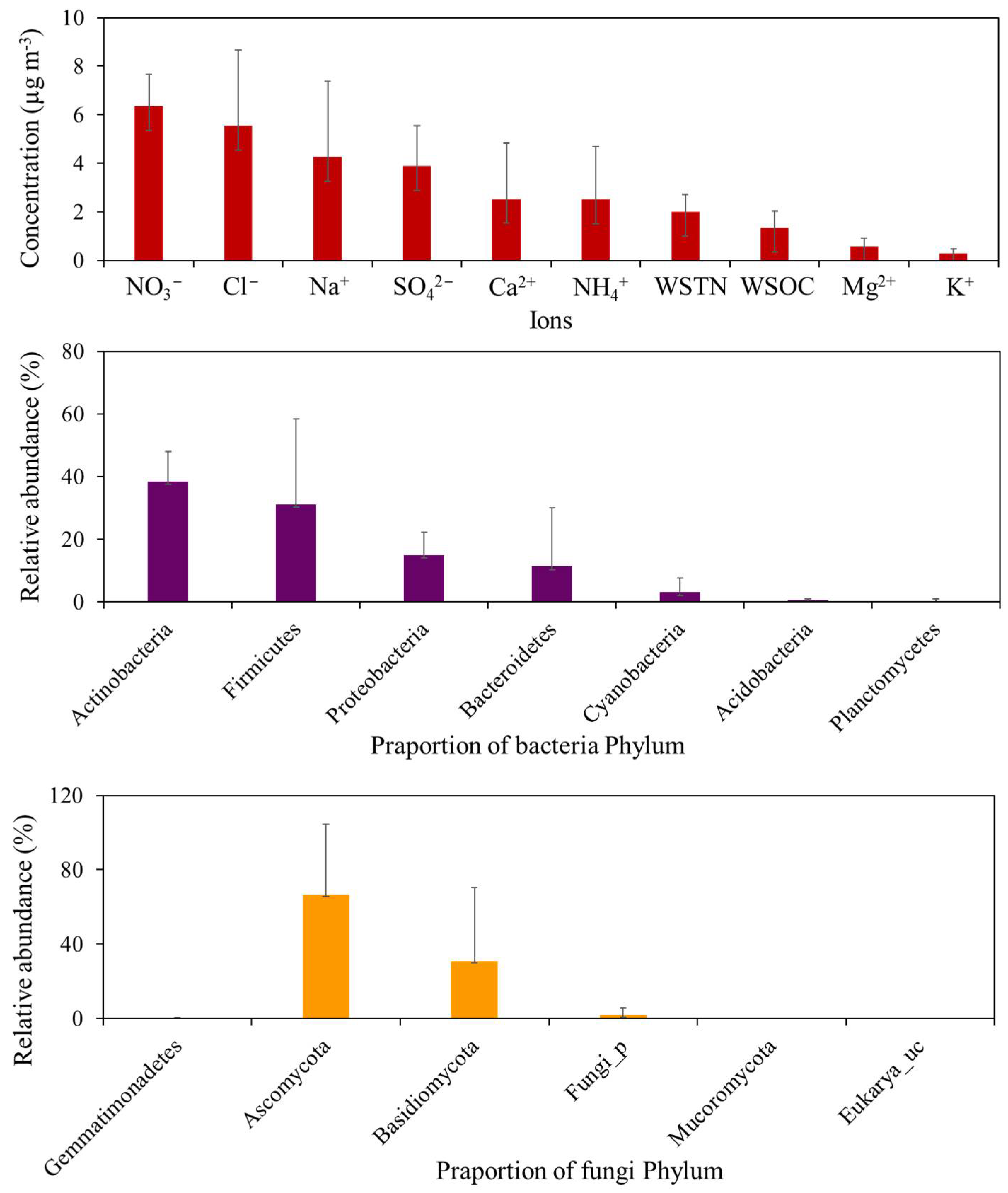
3.3. Chemical Characteristics of the Snowfall Samples
3.4. Temporal Variability of the Snowfall Components
3.5. Potential Sources
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinkler, J.; Hansen, B.U.; Tamstorf, M.P.; Sigsgaard, C.; Petersen, D. Snow and Snow-Cover in Central Northeast Greenland. Adv. Ecol. Res. 2008, 40, 175–195. [Google Scholar]
- Madan, N.J. Snow Ecology: An Interdisciplinary Examination of Snow-covered Ecosystems. J. Ecol. 2001, 89, 1097–1098. [Google Scholar] [CrossRef]
- Lopatina, A.; Medvedeva, S.; Shmakov, S.; Logacheva, M.D.; Krylenkov, V.; Severinov, K. Metagenomic analysis of bacterial communities of antarctic surface snow. Front. Microbiol. 2016, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.; Yamamoto, N. Falling bacterial communities from the atmosphere. Environ. Microbiome 2020, 15, 22. [Google Scholar] [CrossRef]
- Mortazavi, R.; Attiya, S.; Ariya, P.A. Arctic microbial and next-generation sequencing approach for bacteria in snow and frost flowers: Selected identification, abundance and freezing nucleation. Atmos. Chem. Phys. 2015, 15, 6183–6204. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Yang, T.; Su, J.; Qin, Y.; Wang, S.; Gillings, M.; Wang, C.; Ju, F.; Lan, B.; et al. Air pollution could drive global dissemination of antibiotic resistance genes. ISME J. 2020, 15, 270–281. [Google Scholar] [CrossRef]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Honeyman, A.S.; Day, M.L.; Spear, J.R. Regional fresh snowfall microbiology and chemistry are driven by geography in storm-tracked events, Colorado, USA. PeerJ 2018, 6, e5961. [Google Scholar] [CrossRef]
- Segawa, T.; Miyamoto, K.; Ushida, K.; Agata, K.; Okada, N.; Kohshima, S. Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains, Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl. Environ. Microbiol. 2005, 71, 123–130. [Google Scholar] [CrossRef]
- Amoroso, A.; Domine, F.; Esposito, G.; Morin, S.; Savarino, J.; Nardino, M.; Montagnoli, M.; Bonneville, J.-M.; Clement, J.-C.; Ianniello, A.; et al. Microorganisms in Dry Polar Snow Are Involved in the Exchanges of Reactive Nitrogen Species with the Atmosphere. Environ. Sci. Technol. 2009, 44, 714–719. [Google Scholar] [CrossRef]
- Fujii, M.; Takano, Y.; Kojima, H.; Hoshino, T.; Tanaka, R.; Fukui, M. Microbial Community Structure, Pigment Composition, and Nitrogen Source of Red Snow in Antarctica. Microb. Ecol. 2009, 59, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kos, G.; Kanthasami, V.; Adechina, N.; Ariya, P.A. Volatile organic compounds in Arctic snow: Concentrations and implications for atmospheric processes. Environ. Sci. Process. Impacts 2014, 16, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, U. An Electron Microscope Study of Snow Crystal Nuclei. J. Glaciol. 1953, 2, 176–180. [Google Scholar] [CrossRef]
- Cowan, D.A.; Tow, L.A. Endangered Antarctic Environments. Annu. Rev. Microbiol. 2004, 58, 649–690. [Google Scholar] [CrossRef] [PubMed]
- Hanesiak, J.M.; Yackel, J.J.; Barber, D.G. Effect of Melt Ponds on First-Year Sea Ice Ablation—Integration of RADARSAT-1 and Thermodynamic Modelling. Can. J. Remote Sens. 2014, 27, 433–442. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; Luo, X.; Zhao, Z.; Li, X. Seasonal Disparities in Airborne Bacteria and Associated Antibiotic Resistance Genes in PM2.5 between Urban and Rural Sites. Environ. Sci. Technol. Lett. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Odabasi, M.; Dumanoglu, Y.; Ozgunerge Falay, E.; Tuna, G.; Altiok, H.; Kara, M.; Bayram, A.; Tolunay, D.; Elbir, T. Investigation of spatial distributions and sources of persistent organic pollutants (POPs) in a heavily polluted industrial region using tree components. Chemosphere 2016, 160, 114–125. [Google Scholar] [CrossRef]
- Saleeby, S.M.; Cotton, W.R.; Lowenthal, D.; Borys, R.D.; Wetzel, M.A. Influence of cloud condensation nuclei on orographic snowfall. J. Appl. Meteorol. Climatol. 2009, 48, 903–922. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, S.; Wu, Y.; Qin, D. Bacteria in Himalayan glacial ice and its relationship to dust. Biogeosciences 2008, 5, 1741–1750. [Google Scholar] [CrossRef]
- Möhler, O.; Georgakopoulos, D.G.; Morris, C.E.; Benz, S.; Ebert, V.; Hunsmann, S.; Saathoff, H.; Schnaiter, M.; Wagner, R. Heterogeneous ice nucleation activity of bacteria: New laboratory experiments at simulated cloud conditions. Biogeosciences 2008, 5, 1425–1435. [Google Scholar] [CrossRef]
- DeMott, P.J.; Cziczo, D.J.; Prenni, A.J.; Murphy, D.M.; Kreidenweis, S.M.; Thomson, D.S.; Borys, R.; Rogers, D.C. Measurements of the concentration and composition of nuclei for cirrus formation. Proc. Natl. Acad. Sci. USA 2003, 100, 14655–14660. [Google Scholar] [CrossRef]
- Obbard, R.W.; Roscoe, H.K.; Wolff, E.W.; Atkinson, H.M. Frost flower surface area and chemistry as a function of salinity and temperature. J. Geophys. Res. Atmos. 2009, 114, 20305. [Google Scholar] [CrossRef]
- Kaleschke, L.; Richter, A.; Burrows, J.; Afe, O.; Heygster, G.; Notholt, J.; Rankin, A.M.; Roscoe, H.K.; Hollwedel, J.; Wagner, T.; et al. Frost flowers on sea ice as a source of sea salt and their influence on tropospheric halogen chemistry. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef]
- Myers, E.W.; Miller, W. Optimal alignments in linear space. Bioinformatics 1988, 4, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.J.; Eddy, S.R. nhmmer: DNA homology search with profile HMMs. Bioinformatics 2013, 29, 2487–2489. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Jung, J.; Kim, Y.J. Tracking sources of severe haze episodes and their physicochemical and hygroscopic properties under Asian continental outflow: Long-range transport pollution, postharvest biomass burning, and Asian dust. J. Geophys. Res. Atmos. 2011, 116, 2206. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.; Lee, J.; Lee, S. Seasonal Characteristics of Organic Carbon and Elemental Carbon in PM2.5 in Daejeon. J. Korean Soc. Atmos. Environ. 2015, 31, 28–40. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.; Ma, L.; He, C.; Jiang, H.; Sheng, L.; Luo, W. Snow depths’ impact on soil microbial activities and carbon dioxide fluxes from a temperate wetland in Northeast China. Sci. Rep. 2020, 10, 8709. [Google Scholar] [CrossRef] [PubMed]
- Monaco, P.; Divino, F.; Naclerio, G.; Bucci, A. Microbial community analysis with a specific statistical approach after a record breaking snowfall in Southern Italy. Ann. Microbiol. 2020, 70, 63. [Google Scholar] [CrossRef]
- Els, N.; Greilinger, M.; Reisecker, M.; Tignat-Perrier, R.; Baumann-Stanzer, K.; Kasper-Giebl, A.; Sattler, B.; Larose, C. Comparison of Bacterial and Fungal Composition and Their Chemical Interaction in Free Tropospheric Air and Snow Over an Entire Winter Season at Mount Sonnblick, Austria. Front. Microbiol. 2020, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; Pinto, O.H.B.; Šantl-Temkiv, T.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, P.E.A.S. DNA metabarcoding of fungal diversity in air and snow of Livingston Island, South Shetland Islands, Antarctica. Sci. Rep. 2020, 10, 21793. [Google Scholar] [CrossRef]
- Abd Aziz, A.; Lee, K.; Park, B.; Park, H.; Park, K.; Choi, I.G.; Chang, I.S. Comparative study of the airborne microbial communities and their functional composition in fine particulate matter (PM2.5) under non-extreme and extreme PM2.5 conditions. Atmos. Environ. 2018, 194, 82–92. [Google Scholar] [CrossRef]
- Boreson, J.; Dillner, A.M.; Peccia, J. Correlating bioaerosol load with PM2.5 and PM10cf concentrations: A comparison between natural desert and urban-fringe aerosols. Atmos. Environ. 2004, 38, 6029–6041. [Google Scholar] [CrossRef]
- Toom-Sauntry, D.; Barrie, L.A. Chemical composition of snowfall in the high Arctic: 1990–1994. Atmos. Environ. 2002, 36, 2683–2693. [Google Scholar] [CrossRef]
- Fibiger, D.L.; Dibb, J.E.; Chen, D.; Thomas, J.L.; Burkhart, J.F.; Huey, L.G.; Hastings, M.G. Analysis of nitrate in the snow and atmosphere at Summit, Greenland: Chemistry and transport. J. Geophys. Res. Atmos. 2016, 121, 5010–5030. [Google Scholar] [CrossRef]
- Legrand, M.R.; Delmas, R.J. Relative contributions of tropospheric and stratospheric sources to nitrate in Antarctic snow. Tellus B Chem. Phys. Meteorol. 1986, 388, 236249. [Google Scholar] [CrossRef]
- Møller, A.K.; Barkay, T.; Al-Soud, W.A.; Sørensen, S.J.; Skov, H.; Kroer, N. Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the High Arctic. FEMS Microbiol. Ecol. 2011, 75, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. Noaa’s hysplit atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Lai, S.C.; Zou, S.C.; Cao, J.J.; Lee, S.C.; Ho, K.F. Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, South China. J. Environ. Sci. 2007, 19, 939–947. [Google Scholar] [CrossRef]
- Glavas, S.D.; Nikolakis, P.; Ambatzoglou, D.; Mihalopoulos, N. Factors affecting the seasonal variation of mass and ionic composition of PM2.5 at a central Mediterranean coastal site. Atmos. Environ. 2008, 42, 5365–5373. [Google Scholar] [CrossRef]
- Tyagi, P.; Kawamura, K.; Bikkina, S.; Mochizuki, T.; Aoki, K. Hydroxy fatty acids in snow pit samples from Mount Tateyama in central Japan: Implications for atmospheric transport of microorganisms and plant waxes associated with Asian dust. J. Geophys. Res. Atmos. 2016, 121, 13641–13660. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Zhang, R.; Wu, Y.; Zhang, Z.; Zhang, X.; Tang, Y.; Cao, J.; Zhang, Y. Uncertainty assessment of source attribution of PM2.5 and its water-soluble organic carbon content using different biomass burning tracers in positive matrix factorization analysis—A case study in Beijing, China. Sci. Total Environ. 2016, 543, 326–335. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Xie, Z.; Du, S.; Zeng, X.; Hou, J.; Ma, T. Characteristics of microbial activity in atmospheric aerosols and its relationship to chemical composition of PM2.5 in Xi’an, China. J. Aerosol Sci. 2020, 146, 105572. [Google Scholar] [CrossRef]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Zhang, Y.; Fu, P. Variations of bacteria and fungi in PM2.5 in Beijing, China. Atmos. Environ. 2018, 172, 55–64. [Google Scholar] [CrossRef]
- Rousk, K.; Jones, D.L.; DeLuca, T.H. Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front. Microbiol. 2013, 4, 150. [Google Scholar] [CrossRef]
- Murillo, A.A.; Ramírez-Flandes, S.; DeLong, E.F.; Ulloa, O. Enhanced metabolic versatility of planktonic sulfur-oxidizing γ-proteobacteria in an oxygen-deficient coastal ecosystem. Front. Mar. Sci. 2014, 1, 18. [Google Scholar] [CrossRef]
- Harding, T.; Jungblut, A.D.; Lovejoy, C.; Vincent, W.F. Microbes in High Arctic Snow and Implications for the Cold Biosphere. Appl. Environ. Microbiol. 2011, 77, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; Stingl, U. Molecular diversity and ecology of microbial plankton. Nature 2005, 437, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Giudice, A.L.; Mysara, M.; Monsieurs, P.; Raffa, C.; Leys, N.; Amalfitano, S.; Van Houdt, R. Snow Surface Microbiome on the High Antarctic Plateau (DOME C). PLoS ONE 2014, 9, e104505. [Google Scholar] [CrossRef] [PubMed]
- Aller, J.Y.; Kuznetsova, M.R.; Jahns, C.J.; Kemp, P.F. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 2005, 36, 801–812. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kawamura, K.; Aoki, K. Water-Soluble Organic Nitrogen in High Mountain Snow Samples from Central Japan. Aerosol Air Qual. Res. 2016, 16, 632–639. [Google Scholar] [CrossRef]

| Starting Date | End Date | Time (Start) | Time (End) | Sample Code |
|---|---|---|---|---|
| 1 January 2021 | 2 January 2021 | 6:00 p.m. | 9:00 a.m. | DJS-1 |
| 1 January 2021 | 2 January 2021 | 6:00 p.m. | 9:00 a.m. | DJS-2 |
| 6 January 2021 | 7 January 2021 | 6:00 p.m. | 9:00 a.m. | DJS-3 |
| 18 January 2021 | 18 January 2021 | 9:00 a.m. | 12:00 p.m. | DJS-4 |
| 28 January 2021 | 28 January 2021 | 11:30 a.m. | 3:30 a.m. | DJS-5 * |
| Name | Sequence |
|---|---|
| 16S Forward | (5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG 3′) |
| 16S Reverse | (5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-GACTACHVGGGTATCTAATCC 3’) |
| ITS forward | (5′ AATGATACGGCGACCACCGAGATCTACAC-GCATCGATGAAGAACGCAGC 3′) |
| ITS Reverse | (5′ CAAGCAGAAGACGGCATACGAGAT-TCCGCTTATTGATATGC 3′) |
| Snowfall Components | Factor-1 | Factor-2 | Factor-3 | Factor-4 |
|---|---|---|---|---|
| SO42− | 0.85 | 0.40 | 0.01 | 0.34 |
| NO3− | −0.23 | −0.75 | −0.50 | −0.36 |
| NH4+ | 0.98 | 0.17 | 0.10 | 0.00 |
| Na+ | 0.41 | 0.91 | 0.10 | −0.06 |
| Cl− | 0.31 | 0.94 | 0.04 | −0.12 |
| Mg2+ | 0.37 | 0.92 | 0.06 | 0.05 |
| Ca2+ | 0.92 | 0.30 | 0.12 | 0.21 |
| K+ | 0.76 | 0.65 | 0.09 | 0.06 |
| WSOC | 0.98 | 0.17 | 0.09 | 0.03 |
| WSTN | 0.93 | −0.18 | −0.30 | 0.10 |
| Acidobacteria | −0.65 | 0.72 | 0.22 | 0.02 |
| Actinobacteria | 0.80 | 0.43 | 0.03 | 0.41 |
| Bacteroidetes | −0.14 | -0.34 | −0.16 | 0.91 |
| Cyanobacteria | 0.73 | 0.66 | 0.20 | 0.02 |
| Firmicutes | −0.41 | -0.23 | −0.03 | −0.88 |
| Gemmatimonadetes | 0.95 | 0.28 | 0.05 | −0.10 |
| Planctomycetes | −0.30 | −0.32 | 0.82 | −0.37 |
| Proteobacteria | 0.46 | 0.72 | 0.27 | 0.44 |
| Ascomycota | 0.06 | 0.53 | 0.84 | 0.10 |
| Basidiomycota | −0.15 | −0.53 | −0.83 | −0.09 |
| Eukarya_uc | −0.05 | −0.30 | −0.73 | −0.62 |
| Fungi_p | 0.94 | 0.31 | 0.16 | −0.01 |
| Mucoromycota | 0.12 | 0.30 | 0.94 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Z.U.; Nirmalkar, J.; Park, D.; Jung, J.; Kim, S. Uncovering the Fresh Snowfall Microbiome and Its Chemical Characteristics with Backward Trajectories in Daejeon, the Republic of Korea. Atmosphere 2022, 13, 1590. https://doi.org/10.3390/atmos13101590
Hassan ZU, Nirmalkar J, Park D, Jung J, Kim S. Uncovering the Fresh Snowfall Microbiome and Its Chemical Characteristics with Backward Trajectories in Daejeon, the Republic of Korea. Atmosphere. 2022; 13(10):1590. https://doi.org/10.3390/atmos13101590
Chicago/Turabian StyleHassan, Zohaib Ul, Jayant Nirmalkar, Dongju Park, Jinsang Jung, and Seil Kim. 2022. "Uncovering the Fresh Snowfall Microbiome and Its Chemical Characteristics with Backward Trajectories in Daejeon, the Republic of Korea" Atmosphere 13, no. 10: 1590. https://doi.org/10.3390/atmos13101590
APA StyleHassan, Z. U., Nirmalkar, J., Park, D., Jung, J., & Kim, S. (2022). Uncovering the Fresh Snowfall Microbiome and Its Chemical Characteristics with Backward Trajectories in Daejeon, the Republic of Korea. Atmosphere, 13(10), 1590. https://doi.org/10.3390/atmos13101590







